Advances in the Synthesis of Heteroaromatic Hybrid Chalcones
Abstract
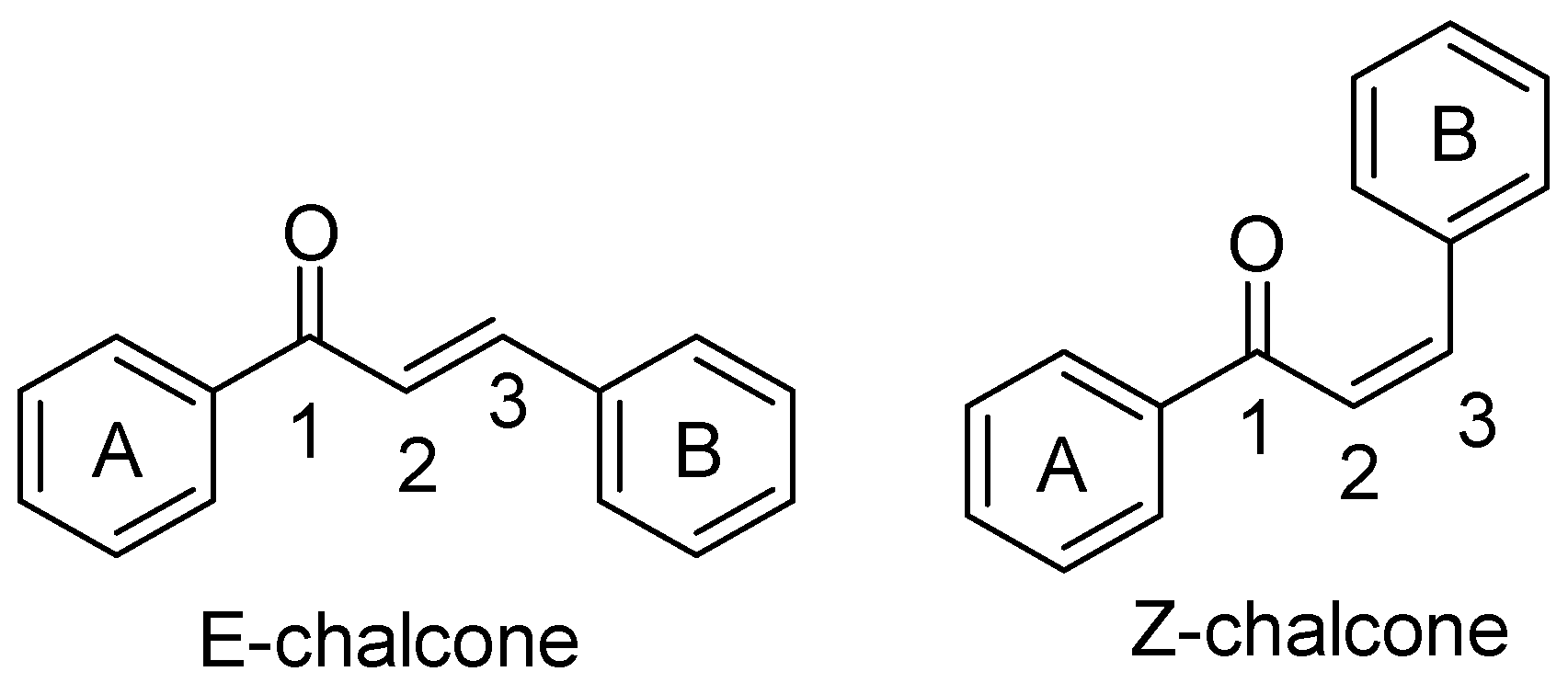
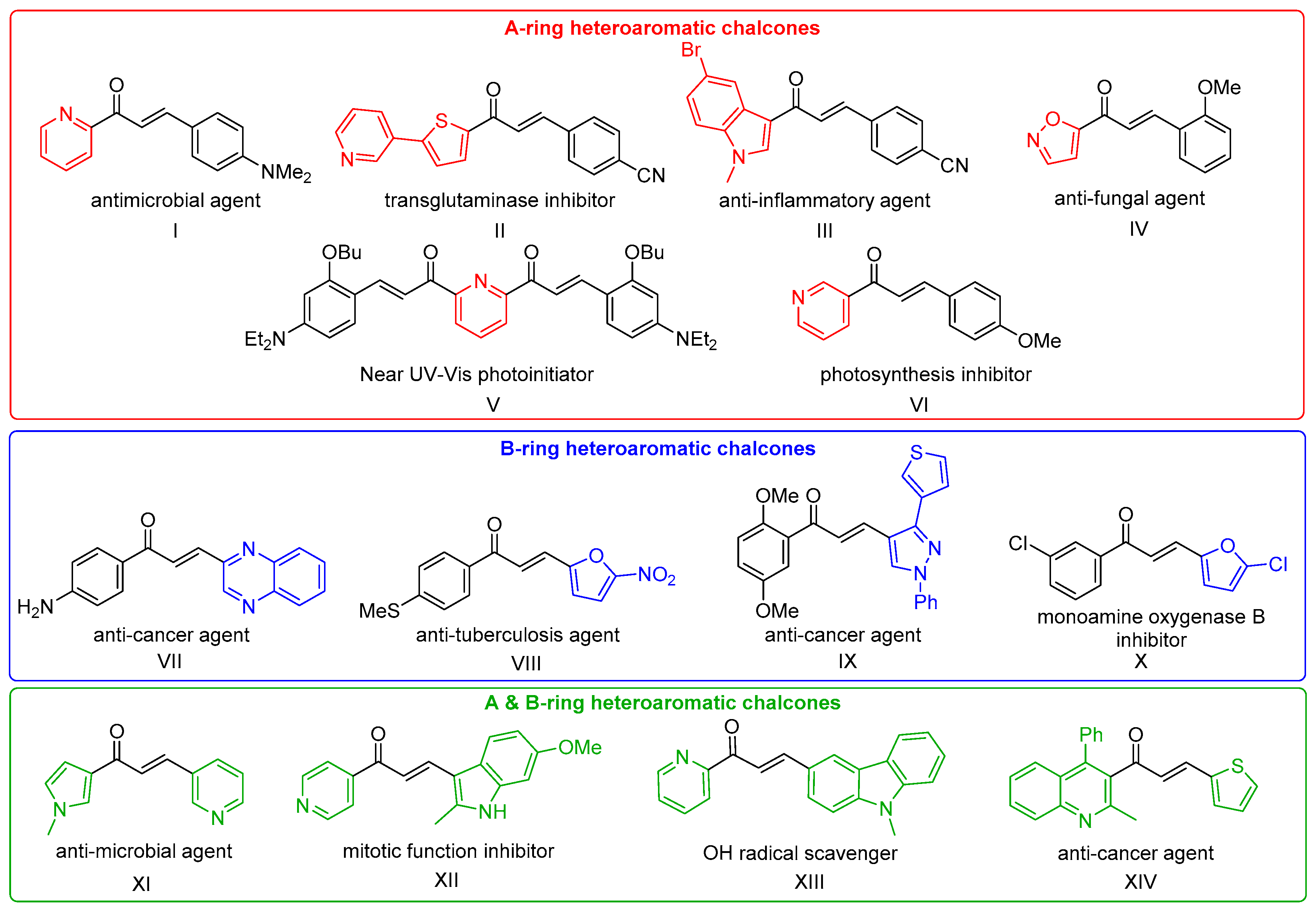
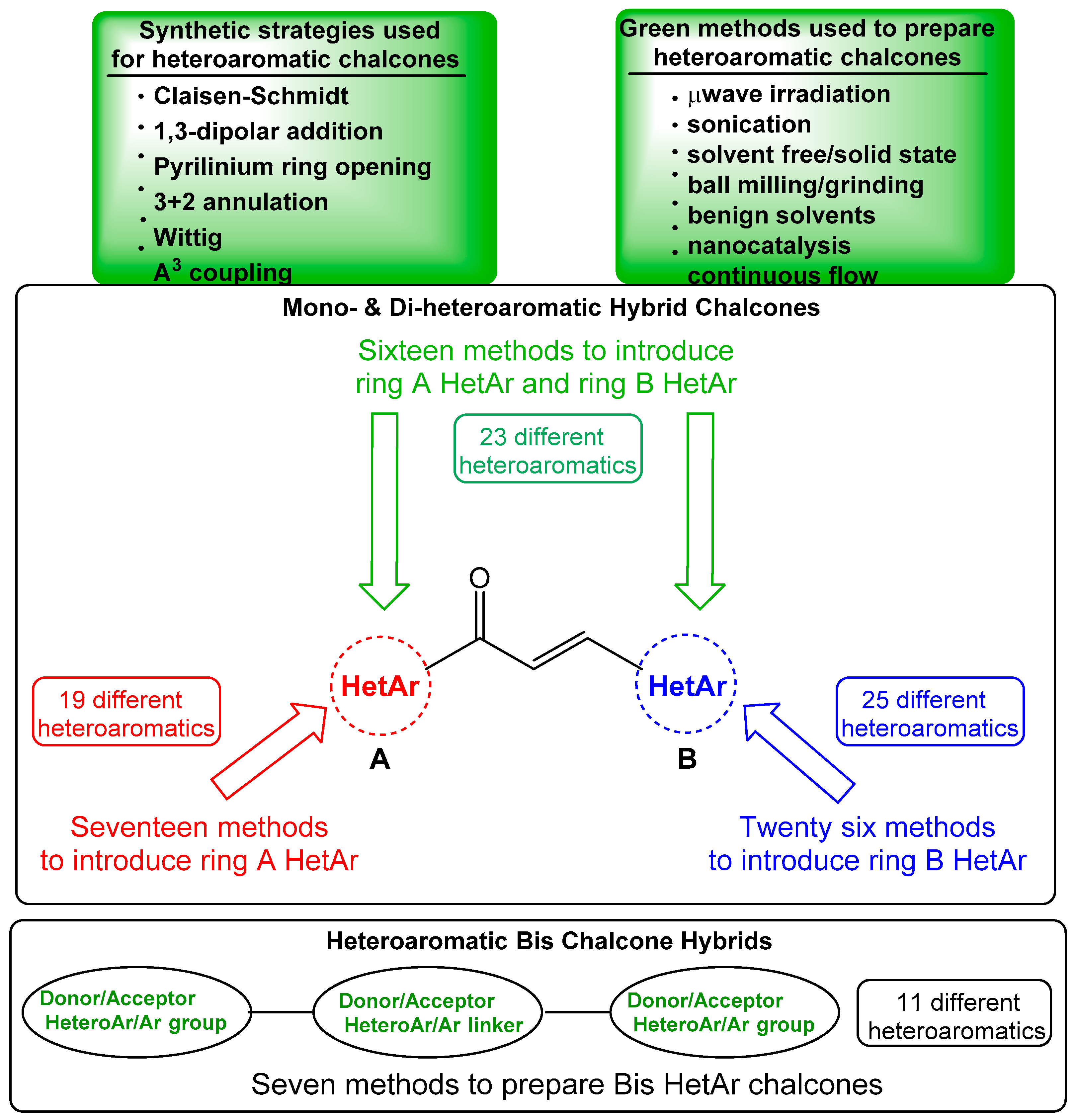
1. Introduction
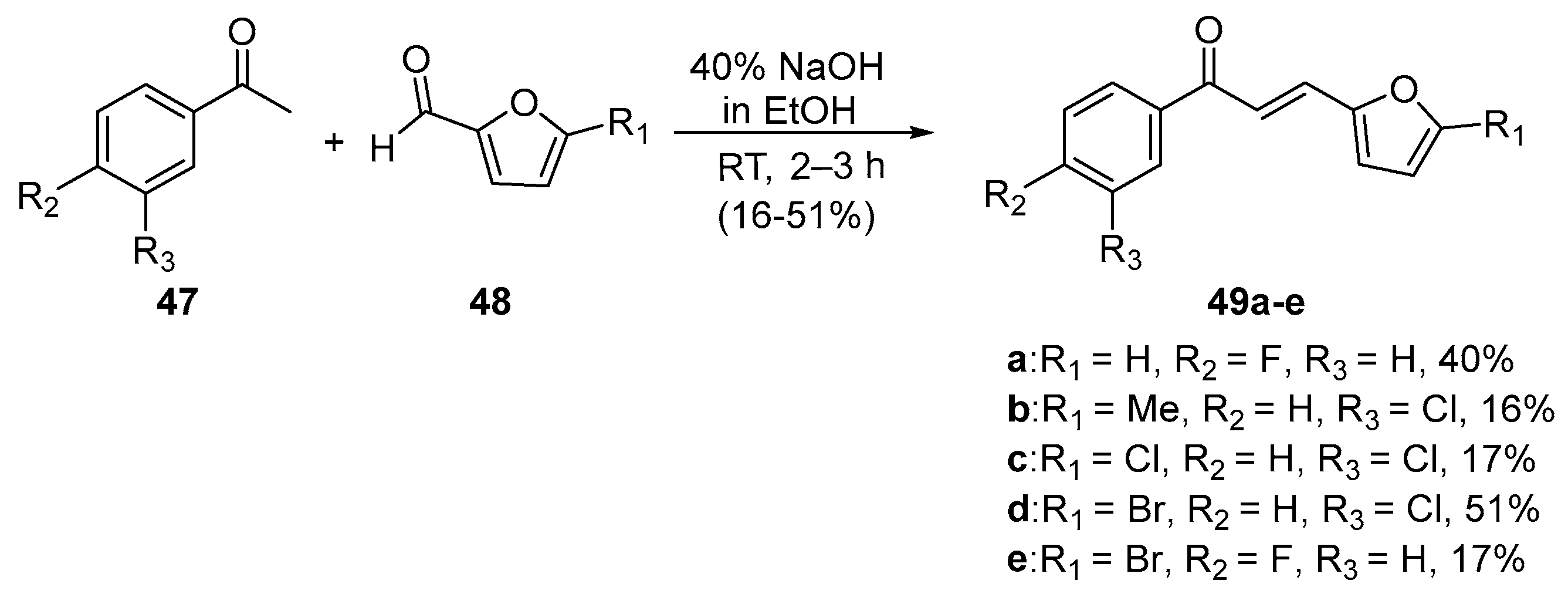
2. A-Ring Heteroaromatic Hybrid Chalcone Synthesis
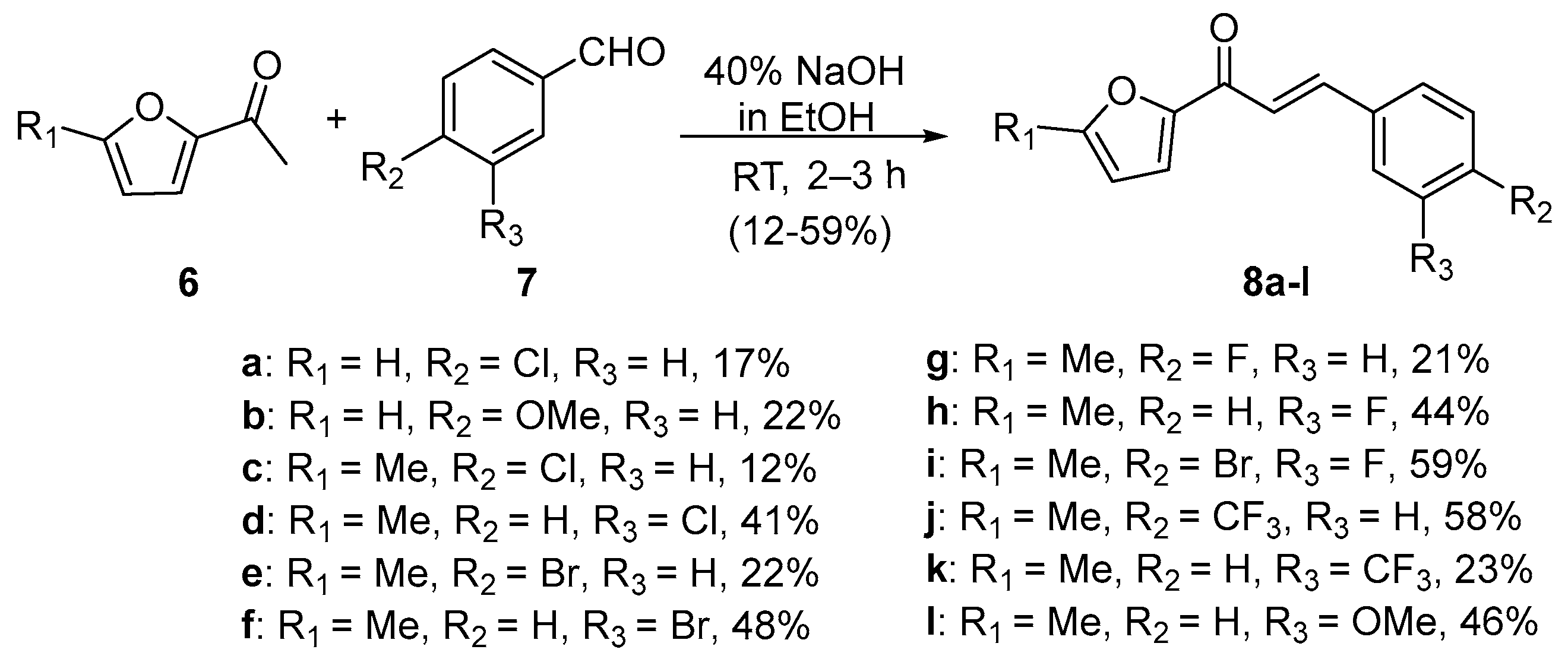
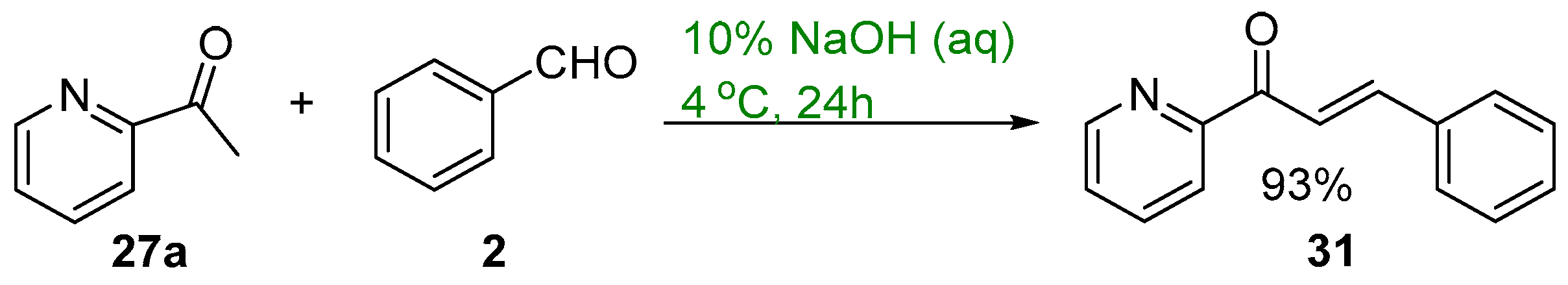
2.1. Claisen–Schmidt Condensations
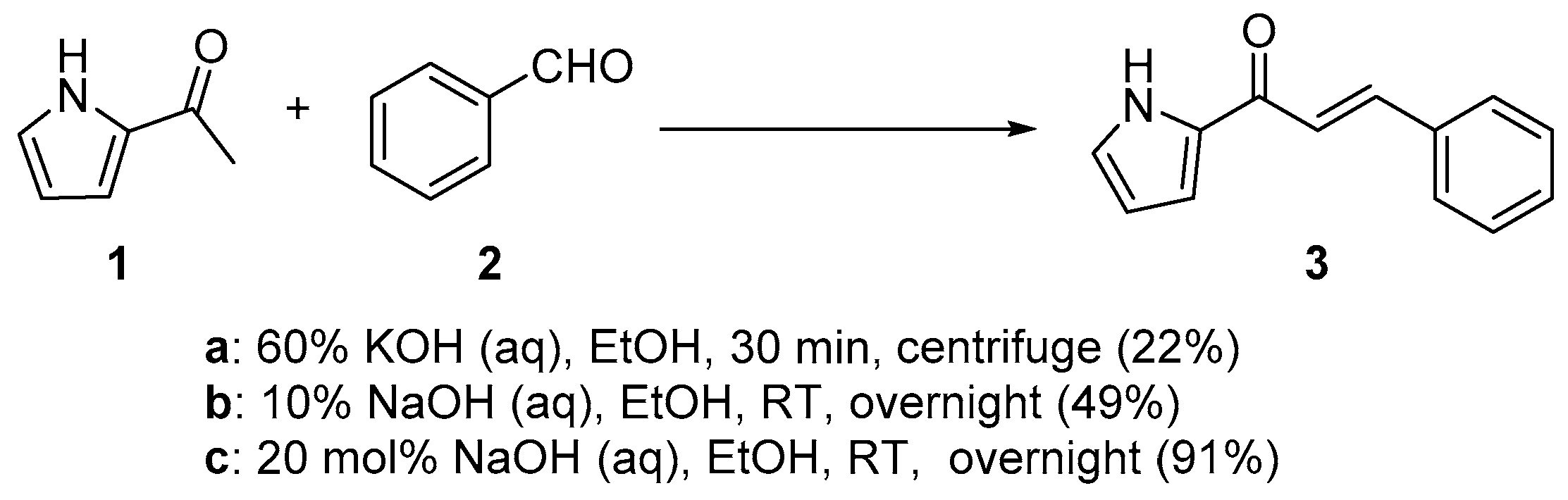
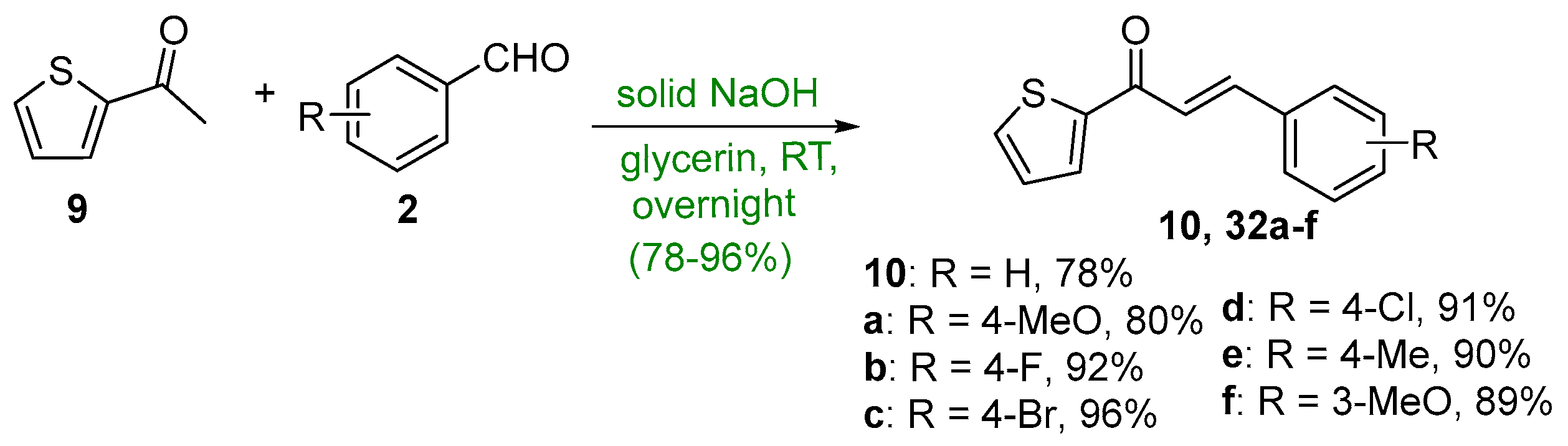
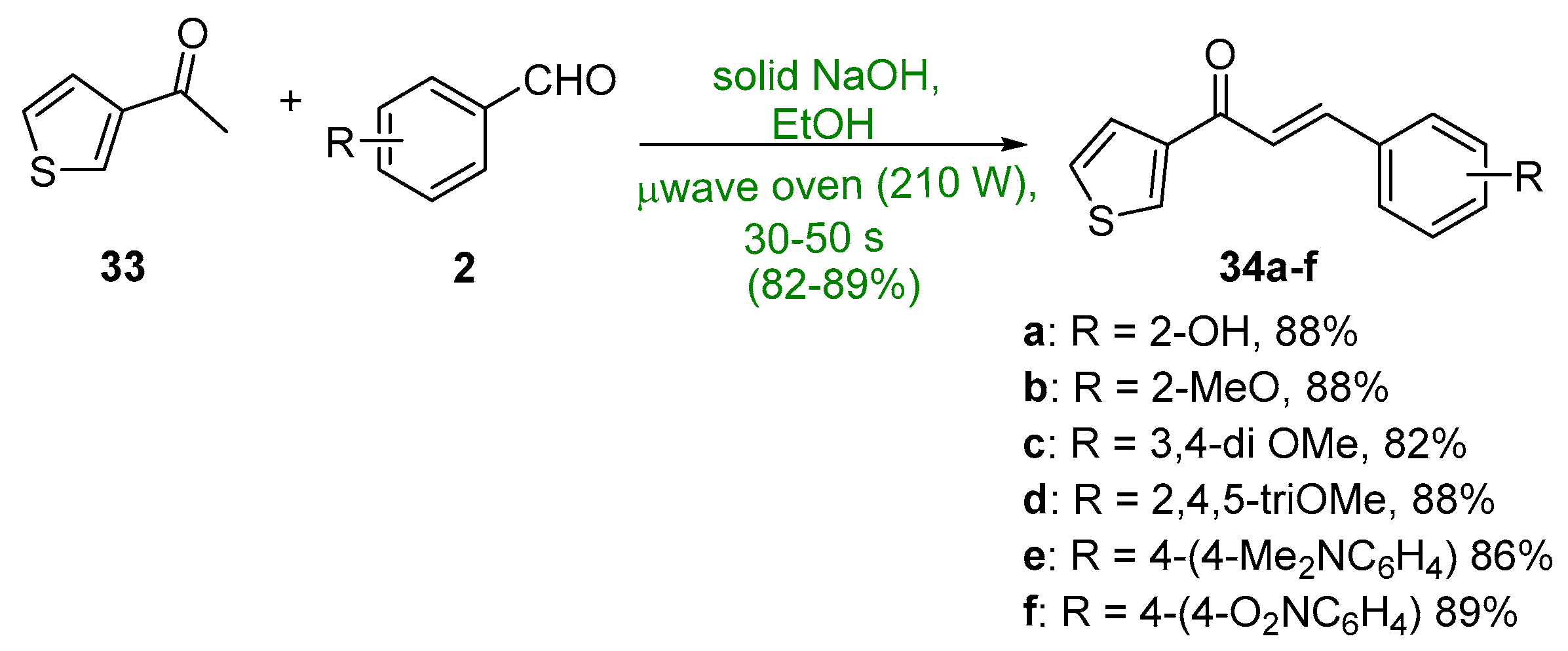
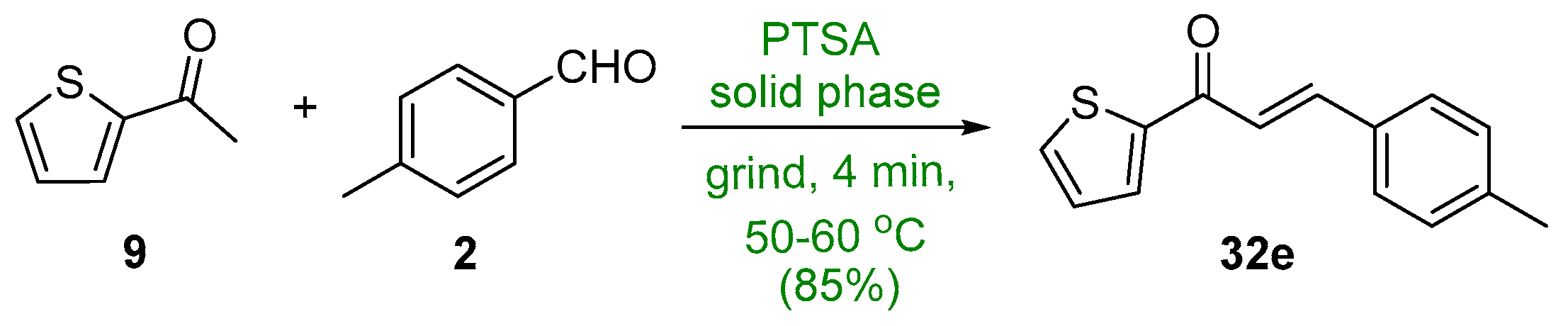
2.1.1. Base-Catalyzed C-S Condensations
2.1.2. Acid-Catalyzed C-S Condensations
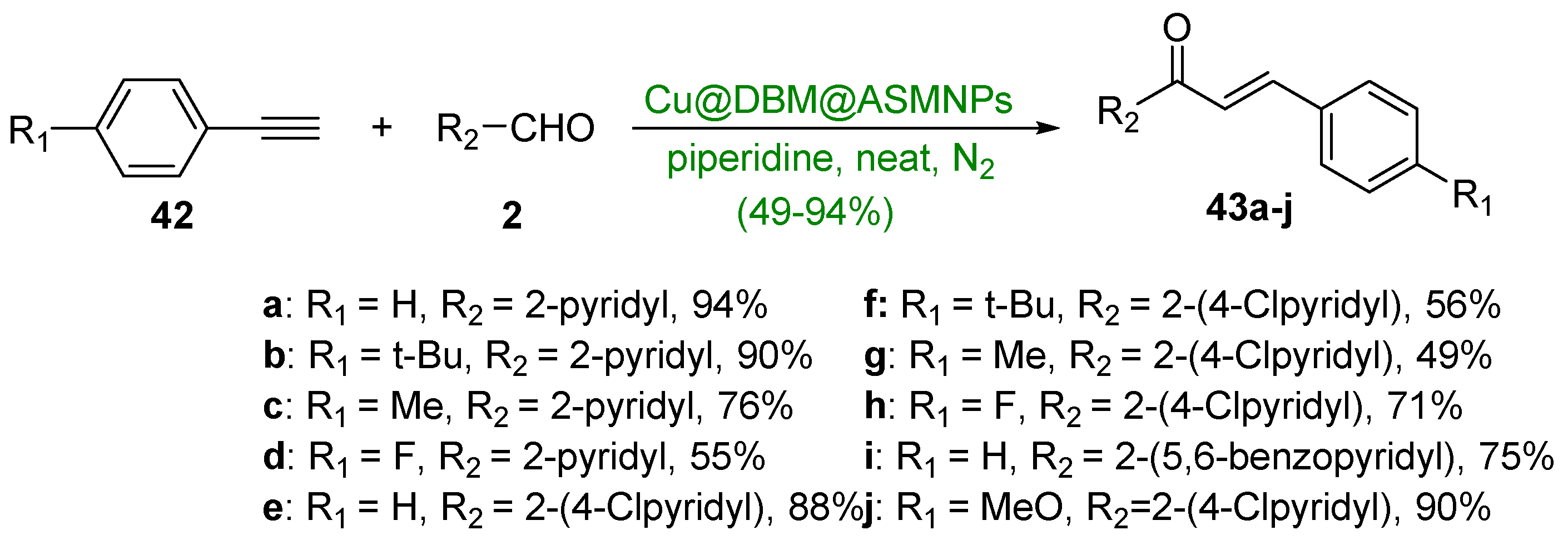
2.2. Non C-S Condensations
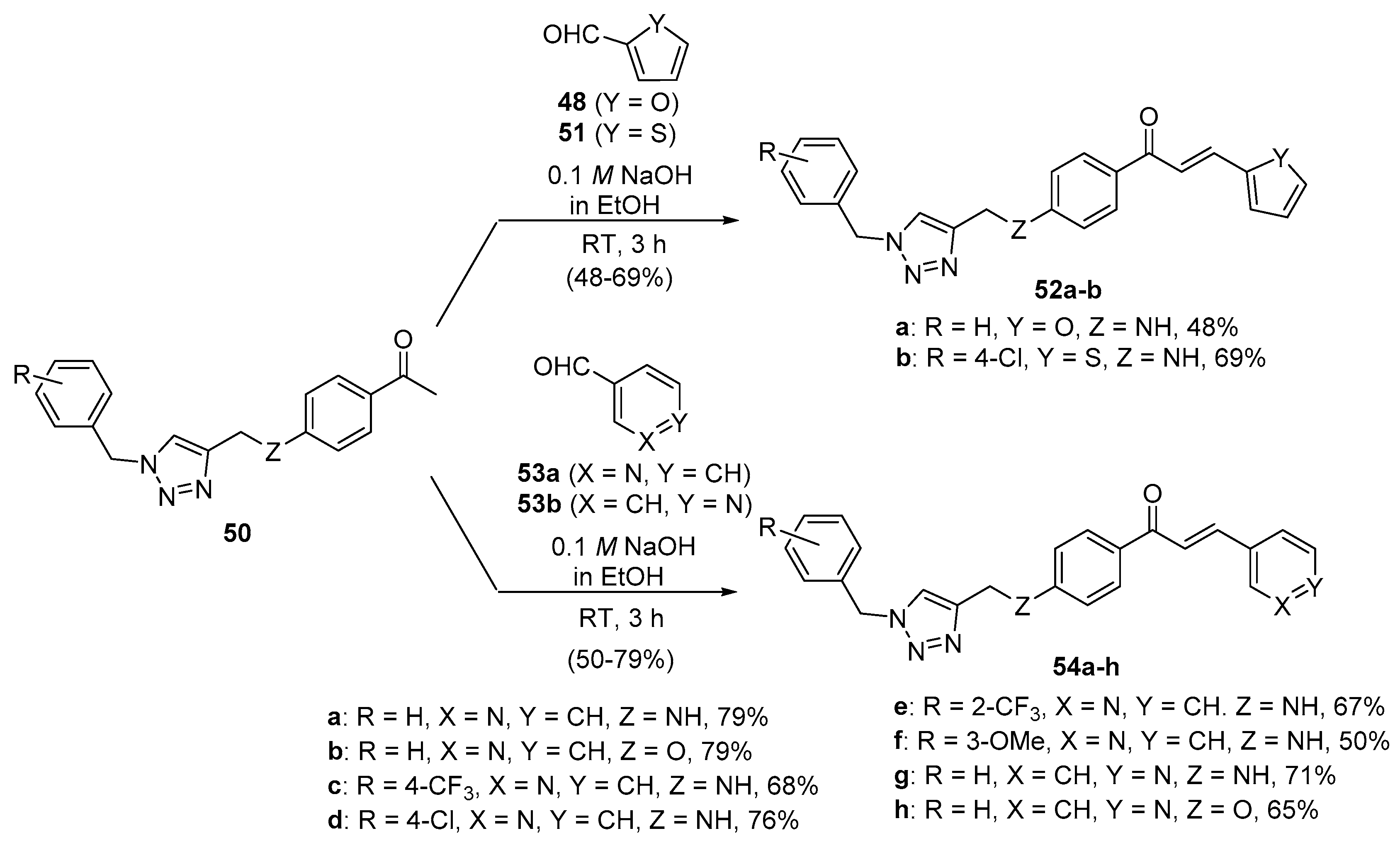
3. B-Ring Heteroaromatic Hybrid Chalcone Synthesis
3.1. Claisen–Schmidt Condensations
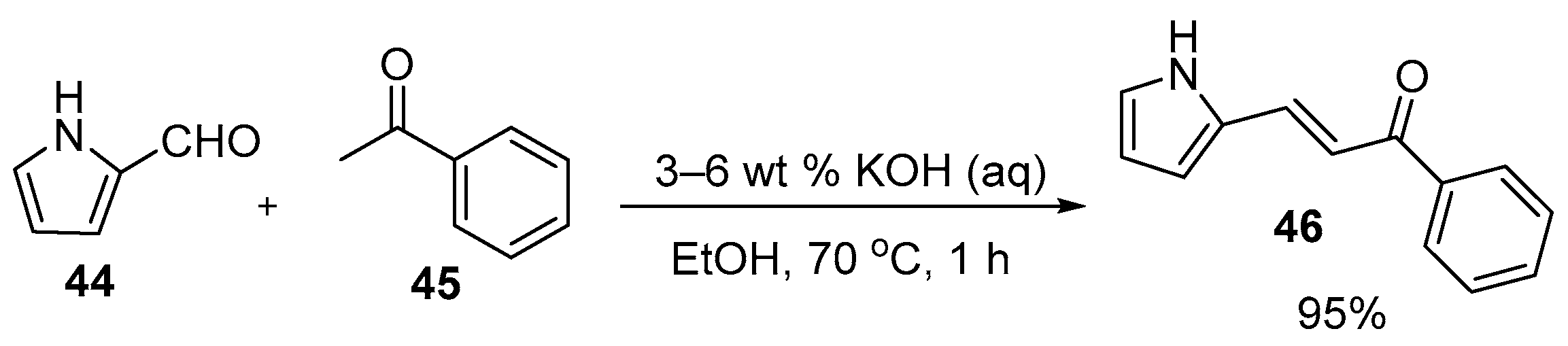
Base-Catalyzed C-S Condensations
3.2. Non C-S Condensations
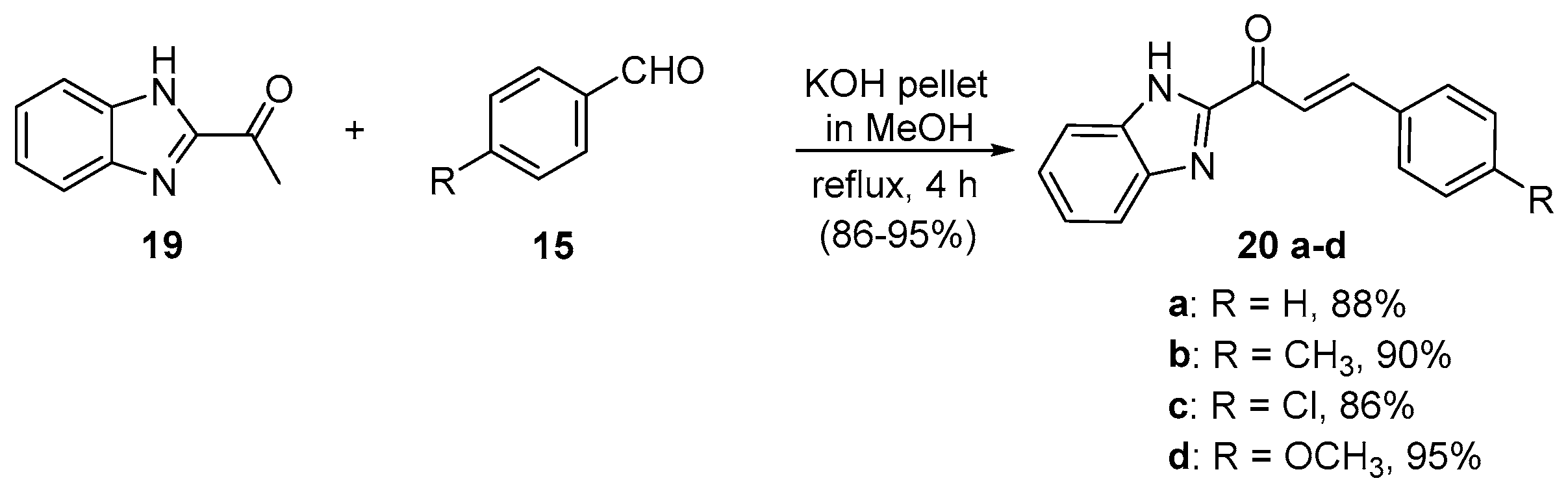
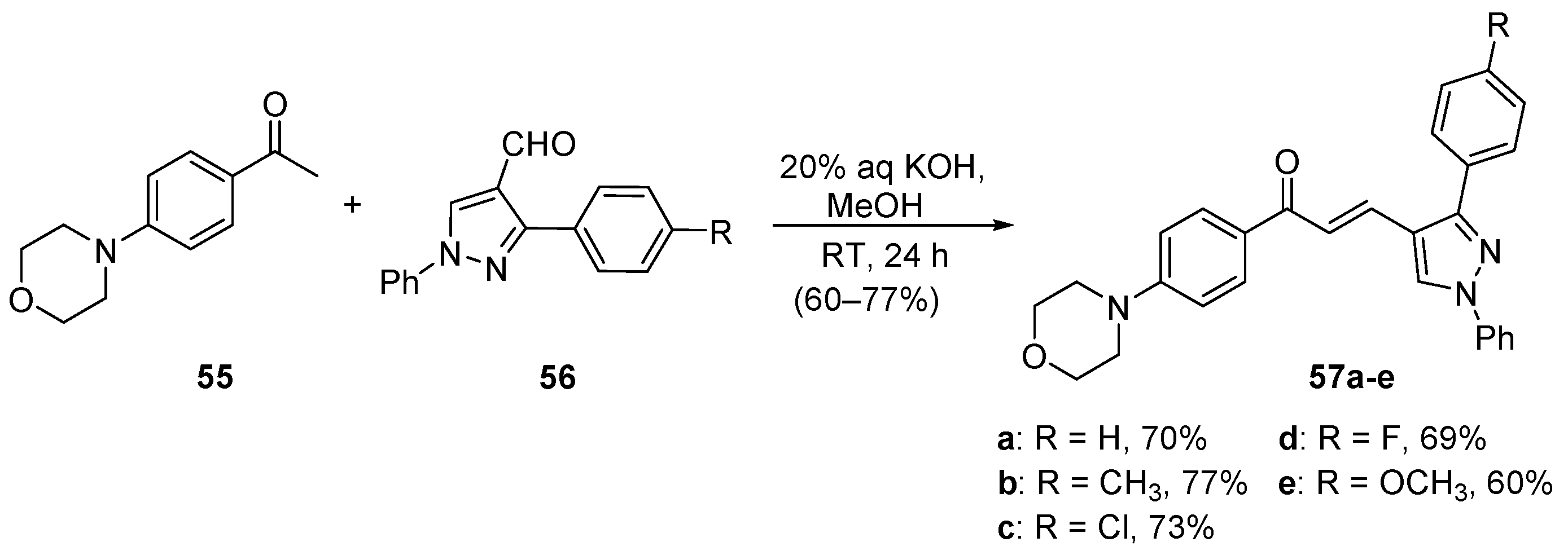
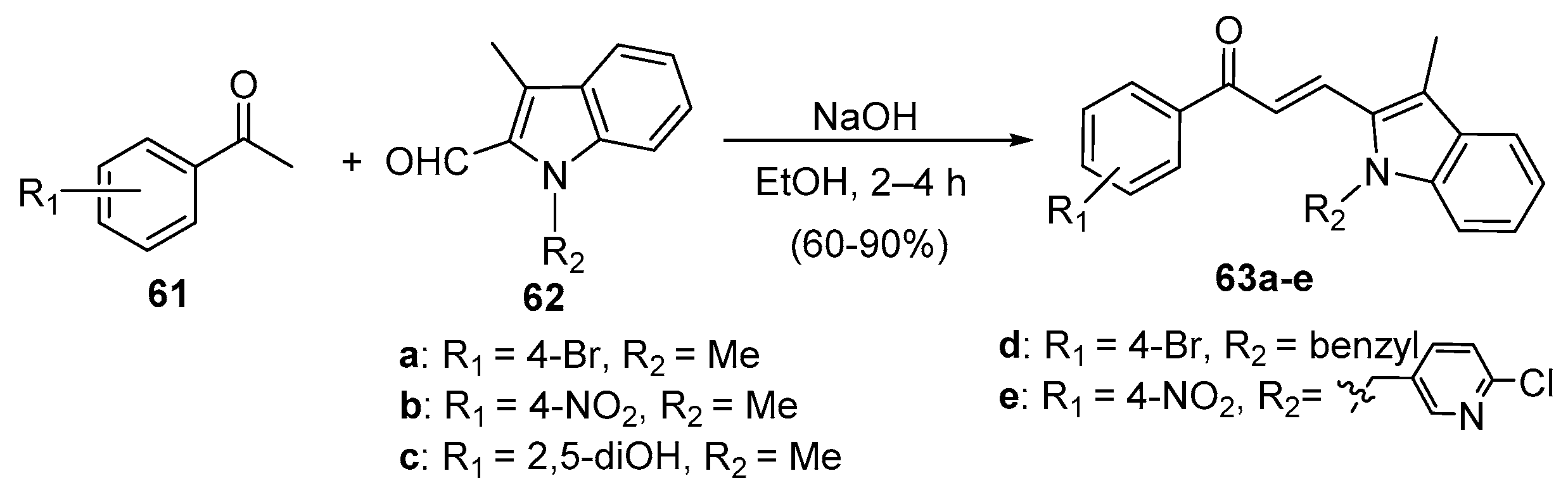
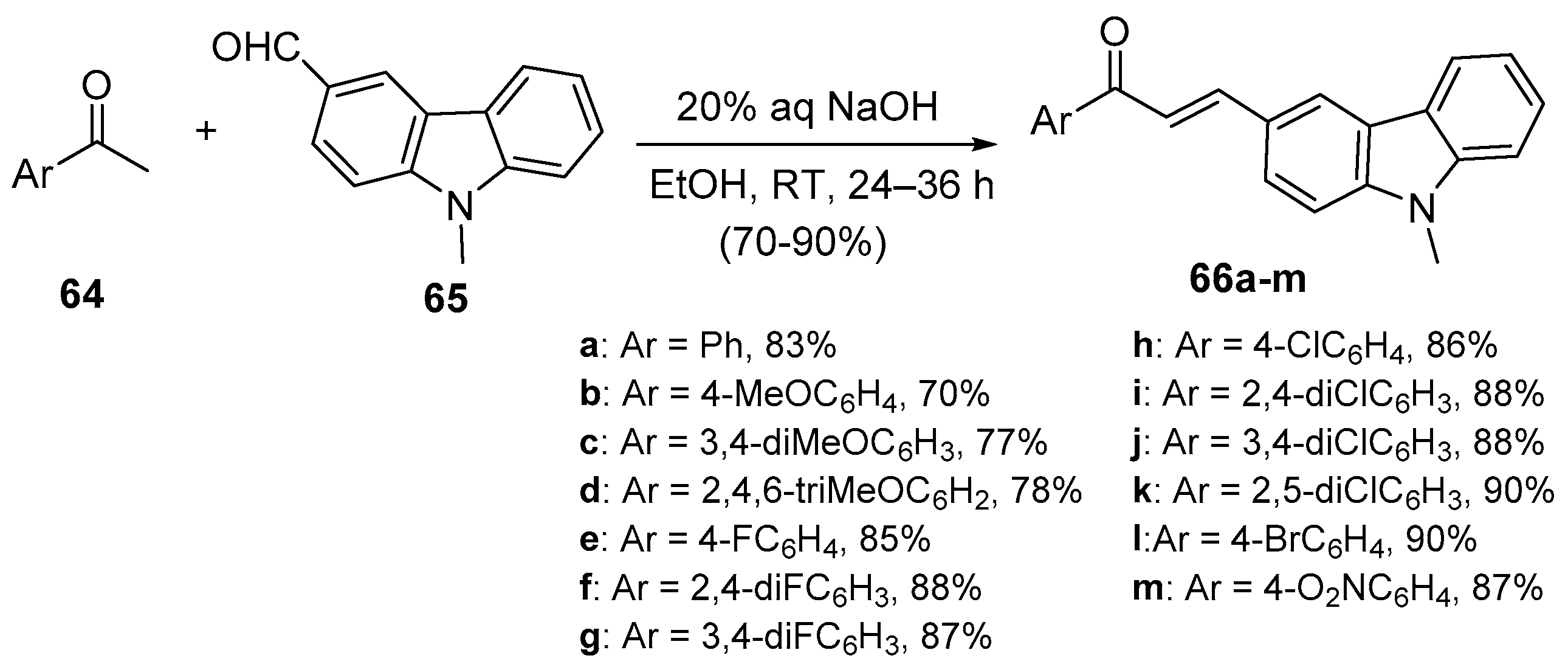
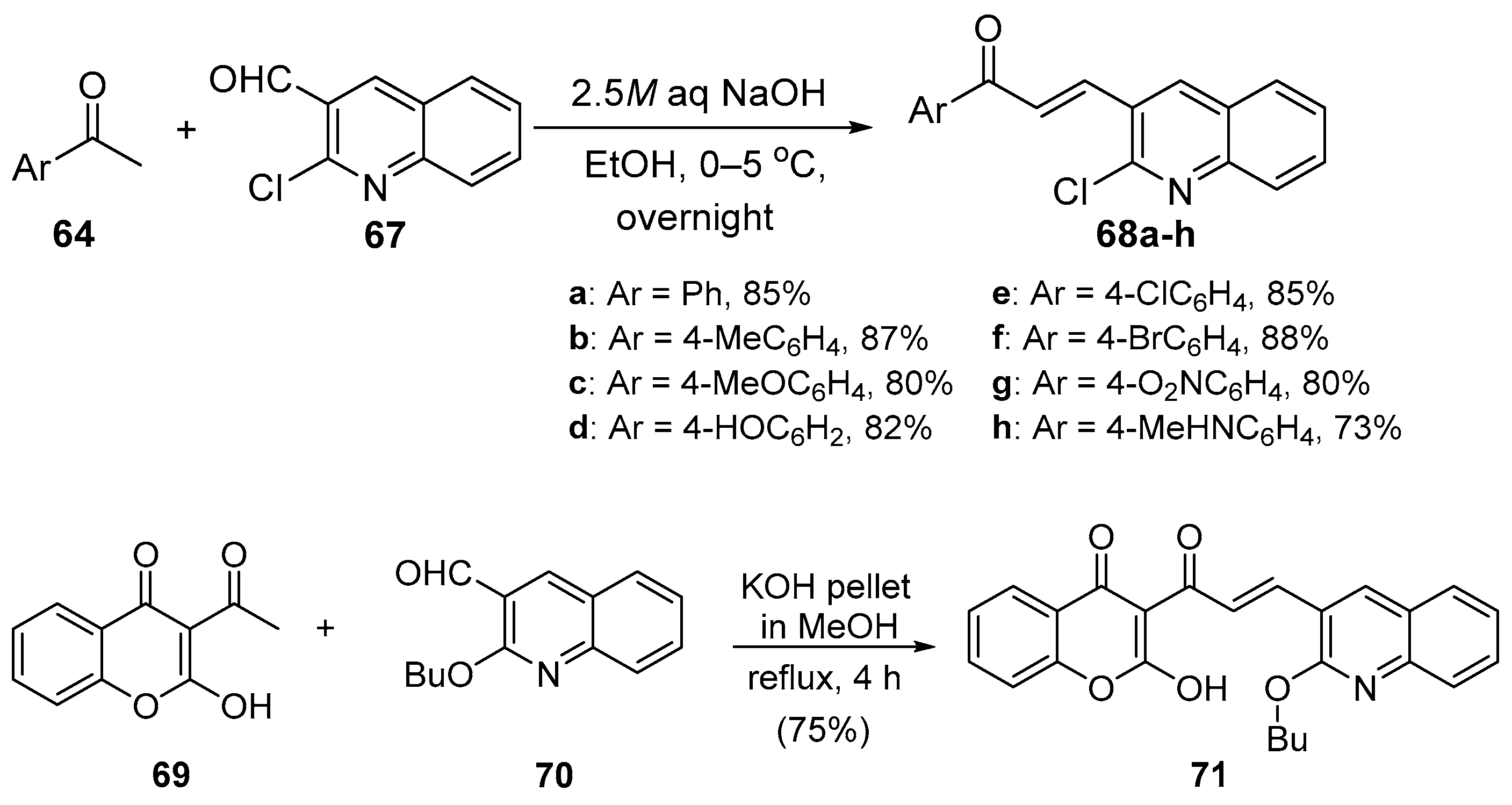
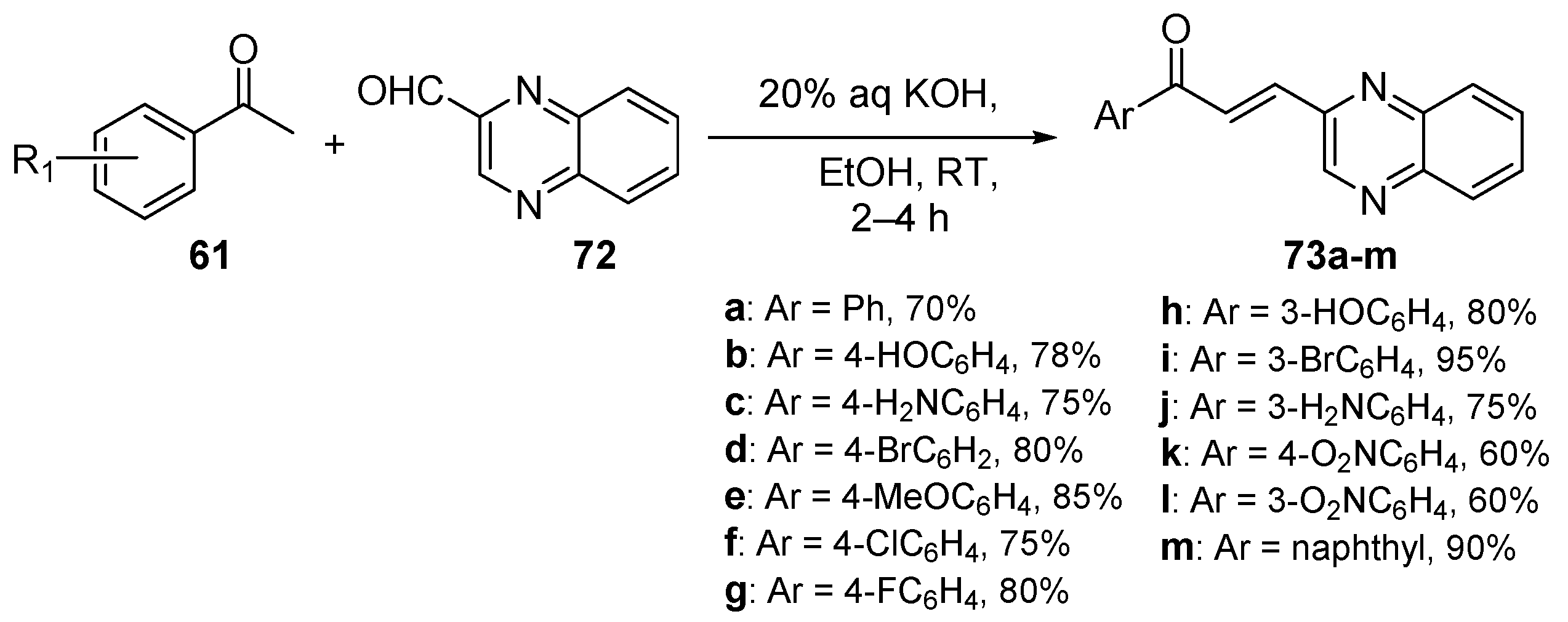
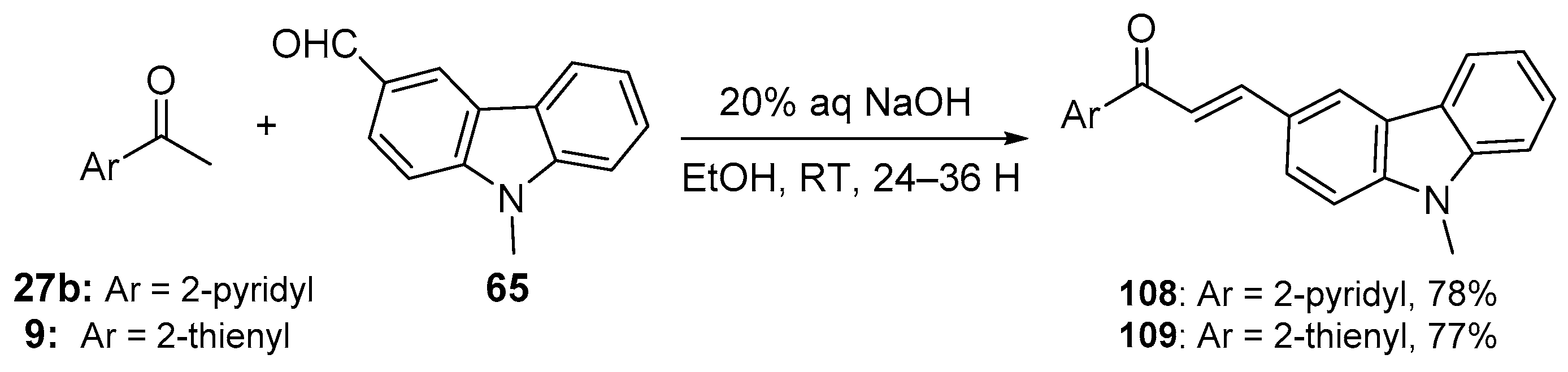
4. A–B Ring Dual Heteroaromatic Hybrid Chalcone Synthesis
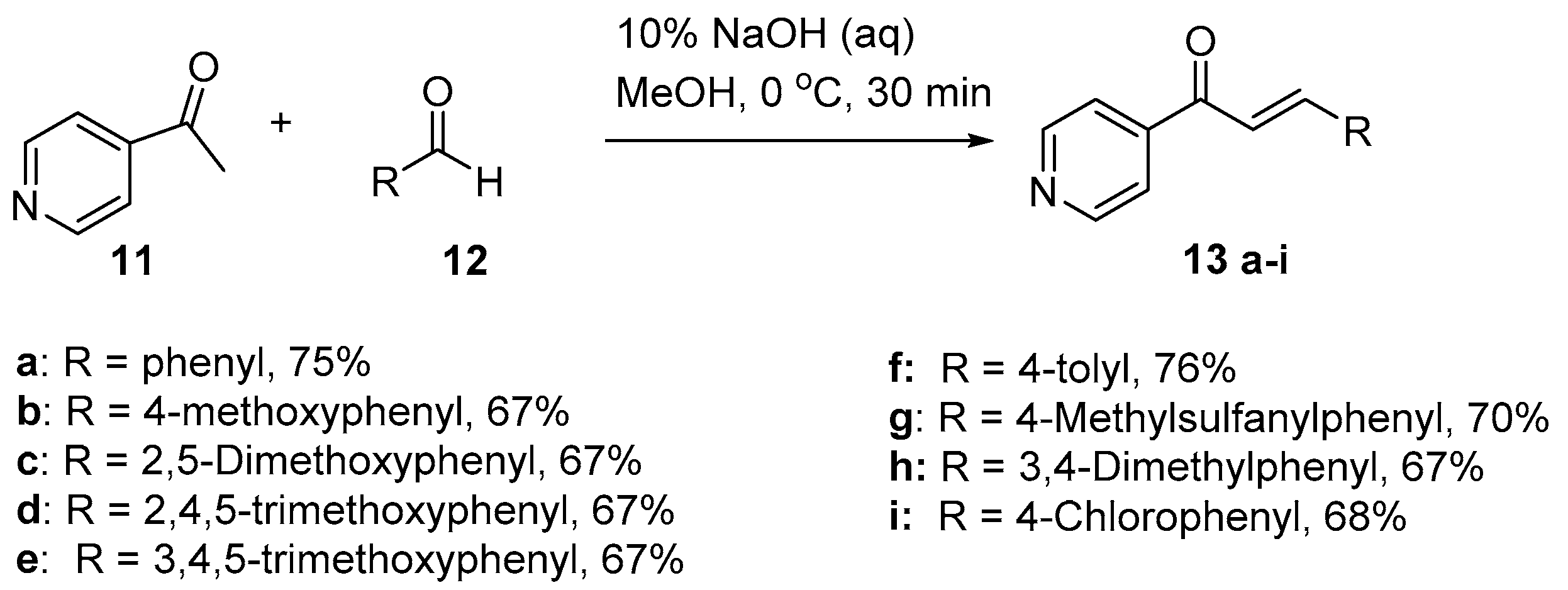
4.1. Claisen–Schmidt Condensations
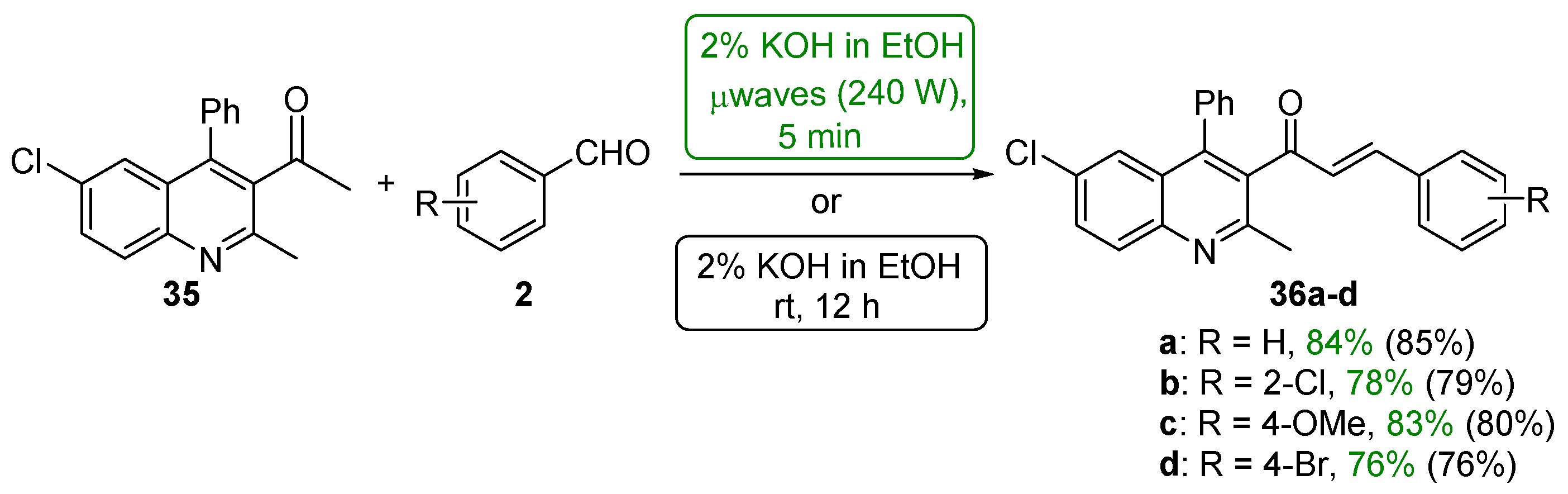
4.1.1. Base-Catalyzed C-S Condensations
4.1.2. Green C-S Condensations
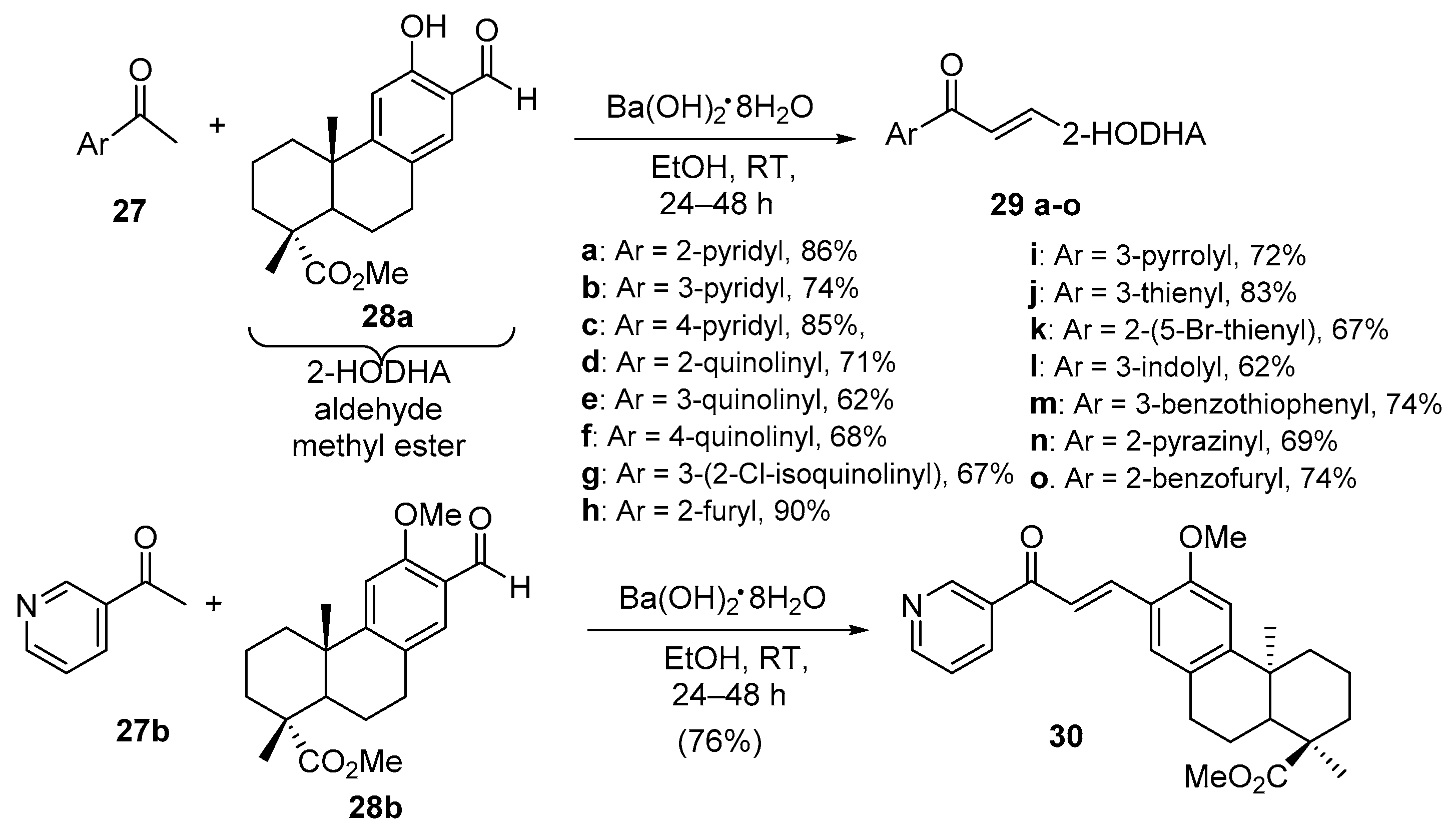
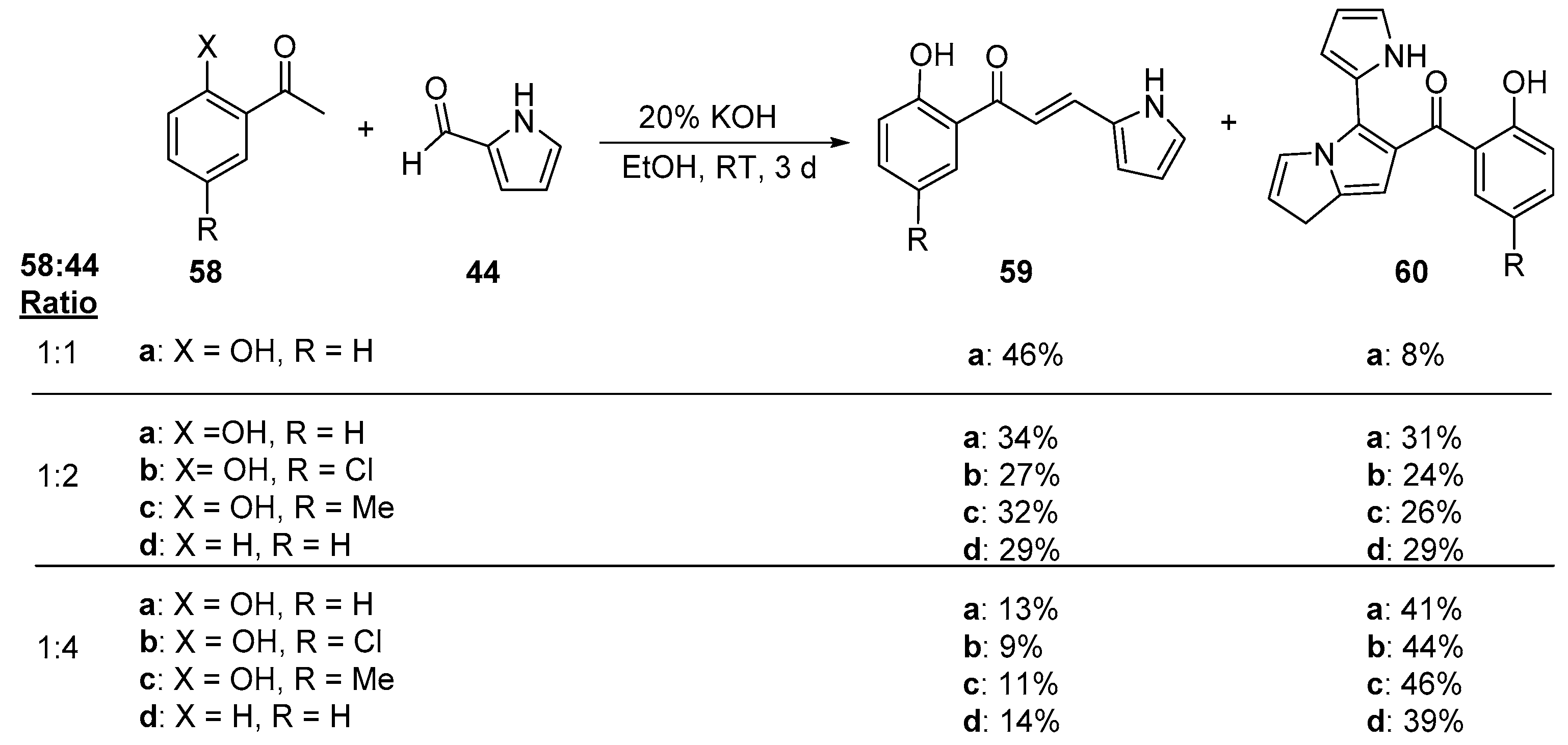
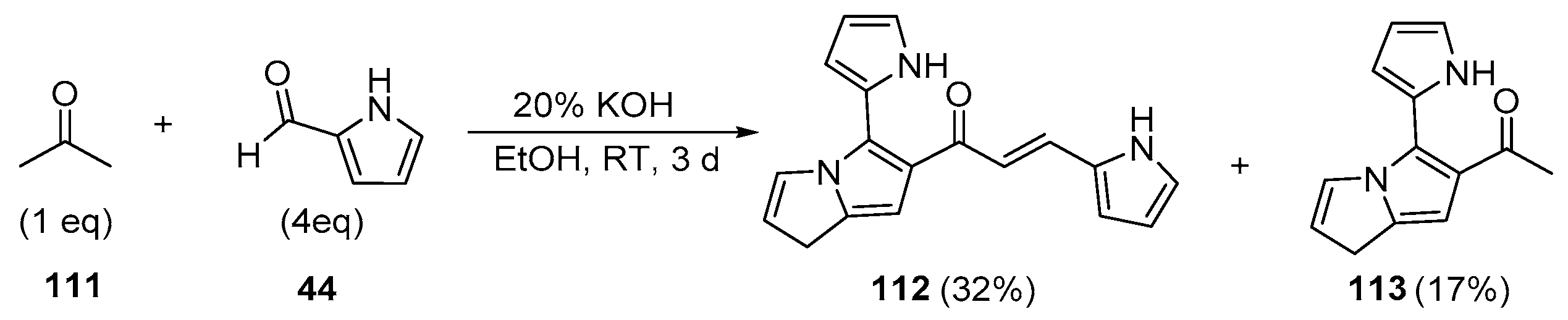
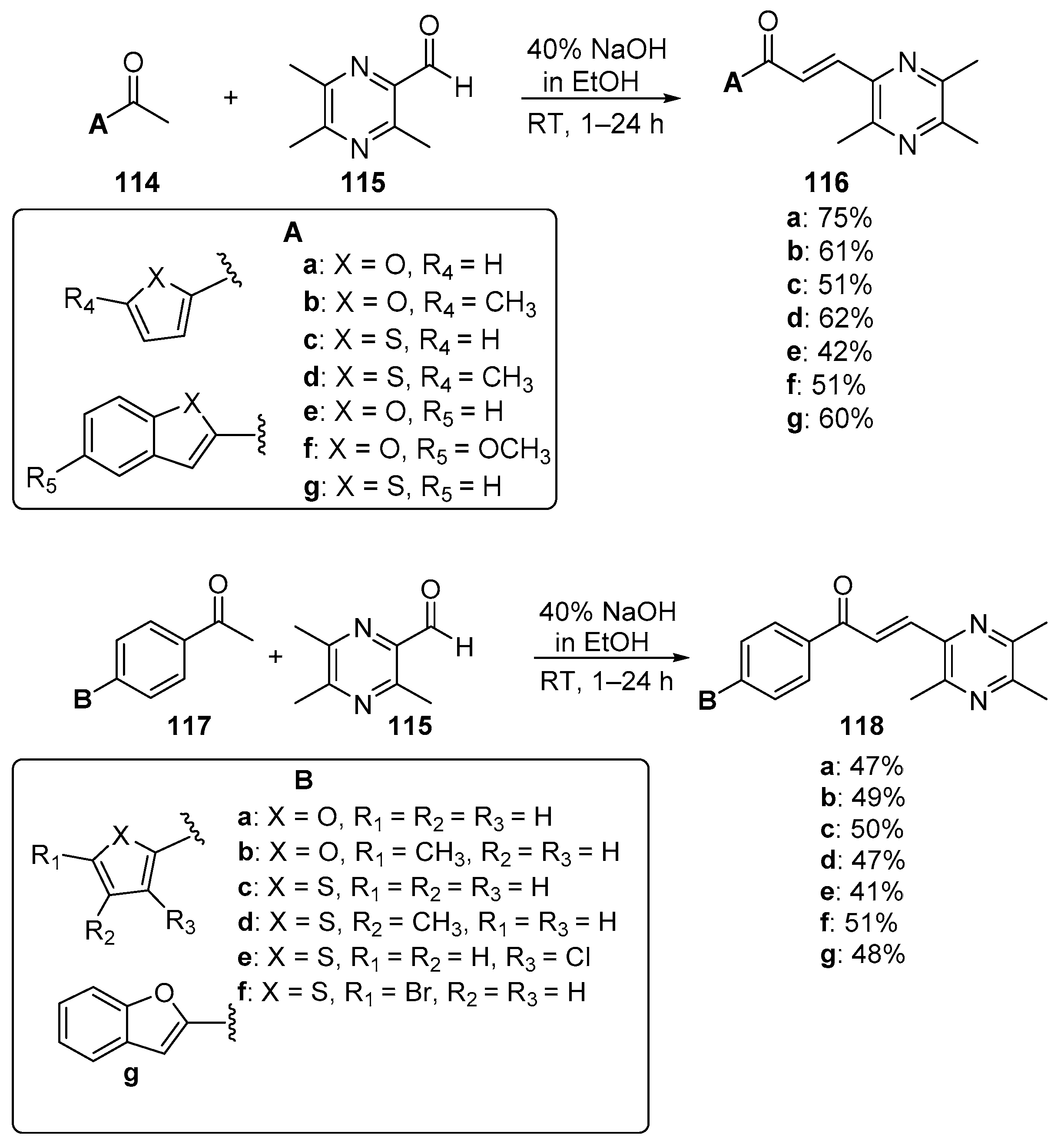
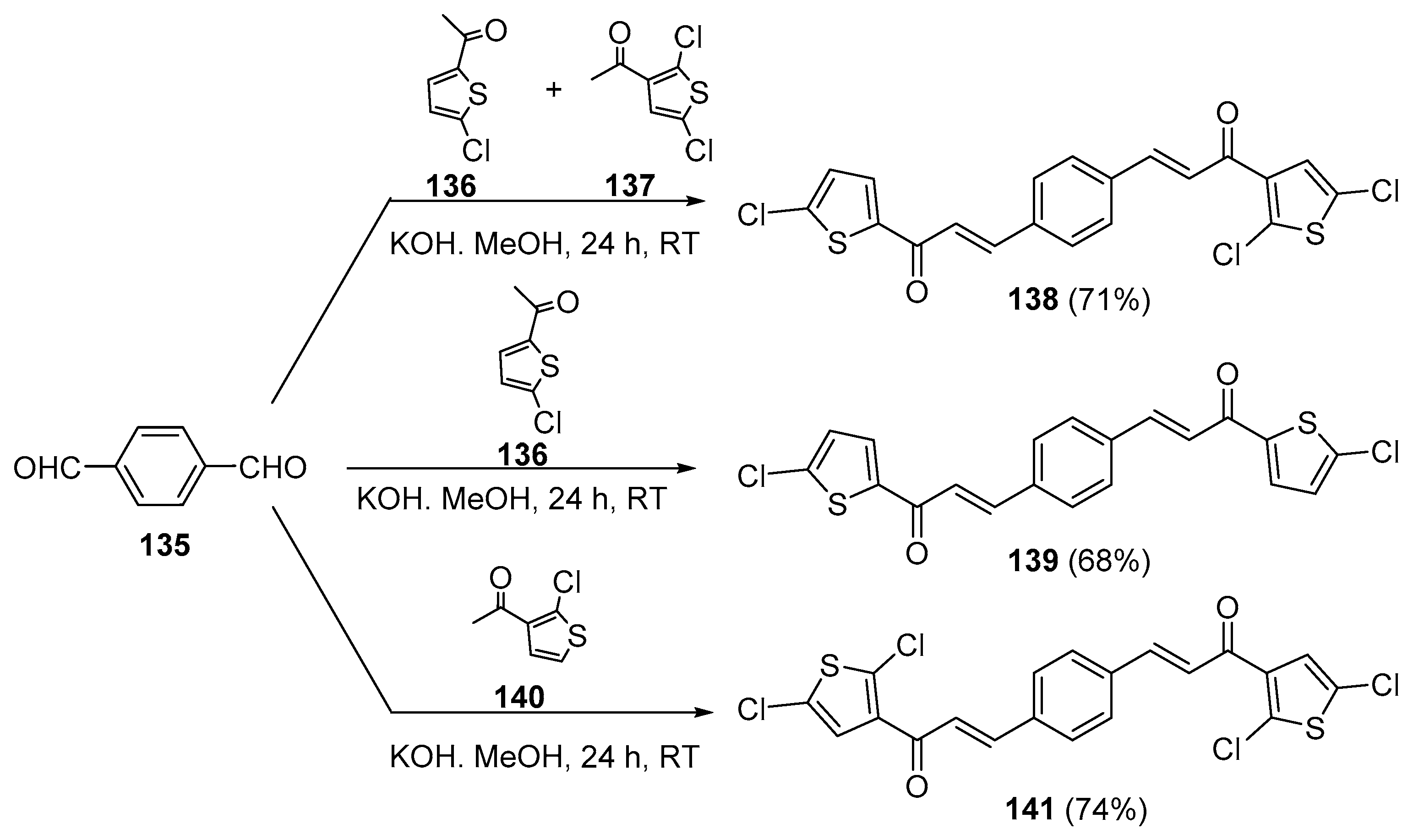
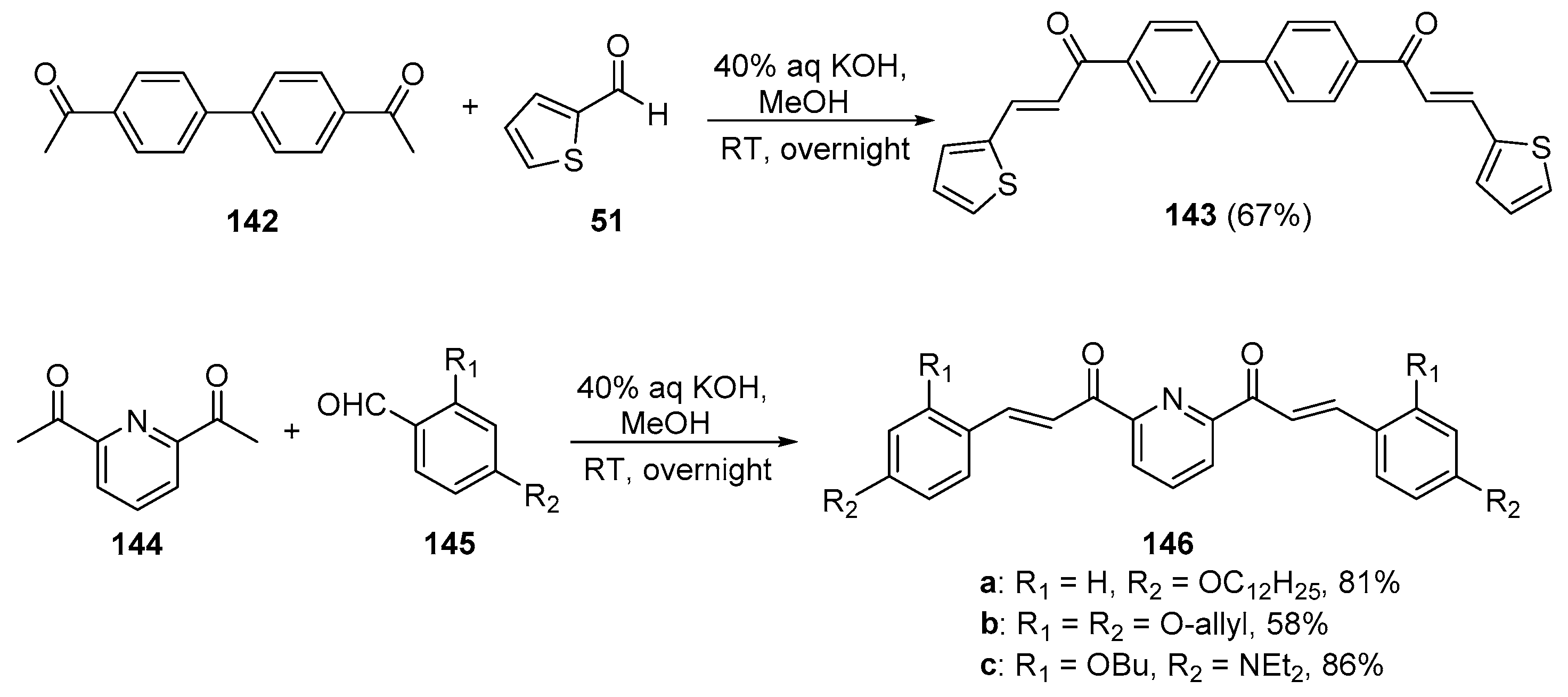
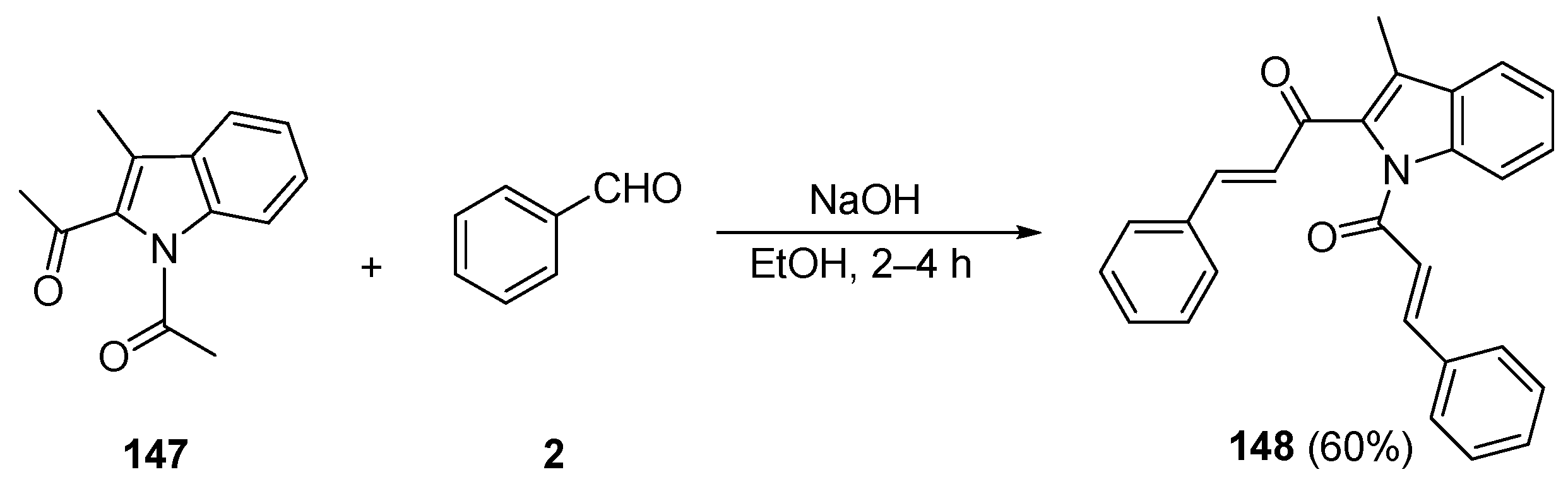
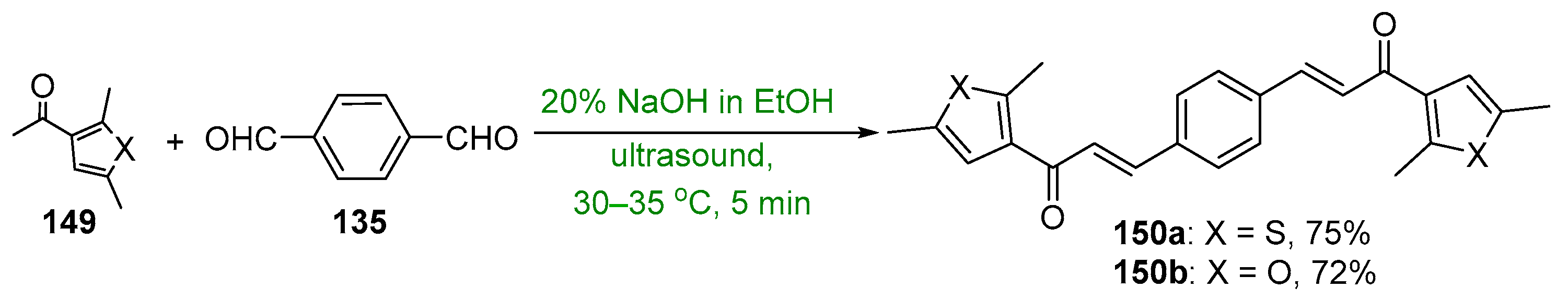
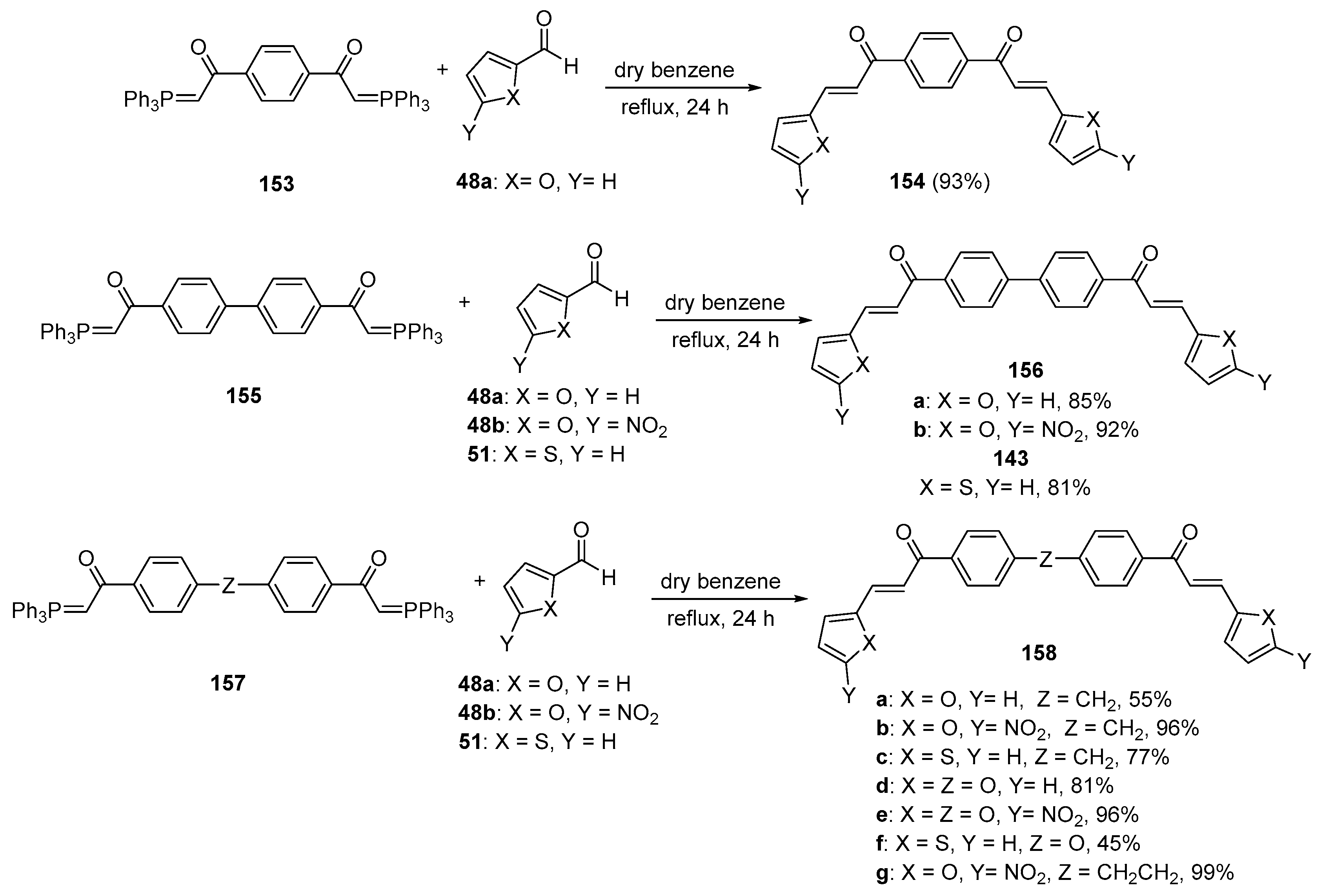
5. Heteroaromatic Bis Chalcone Hybrid Synthesis
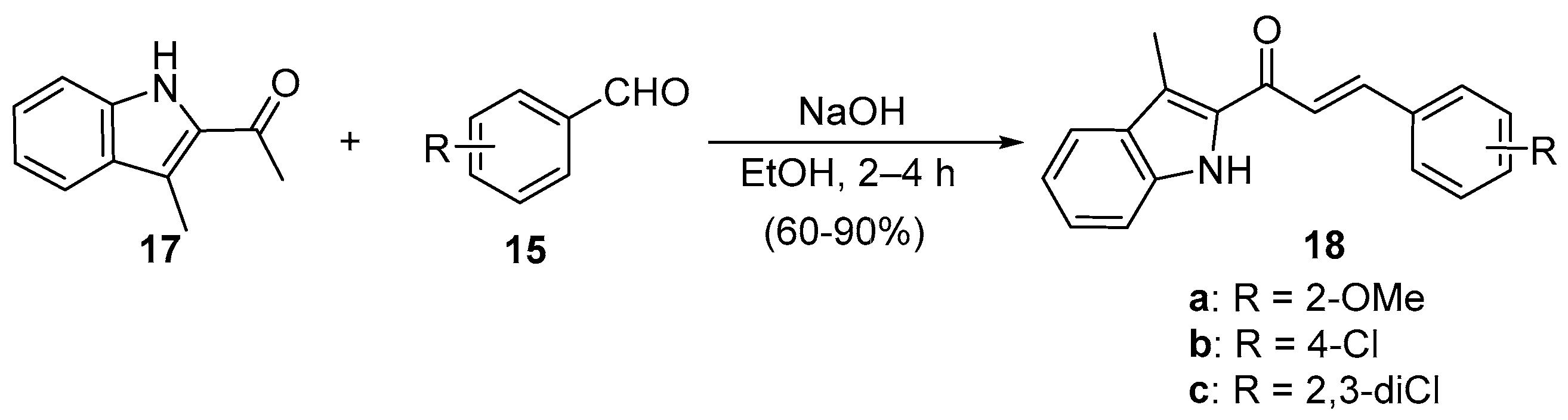
5.1. Claisen–Schmidt Condensations
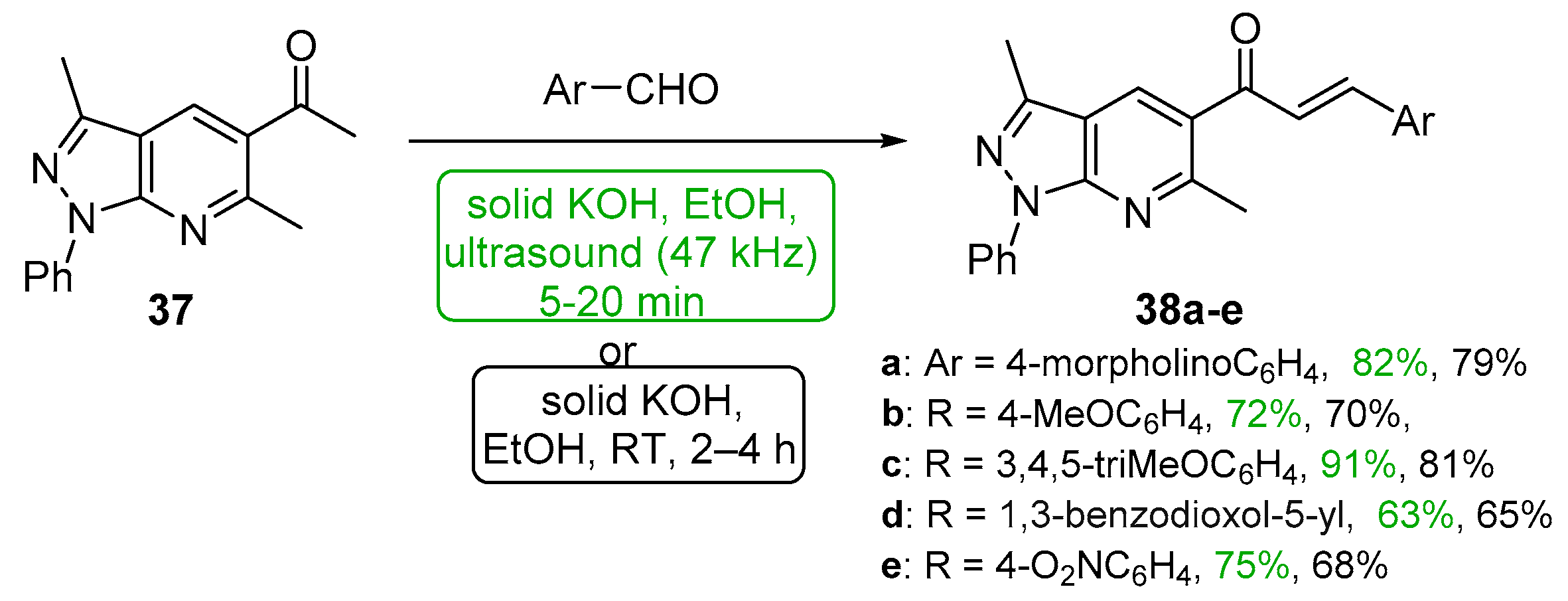
5.1.1. Base-Catalyzed C-S Condensations
5.1.2. Non C-S Condensations
6. Conclusions and Future Directions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kostanecki, S.; Tambor, J. Ueber die sechs isomeren Monooxybenzalacetophenone (Monooxychalkone). J. Chem. Ber. 1899, 32, 1921–1926. [Google Scholar] [CrossRef]
- Faass, M.; Mallia, A.; Sommer, R.; Sloop, J. (Z)-2-(3, 5-Dimethoxybenzylidene) Indan-1-one. CCDC 2081969: Experimental Crystal Structure Determination. CSD Commun. 2021. [Google Scholar] [CrossRef]
