Abstract
Extra virgin olive oils (EVOOs) obtained from five Turkish olive cultivars widely produced in the Aegean and Marmara regions were investigated based on their total antioxidant capacity (TAC), total phenolic content (TPC), pigment contents, fatty acid (FA) profiles, phenolic compounds (PC), volatile compounds (VC), and sensory properties. The results showed that all properties of EVOO samples were significantly affected by the olive cultivar used. The pigment contents in Ayvalık (9.90 mg·kg−1) and Uslu (9.00 mg·kg−1) oils were higher than the others (p < 0.05). The greatest values for oleic acid (74.13%) and TPC (350.6 mg·kg−1) were observed in Gemlik and Domat oils, respectively (p < 0.05). Edincik oil showed the maximum hydroxytyrosol content (48.022 mg·kg−1) and TAC value (515.36 mg TE·kg−1) (p < 0.05). The Edincik, Domat, and Uslu oils were significantly not different for the total content of C6 compounds derived by lipoxygenase, which are the main volatiles responsible for the typical aroma of EVOOs (p > 0.05). Domat oil also exhibited the highest scores for bitterness and pungency perceptions (p < 0.05). The fruitiness scores of the oil samples (except for Ayvalık oil) were close to each other, even if they were statistically different (p < 0.05). Principal component analysis (PCA) indicated that the Ayvalık oil was separated from the others due to its poor-quality characteristics. As a result, it can be stated that Domat olive oil has better quality than the others.
1. Introduction
Extra virgin olive oil (EVOO) is obtained only via mechanical or physical processes from the olive fruit (Olea europaea L.) and is designated as the highest quality in the classification of olive oils (OO) [1]. The main popularity of EVOO comes from its pleasant flavor and various beneficial effects on health [2]. In particular, advances in healthy diets have increased the demand for olive oil globally from year to year.
Nowadays, some comprehensive components, especially the profiles of volatile, phenolic, and pigment constituents, are used in the quality assessment of EVOO. In recent years, these components have been widely used to determine the characterizations [3,4] and authentications [5,6] of monovarietal EVOOs. Volatile compounds (VC) in EVOO are mainly responsible for its typical flavor [4], while PCs are associated with their pharmacological effects on health [7]. VCs are mostly formed by lipoxygenase activity during the initial stage of EVOO extraction [8].
The positive effects of EVOO on health are mainly attributed to its fatty acid (FA) fractions and PC [8]. It is reported that PC in EVOO can both prevent the oxidation of low-density lipoprotein (LDL) and reduce its amount in blood plasma [8]. Therefore, the European Commission (EC, 2012) [9] allowed the health claim on the proposition of the European Food Safety Authority (EFSA) [10] that offers the consumption of an amount containing at least 5 mg of hydroxytyrosol and its derivatives per 20 g of EVOO. These molecules also play crucial roles in the gustatory perception and shelf life of EVOO [2]. Moreover, phenolic compounds contribute to prolonging the shelf life of the EVOO by increasing its oxidative stability [11].
Turkey is one of the countries where olive farming has been carried out for a long time. According to a hypothesis that is generally accepted, the origin of the wild olive tree is a certain region of Turkey (as known Anatolia or Minor Asya), and olive cultivation spread from Syria to Greece and other Mediterranean countries through this area, which includes the Aegean region [12,13]. Additionally, archaeological digs revealed an ancient OO mill dating from 600 BC in Urla, a county of Izmir Province located in this region [12]. In 2021, Turkey was the fourth-largest country in the world in terms of total OO production [14]. Almost half (48%) of its total annual olive production is obtained in the Aegean region [15].
Numerous comparative studies have been conducted on the main properties of different monovarietal EVOO. However, many of them have investigated only a certain class of components or routine quality characteristics of EVOO. Diraman et al. (2010) [16] studied the relationship between FA compositions and geographical origins of 103 Turkish OO samples. In another research, the influences of cultivar, harvest year, and geographical location on FA fractions in a total of 36 VOO samples produced from 18 various olive cultivars from different countries were also investigated by Hmida et al. (2022) [17]. Dogi et al. (2020) determined PC in virgin olive oil (VOO) of six different Albanian olive cultivars [18]. PC and VC in different Italian cultivars of EVOO were determined by Veneziani et al. (2018) [8]. Ilyasoglu et al. (2010) characterized the EVOO of two prominent cultivars of the Aegean region of Turkey based on their VC [19]. Moreover, Kaftan and Elmaci (2011) compared a total of 30 VOO from 2 varieties in terms of VC and legal quality parameters [20].
In the literature, studies on the comprehensive characterization and comparison of monovarietal Turkish EVOO are limited. Although there have been various studies on some features of the country EVOO, these mostly focused on only one of their component groups, such as PC, VC, and FA fractions. In this sense, this study aimed to determine and compare the VC, PC, and FA profiles, TAC, pigment contents, and sensory properties of the EVOO of Turkish olive cultivars widely grown in the Aegean and Marmara regions.
2. Results and Discussion
2.1. Quality Parameters
FFA, PV, K232, and K270 are the basic parameters used in the classification of olive oils. Among these, FFA is a measure of the enzymatic hydrolysis of lipids, while the others are indicators for determining the level of oxidation in lipids [21]. These features are mainly affected by the maturity degree of olives and extraction conditions used in the production of oils, as well as by the variety of olive [22,23]. The FFA, PV, K232, and K270 of the oils ranged from 0.05 ± 0.00 to 0.12 ± 0.01%, 3.50 ± 0.27 to 6.33 ± 0.72 meq O2·kg−1, 1.64 ± 0.01 to 1.99 ± 0.02, and 0.13 ± 0.00 to 0.18 ± 0.02, respectively (Table 1). Based on these ranges, all oil samples were classified as EVOO according to European Commission (EC,2003) [24] and International Olive Council (IOC, 2021) [25]. Additionally, the values observed for the quality parameters of the samples were in agreement with the previous findings on most of the EVOOs of both Turkish [26,27] and other countries [28,29].

Table 1.
Legal quality parameters and pigment contents of EVOOs from five Turkish olive cultivars.
2.2. Pigment Contents
The concentration of chlorophyll and carotenoid in OOs directly affects their color [30]. The OOs with a higher intensity of green color are generally preferred by consumers, as this color is associated with freshness and good quality [23]. The amount of chlorophyll in oils ranged from 1.84 ± 0.12 to 5.52 ± 0.01 mg·kg−1, while the carotenoid content varied from 1.67 ± 0.05 to 4.47 ± 0.02 mg·kg−1 (Table 1). Both chlorophylls and carotenoids contents of Ayvalık and Uslu oils were higher than those of other variety olive oils, while lower these contents were found in Domat and Gemlik oils (p < 0.05).
The amount of these pigments in OOs can vary widely depending on the primarily variety and harvest time [22] and also climatic conditions and processing methods [31]. Jolayemi et al. (2021) [32] reported that chlorophyll and carotenoid contents of a hundred samples of Turkish OOs from different olive cultivars obtained from five different production seasons varied from 0.6 to 5.6 and 0.6 to 3.3 mg·kg−1, respectively, as a wide range. The pigment contents in the current study also showed wide ranges. In a previous study by Jolayemi et al. (2016) [22], it was reported that ranges of chlorophylls and carotenoids in Memecik oils were 1.50–4.55 and 1.11–2.86 mg·kg−1 and in Ayvalık oils were 1.28–2.57 mg·kg−1 and 1.01–1.61 mg·kg−1, respectively. These values reported for Ayvalık oil are lower than those found for the same variety in the present study.
