1. Introduction
In recent years, third-order NLO materials have already had widespread applications in many fields [
1,
2,
3,
4,
5,
6,
7,
8], such as optical switching, optical limiting, optical communications, optical data processing, and mode-locked laser system, etc. To date, multifarious elaborately designed organic/inorganic and hybrid materials have been synthesized for third-order NLO [
9,
10,
11,
12,
13,
14,
15], including semiconductor quantum dots, black phosphorus, carbon nanodots, polymers or conjugated organic molecules (such as porphyrins and phthalocyanines), metal-clusters or metal-oxo clusters and metal-organic frameworks, and so on. It is acknowledged that ionic pair compounds are potentially excellent NLO materials because of their intrinsic attributes, such as high positive/negative charge, ordered self-assembly structures and stability [
16,
17,
18,
19,
20], and the process of electron/charge transfer between anion and cation groups is usually considered as the significant factor for favorable NLO response [
21,
22,
23,
24]. With the enhancement of the covalent or coordinative interactions between anion and cation groups, the third-order NLO properties also increase, which may be because the covalent or coordinative interactions are beneficial to the electron/charge transfer between anion and cation groups [
25,
26].
Very recently, our group has been devoted to research work based on an anionic metal-organic cage (Ti
4L
6 or Zr
4L
6, L = embonate) [
27]. It is an interesting dispersed molecular cage with a tetrahedral symmetry environment, and it can be soluble and stable in water and some common solvents. In the study, we found that it is an excellent building block for stepwise assembly due to its abundant interaction sites (uncoordinated carboxyl oxygen atoms and naphthyls), facilitating the incorporation of metal coordination bonding, π-π stacking, and other noncovalent interactions altogether. By using such ultra-stable cage as a reactive precursor to assembly with metal ions and highly conjugated organic ligands or groups [
28,
29,
30], a few advanced cage-based materials with various dimensional structures were obtained by two-step reaction, including 3D frameworks with hydrocarbons sorption capacities, simple cage compounds with identification and separation functions, and a series of co-crystals with notable third-order NLO performances.
