Analysis of Essential Oils Components from Aromatic Plants Using Headspace Repellent Method against Aedes aegypti Mosquitoes
Abstract
1. Introduction
2. Results
2.1. Distillation and Yield of Essential Oils
2.2. Bioactivity Test of the Essentials Oils against Aedes aegypti Mosquitoes
2.3. Activity Test of the Patchouli and Cinnamon Bark Essential Oils Aroma Using Headspace Repellent Method
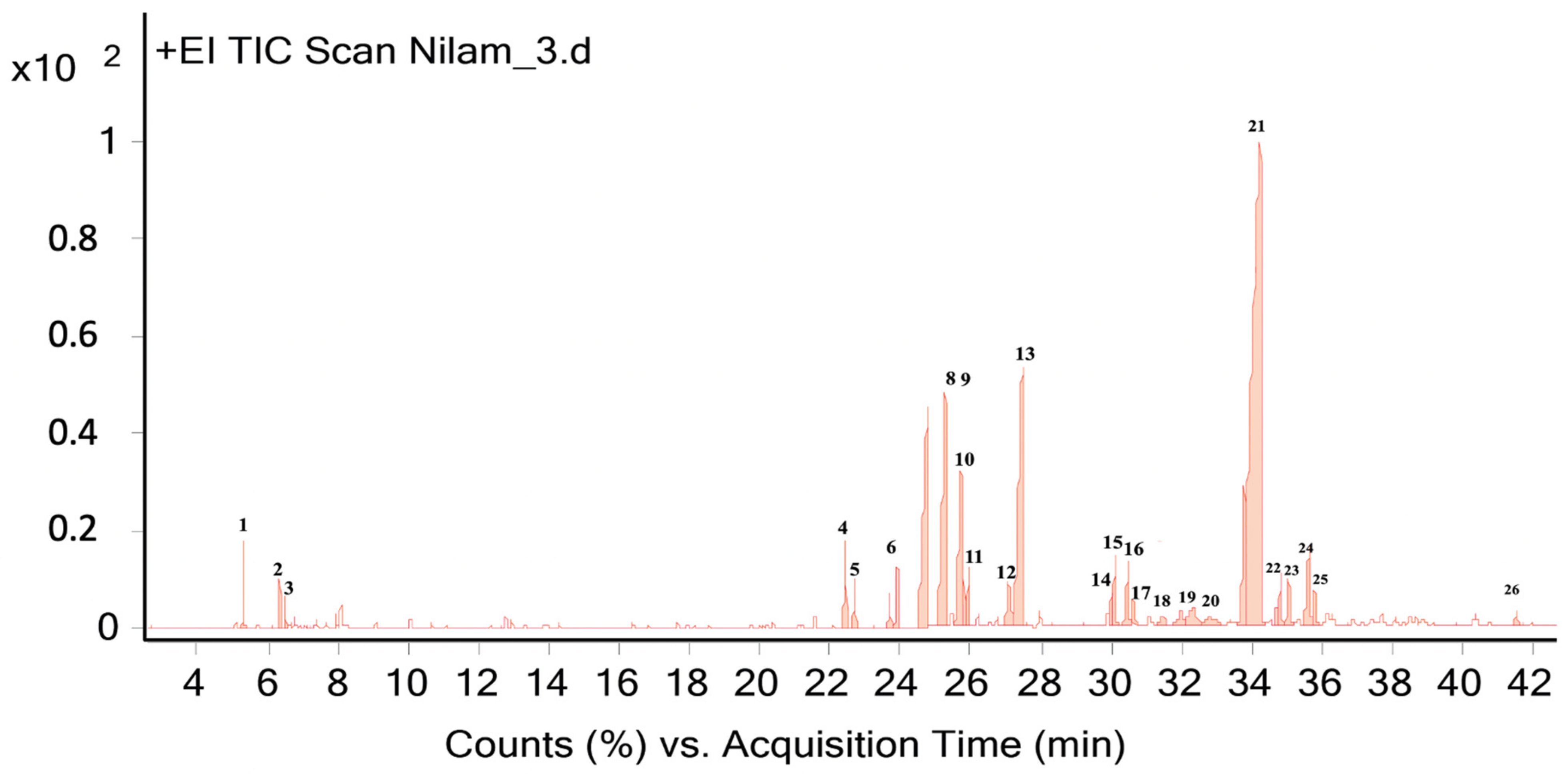
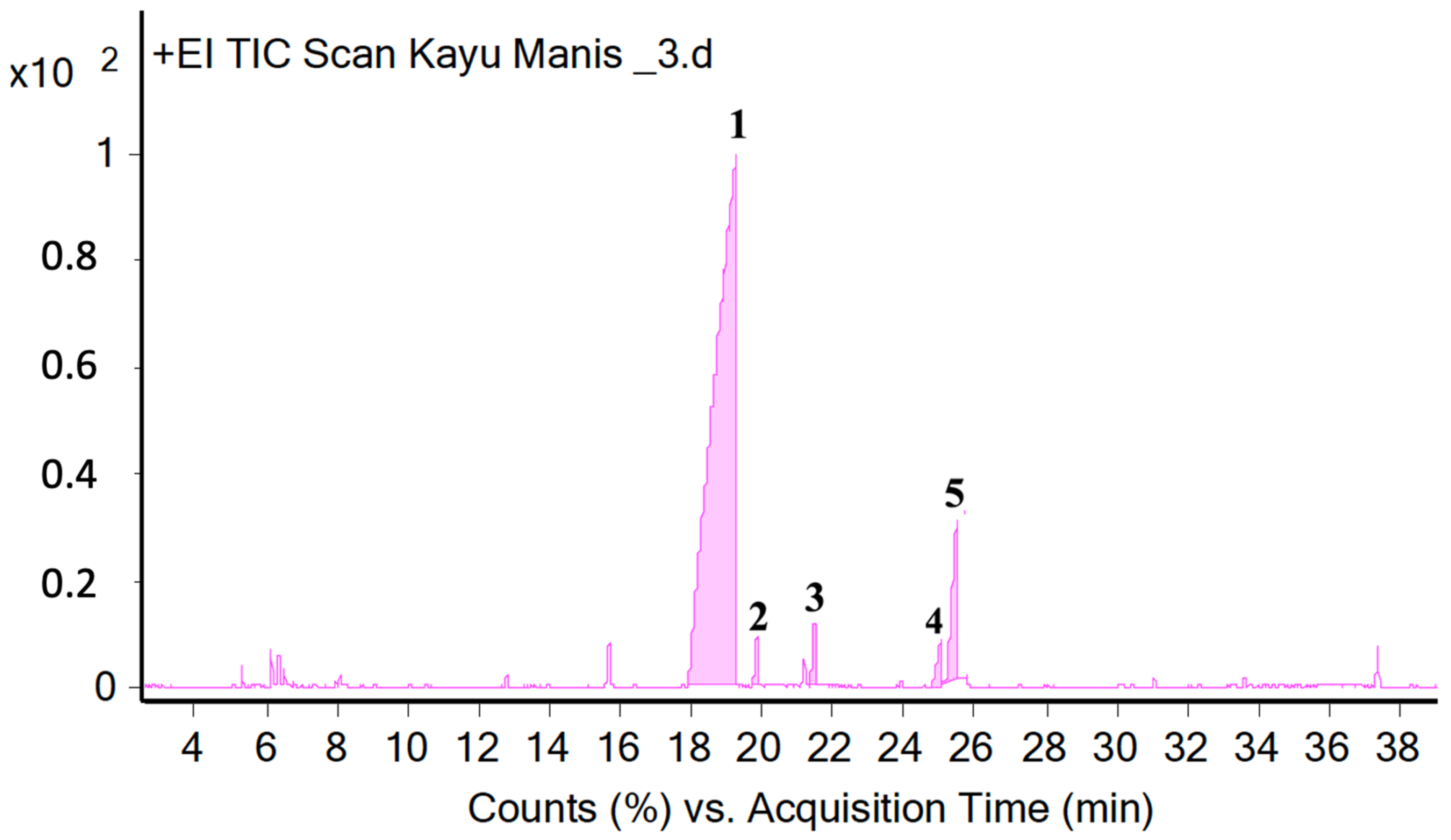
2.4. Analysis of Essential Oils Aromas’ Components Using GC-MS Method
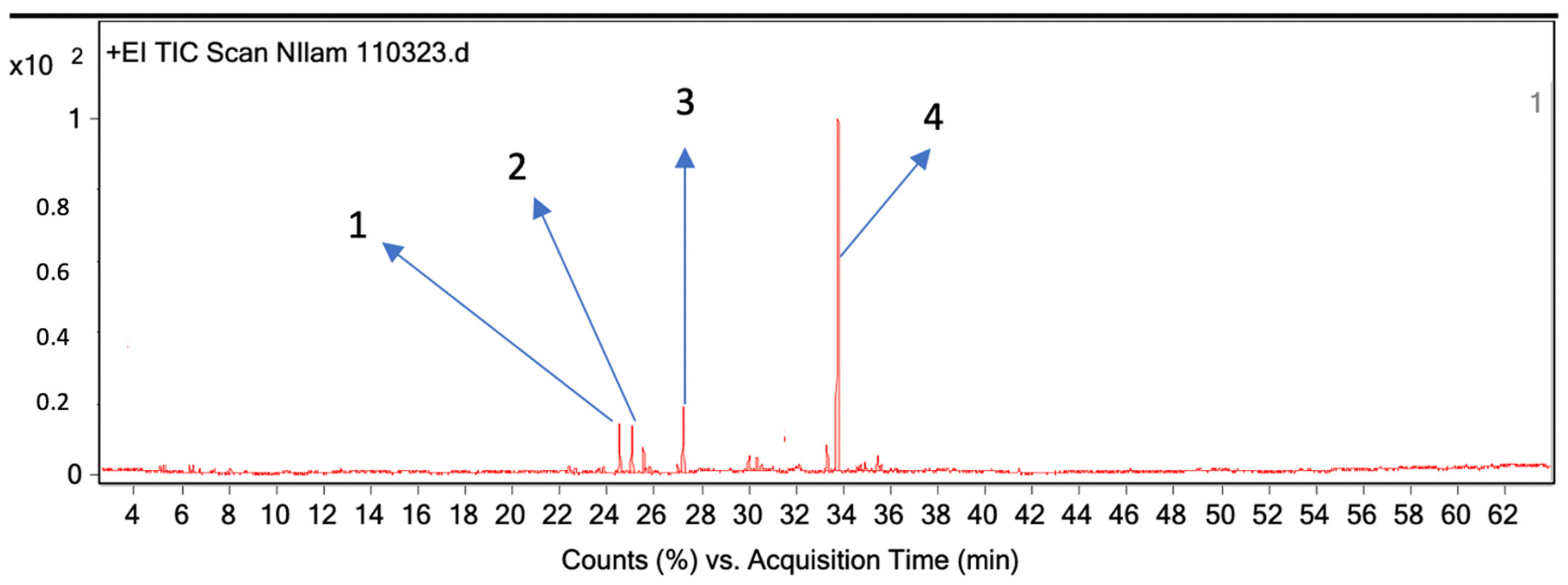
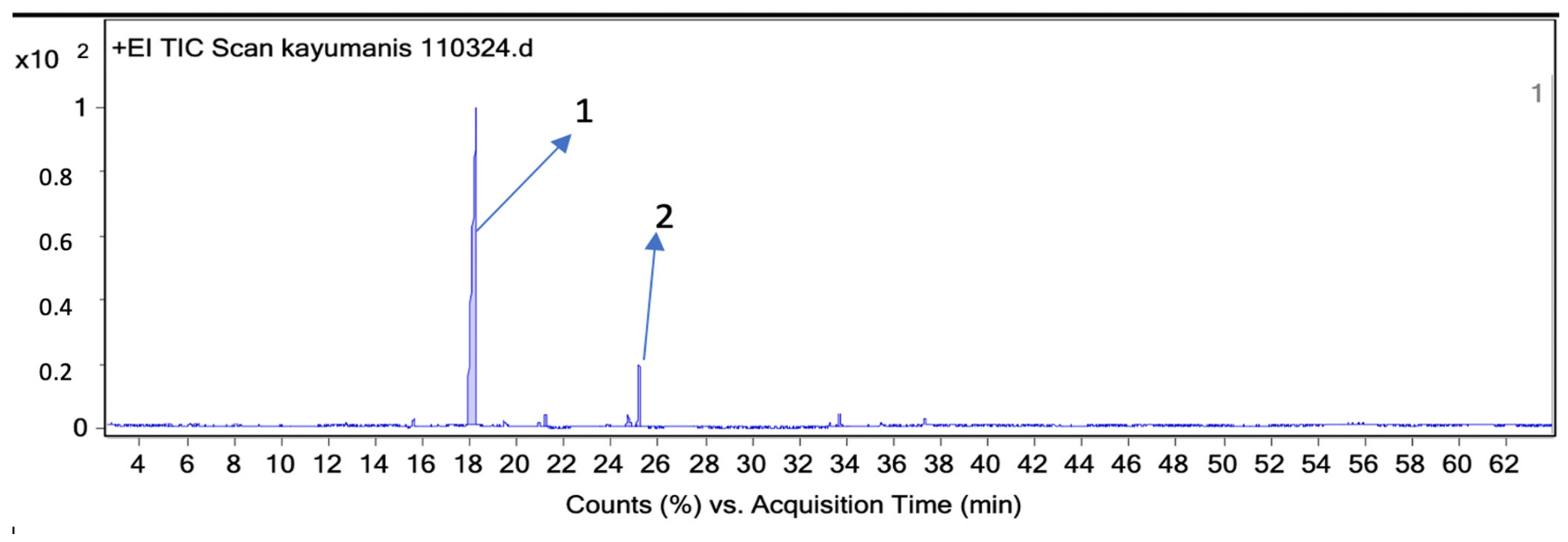
2.5. Analysis of Essential Oils Aromas’ Components Using GC-MS Headspace Repellent Method
3. Discussion
4. Materials and Methods
4.1. Samples
4.2. Essential Oils Isolation
4.3. Activity Evaluation of Essential Oils’ Repellent Power
4.4. Analysis of Essential Oil Aromas’ Components Using Gas Chromatography–Mass Spectrometry (GC-MS)
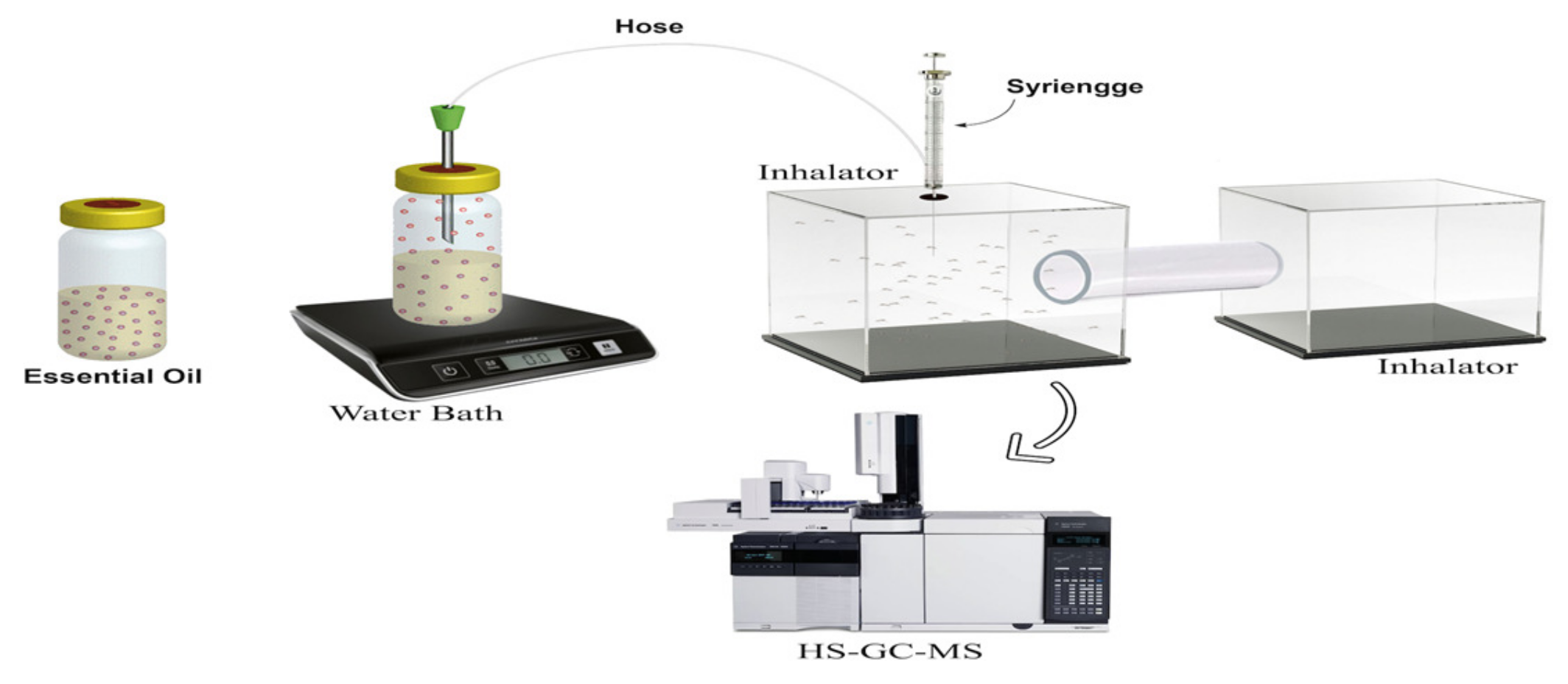
4.5. Equipment Development
4.6. Analysis of Essential Oils Activities Test Using Headspace Repellent Method
4.7. Analysis of Essential Oils Aromas’ Components Using GC-MS Headspace Repellent
4.8. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
Abbreviations
| LRIx: | Linear retention index of component x that is investigated |
| tx: | Retention time of component x (min) |
| tn: | Retention time of standard alkane with n carbon atoms eluted before the retention time of component x |
| tn+1: | Retention time of standard alkane with n+1 carbon atoms eluted before the retention time of component x |
| n: | Number of carbon atoms in the standard alkane eluted before component x |
References
- Leonardi, M.; Ambryszewska, K.E.; Melai, B.; Flamini, G.; Cioni, P.L.; Parri, F.; Pistelli, L. Essential-Oil Composition of Helichrysum italicum (Roth) G.Don ssp. italicum from Elba Island (Tuscany, Italy). Chem. Biodivers. 2013, 10, 343–355. [Google Scholar] [CrossRef]
- Rouis, Z.; Laamari, A.; Abid, N.; Elaissi, A.; Cioni, P.L.; Flamini, G.; Aouni, M. Chemical composition and larvicidal activity of several essential oils from Hypericum species from Tunisia. Parasitol. Res. 2013, 112, 699–705. [Google Scholar] [CrossRef] [PubMed]
- Shelton, D.; Zabaras, D.; Chohan, S.; Wyllie, S.G.; Baverstock, P.; Leach, D.; Henry, R. Isolation and partial characterisation of a putative monoterpene synthase from Melaleuca alternifolia. Plant Physiol. Biochem. 2004, 42, 875–882. [Google Scholar] [CrossRef]
- Sadeghi, H.; Tahery, Y.; Moradi, S. Intra- and inter-specific variation of turpentine composition in Eldar pine (Pinus eldarica Medw.) and black pine (Pinus nigra Arnold). Biochem. Syst. Ecol. 2013, 48, 189–193. [Google Scholar] [CrossRef]
- Del Terra, L.; Lonzarich, V.; Asquini, E.; Navarini, L.; Graziosi, G.; Suggi Liverani, F.; Pallavicini, A. Functional characterization of three Coffea arabica L. monoterpene synthases: Insights into the enzymatic machinery of coffee aroma. Phytochemistry 2013, 89, 6–14. [Google Scholar] [CrossRef] [PubMed]
- Murungi, L.K.; Kirwa, H.; Torto, B. Differences in essential oil content of berries and leaves of Solanum sarrachoides (Solanaceae) and the effects on oviposition of the tomato spider mite (Tetranychus evansi). Ind. Crops Prod. 2013, 46, 73–79. [Google Scholar] [CrossRef]