- Encarnacion-Thomas, E.; Sommer, R.D.; Mallia, V.A.; Sloop, J. (E)-2-(3,5-Dimethoxy-benzylidene)indan-1-one. IUCrData 2020, 5, x200759. [Google Scholar] [CrossRef]
- Makarov, A.; Sorotskaja, L.; Uchuskin, M.; Trushkov, I. Synthesis of quinolines via acid-catalyzed cyclodehydration of 2-(tosylamino)chalcones. Chem. Heterocycl. Comp. 2016, 52, 1087–1091. [Google Scholar] [CrossRef]
- Jin, H.; Jiang, X.; Yoo, H.; Wang, T.; Sung, C.; Choi, U.; Lee, C.-R.; Yu, H.; Koo, S. Synthesis of Chalcone-Derived Heteroaromatics with Antibacterial Activities. ChemistrySelect 2020, 5, 12421–12424. [Google Scholar] [CrossRef]
- Kalirajan, R.; Sivakumar, S.; Jubie, S.; Gowramma, B.; Suresh, B. Synthesis and Biological evaluation of some heterocyclic derivatives of Chalcones. Int. J. ChemTech Res. 2009, 1, 27–34. [Google Scholar] [CrossRef]
- El-Gohary, N. Arylidene Derivatives as Synthons in Heterocyclic Synthesis. Open Access Lib. J. 2014, 1, e367. [Google Scholar] [CrossRef]
- Albuquerque, H.; Santos, C.; Cavaleiro, J.; Silva, A. Chalcones as Versatile Synthons for the synthesis of 5- and 6-membered Nitrogen Heterocycles. Curr. Org. Chem. 2014, 18, 2750–2775. [Google Scholar] [CrossRef]
- Zhuang, C.; Zhang, W.; Sheng, C.; Zhang, W.; Xing, C.; Miao, Z. Chalcone: A Privileged Structure in Medicinal Chemistry. Chem. Rev. 2017, 117, 7762–7810. [Google Scholar] [CrossRef] [PubMed]
- Ardiansah, B. Chalcones bearing N, O, and S-heterocycles: Recent notes on their biological significances. J. Appl. Pharm. Sci. 2019, 9, 117–129. [Google Scholar] [CrossRef]
- Sharshira, E.; Hamada, N. Synthesis and Antimicrobial Evaluation of Some Pyrazole Derivatives. Molecules 2012, 17, 4962–4971. [Google Scholar] [CrossRef] [PubMed]
- Borge, V.V.; Patil, R.M. Comparative Study on Synthesis and Biological, Pharmaceutical Applications of Aromatic Substituted Chalcones. Mini. Rev. Org. Chem. 2023, 20, 260–269. [Google Scholar] [CrossRef]
- Mhaibes, R.M. Antimicrobial and Antioxidant Activity of Heterocyclic Compounds Derived from New Chalcones. J. Med. Chem. Sci. 2023, 6, 931–937. [Google Scholar] [CrossRef]
- Adnan, D.; Singh, B.; Mehta, S.; Kumar, V.; Kataria, R. Simple and solvent free practical procedure for chalcones: An expeditious, mild and greener approach. Curr. Res. Green Sustain. Chem. 2020, 3, 100041. [Google Scholar] [CrossRef]
- Urbonavîcius, A.; Fortunato, G.; Ambrazaitytė, E.; Plytninkienė, E.; Bieliauskas, A.; Milišíunaitė, V.; Luisi, R.; Arbâciauskienė, E.; Krikštolaitytė, S.; Šâckus, A. Synthesis and Characterization of Novel Heterocyclic Chalcones from 1-Phenyl-1H-pyrazol-3-ol. Molecules 2022, 27, 3752. [Google Scholar] [CrossRef]