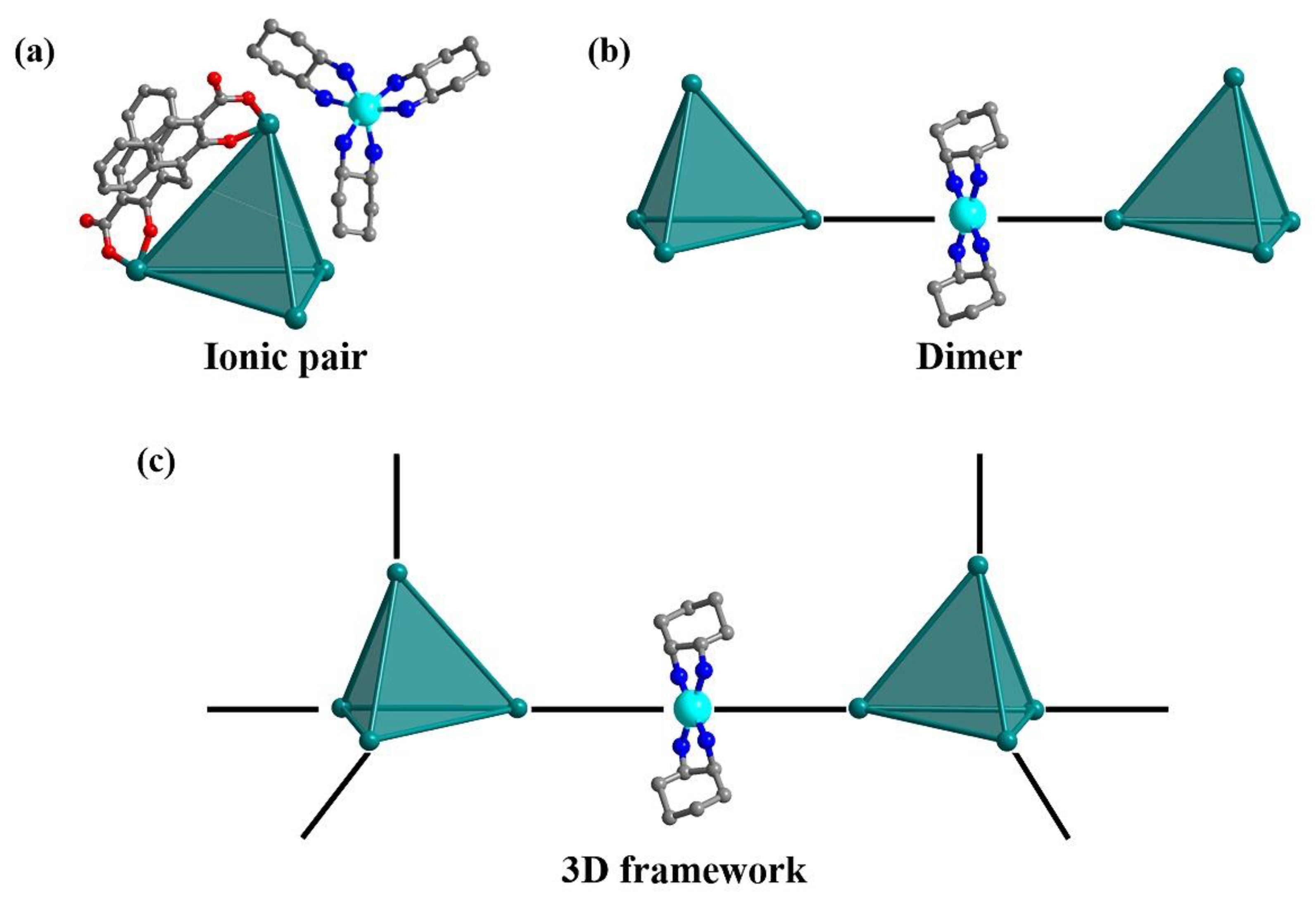
In this work, we investigate the assembly behavior of anionic Zr
4L
6 cage and N, N-chelated transition-metal cations (
Scheme 1), and we explore the effect of charge transfer between them on third-order NLO performances. More specifically, through changing the type and molar ratio of the salt and solvent, the reaction of Cu
+ or Zn
2+ ions, 1,2-diaminocyclohexane, and Zr
4L
6 cages produced a series of novel Zr
4L
6-based architectures, including ion pair structures (
PTC-355 and
PTC-356), dimer (
PTC-357), and 3D frameworks (
PTC-358 and
PTC-359). X-ray single crystal structural analyses show that
PTC-358 exhibits a 2-fold interpenetrating framework with 3,4- connected topology, and
PTC-359 shows a 2-fold interpenetrating framework with a 4-connected
dia network. Both
PTC-358 and
PTC-359 can be stable in air and other common solvents at room temperature. In addition, the phase purity, UV-vis spectra, and photocurrent properties of these materials were also studied. For these cage-assembled compounds
PTC-355–
PTC-359, their emission peaks can be observed at 460, 472, 490, 510, and 501 nm, respectively. Furthermore, by means of a film-making method, we tested the third-order NLO properties of these materials. We found that the efficiency of the charge transfer was greatly enhanced with the increasing coordinative interaction between the anion cage and cation group, leading to the enhancement of NLO response.
2. Results and Discussion
The simple method previously reported was used to synthesize large amounts of PTC-101(Zr) as the source of the Zr
4L
6 cages [
21], which is also described in detail in the experimental section. In the beginning, CuBr and (+/−)-trans-1,2-diaminocyclohexane (
trans-DCH) were added to the DMF/H
2O solution of PTC-101(Zr), which was kept at room temperature for 3 days, yielding blue block crystals of
PTC-355. Single-crystal structural analysis reveals that
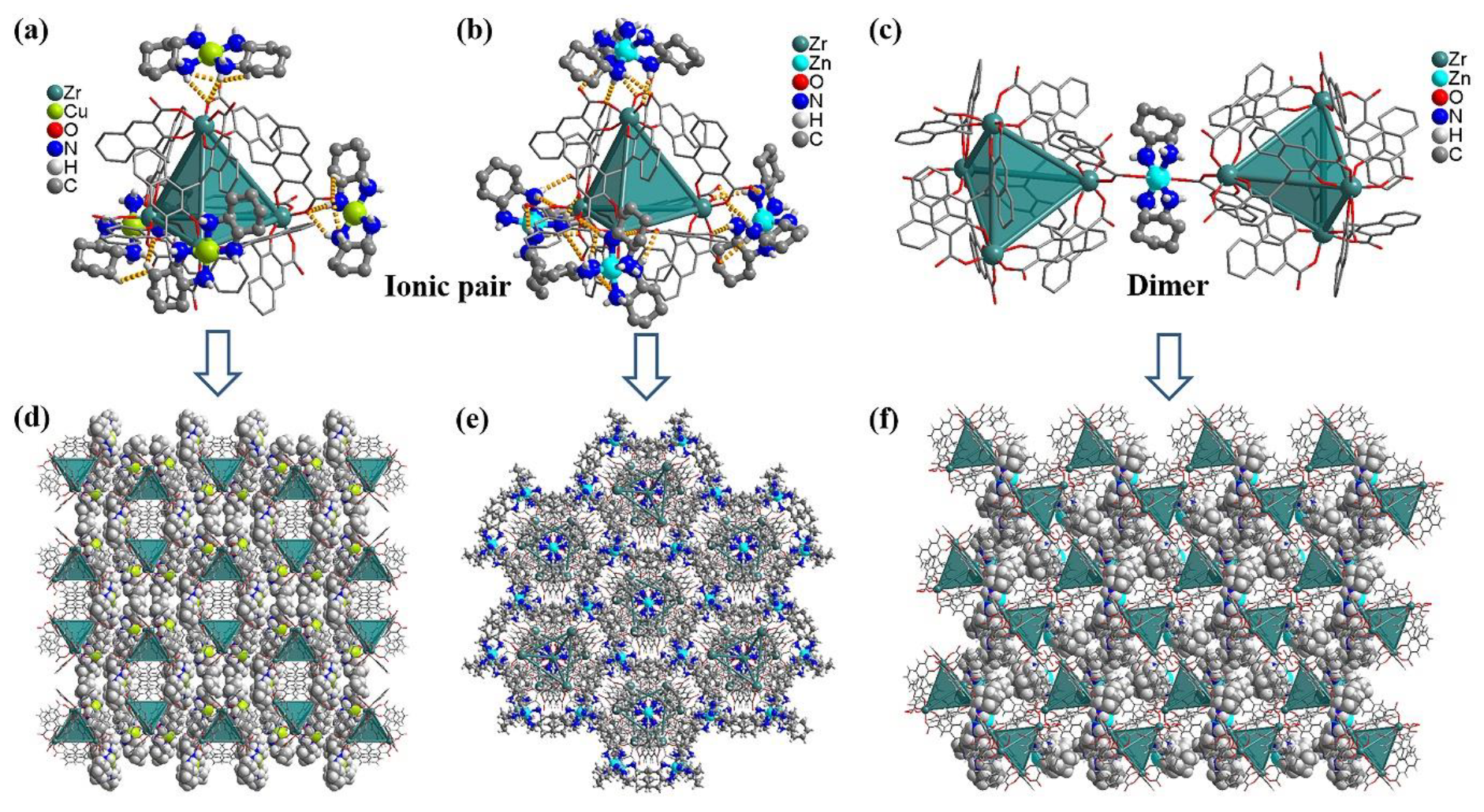
PTC-355 crystallizes in a monoclinic system with space group
C2/
c, and the asymmetric unit contains half of anionic Zr
4L
6 cage, two [Cu(
trans-DCH)
2]
2+ cations, and some solvent molecules, as shown in
Figure S1 in the
Supporting Information. In
PTC-355, the Cu(II) center is chelated by two
trans-DCH ligands (
Figure 1a) and shows [CuN4] planar quadrilateral coordination geometry, giving rise to a [Cu(
