Optimization of the Extraction of Antioxidant Compounds from Roselle Hibiscus Calyxes (Hibiscus sabdariffa), as a Source of Nutraceutical Beverages
Abstract
1. Introduction
2. Results and Discussion
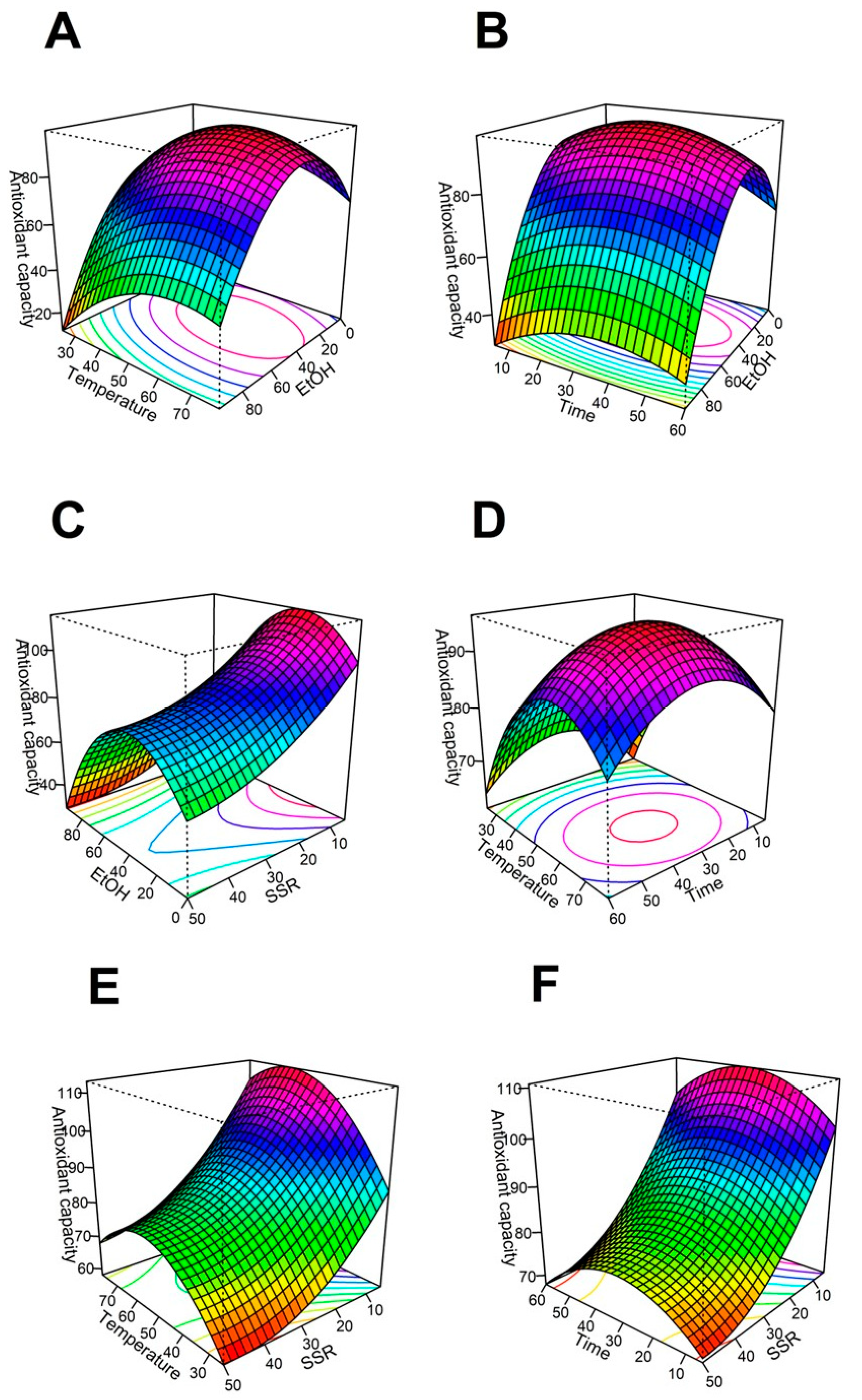
2.1. Effect of Ethanol Content on Antioxidant Capacity
2.2. Effect of Extraction Temperature on Antioxidant Capacity
2.3. Effect of Extraction Time on Antioxidant Capacity
2.4. Effect of Solid/Solvent Ratio on Antioxidant Capacity
2.5. Contribution of Total Polyphenolic Compounds (TPC) and Anthocyanins (ACs) to Antioxidant Activity
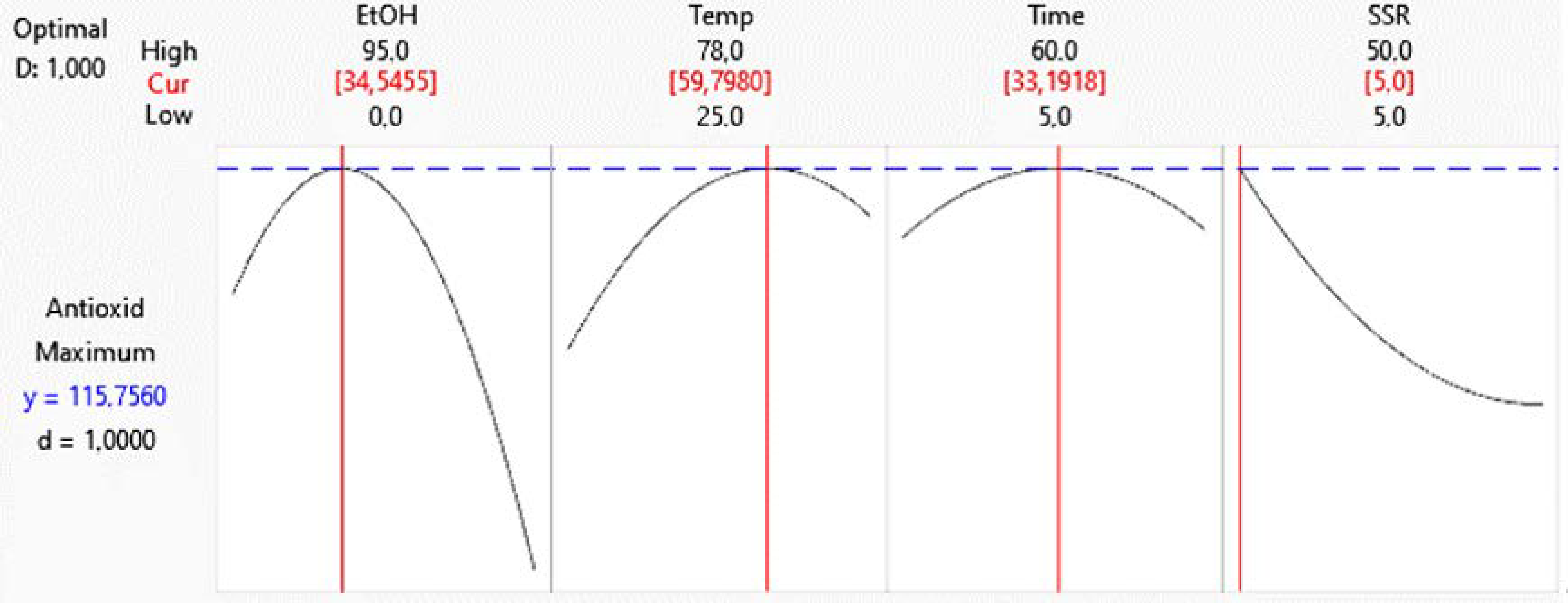
2.6. Study of a Formula Prepared Using Optimized Hibiscus Extract as a Base Component
3. Materials and Methods
3.1. Plant Material Preparation and Maceration
3.2. Antioxidant Activity Determination
3.3. Box-Behnken Design (BBD)
3.4. Total Phenolic Content (TPC) Determination
3.5. Identification and Quantification of Anthocyanins
3.6. Preparation and Characterization of a Nutraceutical Drink Using Optimized Roselle Extract as Raw Material
3.7. Statistical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Benherlal, P.S.; Arumughan, C. Chemical Composition and in Vitro Antioxidant Studies on Syzygium Cumini Fruit. J. Sci. Food Agric. 2007, 87, 2560–2569. [Google Scholar] [CrossRef]
- Subhaswaraj, P.; Sowmya, M.; Bhavana, V.; Dyavaiah, M.; Siddhardha, B. Determination of Antioxidant Activity of Hibiscus sabdariffa and Croton caudatus in Saccharomyces Cerevisiae Model System. J. Food Sci. Technol. 2017, 54, 2728–2736. [Google Scholar] [CrossRef] [PubMed]
- Losada-Barreiro, S.; Bravo-Díaz, C. Free Radicals and Polyphenols: The Redox Chemistry of Neurodegenerative Diseases. Eur. J. Med. Chem. 2017, 133, 379–402. [Google Scholar] [CrossRef] [PubMed]
- Santos-Sánchez, N.F.; Salas-Coronado, R.; Villanueva-Cañongo, C.; Hernández-Carlos, B. Antioxidant Compounds and Their Antioxidant Mechanism. In Antioxidants; Intechopen: London, UK, 2019; pp. 1–28. [Google Scholar] [CrossRef]