- Kitawata, B.; Singha, M.; Kale, R. Solvent Free Synthesis, Characterization, Anticancer, Antibacterial, Antifungal, Antioxidant and SAR Studies of Novel (E)-3-aryl-1-(3-alkyl-2-pyrazinyl)-2-propenone. New J. Chem. 2013, 37, 2541–2550. [Google Scholar] [CrossRef]
- Chen, H.; Noirbent, G.; Liu, S.; Brunel, D.; Graff, B.; Gigmes, D.; Zhang, Y.; Sun, K.; Morlet-Savary, F.; Xiao, P.; et al. Bis-Chalcone Derivatives Derived from Natural Products as Near-UV/Visible Light Sensitive Photoinitiators for 3D/4D Printing. Mater. Chem. Front. 2021, 5, 901–916. [Google Scholar] [CrossRef]
- Mara Silva de Padua, G.; Maria De Souza, J.; Celia Moura Sales, M.; Gomes de Vasconcelos, L.; Luiz Dall’Oglio, E.; Faraggi, T.M.; Moreira Sampaio, O.; Campos Curcino Vieira, L. Evaluation of Chalcone Derivatives as Photosynthesis and Plant Growth Inhibitors. Chem. Biodivers. 2021, 18, e2100226. [Google Scholar] [CrossRef]
- Bukhari, S. Synthesis and evaluation of new chalcones and oximes as anticancer agents. RSC Adv. 2022, 12, 10307–10320. [Google Scholar] [CrossRef]
- Gaber, M.; El-Ghamry, H.A.; Mansour, M.A. Pd(II) and Pt(II) chalcone complexes. Synthesis, spectral characterization, molecular modeling, biomolecular docking, antimicrobial and antitumor activities. J. Photochem. Photobiol. A Chem. 2018, 354, 163–174. [Google Scholar] [CrossRef]
- Baretto, M.; Fuchi, N. Tissue Transglutaminase Inhibitor, Chalcone Derivative, and Pharmaceutical Application Thereof. Japan Patent 2013-180955A, 12 September 2013. [Google Scholar]
- Özdemir, A.; Altıntop, M.D.; Turan-Zitouni, G.; Çiftçi, G.A.; Ertorun, I.; Alataş, Ö.; Kaplancıklı, Z.A. Synthesis and evaluation of new indole-based chalcones as potential antiinflammatory agents. Eur. J. Med. Chem. 2015, 89, 304–309. [Google Scholar] [CrossRef] [PubMed]
- Shaik, A.; Bhandare, R.; Palleapati, K.; Nissankararao, S.; Kancharlapalli, V.; Shaik, S. Antimicrobial, Antioxidant, and Anticancer Activities of Some Novel Isoxazole Ring Containing Chalcone and Dihydropyrazole Derivatives. Molecules 2020, 25, 1047. [Google Scholar] [CrossRef] [PubMed]
- Desai, V.; Desai, S.; Gaonkar, S.N.; Palyekar, U.; Joshi, S.D.; Dixit, S.K. Novel quinoxalinyl chalcone hybrid scaffolds as enoyl ACP reductase inhibitors: Synthesis, molecular docking and biological evaluation. Bioorg. Med. Chem. Lett. 2017, 27, 2174–12180. [Google Scholar] [CrossRef] [PubMed]