2.3. Fatty Acid Profile
Fatty acid compositions of the EVOO samples are given in Table 2. The amounts of fatty acid fractions in all samples fulfilled the legal ranges for EVOO stated in EC (2003) [24] and IOC (2021) [25]. The amounts of oleic acid in all samples were the highest, followed by palmitic, linoleic, and stearic acids. There were significant differences between these major fatty acid levels in the oil samples (p < 0.05). A rich oleic acid content in oils is not only desirable for a long shelf life concerning oxidative stability but is also necessary for health benefits [26]. In fact, the oleic/linoleic acid ratio can be used as an index to measure the stability of olive oil, with a high ratio indicating good oxidative stability for oils [33]. The value of this ratio for Gemlik oil (13.65) was clearly higher than that of the others, which ranged between 6.11(Uslu) and 7.41 (Ayvalık). Because of its higher oleic acid content, Gemlik oil also exhibited the highest MUFA (75.61 ± 0.03%), lowest PUFA content (6.10 ± 0.02) and thus showed a higher MUFA/PUFA ratio (12.4 ± 0.03). Palmitic acid was the main unsaturated fatty acid in all oils, with a range of 12.88 ± 0.01% (Edincik) to 14.58 ± 0.01% (Ayvalık).

Table 2.
Fatty acid profile (%) of EVOOs from five Turkish olive cultivars.
The composition of fatty acids in OOs is mainly affected by genetic variations, geographical conditions (locations, climate, latitude), ripening degree, season, and production conditions [32,34]. The amount of oleic acid in the Ayvalık oil of this study was in agreement with the results of Uluata et al. (2021) [26], who found a range between 52.78 ± 2.03% and 71.22 ± 1.12% for oleic acid levels in different eleven commercial EVOOs from the Ayvalık olive cultivar grown in the Aegean region. Jolayemi et al. (2021) [32] reported that MUFA, PUFA, and MUFA/PUFA values in a hundred OO samples obtained from 8 different olive cultivars (including Ayvalık) from various locations in Aegan region ranged from 66 to 76.5%, from 8.6 to 18.2%, and from 3.6 to 8.8, respectively, in agreement with the findings of this study. Another study on the relationship between the origin, harvest year, and fatty acid profile of Turkish VOOs (Diraman et al., 2010) [16] reported ranges for oleic, palmitic, linoleic, stearic, and linolenic acid percentages as 62.90–77.16, 9.62–18.97, 6.26–17.17, 1.42–3.54, and 0.37–1.00, respectively, in 103 VOOs from numerous olive cultivars, including the Ayvalık, Gemlik, and Domat varieties. These findings are consistent with those in this study. Similar results regarding fatty acid fractions have also been reported for EVOOs from different countries [33,35]. Consequently, the differences between fatty acid fractions in the EVOO samples may not only be due to the variety effect but also to other factors mentioned above.
2.4. Individual Phenolic Compounds and Total Phenolic Content (TPC)
The PCs in EVOO are phenolic alcohols (hydroxytyrosol, tyrosol) and their secoiridoid derivatives, phenolic acids, flavonoids, and lignans [12]. Among these, hydroxytyrosol, tyrosol, and their secoiridoid derivatives, and the lignans are responsible for the pungency and bitterness of EVOO [9,13]. The individual PC contents and TPC in EVOO samples are presented in Table 3. EVOO from Domat contained the highest TPC (350.6 ± 8.770 mg·kg−1), followed by Gemlik (321.905 ± 6.185 mg·kg−1), Edincik (294.090 ± 13.810 mg·kg−1), Uslu (291.145 ± 5.485 mg·kg−1), and Ayvalık (234.71 ± 1.490 mg·kg−1) olives. These values were higher than the TPC levels reported for five different EVOO obtained from the Chemlali variety (117.64 ± 1.23–151.7 ± 1.97 mg GAE·kg−1) grown in different locations [36]. TPC in the Ayvalık oils was close to that found by Uluata et al. (2021) [26], who reported that the TPC in EVOO from Ayvalık olives grown in a location close to that used in this study was 215.29 ± 1.55 mg GAE·kg−1. However, the TPC determined by these authors for EVOO from Uslu (114.22 ± 3.44 mg GAE·mg−1) was lower than that of this study. These differences could mostly be due to the variety [37], maturity [22], and growing conditions of olives [21].

Table 3.
Phenolic compounds (mg·kg−1) in EVOOs from five Turkish olive cultivars.
A total of 11 phenolic compounds were identified and quantified in the oil samples (Table 3). The concentrations of most of them were significantly affected by cultivars (p < 0.05). Many previous studies have also reported that the cultivar affects the phenolic compound content of OOs [27,29]. Phenolic acids were scarce in all oils in comparison with other phenolic groups, as found by previous studies [23,38]. Among the phenolic acids identified in samples, vanillic acid was the most abundant compound, with a range of 1.445 ± 0.220 (Uslu) to 1.991 ± 0.031 mg·kg−1 (Edincik), while caffeic acid had the lowest content (0.009 ± 0.001 to 0.184 ± 0.002 mg·kg−1). The phenolic acid contents determined in all samples are in agreement with those reported in many previous studies [35,39].
Hydroxytyrosol was the predominant PC in the samples, with an amount in the range of 11.621 ± 0.420 to 48.022 ± 1.186 mg·kg−1, except in Ayvalık oil, which contained the greatest amount for pinoresinol (15.247 ± 0.524 mg·kg−1) within the PC. The amount of hydroxytyrosol in Edincik oil (48.022 ± 1.186 mg·kg−1) was significantly higher than that in other oils (p < 0.05). The level of hydroxtyrosol in the EVOO from Ayvalık olive (11.621 ± 0.420 mg·kg−1) was greater than that reported by Jolayemi et al. (2016) [22] for nine EVOO samples obtained by three different malaxation temperatures (27, 37, and 47 °C) from Ayvalık cultivar harvested at three different times. Additionally, the amounts of hydroxytyrosol in their EVOO samples were lower than those of tyrosol, contradicting that of the analyzed oils. The tyrosol contents in all samples were similar to that in the EVOOs of six different Italian olive varieties reported in a previous study [8], whereas the hydroxtyrosol contents observed were higher than theirs. The hydroxytyrosol content in OOs of Moroccan, Spanish, and Tunisian cultivars was reported as 6.65, 14.34, and 22.57 mg GAE.kg−1, respectively, by Negro et al. (2019) [40]. On the other hand, Topi et al. (2020) [18] reported that the hydroxytyrosol contents in five Albanian OO samples were higher than that of tyrosol, similar to the findings of the present study. As is known, hydroxytyrosol and tryrosol are derived by enzymatic hydrolysis from oleuropein and ligstroside, respectively [41]. Additionally, it was reported that the antioxidant activity of hydroxytyrosol is better than that of tyrosol [42,43]. In fact, there was a high correlation between the hydroxytyrosol content and the TAC of the samples (Pearson’s coefficient = 0.979, p < 0.001), but tyrosol was not interestingly correlated with TAC (p > 0.05).
Regarding flavonoids, EVOOs from Domat and Gemlik olives provided higher apigenin contents compared to the other cultivars. Apigenin and luteolin concentrations in the oils ranged from 2.409 ± 0.030 (Edincik) to 12.824 ± 0.010 mg·kg−1 (Gemlik) and from 1.640 ± 0.089 (Edincik) to 2.658 ± 0.080 mg·kg−1 (Ayvalık), respectively. The apigenin contents of the five EVOOs were more than that of two fresh monovarietal EVOOs from Portuguese olive varieties (0.71–1.00 mg·kg−1) reported by Klisović et al. (2022) [44], but the luteolin contents were lower than theirs (3.05–3.73 mg·kg−1). Moreover, the value of luteolin found in the EVOO from Ayvalık (2.658 ± 0.080 mg·kg−1) was partially less than the range (3.65–5.24 mg·kg−1) reported by Jolayemi et al. (2016) [22] for EVOOs of the same variety produced under similar conditions concerning malaxation temperatures and maturity. The amounts of PC in OOs are affected by genetic as well as pre- and post-harvest factors [45]. In a previous study [27], the amount of apigenin in Ayvalık and Gemlik oils varied from 4.74 ± 0.49 to 6.71 ± 0.73 mg·kg−1 and from 1.00 ± 0.04 to 1.65 ± 0.11 mg·kg−1, respectively. These contents are lower than those of the oils from same cultivars investigated in the current study.
As a noticeable point, although the amounts of major secoiridoids (hydroxytyrosol and tyrosol derivatives) in the samples based on their HPLC peaks have not been quantified, it is estimated that most of the TPC measured by spectrophotometry consisted of amounts of these compounds [42]. Essentially, in many previous studies on OOs, TPC values calculated spectrophotometrically were lower than the sum of individual PCs identified by HPLC [29,46,47]. Because of this, it can be concluded that the EVOOs from the olive varieties (except for Ayvalık) fulfilled the value of PC required by the European Commission for the health claim [9], which requires at least 5 mg of hydroxytyrosol and its other secoiridoid derivatives in 20 g of OO or at least 250 mg in 1 kg of OO for these PC as an equivalent concentration.
2.5. Total Antioxidant Capacity (TAC)
Certain PCs in OOs, such as oleuropein derivatives, exhibit strong antioxidant activities based on their radical scavenging abilities, as well as some other useful biological functions. The antioxidant effects of these PCs also contribute to the extended shelf life of OOs against lipid oxidation in comparison with those of other vegetable oils [48]. The TAC of the sample in terms of 2,2-Diphenyl-1-picrylhydrazyl (DPPH) scavenging capacity are shown in Figure 1. The TAC of the Edincik, Domat, Uslu, Gemlik, and Ayvalık oils were 515.36 ± 25.62, 420.98 ± 11.36, 258.63 ± 6.37, 150.66 ± 8.49, and 137.91 ± 16.23 mg TE·kg−1. It can be seen that the TAC of Edincik and Domat oils were notably higher than those of the other three oils.
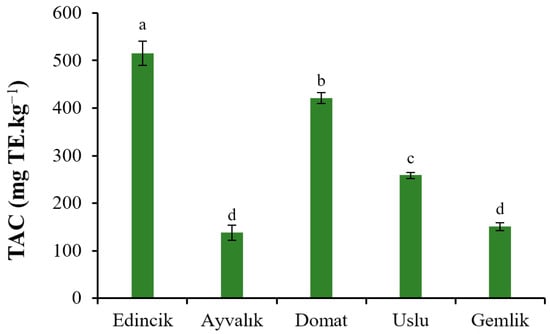
Figure 1.
TAC of EVOOs from five Turkish olive cultivars. Different letters on the bars indicate significant differences between different oil samples (p < 0.05).
In a recent study, Comlekcioglu et al. (2022) [37] reported that differences between the DPPH scavenging capacity of Gemlik (80 ± 3.6%), Ayvalık (75.92 ± 4.2%) and Domat olives (74.00 ± 3.3%) were not significant (p > 0.05), agreeing with the case between the EVOOs of Ayvalık and Gemklik olives. The TAC of Edincik and Ayvalık oils was higher than that reported by Uluata et al. (2021) [26], who found that the TACs (DPPH method) for ten different EVOOs of Ayvalık variety harvested from various locations of the Aegean region were between 58.25 ± 0.00 and 309.03 ± 14.02 mg TE·kg−1. They also reported the TAC of Uslu oil was 123.67 ± 6.23 mg TE·kg−1, lower than that of EVOO from the same variety investigated in this study. Previously, Borges et al. (2019) [49] studied the antioxidant properties (based on DPPH assay) of EVOOs from Hojiblanca and Arbequina olives and found values between 167.69 and 352.91 mg TE·kg−1 and 177.70 and 380.44 mg TE·kg−1, respectively, in agreement with those found in this study. Reboredo-Rodríguez et al. (2018) [50] found the TAC based on the DPPH scavenging activity of EVOO from Brava olives to be 577.91 mg TE·kg−1. This value is similar to the TAC of the Edincik oil but higher than that of oils from other varieties.
2.6. Volatile Compounds (VCs)
The flavor of OO is a remarkable feature to evaluate their quality and is associated with VCs. These VCs are mostly formed during oil extraction and also develop during harvest and storage processes. The positive attributes and organoleptic defects in sensory perception of OO are associated with VCs [51]. Table 4 shows the amount of each VC and their contents of chemical classes in samples. A total of 38 volatile aroma compounds were identified in the EVOO samples. The chemical classes of these VC were as follows: terpenoids (8), alcohols (10), aldehydes (11), esters (2), acids (2), and miscellaneous (5). Almost all of the VCs detected in the EVOO samples have also been reported in many previous studies on OOs. The total concentration of VC in Edincik (83.754 mg·kg−1), Domat (85.077 mg·kg−1), Uslu (87.987 mg·kg−1), and Gemlik (85.044 mg·kg−1) oils were not different significantly from each other (p > 0.05) but were higher than that of Ayvalık (52.368 mg·kg−1) oil (p < 0.05).

Table 4.
Composition of volatile compounds (mg·kg−1) in EVOOs from five Turkish olive cultivars.
The predominant VCs in the samples were C6 and C5 volatiles derived by lipoxygenase (LOX) pathway from linoleic and linolenic acids as unsaturated fatty acids. These VCs identified in oils were as follows: C6 aldehydes (hexanal, 3-hexenal, (E)-2-hexenal and 2,4-hexanedienal), C6 alcohols (1-hexanol, (Z)-3-hexen-1-ol and (E)-2-hexen-1-ol), C5 aldehydes (pentanal, (E)-2-pentenal) and C5 alcohols (1-penten-3-ol, 1-pentanol and (Z)-2-penten-1-ol). These compounds formed by LOX have been also reported previously as the most common volatiles in EVOO and the most responsible for their characteristic aroma [5,6,52]. In addition to these VCs, the two identified esters (hexyl acetate and (E)-3-hexen-1-ol acetate) can be formed sequentially by the LOX pathway. These esters are also associated with the typical green and fruity aroma of EVOO [53].
From the quantitative point of view, the total level of LOX group VC accounted for 65.87%, 21.01%, 70.49%, 57.34%, and 42.46% of the total concentration VC in Edincik, Ayvalık, Domat, Uslu, and Gemlik oils, respectively. Similar results were observed in most of the studies on the VCs of OOs. For instance, the study of Cecchi et al. (2022) [5] revealed that most of the VCs found in 320 VOO samples produced from 9 olive varieties from different geographical areas were compounds derived by LOX, as already reported in other previous papers [35,44]. It was stated that a rich C6 volatiles (both aldehydes and alcohols) content increases the intensity of the desirable green odor of OO [44,54]. The Edincik and the Domat oils had higher total amounts of C6 compounds (71.301 and 69.798 mg·kg−1, respectively) than those of the others, while the Ayvalık oil showed the lowest ones (36.886 mg·kg−1) (Figure 2). Similarly, Ilyasoglu et al. (2011) [19] showed that C6 volatiles were the most abundant VCs in both Ayvalık and Memecik EVOOs they studied.
