Chemical Composition and Biological Activities of Essential Oils of Four Asarum Species Growing in Vietnam
Abstract
1. Introduction
2. Results and Discussion
2.1. Chemical Composition of the Essential Oils
2.2. Antimicrobial Activity of the EOs
2.3. Antioxidant Activity of the EOs
2.4. Anti-Inflammatory Activity of the EOs
3. Materials and Methods
3.1. Plant Materials
3.2. Extraction the EOs
3.3. Analyzing Chemical Constituents of the Essential Oils
3.4. Antimicrobial Assays
3.5. Antioxidant Assays
3.6. Anti-Inflammatory Assays
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Chang, T.L.; Wang, J.C. Three new species of Asarum (section Heterotropa) from Taiwan. Bot. Stud. 2009, 50, 229–240. [Google Scholar]
- Kopyt’ko, Y.F.; Shchurevich, N.N.; Sokol’skaya, T.A.; Markaryan, A.A.; Dargaeva, T.D. Uses, chemical composition, and standardization of plant raw material and medicinal substances from plants of the genus Asarum L. Pharm. Chem. J. 2013, 47, 33. [Google Scholar] [CrossRef]
- Tuan, N.A. Research science basis for conservation, development and use sustain some species of Asarum L. genus of Vietnam. Ph.D. Thesis, University of Science and Technology (VAST), Hanoi, Vietnam, 2015. (In Vietnamese). [Google Scholar]
- Hue, H.T.T.; Ky, L.D.; Hoang, N.H. Analysis of DNA Markers from Vietnamese Asarum L. Species. VNU J. Sci. Nat. Sci. Technol. 2022, 38, 56. [Google Scholar] [CrossRef]
- Hanze, L.; Changhong, W. The genus Asarum: A review on phytochemistry, ethnopharmacology, toxicology and pharmacokinetics. J. Ethnopharmacol. 2022, 282, 114642. [Google Scholar] [CrossRef]
- Liu, G.X.; Xu, F.; Shang, M.Y.; Wang, X.; Cai, S.Q. The relative content and distribution of absorbed volatile organic compounds in rats administered Asari Radix et Rhizoma are different between powder- and decoction-treated groups. Molecules 2020, 25, 4441. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Liu, G.; Shang, M.; Xu, F.; Li, Y.; Zhou, Y.; Xie, D.; Wang, X.; Cai, S. Identification based on HPLC and anti-inflammatory targets as well as related constituents analysis of Asarum heterotropoides var. mandshuricum and A. sieboldii. China J. Chin. Mater. Med. 2020, 45, 1374. [Google Scholar] [CrossRef]
- Haque, A.; Moon, J.N.; Saravana, P.S.; Tilahun, A.; Chun, B.S. Composition of Asarum heterotropoides var. mandshuricum radix oil from different extraction methods and activities against human body odor-producing bacteria. J. Food Drug Anal. 2016, 24, 813. [Google Scholar] [CrossRef]
- Wang, X.; Xu, F.; Zhang, H.; Peng, L.; Zhen, Y.; Wang, L.; Xu, Y.; He, D.; Li, X. Orthogonal test design for optimization of the extraction of essential oil from Asarum heterotropoides var. mandshuricum and evaluation of its antibacterial activity against periodontal pathogens. 3 Biotech. 2018, 8, 1–10. [Google Scholar] [CrossRef]
- Dan, Y.; Liu, H.-Y.; Gao, W.-W.; Chen, S.-L. Activities of essential oils from Asarum heterotropoides var. mandshuricum against five phytopathogens. Crop. Protect. 2010, 29, 295. [Google Scholar] [CrossRef]