- Wong, S.P.; Leong, L.P.; William Koh, J.H. Antioxidant Activities of Aqueous Extracts of Selected Plants. Food Chem. 2006, 99, 775–783. [Google Scholar] [CrossRef]
- Shruthi, V.H.; Ramachandra, C.T.; Nidoni, U.; Hiregoudar, S.; Naik, N.; Kurubar, A.R. Roselle (Hibiscus sabdariffa L.) as a Source of Natural Colour: A Review. Plant Arch. 2016, 16, 515–522. [Google Scholar]
- Da-Costa-Rocha, I.; Bonnlaender, B.; Sievers, H.; Pischel, I.; Heinrich, M. Hibiscus sabdariffa L.—A Phytochemical and Pharmacological Review. Food Chem. 2014, 165, 424–443. [Google Scholar] [CrossRef]
- Sharma, H.K.; Sarkar, M.; Choudhary, S.B.; Kumar, A.A.; Maruthi, R.T.; Mitra, J.; Karmakar, P.G. Diversity Analysis Based on Agro-Morphological Traits and Microsatellite Based Markers in Global Germplasm Collections of Roselle (Hibiscus sabdariffa L.). Ind. Crops Prod. 2016, 89, 303–315. [Google Scholar] [CrossRef]
- Singh, S.; Chunglok, W.; Nwabor, O.F.; Chulrik, W.; Jansakun, C.; Bhoopong, P. Porous Biodegradable Sodium Alginate Composite Fortified with Hibiscus sabdariffa L. Calyx Extract for the Multifarious Biological Applications and Extension of Climacteric Fruit Shelf-Life. J. Polym. Environ. 2023, 31, 922–938. [Google Scholar] [CrossRef]
- Patel, S. Hibiscus sabdariffa: An Ideal yet under-Exploited Candidate for Nutraceutical Applications. Biomed. Prev. Nutr. 2014, 4, 23–27. [Google Scholar] [CrossRef]
- Badreldin, H.A.; Naser Al, W.; Gerald, B. Phytochemical, Pharmacological and Toxicological Aspects of Hibiscus sabdariffa L.: A Review. Phytother. Res. 2005, 19, 369–375. [Google Scholar]
- Kao, E.S.; Yang, M.Y.; Hung, C.H.; Huang, C.N.; Wang, C.J. Polyphenolic Extract from Hibiscus sabdariffa Reduces Body Fat by Inhibiting Hepatic Lipogenesis and Preadipocyte Adipogenesis. Food Funct. 2016, 7, 171–182. [Google Scholar] [CrossRef]
- Ojulari, O.V.; Lee, S.G.; Nam, J.O. Beneficial Effects of Natural Bioactive Compounds from Hibiscus sabdariffa L. on Obesity. Molecules 2019, 24, 210. [Google Scholar] [CrossRef] [PubMed]
