Photoantibacterial Poly(vinyl)chloride Films Applying Curcumin Derivatives as Bio-Based Plasticizers and Photosensitizers
Abstract
1. Introduction
2. Results and Discussion
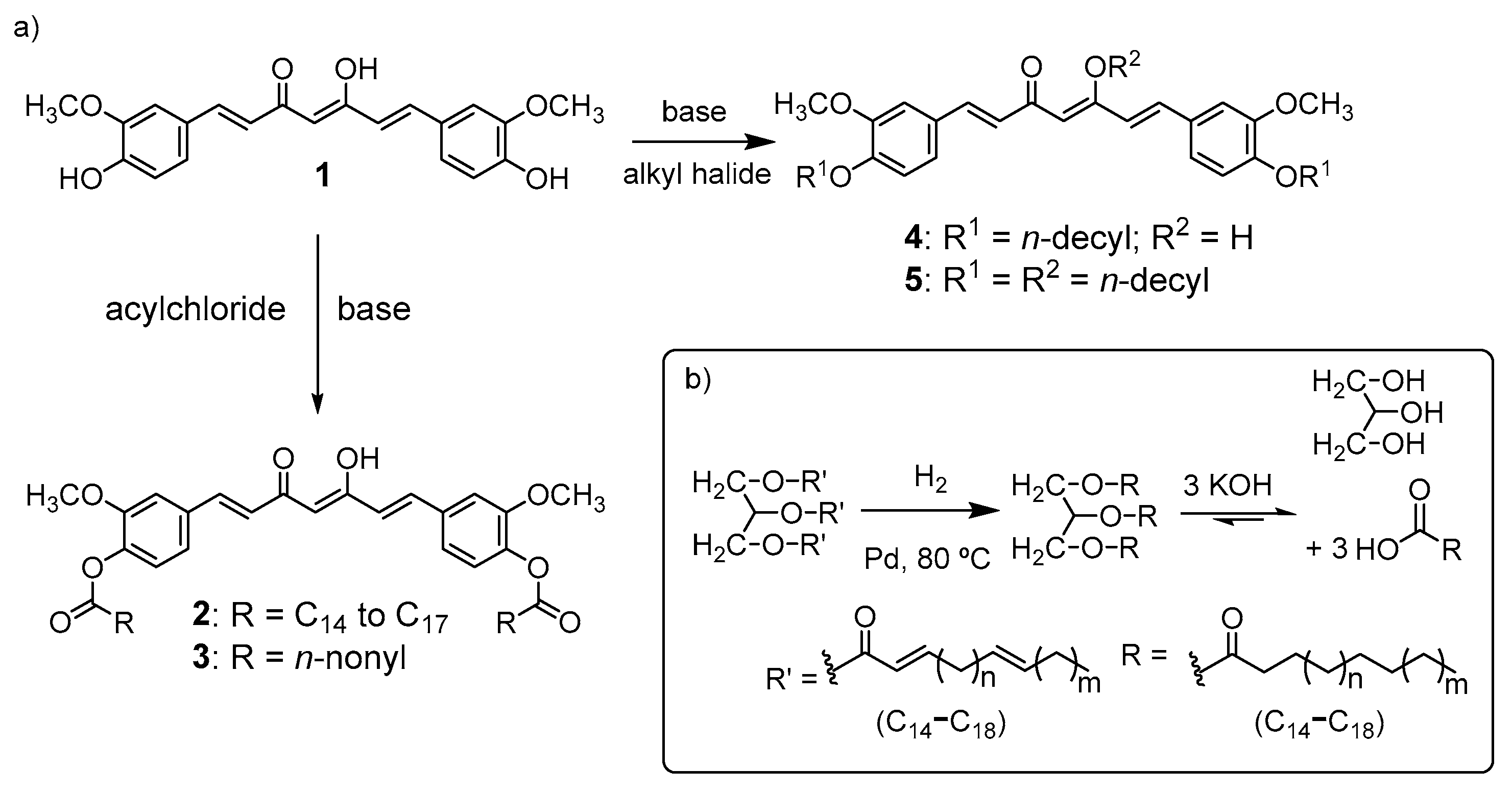
2.1. Synthesis and Characterization of Curcumin-Based Photosensitive Plasticizers
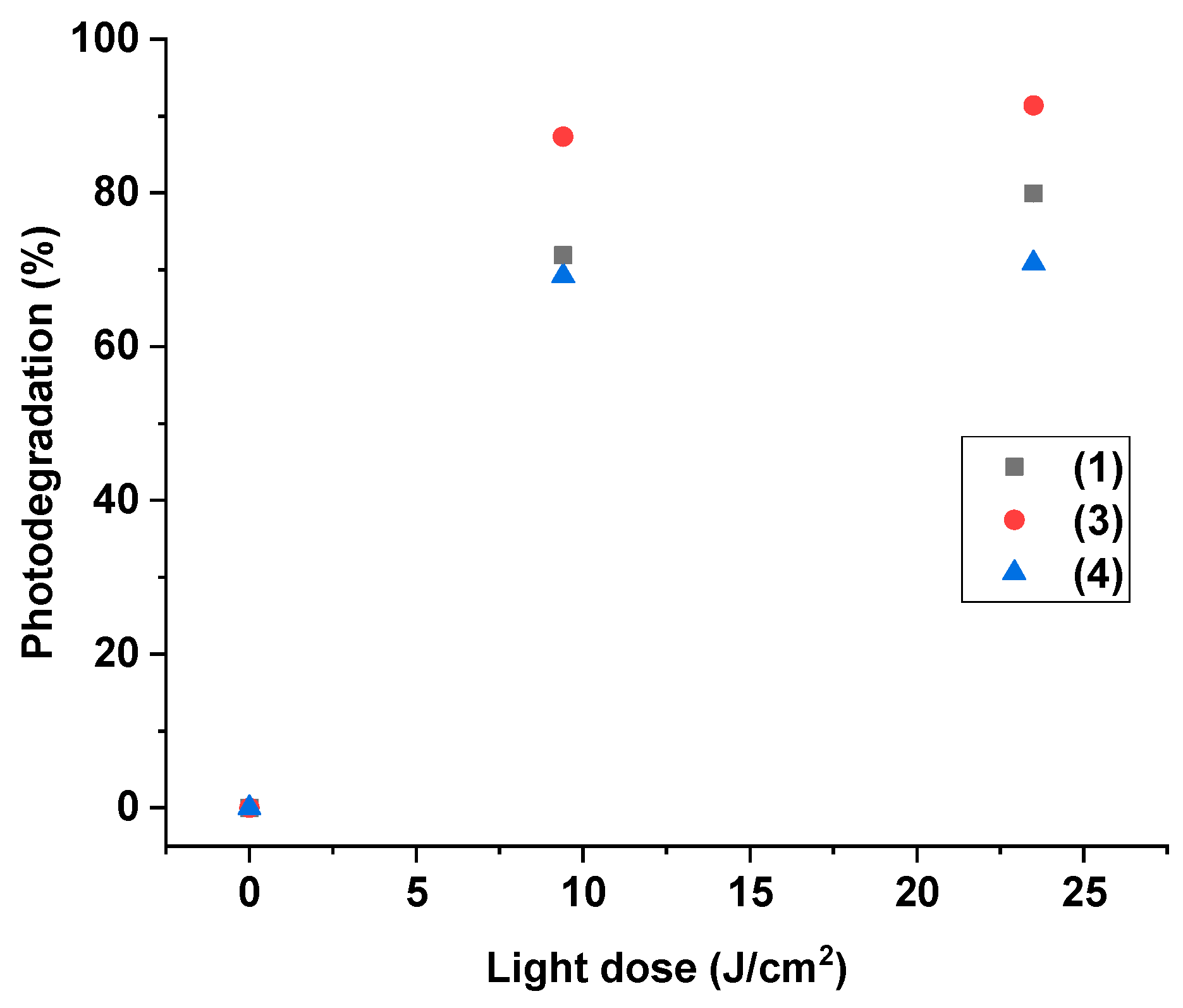
2.2. Photodegradation Evaluation of Curcumin-Based Plasticizers
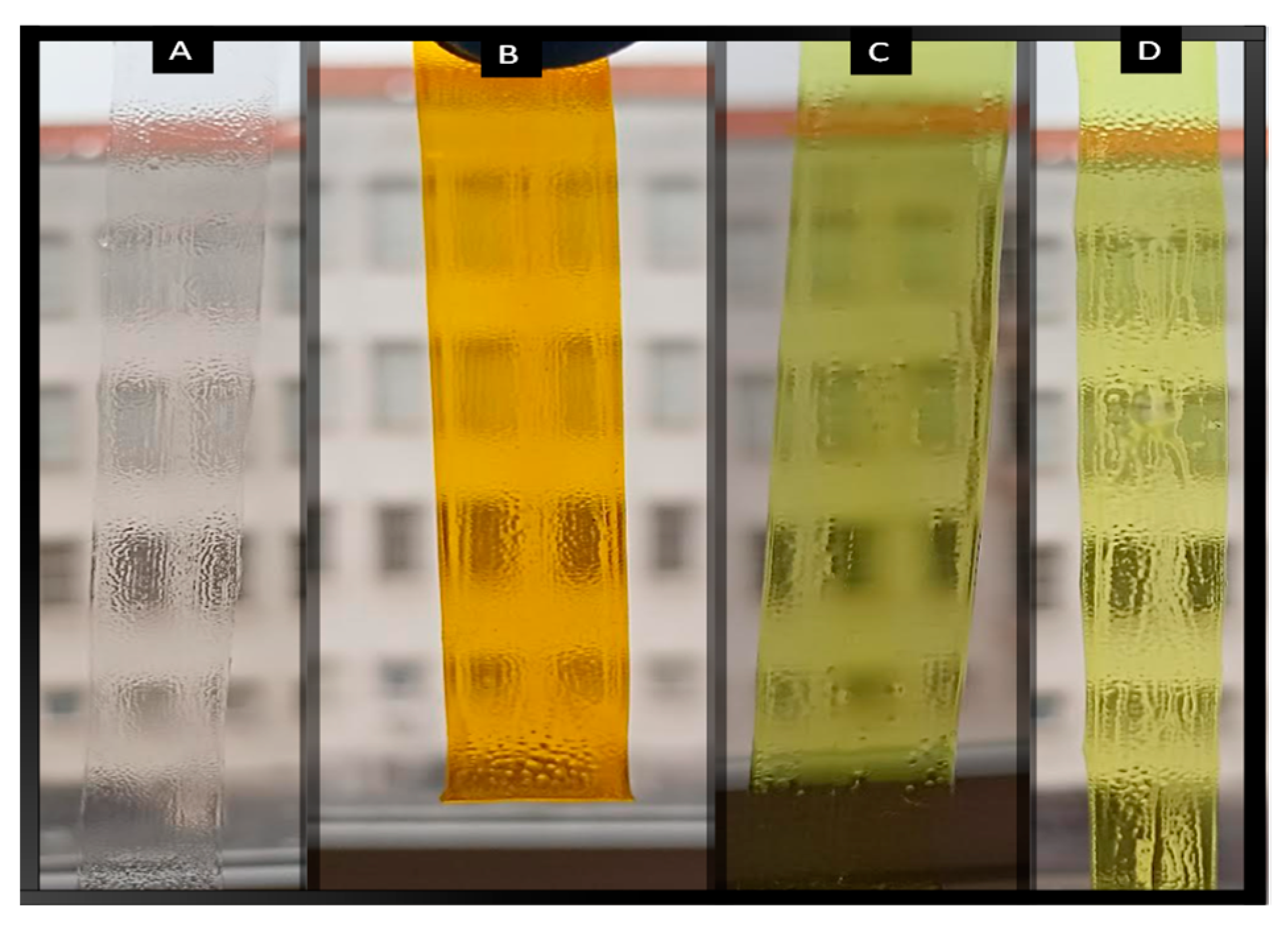
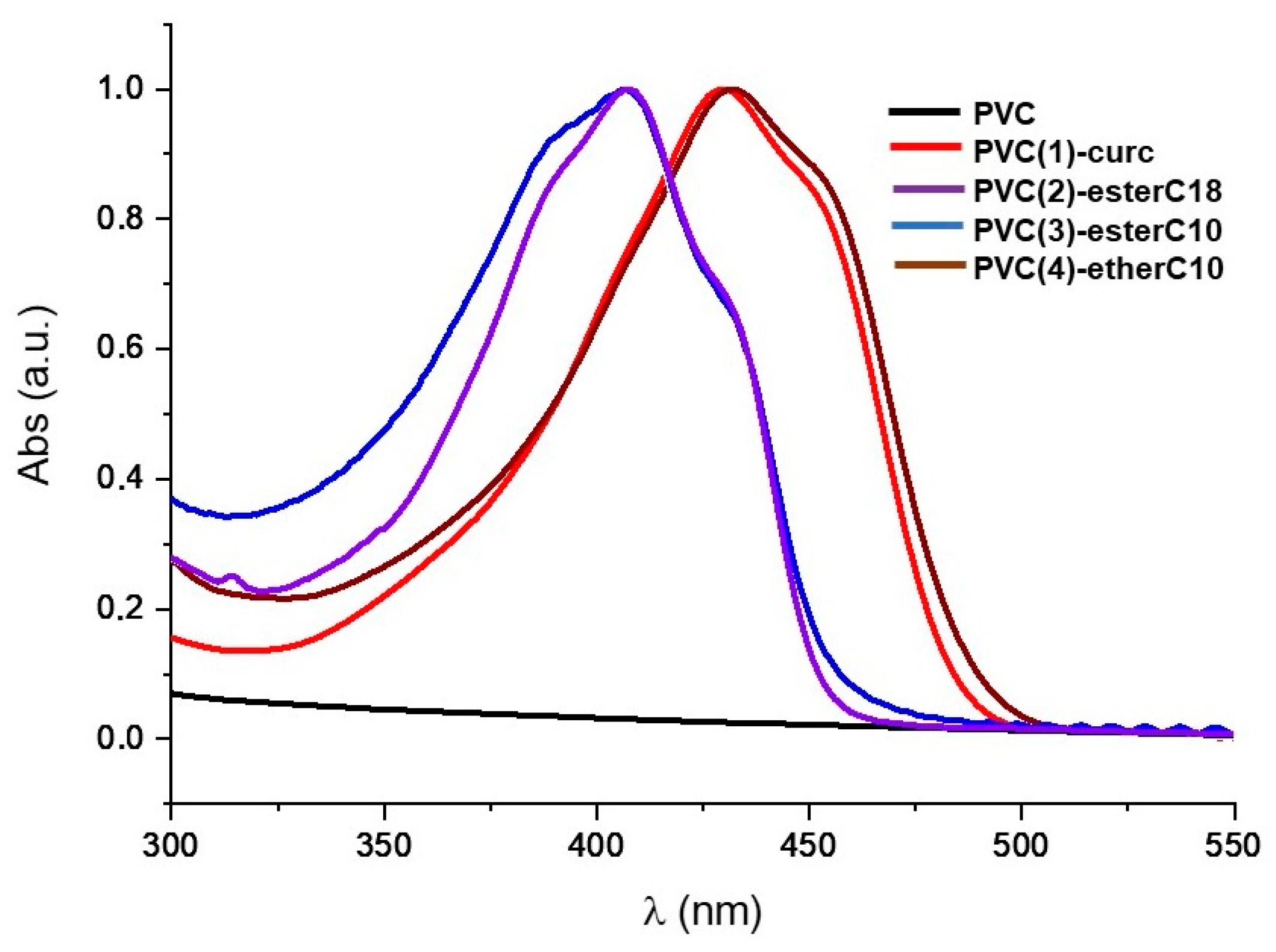
2.3. Preparation and Characterization of PVC–Curcumin-Based Films
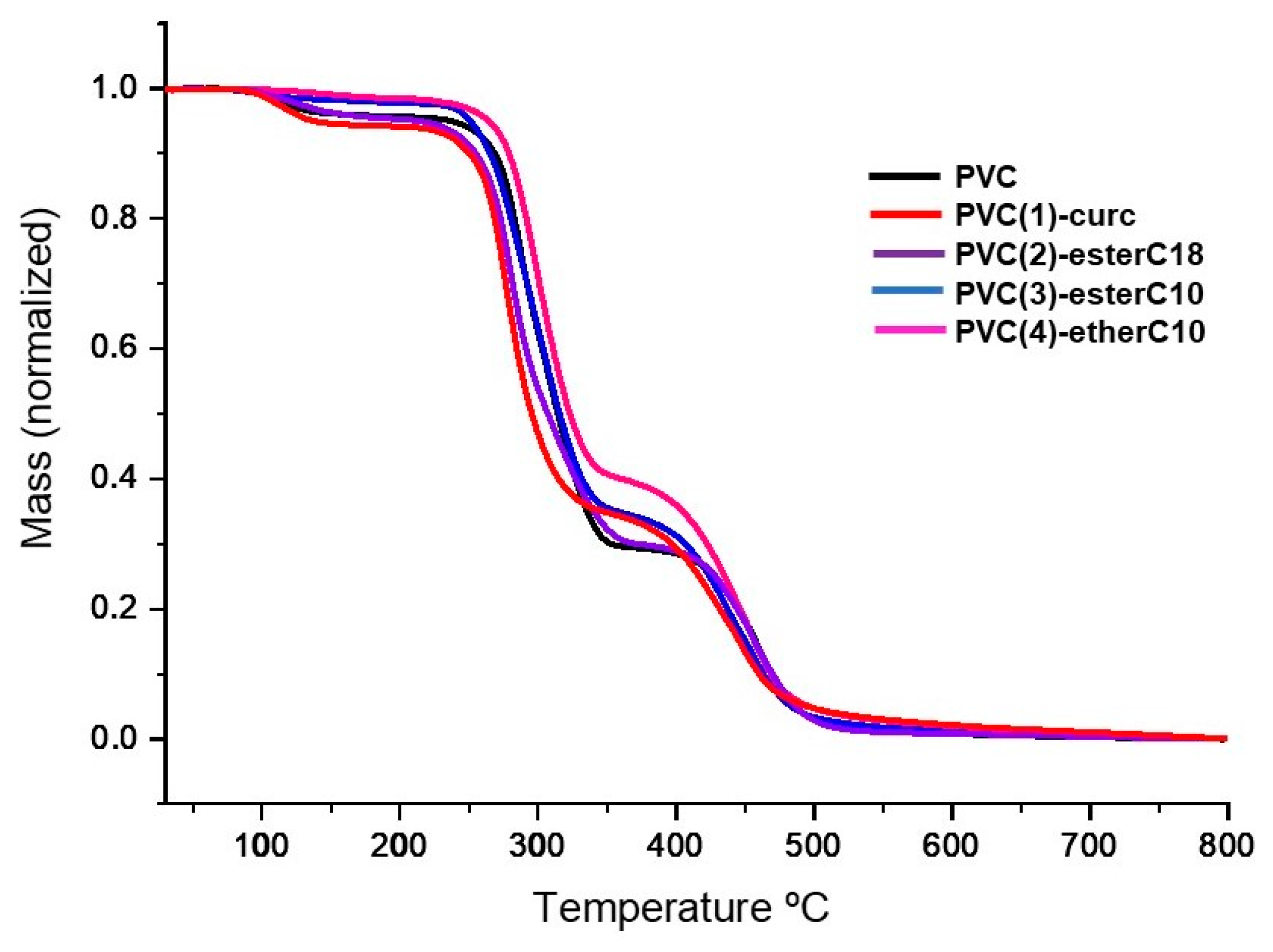
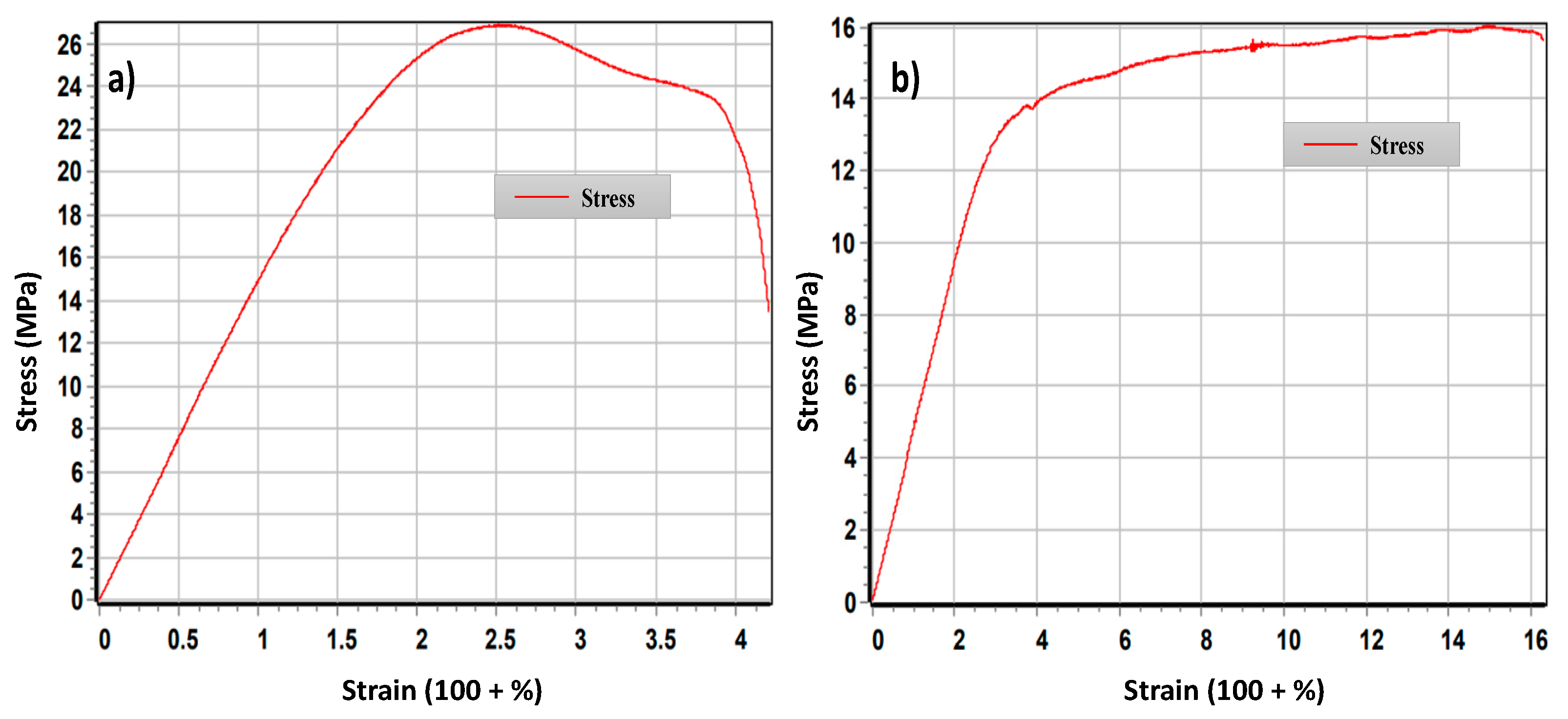
2.4. Thermal Stability and Mechanical Properties
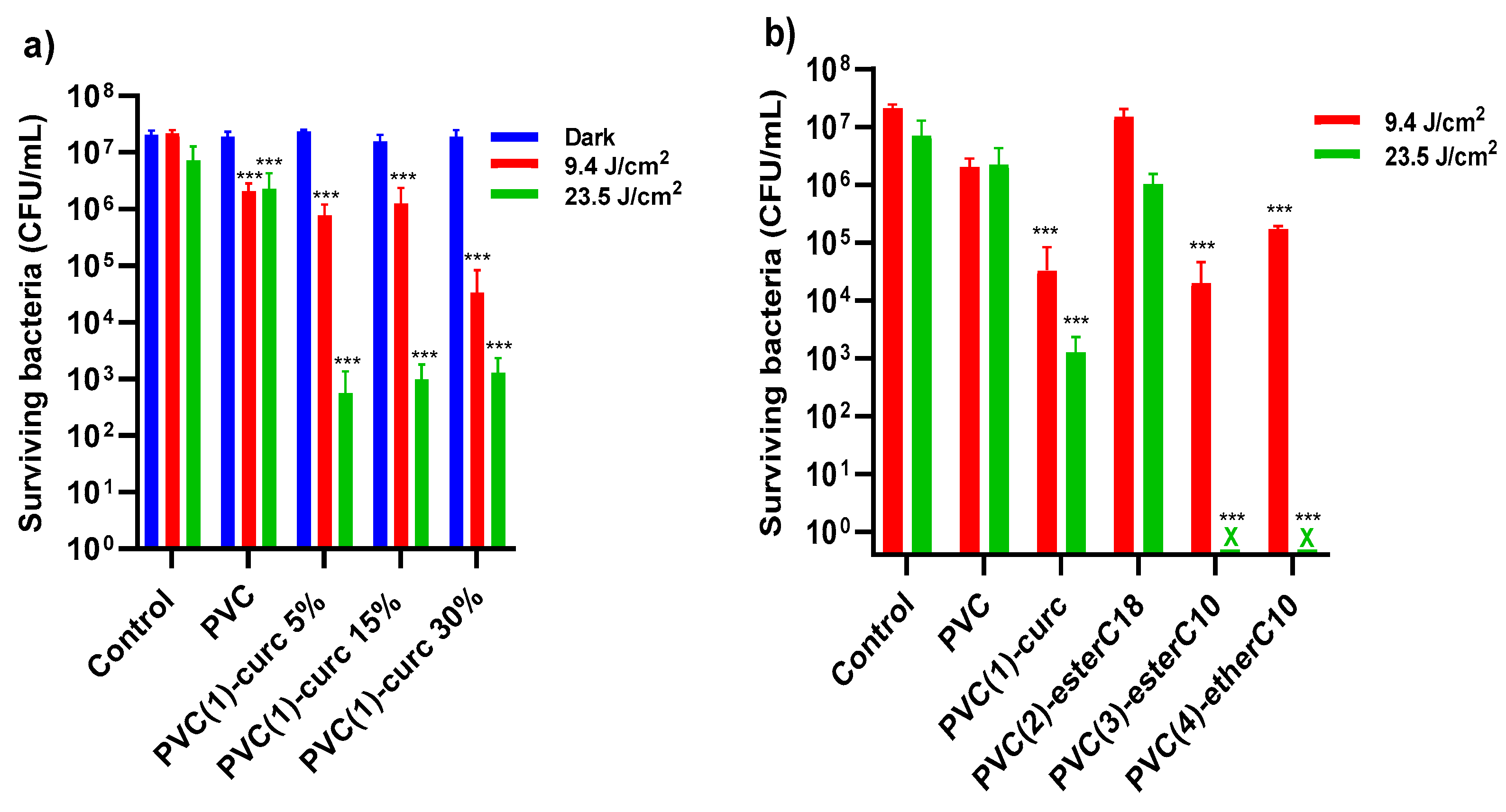
2.5. Antibacterial Photoinactivation Studies
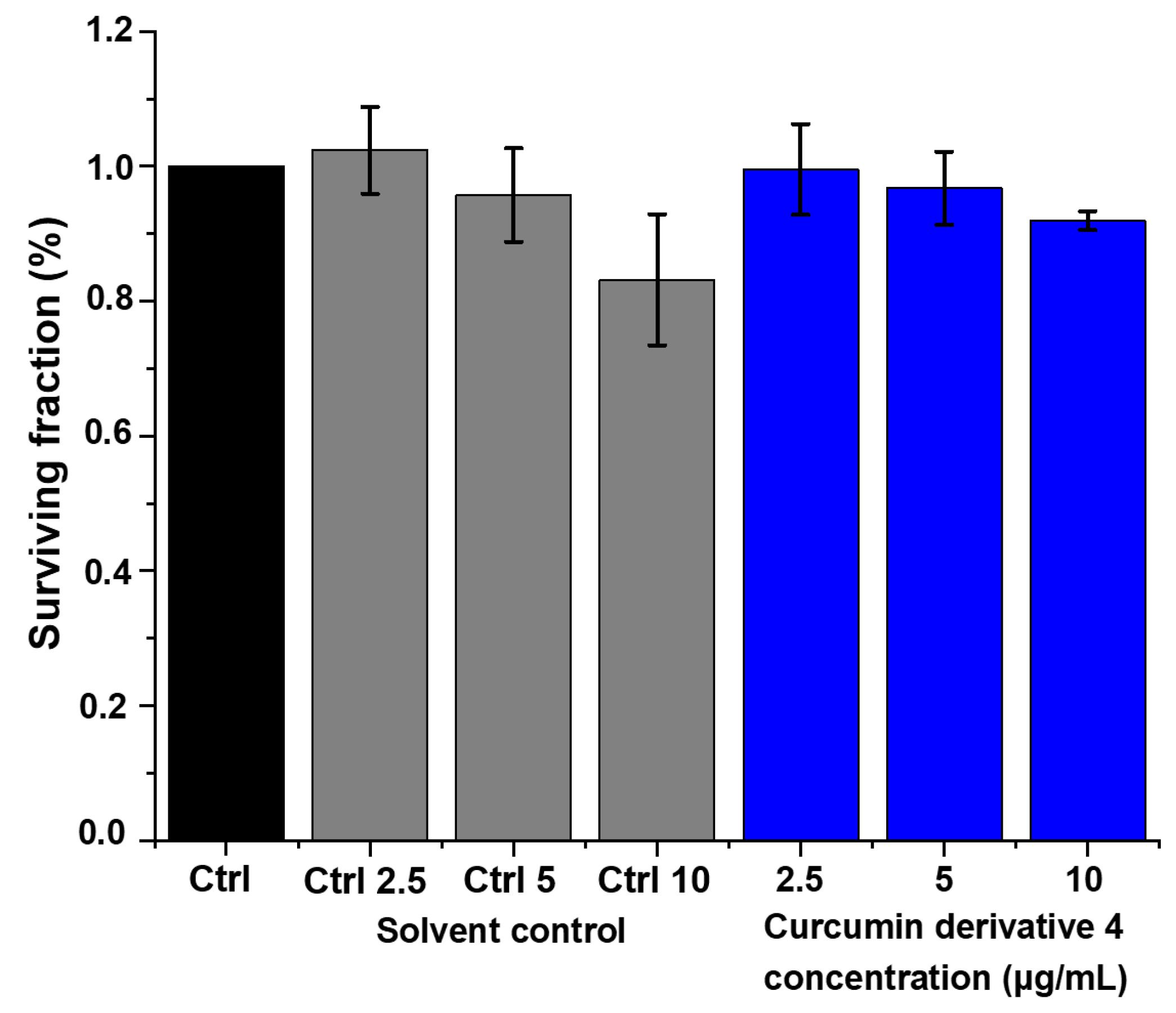
2.6. Cytotoxicity Evaluation
3. Materials and Methods
3.1. Materials and Methods
3.2. Synthesis of Curcumin-Ester-Based Plasticizers
Synthesis of Curcumin-decanoyl Ester Derivative 3
3.3. Synthesis of Curcumin-Ether-Based Plasticizers
3.4. Photophysical and Photochemical Characterization of Curcumin-Based Plasticizers
3.5. Photodegradation Evaluation of Curcumin-Based Plasticizers
3.6. Preparation of PVC–Curcumin-Based Films
3.7. Characterization of PVC–Curcumin-Based Films
3.8. Photoantibacterial Evaluation of Photosensitive PVC–Curcumin-Based Films
3.9. Cytotoxicity Evaluation of Curcumin-Based Plasticizer 4
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Jamarani, R.; Erythropel, H.C.; Nicell, J.A.; Leask, R.L.; Maric, M. How green is your plasticizer? Polymers 2018, 10, 834. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S. Recent developments of biobased plasticizers and their effect on mechanical and thermal properties of poly(vinyl chloride): A review. Ind. Eng. Chem. Res. 2019, 58, 11659–11672. [Google Scholar] [CrossRef]
- Ostle, C.; Thompson, R.C.; Broughton, D.; Gregory, L.; Wootton, M.; Johns, D.G. The rise in ocean plastics evidenced from a 60-year time series. Nat. Commun. 2019, 10, 1622. [Google Scholar] [CrossRef]
- Rowdhwal, S.S.S.; Chen, J. Toxic effects of di-2-ethylhexyl phthalate: An overview. BioMed Res. Int. 2018, 2018, 1750368. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Zhu, H.K.; Kannan, K. A review of biomonitoring of phthalate exposures. Toxics 2019, 7, 21. [Google Scholar] [CrossRef]
- Polyvinyl Chloride (pvc) Market Share, Report nr pm1448. Available online: https://www.polarismarketresearch.com/industry-analysis/polyvinyl-chloride-pvc-market:2019 (accessed on 25 October 2022).
- Velazco-Medel, M.A.; Camacho-Cruz, L.A.; Bucio, E. Modification of relevant polymeric materials for medical applications and devices. Med. Devices Sens. 2020, 3, e10073. [Google Scholar] [CrossRef]
- Dadi, N.C.T.; Radochová, B.; Vargová, J.; Bujdáková, H. Impact of healthcare-associated infections connected to medical devices—An update. Microorganisms 2021, 9, 2332. [Google Scholar] [CrossRef] [PubMed]
- Zangirolami, A.C.; Dias, L.D.; Blanco, K.C.; Vinagreiro, C.S.; Inada, N.M.; Arnaut, L.G.; Pereira, M.M.; Bagnato, V.S. Avoiding ventilator-associated pneumonia: Curcumin-functionalized endotracheal tube and photodynamic action. PNAS 2020, 117, 22967–22973. [Google Scholar] [CrossRef]
- Commission Regulation (EU) 2018/2005 of 17 December 2018 on Registration, Evaluation, Authorisation and Restriction of Chemicals (REACH) as Regards Bis(2-Ethylhexyl) Phthalate (DEHP), Dibutyl phthalate (DBP), Benzyl Butyl Phthalate (BBP) and Diisobutyl Phthalate (DIBP). 2018. Available online: http://data.Europa.Eu/eli/reg/2018/2005/oj (accessed on 1 November 2022).
- Food and Drugs Administration, USA, Guidance for Industry: Limiting the Use of Certain Phthalates as Excipients in Cder-regulated Products. 2012. Available online: https://www.Fda.Gov/files/drugs/published/limiting-the-use-of-certain-phthalates-as-excipients-in-cder-regulated-products_pdf.Pdf (accessed on 1 November 2022).
- Saltos, J.A.; Shi, W.; Mancuso, A.; Sun, C.; Park, T.; Averick, N.; Punia, K.; Fata, J.; Raja, K. Curcumin-derived green plasticizers for poly(vinyl) chloride. RSC Adv. 2014, 4, 54725–54728. [Google Scholar] [CrossRef]
- Galli, F.; Nucci, S.; Pirola, C.; Bianchi, C.L. Epoxy methyl soyate as bio-plasticizer: Two different preparation strategies. Chem. Eng. Trans 2014, 37, 601–606. [Google Scholar]
- Yang, Y.; Huang, J.; Zhang, R.; Zhu, J. Designing bio-based plasticizers: Effect of alkyl chain length on plasticization properties of isosorbide diesters in pvc blends. Mater. Des. 2017, 126, 29–36. [Google Scholar] [CrossRef]
- Sobotta, L.; Skupin-Mrugalska, P.; Piskorz, J.; Mielcarek, J. Porphyrinoid photosensitizers mediated photodynamic inactivation against bacteria. Eur J. Med. Chem. 2019, 175, 72–106. [Google Scholar] [CrossRef] [PubMed]
- Aroso, R.T.; Dias, L.D.; Blanco, K.C.; Soares, J.M.; Alves, F.; da Silva, G.J.; Arnaut, L.G.; Bagnato, V.S.; Pereira, M.M. Synergic dual phototherapy: Cationic imidazolyl photosensitizers and ciprofloxacin for eradication of in vitro and in vivo e. Coli infections. J. Photochem. Photobiol. B Biol. 2022, 233, 112499. [Google Scholar] [CrossRef] [PubMed]
- Aroso, R.T.; Schaberle, F.A.; Arnaut, L.G.; Pereira, M.M. Photodynamic disinfection and its role in controlling infectious diseases. Photochem. Photobiol. Sci. 2021, 20, 1497–1545. [Google Scholar] [CrossRef] [PubMed]
- Vinagreiro, C.S.; Zangirolami, A.; Schaberle, F.A.; Nunes, S.C.C.; Blanco, K.C.; Inada, N.M.; da Silva, G.J.; Pais, A.A.C.C.; Bagnato, V.S.; Arnaut, L.G.; et al. Antibacterial photodynamic inactivation of antibiotic-resistant bacteria and biofilms with nanomolar photosensitizer concentrations. ACS Infect. Dis. 2020, 6, 1517–1526. [Google Scholar] [CrossRef]
- Hu, X.; Huang, Y.-Y.; Wang, Y.; Wang, X.; Hamblin, M.R. Antimicrobial photodynamic therapy to control clinically relevant biofilm infections. Front. Microbiol. 2018, 9, 1299. [Google Scholar] [CrossRef]
- Klausen, M.; Ucuncu, M.; Bradley, M. Design of photosensitizing agents for targeted antimicrobial photodynamic therapy. Molecules 2020, 25, 5239. [Google Scholar] [CrossRef]
- Cieplik, F.; Deng, D.; Crielaard, W.; Buchalla, W.; Hellwig, E.; Al-Ahmad, A.; Maisch, T. Antimicrobial photodynamic therapy—What we know and what we don’t. Crit. Rev. Microbiol. 2018, 44, 571–589. [Google Scholar] [CrossRef]
- Dias, L.D.; Blanco, K.C.; Mfouo-Tynga, I.S.; Inada, N.M.; Bagnato, V.S. Curcumin as a photosensitizer: From molecular structure to recent advances in antimicrobial photodynamic therapy. J. Photochem. Photobiol. C 2020, 45, 100384. [Google Scholar] [CrossRef]
- Polat, E.; Kang, K. Natural photosensitizers in antimicrobial photodynamic therapy. Biomedicines 2021, 9, 584. [Google Scholar] [CrossRef]
- Trigo-Gutierrez, J.K.; Vega-Chacon, Y.; Soares, A.B.; Mima, E.G.D. Antimicrobial activity of curcumin in nanoformulations: A comprehensive review. Int. J. Mol. Sci. 2021, 22, 7130. [Google Scholar] [CrossRef]
- Baptista, J.A.; Eusébio, M.E.S.; Pereira, M.M. New renewable raw materials for thermal energy storage. J. Therm. Anal. Calorim. 2021, 145, 27–37. [Google Scholar] [CrossRef]
- Shieh, W.C.; Dell, S.; Repic, O. 1,8-diazabicyclo [5.4.0]undec-7-ene (dbu) and microwave-accelerated green chemistry in methylation of phenols, indoles, and benzimidazoles with dimethyl carbonate. Org. Lett. 2001, 3, 4279–4281. [Google Scholar] [CrossRef] [PubMed]
- Mondal, S.; Ghosh, S.; Moulik, S.P. Stability of curcumin in different solvent and solution media: Uv–visible and steady-state fluorescence spectral study. J. Photochem. Photobiol. B Biol. 2016, 158, 212–218. [Google Scholar] [CrossRef] [PubMed]
- Robinson, J.W.; Frame, E.S.; Frame II, G.M. Visible and ultraviolet molecular spectroscopy. In Undergraduate Instrumental Analysis, 7th ed.; CRC Press: Boca Raton, FL, USA, 2014. [Google Scholar]
- Zayed, M.E.M.; El-Shishtawy, R.M.; Elroby, S.A.; Obaid, A.Y.; Al-amshany, Z.M. Experimental and theoretical study of o-substituent effect on the fluorescence of 8-hydroxyquinoline. Int. J. Mol. Sci. 2015, 16, 3804–3819. [Google Scholar] [CrossRef]
- Schmidt, R.; Tanielian, C.; Dunsbach, R.; Wolff, C. Phenalenone, a universal reference compound for the determination of quantum yields of singlet oxygen o2(1-delta-g) sensitization. J. Photochem. Photobiol. A 1994, 79, 11–17. [Google Scholar] [CrossRef]
- Eastman, J.W. Quantitative spectrofluorimetry—Fluorescence quantum yield of quinine sulfate. PhotoChem. Photobiol. 1967, 6, 55. [Google Scholar] [CrossRef]
- Han, W.X.; Fu, H.Y.; Xue, T.L.; Liu, T.Z.; Wang, Y.; Wang, T. Facilely prepared blue-green light sensitive curcuminoids with excellent bleaching properties as high performance photosensitizers in cationic and free radical photopolymerization. Polym. Chem. 2018, 9, 1787–1798. [Google Scholar] [CrossRef]
- Cherrington, R.; Liang, J. multifunctionality. In Design and Manufacture of Plastic Components for Multifunctionality; Goodship, V., Middleton, B., Cherrington, R., Eds.; William Andrew Publishing: Oxford, UK, 2016; pp. 19–51. [Google Scholar]
- Angell, C.A. Perspective on the glass transition. J. Phys. Chem. Solids 1988, 49, 863–871. [Google Scholar] [CrossRef]
- Choi, J.; Kwak, S.Y. Hyperbranched poly(epsilon-caprolactone) as a nonmigrating alternative plasticizer for phthalates in flexible pvc. Env. Sci. Technol. 2007, 41, 3763–3768. [Google Scholar] [CrossRef]
- Jia, P.Y.; Bo, C.Y.; Zhang, L.Q.; Hu, L.H.; Zhang, M.; Zhou, Y.H. Synthesis of castor oil based plasticizers containing flame retarded group and their application in poly (vinyl chloride) as secondary plasticizer. J. Ind. Eng. Chem. 2015, 28, 217–224. [Google Scholar] [CrossRef]
- Jia, P.Y.; Xia, H.Y.; Tang, K.H.; Zhou, Y.H. Plasticizers derived from biomass resources: A short review. Polymers 2018, 10, 1303. [Google Scholar] [CrossRef]
- Wypych, G. Handbook of Plasticizers; Chem Tek Publishing: Toronto, ON, Canada, 2004. [Google Scholar]
- Zhang, H.F.; Zhu, F.F.; Fu, Q.H.; Zhang, X.X.; Zhu, X.B. Mechanical properties of renewable plasticizer based on ricinoleic acid for pvc. Polym. Test. 2019, 76, 199–206. [Google Scholar] [CrossRef]
- Pareek, M.; Sunoj, R.B. Mechanistic insights into rhodium-catalyzed enantioselective allylic alkylation for quaternary stereogenic centers. Chem. Sci. 2021, 12, 2527–2539. [Google Scholar] [CrossRef] [PubMed]
- Jensen, R.L.; Arnbjerg, J.; Birkedal, H.; Ogilby, P.R. Singlet oxygen’s response to protein dynamics. J. Am. Chem. Soc. 2011, 133, 7166–7173. [Google Scholar] [CrossRef] [PubMed]
- Carmona-Vargas, C.C.; de, C. Alves, L.; Brocksom, T.J.; de Oliveira, K.T. Combining batch and continuous flow setups in the end-to-end synthesis of naturally occurring curcuminoids. React. Chem. Eng. 2017, 2, 366–374. [Google Scholar] [CrossRef]
- Baptista, J.P.A.R.P. Study on Bee Wax for Potential Application as Phase Change Material, Msc Thesis; University of Coimbra: Coimbra, Portugal, 2019; Available online: http://hdl.handle.net/10316/83115 (accessed on 25 January 2023).
- Davis, J.R. Tensile Testing, 2nd ed.; ASM International: Almere, The Netherlands, 2004. [Google Scholar]
- Jawaid, M.; Thariq, M.; Saba, N. (Eds.) Mechanical and Physical Testing of Biocomposites, Fibre-Reinforced Composites and Hybrid Composites; Woodhead Publishing: Sawston, UK, 2018. [Google Scholar]

| Compound | λmax (nm) | Ɛ (M−1.cm−1) | ΦΔ (a) | ΦF (b) |
|---|---|---|---|---|
| 1 | 422 | 59,359 | 0.372 | 0.101 |
| 2 | 401 | 49,093 | 0.220 | 0.012 |
| 3 | 400 | 43,682 | 0.277 | 0.020 |
| 4 | 421 | 42,695 | 0.333 | 0.117 |
| 5 | 380 | 26,665 | n.d. | n.d. |
| Entry | Samples | Tg [°C] (a) | σM [MPa] | εtB [%] |
|---|---|---|---|---|
| 1 | PVC | 73.1 ± 0.5 | 26 ± 1 | 104 ± 5 |
| 2 | PVC(1)-curc | 65 ± 3 | 22 ± 3 | 101 ± 2 |
| 3 | PVC(2)-esterC18 | 79.0 ± 0.2 | 21 ± 5 | 102.7 ± 0.5 |
| 4 | PVC(3)-esterC10 | 61.1 ± 0.5 | 27.4 ± 0.1 | 103 ± 3 |
| 5 | PVC(4)-etherC10 | 55.2 ± 0.2 | 18 ± 2 | 111 ± 3 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rodrigues, F.M.S.; Tavares, I.; Aroso, R.T.; Dias, L.D.; Domingos, C.V.; de Faria, C.M.G.; Piccirillo, G.; Maria, T.M.R.; Carrilho, R.M.B.; Bagnato, V.S.; et al. Photoantibacterial Poly(vinyl)chloride Films Applying Curcumin Derivatives as Bio-Based Plasticizers and Photosensitizers. Molecules 2023, 28, 2209. https://doi.org/10.3390/molecules28052209
Rodrigues FMS, Tavares I, Aroso RT, Dias LD, Domingos CV, de Faria CMG, Piccirillo G, Maria TMR, Carrilho RMB, Bagnato VS, et al. Photoantibacterial Poly(vinyl)chloride Films Applying Curcumin Derivatives as Bio-Based Plasticizers and Photosensitizers. Molecules. 2023; 28(5):2209. https://doi.org/10.3390/molecules28052209
Chicago/Turabian StyleRodrigues, Fábio M. S., Iúri Tavares, Rafael T. Aroso, Lucas D. Dias, Carolina V. Domingos, Clara M. G. de Faria, Giusi Piccirillo, Teresa M. R. Maria, Rui M. B. Carrilho, Vanderlei S. Bagnato, and et al. 2023. "Photoantibacterial Poly(vinyl)chloride Films Applying Curcumin Derivatives as Bio-Based Plasticizers and Photosensitizers" Molecules 28, no. 5: 2209. https://doi.org/10.3390/molecules28052209
APA StyleRodrigues, F. M. S., Tavares, I., Aroso, R. T., Dias, L. D., Domingos, C. V., de Faria, C. M. G., Piccirillo, G., Maria, T. M. R., Carrilho, R. M. B., Bagnato, V. S., Calvete, M. J. F., & Pereira, M. M. (2023). Photoantibacterial Poly(vinyl)chloride Films Applying Curcumin Derivatives as Bio-Based Plasticizers and Photosensitizers. Molecules, 28(5), 2209. https://doi.org/10.3390/molecules28052209