- Han, J.; Wang, J.; Han, X.; Ji, M. Effects of essential oil from Asarum heterotropoides on toxicity and related enzymes of Tetranychus urticae. Nat. Prod. RD 2012, 24, 525. [Google Scholar] [CrossRef]
- Liu, H.Y.; Gao, W.W.; Fan, Y.; Chen, S.L. Inhibitory effect of essential oil from Asarum heterotropoides Fr. Schmidt var. mandshuricum (Maxim.) Kitag against plant pathogenic fungi. Acta Phytopathol. Sin. 2007, 37, 95. [Google Scholar] [CrossRef]
- Wu, H.; Li, J.; Zhang, F.; Li, L.; Liu, Z.; He, Z. Essential oil components from Asarum sieboldii Miquel are toxic to the house dust mite Dermatophagoides farinae. Parasitol. Res. 2012, 111, 1895. [Google Scholar] [CrossRef] [PubMed]
- Imai, T.; Masuda, R. Insecticidal activities of methyleugenol and β-asarone, from the herbal medicines Saishin and Sekishōkon, and other alkoxy-propenyl-benzene derivatives against the cigarette beetle Lasioderma serricorne (Coleoptera: Anobiidae). Appl. Entomol. Zool. 2017, 52, 183. [Google Scholar] [CrossRef]
- Kang, S.; Chung, Y.J.; Lim, J.A. Antifungal and insecticidal activity of essential oil from Asarum sieboldii against wood contaminant fungi and Lasioderma serricorne L. J. Conserv. Sci. 2012, 28, 395. [Google Scholar] [CrossRef]
- Hashimoto, K.; Yanagisawa, T.; Okui, Y.; Ikeya, Y.; Maruno, M.; Fujita, T. Studies on anti-allergic components in the roots of Asiasarum sieboldi. Planta Med. 1994, 60, 124. [Google Scholar] [CrossRef]
- Hoi, T.M. Chemical composition of essential oil of Asarum caudigerum Hance) in Huong Son, Ha Tinh. J. Biol. 2004, 26, 59–60. (In Vietnamese) [Google Scholar]
- Thai, T.H.; Hien, N.T.; Minh, D.T.; Tuan, N.A. Chemical composition of the essential oil of Asarum glabrum Merr. in Ha Giang, Vietnam. J. Biol. 2010, 32, 94–96. (In Vietnamese) [Google Scholar]
- Thai, T.H.; Hien, N.T.; Thuy, D.T.T.; Tuyen, T.T.; Van, P.T.T. Chemical composition of essential oils of some species in the genus Asarum L. in Vietnam. J. Biol. 2013, 35, 55–60. (In Vietnamese) [Google Scholar] [CrossRef]
- Thai, T.H.; Hien, N.T.; Hoi, T.M.; Tuan, N.A.; Dat, N.T.; Hai, N.T. Chemical composition of essential oils from the leaves and rhizomes, roots of the Asarum geophyllum Merr in Cao Bang province. TNU J. Sci. Technol. 2022, 227, 260–265. (In Vietnamese) [Google Scholar]
- Paul, G.R.; Lei, B.; Anne, L.; Jean, P.; Jean-Marie, D.; Jean, C.; Joseph, C.; Jean-Michel, B.; Liliane, B. (E)-Methylisoeugenol and elemicin: Antibacterial components of Daucus carota L. essential oil against Campylobacter jejuni. J. Agric. Food Chem. 2007, 55, 7332. [Google Scholar] [CrossRef]
- Ana, C.T.; Maria, J.G.; Carlos, C.; Maria, T.C.; Maria, C.L.; Jorge, C.; Lígia, R.S. Essential oil of Daucus carota subsp. halophilus: Composition, antifungal activity and cytotoxicity. J. Ethnopharmacol. 2008, 119, 129. [Google Scholar] [CrossRef]
