Tetrel-Bond Interactions Involving Metallylenes TH2 (T = Si, Ge, Sn, Pb): Dual Binding Behavior
Abstract
1. Introduction
2. Results and Discussion
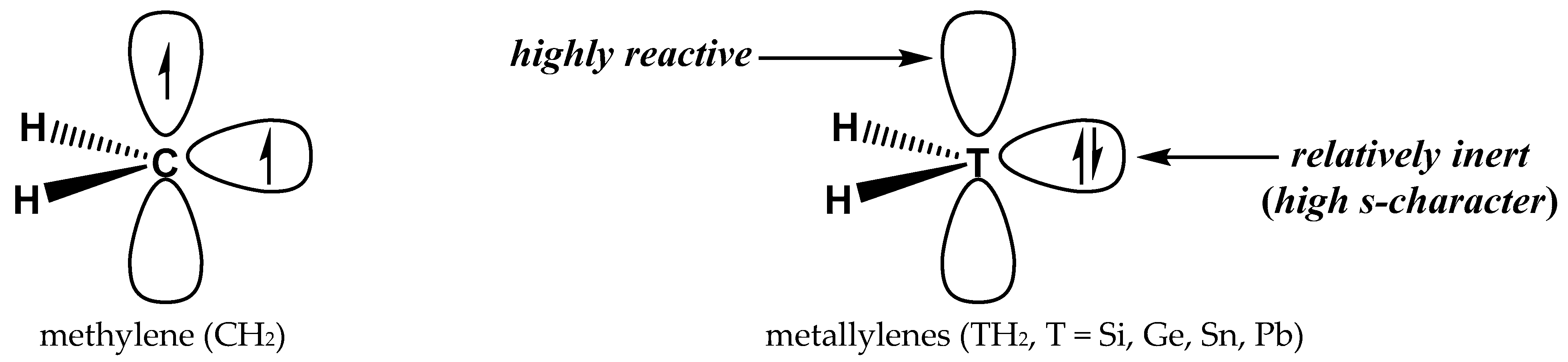
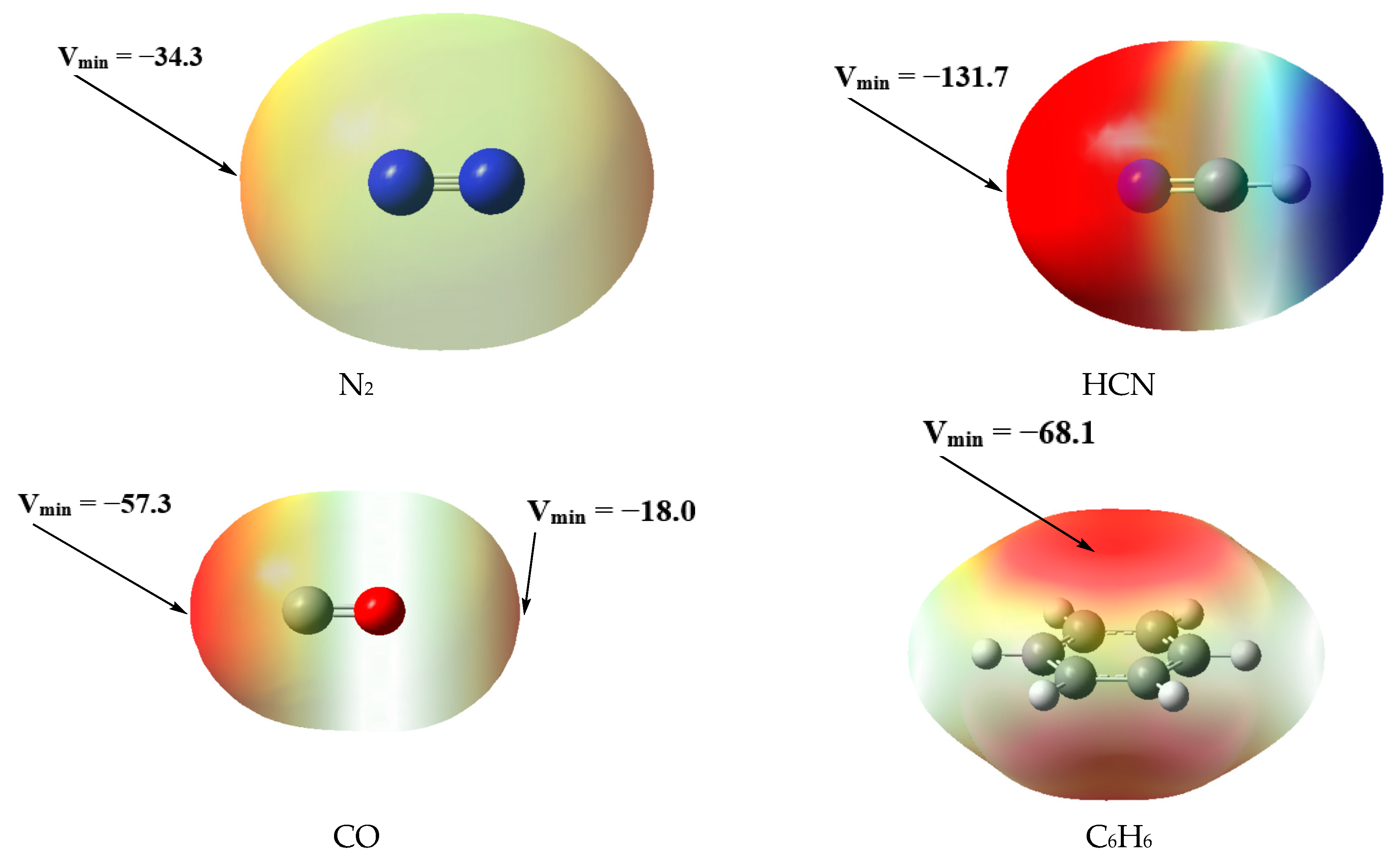
2.1. Geometries and MEP Surfaces of Monomers
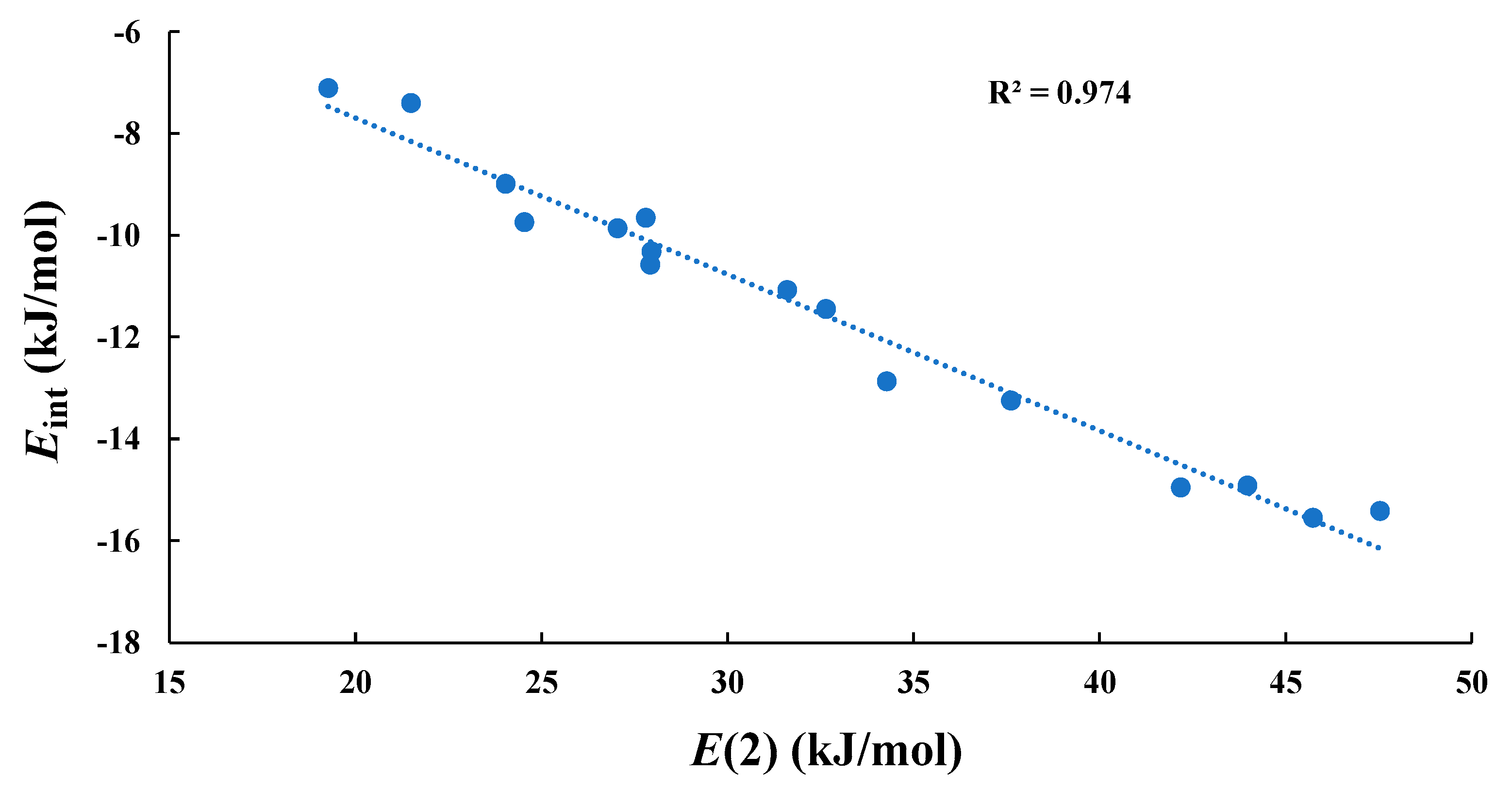
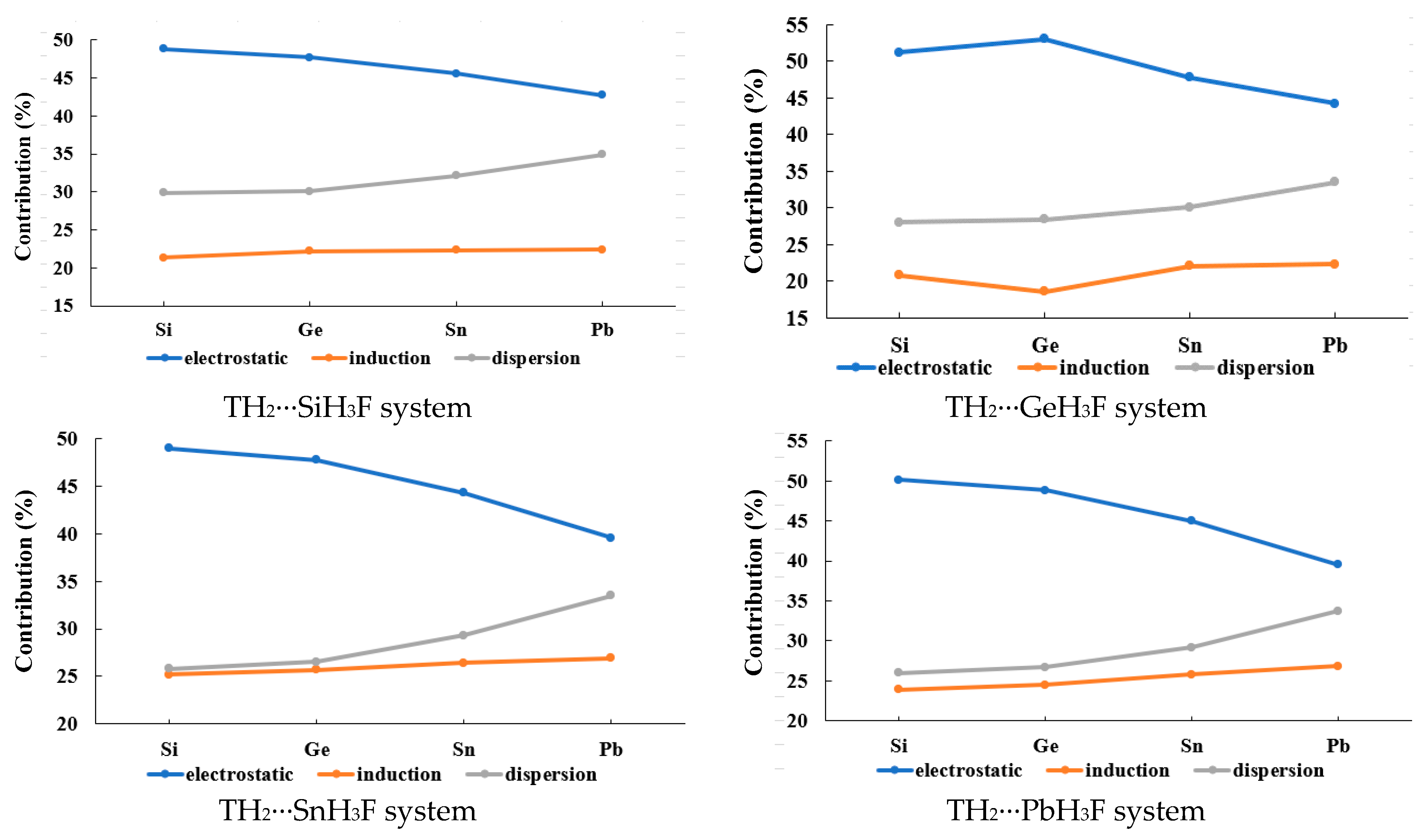
2.2. Type-A (σ-Hole Tetrel Bond) Complexes: TH2 Act as Lewis Bases
2.3. Type-B (π-Hole Tetrel Bond) Complexes: TH2 Act as Lewis Acids
3. Computational Methods
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Wheeler, S.E.; Seguin, T.J.; Guan, Y.; Doney, A.C. Noncovalent interactions in organocatalysis and the prospect of computational atalyst design. Acc. Chem. Res. 2016, 49, 1061–1069. [Google Scholar] [CrossRef]
- Vallavoju, N.; Sivaguru, J. Supramolecular photocatalysis: Combining confinement and non-covalent interactions to control light initiated reactions. Chem. Soc. Rev. 2014, 43, 4084–4101. [Google Scholar] [CrossRef] [PubMed]
- Georgakilas, V.; Tiwari, J.N.; Kemp, K.C.; Perman, J.A.; Bourlinos, A.B.; Kim, K.S.; Zboril, R. Noncovalent functionalization of graphene and graphene oxide for energy materials, biosensing, catalytic, and biomedical applications. Chem. Rev. 2016, 116, 5464–5519. [Google Scholar] [CrossRef] [PubMed]
- Scheiner, S. Hydrogen Bonding, A Theoretical Perspective; Oxford University Press: New York, NY, USA, 1997. [Google Scholar]
- Bauzá, A.; Mooibroek, T.J.; Frontera, A. Tetrel-bonding interaction: Rediscovered supramolecular force? Angew. Chem. Int. Ed. 2013, 52, 12317–12321. [Google Scholar] [CrossRef]
- Alkorta, I.; Rozas, I.; Elguero, J. Molecular complexes between silicon derivatives and electron-rich groups. J. Phys. Chem. A 2001, 105, 743–749. [Google Scholar] [CrossRef]
- Chen, Y.S.; Yao, L.F.; Wang, F. Intermolecular Interactions Involving Heavy Alkenes H2Si=TH2 (T = C, Si, Ge, Sn, Pb) with H2O and HCl: Tetrel Bond and Hydrogen Bond. ACS Omega 2020, 5, 30210–30225. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.S.; Yao, L.F.; Wang, F. Intermolecular interactions between the heavy alkenes H2Si = TH2 (T = C, Si, Ge, Sn, Pb) and acetylene. J. Mol. Model. 2021, 27, 110. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.S.; Yao, L.F.; Wang, F. Intermolecular interactions between the heavy-atom analogues of acetylene T2H2 (T = Si, Ge, Sn, Pb) and HCN. J. Mol. Model. 2023, 29, 52. [Google Scholar] [CrossRef]
- Scheiner, S. Origins and Properties of the Tetrel Bond. Phys. Chem. Chem. Phys. 2021, 23, 5702–5717. [Google Scholar] [CrossRef]
- Scheiner, S. The ditetrel bond noncovalent bond between neutral tetrel atoms. Phys. Chem. Chem. Phys. 2020, 22, 16606–16614. [Google Scholar] [CrossRef]
- Thomas, S.P.; Pavan, M.S.; Row, T.N.G. Experimental evidence for ‘carbon bonding’ in the solid state from charge density analysis. Chem. Commun. 2014, 50, 49–51. [Google Scholar] [CrossRef] [PubMed]
- Mani, D.; Arunan, E. The X-C···π (X = F, Cl, Br, CN) carbon bond. J. Phys. Chem. A 2014, 118, 10081–10089. [Google Scholar] [CrossRef] [PubMed]
- Southern, S.A.; Bryce, D.L. NMR investigations of noncovalent carbon tetrel bonds. Computational assessment and initial experimental observation. J. Phys. Chem. A 2015, 119, 11891–11899. [Google Scholar] [CrossRef] [PubMed]
- Scheiner, S. Comparison of CH···O, SH···O, chalcogen, and tetrel bonds formed by neutral and cationic sulfur-containing compounds. J. Phys. Chem. A 2015, 119, 9189–9199. [Google Scholar] [CrossRef]