trans-DCH)
2]
2+ ion. The Cu-N bond distances vary from 1.96 to 2.03 Å. The calixarene-like oxygen vertices of each Zr
4L
6 cage match very well with four [Cu(
trans-DCH)
2]
2+ units. Through weak C/N−H⋯O (2.9–3.4 Å) and C−H···π (3.6–4.0 Å) interactions, the Zr
4L
6 cages and the [Cu(
trans-DCH)
2]
2+ cations stack alternately into a 3D dense supramolecular structure (
Figure 1d and
Figure S2). By replacing the above copper salt (CuBr) with zinc salt (Zn(NO
3)
2·7H
2O) and changing the solvent type (we used 1,4-dioxane/H
2O/MeCN as solvent), as well as raising the reactive temperature (80 °C), we successfully synthesized yellow block crystals of
PTC-356. Single crystal structural analysis shows that
PTC-356 crystallizes in a trigonal system with space group
R3
c. In the asymmetric unit of
PTC-356, there are one-third of the Zr
4L
6 cage and one and one-third of [Zn(
trans-DCH)
3]
2+ cations (
Figure S3). Unlike
PTC-355, in the structure of
PTC-356, the Zn(II) center in the [Zn(
trans-DCH)
3]
2+ unit is chelated by three
trans-DCH ligands (
Figure 1b) and exhibits [ZnN6] octahedral coordination geometry. The Zn−N bond distances vary from 2.18 to 2.21 Å. Because of its highly symmetrical crystal system,
PTC-356 has an attractive honeycomb-like packing superstructure (
Figure 1e). There is no typical hydrogen bond, and the binding interaction may be due to weak van der Waals force between zirconium cages and cation units. However, when
trans-DCH and Zn(NO
3)
2·7H
2O were replaced with
cis-1,2-diaminocyclohexane (
cis-DCH) and Zn(CH
3COO)
2·2H
2O, respectively, compound
PTC-357 was obtained. Structural analysis displays that
PTC-357 crystallizes in a triclinic system with space group
P-1, and the asymmetric unit includes one Zr
4L
6 cage, half of [Zn(
cis-DCH)
2]
2+ and two [Zn(
cis-DCH)
3]
2+ cations, and one (Me
2NH
2)
+ cation. In the structure of
PTC-357, an interesting dimer can be observed (
Figure 1c), in which two Zr
4L
6 cages are connected by one [Zn(
cis-DCH)
2]
2+ unit. Herein the Zn(II) center is six-coordinated by four N atoms from two
cis-DCH and two carboxyl O atoms from two tetrahedrons (the Zn–O bond distance is 2.44 Å) to build a distorted tetrahedral coordination geometry. The Zn−O bond distances vary from 2.00 to 2.24 Å. These dimers and surrounding [Zn(
cis-DCH)
3]
2+ cations are further packed into a 3D dense architecture (
Figure 1f and
Figure S5).