- Thai, T.H.; Bazzali, O.; Hoi, T.M.; Tuan, N.A.; Tomi, F.; Casanova, J.; Bighelli, A. Chemical composition of the essential oils from two Vietnamese Asarum species: Asarum glabrum and Asarum cordifolium. Nat. Prod. Commun. 2013, 8, 235. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, M.E.; Mohammed, H. Biological evaluation of Safrole oil and Safrole oil Nanoemulgel as antioxidant, antidiabetic, antibacterial, antifungal and anticancer. BMC Complementary Med. Ther. 2021, 21, 159. [Google Scholar] [CrossRef]
- Lucas, F.S.G.; Fernando, P.B.; Maria, F.H.; Murilo, T.G.; Victor, P.R.; De Souza, P.F.; Jacqueline, C.L.C.; Taís, N.C.M.; Carlos, A.H.; Regina, K.T.; et al. Beta-caryophyllene as an antioxidant, anti-inflammatory and re-epithelialization activities in a rat skin wound excision model. Oxidative Med. Cell. Longev. 2022, 2022, 9004014. [Google Scholar] [CrossRef]
- Renieidy, F.C.D.; Allisson, B.J.; Evandro, A.N.; Sergio, A.L.M.; de Alberto, O.; Luis, C.S.C.; Carlos, H.G.M.; da Mylla, S.C.; da Claudio, V.S.; Guilherme, R.O.F.; et al. Chemical Composition of Seasonal Essential Oils from Psidium myrtoides O. Berg Leaves with Antimicrobial, Antiprotozoal, Antioxidant and Anti-inflammatory Potential Activities. Rev. Virtual Quim. 2022, 14, 103. [Google Scholar] [CrossRef]
- James, B.K.; James, P.N.; Harry, L.T.M. Pathogenic Escherichia coli. Nat. Rev. Microbiol. 2004, 2, 123. [Google Scholar] [CrossRef]
- Nerino, A.; Michele, M.; Mikhail, F.A.; Carmine, D.I. Escherichia coli in Europe: An Overview. Int. J. Environ. Res. Public Health 2013, 10, 6235. [Google Scholar] [CrossRef]
- Wood, S.J.; Kuzel, T.M.; Shafikhani, S.H. Pseudomonas aeruginosa: Infections, Animal Modeling, and Therapeutics. Cells 2023, 12, 199. [Google Scholar] [CrossRef]
- Abeer, H.; Baby, T.; Elsayed, F.A. Bacillus subtilis: A plant-growth promoting rhizobacterium that also impacts biotic stress. Saudi J. Biol. Sci. 2019, 26, 1291. [Google Scholar] [CrossRef]
- Rainer, B.; Antoine, D.; Colin, R.H.; Claudine, M.; Eduardo, P.C.R.; Agnieszka, S.; David, V. Bacillus subtilis, the model Gram-positive bacterium: 20 years of annotation refinement. Microb. Biotechnol. 2018, 11, 3. [Google Scholar] [CrossRef]
- Nour, A.M.; Paul, L.; Cassandra, P.; Catherine, D.R.; Albert, S.; Jean, P.L.; Virginie, M. Staphylococcus aureus Toxins: An Update on Their Pathogenic Properties and Potential Treatments. Toxins 2021, 13, 677. [Google Scholar] [CrossRef]
- Narin, A.R.; Nawfal, R.H. Staphylococcus aureus: An Overview of Discovery, Characteristics, Epidemiology, Virulence Factors and Antimicrobial Sensitivity. Eur. J. Mol. Clin. Med. 2021, 8, 1160–1183. [Google Scholar]
- Schuster, E.S.; Dunn-Coleman, N.D.-C.; Jens, F.; Van Dijck, P.W. On the safety of Aspergillus niger—A review. Appl. Microbiol. Biotechnol. 2002, 59, 426. [Google Scholar] [CrossRef] [PubMed]
- Mateusz, F.; Aleksandra, W.; Jadwiga, W.; Jaroslaw, G.; Iwona, G. Atypical Presentation of Aspergillus niger Infection in the Oral Cavity as a Prediction of Invasive Pulmonary Aspergillosis in a Patient with COVID-19: Case Report and Literature Review. Microorganisms 2022, 10, 1630. [Google Scholar] [CrossRef]
- Fravel, D.; Olivain, C.; Alabouvette, C. Fusarium oxysporum and its biocontrol. New Phytol. 2003, 157, 493. [Google Scholar] [CrossRef] [PubMed]
- Da Silva Fernandes, F.; de Souza, É.S.; Carneiro, L.M.; Alves Silva, J.P.; de Souza, J.V.B.; da Silva Batista, J. Current Ethanol Production Requirements for the Yeast Saccharomyces cerevisiae. Int. J. Microbiol. 2022, 2022, 7878830. [Google Scholar] [CrossRef] [PubMed]
- François, L.M.; Duncan, W.; Bernhard, H. Candida albicans pathogenicity mechanisms. Virulence 2013, 4, 119. [Google Scholar] [CrossRef]
- Villa, C.; Robustelli, D.; Cuna, F.S.; Russo, E.; Ibrahim, M.F.; Grignani, E.; Preda, S. MicrowaveAssisted and Conventional Extractions of Volatile Compounds from Rosa × damascena Mill. Fresh Petals for Cosmetic Applications. Molecules 2022, 27, 3963. [Google Scholar] [CrossRef]
- Sandrine, P.; Zoubida, C.D.; Emmanuel, P.; Christian, G.; Farid, C. Downscaling of Industrial Turbo-Distillation to Laboratory Turbo-Clevenger for Extraction of Essential Oils. Application of Concepts of Green Analytical Chemistry. Molecules 2019, 24, 2734. [Google Scholar] [CrossRef]
- Ninh, T.S.; Tuan, A.L.; Dinh, T.T.T.; Dinh, L.N.; Tran, T.T.; Minh, D.H.T.; Manh, H.N. Essential Oils of the Leaf and Stem of Polyalthia viridis Craib and Their Biological Activitie. Nat. Prod. Commun. 2021, 16, 1. [Google Scholar] [CrossRef]
- Mckane, L.; Kandel, J. Microbiology, Essentials and Applications; McGraw-Hill Book Company: New York, NY, USA, 1986. [Google Scholar]
- Cong, N.T.; Nhan, H.T.; Hung, L.V.; Thang, T.D.; Kuo, P.C. Synthesis and Antibacterial Activity of Analogs of 5-Arylidene-3-(4-methylcoumarin-7-yloxyacetylamino)-2-thioxo-1,3-thiazoli-din-4-one. Molecules 2014, 19, 13577. [Google Scholar] [CrossRef] [PubMed]
- Kumar, G.P.; Navyaa, K.; Ramya, E.M.; Venkataramana, M.; Anand, T.; Anilakumar, K.R. DNA damage protecting and free radical scavenging properties of Terminalia arjuna bark in PC-12 cells and plasmid DNA. Free. Radic. Antioxid. 2013, 3, 35. [Google Scholar] [CrossRef]
- Shela, G.; Olga, M.B.; Elena, K.; Antonin, L.; Milan, C.; Nuria, G.M.; Ratiporn, H.; Yong- Seo, P.; Soon-Teck, J.; Simon, T. Bioactive compounds and antioxidant potential in fresh and dried Jaffa sweeties, a new kind of citrus fruit. J. Sci. Food Agric. 2004, 14, 154. [Google Scholar] [CrossRef]
- Alhallaf, W.; Perkins, L.B. The Anti-Inflammatory Properties of Chaga Extracts Obtained by Different Extraction Methods against LPS-Induced RAW 264. Molecules 2022, 27, 4207. [Google Scholar] [CrossRef] [PubMed]
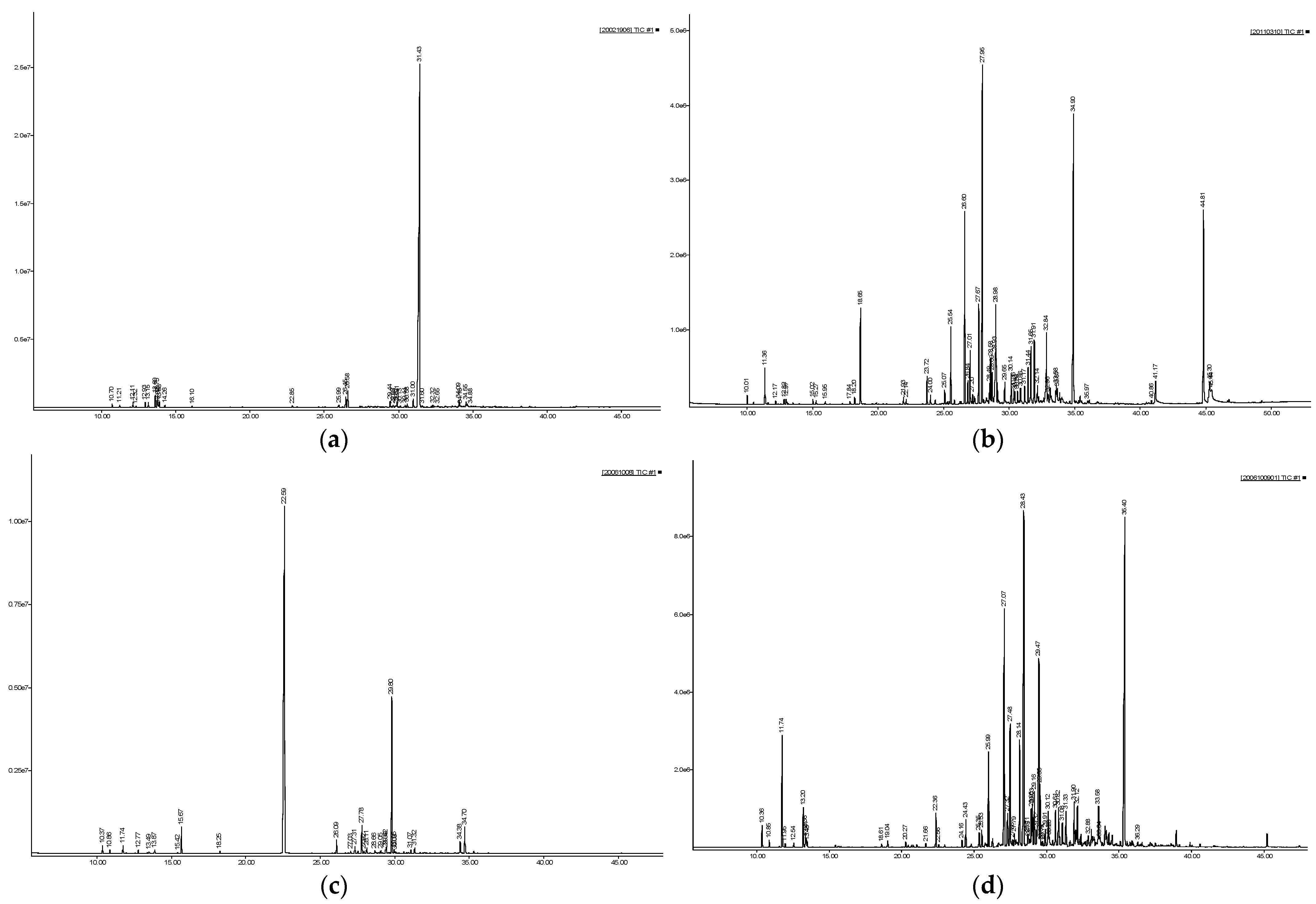
| No. | Chemical Name | Formula | A. geophilum | A. yentunensis | A. splendens | A. cordifolium | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| a RI | Time | b RI | %FID | Time | b RI | %FID | Time | b RI | %FID | Time | b RI | %FID | |||
| 1 | α-pinene | C10H16 | 932 | 10.01 | 938 | 0.33 | 10.37 | 939 | 0.84 | 10.36 | 939 | 0.84 | 10.70 | 939 | 0.56 |
| 2 | camphene | C10H16 | 946 | - | - | - | 10.87 | 955 | 0.39 | 10.86 | 955 | 0.28 | 11.21 | 956 | 0.38 |
| 3 | β-pinene | C10H16 | 986 | 11.36 | 983 | 1.45 | 11.74 | 984 | 0.91 | 11.74 | 984 | 4.71 | 12.11 | 985 | 0.96 |