- Alshamar, H.A.; Dapson, R.W. Use of Roselle Extracted from Hibiscus sabdariffa for Histological Staining: A Critical Review and Rational Stain Formulation. Biotech. Histochem. 2021, 96, 94–101. [Google Scholar] [CrossRef]
- Liu, J.Z.; Lyu, H.C.; Fu, Y.J.; Jiang, J.C.; Cui, Q. Simultaneous Extraction of Natural Organic Acid and Flavonoid Antioxidants from Hibiscus manihot L. Flower by Tailor-Made Deep Eutectic Solvent. LWT 2022, 163, 113533. [Google Scholar] [CrossRef]
- Afshari, K.; Samavati, V.; Shahidi, S.A. Ultrasonic-Assisted Extraction and in-Vitro Antioxidant Activity of Polysaccharide from Hibiscus Leaf. Int. J. Biol. Macromol. 2015, 74, 558–567. [Google Scholar] [CrossRef]
- Chumsri, P.; Sirichote, A.; Itharat, A. Studies on the Optimum Conditions for the Extraction and Concentration of Roselle (Hibiscus sabdariffa Linn.) Extract. Songklanakarin J. Sci. Technol. 2008, 30, 133–139. [Google Scholar]
- Pozos, G.I.P.; Ruiz-López, M.A.; Nátera, J.F.Z.; Moya, C.Á.; Ramírez, L.B.; Silva, M.R.; Macías, R.R.; García-López, P.M.; Cruz, R.G.; Pérez, E.S.; et al. Antioxidant Capacity and Antigenotoxic Effect of Hibiscus sabdariffa L. Extracts Obtained with Ultrasound-Assisted Extraction Process. Appl. Sci. 2020, 10, 560. [Google Scholar] [CrossRef]
- Sindi, H.A.; Marshall, L.J.; Morgan, M.R.A. Comparative Chemical and Biochemical Analysis of Extracts of Hibiscus sabdariffa. Food Chem. 2014, 164, 23–29. [Google Scholar] [CrossRef] [PubMed]
- Tsai, P.J.; McIntosh, J.; Pearce, P.; Camden, B.; Jordan, B.R. Anthocyanin and Antioxidant Capacity in Roselle (Hibiscus sabdariffa L.) Extract. Food Res. Int. 2002, 35, 351–356. [Google Scholar] [CrossRef]
- Cissé, M.; Bohuon, P.; Sambe, F.; Kane, C.; Sakho, M.; Dornier, M. Aqueous Extraction of Anthocyanins from Hibiscus sabdariffa: Experimental Kinetics and Modeling. J. Food Eng. 2012, 109, 16–21. [Google Scholar] [CrossRef]
- Ajiboye, T.O.; Salawu, N.A.; Yakubu, M.T.; Oladiji, A.T.; Akanji, M.A.; Okogun, J.I. Antioxidant and Drug Detoxification Potentials of Hibiscus sabdariffa Anthocyanin Extract. Drug Chem. Toxicol. 2011, 34, 109–115. [Google Scholar] [CrossRef]
- Cid-Ortega, S.; Guerrero-Beltrán, J.A. Roselle Calyces (Hibiscus sabdariffa), an Alternative to the Food and Beverages Industries: A Review. J. Food Sci. Technol. 2015, 52, 6859–6869. [Google Scholar] [CrossRef]
- Shih, M.C.; Yang, K.T.; Kuo, S.T. Optimization Process of Black Soybean Natto Using Response Surface Methodology. J. Food Sci. 2009, 74, 294–301. [Google Scholar] [CrossRef] [PubMed]
- Ramirez-Rodrigues, M.M.; Plaza, M.L.; Azeredo, A.; Balaban, M.O.; Marshall, M.R. Physicochemical and Phytochemical Properties of Cold and Hot Water Extraction from Hibiscus sabdariffa. J. Food Sci. 2011, 76, 429–435. [Google Scholar] [CrossRef] [PubMed]
- Prenesti, E.; Berto, S.; Daniele, P.G.; Toso, S. Antioxidant Power Quantification of Decoction and Cold Infusions of Hibiscus sabdariffa Flowers. Food Chem. 2007, 100, 433–438. [Google Scholar] [CrossRef]
- Cid-Ortega, S.; Guerrero-Beltrán, J.A.; Andzi Barhé, T.; Feuya Tchouya, G.R.; Sindi, H.A.; Marshall, L.J.; Morgan, M.R.A.; Pimentel-Moral, S.; Borrás-Linares, I.; Lozano-Sánchez, J.; et al. Phytochemistry, Antioxidant Capacity, Total Phenolic Content and Anti-Inflammatory Activity of Hibiscus sabdariffa Leaves. Food Chem. 2015, 77, 1055–1060. [Google Scholar] [CrossRef]
- Montgomery, D. Design and Analysis of Experiments, 9th ed.; WILEY: Hoboken, NJ, USA, 2020; ISBN 978-1-119-63842-1. [Google Scholar]
- Elik, A. Response Surface Methodology Based on Central Composite Design for Optimizing Temperature-Controlled Ionic Liquid-Based Microextraction for the Determination of Histamine Residual in Canned Fish Products. J. Food Compos. Anal. 2021, 98, 103807. [Google Scholar] [CrossRef]
- Sáyago-Ayerdi, S.G.; Arranz, S.; Serrano, J.; Goñi, I. Dietary Fiber Content and Associated Antioxidant Compounds in Roselle Flower (Hibiscus sabdariffa L.) Beverage. J. Agric. Food Chem. 2007, 55, 7886–7890. [Google Scholar] [CrossRef]
- Do, Q.D.; Angkawijaya, A.E.; Tran-Nguyen, P.L.; Huynh, L.H.; Soetaredjo, F.E.; Ismadji, S.; Ju, Y.H. Effect of Extraction Solvent on Total Phenol Content, Total Flavonoid Content, and Antioxidant Activity of Limnophila Aromatica. J. Food Drug Anal. 2014, 22, 296–302. [Google Scholar] [CrossRef]
- Kechinski, C.P.; Guimarães, P.V.R.; Noreña, C.P.Z.; Tessaro, I.C.; Marczak, L.D.F. Degradation Kinetics of Anthocyanin in Blueberry Juice during Thermal Treatment. J. Food Sci. 2010, 75, 173–176. [Google Scholar] [CrossRef]
- Moreno, J.; Peinado, R. Polyphenols. In Enological Chemistry; Academic Press: Cambridge, MA, USA, 2012; pp. 53–76. ISBN 9780123884381. [Google Scholar]
- Mattioli, R.; Francioso, A.; Mosca, L.; Silva, P. Anthocyanins: A Comprehensive Review of Their Chemical Properties and Health Effects on Cardiovascular and Neurodegenerative Diseases. Molecules 2020, 25, 3809. [Google Scholar] [CrossRef] [PubMed]
- Singh, M.; Thrimawithana, T.; Shukla, R.; Adhikari, B. Extraction and Characterization of Polyphenolic Compounds and Potassium Hydroxycitrate from Hibiscus sabdariffa. Future Foods 2021, 4, 100087. [Google Scholar] [CrossRef]
- Pimentel-Moral, S.; Borrás-Linares, I.; Lozano-Sánchez, J.; Arráez-Román, D.; Martínez-Férez, A.; Segura-Carretero, A. Microwave-Assisted Extraction for Hibiscus sabdariffa Bioactive Compounds. J. Pharm. Biomed. Anal. 2018, 156, 313–322. [Google Scholar] [CrossRef]
- Duque-Soto, C.; Expósito-Almellón, X.; García, P.; Pando, M.E.; Borrás-Linares, I.; Lozano-Sánchez, J. Extraction, Characterization, and Bioactivity of Ohenolic Compounds-A Case on Hibiscus Genera. Foods 2023, 12, 963. [Google Scholar] [CrossRef]
- Roselló-Soto, E.; Martí-Quijal, F.J.; Cilla, A.; Munekata, P.E.S.; Lorenzo, J.M.; Remize, F.; Barba, F.J. Influence of Temperature, Solvent and PH on the Selective Extraction of Phenolic Compounds from Tiger Nuts by-Products: Triple-TOF-LC-MS-MS Characterization. Molecules 2019, 24, 797. [Google Scholar] [CrossRef] [PubMed]
- Che Sulaiman, I.S.; Basri, M.; Fard Masoumi, H.R.; Chee, W.J.; Ashari, S.E.; Ismail, M. Effects of Temperature, Time, and Solvent Ratio on the Extraction of Phenolic Compounds and the Anti-Radical Activity of Clinacanthus Nutans Lindau Leaves by Response Surface Methodology. Chem. Cent. J. 2017, 11, 54. [Google Scholar] [CrossRef]
- Tan, P.W.; Tan, C.P.; Ho, C.W. Antioxidant Properties: Effects of Solid-to-Solvent Ratio on Antioxidant Compounds and Capacities of Pegaga (Centella asiatica). Int. Food Res. J. 2011, 18, 557–562. [Google Scholar]
- Wong, B.Y.; Tan, C.P.; Ho, C.W. Effect of Solid-to-Solvent Ratio on Phenolic Content and Antioxidant Capacities of “Dukung Anak” (Phyllanthus niruri). Int. Food Res. J. 2013, 20, 325–330. [Google Scholar]
- Segura-Carretero, A.; Puertas-Mejía, M.A.; Cortacero-Ramírez, S.; Beltrán, R.; Alonso-Villaverde, C.; Joven, J.; Dinelli, G.; Fernández-Gutiérrez, A. Selective Extraction, Separation, and Identification of Anthocyanins from Hibiscus sabdariffa L. Using Solid Phase Extraction-Capillary Electrophoresis-Mass Spectrometry (Time-of-Flight/Ion Trap). Electrophoresis 2008, 29, 2852–2861. [Google Scholar] [CrossRef]
- Braithwaite, M.C.; Tyagi, C.; Tomar, L.K.; Kumar, P.; Choonara, Y.E.; Pillay, V. Nutraceutical-Based Therapeutics and Formulation Strategies Augmenting Their Efficiency to Complement Modern Medicine: An Overview. J. Funct. Foods 2014, 6, 82–99. [Google Scholar] [CrossRef]
- Gunjal, S.D. An Overview of Process Parameters and Spray Drying Agents Involved in Spray Drying of Herbal Extracts. Paid. J. 2020, 13, 102–118. [Google Scholar]
- USDA. Antioxidants and Health. Available online: https://www.ars.usda.gov/news-events/news/research-news/2007/data-on-food-antioxidants-aid-research/ (accessed on 24 March 2022).