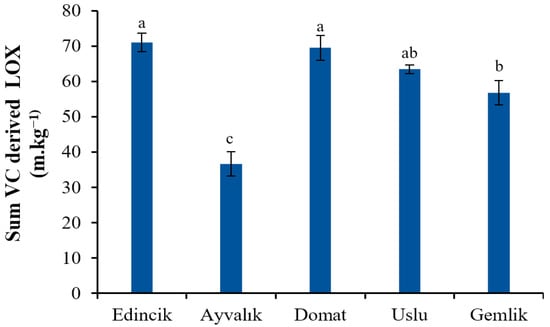
Figure 2.
Total amount of VC derived by LOX in EVOOs from five Turkish olive cultivars. Different letters on the bars indicate significant differences between different oil samples (p < 0.05).
Within C6 compounds, (E)-2-hexenal (green leaf- and almond-like) was the major compound found in all EVOO samples. (E)-2-hexenal and 3-hexenal have been reported to significantly influence the sensory properties of OO, providing its freshness and pleasant aroma [5,55]. (E)-2-hexenal only represented approximately 58%, 18%, 64%, 51% and 40% of the total VC content of Edincik, Ayvalık, Domat, Uslu and Gemlik oils, respectively. Similar percentages of this volatile compound were also obtained by several studies for EVOOs from different countries, such as Croatia [44], Portugal [55], Tunisia [35], Turkey [56], and some others [5]. On the contrary, in a study conducted by Baccouri et al. (2022) [46], (E)-2-hexenal was not found in 2 out of 5 VOO samples from Tunisia.
The amount of 1-penten-3-ol, as an aroma-active compound (grassy, green plants), was higher in Gemlik oil (15.681 mg·kg−1) than that of others (ranging from 0 to 8.797 mg·kg−1). In a previous study [57], the amount of this compound in Gemlik oil was also found to be higher than that of Ayvalık oil, as found in this study. 5-Ethyl-2(5H)-furanone (tomato-like), probably derived by autoxidation from (Z)-3-hexenal [58], was not detected in Gemlik oils. C7-C10 aldehyde VCs, including nonanal and (E)-2-decenal, were found in low amounts in the samples. These compounds are responsible for sensory defects in OOs [53].
It is reported that the effect of variety plays a key role in the variability of VCs in OOs [53]. However, the harvest time [59], conditions during and after the extraction process (i.e., temperature and time) [22,56], agronomic factors [21], planting area or geographical origin [46], and selected method of analysis [3,6] also affect the distribution of VC in OOs.
2.7. Sensory Attributes
The results of the sensory evaluation of EVOO samples are depicted in Figure 3. The sensory attributes of all EVOO were found to be typical for EVOO (fruitiness medians > 0; three defects medians = 0) category (IOC, 2018) [60]. The highest intensity of fruitiness was determined in the EVOO of the Uslu variety, whereas that of the Ayvalık variety was characterized by the lowest intensity of the three positive sensations (p < 0.05). The highest scores for the perception for bitterness and pungency were observed for Domat oil (p < 0.05). Concerning the relationships between the positive attributes, as expected, all three perceptions strongly correlated with each other (Pearson’s coefficients ranged between 0.845 and 0.910, p < 0.001). Furthermore, significant correlations were found between the positive attributes and both TPC and the total concentration of the VC group synthesized by the LOX pathway. These correlations are supported by the findings of Klisović et al. (2022) [44]. Similarly, Caporale et al. (2004) [61] also reported a linear relationship between TPC and bitterness for OOs. The pungency scores of samples were highly correlated with their TPC (Pearson’s coefficients = 0.910, p < 0.001) and the total concentration of VC derived by LOX (Pearson’s coefficients = 0.935, p < 0.001). Additionally, there was also a good correlation between these VC contents (total) and fruitiness (Pearson’s coefficients = 0.647, p < 0.05). These results are in agreement with those discussed in several previous studies [4,17,44]. It was reported that the bitter taste in OOs is attributable to compounds oleuropein aglycone forms, while their pungency is associated with ligstroside aglycone compounds [17].

Figure 3.
Scores of sensory attributes of EVOOs from five Turkish olive cultivars.
2.8. Principal Component Analysis (PCA)
PCA was applied to all data sets (except for sensory properties) to visually reveal the relationships between the variables and the samples. The results of PCA are illustrated in Figure 4. The first three principal components (PCs) accounted for 75.50% of the total variance of the data set. PC1, PC2, and PC3 explained 28.90%, 23.89%, and 21.81% of the total variance, respectively. In PCA, the first five PCs with eigenvalues greater than 1 were obtained. However, only the first three were used for its evaluation as they could explain most of the total variance. The factor loading values and eigenvalues are given in the supplementary material (SM) (Table S1).
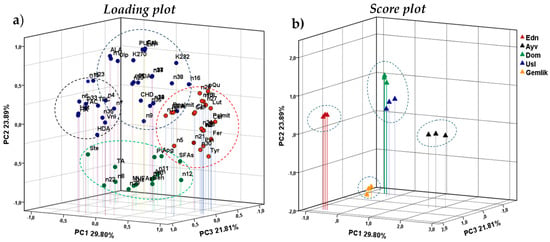
Figure 4.
Loading plot (a) and score plot (b) of the three first components of PCA based on analyzed parameters of samples. Abbreviations: Edn, Edincik; Ayv, Ayvalık; Dom, Domat; Usl, Uslu; FFA, Free fatty acid; MUFA, Monounsaturated fatty acid; PUFA, Polyunsaturated fatty acid; SFA, Saturated fatty acid; PV, Peroxide value; TAC, Total antioxidant capacity; TPC, Total phenolic content; ALA, linolenic acid; PDA, Pentadecanoic acid; Palmit, Palmitic acid; Dpalmit, 9-Hexadecenoic acid; HDA, Heptadecanoic acid, CHD, 10-Heptadecanoic acid, Ste, Stearic acid; Ole, Oleic acid; Lin, Linoleic Acid, Ach, Eicosanoic acid; CEA, 11-Eicosenoic acid; HA, Heneicosanoic acid; Beh, Docosanoic acid; n, Compound numbered from 1 to 38 in Table 4. The components/properties in the red, green, and black cycles in the Loading plot contributed to the separation of Ayvalık, Gemlik and Edincik oils, respectively, while those in the blue circle contributed to the separation of Domat and Uslu oils from others.
Figure 4 shows that PC1 differentiates the Ayvalık oil from the oils of other varieties according to higher VC (β-sesquiphellandrene, pyran aldehyde, α-bergamotene, (E)-3-hexen-1-ol acetate, hexyl acetate, etc.) and PC (tyrosol, t-ferulic acid, caffeic acid, pinoresinol, etc.) contents (Figure 4a,b). Similarly, this oil separated from the other samples due to its lower TPC and (E)-2-hexenal concentration. Although Uslu, Domat, and Edincik oils separated from the other samples along PC2, the first two are located closer to each other on the score plot because of their similar chemical compositions, and the latter (Edincik) differs by PC3. Edincik oil is characterized predominantly by higher hydroxtyrosol, heptadecanoic acid, α-muurolene, decanoic acid, and heneicosanoic acid. Gemlik oil discriminated from other samples along PC2 mainly based on its lower linoleic acid PUFAs contents and higher oleic acid, 3-hexenal, MUFAs, and 1-penten-3-ol contents.