| 4 | myrcene | C10H16 | 988 | - | - | - | - | - | - | 11.95 | 991 | 0.15 | 12.32 | 992 | 0.21 |
| 5 | α-phelandren | C10H16 | 1010 | 12.17 | 1009 | 0.13 | - | - | - | 12.55 | 1010 | 0.15 | 12.93 | 1010 | 0.79 |
| 6 | δ-3-carene | C10H16 | 1015 | - | - | - | 12.77 | 1016 | 0.39 | - | - | - | 13.15 | 1017 | 0.83 |
| 7 | o-cymene | C10H14 | 1022 | 12.82 | 1028 | 0.17 | - | - | - | 13.20 | 1029 | 1.22 | 13.60 | 1030 | 1.80 |
| 8 | limonene | C10H16 | 1030 | 12.97 | 1033 | 0.18 | - | - | - | 13.36 | 1034 | 0.55 | 13.75 | 1034 | 1.87 |
| 9 | β-phelandrene | C10H16 | 1031 | - | - | - | - | - | - | 13.41 | 1035 | 0.33 | 13.82 | 1036 | 0.31 |
| 10 | eucalyptol | C10H18O | 1032 | - | - | - | - | - | - | 13.48 | 1037 | 0.16 | - | - | - |
| 11 | (Z)-β-ocimene | C10H16 | 1039 | - | - | - | 13.49 | 1038 | 0.13 | - | - | - | 13.88 | 1038 | 1.33 |
| 12 | (E)-β-ocimene | C10H16 | 1050 | - | - | - | 13.87 | 1049 | 0.42 | - | - | - | 14.26 | 1049 | 0.34 |
| 13 | terpinolene | C10H16 | 1094 | 15.02 | 1093 | 0.20 | 15.43 | 1094 | 0.13 | - | - | - | |||
| 14 | linalool | C10H18O | 1095 | 15.27 | 1101 | 0.18 | 15.67 | 1101 | 3.19 | - | - | - | 16.10 | 1102 | 0.20 |
| 15 | exo-fenchol | C10H18O | 1118 | 15.95 | 1120 | 0.13 | - | - | - | - | - | - | - | - | - |
| 16 | borneol | C10H18O | 1171 | 17.84 | 1174 | 0.15 | 18.25 | 1175 | 0.31 | - | - | - | - | - | - |
| 17 | terpinen-4-ol | C10H18O | 1184 | 18.20 | 1184 | 0.30 | - | - | - | 18.61 | 1185 | 0.12 | - | - | - |
| 18 | α-terpineol | C10H18O | 1195 | 18.65 | 1197 | 4.07 | - | - | - | 19.04 | 1197 | 0.26 | - | - | - |
| 19 | thymol methyl ether | C11H16O | 1232 | - | - | - | - | - | - | 20.27 | 1233 | 0.13 | - | - | - |
| 20 | geranial | C10H16O | 1271 | - | - | - | - | - | - | 21.66 | 1273 | 0.18 | - | - | - |
| 21 | bornyl acetate | C12H20O2 | 1289 | 21.94 | 1293 | 0.23 | - | - | - | 22.36 | 1294 | 1.08 | 22.85 | 1295 | 0.23 |
| 22 | safrole | C10H10O2 | 1293 | - | - | - | 22.59 | 1300 | 64.74 | - | - | - | - | - | - |
| 23 | n-tridecane | C13H28 | 1300 | 22.14 | 1299 | 0.18 | - | - | - | 22.56 | 1299 | 0.14 | - | - | - |
| 24 | δ-elemene | C15H24 | 1343 | 23.72 | 1347 | 0.81 | - | - | - | 24.16 | 1348 | 0.30 | - | - | - |
| 25 | α-terpinyl acetate | C12H20O2 | 1349 | 24.00 | 1355 | 0.35 | - | - | - | - | - | - | |||
| 26 | endo-isocamphanyl acetate | C11H18O2 | 1350 | - | - | - | - | - | - | 24.43 | 1356 | 1.01 | - | - | - |
| 27 | geranyl acetate | C12H20O2 | 1379 | - | - | - | - | - | - | 25.35 | 1384 | 0.51 | - | - | - |
| 28 | α-copaene | C15H24 | 1394 | 25.07 | 1388 | 0.42 | - | - | - | 25.53 | 1389 | 0.46 | 26.00 | 1390 | 0.50 |
| 29 | cis-β-elemene | C15H24 | 1396 | 25.54 | 1402 | 2.75 | - | - | - | 25.99 | 1404 | 3.48 | 26.45 | 1404 | 1.84 |
| 30 | methyl eugenol | C11H14O2 | 1403 | - | - | - | 26.09 | 1407 | 1.22 | - | - | - | 26.58 | 1408 | 2.16 |
| 31 | β-caryophyllene | C15H24 | 1434 | 26.60 | 1436 | 8.05 | 27.03 | 1437 | 0.29 | 27.07 | 1438 | 9.52 | |||
| 32 | γ-elemene | C15H24 | 1440 | 26.84 | 1444 | 0.68 | - | - | - | - | - | - | - | - | - |