Encouraged by the above results, more synthetic experiments were explored. By replacing Zn(CH
3COO)
2·2H
2O with ZnSO
4·7H
2O and reducing the amount of
cis-DCH in the above synthetic procedure of
PTC-357,
PTC-358 was prepared. Compound
PTC-358 has a highly symmetric cubic chiral space group
I23, and its asymmetric unit possesses five out of six Zr
4L
6 cages and one [Zn(
cis-DCH)
2]
2+ cation (
Figure S6). Interestingly, such in situ generated [Zn(
cis-DCH)
2]
2+ unit further connects two adjacent Zr
4L
6 cages through coordination bonds (one Zn–O bond distance is 2.197 Å and another is the weak force of 2.827 Å), and the Zr
4L
6 cages are coordinated by three or four [Zn(
cis-DCH)
2]
2+ units (
Figure 2a), giving rise to a 3D framework possessing large cavities (
Figure 2b). The large intraframework spaces are occupied by another identical but independent framework, giving a 2-fold interpenetrating structure (
Figure 2c). Considering the bridging [Zn(
cis-DCH)
2]
2+ units, the Zr
4L
6 cages act as three- or four-connected nodes, so the network topology of
PTC-358 can be described as a (3,4)-connected net. However, by reducing the amount of Zn(CH
3COO)
2·2H
2O and
cis-DCH in the above synthetic procedure of
PTC-357, we obtain compound
PTC-359. Structural analysis shows that
PTC-359 crystallizes in a monoclinic system with space group
P2
1/
n and the asymmetric unit involves two Zr
4L
6 cages, four [Zn(
cis-DCH)
2]
2+ cations, and one (Me
2NH
2)
+ cation.
PTC-359 exhibits a 4-connected
dia-type network of Zr
4L
6 tetrahedra linked together by [Zn(
cis-DCH)
2]
2+ units (
Figure 2d,e). In
PTC-359, a larger diamondoid cage is constructed by ten Zr
4L
6 cages and twelve [Zn(
cis-DCH)
2]
2+ units (
Figure 2f). Similarly, because the potential space is large enough to fill two independent frameworks, forming a 2-fold interpenetrated structure. This is a very interesting phenomenon in the field of supramolecular self-assembly, which also shows that the reaction conditions have a significant effect on the assembly behavior of titanium cage and zinc units.
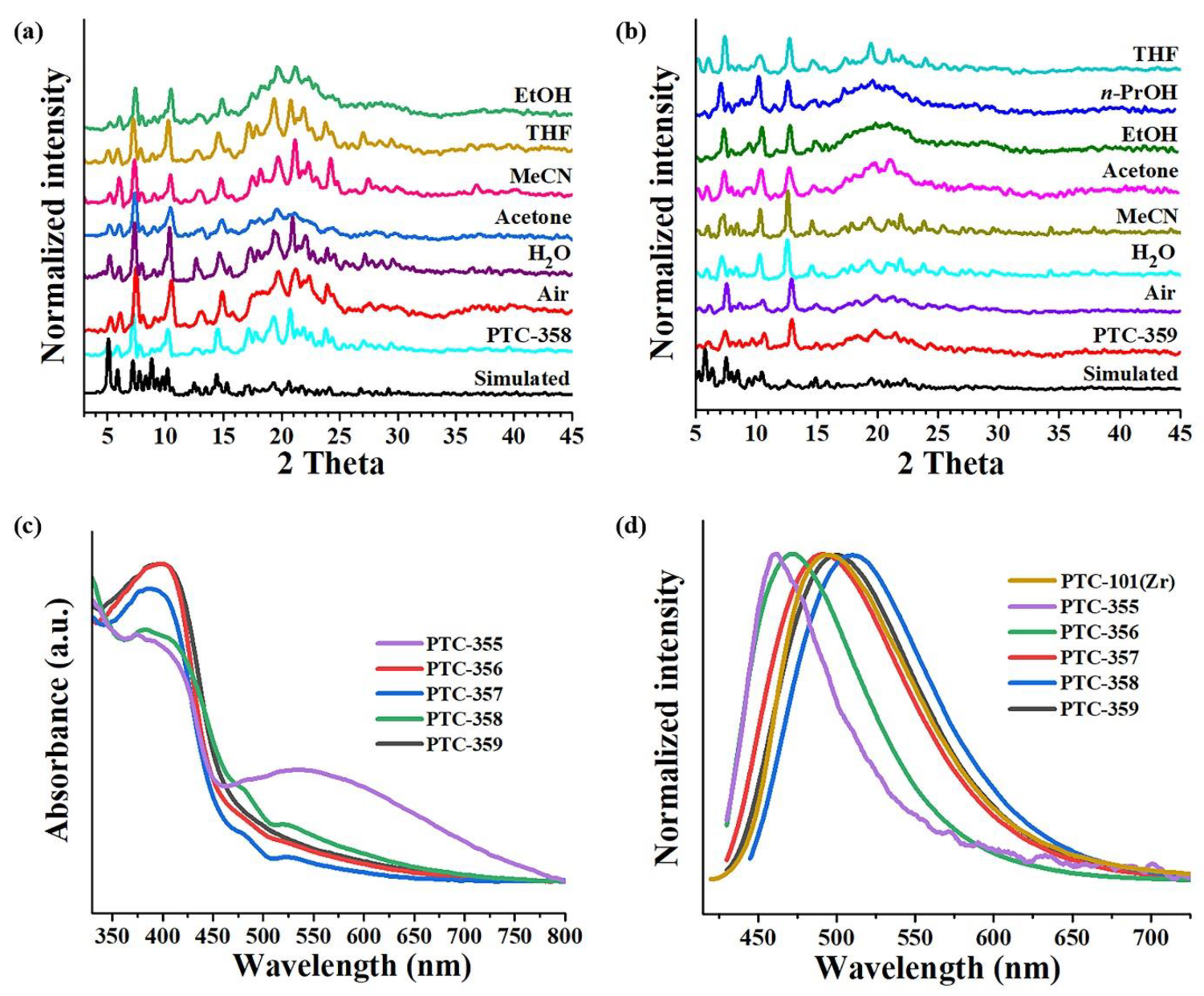
The powder X-Ray Diffraction (XRD) of
PTC-355–
PTC-359 confirmed the phase purities of these samples (
Figure 3a,b and
Figures S13–S15) because their XRD patterns are very similar to those simulated from their single crystal data. The above results show that the self-assembly of Zr
4L
6 cages with N, N-chelated zinc units into
PTC-356–
PTC-359 under certain conditions is quite stable and directed. Thermal gravimetric analyses (TGA) curves of these materials were measured, as shown in
Figures S8–12 in the
Supporting Information. The TGA curve of
PTC-355 shows a distinct weightless platform before 250 °C. For other compounds, the solvents were gradually lost with increasing temperature, followed by the decomposition of their structures. The residues after decomposition may be ZrO