- Azofra, L.M.; Scheiner, S. Tetrel, chalcogen, and CH···O hydrogen bonds in complexes pairing carbonyl-containing molecules with 1, 2, and 3 molecules of CO2. J. Chem. Phys. 2015, 142, 034307. [Google Scholar] [CrossRef]
- Bauzá, A.; Mooibroek, T.J.; Frontera, A. Tetrel bonding interactions. Chem. Rec. 2016, 16, 473–487. [Google Scholar] [CrossRef]
- Bauzá, A.; Frontera, A.; Mooibroek, T.J. 1,1,2,2-Tetracyanocyclopropane (TCCP) as supramolecular synthon. Phys. Chem. Chem. Phys. 2016, 18, 1693–1698. [Google Scholar] [CrossRef]
- Liu, M.X.; Li, Q.Z.; Cheng, J.B.; Li, W.Z.; Li, H.B. Tetrel bond of pseudohalide anions with XH3F (X = C, Si, Ge, and Sn) and its role in SN2 reaction. J. Chem. Phys. 2016, 145, 224310. [Google Scholar] [CrossRef]
- Liu, M.X.; Li, Q.Z.; Scheiner, S. Comparison of tetrel bonds in neutral and protonated complexes of pyridineTF3 and furanTF3 (T = C, Si, and Ge) with NH3. Phys. Chem. Chem. Phys. 2017, 19, 5550–5559. [Google Scholar] [CrossRef]
- Legon, A.C. Tetrel, pnictogen and chalcogen bonds identified in the gas phase before they had names: A systematic look at non-covalent interactions. Phys. Chem. Chem. Phys. 2017, 19, 14884–14896. [Google Scholar] [CrossRef]
- Scheiner, S. Comparison of various means of evaluating molecular electrostatic potentials for noncovalent interactions. J. Comput. Chem. 2018, 39, 500–510. [Google Scholar] [CrossRef] [PubMed]
- Zierkiewicz, W.; Michalczyk, M.; Scheiner, S. Implications of monomer deformation for tetrel and pnicogen bonds. Phys. Chem. Chem. Phys. 2018, 20, 8832–8841. [Google Scholar] [CrossRef]
- Remyaa, K.; Suresh, C.H. Intermolecular carbon–carbon, nitrogen–nitrogen and oxygen–oxygen non-covalent bonding in dipolar molecules. Phys. Chem. Chem. Phys. 2015, 17, 18380–18392. [Google Scholar] [CrossRef]
- Varadwaj, P.R.; Varadwaja, A.; Jin, B.Y. Significant evidence of C···O and C···C long-range contacts in several heterodimeric complexes of CO with CH3–X, should one refer to them as carbon and dicarbon bonds! Phys. Chem. Chem. Phys. 2014, 16, 17238–17252. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.Z.; Zhuo, H.Y.; Li, H.B.; Liu, Z.B.; Li, W.Z.; Cheng, J.B. Tetrel-hydride interaction between XH3F (X = C, Si, Ge, Sn) and HM (M = Li, Na, BeH, MgH). J. Phys. Chem. A 2015, 119, 2217–2224. [Google Scholar] [CrossRef] [PubMed]
- Sethio, D.; Oliveira, V.; Kraka, E. Quantitative assessment of tetrel bonding utilizing vibrational spectroscopy. Molecules 2018, 23, 2763. [Google Scholar] [CrossRef] [PubMed]
- Scheiner, S. Systematic Elucidation of Factors That Influence the Strength of Tetrel Bonds. J. Phys. Chem. A 2017, 121, 5561–5568. [Google Scholar] [CrossRef]
- Clark, T.; Hennemann, M.; Murray, J.S.; Politzer, P. Halogen bonding: The sigma-hole. Proceedings of “Modeling interactions in biomolecules II”, Prague, 5–9 September 2005. J. Mol. Model. 2007, 13, 291–296. [Google Scholar] [CrossRef]
- Murray, J.S.; Lane, P.; Politzer, P. Expansion of the sigma-hole concept. J. Mol. Model. 2009, 15, 723–729. [Google Scholar] [CrossRef]
- Li, Q.Z.; Guo, X.; Yang, X.; Li, W.Z.; Cheng, J.B.; Li, H.B. A σ-hole interaction with radical species as electron donors: Does single-electron tetrel bonding exist? Phys. Chem. Chem. Phys. 2014, 16, 11617–11625. [Google Scholar] [CrossRef]
- McDowell, S.A.C.; Joseph, J.A. The effect of atomic ions on model σ-hole bonded complexes of AH3Y(A = C, Si, Ge; Y = F, Cl, Br). Phys. Chem. Chem. Phys. 2014, 16, 10854–10860. [Google Scholar] [CrossRef]
- Quiñonero, D. Sigma-hole carbon-bonding interactions in carbon–carbon double bonds: An unnoticed contact. Phys. Chem. Chem. Phys. 2017, 19, 15530–15540. [Google Scholar] [CrossRef] [PubMed]