- Kodama, D.H.; Gonçalves, A.E.D.S.S.; Lajolo, F.M.; Genovese, M.I. Flavonoids, Total Phenolics and Antioxidant Capacity: Comparison between Commercial Green Tea Preparations. Ciência E Tecnol. De Aliment. 2010, 30, 1077–1082. [Google Scholar] [CrossRef]
- Prior, R.L.; Cao, G. Antioxidant Capacity and Polyphenolic Components of Teas: Implications for Altering In Vivo Antioxidant Status. Proc. Soc. Exp. Biol. Med. 1999, 220, 255–261. [Google Scholar] [CrossRef]
- Intipunya, P.; Bhandari, B.R. Chemical Deterioration and Physical Instability of Food Powders; Woodhead Publishing Limited: Sawston, UK, 2010; ISBN 9781845694951. [Google Scholar]
- Vega-López, B.; Carvajal-Miranda, Y.; Brenes-Peralta, L.; Gamboa-Murillo, M.; Venegas-Padilla, J.; Rodríguez, G.; Jiménez-Bonilla, P.; Álvarez-Valverde, V. Phytonutraceutical Evaluation of Five Varieties of Tomato (Solanum lycopersicum) during Ripening and Processing. LWT 2022, 164, 113592. [Google Scholar] [CrossRef]
- Prior, R.L.; Hoang, H.; Gu, L.; Wu, X.; Bacchiocca, M.; Howard, L.; Hampsch-Woodill, M.; Huang, D.; Ou, B.; Jacob, R. Assays for Hydrophilic and Lipophilic Antioxidant Capacity (Oxygen Radical Absorbance Capacity (ORACFL)) of Plasma and Other Biological and Food Samples. J. Agric. Food Chem. 2003, 51, 3273–3279. [Google Scholar] [CrossRef] [PubMed]
- Sánchez-Rangel, J.C.; Benavides, J.; Heredia, J.B.; Cisneros-Zevallos, L.; Jacobo-Velázquez, D. The Folin-Ciocalteu Assay Revisited: Improvement of Its Specificity for Total Phenolic Content Determination. Anal. Methods 2013, 5, 5990–5999. [Google Scholar] [CrossRef]
- DIN (German Institute for Standardization). Determining the Gross Calorific Value of Solid and Liquid Fuels Using the Bomb Calorimeter, and Calculation of Net Calorific Value—Part 1 General Information; DIN 51900-1; German Institute for Standardization: Berlin, Germany, 2000. [Google Scholar]
- Nielsen, S. Food Analysis Laboratory Manual- Ash Content Determination. In Food Analysis Laboratory Manual; Springer: Cham, Switzerland, 2017; pp. 117–119. ISBN 978-3-319-44125-2. [Google Scholar]
- AOAC. Official Methods of Analysis of the Association of Analytical Chemists International; Association of Analytical Chemists International: Rockville, MD, USA, 2000. [Google Scholar]

| Experiment Number | X1 | X2 | X3 | X4 | Antioxidant Capacity (µmol TE/ g DM ± SD) | Adjust |
|---|---|---|---|---|---|---|
| 1 | −1 | −1 | 0 | 0 | 68.24 ± 2.46 | 71.97 |
| 2 | 1 | −1 | 0 | 0 | 20.05 ± 1.72 | 19.37 |
| 3 | −1 | 1 | 0 | 0 | 72.45 ± 1.73 | 75.34 |
| 4 | 1 | 1 | 0 | 0 | 53.22 ± 3.55 | 52.13 |
| 5 | 0 | 0 | −1 | −1 | 95.08 ± 13.37 | 97.17 |
| 6 | 0 | 0 | 1 | −1 | 102.46 ± 1.58 | 97.17 |
| 7 | 0 | 0 | −1 | 1 | 65.14 ± 0.17 | 65.91 |
| 8 | 0 | 0 | 1 | 1 | 65.60 ± 2.03 | 65.91 |
| 9 | 0 | 0 | 0 | 0 | 106.35 ± 25.55 | 103.61 |
| 10 | −1 | 0 | 0 | −1 | 114.10 ± 19.46 | 99.49 |
| 11 | 1 | 0 | 0 | −1 | 66.33 ± 1.36 | 60.76 |
| 12 | −1 | 0 | 0 | 1 | 58.47 ± 2.46 | 68.23 |