2 and CuO or ZnO. In addition, the stabilities of
PTC-358 and
PTC-359 were also studied. At room temperature, some crystals of
PTC-358 and
PTC-359 were exposed to air for 12 h or immersed in different solvents for 1 day, respectively, and then their XRD patterns were examined. As shown in
Figure 3a,b, the main peaks of their XRD patterns are maintained, although very individual peaks widen or disappear. Obviously, both of them can be stable in air, H
2O, and other common solvents, such as acetonitrile (MeCN), ethanol (EtOH), tetrahydrofuran (THF) and acetone, etc. In contrast,
PTC-355 and
PTC-356 have poor air and solvent stabilities. Herein the coordination and interpenetrating frameworks have good stabilities compared to the supramolecular packing frameworks. Diffuse reflectance spectroscopy was used to study the UV/Vis absorption of these compounds. According to the Kubelka–Munk function, they exhibit relatively low bandgaps (
Figure 3c). To understand the structure–function relationship, the electronic properties and frontier orbitals of
PTC-359 were calculated based on the density function theory (DFT). From
Figure S26, the highest occupied molecular orbital (HOMO) is mainly provided by the embonate (L) ligand and Zr atom, and the lowest unoccupied molecular orbital (LUMO) is mainly provided by another L and [Zn(cis-DCH)
2]
2+ unit. The electron transfers between HOMO and LUMO are mainly ligand-to-ligand charge transition (LLCT) and a small part of metal-to-ligand charge transition (MLCT). Under the electrostatic interaction of anion Zr
4L
6 cage and [Zn(cis-DCH)
2]
2+ cationic unit, it is beneficial for the charge transfer from L in Zr
4L
6 cage to L combined with [Zn(cis-DCH)
2]
2+ unit, which may lead to good NLO performance.
We also studied the solid-state excitation and emission spectra of compounds
PTC-355–
PTC-359 at room temperature. Since the π-conjugated L (embonate) ligand possesses a strong absorbing chromophore, the Zr
4L
6 cages themselves have good photoluminescent properties. As shown in
Figure S16, upon excitation at 363 nm, the L ligand displays an emission band at 420–700 nm (
Figure S17), which may belong to the π→π* transition. When excited at 430 nm, PTC-101(Zr) shows a similar emission peak near 495 nm (
Figure 3d). For these cage-assembled compounds
PTC-355–
PTC-359, their emission peaks can be observed at 460, 472, 490, 510, and 501 nm, respectively. Their excitation spectra are shown in
Figures S19–23. Remarkably,
PTC-355 and
PTC-356 exhibit significant blue shifts (ca. 20–40 nm) in comparison with PTC-101(Zr), and
PTC-358 displays a blue shift of 15 nm. In this case, the ligand-based luminescence is in a dominating place, and the blue-shifted or red-shifted phenomenon may be attributed to the metal–ligand/cage coordinative interactions [
31,
32].