- Gomes, M.N.; Braga, R.C.; Grzelak, E.M.; Neves, B.J.; Muratov, E.N.; Ma, R.; Klein, L.K.; Cho, S.; Oliveira, G.R.; Franzblau, S.G.; et al. QSAR-driven design, synthesis and discovery of potent and selective chalcone derivatives with antitubercular activity. Eur. J. Med. Chem. 2017, 137, 126–138. [Google Scholar] [CrossRef]
- Hawash, M.; Kahraman, D.C.; Eren, F.; Atalay, R.C.; Baytas, S.N. Synthesis and biological evaluation of novel pyrazolic chalcone derivatives as novel hepatocellular carcinoma therapeutics. Eur. J. Med. Chem. 2017, 129, 12–26. [Google Scholar] [CrossRef]
- Minders, C.; Petzer, J.; Petzer, A.; Lourens, A. Monoamine oxidase inhibitory activities of heterocyclic chalcones. Bioorg. Med. Chem. Lett. 2015, 25, 5270–5276. [Google Scholar] [CrossRef]
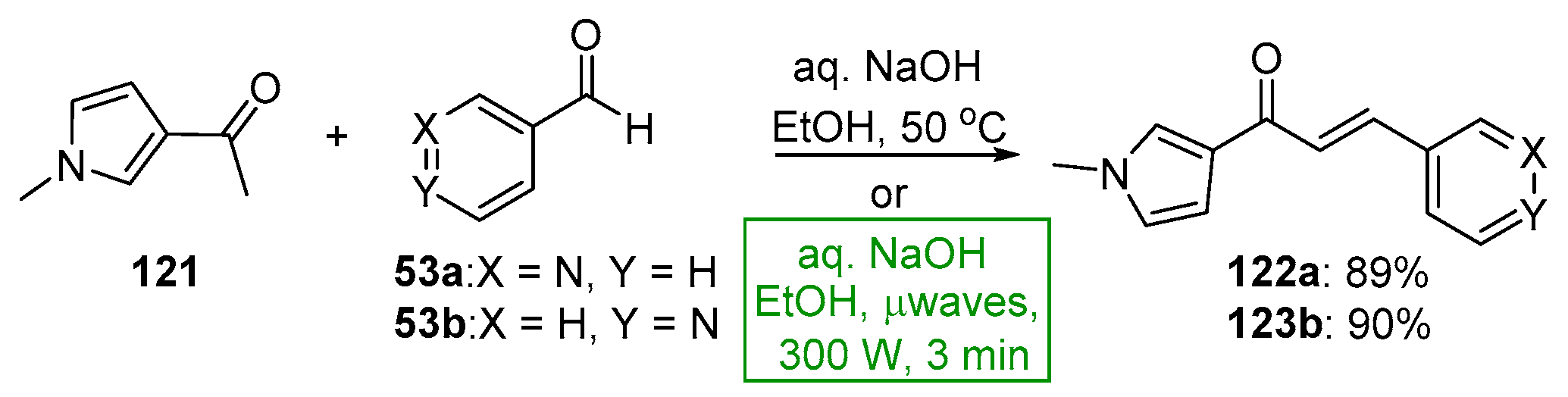
- Usta, A.; Oztürk, E.; Beriş, F. Microwave-assisted preparation of azachalcones and their N-alkyl derivatives with antimicrobial activities. Nat. Prod. Res. 2014, 28, 483–487. [Google Scholar] [CrossRef]
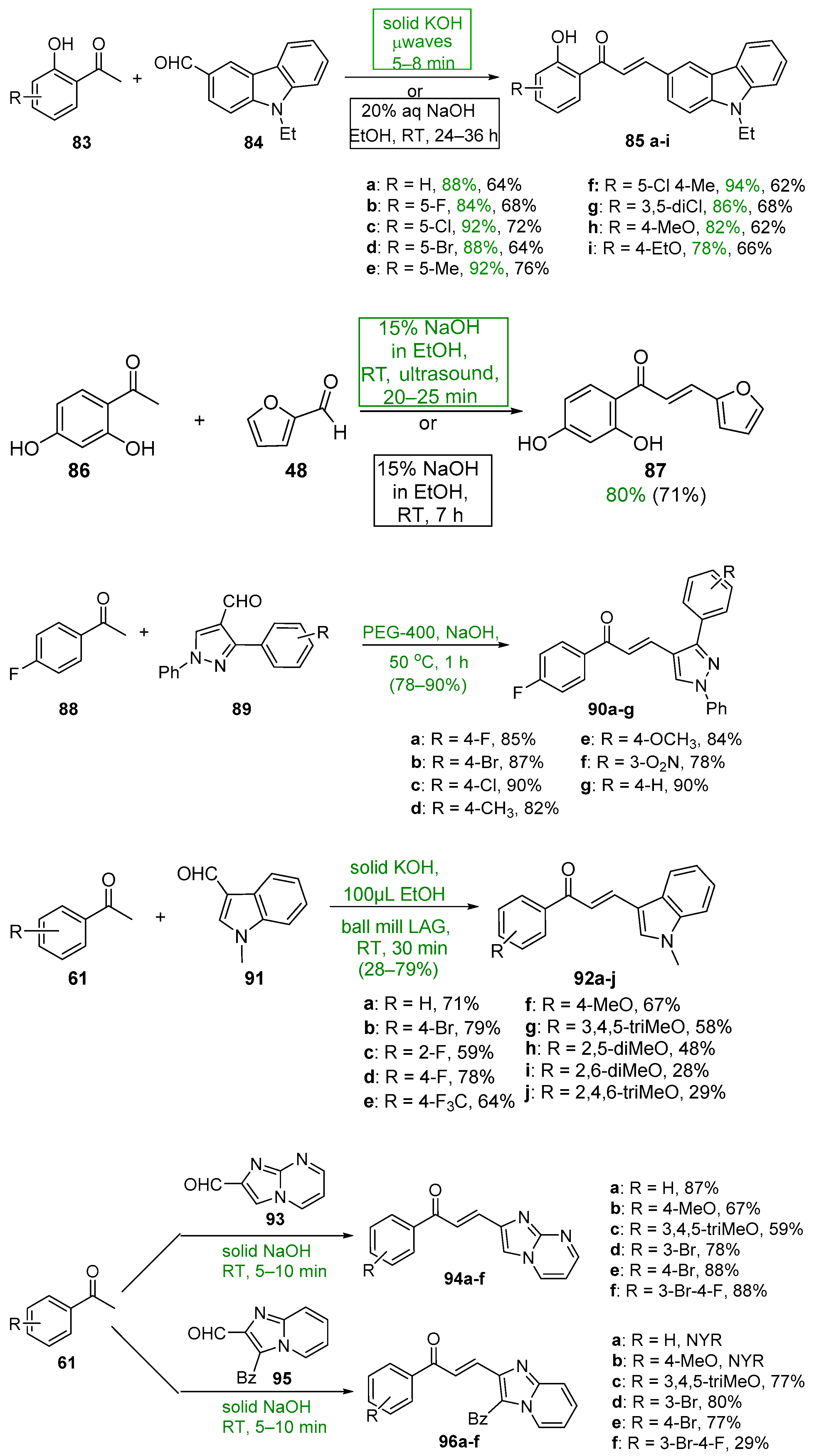
- Li, P.; Jiang, H.; Zhang, W.; Li, Y.; Zhao, M.; Zhou, W. Synthesis of carbazole derivatives containing chalcone analogs as non-intercalative topoisomerase II catalytic inhibitors and apoptosis inducers. Eur. J. Med. Chem. 2018, 145, 498–510. [Google Scholar] [CrossRef]
- Bandgar, B.; Adsul, L.; Lonikar, S.; Chavan, H.; Shringare, S.; Patil, S.; Jalde, S.; Koti, B.; Dhole, N.; Gacche, R.; et al. Synthesis of novel carbazole chalcones as radical scavenger, antimicrobial and cancer chemopreventive agents. J. Enzym. Inhib. Med. Chem. 2013, 28, 593–600. [Google Scholar] [CrossRef]
- Sweeting, S.; Hall, C.; Potticary, J.; Pridmore, N.; Warren, S.; Cremeens, M.; D’Ambruoso, G.; Matsumoto, M.; Hall, S. The solubility and stability of heterocyclic chalcones compared with transchalcone. Acta Cryst. B 2020, B76, 13–17. [Google Scholar] [CrossRef]
- Robinson, T.P.; Hubbard, R.B.; Ehlers, T.J.; Arbiser, J.L.; Goldsmith, D.J.; Bowen, J.P. Synthesis and biological evaluation of aromatic enones related to curcumin. Bioorg. Med. Chem. 2005, 13, 4007–4013. [Google Scholar] [CrossRef] [PubMed]
- Song, T.; Duan, Y.; Yang, Y. Chemoselective transfer hydrogenation of α,β-unsaturated carbonyls catalyzed by a reusable supported Pd nanoparticles on biomass-derived carbon. Catal. Commun. 2019, 120, 80–85. [Google Scholar] [CrossRef]
- Lokeshwari, D.M.; Rekha, N.D.; Srinivasan, B.; Vivek, H.K.; Kariyappa, A.K. Design, synthesis of novel furan appended benzothiazepine derivatives and in vitro biological evaluation as potent VRV-PL-8a and H+/K+ ATPase Inhibitors. Bioorg. Med. Chem. Lett. 2017, 27, 3048–3054. [Google Scholar] [CrossRef]
