Production and Characterization of New Biosurfactants/Bioemulsifiers from Pantoea alhagi and Their Antioxidant, Antimicrobial and Anti-Biofilm Potentiality Evaluations
Abstract
1. Introduction
2. Results
2.1. Bacterial Identification
2.2. Biosurfactants Production
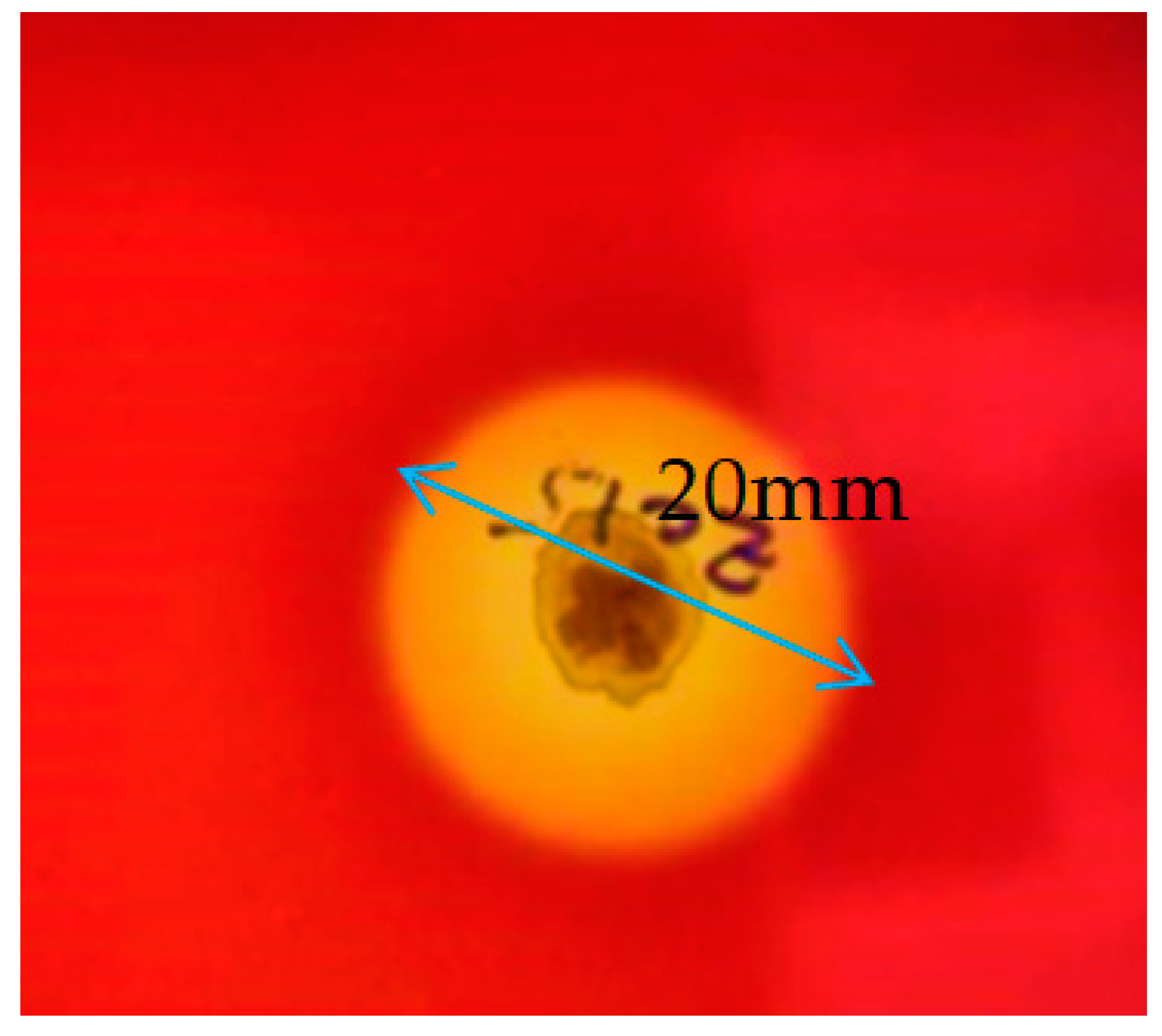
2.2.1. Hemolytic Activity
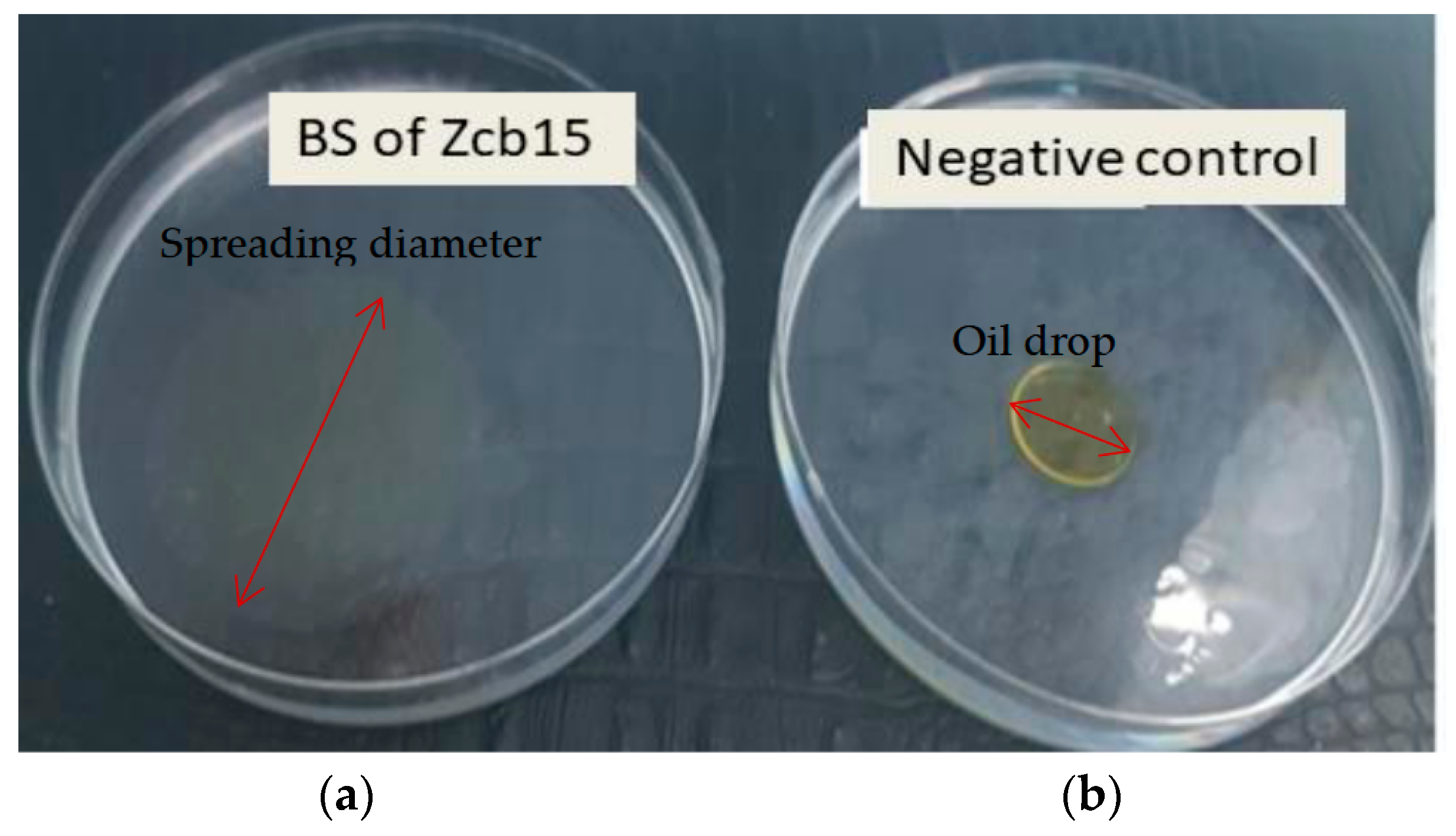
2.2.2. The Oil Spreading Test
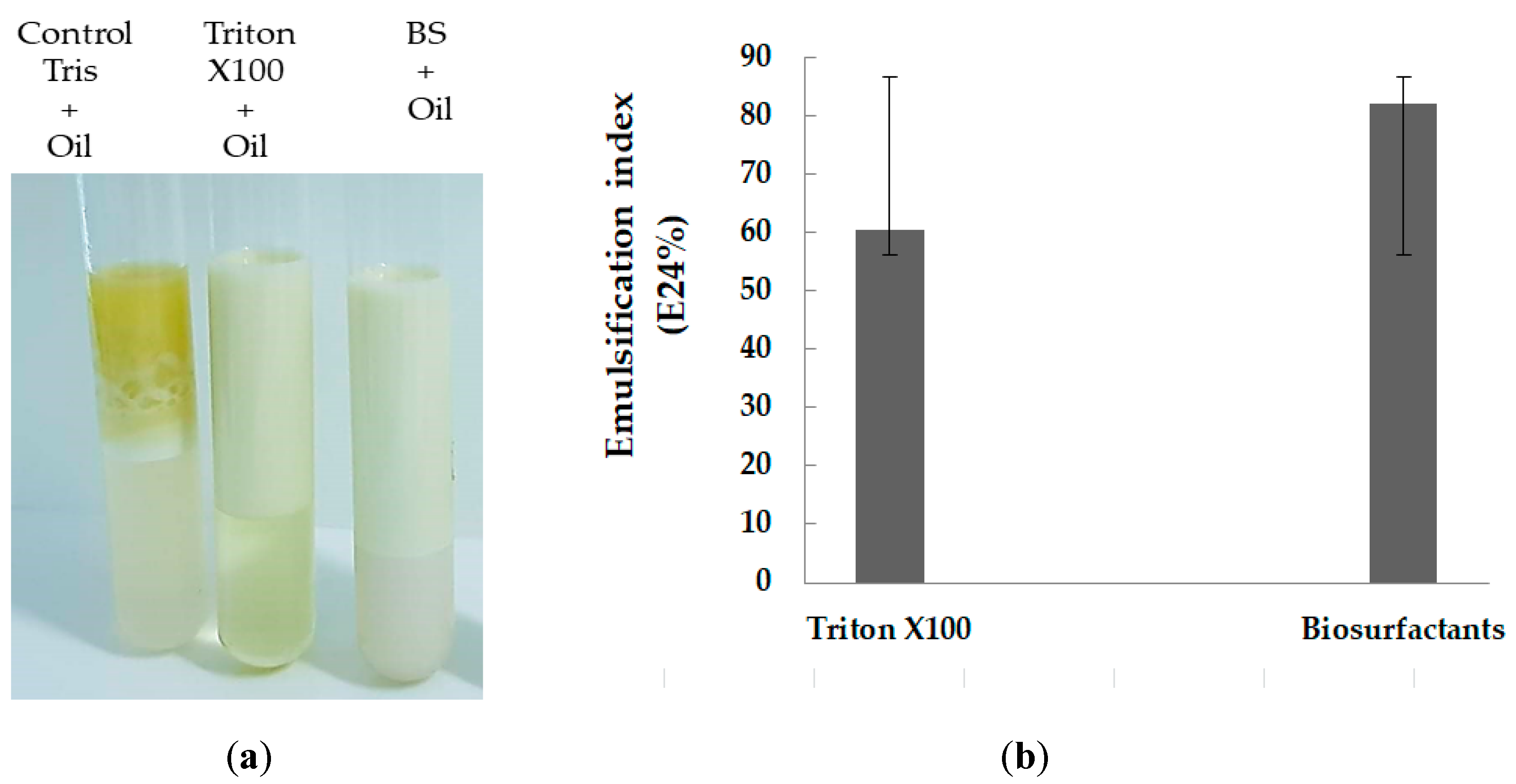
2.2.3. The Emulsification Activity
2.3. Biosurfactants Production Optimization
2.4. FTIR Analysis
2.5. Fatty Acid Analysis
2.5.1. Fatty Acid Composition of Biosurfactants and Cell Membrane of Pantoea alhagi Strain Zcb15
2.5.2. Fatty Acid Comparison of Biosurfactants
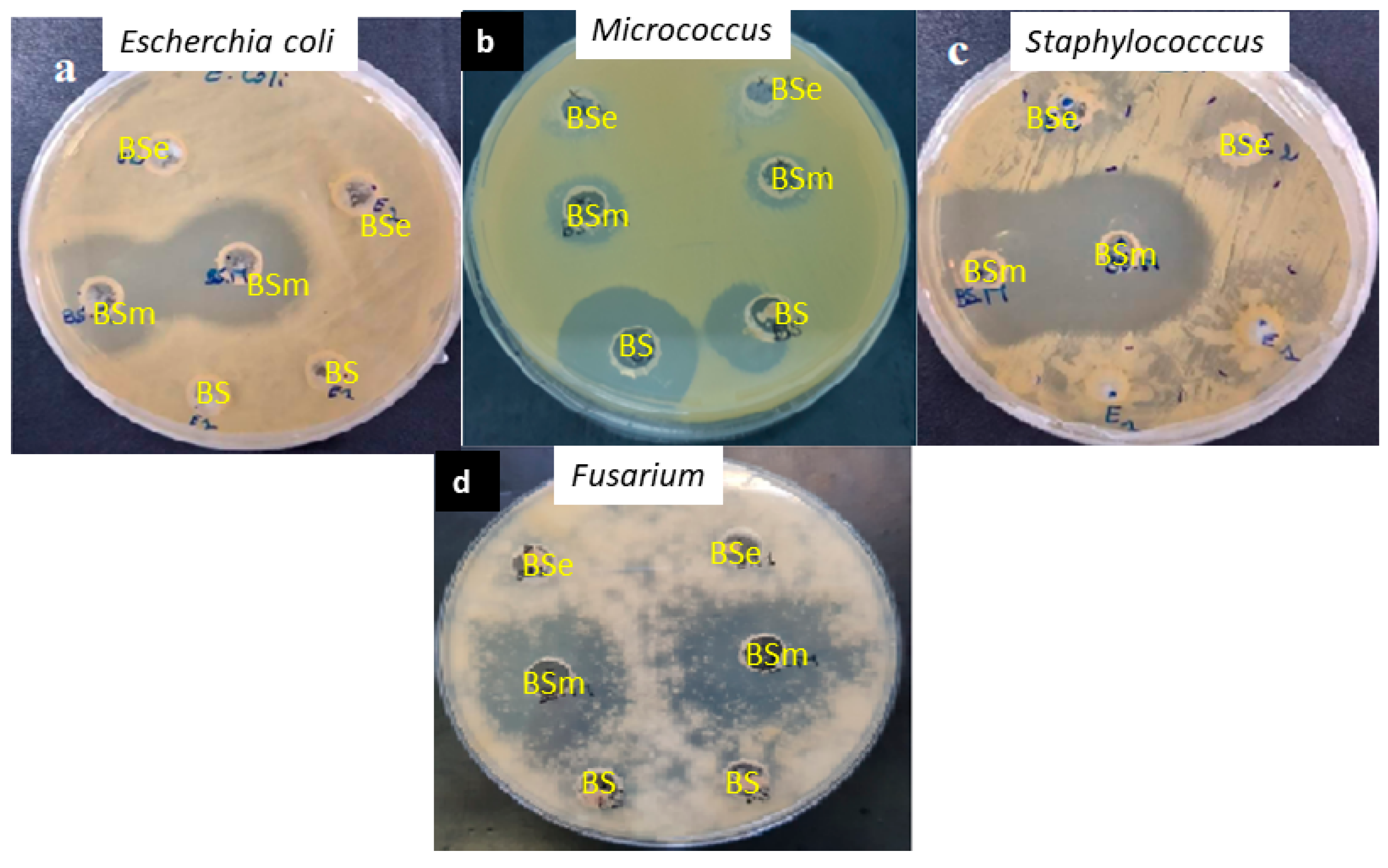
2.6. Antimicrobial and Anti-Biofilm Potentialities of the Biosurfactants
2.7. Antioxidant Activity
3. Discussion
4. Materials and Methods
4.1. Bacteria Isolation and Phylogenetic Identification
4.2. Biosurfactants Production
4.2.1. Hemolytic Activity
4.2.2. Oil Spreading Test or Oil Displacement Test
4.2.3. Emulsification Index (E24)
4.2.4. Emulsification Assay
4.2.5. Carbon Source Effects on Biosurfactants Production
4.3. Fourier-Transform Infrared (FTIR) Analysis of Biosurfactants
4.4. Extraction of Biosurfactants
4.5. Fatty Acid Analysis of Cell Membrane of Pantoea Alhagi Strain Zcb15 and Its Biosurfactants
4.6. Antimicrobial Acticity of BS
4.6.1. Agar Well Diffusion Method
4.6.2. Anti-Biofilm Activity
4.7. DPPH Radical Scavenging Activity
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Plaza, G.; Achal, V. Biosurfactants: Ecofriendly and Innovative Biocides against Bio-corrosio. Int. J. Mol.Sci. 2020, 21, 2152. [Google Scholar] [CrossRef] [PubMed]
- Bjerk, T.R.; Severino, P.; Jain, S.; Marques, C.; Silva, A.M.; Pashirova, T.; Souto, E.B. Biosurfactants: Properties and Applications in Drug Delivery. Biotechnol. Ecotoxicol. Bioeng. 2021, 8, 115. [Google Scholar] [CrossRef] [PubMed]
- Biniarz, P.; Tukaszewicz, M. Direct quantification of lipopeptide biosurfactants in biological samples via HPLC and UPLC-MS requires sample modification with an organic solvent. Appl. Microbiol. Biotechnol. 2017, 101, 4747–4759. [Google Scholar] [CrossRef] [PubMed]
- Akbari, S.; Addurahman, N.H.; Yunus, R.M.; Fayaz, F.; Alara, O.R. Biosurfactants a new frontier for social and environmental safety: A mini review. Biotechnol. Res. Innov. 2018, 2, 81–90. [Google Scholar] [CrossRef]
- Santos, D.K.F.; Rufino, R.D.; Luna, J.M.; Santos, V.A.; Sarubbo, L.A. Biosurfactants: Multifunctional biomolecules of the 21st century. Int. J. Mol. Sci. 2016, 17, 401. [Google Scholar] [CrossRef]
- Raval, H.D.; Raviya, M.R.; Rathod, H.C. Polyamide-surfactant interaction: Exploration of new avenues for reverse osmosis separation applications. Adv. Polym Technol. 2018, 37, 3106–3114. [Google Scholar] [CrossRef]
- Shekhar, S.; Sundaramanickam, A.; Balasubramanian, T. Biosurfactant producing Micorbes and their Potential Applications: A review. Crit. Rev. Environ. Sci. Tehcnology 2015, 45, 1522–1554. [Google Scholar] [CrossRef]
- Jimoh, A.A.; Lin, J. Biosurfactant: A new frontier for greener technology and environmental sstainability. Ecotoxicol. Environ. Saf. 2019, 184, 109607. [Google Scholar] [CrossRef]
- Dos Santos, R.A.; Rodriguez, D.M.; Da Silca, L.A.R.; de Almeida, S.M.; De Campos Takaki, G.M.; de Lima, M.A.B. Enhanced production of prodigiosin by Serratia marcescens UCP1549 using agrosubstartes in soil –state fermentation. Arch. Microbiol. 2021, 203, 4091–4100. [Google Scholar] [CrossRef]
- Araujo, H.W.C.; Andrade, R.F.S.; Monero-Rodriguez, D.; Rubio-Ribeaux, D.; da Silva, C.A.A.; Campos-Takaki, G.M. Sustaianble biosurfactant produced by Serratia marcesences UCP1549 and its suitability for agriculturla and marine bioremediation applications. Microb. Cell. Fact. 2019, 18, 2. [Google Scholar] [CrossRef]
- Martinez, M.; Schujman, G.; Gramajo, H.; de Mendoza, D. Regulation of Membrane Lipid Homeostasis in Bacteria. In Handbook of Hydrocarbon and Lipid Microbiology; Timmis, K., Ed.; Springer: Berlin/Heidelberg, Germany, 2010; pp. 510–517. [Google Scholar]
- Aguilar, P.; Hernandez-Arriaga, A.M.; Cybulski, L.E.; Erazo, A.C.; de Mendoza, D. Molecular basis of thermosensing: A two-component signal transduction thermometer in Bacillus subtilis. EMBO J. 2001, 20, 1681–1691. [Google Scholar] [CrossRef] [PubMed]
- Sohlenkamp, C.; Geiger, O. Bacterial membrane lipids: Diversity in structures and pathways. FEMS Microbiol. Rev. 2016, 40, 133–159. [Google Scholar] [CrossRef] [PubMed]
- Horikoshi, K.; Bull, A.T. Prologue: Definition, categories, distribution, orign and evolution, poneering studies, and emerging fields of Extremophiles. In Extremophiles Handbook; Springer: Tokyo, Japan, 2011; pp. 3–15. [Google Scholar]
- Schultz, J.; Rosado, A.S. Extreme environments: A source of biosurfactants for biotechnological applications Júnia Schultz1 Alexandre Soares Rosado. Extremophiles 2020, 24, 189–206. [Google Scholar] [CrossRef] [PubMed]
- Iqbal, E.; Salim, K.A.; Lim, L.B.L. Phytochemical screening, Total phenolics and Antioxidant Activities of Bark and Leaf extracts of Goniothalamus velutinus (Airy Shaw) from Brunei Darussalam. J. King Saud Univ. Sci. 2015, 27, 224–232. [Google Scholar] [CrossRef]
- Singh, P.; Tiwary, B.N. Isolation and characterization of glycolipid biosurfactant produced by a Pseudomonas otitidis strain isolated from Chirimiri coal mines, India. Bioresour. Bioprocess. 2016, 3, 42. [Google Scholar] [CrossRef]
- Duncan, K.E.; Ferguson, N.; Kimura, K.; Zhou, X.; Istock, C.A. Fine-scale genetic and phenotypic structures in natural populations of Bacillus subtilis and Bacillus licheniformis: Important implications for bacterial evolution and speciation. Evolution 1994, 48, 2002–2025. [Google Scholar]
- Istock, C.A.; Ferguson, N.; Istock, N.L.; Duncan, K.E. Geographical diversity of genomic lineages in Bacillus subtilis (Ehrenberg) Cohn sensu lato. Org. Divers. Evol. 2001, 1, 179–191. [Google Scholar] [CrossRef]
- Roberts, M.S.; Nakamura, L.K.; Cohen, F.M. Bacillus mojavenesis sp. nov., distinguished from Bacillus subtilis by sexual isolation, divergence in DNA sequence, and differences in fatty acid composition. Int. J. Syst. Bact. 1994, 44, 256–264. [Google Scholar] [CrossRef]
- Marzban, A.; Ebrahimipour, G.; Danesh, A. Bioactivity of a Novel Glycolipid Produced by a Halophilic Buttiauxella sp. and Improving Submerged Fermentation Using a Response Surface Method. Molecules 2016, 21, 1256. [Google Scholar] [CrossRef]
- Redman, R.S.; Kim, Y.O.; Woodward, C.J.; Greer, C.; Espino, L.; Doty, S.L.; Rodriguez, R.J. Increased fitness of rice plants to abiotic stree via habitat adapted symbiosis; a strategy for mitigating imapcts of climate change. LOS One 2011, 6, e14823. [Google Scholar] [CrossRef]
- Coleman-Derr, D.; Tringe, S.G. Building the crops of tomorrow; advantages of symbiont-based approaches to improving abiotic stress tolerance. Front Microbiol. 2014, 5, 283. [Google Scholar] [CrossRef] [PubMed]
- Pacwa-Płociniczak, M.; Płaza, G.A.; Piotrowska-Seget, Z.; Cameotra, S.S. Environmental applications of biosurfactants: Recent advances. Int. J. Mol. Sci. 2011, 12, 633–654. [Google Scholar] [CrossRef] [PubMed]
- Angelin, J.; Kavitha, M. Exopolysaccharides from probiotic bacteria and their health potential. Int. J. Biol. Macromol. 2020, 162, 853–865. [Google Scholar] [CrossRef] [PubMed]
- Poli, Z.; Anzelmo, G.; Nicolaus, B. Bacterial exopolysaccharides from extreme marine habitats: Production, characterization and biological activities. Mar Drugs 2010, 8, 1779–1802. [Google Scholar] [CrossRef]
- Chen, C.; Xin, K.; Liu, H.; Cheng, J.; Shen, X.; Wang, Y.; Zhang, L. Pantoea alhagi a novel endophytic bacterium with ability to improve growth and drought tolerance in wheat. Sci. Rep. 2017, 7, 41564. [Google Scholar] [CrossRef] [PubMed]
- Rahman, P.K.S.M.; Pasirayi, G.; Auger, V.; Ali, Z. Production of rhamnolipid biosurfactants by Pseudomonas aeruginosa DS10–129 in a microfluidic bioreactor. Biotechnol. Appl. Biochem. 2010, 55, 45–52. [Google Scholar] [CrossRef]
- Plaza, G.A.; Zjawiony, I.M.; Banat, I. Use of different methods for detection of thermophilic biosurfactants-producing bacteria from hydrocarbon contaminated and bioremediated soils. J. Pet.Sci. Eng. 2006, 50, 71–77. [Google Scholar] [CrossRef]
- Mnif, I.; Ellouz-Chaabouni, S.; Ghribi, D. Glycolipid Biosurfactants, Main classes, Functional properties and related potential applications in Environmental Biotechnology. J. Polym. Environ. 2018, 26, 2192–2206. [Google Scholar] [CrossRef]
- Gagelidze, N.A.; Amiranashvili, L.L.; Varsimashvili, K.I.; Tinikashvili, L.M.; Tolordava, L.L.; Sadunishvili, T.A. Selection of effective biosurfactants producers among Bacillus strains isolated from soild of Georgia. Ann. Algrarian Sci. 2016, 14, 72–75. [Google Scholar] [CrossRef]
- Banat, I.M.; Makkar, R.S.; Cameotra, S.S. Potential commercial applications of microbial surfactants. Appl. Microbiol. Biotechnol. 2000, 53, 495–508. [Google Scholar] [CrossRef]
- Wang, J.; Ji, G.; Tian, J.; Zhang, H.; Dong, H.; Yu, L. Functional characterization of a biosurfacatant producing thermos-tolerant bacteria isolated from an oil reservoir. Pest. Sci. 2011, 8, 353–356. [Google Scholar] [CrossRef]
- Nayarisseri, A.; Sing, P.; Singh, S.K. Screening isolation and characterization of biosurfacatant producing Bacillus subtilis strain ANSKLAB03. Bioinformation 2018, 14, 304–314. [Google Scholar] [CrossRef]
- Bodour, A.A.; Guerrero-Barajas, C.; Jiorle, B.V.; Malcomson, M.E.; Paull, A.K.; Somogyi, A.; Trinh, L.N.; Bates, R.B.; Maier, R.M. Structure and characterization of Flavolipids, a novel class of biosurfactants produced by Flavobacterium sp. strain MTN 11. Appl. Environ. Microbiol 2004, 70, 114–120. [Google Scholar] [CrossRef] [PubMed]
- Morikawa, M. Hirata, Y.; Imanaka, T. A study on the structure-function relationship of lipopeptide biosurfactants. Biochim. Biophys. Acta 2000, 1488, 211–218. [Google Scholar] [CrossRef] [PubMed]
- Xu, H.M.; Rong, Y.J.; Zhao, M.X.; Song, B.; Chi, Z.M. Antibacterial activity of the lipopeptides produced by Bacillus amyloliquefaciens M1 against multidrug-resistant Vibrio spp. isolated from diseased marine animals. Appl. Microbiol. Biotechnol. 2014, 98, 127–136. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, E.F.; Hassan, S.S. Antimicrobial activity of a bioemulsifier produce by Serratia marcescens S10. J. Al-Nahrain Univ. 2013, 16, 147–155. [Google Scholar] [CrossRef]
- Patowary, K.; Patowary, R.; Kalita, M.C.; Deka, S. Characterization of Biosurfactant Produced during Degradation of Hydrocarbons Using Crude Oil As Sole Source of Carbon. Front. Microbiol. 2017, 8, 279. [Google Scholar] [CrossRef]
- Jemil, N.; Ben Ayed, H.; Manresa, A.; Nasri, M.; Hmidet, N. Antioxidant properties, antimicrobial and anti-adhesive activities of DCS1 lipopeptides from Bacillus methylotrophicus DCS1. BMC Microbiol. 2017, 17, 144. [Google Scholar] [CrossRef]
- Radhakrishanan, E.K.; Mathew, J. Characterization of biosurfactants produced by the endophyte Burkhorderia sp WYAT7 and evaluation of its antibacterial and anitbiofilm potentials. J. biotechnol. 2020, 31, 1–10. [Google Scholar]
- Coronel-Leon, J.; Marques, A.M.; Bastida, J.; Maneresa, A. Optimized the production of the biosurfactants lichenysin and its application in biofilm control. J. Appl. Microbiol. 2016, 120, 99–111. [Google Scholar] [CrossRef]
- Yalçin, E.; Ҫavuşoǧlu, K. Structural analysis and antioxidant activity of a biosurfactants obtained from Bacillus subtilis RW1. Turk J. Biochem. 2010, 35, 243–247. [Google Scholar]
- Zhao, H.; Xia, J.; Wang, J.; Yan, X.; Wang, C.; Lei, T.; Xian, M.; Zhang, H. Production of bacterial cellulose using polysaccharide fermentation wastewater as inexpensive nutrient sources. Biotechnol. Biotechnol. Equip. 2018, 32, 350–356. [Google Scholar] [CrossRef]
- Rani, M.; Weadge, J.; Jabaji, S. Isolation and characterization of Biosurfactants producing Bacteria from Oil Well Batteries with antimicrobial activities against Food Borne and Plant Pathogens. Front Microbiol. 2020, 27, 64. [Google Scholar] [CrossRef] [PubMed]
- Zarinviarsagh, M.; Ebrahimipour, G.; Sadeghi, H. Lipase and biosurfactant from Ochrobactrum intermedium strain MZV101 isolated by washing powder for detergent application. Lipids Health Dis. 2017, 18, 177. [Google Scholar] [CrossRef]
- Ferhat, S.; Alouaoui, R.; Badis, A.; Moulai-Mostefa, N. Production and characterization of biosurfacatant by free and immobilized cells from Ochrobactum intermedium isolated from the soil of southern Algeria with a view to environmental application. Biotechnol. Biotechnol. Equip. 2017, 31, 733–742. [Google Scholar] [CrossRef]
- Shoeb, E.; Ahmed, N.; Akhter, J.; Badar, U.; Siddiqui, K.; Ansari, F.A.; Waqar, M.; Imtiaz, S.; Akhtar, N.; Shaikh, Q.U.A.; et al. Screening and characterization of biosurfactant-producing bacteria isolated from the Arabian Sea coast of Karachi. Turk. J. Biol. 2015, 39, 210–216. [Google Scholar] [CrossRef]
- Kanamarlapudi, S.L.R.K.; Muddada, S. Characteization of Exopolysaccharides Produced by Streptococcus thermophilus CC30. BioMed Res. Int. 2017, 2017, 4201809. [Google Scholar] [CrossRef]
- Dagher, F.; Deziel, E.; Lirette, P.; Bisailon, J.; Villemur, R. Com parative study of five polycyclic arom atic hydrocarbon degrading bacterial strains isolated from contam inated soils. Can. J. Microbiol. 1997, 43, 368–377. [Google Scholar] [CrossRef]
- Sasser, M. Identification of Bacteria by Gas Chromatography of Cellular Fatty Acids; MIDI Technical Note 1011990; MIDI Inc.: Newark, DE, USA; Available online: http://www.microbialid.com/PDF/TechNote_101.pdf (accessed on 13 February 2023).
- Essghaier, B.; Dridi, R.; Arouri, A.; Zid, M.F. Synthesis, structural characterization and prospects for a new tris (5-methylbenzimidazole) tris (oxalato) ferrate(III) trihydrate complex as a promising antibacterial and antifungal agent. Polyhedron 2021, 208, 115420. [Google Scholar] [CrossRef]
- Kim, M.K.; Zhao, A.; Wang, A.; Brown, Z.Z.; Muir, T.W.; Stone, H.A.; Bassler, B.L. Surface attached molecules control Staphylococcus aureus quorum sensing and biofilm development. Nat. Microbiol. 2017, 2, 17080. [Google Scholar] [CrossRef]
- Gulati, M.; Lohse, M.B.; Ennis, C.L.; Gonzalez, C.L.; Perry, A.M.; Bapat, P.; Arevalo, A.V.; Rodriguez, D.L.; Nobile, C.J. In vitro culturing and screening of Candida albicans Biofilms. Curr. Protoc.Microbiol. 2018, 50, e60. [Google Scholar] [CrossRef] [PubMed]
- Chan, E.W.C.; Lim, Y.Y.; Omar, M. Antioxidant and antibacterial activity of leaves of Etlingera Species (Zingiberaceae) in Penin-sular Malaysia. Food Chem. 2007, 104, 1586–1593. [Google Scholar] [CrossRef]



| Vegetable Oil Types | Biosurfactant Activity (UA/mL) * |
|---|---|
| LB + oil corn | 0.0152 ± 0.7 |
| LB + sunflower oil | 0.0202 ± 0.5 |
| LB + Tween 80 | 0.0141 ± 0.48 |
| LB olive oil | 0.0151 ± 0.47 |
| Fatty Acid | Formula | BS | Cell Membrane |
|---|---|---|---|
| Methyl 2-hydroxydecanoate | 2-OHC10:0 | ND | 0.46 |
| Undecylic acid | C11 | ND | 0.02 |
| Lauric acid | C12:0 | ND | 0.25 |
| Methyl 2-hydroxydodecanoate | 2-OHC12:0 | ND | 67.00 |
| Tridecyl acid | C13:0 | ND | 0.09 |
| Myristic acid | C14:0 | 0.21 | 0.00 |
| Methyl 2-hydroxytetradecanoate | 2-OHC14:0 | 0.44 | 2.63 |
| Methyl 3-hydroxytetradecanoate | 3-OHC14:0 | ND | 0.04 |
| Methyl 13-methyltetradecanoate | i-C15:0 | 0.03 | 0.10 |
| Methyl 12-methyltetradecanoate | a-C15:0 | 10.93 | 6.73 |
| Palmitic acid | C16:0 | 0.26 | 0.06 |
| Methyl 14-methylpentadecanoate | i C16:0 | 0.05 | 0.41 |
| Methyl 2-hydroxyhexadecanoate | 2-OHC16:0 | 0.05 | 0.07 |
| Palmitoleic acid | C16:1n-9 | 0.03 | 0.02 |
| Margaric acid | C17:0 | ND | 0.01 |
| Methyl 14-methylhexadecanoate | i-C17:0 | ND | 12.05 |
| Methyl cis-9,10-methylenehexadecanoate | C17:0d | ND | 1.08 |
| Stearic acid | C18:0 | 8.60 | 1.72 |
| Oleic acid | C18:1n-9 | 76.26 | 3.06 |
| Vaccenic acid | C18:1n-11 | 2.29 | 3.73 |
| Nonadecylic acid | C19:0 | 0.05 | 0.12 |
| Arachidic acid | C20:0 | 0.26 | 0.08 |
| Strains [Ref] | Fatty Acid Composition (%) | ||||||
|---|---|---|---|---|---|---|---|
| C13 | C14 | C15 | C16 | C18 | C19 | C20 | |