Considering ionic pairs are potentially excellent nonlinear optical materials due to their intrinsic attributes, such as high positive/negative charge and ordered self-assembled structures, we decided to study the third-order NLO property of
PTC-355–
PTC-359. In order to ensure the stability of its structure and expand its practical optical applications, these crystal materials were evenly dispersed into PDMS (polydimethylsiloxane), and then flexible and transparent composite films were obtained and named
PDMS-PTC-355,
PDMS-PTC-356,
PDMS-PTC-357,
PDMS-PTC-358, and
PDMS-PTC-359 (
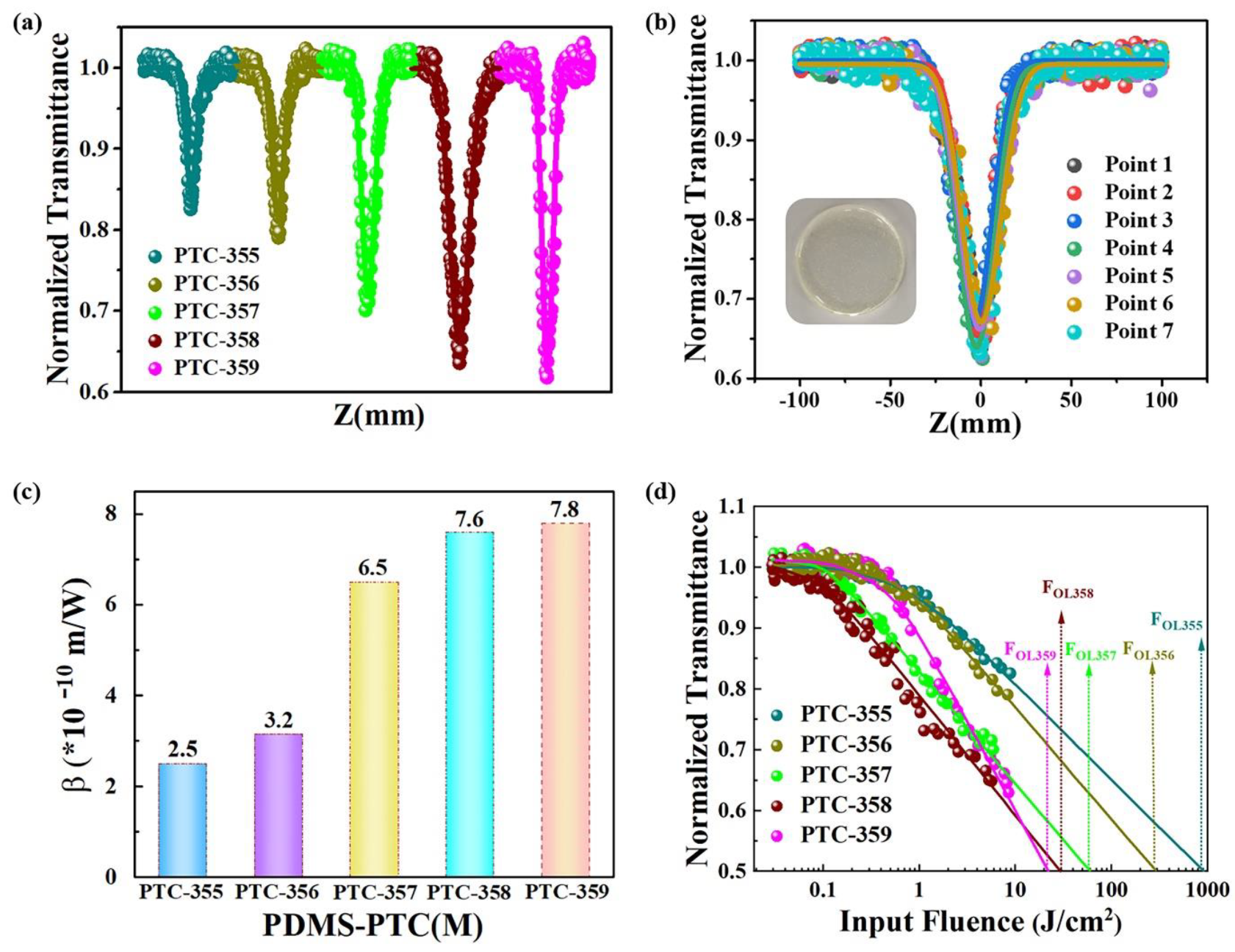
Figure S24). The third-order NLO properties of these composite films were studied with a nanosecond laser using a typical open-hole Z-scanning system at 532 nm. We fixed the laser energy at 80 μJ, and the composite film thickness was controlled at 800 μm. The test results show that their transmittances are 0.80, 0.78, 0.49, 0.78, and 0.87, respectively (
Table S1). The experimental results show that all composite films exhibit typical reverse saturation absorption (RSA) response, and from
PDMS-PTC-355 to
PDMS-PTC-359, they exhibit successively enhanced optical limiting response (
Figure 4a). At Z = 0, the minimum normalized transmission (Tmin) of
PDMS-PTC-355,
PDMS-PTC-356,
PDMS-PTC-357,
PDMS-PTC-358, and
PDMS-PTC-359 membranes are 0.82, 0.79, 0.70, 0.64 and 0.62, respectively (
Figure 4a). To demonstrate the stability and homogeneity of these prepared films, we performed seven rounds of testing on the same
PDMS-PTC-359 film, but different test points were selected (
Figure 4b). The results show that the Tmin of these different points are nearly identical.
Figure 4d shows the change between the normalized transmittance of the samples and the laser input flux. It can be seen that the normalized transmittance decreases significantly with increasing laser energy, indicating that these samples have a significant nonlinear light limit effect.