3. Materials and Methods
3.1. Samples
The samples of monovarietal EVOO used were obtained from olive cultivars of Aegean and Marmara regions (Turkey): Edincik (Edincik, Marmara), Ayvalık (Ayvalık, Marmara), Domat (Akhisar, Aegean), Uslu (Akhisar, Aegean) and Gemlik (synonym Trilye) (Gemlik, Marmara) (Figure 5) The morphological and quality characteristics [62] and phenotypes [63] of the olive varieties are given in the SM (Table S2 and Figure S1). About 300 kg of olive fruits of each cultivar were harvested in the last week of October of the crop season 2020/2021. Their maturities indices were between 1.0 and 1.2, (yellow-green) as reported by IOC (2011) [64]. These olive fruits of cultivars were processed separately with a two-phase system (MORI-TEM srl 1000 3GV 400, Florence, Italia) within 8 h after harvest to obtain the EVOO samples. The temperature and time of malaxation were 25 ± 1 °C and 30 min, respectively. After the extraction, 1 L of each EVOO was stored in dark glass bottles (100 mL) at −20 °C until analysis. For each olive cultivar used, oil extraction and all analyses were performed in triplicate.
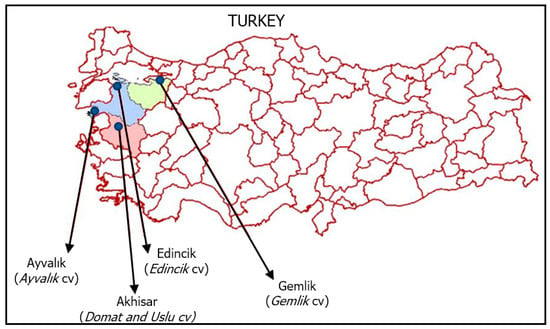
Figure 5.
The geographical distribution of olive cultivars used for oil samples.
3.2. Chemicals
Sodium hydroxide, sodium carbonate, potassium chromate, potassium iodide, phosphoric acid, methanol, ethanol, acetic acid (glacial), diethyl ether, phenolphthalein, chloroform, sodium thiosulfate, cyclohexane, acetonitrile, Folin–Ciocalteu reagent and standards of oleuropein, hydroxytyrosol, tyrosol, pinoresinol, p-qumaric acid, caffeic acid, syringic acid, vanillic acid, ferulic acid, gallic acid, luteolin, apigenin, isobutyl acetate, DPPH, Trolox and fatty acid methyl esters (FAMEs) mixture were purchased from Sigma-Aldrich (Darmstadt, Germany).
3.3. Quality Parameters Analysis
The FFA, PV, and extinction coefficients (K270 and K270) of the samples were determined according to the Turkish Official Methods (2014) [65]. FFA and PV were expressed as % oleic acid and meq O2·kg−1, respectively.
3.4. Extraction of Phenolic Compounds (PC)
The extraction of PC in the samples was carried out based on the method of IOC (2017) [66], as described by Rodrigues et al. (2019) [67], with slight modifications. Measures of 3 g of EVOO and 250 µL of syringic acid solution (0.15 mg·mL−1) prepared in methanol:water (80:20, v.v−1) were mixed and shaken in a 12 mL tube. Then, 3 mL of methanol:water was added and vortexed for 30 s. Thereafter, this mixture was centrifuged at 500 rmp at 4 °C for 5 min. The lower phase was transferred to another tube and the extraction was repeated two more times. Then, the collected methanolic phases were washed with 1.5 hexane two times to remove the oil residues. The lower phase was taken and used as the phenolic extract for the analysis of TPC, TAC, and PC.
3.5. Total Chlorophyll and Carotenoid Content Analysis
The total amounts of chlorophyll and carotenoid in samples were determined according to the method of Mínguez-Mosquera et al. (1991) [30]. Briefly, 7.5 g of EVOO was weighed in a tube and the volume was adjusted to 25 mL with cyclohexane, followed by vortexing for one minute. Then, the absorbances of this mixture were measured with a Biochrom Libra S70 Dual UV–vis spectrophotometer (Harvard Bioscience Co. Shanghai, China) at 470 nm and 670 nm against cyclohexane for chlorophylls and carotenoids, respectively. The total chlorophyll and carotenoid contents were calculated using the following Equations (1) and (2) and expressed as mg·kg−1 of pheophytin and lutein, respectively.
where Abs470 and Abs670 are the absorbance values read at these wavelengths, and d is the length of the optical path (1 cm).
Carotenoids = (Abs470 × 106) / (2000 × 100 × d)
Chlorophyl = (Abs670 × 106) / (613 × 100 × d)
3.6. Total Phenolic Content (TPC) Analysis
The TPCs of samples were determined by using the Folin–Ciocalteu method, as adopted by Capanoglu et al. (2013) [68]. Measures of 100 µL of the phenolic extract, 900 µL of deionized water, and 5 mL of Folin–Ciocalteu reagent (0.2 N) were mixed in a tube and kept for 8 min. Then, 5 mL of sodium carbonate was added and vortexed for 30 s. This mixture was left in the dark at the room temperature for 2 h and then its absorbance was measured with a UV–vis spectrophotometer (Biochrom Libra S70 Dual) at 765 nm. The results were calculated from a calibration curve created with different solutions of gallic acid as standard and expressed as mg gallic acid equivalent (GAE) per kg of samples.
3.7. Total Antioxidant Capacity (TAC) Analysis
The TAC of the samples was determined using the DPPH radical scavenging capacity of the metabolic extracts based on the method reported by Osei et al. (2022) [69], with some modifications. A measure of 3 mL of 60 µM DPPH in methanol was added to 0.5 mL of the phenolic extract and the resulting mixture was incubated in dark for 30 min at room temperature. Then, the absorbance of this solution was recorded at 517 nm against methanol as the blank using a UV–vis spectrophotometer (Biochrom Libra S70 Dual). For the control sample, a methanol:water solution (80:20 v/v) was used instead of the phenolic extract. The percentage of DPPH inhibition of each phenolic extract of samples was calculated following Equation (3):
where Abscontrol and Abssample were absorbances recorded at 517 nm for the control and phenolic extract of the sample, respectively.
DPPH inhibition (%) = (Abscontrol−Abssample) / (Abscontrol)
The value of IC50 of each sample, which corresponds to the concentration of extract reducing half of the amount of DPPH radical, was calculated from the regression curve obtained using five different phenolic extract solutions diluted by methanol:water solution. Trolox was used as standard and the results were expressed as mg Trolox equivalent (TE) per kg sample.
3.8. Fatty Acid Composition Analysis
The analysis of FA composition in samples was carried out by a gas chromatography and flame ionization detector (GC-FID) system (Shimadzu QP2020, Shimadzu Corp., Kyoto, Japan) equipped with an Rtx-2330 capillary column (0.20 µm, 60 m × 0.25 mm, Restek, Bad Homburg, Germany) [65]. Approximately 0.1 g of oil sample was added to 10 mL of hexane and shaken vigorously. To obtain FAMEs, 0.5 mL of the solution of potassium hydroxide (2N) in methanol was added to this mixture and vortexed for 20 s. After holding in the dark for 2 h, 1 µL of this solution was injected into the GC with a split mode (1:100). The temperatures of the injection port and detector were set at 250 °C. The oven temperature was first set at 140 for 5 min. Then, it was increased to 240 °C at a rate of 4 °C/min and maintained at isotherm for 12 min. The carrier gas was helium at a flow rate of 1 mL/min. The peak identifications and calculation of their areas as relative percentages were performed by using the mixture standards of FAMEs.