- Liu, W.; Shi, H.-M.; Jin, H.; Zhao, H.-Y.; Zhou, G.-P.; Wen, F.; Yu, Z.-Y.; Hou, T.-P. Design, Synthesis and Antifungal Activity of a Series of Novel Analogs Based on Diphenyl Ketones. Chem. Biol. Drug. Des. 2009, 73, 661–667. [Google Scholar] [CrossRef] [PubMed]
- Robinson, S.J.; Petzer, J.P.; Petzer, A.; Bergh, J.J.; Lourens, A.C.U. Selected furanochalcones as inhibitors of monoamine oxidase. Bioorg. Med. Chem. Lett. 2013, 23, 4985–4989. [Google Scholar] [CrossRef]
- Parveen, H.; Iqbal, P.F.; Azam, A. Synthesis and Characterization of a New Series of Hydroxy Pyrazolines. Synth. Commun. 2008, 38, 3973–3983. [Google Scholar] [CrossRef]
- Sunduru, N.; Agarwal, A.; Katiyar, S.B.; Goyal, N.; Gupta, S.; Chauhana, P.M.S. Synthesis of 2,4,6-trisubstituted pyrimidine and triazine heterocycles as antileishmanial agents. Bioorg. Med. Chem. 2006, 14, 7706–7715. [Google Scholar] [CrossRef]
- Sinha, S.; Manju, S.; Doble, M. Chalcone-Thiazole Hybrids: Rational Design, Synthesis, and Lead Identification against 5-Lipoxygenase. Med. Chem. Lett. 2019, 10, 1415–1422. [Google Scholar] [CrossRef]
- Zhao, X.; Dong, W.; Gao, Y.; Shin, D.-S.; Ye, Q.; Su, L.; Jiang, F.; Zhao, B.; Miao, J. Novel indolyl-chalcone derivatives inhibit A549 lung cancer cell growth through activating Nrf-2/HO-1 and inducing apoptosis in vitro and in vivo. Sci. Rep. 2017, 7, 3919. [Google Scholar] [CrossRef]
- Hsieh, C.-Y.; Ko, P.-W.; Chang, Y.-J.; Kapoor, M.; Liang, Y.-C.; Chu, H.-L.; Lin, H.-H.; Horng, J.-C.; Hsu, M.-H. Design and Synthesis of Benzimidazole-Chalcone Derivatives as Potential Anticancer Agents. Molecules 2019, 24, 3259. [Google Scholar] [CrossRef]
- Hsieh, C.; Kuiying Xu, K.; Lee, I.; Graham, T.; Tu, Z.; Dhavale, D.; Kotzbauer, P.; Mach, R. Chalcones and Five-Membered Heterocyclic Isosteres Bind to Alpha Synuclein Fibrils in Vitro. ACS Omega 2018, 3, 4486–4493. [Google Scholar] [CrossRef] [PubMed]
- Saito, Y.; Kishimoto, M.; Yoshikawa, Y.; Kawaii, S. Synthesis and Structure–Activity Relationship Studies of Furan-ring Fused Chalcones as Antiproliferative Agents. Anticancer Res. 2015, 35, 811–818. [Google Scholar] [PubMed]
- Grigoropoulou, S.; Manou, D.; Antoniou, A.I.; Tsirogianni, A.; Siciliano, C.; Theocharis, A.D.; Athanassopoulos, C.M. Synthesis and Antiproliferative Activity of Novel Dehydroabietic Acid-Chalcone Hybrids. Molecules 2022, 27, 3623. [Google Scholar] [CrossRef] [PubMed]