In order to quantitatively evaluate the NLO performance of the sample, the nonlinear absorption coefficient of the sample was obtained from the open-hole Z-scan test results of the fitting test(β). Calculations of
PDMS-PTC-355,
PDMS-PTC-356,
PDMS-PTC-357,
PDMS-PTC-358, and
PDMS-PTC-359 composite films β Values are 2.5 × 10
−10, 3.2 × 10
−10, 6.5 × 10
−10, 7.6 × 10
−10 and 7.8 × 10
−10 m/W (
Figure 4c), respectively, which are obtained by comparison β. The values increase in turn. From
Figure 4d, the OL values of the sample (Fol, the input flux whose transmittance is half of the linear transmittance) are 903.00 J/cm
2(F
OL355), 295.00 J/cm
2(F
OL356), 60.87 J/cm
2(F
OL357), 28.53 J/cm
2(F
OL358), 22.49 J/cm
2(F
OL359), respectively. Its OL value decreases in turn. Comparing the β Values and OL values show that
PDMS-PTC-359 has the highest β Value and the lowest OL value, indicating that its NLO performance is the highest.
PDMS-PTC-355 has the lowest β Value and the highest OL value, indicating that it has the lowest NLO performance. For better comparison, we also tested the third-order NLO properties of the starting material PDMS-PTC-101 (Zr) by the same method of film preparation. The result shows that it has no obvious optical limiting effect (
Figure S25). Obviously, the self-assembly of anionic Zr
4L
6 cages with N, N-chelated transition-metal cations results in the activation and amplification of the NLO response. As described above, the increase in coordination bonds facilitates charge transfer between anion and cation groups, thus improving the third-order NLO performance. Herein, these assembled materials range from ion pair structure and dimer to 3,4-connected, 4-connected 3D frameworks; they have increasing coordinative interactions in turn, which may cause enhanced optical limiting effects.
3. Experimental Procedure
3.1. Materials and Methods
Materials and instrumentation. All reagents were purchased commercially and used without further purification. PTC-101(Zr), as the starting material of Zr
4L
6 cages, was massively synthesized by the method reported in our previous work [
21]. Thermal gravimetric analyses (TGA) curves of these materials were carried out on a NETSCHZ STA-449C thermoanalyzer with a heating rate of 10 °C/min under a nitrogen atmosphere. Powder X-ray diffraction (XRD) patterns were recorded on a Rigaku Dmax/2500 X-ray diffractometer operating at 40 kV and 100 mA, using Cu-Kα or Ga-Kα radiation (λ = 1.54056 or 1.3405Å). The patterns were scanned over an angular range of 5–45° (2 theta) with a step length of 0.05° (2theta). The UV diffuse reflection data were recorded at room temperature using a powder sample with BaSO
4 as a standard (100% reflectance) on a PerkinElmer Lamda-950 UV spectrophotometer. Fluorescence spectra were measured with a HORIBA Jobin-Yvon FluoroMax-4 spectrometer.
X-ray Crystallography. A high-quality single crystal was selected for the determination of single crystal structure. Crystallographic data of
PTC-355–
PTC-359 were collected on a Supernova single crystal diffractometer equipped with graphite-monochromatic Cu-Kα or Ga-Kα radiation (λ = 1.54056 or 1.3405 Å) at 100 K. Absorption correction was applied using SADABS.
2 Structure was solved by direct method and refined by full-matrix least-squares on F using SHELXTL. In these structures, some cations/anions and free guest molecules were highly disordered and could not be located. The diffused electron densities resulting from these residual cations/anions and guest molecules were removed from the data set using the SQUEEZE routine of PLATON and refined further using the data generated. Crystal data and details of data collection and refinement of
PTC-355–
PTC-359 were summarized in
Table 1 and
Table 2. CCDC 2220530-2220534 contains the supplementary crystallographic data for this paper. These data are provided free of charge by The Cambridge Crystallographic Data Centre.
3.2. Synthesis of PTC-355-PTC-359
The simple method previously reported was used to synthesize large amounts of PTC-101(Zr) as the source of the Zr
4L
6 cages [
21]. The specific synthesis method is as follows: Zr(O
nPr)
4 (160 μL, 0.5 mmol), H
4L (155 mg, 0.4 mmol), and 2 drops of ethylenediamine (en) were added to 6 mL of n-propanol/DMF (2:1,
v/
v) and mixed at room temperature (in order to improve the solubility of the reactive raw material, the mixture was treated with ultrasound). The above mixture was heated at 100 °C for 3 days. After cooling to room temperature, yellow polyhedral crystals of PTC-101(Zr) (Molecular formula: (Me
2NH
2)
8[(Zr
4L
6)]·Guests) were obtained and then washed in the mother liquor. The final product was dried in the air and kept in the bottle. The yield of the final product was ~75% based on H
4L.