3.9. Phenolic Compounds (PC) Analysis
The analysis of phenolic fractions in samples was carried out using a Water Alliance e2695 HPLC (Waters, Milford, MA, USA) system, consisting of a photodiode array detector (PDA) (Waters 2996, Milford, MA, USA) and an inertSustain C18 (5 µm, 4.6 × 250 mm, GL Sciences, Tokyo, Japan). The phenolic extract was filtered through a 0.45 μm polyvinylidine fluoride (PVDF) syringe filter before the injection into the system. The operational procedures of the HPLC were performed as described by Veneziani et al. (2018) [8], with some modifications. The results are expressed as mg·kg−1. Details of the analysis are presented in the SM.
3.10. Volatile Compound (VC) Analysis
The VCs in samples were isolated by solid phase micro-extraction (SPME) and analyzed with a gas chromatography-mass detector (GC-MS) (Shimadzu QP2020, Shimadzu Corp., Kyoto, Japan) system coupled to an autosampler (AOC 5000 Plus, CTC, Switzerland), according to Genovese et al. (2015) [54] and Korkmaz et al. (2020) [70], with some modifications. Details of the analysis are presented in SM.
3.11. Sensory Analysis
The sensory properties of samples were evaluated by a trained sensory panel of ten assessors from the Central Laboratory of Mardin Artuklu University (Mardin, Turkey) according to the Turkish Official Methods (2014) [65] adapted from the procedure of IOC (2018) [60]. The quantitative intensity of positive attributes (fruitiness, bitterness, and pungency) and defects (musty, fusty, winey–vinegary) were determined by marking the scale from 0 (no perception) to 10 (the highest intensity) cm on the original profile sheet of the method used. The results were expressed as median values of assessor perception scores.
3.12. Statistical Analysis
The significance of differences among the values of all parameters of samples was determined by one-way analysis of variance (ANOVA) followed by Duncan’s multi-comparison test (p < 0.05). Principal component analysis (PCA) as a multivariate analysis was also performed to compare the sample based on their investigated properties. All statistical analyses were carried out using the SPSS (version 16.0, Chicago, IL, USA) software package.
4. Conclusions
The monovarietal EVOOS from five different Turkish olive varieties were characterized in terms of their FA, PC, and VC profiles as well as TPC, TAC, pigment contents, and sensory properties. Additionally, the EVOO samples were compared with each other based on these properties. There were significant differences between the major components in FA, PC, and VC profiles of the oil samples. The highest TAC was obtained for the EVOO from the Edincik variety, possibly due to it having the highest hydroxytyrosol content. The Domat oil exhibited the highest TPC and, therefore, probably also had higher scores for bitterness and pungency. The scores obtained for the fruitiness perceptions of the oils (except for the Ayvalık oil) were close to each other. This was also confirmed by the relationship between their total content of VCs derived from LOX. The result of PCA showed that the EVOO from Ayvalık olives is distinct from the oils from other varieties due to its lower values for many quality attributes. Based on this study, it can be concluded that the EVOO from Domat olives had the best characteristics sensory quality parameters and chemical properties affecting them. In the future, studies should be conducted on the storage stability of these oils.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/molecules28031483/s1, Table S1: Rotated factor loadings, eigenvalues and variances explained of the three first principal components; Table S2: Morphological and quality characteristics of the olive plant/fruit variety used in the study; Figure S1: Fruits, leaves and seeds of olive varieties used.
Funding
This research was funded by the Ministry of Industry and Technology of Republic of the Turkey-Southeastern Anatolia Project (GAP) Regional Development Administration and Derik (Mardin) Municipality (Grant number MAÜ-GAP-20-SBF-005).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Acknowledgments
I thank Birsen Can Pehlivan for her support in obtaining the olive cultivar and EVOO samples used in this study. I also thank Ahmet Ferit Atasoy and Tuğba Aslan for their help with the analysis of regal parameters and the spectrophotometric measurements within the scope of the related project.
Conflicts of Interest
The author declares no conflict of interest.
Sample Availability
Samples of the compounds are not available from the author.
References
- European Commission (EC). Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/PDF/?uri=CELEX:01991R2568-20161204&from=EN (accessed on 15 November 2022).
- Marx, Í.M.G.; Casal, S.; Rodrigues, N.; Pinho, T.; Veloso, A.C.A.; Pereira, J.A.; Peres, A.M. Impact of the Malaxation Temperature on the Phenolic Profile of Cv. Cobrançosa Olive Oils and Assessment of the Related Health Claim. Food Chem. 2021, 337, 127726. [Google Scholar] [CrossRef] [PubMed]
- Ríos-Reina, R.; Aparicio-Ruiz, R.; Morales, M.T.; García-González, D.L. Contribution of Specific Volatile Markers to Green and Ripe Fruity Attributes in Extra Virgin Olive Oils Studied with Three Analytical Methods. Food Chem. 2023, 399, 133942. [Google Scholar] [CrossRef]
- Žanetić, M.; Jukić Špika, M.; Ožić, M.M.; Brkić Bubola, K. Comparative Study of Volatile Compounds and Sensory Characteristics of Dalmatian Monovarietal Virgin Olive Oils. Plants 2021, 10, 1995. [Google Scholar] [CrossRef]
- Cecchi, L.; Migliorini, M.; Giambanelli, E.; Cane, A.; Zanoni, B.; Canuti, V.; Mulinacci, N.; Melani, F. Is the Volatile Compounds Profile a Suitable Tool for Authentication of Virgin Olive Oils (Olea Europaea L.) according to Cultivars? a Study by Using HS-SPME-GC-MS and Chemometrics. Food Control 2022, 139, 109092. [Google Scholar] [CrossRef]
- Spadafora, N.D.; Mascrez, S.; McGregor, L.; Purcaro, G. Exploring Multiple-Cumulative Trapping Solid-Phase Microextraction Coupled to Gas Chromatography-Mass Spectrometry for Quality and Authenticity Assessment of Olive Oil. Food Chem. 2022, 383, 132438. [Google Scholar] [CrossRef] [PubMed]
- Visioli, F.; Davalos, A.; López de las Hazas, M.; Crespo, M.C.; Tomé-Carneiro, J. An overview of the Pharmacology of Olive Oil and Its Active Ingredients. Br. J. Pharmacol. 2020, 177, 1316–1330. [Google Scholar] [CrossRef]
- Veneziani, G.; Esposto, S.; Taticchi, A.; Urbani, S.; Selvaggini, R.; Sordini, B.; Servili, M. Characterization of Phenolic and Volatile Composition of Extra Virgin Olive Oil Extracted from Six Italian Cultivars Using a Cooling Treatment of Olive Paste. LWT 2018, 87, 523–528. [Google Scholar] [CrossRef]
- European Commission (EC). Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/PDF/?uri=CELEX:32012R0432&from=EN (accessed on 10 December 2022).
- European Food Safety Authority (EFSA). Available online: https://efsa.onlinelibrary.wiley.com/doi/epdf/10.2903/j.efsa.2012.2848 (accessed on 10 October 2022).
- Anna, R.; Russo, M.; Cacciola, F.; Salafia, F.İ.; Polkowska, Z.A.; Dugo, P.; Mondello, L. Concentration of Potentially Bioactive Compounds in Italian Extra Virgin Olive Oils from Various Sources by Using LC-MS and Multivariate Data Analysis. Foods 2020, 9, 1120. [Google Scholar] [CrossRef]
- Uylaşer, V.; Yildiz, G. The Historical Development and Nutritional Importance of Olive and Olive Oil Constituted an Important Part of the Mediterranean Diet. Crit. Rev. Food Sci. Nutr. 2014, 54, 1092–1101. [Google Scholar] [CrossRef] [PubMed]
- International Olive Council (IOC). The Olive Tree. Available online: https://www.internationaloliveoil.org/olive-world/olive-tree/ (accessed on 7 December 2022).