- Mubofu, E.B.; Engberts, J.B.F.N. Specific acid catalysis and Lewis acid catalysis of Diels–Alder reactions in aqueous media. J. Phys. Org. Chem. 2004, 17, 180–186. [Google Scholar] [CrossRef]
- Jianga, X.; Jina, H.; Wanga, T.; Yoob, H.; Koo, S. Synthesis of Phenyl-2,2-bichalcophenes and Their Aza-Analogues by Catalytic Oxidative Deacetylation. Synthesis 2019, 51, 3259–3268. [Google Scholar] [CrossRef]
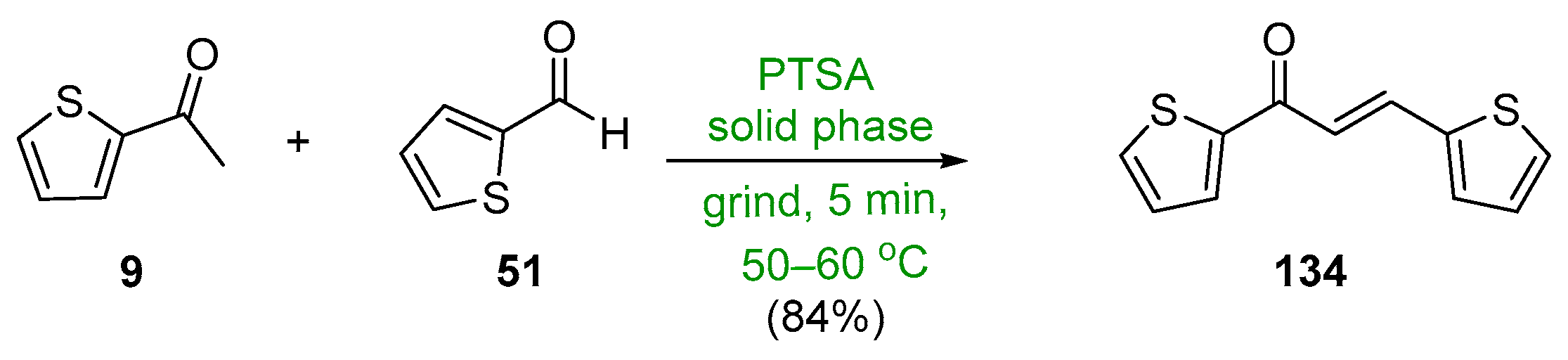
- Ritter, M.; Martins, R.; Rosa, S.; Malavolta, J.; Lund, R.; Flores, A.; Pereira, C. Green Synthesis of Chalcones and Microbiological Evaluation. J. Braz. Chem. Soc. 2015, 26, 1201–1210. [Google Scholar] [CrossRef]
- Khan, S.; Asiri, A. Green Synthesis, Characterization and biological evaluation of novel chalcones.as anti bacterial agents. Arab. J. Chem. 2013, 10, S2890–S2895. [Google Scholar] [CrossRef]
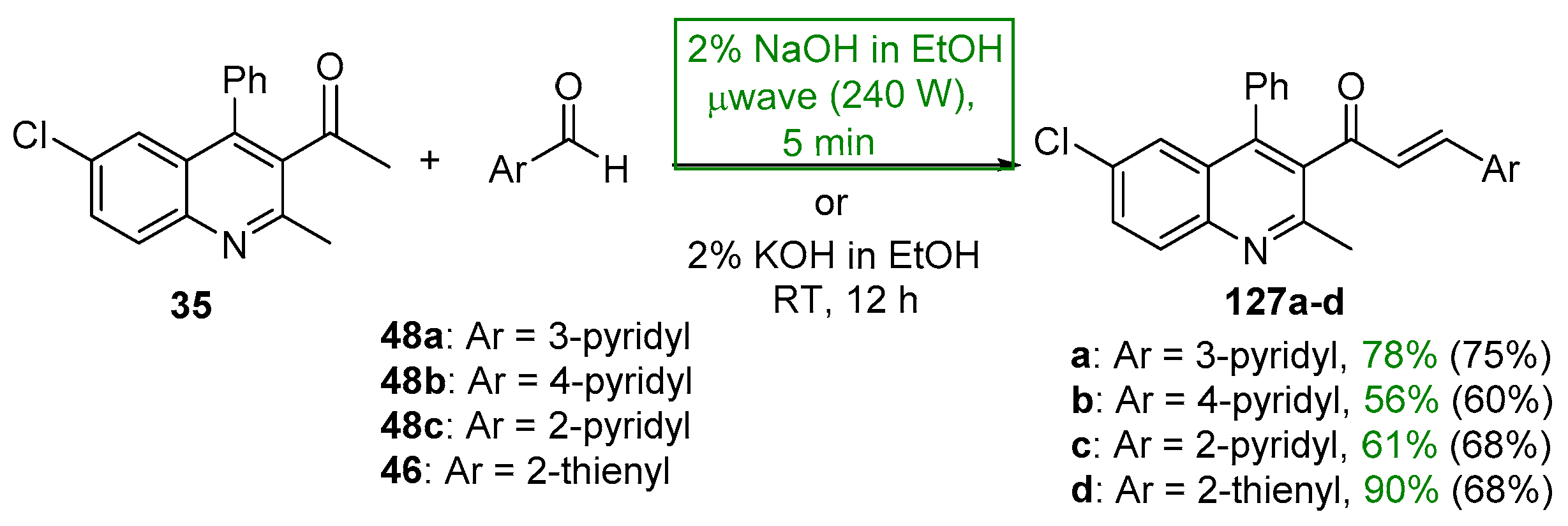
- Sarveswari, S.; Vijayakumar, V. A rapid microwave assisted synthesis of 1-(6-chloro-2-methyl-4-phenylquinolin-3-yl)-3-(aryl)prop-2-en-1-ones and their anti bacterial and anti fungal evaluation. Arab. J. Chem. 2016, 9, S35–S40. [Google Scholar] [CrossRef]
- Polo, E.; Ferrer-Pertuz, K.; Trilleras, J.; Quiroga, J.; Guti’errez, M. Microwave-assisted one-pot synthesis in water of carbonylpyrazolo [3,4-b]pyridine derivatives catalyzed by InCl3 and sonochemical assisted condensation with aldehydes to obtain new chalcone derivatives containing the pyrazolopyridinic moiety. RSC Adv. 2017, 7, 50044–50050. [Google Scholar] [CrossRef]
- Yadav, P.; Yadav, M.; Gaur, R.; Gupta, R.; Arora, G.; Rana, P.; Srivastava, A.; Sharma, R.K. Fabrication of copper-based silica-coated magnetic nanocatalyst for efficient one-pot synthesis of chalcones via A3 coupling of aldehydes-alkynes-amines. Chem. Cat. Chem. 2020, 12, 2488–2496. [Google Scholar] [CrossRef]
- Fu, D.-J.; Zhang, S.-Y.; Liu, Y.-C.; Yue, X.-X.; Liu, J.-J.; Song, J.; Zhao, R.-H.; Li, F.; Sun, H.-H.; Zhang, Y.-B.; et al. Design, synthesis and antiproliferative activity studies of 1,2,3-triazole–chalcones. Med. Chem. Commun. 2016, 7, 1664–1671. [Google Scholar] [CrossRef]
- Gadhave, A.G.; Uphade, B.K. Synthesis of Some Pyrazole Containing Chalcones and Pyridine-3-Carbonitriles and Study of their Anti-inflammatory Activity. Orient. J. Chem. 2017, 33, 219–225. [Google Scholar] [CrossRef]
- Mallik, A.; Dey, S.; Chattopadhyay, F.; Patra, A. Novel formation of 6-acyl-5-(2-pyrrolyl)-3H-pyrrolizines bym base-catalysed condensation of pyrrole-2-aldehyde with methyl ketones. Tetrahedron Lett. 2000, 43, 1295–1297. [Google Scholar] [CrossRef]