Synthesis of [(Zr4L6)(Cu(trans-DCH)2)4]·Guests (PTC-355). PTC-101(Zr) (0.02 mmol, 80 mg), CuBr (0.12 mmol, 18 mg), and 8 drops of (+/−)-trans-1,2-diaminocyclohexane (trans-DCH) were dissolved to 6 mL of DMF/H2O (1:4, v/v) solvents and mixed at room temperature (In order to improve the solubility of the reactive raw material, the mixture was treated with ultrasound.). The above mixture was kept for 3 days at room temperature. Blue block crystals of PTC-355 were produced and then washed and stored in the mother liquor. The yield of the final product was ~40% based on PTC-101(Zr).
Synthesis of [(Zr4L6)(Zn(trans-DCH)3)4]·Guests (PTC-356). PTC-101(Zr) (0.02 mmol, 80 mg), Zn(NO3)2·7H2O (0.18 mmol, 40 mg), and 2 drops of trans-DCH were dissolved in 6 mL of 1,4-dioxane/H2O/MeCN (2:2:2, v/v/v) solvents and mixed at room temperature (In order to improve the solubility of the reactive raw material, the mixture was treated with ultrasound.). The above mixture was heated at 80 °C for 3 days. After cooling to room temperature, yellow block crystals of PTC-356 were produced and then washed and stored in the mother liquor. The yield of the final product was ~55% based on PTC-101(Zr).
Synthesis of (Me2NH2)[(Zr4L6)(Zn1/2(cis-DCH))(Zn(cis-DCH)3)2]·Guests (PTC-357). PTC-101(Zr) (0.02 mmol, 80 mg), Zn(CH3COO)2·2H2O (0.28 mmol, 60 mg), and 8 drops of cis-1,2-diaminocyclohexane (cis-DCH) were dissolved to 6 mL of DMF/H2O/MeCN (1:2:3, v/v/v) solvents and mixed at room temperature (In order to improve the solubility of the reactive raw material, the mixture was treated with ultrasound.). The above mixture was heated at 60 °C for 3 days. After cooling to room temperature, yellow block crystals of PTC-357 were produced and then washed and stored in the mother liquor. The yield of the final product was ~45% based on PTC-101(Zr).
Synthesis of [(Me2NH2)8/3[(Zr4L6)5/6(Zn(cis-DCH)2)]·Guests (PTC-358). PTC-101(Zr) (0.02 mmol, 80 mg), ZnSO4·7H2O (0.09 mmol, 26 mg), and 2 drops of cis-DCH were dissolved to 6 mL of DMF/H2O/MeCN (1:2:3, v/v/v) solvents and mixed at room temperature (In order to improve the solubility of the reactive raw material, the mixture was treated with ultrasound.). The above mixture was heated at 60 °C for 3 days. After cooling to room temperature, yellow block crystals of PTC-358 were produced and then washed and stored in the mother liquor. The yield of the final product was ~32% based on PTC-101(Zr).
Synthesis of [(Me2NH2)8[(Zr4L6)2(Zn(cis-DCH)2)4]·Guests (PTC-359). PTC-101(Zr) (0.02 mmol, 80 mg), Zn(CH3COO)2·2H2O (0.09 mmol, 20 mg), and 5 drops of cis-DCH were dissolved in 6 mL of DMF/H2O/MeCN (1:2:3, v/v/v) solvents and mixed at room temperature (In order to improve the solubility of the reactive raw material, the mixture was treated with ultrasound.). The above mixture was heated at 60 °C for 3 days. After cooling to room temperature, yellow block crystals of PTC-359 were produced and then washed and stored in the mother liquor. The yield of the final product was ~ 50% based on PTC-101(Zr).
3.3. Manufacture Fabrication
Manufacture of PTCs dispersed PDMS films. PTCs refer to complex PTC-355–PTC-359. The crystals of PTC-355–PTC-359 need to be dried in the air before sampling, respectively. First, 6 mg of the sample was mixed with 2 g PDMS (polydimethylsiloxane), and the sample was evenly dispersed by magnetic stirring for several hours. The second step is to add 1/10 mass of specific curing agent and continue to stir evenly for about 10 min. The third step is to take 1 g of the mixture and put it into a specific membrane. Under the action of gravity, the mixture is paved in the mold and then placed at room temperature for 0.5 h to eliminate bubbles. Finally, put the membrane utensil into a 60 °C oven for 5 h to obtain films for testing.
Z-scan measurements. The third-order NLO properties of the above sample were evaluated by using the Z-scan technique. The excitation light source was an Nd:YAG laser with a repetition rate of 5 Hz. The laser pulse (period, 5 ns; wavelength, 532 nm) was split into two beams with a mirror. The pulse energies at the front and back of the samples were monitored using energy detectors 1 and 2. All of the measurements were conducted at room temperature. The sample was mounted on a computer-controlled translation stage that shifted each sample along the z-axis.
Calculation of the nonlinear optical parameters. The relationship of the sample transmission and input fluence can be plotted from the open-aperture Z-scan curve. From the input laser pulse energy Ein and beam radius ω(z), the light fluence Fin(z) at any position can be obtained.
Fin(
z) is defined as:
where
ω(
z) is defined as:
where
ω0 and
z0 are the light beam radius and the Rayleigh range, respectively, and z
0 is defined as:
where
k is defined as: