Bioactive Compounds, Antioxidant Activity and Sensory Properties of Northern Red Oak (Quercus rubra L., syn. Q. borealis F. Michx) Seeds Affected by Roasting Conditions
Abstract
1. Introduction
2. Results and Discussion
2.1. Effect of Roasting Conditions on Bioactive Compounds Profile in Q. rubra Samples
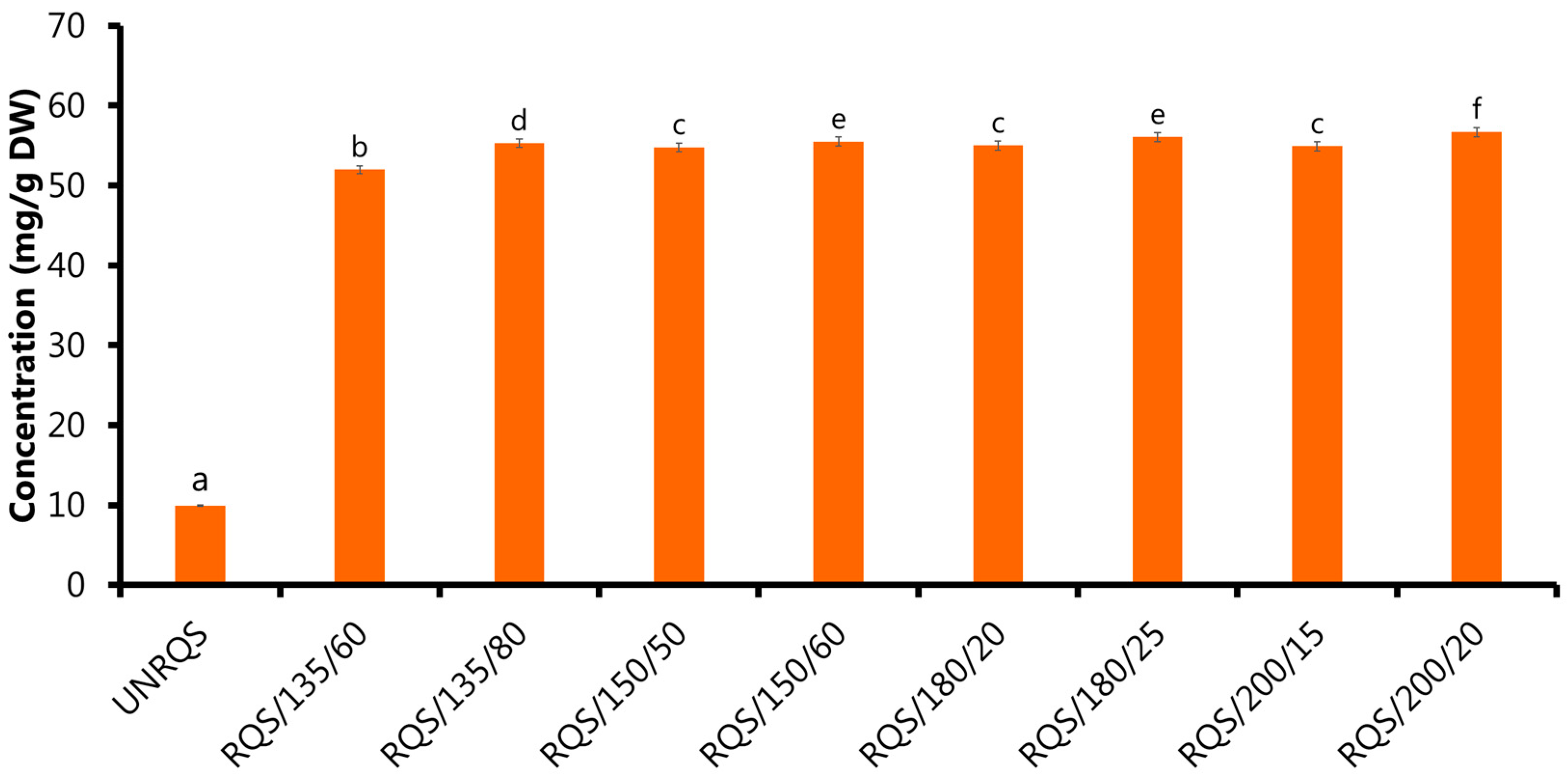
2.1.1. Effect of Roasting Conditions on Phenolic Compounds
2.1.2. Effect of Roasting Conditions on Melanoidins
2.2. Effect of Roasting Conditions on Antioxidant Properties of Q. rubra Samples
2.3. Effect of Roasting Conditions on Color of Q. rubra Samples
2.4. Effect of Roasting Conditions on Taste of Q. rubra Samples
3. Materials and Methods
3.1. Chemical Reagents and Materials
3.2. Plant Materials
3.3. Phenolic Compounds Determination
3.4. Melanoidins Determination
3.5. Antioxidant Activity Analysis
3.5.1. Determination of Free Radical-Scavenging Capacity
3.5.2. Determination of Ferric Reducing Antioxidant Power
3.5.3. Determination of Ferrous Ion Chelating Activity
3.6. CIE L*a*b* Color and Browning Index Determination
3.7. Determination of pH
3.8. Proximate Analysis
3.9. Determination of Free Sugars by HPLC
3.10. Taste Evaluation by Electronic Tongue
3.11. Statistical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Vinha, A.F.; Barreira, J.C.M.; Costa, A.S.G.; Oliveira, M.B.; Beatriz, P.P. A new age for Quercus spp. fruits: Review on nutritional and phytochemical composition and related biological activities of acorns. Compr. Rev. Food Sci. Food Saf. 2016, 15, 947–981. [Google Scholar] [CrossRef] [PubMed]
- Rakić, S.; Petrović, S.; Kukić, J.; Jadranin, M.; Tešević, V.; Povrenović, D.; Šiler-Marinković, S. Influence of thermal treatment on phenolic compounds and antioxidant properties of oak acorns from Serbia. Food Chem. 2007, 104, 830–834. [Google Scholar] [CrossRef]
- Marc, R.A.; Niculae, M.; Páll, E.; Mureșan, V.; Mureșan, A.; Tanislav, A.; Pușcaș, A.; Mureșan, C.C.; Cerbu, C. Red Oak (Quercus rubra L.) fruits as potential alternative for cocoa powder: Optimization of roasting conditions, antioxidant, and biological properties. Forests 2021, 12, 1088. [Google Scholar] [CrossRef]
- Pinto, D.; Franco, S.D.; Silva, A.M.; Cupara, S.; Koskovac, M.; Kojicic, K.; Soares, S.; Rodrigues, F.; Sut, S.; Dall’Acqua, S.; et al. Chemical characterization and bioactive properties of a coffee-like beverage prepared from Quercus cerris kernels. Food Funct. 2019, 10, 2050–2060. [Google Scholar] [CrossRef] [PubMed]
- Coelho, M.; Silva, S.; Rodríguez-Alcalá, L.M.; Oliveira, A.; Costa, E.M.; Borges, A.; Martins, C.; Rodrigues, A.S.; Pintado, M.M.E. Quercus based coffee-like beverage: Effect of roasting process and functional characterization. J. Food Meas. Charact. 2018, 12, 471–479. [Google Scholar] [CrossRef]
- Ferreira-Dias, S.; Valente, D.G.; Abreu, J.M.F. Pattern recognition of acorns from different Quercus species based on oil content and fatty acid profile. Grasas Aceites 2003, 54, 384–391. [Google Scholar] [CrossRef]
- Górnaś, P.; Rudzinska, M.; Grygier, A.; Ying, Q.; Mišina, I.; Urvaka, E.; Rungis, D. Sustainable valorization of oak acorns as a potential source of oil rich in bioactive compounds. Process Saf. Environ. Prot. 2019, 128, 244–250. [Google Scholar] [CrossRef]
- Górnaś, P. Oak Quercus rubra L. and Quercus robur L. acorns as an unconventional source of gamma- and beta-tocopherol. Eur. Food Res. Technol. 2019, 245, 257–261. [Google Scholar] [CrossRef]
- Cantos, E.; Espín, J.C.; López-Bote, C.; de la Hoz, L.; Ordóñez, J.A.; Tomás-Barberán, F.A. Phenolic compounds and fatty acids from acorns (Quercus spp.), the main dietary constituent of free-ranged Iberian pigs. J. Agric. Food Chem. 2003, 51, 6248–6255. [Google Scholar] [CrossRef]
- Oracz, J.; Żyżelewicz, D.; Pacholczyk-Sienicka, B. UHPLC-DAD-ESI-HRMS/MS profile of phenolic compounds in northern red oak (Quercus rubra L., syn. Q. borealis F. Michx) seeds and its transformation during thermal processing. Ind. Crops Prod. 2022, 189, 115860. [Google Scholar] [CrossRef]
- López-Hidalgo, C.; Trigueros, M.; Menéndez, M.; Jorrin-Novo, J.V. Phytochemical composition and variability in Quercus ilex acorn morphotypes as determined by NIRS and MS-based approaches. Food Chem. 2021, 338, 127803. [Google Scholar] [CrossRef] [PubMed]
- Abdalla, S.; Pizzi, A.; Bahabri, F.; Ganash, A. Analysis of valonia oak (Quercus aegylops) acorn tannin and wood adhesives application. BioResources 2015, 10, 7165–7177. [Google Scholar] [CrossRef]
- Rakić, S.; Petrović, S.; Tešević, V.; Simić, M.; Maletić, R. Oak acorn, polyphenols and antioxidant activity in functional food. J. Food Eng. 2006, 74, 416–423. [Google Scholar] [CrossRef]
- Gul, F.; Khan, K.M.; Adhikari, A.; Zafar, S.; Akram, M.; Khan, H.; Saeed, M. Antimicrobial and antioxidant activities of a new metabolite from Quercus incana. Nat. Prod. Res. 2017, 31, 1901–1909. [Google Scholar] [CrossRef] [PubMed]
- Soares, S.; Silva, M.S.; García-Estevez, I.; Groβmann, P.; Brás, N.; Brandão, E.; Mateus, N.; de Freitas, V.; Beherens, M.; Meyerhof, W. Human bitter taste receptors are activated by different classes of polyphenols. J. Agric. Food Chem. 2018, 66, 8814–8823. [Google Scholar] [CrossRef]
- Rodríguez-Bencomo, J.J.; Kelebek, H.; Sonmezdag, A.S.; Rodríguez-Alcalá, L.M.; Fontecha, J.; Selli, S. Characterization of the aroma-active, phenolic, and lipid profiles of the pistachio (Pistacia vera L.) nut as affected by the single and double roasting process. J. Agric. Food Chem. 2015, 63, 7830–7839. [Google Scholar] [CrossRef]
- Chandrasekara, N.; Shahidi, F. Effect of Roasting on Phenolic Content and Antioxidant Activities of Whole Cashew Nuts, Kernels, and Testa. J. Agric. Food Chem. 2011, 59, 5006–5014. [Google Scholar] [CrossRef]
- Sun, K.; Dai, Z.; Hong, W.; Zhao, J.; Zhao, H.; Luo, J.; Xie, G. Effects of Maillard Reaction on Volatile Compounds and Antioxidant Capacity of Cat Food Attractant. Molecules 2022, 27, 7239. [Google Scholar] [CrossRef]
- Sun, J.; Shi, W.; Wu, Y.; Ji, J.; Feng, J.; Zhao, J.; Shi, X.; Du, C.; Chen, W.; Liu, J.; et al. Variations in acorn traits in two oak species: Quercus mongolica Fisch. ex Ledeb. and Quercus variabilis Blume. Forests 2021, 12, 1755. [Google Scholar] [CrossRef]
- Wesołowski, T.; Rowiński, P.; Maziarz, M. Interannual variation in tree seed production in a primeval temperate forest: Does masting prevail? Eur. J. For. Res. 2015, 134, 99–112. [Google Scholar] [CrossRef]
- Marquart, T.J.; Scholes, C.M.; Chapman, J.M. Distribution, quantification and identification of tannins in acorns from red and white oak trees. In Proceedings of the Midwest Regional Meeting, Kansas, MO, USA, 7–10 November 2007. [Google Scholar]
- Yoshida, T.; Yoshimura, M.; Amakura, Y. Chemical and biological significance of oenothein B and related ellagitannin oligomers with macrocyclic structure. Molecules 2018, 23, 552. [Google Scholar] [CrossRef] [PubMed]
- Górnaś, P.; Juhņeviča-Radenkova, K.; Radenkovs, V.; Mišina, I.; Pugajeva, I.; Soliven, A.; Segliņa, D. The impact of different baking conditions on the stability of the extractable polyphenols in muffins enriched by strawberry, sour cherry, raspberry or black currant pomace. LWT Food Sci. Technol. 2016, 65, 946–953. [Google Scholar] [CrossRef]
- González, M.J.; Torres, J.L.; Medina, I. Impact of thermal processing on the activity of gallotannins and condensed tannins from Hamamelis virginiana used as functional ingredients in seafood. J. Agric. Food Chem. 2010, 58, 4274–4283. [Google Scholar] [CrossRef] [PubMed]