- Bindu, P.; Mahadevan, K.; Naik, T.; Harish, B. Synthesis, DNA binding, docking and photocleavage studies of quinolinyl chalcones. Med. Chem. Commun. 2014, 5, 1708–1717. [Google Scholar] [CrossRef]
- Abonia, R.; Gutiérrez, L.; Quiroga, J.; Insuasty, B. (E)-3-[3-(2-Butoxyquinolin-3-yl)acryloyl]-2-hydroxy-4H-chromen-4-one. Molbank 2018, 2018, M1001. [Google Scholar] [CrossRef]
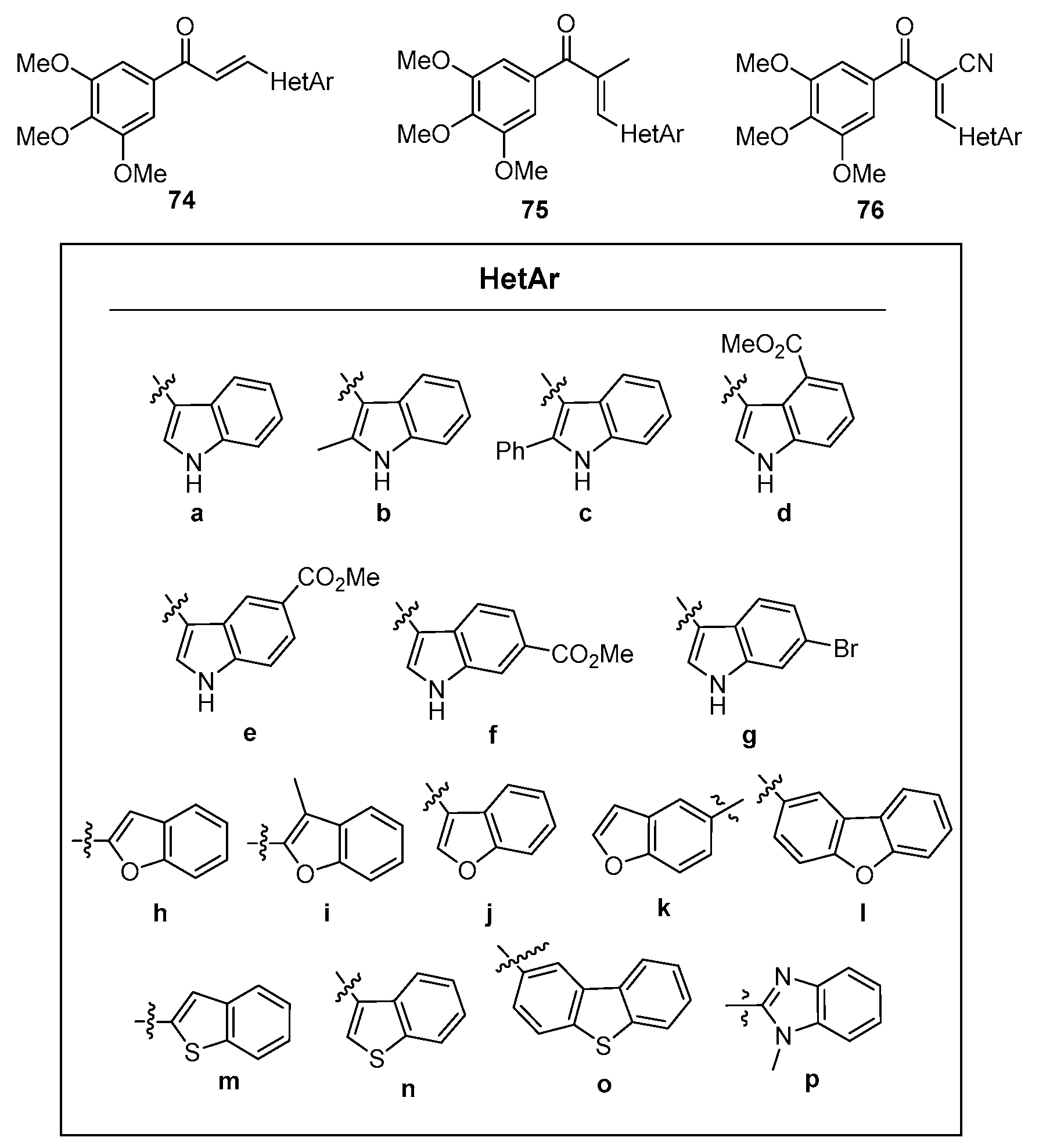
- Sun, M.; Yuyang, W.; Minghua, Y.; Qing, Z.; Yixin, Z.; Yongfang, Y.; Yongtao, D. Angiogenesis, Anti-Tumor, and Anti-Metastatic Activity of Novel α-Substituted Hetero-Aromatic Chalcone Hybrids as Inhibitors of Microtubule Polymerization. Front. Chem. 2021, 9, 766201. [Google Scholar] [CrossRef]
- Ashok, D.; Ravi, S.; Ganesh, A.; Lakshmi, B.; Adam, S.; Murthy, S. Microwave-assisted synthesis and biological evaluation of carbazole-based chalcones, aurones and flavones. Med. Chem. Res. 2016, 25, 909–922. [Google Scholar] [CrossRef]
- Bhatt, K.; Vishal Rana, V.; Patel, N.; Parikh, J.; Pillai, S. Novel Oxygen Fused Bicyclic Derivatives and Antioxidant Labelling: Bioactive Chalcone Based Green Synthesis. Biointerface Res. Appl. Chem. 2023, 13, 130. [Google Scholar] [CrossRef]
- Jadhava, S.; Peerzadeb, N.; Gawalia, R.; Bhosaleb, R.; Kulkarnic, A.; Varpe, B. Green synthesis and biological screening of some fluorinated pyrazole chalcones in search of potent anti-inflammatory and analgesic agents. Egypt Pharmaceut. J. 2020, 19, 172–181. [Google Scholar] [CrossRef]
- Kudlickova, Z.; Stahorsky, M.; Michalkova, R.; Vilkova, M.; Balaz, M. Mechanochemical synthesis of indolyl chalcones with antiproliferative activity. Green Chem. Lett. Rev. 2022, 15, 2089061. [Google Scholar] [CrossRef]
- Nimmala, S.; Chepyala, K.; Balram, B.; Ram, B. Synthesis and antibacterial activity of novel imidazo [1,2-a]pyrimidine and imidazo [1,2-a]pyridine chalcone derivatives. Der Pharma Chem. 2012, 4, 2408–2415. [Google Scholar]
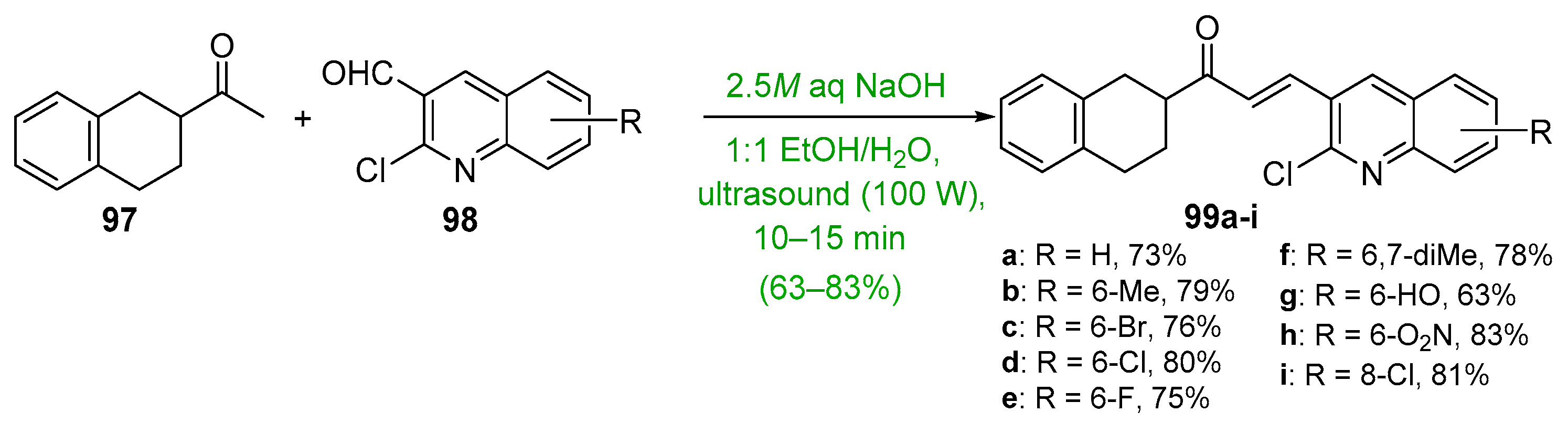
- Joshi, H.; Saglani, M. Rapid and greener ultrasound assisted synthesis of series (2-((substituted 2-Chloroquinolin-3-yl) methylene)-3,4-dihydronaphthalen-1(2h)-one) derivatives and their biological activity. J. Adv. Sci. Res. 2020, 11, 147–152. [Google Scholar]
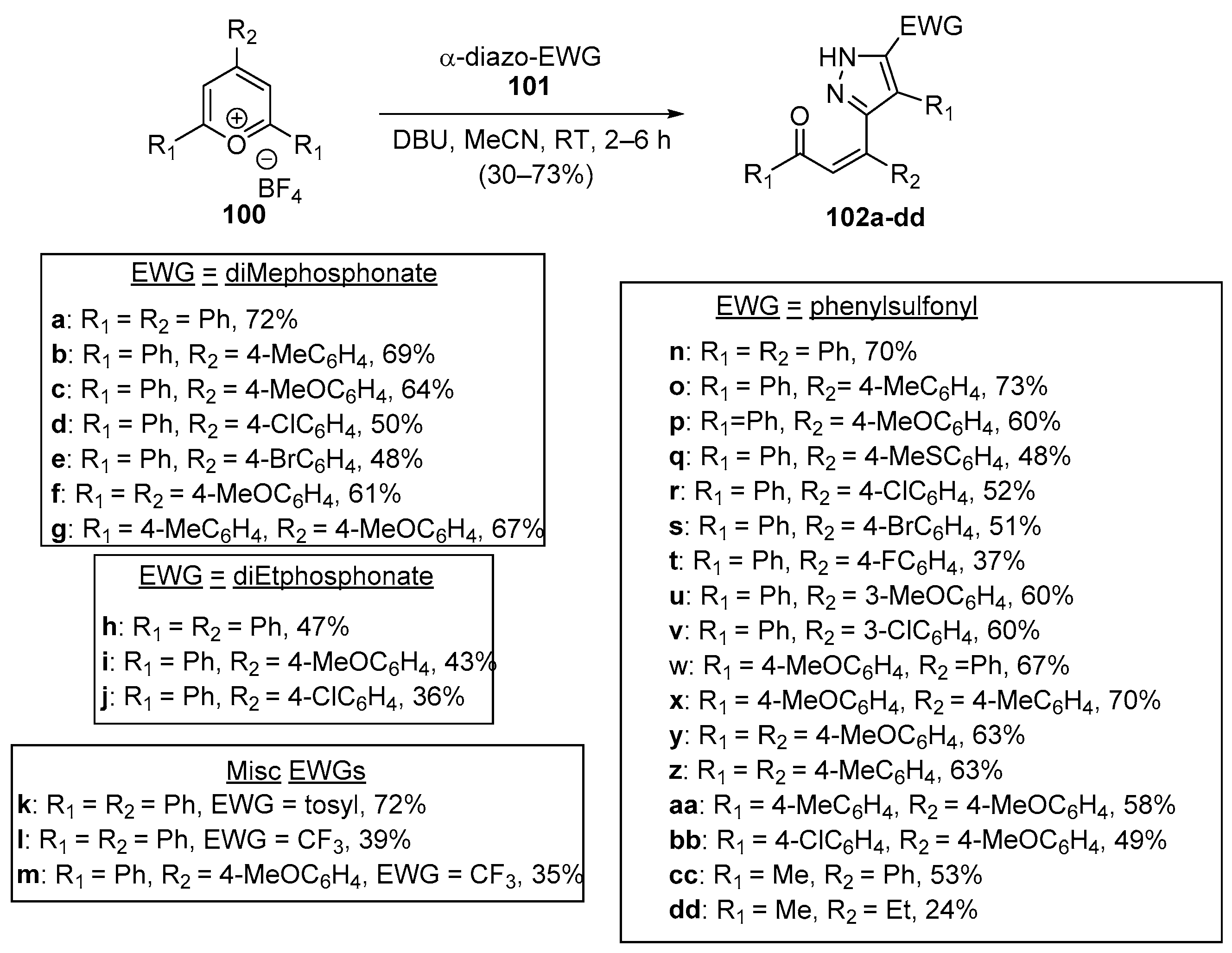
- Devi, L.; Sharma, G.; Kant, R.; Shukla, S.; Rastogi, N. Regioselective Synthesis of Functionalized Pyrazole-Chalcones via Base Mediated Reaction of Diazo Compounds with Pyrylium Salts. Org. Biomol. Chem. 2021, 19, 4132–4136. [Google Scholar] [CrossRef] [PubMed]
- Tan, P.; Wang, S.R. Reductive (3 + 2) Annulation of Benzils with Pyrylium Salts: Stereoselective Access to Furyl Analogues of Cis-Chalcones. Org. Lett. 2019, 21, 6029–6033. [Google Scholar] [CrossRef] [PubMed]
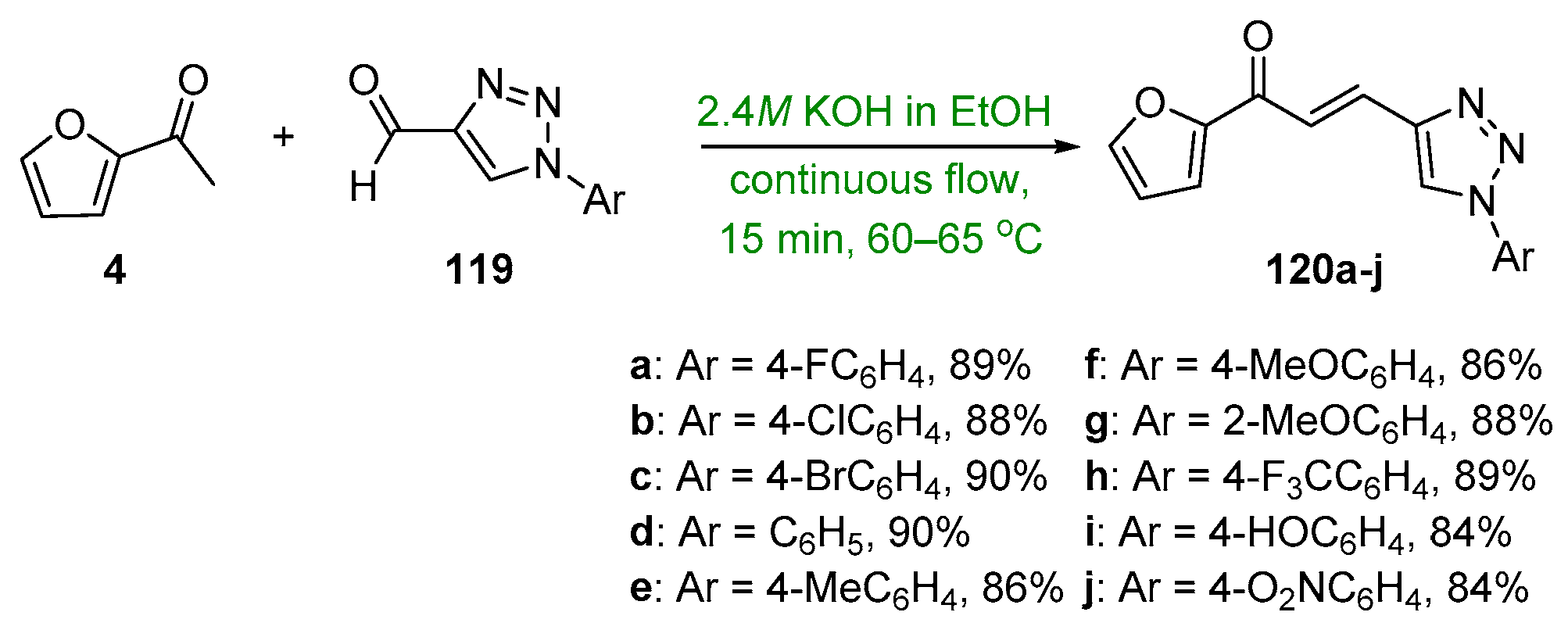
- Kumar, K.; Siddaiah, V.; Lilakar, J.; Ganesh, A. An efficient continuous-flow synthesis and evaluation of antimicrobial activity of novel 1,2,3-Triazole-Furan hybrid chalcone derivatives. Chem. Data Collect. 2020, 28, 100457. [Google Scholar] [CrossRef]
- Kuamr, D.; Kumar, N.M.; Tantak, M.P.; Ogura, M.; Eriko Kusaka, E.; Ito, T. Synthesis and identification of α-cyano bis(indolyl)chalcones as novel anticancer agents. Bioorg. Med. Chem. Lett. 2014, 24, 5170–5174. [Google Scholar] [CrossRef]
- Alidmat, M.; Khairuddean, M.; Salhimi, S.; Al-Amin, M. Docking studies, synthesis, characterization, and cytotoxicity activity of new bis-chalcone derivatives. Biomed. Res. Ther. 2021, 8, 4294–4306. [Google Scholar] [CrossRef]
- Asiri, A.; Marwani, H.; Alamry, K.; Al-Amoudi, M.; Khan, S.; El-Daly, S. Green Synthesis, Characterization, Photophysical and Electrochemical Properties of Bis-chalcones. Int. J. Electrochem. Sci. 2014, 9, 799–809. [Google Scholar]
- Abdel-Aziz, H.A.; Al-Rashood, K.A.; ElTahir, K.E.H.; Ibrahim, H.S. Microwave-assisted Synthesis of Novel 3,4-Bis-chalcone-N-arylpyrazoles and Their Anti-inflammatory Activity. J. Chin. Chem. Soc. 2011, 58, 863–868. [Google Scholar] [CrossRef]
- Saikachi, H.; Muto, H. Preparation of Resonance-Stabilized Bisphophoranes. Chem. Pharm. Bull. 1971, 19, 2262–2270. [Google Scholar] [CrossRef]

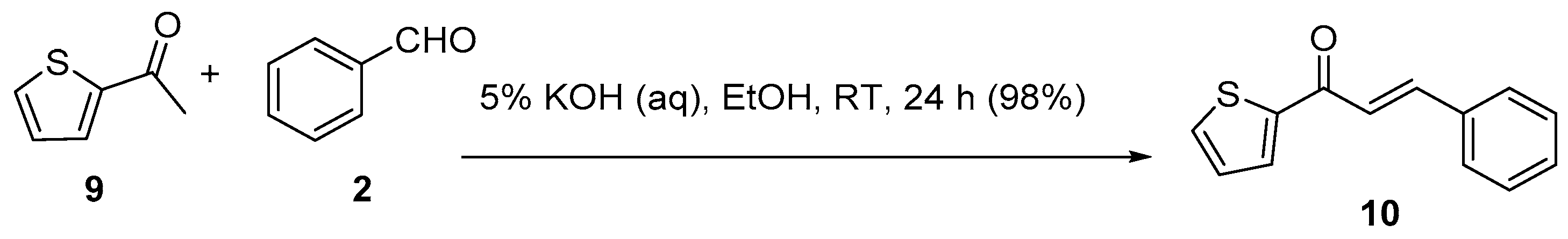



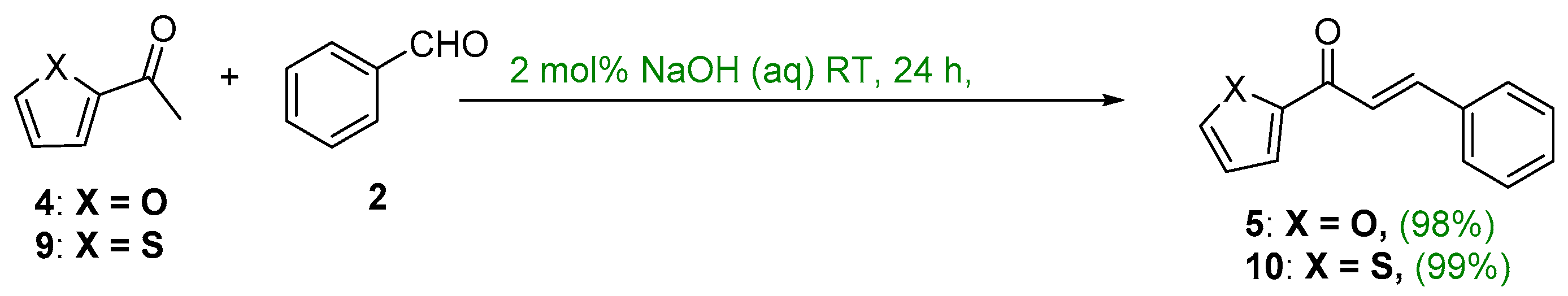
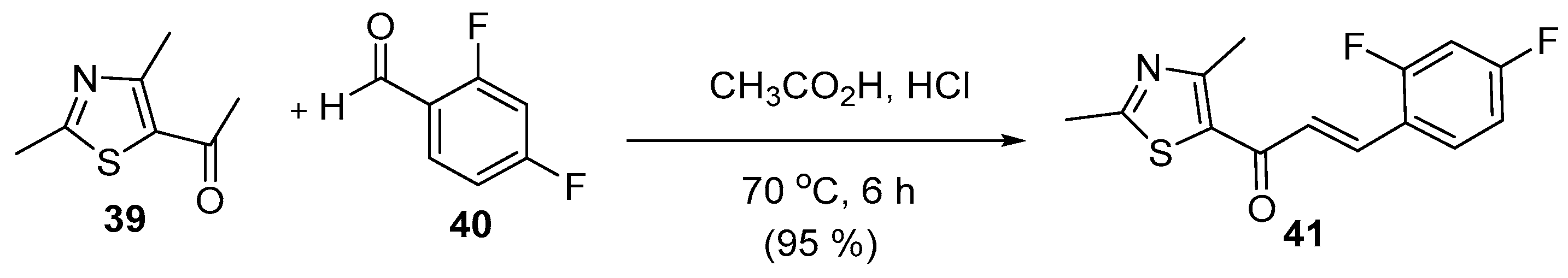




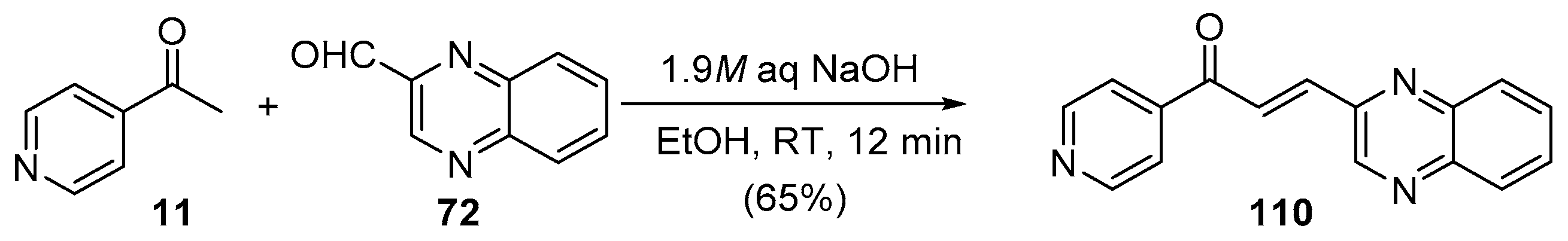




Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mallia, A.; Sloop, J. Advances in the Synthesis of Heteroaromatic Hybrid Chalcones. Molecules 2023, 28, 3201. https://doi.org/10.3390/molecules28073201
Mallia A, Sloop J. Advances in the Synthesis of Heteroaromatic Hybrid Chalcones. Molecules. 2023; 28(7):3201. https://doi.org/10.3390/molecules28073201
Chicago/Turabian StyleMallia, Ajay, and Joseph Sloop. 2023. "Advances in the Synthesis of Heteroaromatic Hybrid Chalcones" Molecules 28, no. 7: 3201. https://doi.org/10.3390/molecules28073201
APA StyleMallia, A., & Sloop, J. (2023). Advances in the Synthesis of Heteroaromatic Hybrid Chalcones. Molecules, 28(7), 3201. https://doi.org/10.3390/molecules28073201