| 13 | 1 | 0 | 0 | 1 | 27.00 ± 3.68 | 29.50 |
| 14 | 0 | −1 | −1 | 0 | 73.72 ± 0.88 | 76.51 |
| 15 | 0 | 1 | −1 | 0 | 99.23 ± 4.84 | 95.34 |
| 16 | 0 | −1 | 1 | 0 | 75.61 ± 6.94 | 76.51 |
| 17 | 0 | 1 | 1 | 0 | 96.10 ± 3.98 | 95.34 |
| 18 | 0 | 0 | 0 | 0 | 101.81 ± 10.57 | 110.69 |
| 19 | 0 | −1 | 0 | −1 | 81.44 ± 3.66 | 76.45 |
| 20 | 0 | 1 | 0 | −1 | 31.96 ± 2.72 | 37.72 |
| 21 | 0 | −1 | 0 | 1 | 73.23 ± 5.17 | 76.45 |
| 22 | 0 | 1 | 0 | 1 | 38.62 ± 0.51 | 37.72 |
| 23 | −1 | 0 | −1 | 0 | 74.69 ± 2.91 | 80.24 |
| 24 | 1 | 0 | −1 | 0 | 93.61 ± 2.41 | 99.08 |
| 25 | −1 | 0 | 1 | 0 | 61.27 ± 2.46 | 48.98 |
| 26 | 1 | 0 | 1 | 0 | 70.43 ± 1.72 | 67.82 |
| 27 | 0 | 0 | 0 | 0 | 100.23 ± 1.73 | 101,04 |
| Source | DF | SSq Adjust | MC Adjust | F-Value | p-Value |
|---|---|---|---|---|---|
| Model | 9 | 15,361.9 | 1706.9 | 36.2 | <0.0001 |
| X1 | 1 | 4291.9 | 4291.9 | 91.0 | <0.0001 |
| X2 | 1 | 975.3 | 975.3 | 20.7 | <0.0001 |
| X4 | 1 | 3719.4 | 3719.4 | 78.9 | <0.0001 |
| X1X1 | 1 | 7174.4 | 7174.4 | 152.1 | <0.0001 |
| X2X2 | 1 | 1381.3 | 1381.3 | 29.3 | <0.0001 |
| X3X3 | 1 | 574.6 | 574.5 | 12.2 | 0.003 |
| X1X2 | 1 | 216.7 | 216.7 | 4.6 | 0.047 |
| Lack of fit | 16 | 1400.0 | 87.5 | 8.7 | 0.108 |
| R-Squared | 0.9504 | ||||
| Adjusted R-Squared | 0.9241 | ||||
| Predicted R-Squared | 0.8747 | ||||
| Factor | Symbol | Level | ||
|---|---|---|---|---|
| −1 | 0 | 1 | ||
| Ethanol:water | X1 | 5:95 | 50:50 | 0:100 |
| Temperature (°C) | X2 | 25 | 50 | 78 |
| Time (min) | X3 | 5 | 30 | 60 |
| Solid/Solvent ratio | X4 | 1/100 | 1/50 | 1/10 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Villalobos-Vega, M.J.; Rodríguez-Rodríguez, G.; Armijo-Montes, O.; Jiménez-Bonilla, P.; Álvarez-Valverde, V. Optimization of the Extraction of Antioxidant Compounds from Roselle Hibiscus Calyxes (Hibiscus sabdariffa), as a Source of Nutraceutical Beverages. Molecules 2023, 28, 2628. https://doi.org/10.3390/molecules28062628
Villalobos-Vega MJ, Rodríguez-Rodríguez G, Armijo-Montes O, Jiménez-Bonilla P, Álvarez-Valverde V. Optimization of the Extraction of Antioxidant Compounds from Roselle Hibiscus Calyxes (Hibiscus sabdariffa), as a Source of Nutraceutical Beverages. Molecules. 2023; 28(6):2628. https://doi.org/10.3390/molecules28062628
Chicago/Turabian StyleVillalobos-Vega, María José, Gerardo Rodríguez-Rodríguez, Orlando Armijo-Montes, Pablo Jiménez-Bonilla, and Víctor Álvarez-Valverde. 2023. "Optimization of the Extraction of Antioxidant Compounds from Roselle Hibiscus Calyxes (Hibiscus sabdariffa), as a Source of Nutraceutical Beverages" Molecules 28, no. 6: 2628. https://doi.org/10.3390/molecules28062628
APA StyleVillalobos-Vega, M. J., Rodríguez-Rodríguez, G., Armijo-Montes, O., Jiménez-Bonilla, P., & Álvarez-Valverde, V. (2023). Optimization of the Extraction of Antioxidant Compounds from Roselle Hibiscus Calyxes (Hibiscus sabdariffa), as a Source of Nutraceutical Beverages. Molecules, 28(6), 2628. https://doi.org/10.3390/molecules28062628