- Murray, J.S.; Lane, P.; Clark, T.; Riley, K.E.; Politzer, P. σ-Holes, π-holes and electrostatically-driven interactions. J. Mol. Model. 2012, 18, 541–548. [Google Scholar] [CrossRef]
- Wei, Y.X.; Li, Q.Z.; Scheiner, S. The π-tetrel bond and its influence on hydrogen bonding and proton transfer. ChemPhysChem 2018, 19, 736–743. [Google Scholar] [CrossRef] [PubMed]
- Zierkiewicz, W.; Michalczyk, M.; Scheiner, S. Comparison between Tetrel Bonded Complexes Stabilized by σ and π Hole Interactions. Molecules 2018, 23, 1416. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Zeng, Y.; Li, X.; Sun, Z.; Meng, L. Insight into the pseudo π-hole interactions in the M3H6(NCF)n (M = C, Si, Ge, Sn, Pb; n = 1, 2, 3) complexes. Phys. Chem. Chem. Phys. 2016, 18, 24672–24680. [Google Scholar] [CrossRef] [PubMed]
- Yourdkhani, S.; Korona, T.; Hadipour, N.L. Interplay between tetrel and triel bonds in RC6H4CN···MF3CN···BX3 complexes: A combined symmetry-adapted perturbation theory, Møller-Plesset, and quantum theory of atoms-in-molecules study. J. Comput. Chem. 2015, 36, 2412–2428. [Google Scholar] [CrossRef] [PubMed]
- Marín-Luna, M.; Alkorta, I.; Elguero, J. Cooperativity in tetrel bonds. J. Phys. Chem. A 2016, 120, 648–656. [Google Scholar] [CrossRef]
- Esrafili, M.D.; Mohammadian-Sabet, F. Cooperativity of tetrel bonds tuned by substituent effects. Mol. Phys. 2016, 114, 1528–1538. [Google Scholar] [CrossRef]
- Rezaei, Z.; Solimannejad, M.; Esrafili, M.D. Interplay between hydrogen bond and single-electron tetrel bond: H3C···COX2···HY and H3C···CSX2···HY (X = F, Cl; Y = CN, NC) complexes as a working model. Comput. Theor. Chem. 2015, 1074, 101–106. [Google Scholar] [CrossRef]
- Xu, H.L.; Cheng, J.B.; Yang, X.; Liu, Z.B.; Xiao, B.; Li, Q.Z. Interplay between the σ-tetrel bond and σ-halogen bond in PhSiF3 ···4-iodopyridine···N-base. RSC Adv. 2017, 7, 21713–21720. [Google Scholar] [CrossRef]
- Chen, Y.S.; Yao, L.F.; Wang, F. Synergistic and antagonistic interplay between tetrel bond and pnicogen bond in complexes involving ring compounds. J. Mol. Model. 2019, 25, 351. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.X.; Yang, L.; Li, Q.Z.; Li, W.Z.; Cheng, J.B.; Xiao, B.; Yu, X.F. Modulating the strength of tetrel bonding through beryllium bonding. J. Mol. Model. 2016, 22, 192. [Google Scholar] [CrossRef]
- McDowell, S.A.C. Sigma-hole cooperativity in anionic [FX···CH3···YF]− (X, Y = Cl, Br) complexes. Chem. Phys. Lett. 2014, 598, 1–4. [Google Scholar] [CrossRef]
- Esrafili, M.D.; Mohammadirad, N.; Solimannejad, M. Tetrel bond cooperativity in open-chain (CH3CN)n and (CH3NC)n clusters (n = 2–7): An ab initio study. Chem. Phys. Lett. 2015, 628, 16–20. [Google Scholar] [CrossRef]
- Solimannejad, M.; Orojloo, M.; Amani, S. Effect of cooperativity in lithium bonding on the strength of halogen bonding and tetrel bonding: (LiCN)n···ClYF3 and (LiCN)n···YF3Cl (Y = C, Si and n = 1-5) complexes as a working model. J. Mol. Model. 2015, 21, 183. [Google Scholar] [CrossRef] [PubMed]
- Scheiner, S. Relative Strengths of a Pnicogen and a Tetrel Bond and Their Mutual Effects upon One Another. J. Phys. Chem. A 2021, 125, 2631–2641. [Google Scholar] [CrossRef]
- Liu, M.X.; Li, Q.Z.; Li, W.Z.; Cheng, J.B. Carbene tetrel-bonded complexes. Struct. Chem. 2017, 28, 823–831. [Google Scholar] [CrossRef]
- Bene, J.E.D.; Alkorta, I.; Elguero, J. Carbon-Carbon Bonding between Nitrogen Heterocyclic Carbenes and CO2. J. Phys. Chem. A 2017, 121, 8136–8146. [Google Scholar] [CrossRef]
- Mizuhata, Y.; Sasamori, T.; Tokitoh, N. Stable Heavier Carbene Analogues. Chem. Rev. 2009, 109, 3479–3511. [Google Scholar] [CrossRef]
- Guo, Y.; Fikri, M.; Friedrichs, G.; Temps, F. An Extended Simultaneous Kinetics and Ringdown Model: Determination of the Rate Constant for the Reaction SiH2 + O2. Phys. Chem. Chem. Phys. 2003, 5, 4622–4630. [Google Scholar] [CrossRef]
- Becerra, R.; Goldberg, N.; Cannady, J.P.; Almond, M.J.; Ogden, J.S.; Walsh, R. Experimental and Theoretical Evidence for Homogeneous Catalysis in the Gas-Phase Reaction of SiH2 with H2O (and D2O): A Combined Kinetic and Quantum Chemical Study. J. Am. Chem. Soc. 2004, 126, 6816–6824. [Google Scholar] [CrossRef] [PubMed]
- Becerra, R.; Cannady, J.P.; Walsh, R. Time-Resolved GasPhase Kinetic and Quantum Chemical Studies of Reactions of Silylene with Chlorine-Containing Species. 1. HCl. J. Phys. Chem. A 2004, 108, 3987–3993. [Google Scholar] [CrossRef]
- Becerra, R.; Cannady, J.P.; Walsh, R. Reactions of Silylene with Unreactive Molecules. I: Carbon Dioxide; Gas-Phase Kinetic and Theoretical Studies. J. Phys. Chem. A 2002, 106, 4922–4927. [Google Scholar] [CrossRef]
- Becerra, R.; Cannady, J.P.; Walsh, R. The Gas-Phase Reaction of Silylene with Acetaldehyde. 2. Theoretical Calculations of Isotope Effects for SiH2 versus SiD2 Addition. J. Phys. Chem. A 2002, 106, 11558–11564. [Google Scholar] [CrossRef]
- Becerra, R.; Carpenter, I.W.; Gutsche, G.J.; King, K.D.; Lawrance, W.D.; Staker, W.S.; Walsh, R. The Gas-Phase Reaction of SiH2 with Dimethylether: Kinetic Evidence for Direct Formation of a Donor-Acceptor Adduct. Chem. Phys. Lett. 2001, 333, 83–90. [Google Scholar] [CrossRef]
- Friedrichs, G.; Fikri, M.; Guo, Y.; Temps, F. Time-Resolved Cavity Ringdown Measurements and Kinetic Modeling of the Pressure Dependences of the Recombination Reactions of SiH2 with the Alkenes C2H4, C3H6, and t-C4H8. J. Phys. Chem. A 2008, 112, 5636–5646. [Google Scholar] [CrossRef]
- Al-Rubaiey, N.; Becerra, R.; Walsh, R. A Gas-Phase Kinetic Study of the Silylene Addition Reaction SiH2 + C2D4 as a Function of Temperature and Pressure: Isotope Effects and Mechanistic Complexities. Phys. Chem. Chem. Phys. 2002, 4, 5072–5078. [Google Scholar] [CrossRef]
- Becerra, R.; Cannady, J.P.; Dormer, G.; Walsh, R. The Addition Reaction between Silylene and Ethyne: Further Isotope Studies, Pressure Dependence Studies, and Quantum Chemical Calculations. J. Phys. Chem. A 2008, 112, 8665–8677. [Google Scholar] [CrossRef]
- Maier, G.; Reisenauer, H.P.; Glatthaar, J.; Zetzmann, R. Complex of Silylene with Nitrogen: A Combined Matrix-Spectroscopic and Density Functional Theory Study. Chem.—Asian J. 2006, 1, 195–202. [Google Scholar] [CrossRef]
- Becerra, R.; Bowes, S.-J.; Ogden, J.S.; Cannady, J.P.; Almond, M.J.; Walsh, R. Time-Resolved Gas-Phase Kinetic and Quantum Chemical Studies of the Reaction of Silylene with Nitric Oxide. J. Phys. Chem. A 2005, 109, 1071–1080. [Google Scholar] [CrossRef] [PubMed]
- Becerra, R.; Carpenter, I.W.; Gordon, M.S.; Roskop, L.; Walsh, R. Gas Phase Kinetic and Quantum Chemical Studies of the Reactions of Silylene with the Methylsilanes. Absolute Rate Constants, Temperature Dependences, RRKM Modelling and Potential Energy Surfaces. Phys. Chem. Chem. Phys. 2007, 9, 2121–2129. [Google Scholar] [CrossRef] [PubMed]
- Becerra, R.; Boganov, S.E.; Egorov, M.P.; Faustov, V.I.; Promyslov, V.M.; Nefedov, O.M.; Walsh, R. An Investigation of the Prototype Germylene Addition Reaction, GeH2 + C2H4: Time-Resolved Gas-Phase Kinetic Studies and Quantum Chemical Calculations of the Reaction Energy Surface. Phys. Chem. Chem. Phys. 2002, 4, 5079–5087. [Google Scholar] [CrossRef]
- Bharatam, P.V.; Moudgil, R.; Kaur, D. Lewis Donor and Acceptor Interactions of Silylenes: A Theoretical Study. Organometallics 2002, 21, 3683–3690. [Google Scholar] [CrossRef]
- Matczak, P. Theoretical insight into the interaction between SnX2 (X=H, F, Cl, Br, I) and benzene. J. Mol. Model. 2016, 22, 208. [Google Scholar] [CrossRef] [PubMed]
- Grabowski, S.J. π-Hole Tetrel Bonds—Lewis Acid Properties of Metallylenes. Crystals 2022, 12, 112. [Google Scholar] [CrossRef]
- Bankiewicz, B.; Matczak, P. Controlling the preferred nitrogen site in 1,2,3-triazine to bind with stannylenes. Polyhedron 2022, 225, 116056. [Google Scholar] [CrossRef]
- Chen, Y.S.; Yao, L.F.; Wang, F. Hydrogen-bonding interactions involving the Imidazol-2-ylidene and its Heavy-atom analogues, Comput. Theor. Chem. 2023, 1220, 114020. [Google Scholar] [CrossRef]
- Buralli, G.J.; Petelski, A.N.; Peruchena, N.M.; Sosa, G.L.; Duarte, D.J.R. Multicenter (FX)n/NH3 Halogen Bonds (X = Cl, Br and n = 1–5). QTAIM Descriptors of the Strength of the X∙∙∙N Interaction. Molecules 2017, 22, 2034. [Google Scholar] [CrossRef]
- Blanco, F.; Alkorta, I.; Solimannejad, J.; Elguero, J. Theoretical Study of the 1:1 Complexes between Carbon Monoxide and Hypohalous Acids. J. Phys. Chem. A 2009, 113, 3237–3244. [Google Scholar] [CrossRef]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Mennucci, B.; Petersson, G.A.; et al. Gaussian 09, Revision D.01; Gaussian, Inc.: Wallingford, UK, 2013. [Google Scholar]
- Feller, D. The role of databases in support of computational chemistry calculations. J. Comput. Chem. 1996, 17, 1571–1586. [Google Scholar] [CrossRef]
- Boys, S.F.; Bernardi, F. The calculation of small molecular interactions by the differences of separate total energies. Some procedures with reduced errors. Mol. Phys. 1970, 19, 553–566. [Google Scholar] [CrossRef]
- Bader, R.F.W.; Carroll, M.T.; Cheeseman, J.R.; Chang, C. Properties of atoms in molecules: Atomic volumes. J. Am. Chem. Soc. 1987, 109, 7968–7979. [Google Scholar] [CrossRef]
- Lu, T.; Chen, F.W. Multiwfn: A multifunctional wavefunction analyzer. J. Comput. Chem. 2012, 33, 580–592. [Google Scholar] [CrossRef] [PubMed]
- Dennington, R.; Keith, T.; Millam, J.S. GaussView, Version 5; Semichem Inc.: Shawnee Mission, KS, USA, 2009. [Google Scholar]
- Reed, A.E.; Curtiss, L.A.; Weinhold, F. Intermolecular interactions from a natural bond orbital, donor-acceptor viewpoint. Chem. Rev. 1988, 88, 899–926. [Google Scholar] [CrossRef]
- Hohenstein, E.G.; Sherrill, C.D. Density fitting and Cholesky decomposition approximations in symmetry-adapted perturbation theory: Implementation and application to probe the nature of pi-pi interactions in linear acenes. J. Chem. Phys. 2010, 132, 184111–184120. [Google Scholar] [CrossRef]
- Turney, J.M.; Simmonett, A.C.; Parrish, R.M.; Hohenstein, E.G.; Evangelista, F.A.; Fermann, J.T.; Mintz, B.J.; Burns, L.A.; Wilke, J.J.; Abrams, M.L.; et al. PSI4: An open-source ab initio electronic structure program. Wiley Interdiscip. Rev. Comput. Mol. Sci. 2012, 2, 556–565. [Google Scholar] [CrossRef]



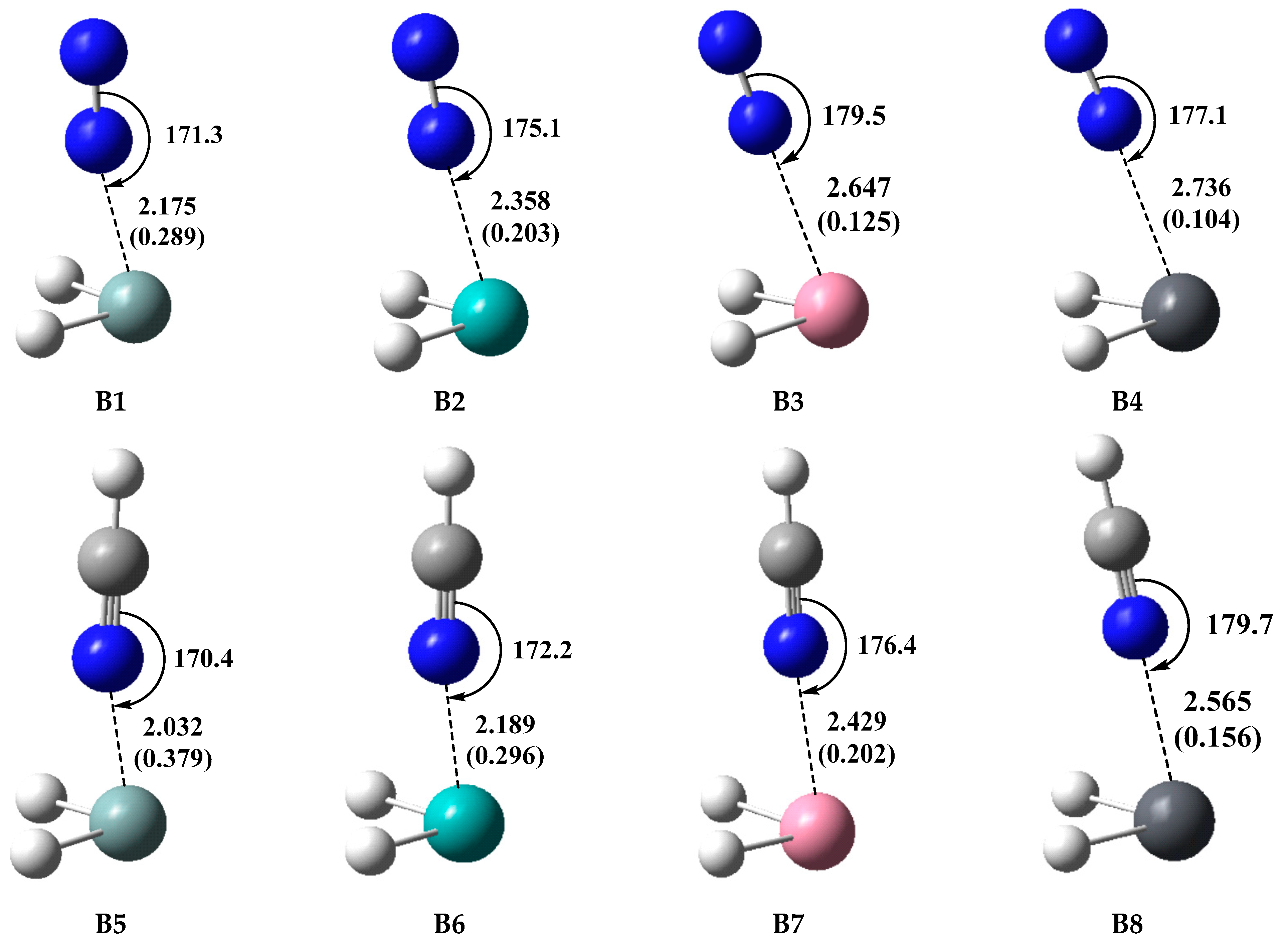
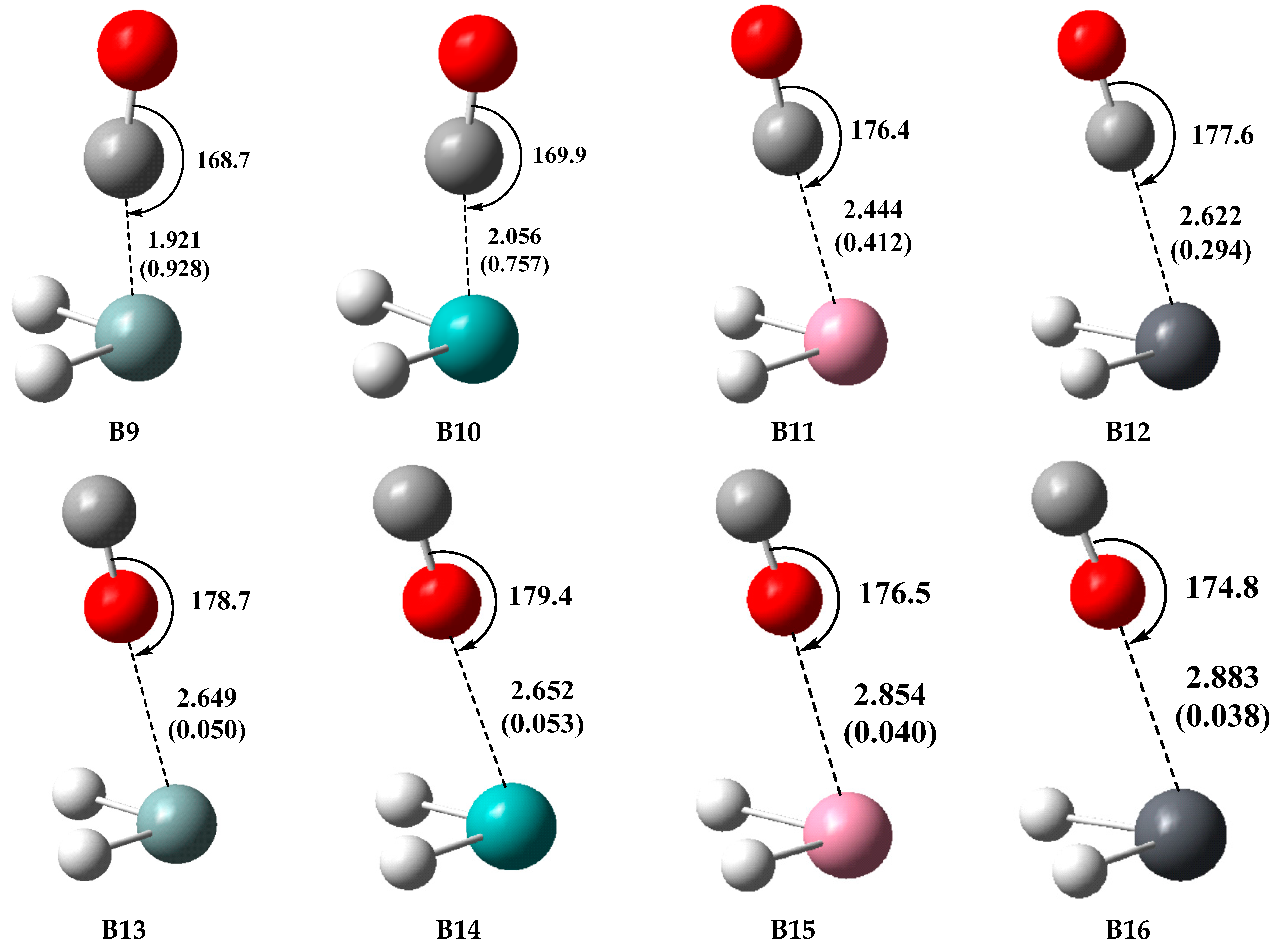

| Complex | R | Eint a | |||
|---|---|---|---|---|---|
| L1 | L2 | L3 | L4 | ||
| A1 (SiH2···SiH3F) | 3.618 (3.505) | −9.07 | −10.58 | −10.91 | −9.95 |
| A2 (SiH2···GeH3F) | 3.632 (3.516) | −10.37 | −11.45 | −12.00 | −10.78 |
| A3 (SiH2···SnH3F) | 3.624 (3.493) | −14.25 | −15.55 | −17.68 | −15.68 |
| A4 (SiH2···PbH3F) | 3.683 (3.575) | −14.46 | −15.42 | −17.68 | −15.38 |
| A5 (GeH2···SiH3F) | 3.591 (3.437) | −8.32 | −10.32 | −10.53 | −9.41 |
| A6 (GeH2···GeH3F) | 3.618 (3.465) | −9.53 | −11.08 | −11.45 | −10.07 |
| A7 (GeH2···SnH3F) | 3.638 (3.463) | −13.13 | −14.96 | −16.89 | −14.92 |
| A8 (GeH2···PbH3F) | 3.695 (3.545) | −13.38 | −14.92 | −17.01 | −14.71 |
| A9 (SnH2···SiH3F) | 3.770 (3.634) | −6.94 | −8.99 | −8.82 | −7.90 |
| A10 (SnH2···GeH3F) | 3.790 (3.660) | −7.98 | −9.66 | −9.66 | −8.53 |
| A11 (SnH2···SnH3F) | 3.852 (3.672) | −10.91 | −12.87 | −13.88 | −12.29 |
| A12 (SnH2···PbH3F) | 3.887 (3.709) | −11.45 | −13.25 | −14.67 | −12.54 |
| A13 (PbH2···SiH3F) | 3.742 (3.537) | −4.68 | −7.11 | −5.81 | −4.93 |
| A14 (PbH2···GeH3F) | 3.775 (3.556) | −5.27 | −7.40 | −5.64 | −4.51 |
| A15 (PbH2···SnH3F) | 3.875 (3.661) | −7.40 | −9.74 | −9.82 | −8.53 |
| A16 (PbH2···PbH3F) | 3.910 (3.700) | −7.69 | −9.86 | −10.49 | −8.61 |
| Complex | Orbital Interaction | E(2) | qCT | ρ | 2ρ | H | G | V |
|---|---|---|---|---|---|---|---|---|
| A1 (SiH2···SiH3F) | LP (Si)→σ* (Si-F) | 27.92 | 0.0340 | 0.0077 | 0.0179 | 0.0005 | 0.0040 | −0.0035 |
| A2 (SiH2···GeH3F) | LP (Si)→σ* (Ge-F) | 32.65 | 0.0389 | 0.0080 | 0.0183 | 0.0005 | 0.0041 | −0.0036 |
| A3 (SiH2···SnH3F) | LP (Si)→σ* (Sn-F) | 45.73 | 0.0614 | 0.0098 | 0.0204 | 0.0004 | 0.0047 | −0.0043 |
| A4 (SiH2···PbH3F) | LP (Si)→σ* (Pb-F) | 47.53 | 0.0595 | 0.0099 | 0.0219 | 0.0006 | 0.0048 | −0.0043 |
| A5 (GeH2···SiH3F) | LP (Ge)→σ* (Si-F) | 27.96 | 0.0355 | 0.0082 | 0.0189 | 0.0005 | 0.0043 | −0.0038 |
| A6 (GeH2···GeH3F) | LP (Ge)→σ* (Ge-F) | 31.60 | 0.0393 | 0.0083 | 0.0190 | 0.0005 | 0.0042 | −0.0037 |
| A7 (GeH2···SnH3F) | LP (Ge)→σ* (Sn-F) | 42.18 | 0.0587 | 0.0097 | 0.0203 | 0.0004 | 0.0047 | −0.0042 |
| A8 (GeH2···PbH3F) | LP (Ge)→σ* (Pb-F) | 43.97 | 0.0571 | 0.0098 | 0.0219 | 0.0006 | 0.0049 | −0.0043 |
| A9 (SnH2···SiH3F) | LP (Sn)→σ* (Si-F) | 24.04 | 0.0339 | 0.0074 | 0.0162 | 0.0004 | 0.0036 | −0.0032 |
| A10 (SnH2···GeH3F) | LP (Sn)→σ* (Ge-F) | 27.80 | 0.0385 | 0.0076 | 0.0163 | 0.0004 | 0.0036 | −0.0032 |
| A11 (SnH2···SnH3F) | LP (Sn)→σ* (Sn-F) | 34.28 | 0.0540 | 0.0083 | 0.0162 | 0.0004 | 0.0037 | −0.0033 |
| A12 (SnH2···PbH3F) | LP (Sn)→σ* (Pb-F) | 37.62 | 0.0555 | 0.0086 | 0.0180 | 0.0005 | 0.0040 | −0.0035 |
| A13 (PbH2···SiH3F) | LP (Pb)→σ* (Si-F) | 19.27 | 0.0298 | 0.0074 | 0.0173 | 0.0005 | 0.0039 | −0.0034 |
| A14 (PbH2···GeH3F) | LP (Pb)→σ* (Ge-F) | 21.49 | 0.0327 | 0.0075 | 0.0171 | 0.0005 | 0.0038 | −0.0033 |
| A15 (PbH2···SnH3F) | LP (Pb)→σ* (Sn-F) | 24.54 | 0.0423 | 0.0077 | 0.0161 | 0.0004 | 0.0036 | −0.0032 |
| A16 (PbH2···PbH3F) | LP (Pb)→σ* (Pb-F) | 27.04 | 0.0437 | 0.0080 | 0.0179 | 0.0006 | 0.0039 | −0.0034 |
| Complex | Eele | %Eele | Eind | %Eind | Edisp | %Edisp | Eex | Etot |
|---|---|---|---|---|---|---|---|---|
| A1 (SiH2···SiH3F) | −20.52 | 48.8 | −8.95 | 21.3 | −12.58 | 29.9 | 31.94 | −10.12 |
| A2 (SiH2···GeH3F) | −23.03 | 51.2 | −9.36 | 20.8 | −12.58 | 28.0 | 33.94 | −11.04 |
| A3 (SiH2···SnH3F) | −26.75 | 49.0 | −13.75 | 25.2 | −14.04 | 25.8 | 38.87 | −15.68 |
| A4 (SiH2···PbH3F) | −25.41 | 50.1 | −12.12 | 23.9 | −13.21 | 26.0 | 35.11 | −15.63 |
| A5 (GeH2···SiH3F) | −21.23 | 47.7 | −9.86 | 22.2 | −13.38 | 30.1 | 34.82 | −9.66 |
| A6 (GeH2···GeH3F) | −24.49 | 53.0 | −8.61 | 18.6 | −13.13 | 28.4 | 35.82 | −10.41 |
| A7 (GeH2···SnH3F) | −25.33 | 47.8 | −13.63 | 25.7 | −14.04 | 26.5 | 38.29 | −14.71 |
| A8 (GeH2···PbH3F) | −24.12 | 48.8 | −12.12 | 24.5 | −13.21 | 26.7 | 34.74 | −14.71 |
| A9 (SnH2···SiH3F) | −19.06 | 45.6 | −9.32 | 22.3 | −13.42 | 32.1 | 33.61 | −8.19 |
| A10 (SnH2···GeH3F) | −21.07 | 47.8 | −9.74 | 22.1 | −13.29 | 30.1 | 35.28 | −8.82 |
| A11 (SnH2···SnH3F) | −20.36 | 44.3 | −12.12 | 26.4 | −13.46 | 29.3 | 33.61 | −12.33 |
| A12 (SnH2···PbH3F) | −20.06 | 45.0 | −11.50 | 25.8 | −13.00 | 29.2 | 31.94 | −12.62 |
| A13 (PbH2···SiH3F) | −16.22 | 42.7 | −8.49 | 22.4 | −13.25 | 34.9 | 32.02 | −5.94 |
| A14 (PbH2···GeH3F) | −17.01 | 44.2 | −8.61 | 22.3 | −12.92 | 33.5 | 32.35 | −6.19 |
| A15 (PbH2···SnH3F) | −14.67 | 39.6 | −9.99 | 26.9 | −12.41 | 33.5 | 28.38 | −8.69 |
| A16 (PbH2···PbH3F) | −14.13 | 39.5 | −9.57 | 26.8 | −12.04 | 33.7 | 26.96 | −8.78 |
| Complex | R | Eint a | |||
|---|---|---|---|---|---|
| L1 | L2 | L3 | L4 | ||
| B1 (SiH2···N2) | 2.175 (2.042) | −24.95 | −26.08 | −35.70 | −25.83 |
| B2 (GeH2···N2) | 2.358 (2.211) | −19.98 | −19.73 | −25.16 | −17.10 |
| B3 (SnH2···N2) | 2.647 (2.549) | −16.34 | −15.84 | −20.06 | −14.55 |
| B4 (PbH2···N2) | 2.736 (2.643) | −14.50 | −14.59 | −17.77 | −12.67 |
| B5 (SiH2···HCN) | 2.032 (1.951) | −68.09 | −71.77 | −85.06 | −73.36 |
| B6 (GeH2···HCN) | 2.189 (2.087) | −55.22 | −55.64 | −65.42 | −54.97 |
| B7 (SnH2···HCN) | 2.429 (2.373) | −47.23 | −46.77 | −54.51 | −46.61 |
| B8 (PbH2···HCN) | 2.565 (2.485) | −40.46 | −40.13 | −45.85 | −38.54 |
| B9 (SiH2···CO) | 1.921 (1.889) | −93.30 | −98.44 | −113.11 | −97.77 |
| B10 (GeH2···CO) | 2.056 (1.989) | −64.83 | −68.55 | −81.26 | −67.26 |
| B11 (SnH2···CO) | 2.444 (2.347) | −38.00 | −40.80 | −48.53 | −40.00 |
| B12 (PbH2···CO) | 2.622 (2.498) | −29.80 | −32.10 | −37.29 | −29.93 |
| B13 (SiH2···OC) | 2.649 (2.581) | −8.03 | −9.45 | −9.95 | −9.03 |
| B14 (GeH2···OC) | 2.652 (2.593) | −7.86 | −9.61 | −9.66 | −8.95 |
| B15 (SnH2···OC) | 2.854 (2.769) | −7.65 | −9.70 | −9.24 | −8.95 |
| B16 (PbH2···OC) | 2.883 (2.785) | −7.65 | −10.07 | −9.28 | −8.86 |
| B17 (SiH2···C6H6) | 2.452 (2.381) | −47.15 | −43.64 | −57.06 | −42.64 |
| B18 (GeH2···C6H6) | 2.537 (2.446) | −42.76 | −39.29 | −51.21 | −36.74 |
| B19 (SnH2···C6H6) | 2.761 (2.700) | −37.75 | −34.99 | −44.73 | −32.65 |
| B20 (PbH2···C6H6) | 2.799 (2.746) | −34.49 | −32.35 | −41.72 | −28.47 |
| Complex | Orbital Interaction | E(2) | qCT | ρ | 2ρ | H | G | V |
|---|---|---|---|---|---|---|---|---|
| B1 (SiH2···N2) | LP (N)→LP* (Si) | 362.57 | −0.0592 | 0.0401 | 0.0605 | −0.0124 | 0.0275 | −0.0399 |
| B2 (GeH2···N2) | LP (N)→LP* (Ge) | 238.01 | −0.0538 | 0.0341 | 0.1253 | −0.0021 | 0.0334 | −0.0354 |
| B3 (SnH2···N2) | LP (N)→LP* (Sn) | 135.10 | −0.0384 | 0.0233 | 0.0880 | 0.0007 | 0.0213 | −0.0205 |
| B4 (PbH2···N2) | LP (N)→LP* (Pb) | 113.78 | −0.0346 | 0.0226 | 0.0895 | 0.0017 | 0.0207 | −0.0191 |
| B5 (SiH2···HCN) | LP (N)→LP* (Si) | 520.33 | −0.0898 | 0.0522 | 0.1647 | −0.0144 | 0.0556 | −0.0700 |
| B6 (GeH2···HCN) | LP (N)→LP* (Ge) | 379.46 | −0.0860 | 0.0514 | 0.1808 | −0.0097 | 0.0549 | −0.0645 |
| B7 (SnH2···HCN) | LP (N)→LP* (Sn) | 229.69 | −0.0639 | 0.0387 | 0.1529 | −0.0014 | 0.0397 | −0.0411 |
| B8 (PbH2···HCN) | LP (N)→LP* (Pb) | 178.74 | −0.0534 | 0.0343 | 0.1357 | 0.0007 | 0.0332 | −0.0325 |
| B9 (SiH2···CO) | LP (C)→LP* (Si) | 1488.16 | −0.1009 | 0.0762 | 0.3377 | −0.0215 | 0.1059 | −0.1274 |
| B10 (GeH2···CO) | LP (C)→LP* (Ge) | 1092.61 | −0.1294 | 0.0790 | 0.2162 | −0.0305 | 0.0846 | −0.1151 |
| B11 (SnH2···CO) | LP (C)→LP* (Sn) | 465.11 | −0.1125 | 0.0424 | 0.1484 | −0.0038 | 0.0409 | −0.0446 |
| B12 (PbH2···CO) | LP (C)→LP* (Pb) | 317.68 | −0.0973 | 0.0341 | 0.1218 | −0.0001 | 0.0306 | −0.0307 |
| B13 (SiH2···OC) | LP (O)→LP* (Si) | 63.16 | −0.0187 | 0.0147 | 0.0385 | −0.0012 | 0.0108 | −0.0119 |
| B14 (GeH2···OC) | LP (O)→LP* (Ge) | 68.01 | −0.0197 | 0.0158 | 0.0568 | 0.0003 | 0.0139 | −0.0137 |
| B15 (SnH2···OC) | LP (O)→LP* (Sn) | 49.78 | −0.0150 | 0.0130 | 0.0493 | 0.0007 | 0.0116 | −0.0109 |
| B16 (PbH2···OC) | LP (O)→LP* (Pb) | 49.45 | −0.0148 | 0.0142 | 0.0574 | 0.0010 | 0.0133 | −0.0123 |
| B17 (SiH2···C6H6) | π (C = C)→LP* (Si) | 221.92 | −0.0726 | 0.0374 | 0.0137 | −0.0104 | 0.0139 | −0.0243 |
| B18 (GeH2···C6H6) | π (C = C)→LP* (Ge) | 185.55 | −0.0719 | 0.0335 | 0.0501 | −0.0053 | 0.0178 | −0.0231 |
| B19 (SnH2···C6H6) | π (C = C)→LP* (Sn) | 106.21 | −0.0611 | 0.0249 | 0.0518 | −0.0019 | 0.0149 | −0.0168 |
| B20 (PbH2···C6H6) | π (C = C)→LP* (Pb) | 82.85 | −0.0586 | 0.0251 | 0.0634 | −0.0010 | 0.0169 | −0.0179 |
| Complex | Eele | %Eele | Eind | %Eind | Edisp | %Edisp | Eex | Etot |
|---|---|---|---|---|---|---|---|---|
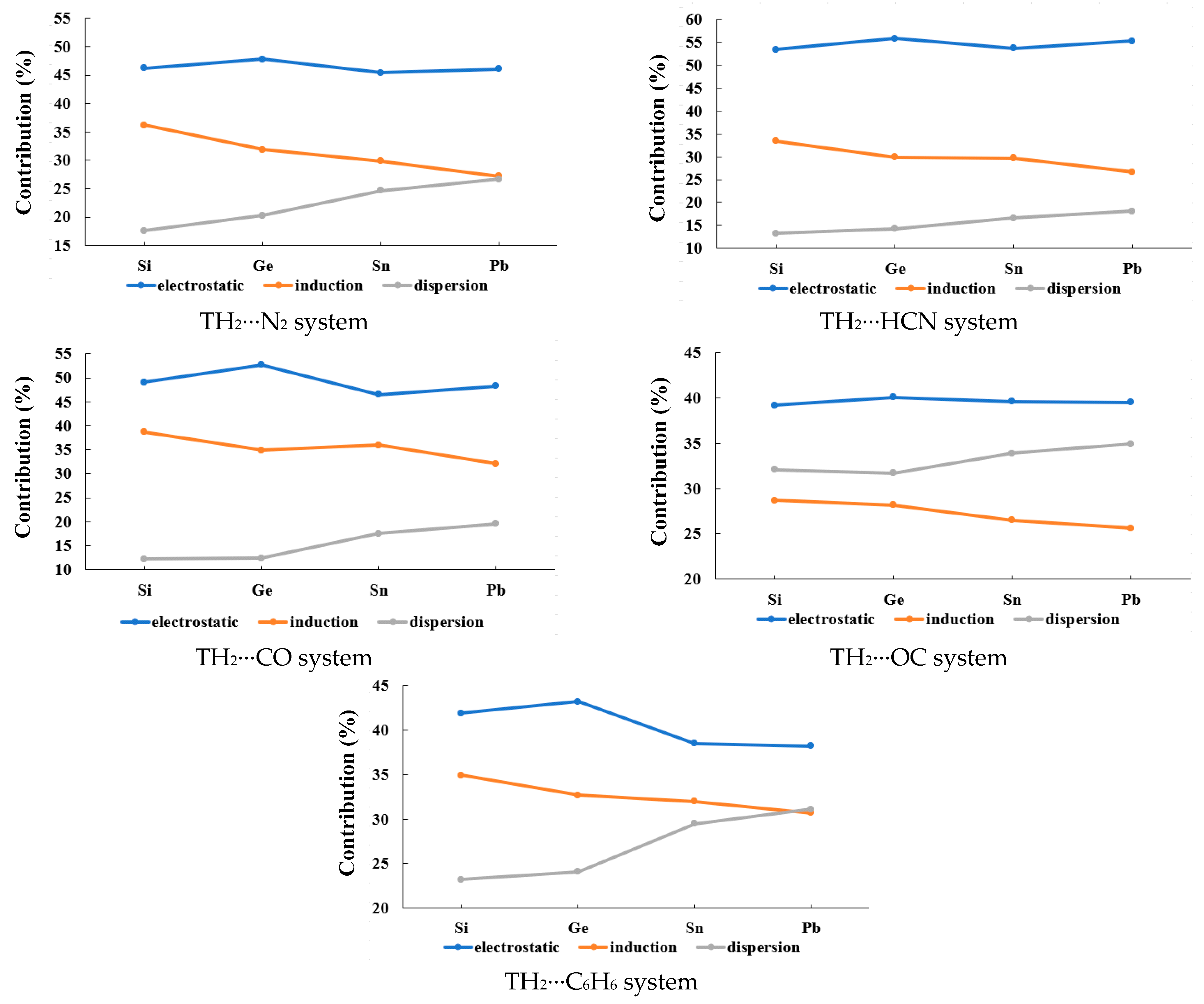
| B1 (SiH2···N2) | −98.06 | 46.2 | −76.66 | 36.2 | −37.24 | 17.6 | 187.68 | −24.29 |
| B2 (GeH2···N2) | −66.13 | 47.8 | −44.06 | 31.9 | −28.13 | 20.3 | 118.96 | −19.35 |
| B3 (SnH2···N2) | −36.74 | 45.4 | −24.16 | 29.9 | −20.02 | 24.7 | 64.33 | −16.59 |
| B4 (PbH2···N2) | −30.68 | 46.1 | −18.14 | 27.2 | −17.81 | 26.7 | 52.04 | −14.59 |
| B5 (SiH2···HCN) | −207.75 | 53.4 | −129.79 | 33.4 | −51.54 | 13.2 | 323.70 | −65.38 |
| B6 (GeH2···HCN) | −158.71 | 55.8 | −85.23 | 29.9 | −40.76 | 14.3 | 231.74 | −52.96 |
| B7 (SnH2···HCN) | −101.24 | 53.7 | −55.97 | 29.7 | −31.22 | 16.6 | 140.82 | −47.61 |
| B8 (PbH2···HCN) | −78.58 | 55.3 | −37.87 | 26.6 | −25.71 | 18.1 | 101.57 | −40.59 |
| B9 (SiH2···CO) | −301.29 | 49.1 | −237.63 | 38.7 | −74.40 | 12.2 | 516.90 | −96.43 |
| B10 (GeH2···CO) | −256.48 | 52.7 | −169.50 | 34.9 | −60.53 | 12.4 | 419.05 | −67.47 |
| B11 (SnH2···CO) | −95.51 | 46.5 | −74.03 | 36.0 | −35.91 | 17.5 | 162.77 | −42.68 |
| B12 (PbH2···CO) | −68.43 | 48.3 | −45.52 | 32.1 | −27.76 | 19.6 | 108.72 | −32.98 |
| B13 (SiH2···OC) | −15.80 | 39.2 | −11.58 | 28.7 | −12.92 | 32.1 | 32.27 | −8.03 |
| B14 (GeH2···OC) | −16.97 | 40.1 | −11.91 | 28.2 | −13.42 | 31.7 | 34.53 | −7.77 |
| B15 (SnH2···OC) | −13.25 | 39.6 | −8.86 | 26.5 | −11.33 | 33.9 | 25.67 | −7.77 |
| B16 (PbH2···OC) | −12.54 | 39.5 | −8.11 | 25.6 | −11.08 | 34.9 | 24.08 | −7.65 |
| B17 (SiH2···C6H6) | −113.99 | 41.9 | −94.84 | 34.9 | −63.03 | 23.2 | 231.70 | −40.17 |
| B18 (GeH2···C6H6) | −104.88 | 43.2 | −79.55 | 32.7 | −58.44 | 24.1 | 206.24 | −36.62 |
| B19 (SnH2···C6H6) | −65.04 | 38.5 | −54.21 | 32.0 | −49.91 | 29.5 | 135.06 | −34.11 |
| B20 (PbH2···C6H6) | −58.31 | 38.2 | −46.86 | 30.7 | −47.36 | 31.1 | 121.89 | −30.64 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, Y.; Yao, L.; Wang, F. Tetrel-Bond Interactions Involving Metallylenes TH2 (T = Si, Ge, Sn, Pb): Dual Binding Behavior. Molecules 2023, 28, 2577. https://doi.org/10.3390/molecules28062577
Chen Y, Yao L, Wang F. Tetrel-Bond Interactions Involving Metallylenes TH2 (T = Si, Ge, Sn, Pb): Dual Binding Behavior. Molecules. 2023; 28(6):2577. https://doi.org/10.3390/molecules28062577
Chicago/Turabian StyleChen, Yishan, Lifeng Yao, and Fan Wang. 2023. "Tetrel-Bond Interactions Involving Metallylenes TH2 (T = Si, Ge, Sn, Pb): Dual Binding Behavior" Molecules 28, no. 6: 2577. https://doi.org/10.3390/molecules28062577
APA StyleChen, Y., Yao, L., & Wang, F. (2023). Tetrel-Bond Interactions Involving Metallylenes TH2 (T = Si, Ge, Sn, Pb): Dual Binding Behavior. Molecules, 28(6), 2577. https://doi.org/10.3390/molecules28062577