- Bakkalbaşi, E.; Menteş, O.; Artik. Food ellagitannins-occurrence, effects of processing and storage. Crit. Rev. Food Sci. 2009, 49, 283–298. [Google Scholar] [CrossRef]
- Hager, T.J.; Howard, L.R.; Prior, R.L. Processing and storage effects on the ellagitannin composition of processed blackberry products. J. Agric. Food Chem. 2010, 58, 11749–11754. [Google Scholar] [CrossRef]
- Sanz, M.; Cadahía, E.; Esteruelas, E.; Muñoz, A.M.; de Simón, B.F.; Hernández, T.; Estrella, I. Phenolic compounds in chestnut (Castanea sativa Mill.) heartwood. Effect of toasting at cooperage. J. Agric. Food Chem. 2010, 58, 9631–9640. [Google Scholar] [CrossRef]
- Platzer, M.; Kiese, S.; Herfellner, T.; Schweiggert-Weisz, U.; Eisner, P. How Does the Phenol Structure Influence the Results of the Folin-Ciocalteu Assay? Antioxidants 2021, 10, 811. [Google Scholar] [CrossRef]
- Liao, Y.-C.; Kim, T.; Silva, J.L.; Hu, W.-Y.; Chen, B.-Y. Effects of roasting degrees on phenolic compounds and antioxidant activity in coffee beans from different geographic origins. LWT Food Sci. Technol. 2022, 168, 113965. [Google Scholar] [CrossRef]
- Oracz, J.; Nebesny, E.; Żyżelewicz, D. Identification and quantification of free and bound phenolic compounds contained in the high-molecular weight melanoidin fractions derived from two different types of cocoa beans by UHPLC-DAD-ESI-HR-MSn. Food Res. Int. 2019, 15, 135–149. [Google Scholar] [CrossRef]
- Bekedam, E.K.; Schols, H.A.; Van Boekel, M.A.J.S.; Smit, G. High molecular weight melanoidins from coffee brew. J. Agric. Food Chem. 2006, 54, 7658–7666. [Google Scholar] [CrossRef]
- Moreira, A.S.; Nunes, F.M.; Domingues, M.R.; Coimbra, M.A. Coffee melanoidins: Structures, mechanisms of formation and potential health impacts. Food Funct. 2012, 3, 903–915. [Google Scholar] [CrossRef] [PubMed]
- Nunes, F.M.; Coimbra, M.A. Melanoidins from coffee infusions. Fractionation, chemical characterization, and effect of the degree of roast. J. Agric. Food Chem. 2007, 55, 3967–3977. [Google Scholar] [CrossRef] [PubMed]
- Moreira, A.S.; Nunes, F.M.; Simões, C.; Maciel, E.; Domingues, P.; Domingues, M.R.; Coimbra, M.A. Transglycosylation reactions, a main mechanism of phenolics incorporation in coffee melanoidins: Inhibition by Maillard reaction. Food Chem. 2017, 227, 422–431. [Google Scholar] [CrossRef] [PubMed]
- Sacchetti, G.; Ioannone, F.; De Gregorio, M.; Di Mattia, C.; Serafini, M.; Mastrocola, D. Non enzymatic browning during cocoa roasting as affected by processing time and temperature. J. Food Eng. 2016, 169, 44–52. [Google Scholar] [CrossRef]