| 33 | α-trans-bergamotene | C15H24 | 1443 | - | - | - | 27.31 | 1446 | 0.67 | 27.32 | 1446 | 1.71 | - | - | - |
| 34 | β-gurjunene | C15H24 | 1446 | 27.01 | 1449 | 1.66 | - | - | - | 27.48 | 1451 | 4.06 | - | - | - |
| 35 | aromadendrene | C15H24 | 1449 | 27.20 | 1455 | 0.61 | - | - | - | 27.66 | 1457 | 0.60 | - | - | - |
| 36 | (Z)-β-farnesene | C15H24 | 1451 | - | - | - | 27.78 | 1461 | 2.81 | 27.79 | 1461 | 0.23 | - | - | - |
| 37 | (E)-β-farnesene | C15H24 | 1460 | - | - | - | 27.92 | 1465 | 0.19 | - | - | - | - | - | - |
| 38 | α-humulene | C15H24 | 1471 | 27.67 | 1470 | 3.87 | 28.11 | 1471 | 0.55 | 28.14 | 1472 | 3.72 | - | - | - |
| 39 | 9-epi-(E)-caryophyllene | C15H24 | 1474 | 27.95 | 1479 | 18.43 | - | - | - | 28.43 | 1481 | 15.76 | - | - | - |
| 40 | γ-curcumene | C15H24 | 1481 | - | - | - | 28.66 | 1488 | 0.24 | - | - | - | - | - | - |
| 41 | γ-muurolene | C15H24 | 1483 | - | - | - | - | - | - | 28.71 | 1490 | 0.59 | - | - | - |
| 42 | ar-curcumene | C15H24 | 1484 | - | - | - | - | - | - | 28.75 | 1491 | 0.63 | - | - | - |
| 43 | germacrene D | C15H24 | 1496 | 28.49 | 1496 | 0.56 | - | - | - | 28.95 | 1498 | 1.21 | 29.44 | 1499 | 1.00 |
| 44 | n-pentadecane | C15H32 | 1500 | 28.58 | 1499 | 1.10 | - | - | - | 29.03 | 1500 | 0.87 | - | - | - |
| 45 | (E)-methyl isoeugenol | C11H14O2 | 1501 | - | - | - | 29.05 | 1501 | 0.27 | - | - | - | - | - | - |
| 46 | β-selinene | C15H24 | 1502 | 28.69 | 1503 | 0.60 | - | - | - | 29.16 | 1505 | 1.71 | 29.62 | 1505 | 0.17 |
| 47 | δ-selinene | C15H24 | 1503 | - | - | - | - | - | - | - | - | - | 29.65 | 1507 | 0.17 |
| 48 | trans-muurola-4(14),5-diene | C15H24 | 1503 | - | - | - | - | - | - | 29.32 | 1510 | 0.94 | - | - | - |
| 49 | viridiflorene | C15H24 | 1505 | 28.93 | 1511 | 1.48 | - | - | - | - | - | - | - | - | - |
| 50 | γ-amorphene | C15H24 | 1508 | - | - | - | - | - | - | - | - | - | 29.82 | 1512 | 0.33 |
| 51 | cis-bicyclogermacrene | C15H24 | 1510 | 28.98 | 1512 | 4.46 | - | - | - | - | - | - | 29.91 | 1515 | 0.73 |
| 52 | (E,E)-α-farnesene | C15H24 | 1511 | - | - | - | 29.39 | 1512 | 0.36 | - | - | - | - | - | - |
| 53 | trans-bicyclogermacrene | C15H24 | 1512 | - | - | - | 29.42 | 1513 | 0.73 | 29.47 | 1515 | 7.50 | - | - | - |
| 54 | β-bisabolene | C15H24 | 1514 | - | - | - | - | - | - | 29.55 | 1518 | 1.65 | - | - | - |
| 55 | α-bulnesene | C15H24 | 1517 | - | - | - | - | - | - | 29.64 | 1521 | 0.14 | 30.13 | 1522 | 0.20 |
| 56 | cuparene | C15H22 | 1518 | - | - | - | - | - | - | 29.74 | 1524 | 0.16 | - | - | - |
| 57 | sesquicineole | C15H26O | 1521 | - | - | - | 29.80 | 1526 | 15.34 | - | - | - | - | - | - |
| 58 | γ-cadinene | C15H24 | 1524 | - | - | - | - | - | - | 29.91 | 1530 | 0.49 | 30.44 | 1533 | 0.27 |
| 59 | myristicin | C11H14O2 | 1529 | - | - | - | 29.95 | 1531 | 0.31 | - | - | - | - | - | - |
| 60 | β-sesquiphellandrene | C15H24 | 1531 | - | - | - | 30.03 | 1534 | 0.16 | - | - | - | - | - | - |
| 61 | δ-cadinene | C15H24 | 1533 | 29.65 | 1535 | 0.60 | - | - | - | 30.12 | 1537 | 0.72 | 30.58 | 1538 | 0.41 |