- Wang, S.; Alseekh, S.; Fernie, A.R.; Luo, J. The Structure and Function of Major Plant Metabolite Modifications. Mol. Plant 2019, 12, 899–919. [Google Scholar] [CrossRef]
- Hakimi, Y.; Fatahi, R.; Shokrpour, M.; Naghavi, M.R. Investigation of Germination Characteristics of Four Medicinal Plants Seed (Lavender, Hyssop, Black cumin and Scrophularia) Under Interaction between Salinity Stress and Temperature Levels. J. Genet. Resour. 2022, 8, 35–45. [Google Scholar] [CrossRef]
- Kaur, G.; Ganjewala, D.; Bist, V.; Verma, P.C. Antifungal and larvicidal activities of two acyclic monoterpenes; citral and geraniol against phytopathogenic fungi and insects. Arch. Phytopathol. Plant Prot. 2019, 52, 458–469. [Google Scholar] [CrossRef]
- Bora, H.; Kamle, M.; Mahato, D.K.; Tiwari, P.; Kumar, P. Citrus Essential Oils (CEOs) and Their Applications in Food: An Overview. Plants 2020, 9, 357. [Google Scholar] [CrossRef]
- Islam, M.T.; da Mata, A.M.O.F.; de Aguiar, R.P.S.; Paz, M.F.C.J.; de Alencar, M.V.O.B.; Ferreira, P.M.P.; de Carvalho Melo-Cavalcante, A.A. Therapeutic Potential of Essential Oils Focusing on Diterpenes. Phytother. Res. 2016, 30, 1420–1444. [Google Scholar] [CrossRef]
- Rafie, S.; Namjoyan, F.; Golfakhrabadi, F.; Yousefbeyk, F.; Hassanzadeh, A. Effect of lavender essential oil as a prophylactic therapy for migraine: A randomized controlled clinical trial. J. Herb. Med. 2016, 6, 18–23. [Google Scholar] [CrossRef]
- Cao, H.; Urban, J.F., Jr.; Anderson, R.A. Cinnamon Polyphenol Extract Affects Immune Responses by Regulating Anti- and Proinflammatory and Glucose Transporter Gene Expression in Mouse Macrophages. J. Nutr. 2008, 138, 833–840. [Google Scholar] [CrossRef]
- Duque, J.; Martins, M.; Anjos, A.; Kuwabara, E.; Navarro-Silva, M. Susceptibility of Aedes aegypti to temephos and cypermethrin insecticides, Brazil [Susceptibilidade de Aedes aegypti aos inseticidas temephos e cipermetrina, Brasil]. Rev. Saúde Pública 2005, 38, 842–843. [Google Scholar] [CrossRef]
- Magalhães, F.J.R.; da Silva, J.G.; Ribeiro-Andrade, M.; Pinheiro, J.W.; Aparecido Mota, R. High prevalence of toxoplasmosis in free-range chicken of the Fernando de Noronha Archipelago, Brazil. Acta Trop. 2016, 159, 58–61. [Google Scholar] [CrossRef]
- Setlur, A.S.; Karunakaran, C.; Pandey, S.; Sarkar, M.; Niranjan, V. Molecular interaction studies of thymol via molecular dynamic simulations and free energy calculations using multi-target approach against Aedes aegypti proteome to decipher its role as mosquito repellent. Mol. Simul. 2023, 49, 325–340. [Google Scholar] [CrossRef]
- Tapondjou, L.A.; Adler, C.; Bouda, H.; Fontem, D.A. Efficacy of powder and essential oil from Chenopodium ambrosioides leaves as post-harvest grain protectants against six-stored product beetles. J. Stored Prod. Res. 2002, 38, 395–402. [Google Scholar] [CrossRef]
- Nerio, L.S.; Olivero-Verbel, J.; Stashenko, E. Repellent activity of essential oils: A review. Bioresour. Technol. 2010, 101, 372–378. [Google Scholar] [CrossRef]
- Katerinopoulos, H.E.; Pagona, G.; Afratis, A.; Stratigakis, N.; Roditakis, N. Composition and insect attracting activity of the essential oil of Rosmarinus officinalis. J. Chem. Ecol. 2005, 31, 111–122. [Google Scholar] [CrossRef]
- Tu, X.-F.; Hu, F.; Thakur, K.; Li, X.-L.; Zhang, Y.-S.; Wei, Z.-J. Comparison of antibacterial effects and fumigant toxicity of essential oils extracted from different plants. Ind. Crops Prod. 2018, 124, 192–200. [Google Scholar] [CrossRef]
- Hernández-Lambraño, R.; Caballero-Gallardo, K.; Olivero-Verbel, J. Toxicity and antifeedant activity of essential oils from three aromatic plants grown in Colombia against Euprosterna elaeasa and Acharia fusca (Lepidoptera: Limacodidae). Asian Pac. J. Trop. Biomed. 2014, 4, 695–700. [Google Scholar] [CrossRef]
- Gunderson, R.; Stuart, D.; Petersen, B. Materialized ideology and environmental problems: The cases of solar geoengineering and agricultural biotechnology. Eur. J. Soc. Theory 2019, 23, 389–410. [Google Scholar] [CrossRef]
- Kannan, M.; Padmanaban, B.; Ashif, K. Evaluation of Biopesticide Formulations against Banana Stem Weevil Odoiporus longicollis (Olivier). Indian J. Entomol. 2021, 84, 690–692. [Google Scholar] [CrossRef]
- Zahra, S.; Amir, A.; Hamid Reza, B.; Seyed Hassan Moosa, K.; Mohammad Mehdi, S.; Kamal, A.; Majid, A.; Fatemehzahra, A. Repellent Efficacy of Eucalyptus globulus and Syzygium aromaticum Essential Oils against Malaria Vector, Anopheles stephensi (Diptera: Culicidae). Iran. J. Public Health 2021, 50, 1668. [Google Scholar] [CrossRef]
- Cansian, R.; Astolfi, V.; Cardoso, R.I.; Paroul, N.; Roman, S.S.; Mielniczki-Pereira, A.A.; Pauletti, G.; Mossi, A. Insecticidal and repellent activity of the essential oil of Cinnamomum camphora var. linaloolifera Y. Fujita (Ho-Sho) and Cinnamomum camphora (L.) J Presl. var. hosyo (Hon-Sho) on Sitophilus zeamais Mots. (Coleoptera, Curculionedae). Rev. Bras. Plantas Med. 2015, 17, 769–773. [Google Scholar] [CrossRef]
- Du, S.-S.; Yang, K.; Wang, C.-F.; You, C.-X.; Geng, Z.-F.; Guo, S.-S.; Deng, Z.-W.; Liu, Z.-L. Chemical Constituents and Activities of the Essential Oil from Myristica fragrans against Cigarette Beetle Lasioderma serricorne. Chem. Biodivers. 2014, 11, 1449–1456. [Google Scholar] [CrossRef]
- Ibáñez, M.D.; Blázquez, M.A. Curcuma longa L. Rhizome Essential Oil from Extraction to Its Agri-Food Applications. A Review. Plants 2021, 10, 44. [Google Scholar] [CrossRef]
- Burfield, T.; Reekie, S.L. Mosquitoes, malaria and essential oils. Int. J. Aromather. 2005, 15, 30–41. [Google Scholar] [CrossRef]
- Diabate, S.; Martin, T.; Murungi, L.K.; Fiaboe, K.K.M.; Subramanian, S.; Wesonga, J.; Deletre, E. Repellent activity of Cymbopogon citratus and Tagetes minuta and their specific volatiles against Megalurothrips sjostedti. J. Appl. Entomol. 2019, 143, 855–866. [Google Scholar] [CrossRef]
- Gokulakrishnan, J.; Kuppusamy, E.; Shanmugam, D.; Appavu, A.; Kaliyamoorthi, K. Pupicidal and repellent activities of Pogostemon cablin essential oil chemical compounds against medically important human vector mosquitoes. Asian Pac. J. Trop. Dis. 2013, 3, 26–31. [Google Scholar] [CrossRef]
- Noel, J.P.; Austin, M.B.; Bomati, E.K. Structure–function relationships in plant phenylpropanoid biosynthesis. Curr. Opin. Plant Biol. 2005, 8, 249–253. [Google Scholar] [CrossRef]
- Bergman, M.E.; Davis, B.; Phillips, M.A. Medically Useful Plant Terpenoids: Biosynthesis, Occurrence, and Mechanism of Action. Molecules 2019, 24, 3961. [Google Scholar] [CrossRef]
- Amer, A.; Mehlhorn, H. Repellency effect of forty-one essential oils against Aedes, Anopheles, and Culex mosquitoes. Parasitol. Res. 2006, 99, 478–490. [Google Scholar] [CrossRef]
- Rodríguez-Maecker, R.; Vyhmeister, E.; Meisen, S.; Rosales Martinez, A.; Kuklya, A.; Telgheder, U. Identification of terpenes and essential oils by means of static headspace gas chromatography-ion mobility spectrometry. Anal. Bioanal. Chem. 2017, 409, 6595–6603. [Google Scholar] [CrossRef]
- Bicchi, C.; Joulain, D. Review headspace-gas chromatographic analysis of medicinal and aromatic plants and flowers. Flavour Fragr. J. 1990, 5, 131–145. [Google Scholar] [CrossRef]
- Masike, K.; Stander, M.A.; de Villiers, A. Recent applications of ion mobility spectrometry in natural product research. J. Pharm. Biomed. Anal. 2021, 195, 113846. [Google Scholar] [CrossRef]
- Gallegos, J.; Garrido-Delgado, R.; Arce, L.; Medina, L.M. Volatile Metabolites of Goat Cheeses Determined by Ion Mobility Spectrometry. Potential Applications in Quality Control. Food Anal. Methods 2015, 8, 1699–1709. [Google Scholar] [CrossRef]
- Gavahian, M.; Chu, Y.-H. Ohmic accelerated steam distillation of essential oil from lavender in comparison with conventional steam distillation. Innov. Food Sci. Emerg. Technol. 2018, 50, 34–41. [Google Scholar] [CrossRef]
- Sharma, S. Aroma Therapy; Sterling Publishers Private Limited: New Delhi, India, 2012; Volume 1, p. 96. [Google Scholar]
- Adams, R.P. Identification of Essential Oil Components by Gas Chromatography/Mass Spectrometry; Allured Publishing Corporation: Carol Steam, IL, USA, 2007; Volume 4. [Google Scholar]
- Adams, R.P. Identification of Essential Oil Components by Gas Chromatography/Mass Spectrometry; Allured Publishing Corporation: Carol Stream, IL, USA, 1995; Volume 4. [Google Scholar]
- Buchin, S.; Salmon, J.C.; Carnat, A.P.; Berger, T.; Bugaud, C.; Bosset, J.O. Identification de composés monoterpéniques, sesquiterpéniques et benzéniques dans un lait d’alpage très riche en ces substances. Mitt Lebensm. 2002, 93, 199–216. [Google Scholar]
- Lucero, M.E.; Estell, R.E.; Fredrickson, E.L. The Essential Oil Composition of Psorothamnus scoparius (A. Gray) Rydb. J. Essent. Oil Res. 2003, 15, 108–111. [Google Scholar] [CrossRef]
- Choi, H.S. Character impact odorants of Citrus Hallabong [(C. unshiu Marcov x C. sinensis Osbeck) × C. reticulata Blanco] cold-pressed peel oil. J Agric Food Chem 2003, 51, 2687–2692. [Google Scholar] [CrossRef] [PubMed]
- Martins, F.T.; Santos, M.H.; Polo, M.; Barbosa, L.C.A. Effects of the interactions among macronutrients, plant age and photoperiod in the composition of Hyptis suaveolens (L.) Poit essential oil from Alfenas (MG), Brazil. Flavour Fragr. J. 2007, 22, 123–129. [Google Scholar] [CrossRef]
- Acosta, S.; Chiralt, A.; Santamarina, P.; Rosello, J.; González-Martínez, C.; Cháfer, M. Antifungal films based on starch-gelatin blend, containing essential oils. Food Hydrocoll. 2016, 61, 233–240. [Google Scholar] [CrossRef]
- Sri Sulasmi, E.; Indriwati, S.E.; Suarsini, E. Preparation of Various Type of Medicinal Plants Simplicia as Material of Jamu Herbal. In Proceedings of the Inter Conference on Education, Malang, East Java, Indonesia, 22–24 November 2016; pp. 1014–1024. [Google Scholar]
- Harbourne, N.; Marete, E.; Jacquier, J.C.; O’Riordan, D. Effect of drying methods on the phenolic constituents of meadowsweet (Filipendula ulmaria) and willow (Salix alba). LWT—Food Sci. Technol. 2009, 42, 1468–1473. [Google Scholar] [CrossRef]
- Jugreet, B.S.; Suroowan, S.; Rengasamy, R.R.K.; Mahomoodally, M.F. Chemistry, bioactivities, mode of action and industrial applications of essential oils. Trends Food Sci. Technol. 2020, 101, 89–105. [Google Scholar] [CrossRef]
- Domingues, R.M.A.; Sousa, G.D.A.; Silva, C.M.; Freire, C.S.R.; Silvestre, A.J.D.; Neto, C.P. High value triterpenic compounds from the outer barks of several Eucalyptus species cultivated in Brazil and in Portugal. Ind. Crops Prod. 2011, 33, 158–164. [Google Scholar] [CrossRef]
- de Elguea-Culebras, G.O.; Bravo, E.M.; Sánchez-Vioque, R. Potential sources and methodologies for the recovery of phenolic compounds from distillation residues of Mediterranean aromatic plants. An approach to the valuation of by-products of the essential oil market—A review. Ind. Crops Prod. 2022, 175, 114261. [Google Scholar] [CrossRef]
- Sabry, B.A.; Farouk, A.; Badr, A.N. Bioactivity evaluation for volatiles and water extract of commercialized star anise. Heliyon 2021, 7, e07721. [Google Scholar] [CrossRef]
- Gleiser, R.M.; Bonino, M.A.; Zygadlo, J.A. Repellence of essential oils of aromatic plants growing in Argentina against Aedes aegypti (Diptera: Culicidae). Parasitol. Res. 2011, 108, 69–78. [Google Scholar] [CrossRef]
- Luo, C.; Li, Y.; Wang, H.; Cui, Y.; Feng, Z.; Li, H.; Li, Y.; Wang, Y.; Wurtz, K.; Weber, P.; et al. Hydroxytyrosol promotes superoxide production and defects in autophagy leading to anti-proliferation and apoptosis on human prostate cancer cells. Curr. Cancer Drug Targets 2013, 13, 625–639. [Google Scholar] [CrossRef]
- Vickers, N.J. Animal Communication: When I’m Calling You, Will You Answer Too? Curr. Biol. 2017, 27, R713–R715. [Google Scholar] [CrossRef]
- Jain, P.L.B.; Patel, S.R.; Desai, M.A. Patchouli oil: An overview on extraction method, composition and biological activities. J. Essent. Oil Res. 2022, 34, 1–11. [Google Scholar] [CrossRef]
- Pavela, R. Lethal and Sublethal Effects of Thyme Oil (Thymus vulgaris L.) on the House Fly (Musca domestica Lin.). J. Essent. Oil Bear. Plants 2013, 10, 346–356. [Google Scholar] [CrossRef]
- Wu, X.L.; Ju, D.H.; Chen, J.; Yu, B.; Liu, K.L.; He, J.X.; Dai, C.Q.; Wu, S.; Chang, Z.; Wang, Y.P.; et al. Immunologic mechanism of Patchouli alcohol anti-H1N1 influenza virus may through regulation of the RLH signal pathway in vitro. Curr. Microbiol. 2013, 67, 431–436. [Google Scholar] [CrossRef]
- Jeong, J.B.; Shin, Y.K.; Lee, S.H. Anti-inflammatory activity of patchouli alcohol in RAW264.7 and HT-29 cells. Food Chem. Toxicol. 2013, 55, 229–233. [Google Scholar] [CrossRef]
- Cheng, Z.; Liang, P.; Yu, X.; Han, Z.; Liu, F.; Yu, J.; Li, X. Percutaneous microwave ablation for benign focal liver lesions: Initial clinical results. Oncol. Lett. 2017, 13, 429–434. [Google Scholar] [CrossRef]
- Zheng, Y.-F.; Xie, J.-H.; Xu, Y.-F.; Liang, Y.-Z.; Mo, Z.-Z.; Jiang, W.-W.; Chen, X.-Y.; Liu, Y.-H.; Yu, X.-D.; Huang, P.; et al. Gastroprotective effect and mechanism of patchouli alcohol against ethanol, indomethacin and stress-induced ulcer in rats. Chem.-Biol. Interact. 2014, 222, 27–36. [Google Scholar] [CrossRef]
- Qu, C.; Yuan, Z.W.; Yu, X.T.; Huang, Y.F.; Yang, G.H.; Chen, J.N.; Lai, X.P.; Su, Z.R.; Zeng, H.F.; Xie, Y.; et al. Patchouli alcohol ameliorates dextran sodium sulfate-induced experimental colitis and suppresses tryptophan catabolism. Pharmacol. Res. 2017, 121, 70–82. [Google Scholar] [CrossRef]
- Wu, J.; Gan, Y.; Li, M.; Chen, L.; Liang, J.; Zhuo, J.; Luo, H.; Xu, N.; Wu, X.; Wu, Q.; et al. Patchouli alcohol attenuates 5-fluorouracil-induced intestinal mucositis via TLR2/MyD88/NF-kB pathway and regulation of microbiota. Biomed Pharmacother. 2020, 124, 109883. [Google Scholar] [CrossRef]
- De Oliveira, M.M.M.; Brugnera, D.F.; do Nascimento, J.A.; Batista, N.N.; Piccoli, R.H. Cinnamon essential oil and cinnamaldehyde in the control of bacterial biofilms formed on stainless steel surfaces. Eur. Food Res. Technol. 2012, 234, 821–832. [Google Scholar] [CrossRef]
- Golmohammad, F.; Eikani, M.H.; Maymandi, H.M. Cinnamon Bark Volatile Oils Separation and Determination Using Solid-Phase Extraction and Gas Chromatography. Procedia Eng. 2012, 42, 247–260. [Google Scholar] [CrossRef]
- Moghimi, R.; Aliahmadi, A.; Rafati, H. Ultrasonic nanoemulsification of food grade trans-cinnamaldehyde: 1,8-Cineol and investigation of the mechanism of antibacterial activity. Ultrason. Sonochem. 2017, 35, 415–421. [Google Scholar] [CrossRef] [PubMed]
- Shen, S.; Zhang, T.; Yuan, Y.; Lin, S.; Xu, J.; Ye, H. Effects of cinnamaldehyde on Escherichia coli and Staphylococcus aureus membrane. Food Control 2015, 47, 196–202. [Google Scholar] [CrossRef]
- Zhou, Z.; Qiao, J.X.; Shetty, A.; Wu, G.; Huang, Y.; Davidson, N.E.; Wan, Y. Regulation of estrogen receptor signaling in breast carcinogenesis and breast cancer therapy. Cell. Mol. Life Sci. 2014, 71, 1549. [Google Scholar] [CrossRef]
- Zhu, R.; Chen, B.; Bai, Y.; Miao, T.; Rui, L.; Zhang, H.; Xia, B.; Li, Y.; Gao, S.; Wang, X.D.; et al. Lycopene in protection against obesity and diabetes: A mechanistic review. Pharmacol. Res. 2020, 159, 104966. [Google Scholar] [CrossRef]
- Xu, J.; Lin, Q.; Sheng, M.; Ding, T.; Li, B.; Gao, Y.; Tan, Y. Antibiofilm Effect of Cinnamaldehyde-Chitosan Nanoparticles against the Biofilm of Staphylococcus aureus. Antibiotics 2022, 11, 1403. [Google Scholar] [CrossRef]
- Lu, L.; Xiong, Y.; Zhou, J.; Wang, G.; Mi, B.; Liu, G. The Therapeutic Roles of Cinnamaldehyde against Cardiovascular Diseases. Oxid. Med. Cell. Longev. 2022, 2022, 9177108. [Google Scholar] [CrossRef]
- Muchtaridi; Diantini, A.; Subarnas, A. Analysis of Indonesian Spice Essential Oil Compounds That Inhibit Locomotor Activity in Mice. Pharmaceuticals 2011, 4, 590–602. [Google Scholar] [CrossRef]
- Zhu, B.C.; Henderson, G.; Yu, Y.; Laine, R.A. Toxicity and repellency of patchouli oil and patchouli alcohol against Formosan subterranean termites Coptotermes formosanus Shiraki (Isoptera: Rhinotermitidae). J. Agric. Food Chem. 2003, 51, 4585–4588. [Google Scholar] [CrossRef]
- Yunus, R.; Supiati, S.; Suwarni, S.; Afrini, I.M.; Mubarak, M. Larvicidal and Repellent Potential of Patchouli Extract (Pogostemon cablin) Varieties of Southeast Sulawesi for Aedes Aegypti Vector. Egypt. J. Chem. 2023, 66, 89–98. [Google Scholar] [CrossRef]
- Nakasen, K. Bio efficacy of Cinnamaldehyde from Cinnamomum verum essential oil against Culex quinquefasciatus (Diptera: Culicidae). J. Entomol. Acarol. Res. 2021, 53, 9400. [Google Scholar] [CrossRef]
- Chansang, A.; Champakaew, D.; Junkum, A.; Amornlerdpison, D.; Chaithong, U.; Jitpakdi, A.; Riyong, D.; Wannasan, A.; Intirach, J.; Muangmoon, R.; et al. Potential of natural essential oils and cinnamaldehyde as insecticides against the dengue vector aedes aegypti (Diptera: Culicidae). Southeast Asian J. Trop. Med. Pub. Health 2018, 49, 6–22. [Google Scholar]
- Babushok, V.I.; Linstrom, P.J. On the Relationship between Kováts and Lee Retention Indices. Chromatographia 2004, 60, 725–728. [Google Scholar] [CrossRef]
- Castello, G.; Testini, G. Determination of retention indices of polychlorobiphenyls by using other compounds detectable by electrondashcapture detection or selected polychlorobiphenyls as the reference series. J. Chromatogr. A 1996, 741, 241–249. [Google Scholar] [CrossRef]
| Essential Oils | Repellent Power at Hour (%) | |||||||
|---|---|---|---|---|---|---|---|---|
| 0 | 1 | 2 | 3 | 4 | 5 | 6 | Average | |
| Patchouli | 98.5 ± 0.1 | 95.8 ± 3.4 | 96.3 ± 2.8 | 96.3 ± 0.5 | 94.8 ± 2.5 | 91.2 ± 3.1 | 93.2 ± 1 | 95.1 ± 2.19 |
| Cinnamon bark | 100 ± 0 | 99.6 ± 0.57 | 96.7 ± 2.3 | 90.6 ± 4.0 | 90.5 ± 7.5 | 93.7 ± 2 | 92 ± 1.5 | 94.7 ± 3.75 |
| Nutmeg | 97.5 ± 1.7 | 93.8 ± 0.57 | 86.3 ± 1.52 | 83.5 ± 6.80 | 74.5 ± 8.38 | 71.1 ± 5.56 | 88.6 ± 7.81 | 85 ± 8.89 |
| Turmeric | 89.8 ± 22.1 | 84.3 ± 4.0 | 82.3 ± 10.9 | 81.1 ± 3.5 | 85.2 ± 2.0 | 81.8 ± 16.8 | 82 ± 10.8 | 83.8 ± 2.8 |
| Clove flowers | 100 ± 0 | 98.4 ± 2 | 78.7 ± 3.6 | 77.5 ± 4 | 69 ± 6.4 | 72.3 ± 23.8 | 66.7 ± 22.9 | 80.4 ± 12.5 |
| Citronella grass | 98.7 ± 1.5 | 65.7 ± 26.8 | 67.7 ± 19.2 | 63.5 ± 6.5 | 70.5 ± 13.6 | 69.8 ± 12.7 | 69.1 ± 4.5 | 72.2 ± 11 |
| Lemongrass | 100 ± 0 | 93.5 ± 6.0 | 78.7 ± 13.8 | 59.3 ± 31.4 | 63.4 ± 11.0 | 59.4 ± 10.5 | 46 ± 9.6 | 71.4 ± 18.3 |
| Essential Oils | Repellent Power (%) | |||
|---|---|---|---|---|
| 1 | 2 | 3 | Average | |
| Patchouli | 96 ± 1.0 | 92 ± 2.0 | 96 ± 2.0 | 96 ± 2.0 |
| Cinnamon bark | 100 ± 0.1 | 92 ± 2.0 | 92 ± 2.0 | 94 ± 1.7 |
| Peak | RT | LRI Exp | LRI Ref | Component | Area % | % Concentration |
|---|---|---|---|---|---|---|
| 1. | 5.30 | 1004 | 1011 [40] | 3-Carene | 272 | 1.05 |
| 2. | 6.34 | 1018 | 1031 [41] | beta-Phellandrene | 162 | 0.63 |
| 3. | 6.49 | 1019 | 1032 [42] | Cyclohexene 4-methylene-1-(1-methylethyl) | 102 | 0.39 |
| 4. | 22.44 | 1337 | 1380 [41] | beta-patchoulene | 454 | 1.76 |
| 5. | 22.70 | 1383 | 1392 [43] | Cyclohexane. 1-ethenyl-1-methyl-2,4-bis(1-methylethenyl)-, [1S-(1α,2β,4β)] | 244 | 0.95 |
| 6. | 23.91 | 1422 | 1428 [44] | Beta-Caryophyllene | 329 | 1.28 |
| 7. | 24.75 | 1452 | 1439 [41] | α-Guaiene | 2368 | 9.22 |
| 8. | 25.26 | 1458 | 1446 [40] | Seychellene | 2104 | 8.19 |
| 9. | 25.71 | 1462 | 1443 [40] | 8-β-cedrane | 1165 | 4.53 |
| 10. | 25.79 | 1469 | 1431 [40] | β -Gurjunene | 223 | 0.86 |
| 11. | 27.05 | 1497 | 1501 [40] | Aciphyllene | 449 | 1.74 |
| 12. | 27.45 | 1512 | 1505 [45] | Azulene. 1,2,3,5,6,7,8,8a-octahydro-1,4-dimethyl-7-(1-methylethenyl)-, [1S-(1α,7α,8aβ)] | 2795 | 10.8 |
| 13. | 30.00 | 1591 | - | n.d | 178 | 0.69 |
| 14. | 30.11 | 1632 | - | n.d | 452 | 1.76 |
| 15. | 30.47 | 1549 | 1582 [40] | Caryophyllene oxide | 434 | 1.69 |
| 16. | 30.60 | 1598 | - | 1,1,4,7-Tetramethyldecahydro-1H-cyclopropa[e]azulene-4,7-diol | 199 | 0.77 |
| 17. | 31.48 | 1424 | - | n.d | 108 | 0.42 |
| 18. | 31.97 | 1055 | - | n.d | 176 | 0.68 |
| 19. | 32.34 | 1628 | - | n.d | 334 | 1.3 |
| 20. | 32.79 | 1655 | - | n.d | 147 | 0.57 |
| 21. | 34.22 | 1668 | 1658 [40] | Patchouli alcohol | 10,000 | 42.7 |
| 22. | 34.69 | 1695 | - | n.d | 102 | 0.39 |
| 23. | 34.80 | 1789 | - | n.d | 270 | 1.05 |
| 24. | 35.03 | 1379 | - | n.d | 264 | 1.02 |
| 25. | 35.63 | 1652 | - | 4-Hydroxy-6-methyl-3-(4-methylpentanoyl)-2H-pyran-2-one | 639 | 2.48 |
| 26. | 41.52 | 1867 | - | (E)-Atlantone | 105 | 0.4 |
| Peak | RT | LRI Exp | LRI Ref [40] | Component | % Concentration |
|---|---|---|---|---|---|
| 1 | 19.26 | 1206 | 1270 | E-Cinnamaldehyde | 73.0 |
| 2 | 19.84 | 1220 | 1259 | 3-Phenyl-2-Propen-1-ol | 0.96 |
| 3 | 21.46 | 1328 | 1356 | Eugenol | 1.22 |
| 4 | 25.02 | 1408 | 1429 | Coumarin | 1.51 |
| 5 | 25.46 | 1410 | 1440 | Z-Cinnamyl acetate | 5.85 |
| Peak | RT | LRI Exp | Area % | % Conc. | Component |
|---|---|---|---|---|---|
| 1 | 24.51 | 1449 | 994 | 5.2 | α-Guaiene: |
| 2 | 25.03 | 1440 | 1005 | 5.2 | Seychellene |
| 3 | 27.21 | 1501 | 1371 | 5.2 | Azulene, 1,2,3,5,6,7,8,8a-octahydro-1,4-dimethyl-7-(1-methylethenyl)-, [1S-(1α,7α,8aβ)] |
| 4 | 33.77 | 1640 | 10,000 | 52.5 | Patchouli alcohol |
| Peak | RT | LRI Exp | Area % | % Concentration | Component |
|---|---|---|---|---|---|
| 3 | 18.26 | 1190 | 10,000 | 86.1 | Cinnamaldehyde |
| 5 | 25.17 | 1389 | 622 | 5.3 | Z-Cinnamyl acetate |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mustapa, M.A.; Guswenrivo, I.; Zurohtun, A.; Khairul Ikram, N.K.; Muchtaridi, M. Analysis of Essential Oils Components from Aromatic Plants Using Headspace Repellent Method against Aedes aegypti Mosquitoes. Molecules 2023, 28, 4269. https://doi.org/10.3390/molecules28114269
Mustapa MA, Guswenrivo I, Zurohtun A, Khairul Ikram NK, Muchtaridi M. Analysis of Essential Oils Components from Aromatic Plants Using Headspace Repellent Method against Aedes aegypti Mosquitoes. Molecules. 2023; 28(11):4269. https://doi.org/10.3390/molecules28114269
Chicago/Turabian StyleMustapa, Mohammad Adam, Ikhsan Guswenrivo, Ade Zurohtun, Nur Kusaira Khairul Ikram, and Muchtaridi Muchtaridi. 2023. "Analysis of Essential Oils Components from Aromatic Plants Using Headspace Repellent Method against Aedes aegypti Mosquitoes" Molecules 28, no. 11: 4269. https://doi.org/10.3390/molecules28114269
APA StyleMustapa, M. A., Guswenrivo, I., Zurohtun, A., Khairul Ikram, N. K., & Muchtaridi, M. (2023). Analysis of Essential Oils Components from Aromatic Plants Using Headspace Repellent Method against Aedes aegypti Mosquitoes. Molecules, 28(11), 4269. https://doi.org/10.3390/molecules28114269