- Nicoli, M.C.; Anese, M.; Manzocco, L.; Lerici, C.R. Antioxidant Properties of Coffee Brews in Relation to the Roasting Degree. LWT Food Sci. Technol. 1997, 30, 292–297. [Google Scholar] [CrossRef]
- Le Bourvellec, C.; Renard, C.M. Interactions between polyphenols and macromolecules: Quantification methods and mechanisms. Crit. Rev. Food Sci. Nutr. 2012, 52, 213–248. [Google Scholar] [CrossRef] [PubMed]
- Mesías, M.; Delgado-Andrade, C. Melanoidins as a potential functional food ingredient. Curr. Opin. Food Sci. 2017, 14, 37–42. [Google Scholar] [CrossRef]
- Jiménez-Zamora, A.; Pastoriza, S.; Rufián-Henares, J.A. Revalorization of coffee by-products. Prebiotic, antimicrobial and antioxidant properties. LWT Food Sci. Technol. 2015, 61, 12–18. [Google Scholar] [CrossRef]
- Baliyan, S.; Mukherjee, R.; Priyadarshini, A.; Vibhuti, A.; Gupta, A.; Pandey, R.P.; Chang, C.M. Determination of Antioxidants by DPPH Radical Scavenging Activity and Quantitative Phytochemical Analysis of Ficus religiosa. Molecules 2022, 27, 1326. [Google Scholar] [CrossRef]
- Birasuren, B.; Kim, N.Y.; Jeon, H.L.; Kim, M.R. Evaluation of the Antioxidant Capacity and Phenolic Content of Agriophyllum pungens Seed Extracts from Mongolia. Prev. Nutr. Food Sci. 2013, 18, 188–195. [Google Scholar] [CrossRef]
- Zafrilla, P.; Ferreres, F.; Tomás-Barberán, F.A. Effect of processing and storage on the antioxidant ellagic acid derivatives and flavonoids of red raspberry (Rubus idaeus) jams. J. Agric. Food Chem. 2001, 49, 3651–3655. [Google Scholar] [CrossRef] [PubMed]
- Oracz, J.; Żyżelewicz, D. In Vitro Antioxidant Activity and FTIR Characterization of High-Molecular Weight Melanoid Fractions from Different Types of Cocoa Beans. Antioxidants 2019, 8, 560. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Zhang, H.; Troise, A.D.; Fogliano, V. Melanoidins from Coffee, Cocoa, and Bread Are Able to Scavenge α-Dicarbonyl Compounds under Simulated Physiological Conditions. J. Agric. Food Chem. 2019, 67, 10921–10929. [Google Scholar] [CrossRef] [PubMed]
- Żyżelewicz, D.; Krysiak, W.; Nebesny, E.; Budryn, G. Application of various methods for determination of the color of cocoa beans roasted under variable process parameters. Eur. Food Res. Technol. 2014, 238, 549–563. [Google Scholar] [CrossRef]
- Bessa-Pereira, C.; Dias, R.; Brandão, E.; Mateus, N.; de Freitas, V.; Soares, S.; Pérez-Gregorio, R. Eat Tasty and Healthy: Role of Polyphenols in Functional Foods. In Functional Foods—Phytochemicals and Health Promoting Potential; Arshad, M.S., Ahmad, M.H., Eds.; IntechOpen: London, UK, 2021. [Google Scholar] [CrossRef]
- Soares, S.; Brandão, E.; Guerreiro, C.; Soares, S.; Mateus, N.; de Freitas, V. Tannins in Food: Insights into the Molecular Perception of Astringency and Bitter Taste. Molecules 2020, 25, 2590. [Google Scholar] [CrossRef] [PubMed]
- Dong, W.; Hu, R.; Long, Y.; Li, H.; Zhang, Y.; Zhu, K.; Chu, Z. Comparative evaluation of the volatile profiles and taste properties of roasted coffee beans as affected by drying method and detected by electronic nose, electronic tongue, and HS-SPME-GC-MS. Food Chem. 2019, 30, 723–731. [Google Scholar] [CrossRef]
- Yoon, S.; Jeong, H.; Jo, S.M.; Hong, S.J.; Kim, Y.J.; Kim, J.K.; Shinource, E.-C. Chemosensoric approach for microwave- or oven-roasted Coffea arabica L. (cv. Yellow Bourbon) using electronic sensors. LWT Food Sci. Technol. 2022, 167, 113844. [Google Scholar] [CrossRef]
- Łuczaj, Ł.; Adamczak, A.; Duda, M. Tannin content in acorns (Quercus spp.) from Poland. Dendrobiology 2014, 72, 103–111. [Google Scholar] [CrossRef]
- Galvan, J.V.; Novo, J.J.J.; Cabrera, A.G.; Ariza, D.; García-Olmo, J.; Cerrillo, R.M.N. Population variability based on the morphometry and chemical composition of the acorn in Holm oak (Quercus ilex subsp. ballota [Desf.] Samp.). Eur. J. For. Res. 2012, 131, 893–904. [Google Scholar] [CrossRef]
- Wu, J.; Jin, Y.; Zhang, M. Evaluation on the physicochemical and digestive properties of melanoidin from black garlic and their antioxidant activities In Vitro. Food Chem. 2021, 340, 127934. [Google Scholar] [CrossRef]
- Diviš, P.; Pořízka, J.; Kříkala, J. The effect of coffee beans roasting on its chemical composition. Potr. Slovak J. Food Sci. 2019, 13, 344–350. [Google Scholar] [CrossRef]
- Utami, R.; Wijaya, C.H.; Lioe, H.N. Taste of Water-Soluble Extracts Obtained from Over-Fermented Tempe. Int. J. Food Prop. 2016, 19, 2063–2073. [Google Scholar] [CrossRef]
- Kabelitz, T.; Hassenberg, K. Control of apple surface microflora for fresh-cut produce by post-harvest hot-water treatment. LWT Food Sci. Technol. 2018, 98, 492–499. [Google Scholar] [CrossRef]
- Purabdolah, H.; Sadeghi, A.; Ebrahimi, M.; Kashaninejad, M.; Tabarestani, S.T.; Mohamadzadeh, J. Techno-functional properties of the selected antifungal predominant LAB isolated from fermented acorn (Quercus persica). J. Food Meas. Charact. 2020, 14, 1754–1764. [Google Scholar] [CrossRef]
- AOAC. Official Methods of Analysis, 18th ed.; Association of Official Analytical Chemists: Washington, WA, USA, 2005. [Google Scholar]
- Thiex, N.; Novotny, L.; Crawford, A. Determination of ash in animal feed: AOAC official method 942.05 revisited. J. AOAC Int. 2012, 95, 1392–1397. [Google Scholar] [CrossRef] [PubMed]
- Oracz, J.; Nebesny, E. Effect of roasting parameters on the physicochemical characteristics of high-molecular weight Mailard reaction products isolated from cocoa beans of different Theobroma cacao L. groups. Eur. Food Res. Technol. 2019, 245, 118–128. [Google Scholar] [CrossRef]
- Kowalski, S.; Oracz, J.; Skotnicka, M.; Mikulec, A.; Gumul, D.; Mickowska, B.; Mazurek, A.; Sabat, R.; Wywrocka-Gurgul, A.; Żyżelewicz, D. Chemical Composition, Nutritional Value, and Acceptance of Nut Bars with the Addition of Edible Insect Powder. Molecules 2022, 27, 8472. [Google Scholar] [CrossRef] [PubMed]



| Samples | DPPH (μM TE/g DW) | FRAP (μM Fe(II)/g DW) | Fe(II) Chelating Ability (mg EDTA/g DW) |
|---|---|---|---|
| UNRQS | 5182.40 ± 3.98 g | 1703.76 ± 2.70 f | 32.40 ± 0.27 g |
| RQS/135/60 | 3789.36 ± 3.12 d,e | 1646.61 ± 2.56 e | 29.23 ± 0.28 f |
| RQS/135/80 | 3505.76 ± 2.98 c | 1286.08 ± 2.39 b | 28.09 ± 0.31 d,e |
| RQS/150/50 | 3316.91 ± 4.09 b | 1365.33 ± 2.45 c | 26.48 ± 0.19 a,b |
| RQS/150/60 | 3144.22 ± 3.72 a | 1179.78 ± 2.71 a | 26.00 ± 0.21 a |
| RQS/180/20 | 3716.70 ± 3.19 d | 1500.64 ± 2.38 d | 28.58 ± 0.17 e,f |
| RQS/180/25 | 3827.15 ± 3.45 e | 1773.83 ± 2.25 g | 26.98 ± 0.23 b,c |
| RQS/200/15 | 3562.03 ± 2.87 c | 1488.90 ± 2.08 d | 28.79 ± 0.18 e,f |
| RQS/200/20 | 3943.94 ± 3.09 f | 1472.40 ± 2.62 d | 27.71 ± 0.25 c,d |
| Samples | L* | a* | b* | ΔE | BI |
|---|---|---|---|---|---|
| UNRQS | 80.30 ± 0.23 g | 1.97 ± 0.11 a | 16.71 ± 0.10 a | - | 3.82 ± 0.10 a |
| RQS/135/60 | 62.79 ± 0.28 f | 8.15 ± 0.08 b | 26.51 ± 0.06 c | 21.00 ± 0.11 a | 13.44 ± 0.11 b |
| RQS/135/80 | 58.51 ± 0.19 c | 9.36 ± 0.09 d | 27.50 ± 0.07 e | 25.42 ± 0.13 d | 16.03 ± 0.13 e |
| RQS/150/50 | 57.97 ± 0.25 c | 9.41 ± 0.08 d | 27.41 ± 0.11 d,e | 25.86 ± 0.10 e | 16.22 ± 0.10 f |
| RQS/150/60 | 53.34 ± 0.26 b | 10.38 ± 0.13 f | 27.20 ± 0.12 d | 30.13 ± 0.09 g | 18.82 ± 0.11 h |
| RQS/180/20 | 59.41 ± 0.28 d | 9.33 ± 0.10 d | 27.65 ± 0.08 e | 24.70 ± 0.12 c | 15.78 ± 0.10 d |
| RQS/180/25 | 53.83 ± 0.16 b | 10.23 ± 0.07 e | 27.19 ± 0.10 d | 29.65 ± 0.11 f | 18.46 ± 0.12 g |
| RQS/200/15 | 61.26 ± 0.25 e | 8.60 ± 0.09 c | 26.78 ± 0.13 c | 22.53 ± 0.10 b | 14.33 ± 0.13 c |
| RQS/200/20 | 47.64 ± 0.29 a | 10.44 ± 0.11 f | 25.02 ± 0.10 b | 34.75 ± 0.14 h | 20.65 ± 0.14 i |
| Samples | pH | Free Sugars (mg/g DW) | |||
|---|---|---|---|---|---|
| Fructose | Glucose | Sucrose | Total | ||
| UNRQS | 4.97 ± 0.03 f | 7.67 ± 0.06 a | 20.05 ± 0.07 c | 122.13 ± 0.09 g | 149.85 ± 0.12 g |
| RQS/135/60 | 4.65 ± 0.04 d | 13.40 ± 0.07 f | 21.15 ± 0.06 e | 85.38 ± 0.08 f | 119.93 ± 0.14 f |
| RQS/135/80 | 4.61 ± 0.02 c | 12.28 ± 0.04 e | 15.89 ± 0.09 a | 67.98 ± 0.07 c | 96.15 ± 0.13 b |
| RQS/150/50 | 4.57 ± 0.05 b | 14.76 ± 0.03 h | 20.64 ± 0.07 d | 69.52 ± 0.04 d | 104.92 ± 0.16 c |
| RQS/150/60 | 4.55 ± 0.03 b | 15.48 ± 0.05 i | 23.44 ± 0.04 f | 57.40 ± 0.05 b | 96.32 ± 0.15 b |
| RQS/180/20 | 4.68 ± 0.07 e | 11.02 ± 0.06 c | 19.09 ± 0.05 b | 75.29 ± 0.08 e | 105.40 ± 0.10 c |
| RQS/180/25 | 4.63 ± 0.04 c,d | 14.49 ± 0.05 g | 24.87 ± 0.06 g | 68.35 ± 0.09 c | 107.71 ± 0.14 e |
| RQS/200/15 | 4.61 ± 0.03 c | 11.74 ± 0.07 d | 19.13 ± 0.09 b | 75.35 ± 0.07 e | 106.22 ± 0.12 d |
| RQS/200/20 | 4.52 ± 0.05 a | 10.05 ± 0.04 b | 23.47 ± 0.08 f | 44.27 ± 0.06 a | 77.79 ± 0.13 a |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Oracz, J.; Prejzner, M.; Grzelczyk, J.; Kowalska, G.; Żyżelewicz, D. Bioactive Compounds, Antioxidant Activity and Sensory Properties of Northern Red Oak (Quercus rubra L., syn. Q. borealis F. Michx) Seeds Affected by Roasting Conditions. Molecules 2023, 28, 2299. https://doi.org/10.3390/molecules28052299
Oracz J, Prejzner M, Grzelczyk J, Kowalska G, Żyżelewicz D. Bioactive Compounds, Antioxidant Activity and Sensory Properties of Northern Red Oak (Quercus rubra L., syn. Q. borealis F. Michx) Seeds Affected by Roasting Conditions. Molecules. 2023; 28(5):2299. https://doi.org/10.3390/molecules28052299
Chicago/Turabian StyleOracz, Joanna, Monika Prejzner, Joanna Grzelczyk, Gabriela Kowalska, and Dorota Żyżelewicz. 2023. "Bioactive Compounds, Antioxidant Activity and Sensory Properties of Northern Red Oak (Quercus rubra L., syn. Q. borealis F. Michx) Seeds Affected by Roasting Conditions" Molecules 28, no. 5: 2299. https://doi.org/10.3390/molecules28052299
APA StyleOracz, J., Prejzner, M., Grzelczyk, J., Kowalska, G., & Żyżelewicz, D. (2023). Bioactive Compounds, Antioxidant Activity and Sensory Properties of Northern Red Oak (Quercus rubra L., syn. Q. borealis F. Michx) Seeds Affected by Roasting Conditions. Molecules, 28(5), 2299. https://doi.org/10.3390/molecules28052299