| Pantoea alhagi [this work] | nd | (C14:0) 0.21 | (a-C15:0) 10.93 | (C16:0) 0.26 | (C18:0) 8.6 (C18:1n-9) 76.26 | (C19:9) 0.515 | (C20:0) 0.26 |
| Bacillus subtilis T89-15 [18,19] | 3 | 2.4 | 67.7 | 22.7 | 3.4 | ||
| Bacillus mojavensis JF-2 [20] | 1.3 | 49.6 | 11.3 | 24.4 | |||
| Bacillus subtilis sp. spizizennii T89-3 [18,19] | 1.35 | 6.8 | 49.7 | 35.5 | 6.2 | ||
| Buttiauxella [21] | C14:0 | C16:0 | C18:0 C18:9 | ||||
| Biosurfactants in Ethyl Acetate | Biosurfactants in Methanol | Biosurfactant Pellet | |
|---|---|---|---|
| Escherchia coli | 0 ± 0 b | 29.50 ± 1.00 a | 0 ± 0 b |
| Staphylococcus aureus | 23.75 ± 10 b | 36.00 ± 0.25 a | 0 ± 0 c |
| Micrococcus luteus | 16.00 ± 0.2 b | 15.75 ± 0.20 c | 21.75 ± 0.20 a |
| Fusarium sp. | 13.75 ± 0.57 c | 31.00 ± 0.50 a | 15.5 ± 0.5 b |
| DPPH (Inhibition %) | |
|---|---|
| Biosurfactants in methanol (2 mg/mL) | 78.07% a |
| Ascorbic acid (0.5 mg/mL) | 29.89 b |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Essghaier, B.; Mallat, N.; Khwaldia, K.; Mottola, F.; Rocco, L.; Hannachi, H. Production and Characterization of New Biosurfactants/Bioemulsifiers from Pantoea alhagi and Their Antioxidant, Antimicrobial and Anti-Biofilm Potentiality Evaluations. Molecules 2023, 28, 1912. https://doi.org/10.3390/molecules28041912
Essghaier B, Mallat N, Khwaldia K, Mottola F, Rocco L, Hannachi H. Production and Characterization of New Biosurfactants/Bioemulsifiers from Pantoea alhagi and Their Antioxidant, Antimicrobial and Anti-Biofilm Potentiality Evaluations. Molecules. 2023; 28(4):1912. https://doi.org/10.3390/molecules28041912
Chicago/Turabian StyleEssghaier, Badiaa, Nesrine Mallat, Khaoula Khwaldia, Filomena Mottola, Lucia Rocco, and Hédia Hannachi. 2023. "Production and Characterization of New Biosurfactants/Bioemulsifiers from Pantoea alhagi and Their Antioxidant, Antimicrobial and Anti-Biofilm Potentiality Evaluations" Molecules 28, no. 4: 1912. https://doi.org/10.3390/molecules28041912
APA StyleEssghaier, B., Mallat, N., Khwaldia, K., Mottola, F., Rocco, L., & Hannachi, H. (2023). Production and Characterization of New Biosurfactants/Bioemulsifiers from Pantoea alhagi and Their Antioxidant, Antimicrobial and Anti-Biofilm Potentiality Evaluations. Molecules, 28(4), 1912. https://doi.org/10.3390/molecules28041912