| 62 | zonarene | C15H24 | 1537 | - | - | - | - | - | - | 30.20 | 1540 | 0.24 | - | - | - |
| 63 | α-bisabolene | C15H24 | 1544 | - | - | - | - | - | - | - | - | - | 31.00 | 1552 | 1.18 |
| 64 | selina-4(15),7(11)-diene | C15H24 | 1545 | 30.15 | 1552 | 1.02 | - | - | - | 30.61 | 1553 | 1.18 | - | - | - |
| 65 | selina-3,7(11)-diene | C15H24 | 1560 | 30.36 | 1559 | 0.41 | - | - | - | 30.82 | 1561 | 1.46 | - | - | - |
| 66 | elemol | C15H26O | 1562 | 30.43 | 1561 | 0.41 | - | - | - | - | - | - | - | - | - |
| 67 | elemicin | C12H16O2 | 1565 | - | - | - | - | - | - | - | - | - | 31.43 | 1566 | 77.20 |
| 68 | (E)-nerolidol | C15H26O | 1569 | 30.64 | 1568 | 0.47 | 31.07 | 1569 | 0.41 | 31.08 | 1569 | 0.79 | 31.60 | 1572 | 0.32 |
| 69 | germacrene B | C15H24 | 1572 | 30.86 | 1575 | 0.43 | - | - | - | 31.33 | 1578 | 0.60 | - | - | - |
| 70 | isoelemicin | C12H16O2 | 1577 | - | - | - | 31.33 | 1578 | 0.32 | - | - | - | - | - | - |
| 71 | 4-epi-maaliol | C15H26O | 1577 | 31.17 | 1586 | 0.67 | - | - | - | - | - | - | - | - | - |
| 72 | scapanol | C15H26O | 1580 | - | - | - | - | - | - | - | - | - | 32.32 | 1596 | 0.33 |
| 73 | spathulenol | C15H24O | 1593 | 31.44 | 1595 | 1.24 | - | - | - | 31.90 | 1597 | 1.21 | - | - | - |
| 74 | viridiflorol | C15H26O | 1595 | 31.65 | 1602 | 2.16 | - | - | - | - | - | - | - | - | - |
| 75 | caryophyllene oxide | C15H24O | 1599 | - | - | - | - | - | - | 32.12 | 1604 | 1.31 | 32.65 | 1607 | 0.23 |
| 76 | cubeban-11-ol | C15H26O | 1601 | 31.91 | 1611 | 2.27 | - | - | - | - | - | - | - | - | - |
| 77 | rosifoliol | C15H26O | 1615 | 32.14 | 1620 | 0.62 | - | - | - | - | - | - | - | - | - |
| 78 | humulene epoxideII | C15H24O | 1620 | - | - | - | - | - | - | 32.88 | 1631 | 0.14 | - | - | - |
| 79 | γ-eudesmol | C15H26O | 1646 | 32.96 | 1648 | 0.29 | - | - | - | 33.58 | 1656 | 1.31 | - | - | - |
| 80 | α-asarone | C12H16O3 | 1650 | - | - | - | - | - | - | - | - | - | 34.09 | 1658 | 0.73 |
| 81 | α-muurolol | C15H26O | 1654 | - | - | - | - | - | - | - | - | - | 34.18 | 1661 | 0.24 |
| 82 | α-cadinol | C15H26O | 1665 | 33.58 | 1670 | 0.74 | - | - | - | 33.64 | 1658 | 0.16 | 34.55 | 1674 | 0.84 |
| 83 | neo-intermedeol | C15H26O | 1670 | 33.67 | 1674 | 1.01 | - | - | - | - | - | - | - | - | - |
| 84 | β-asarone | C12H16O3 | 1678 | - | - | - | 34.38 | 1684 | 0.98 | - | - | - | 34.88 | 1686 | 0.14 |
| 85 | eudesm-7(11)-en-4-ol | C15H26O | 1709 | 34.90 | 1718 | 13.41 | - | - | - | 35.40 | 1721 | 14.21 | - | - | - |
| 86 | (Z)-ligustilide | C12H14O2 | 1741 | - | - | - | - | - | - | 36.29 | 1755 | 0.48 | - | - | - |
| 87 | n-tetradecanoic acid | C14H28O | 1759 | 35.97 | 1758 | 0.18 | - | - | - | - | - | - | - | - | - |
| 88 | isophytol | C20H40O | 1946 | 40.86 | 1949 | 0.12 | - | - | - | - | - | - | - | - | - |
| 89 | n-hexadecanoic acid | C16H32O2 | 1959 | 41.17 | 1962 | 1.31 | - | - | - | - | - | - | - | - | - |
| 90 | phytol | C20H40O | 2122 | 44.81 | 2117 | 7.23 | - | - | - | - | - | - | - | - | - |
| 91 | linoleic acid | C18H32O | 2132 | 45.30 | 2139 | 2.45 | - | - | - | - | - | - | - | - | - |
| 92 | linolenic acid | C18H32O | 2143 | 45.44 | 2145 | 2.31 | - | - | - | - | - | - | - | - | - |
| Monoterpene hydrocarbons | 5 (2.46%) | 7 (3.21%) | 7 (7.01%) | 10 (7.58%) | |||||||||||
| Oxigenated monoterpenes | 7 (5.41%) | 2 (3.5%) | 8 (3.45%) | 2 (0.43%) | |||||||||||
| Sesquiterpene hydrocarbons | 17 (46.84%) | 9 (6.0%) | 25 (59.06%) | 11 (6.53%) | |||||||||||
| Oxigenated sesquiterpenes | 11 (23.29%) | 2 (15.75%) | 7 (19.13%) | 5 (1.96%) | |||||||||||
| Derivatives of benzene (benzenoids) | 1 (0.17%) | 6 (67.84%) | 1 (1.22%) | 5 (82.03%) | |||||||||||
| Others | 8 (14.88%) | - | 3 (1.49%) | - | |||||||||||
| Total | 93.05% | 96.30% | 91.36% | 98.78% | |||||||||||
| Sample | MIC (μg/mL) | |||||||
|---|---|---|---|---|---|---|---|---|
| EC | PA | BS | SA | AN | FO | SC | CA | |
| A. geophilum EO | 200 | 200 | - | - | - | 200 | 200 | - |
| A. yentunensis EO | 200 | - | - | 100 | 200 | - | - | 200 |
| A. splendens EO | 200 | - | - | 200 | - | 200 | - | - |
| A. cordifolium EO | 200 | - | 100 | - | - | - | - | 200 |
| No. | Sample | SC(%) Values | SC50 Values (µg/mL) |
|---|---|---|---|
| Positive control: ascorbic acid | 81.55 ± 0.9 | 13.26 | |
| Negative control [DPPH/EtOH+ DMSO] | 0.0 ± 0.0 | - | |
| 1 | A. geophilum EO | 63.34 ± 1.0 | 28.57 |
| 2 | A. yentunensis EO | 51.58 ± 0.5 | 50.24 |
| 3 | A. splendens EO | 34.24 ± 1.4 | >100 |
| 4 | A. cordifolium EO | 57.86 ± 0.8 | 39.62 |
| No. | Sample * | The Percentages of Inhibition of NO Production (%) | The Percentages of Cell Life (%) | IC50 Values |
|---|---|---|---|---|
| Positive control: Cardamonin | 85.40 ± 0.7 | 71.80 ± 0.5 | 2.33 µM | |
| Negative control | 100.00 ± 0.8 | 100.99 ± 1.0 | - | |
| LPS | 0.00 ± 0.0 | - | - | |
| 1 | A. geophilum EO | 58.20 ± 0.4 | 78.05 ± 0.8 | 40.35 µg/mL |
| 2 | A. yentunensis EO | 53.14 ± 1.6 | 66.87 ± 1.5 | 49.87 µg/mL |
| 3 | A. splendens EO | 69.58 ± 1.3 | 81.85 ± 0.9 | 21.68 µg/mL |
| 4 | A. cordifolium EO | 40.87 ± 0.6 | 60.65 ± 1.4 | 66.37 µg/mL |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Minh, P.T.H.; Tuan, N.T.; Van, N.T.H.; Bich, H.T.; Lam, D.T. Chemical Composition and Biological Activities of Essential Oils of Four Asarum Species Growing in Vietnam. Molecules 2023, 28, 2580. https://doi.org/10.3390/molecules28062580
Minh PTH, Tuan NT, Van NTH, Bich HT, Lam DT. Chemical Composition and Biological Activities of Essential Oils of Four Asarum Species Growing in Vietnam. Molecules. 2023; 28(6):2580. https://doi.org/10.3390/molecules28062580
Chicago/Turabian StyleMinh, Pham Thi Hong, Nguyen Thuong Tuan, Nguyen Thi Hong Van, Hoang Thi Bich, and Do Tien Lam. 2023. "Chemical Composition and Biological Activities of Essential Oils of Four Asarum Species Growing in Vietnam" Molecules 28, no. 6: 2580. https://doi.org/10.3390/molecules28062580
APA StyleMinh, P. T. H., Tuan, N. T., Van, N. T. H., Bich, H. T., & Lam, D. T. (2023). Chemical Composition and Biological Activities of Essential Oils of Four Asarum Species Growing in Vietnam. Molecules, 28(6), 2580. https://doi.org/10.3390/molecules28062580