- International Olive Oil Council (IOC). Available online: http://www.internationaloliveoil.org/estaticos/view/131-world-olive-oil-figures (accessed on 6 December 2022).
- Turkish Statistical Institute (TUIK). Available online: https://biruni.tuik.gov.tr/medas/?kn=92&locale=tr (accessed on 8 July 2022).
- Diraman, H.; Saygi, H.; Hisil, Y. Relationship between Geographical Origin and Fatty Acid Composition of Turkish Virgin Olive Oils for Two Harvest Years. JAOCS 2010, 87, 781–789. [Google Scholar] [CrossRef]
- Ben Hmida, R.; Gargouri, B.; Chtourou, F.; Sevim, D.; Bouaziz, M. Fatty Acid and Triacyglycerid As Markers of Virgin Olive Oil from Mediterranean Region: Traceability and Chemometric Authentication. Eur. Food Res. Technol. 2022, 248, 1749–1764. [Google Scholar] [CrossRef]
- Topi, D.; Guclu, G.; Kelebek, H.; Selli, S. Comparative Elucidation of Phenolic Compounds in Albanian Olive Oils Using LC-DAD-ESI-MS/MS. J. Liq. Chromatogr. Relat. Technol. 2020, 43, 203–212. [Google Scholar] [CrossRef]
- Ilyasoglu, H.; Ozcelik, B.; Van Hoed, V.; Verhe, R. Cultivar Characterization of Aegean Olive Oils with Respect to Their Volatile Compounds. Sci. Hortic. 2011, 129, 279–282. [Google Scholar] [CrossRef]
- Kaftan, A.; Elmaci, Y. Aroma Characterization of Virgin Olive Oil from Two Turkish Olive Varieties by SPME/GC/MS. Int. J. Food Prop. 2011, 14, 1160–1169. [Google Scholar] [CrossRef]
- Stefanoudaki, E.; Williams, M.; Chartzoulakis, K.; Harwood, J. Effect of Irrigation on Quality Attributes of Olive Oil. J. Agric. Food Chem. 2009, 57, 7048–7055. [Google Scholar] [CrossRef]
- Jolayemi, O.S.; Tokatli, F.; Ozen, B. Effects of Malaxation Temperature and Harvest Time on the Chemical Characteristics of Olive Oils. Food Chem. 2016, 211, 776–783. [Google Scholar] [CrossRef]
- Yu, L.; Wang, Y.; Wu, G.; Jin, J.; Jin, Q.; Wang, X. Chemical and volatile characteristics of olive oils extracted from four varieties grown in southwest of China. Int. Food Res. J. 2021, 140, 109987. [Google Scholar] [CrossRef]
- European Commission (EC). Available online: https://eur-lex.europa.eu/LexUriServ/LexUriServ.do?uri=OJ:L:2003:295:0057:0077:en:PDF (accessed on 11 December 2022).
- International Olive Council (IOC). Available online: https://www.internationaloliveoil.org/wp-content/uploads/2021/11/COI-T15-NC3-REV-17_ENK.pdf (accessed on 8 October 2022).
- Uluata, S.; Altuntaş, U.; Özçelik, B. Characterization of Turkish Extra Virgin Olive Oils and Classification Based on Their Growth Regions Coupled with Multivariate Analysis. Food Anal. Methods 2021, 14, 1682–1694. [Google Scholar] [CrossRef]
- Kelebek, H.; Kesen, S.; Selli, S. Comparative Study of Bioactive Constituents in Turkish Olive Oils by LC-ESI/MS/MS. Int. J. Food Prop. 2015, 18, 2231–2245. [Google Scholar] [CrossRef]
- Rodrigues, N.; Peres, F.; Casal, S.; Santamaria-Echart, A.; Barreiro, F.; Peres, A.M. Alberto Pereira Geographical Discrimination of Olive Oils from Cv. ‘Galega Vulgar’. Food Chem. 2023, 398, 133945. [Google Scholar] [CrossRef]
- Antonini, E.; Farina, A.; Leone, A.; Mazzara, E.; Urbani, S.; Selvaggini, R.; Servili, M.; Ninfali, P. Phenolic Compounds and Quality Parameters of Family Farming Versus Protected Designation of Origin (PDO) Extra-Virgin Olive Oils. J. Food Compos. Anal. 2015, 43, 75–81. [Google Scholar] [CrossRef]
- Isabel Minguez-Mosquera, M.; Rejano-Navarro, L.; Gandul-Rojas, B.; SanchezGomez, A.H.; Garrido-Fernandez, J. Color-Pigment Correlation in Virgin Olive Oil. JAOCS 1991, 68, 332–336. [Google Scholar] [CrossRef]
- Ramos-Escudero, F.; Morales, M.T.; Asuero, A.G. Characterization of Bioactive Compounds from Monovarietal Virgin Olive Oils: Relationship between Phenolic Compounds-Antioxidant Capacities. Int. J. Food Prop. 2015, 18, 348–358. [Google Scholar] [CrossRef]
- Jolayemi, O.S.; Tokatli, F.; Ozen, B. UV-Vis Spectroscopy for the Estimation of Variety and Chemical Parameters of Olive Oils. J. Food Meas. Charact. 2021, 15, 4138–4149. [Google Scholar] [CrossRef]
- Yang, Y.; Ferro, M.D.; Cavaco, I.; Liang, Y. Detection and Identification of Extra Virgin Olive Oil Adulteration by GC-MS Combined with Chemometrics. J. Agric. Food Chem. 2013, 61, 3693–3702. [Google Scholar] [CrossRef]
- Haddada, F.M.; Krichène, D.; Manai, H.; Oueslati, I.; Daoud, D.; Zarrouk, M. Analytical Evaluation of Six Monovarietal Virgin Olive Oils from Northern Tunisia. Eur. J. Lipid Sci. Technol 2008, 110, 905–913. [Google Scholar] [CrossRef]
- Chtourou, F.; Valli, E.; Ben Mansour, A.; Bendini, A.; Gallina Toschi, T.; Bouaziz, M. Characterization of Virgin Olive Oils Obtained from Minor Tunisian Varieties for Their Valorization. J. Food Meas. Charact. 2021, 15, 5060–5070. [Google Scholar] [CrossRef]
- Gargouri, B.; Ammar, S.; Zribi, A.; Mansour, A.B.; Bouaziz, M. Effect of Growing Region on Quality Characteristics and Phenolic Compounds of Chemlali Extra-Virgin Olive Oils. Acta Physiol. Plant. 2013, 35, 2801–2812. [Google Scholar] [CrossRef]
- Comlekcioglu, S.; Elgudayem, F.; Nogay, G.; Kafkas, N.E.; Ayed, R.B.; Ercisli, S.; Assouguem, A.; Almeer, R.; Najda, A. Biochemical Characterization of Six Traditional Olive Cultivars: A Comparative Study. Horticulture 2022, 8, 416. [Google Scholar] [CrossRef]
- Dorota, D.; Rupert, M.; Wołosiak, R.; Bzducha-Wróbel, A.; Ścibisz, I.; Matuszewska-Janica, A. Volatiles as Markers of Bioactive Components Found in Croatian Extra Virgin Olive Oils. LWT 2021, 139, 110532. [Google Scholar] [CrossRef]
- Kıvrak, Ş.; Kıvrak, İ. Ultrasonic-assisted Extraction Method of Phenolic Compounds in Extra-Virgin Olive Oils (EVOOs) by Ultra Performance Liquid Chromatography-Tandem Mass Spectrometry (UPLC–MS/MS). Sep. Sci. Technol. 2021, 56, 322–329. [Google Scholar] [CrossRef]
- Negro, C.; Aprile, A.; Luvisi, A.; Nicolì, F.; Nutricati, E.; Vergine, M.; Miceli, A.; Blando, F.; Sabella, E.; De Bellis, L. Phenolic Profile and Antioxidant Activity of Italian Monovarietal Extra Virgin Olive Oils. Antioxidants 2019, 8, 161. [Google Scholar] [CrossRef] [PubMed]
- Taticchi, A.; Esposto, S.; Veneziani, G.; Urbani, S.; Selvaggini, R.; Servili, M. The Influence of the Malaxation Temperature on the Activity of Polyphenoloxidase and Peroxidase and on the Phenolic Composition of Virgin Olive Oil. Food Chem. 2013, 136, 975–983. [Google Scholar] [CrossRef] [PubMed]
- Servili, M.; Selvaggini, R.; Esposto, S.; Taticchi, A.; Montedoro, G.; Morozzi, G. Health and sensory Properties of Virgin Olive Oil Hydrophilic Phenols: Agronomic and Technological Aspects of Production That Affect Their Occurrence in The Oil. J. Chromatogr. A 2004, 1054, 113–127. [Google Scholar] [CrossRef]
- Bayram, B.; Esatbeyoglu, T.; Schulze, N.; Ozcelik, B.; Frank, J.; Rimbach, G. Comprehensive Analysis of Polyphenols in 55 Extra Virgin Olive Oils by HPLC-ECD and Their Correlation with Antioxidant Activities. Plant Foods Hum. Nutr. 2012, 67, 326–336. [Google Scholar] [CrossRef]
- Klisović, D.; Novoselić, A.; Lukić, I.; Brkić Bubola, K. Extra Virgin Olive Oil under Simulated Consumption Conditions: Evaluation of Quality, Health, and Flavour Properties. J. Food Compos. Anal. 2022, 110, 104570. [Google Scholar] [CrossRef]
- Esposto, S.; Taticchi, A.; Servili, M.; Urbani, S.; Sordini, B.; Veneziani, G.; Daidone, L.; Selvaggini, R. Overall Quality Evolution of Extra Virgin Olive Oil Exposed to Light for 10 Months in Different Containers. Food Chem. 2021, 351, 129297. [Google Scholar] [CrossRef]
- Baccouri, B.; Rajhi, I.; Zarrouk, M. Bioactive compounds and Oxidative Stability of Feral Olive Oils from Tunisian Amazigh Mountains using LC-ESI-QTOF-MS Approach for the Development of Innovative Food Products. Eur. Food Res. Technol. 2022, 248, 2843–2855. [Google Scholar] [CrossRef]
- Stefanoudaki, E.; Williams, M.; Harwood, J. Changes in Virgin Olive Oil Characteristics during Different Storage Conditions. Eur. J. Lipid Sci. Technol. 2010, 112, 906–914. [Google Scholar] [CrossRef]
- Bendini, A.; Cerretani, L.; Carrasco-Pancorbo, A.; Gómez-Caravaca, A.; Segura-Carretero, A.; Fernández-Gutiérrez, A.; Lercker, G. Phenolic Molecules in Virgin Olive Oils: A Survey of Their Sensory Properties, Health Effects, Antioxidant Activity and Analytical Methods. An Overview of the Last Decade Alessandra. Molecules 2007, 12, 1679–1719. [Google Scholar] [CrossRef]
- Borges, T.H.; Serna, A.; López, L.C.; Lara, L.; Nieto, R.; Seiquer, I. Composition and Antioxidant Properties of Spanish Extra Virgin Olive Oil regarding Cultivar, Harvest Year and Crop Stage. Antioxidants 2019, 8, 217. [Google Scholar] [CrossRef]
- Reboredo-Rodríguez, P.; González-Barreiro, C.; Cancho-Grande, B.; Forbes-Hernández, T.Y.; Gasparrini, M.; Afrin, S.; Cianciosi, D.; Carrasco-Pancorbo, A.; Simal-Gándara, J.; Giampieri, F.; et al. Characterization of Phenolic Extracts from Brava Extra Virgin Olive Oils and Their Cytotoxic Effects on MCF-7 Breast Cancer Cells. Food Chem. Toxicol. 2018, 119, 73–85. [Google Scholar] [CrossRef]
- Kalua, C.M.; Allen, M.S.; Bedgood, D.R.; Bishop, A.G.; Prenzler, P.D.; Robards, K. Olive Oil Volatile Compounds, Flavour Development and Quality: A Critical Review. Food Chem. 2007, 100, 273–286. [Google Scholar] [CrossRef]
- Veneziani, G.; Esposto, S.; Taticchi, A.; Selvaggini, R.; Urbani, S.; Di Maio, I.; Sordini, B.; Servili, M. Flash Thermal Conditioning of Olive Pastes during the Oil Mechanical Extraction Process: Cultivar Impact on the Phenolic and Volatile Composition of Virgin Olive Oil. J. Agric. Food Chem. 2015, 63, 6066–6074. [Google Scholar] [CrossRef]
- Tomé-Rodríguez, S.; Ledesma-Escobar, C.A.; Penco-Valenzuela, J.M.; Priego-Capote, F. Cultivar Influence on the Volatile Components of Olive Oil Formed in the Lipoxygenase Pathway. LWT 2021, 147, 111485. [Google Scholar] [CrossRef]
- Genovese, A.; Caporaso, N.; Sacchi, R. Temporal Changes of Virgin Olive Oil Volatile Compounds in a Model System Simulating Domestic Consumption: The Role of Biophenols. Int. Food Res. J. 2015, 77, 670–674. [Google Scholar] [CrossRef]
- Marx, Í.M.G.; Casal, S.; Rodrigues, N.; Cruz, R.; Peres, F.; Veloso, A.C.A.; Pereira, J.A.; Peres, A.M. Impact of fresh Olive Leaves Addition during the Extraction of Arbequina Virgin Olive Oils on the Phenolic and Volatile Profiles. Food Chem. 2022, 393, 133327. [Google Scholar] [CrossRef]
- Ergönül, P.G.; Aydar, A.Y.; Göldeli, T.; Mentana, A.; Quinto, M. Changes in Volatile Compounds of Ayvalık (Edremit) and Uslu Olive Oils Depending on Conditions and Time of Storage. Ukr. Food J. 2021, 10, 717–735. [Google Scholar] [CrossRef]
- Kesen, S.; Kelebek, H.; Sen, K.; Ulas, M.; Selli, S. GC-MS-Olfactometric Characterization of the Key Aroma Compounds in Turkish Olive Oils by Application of the Aroma Extract Dilution Analysis. Int. Food Res. J. 2013, 54, 1987–1994. [Google Scholar] [CrossRef]
- Cecchi, T.; Alfei, B. Volatile Profiles of Italian Monovarietal Extra Virgin Olive Oils Via HS-SPME-GC-MS: Newly Identified Compounds, Flavors Molecular Markers, and Terpenic Profile. Food Chem. 2013, 141, 2025–2035. [Google Scholar] [CrossRef]
- Karagoz, S.G.; Yilmazer, M.; Ozkan, G.; Carbonell-Barrachina, Á.A.; Kiralan, M.; Ramadan, M.F. Effect of Cultivar and Harvest Time on C6 and C5 Volatile Compounds of Turkish Olive Oils. Eur. Food Res. Technol. 2017, 243, 1193–1200. [Google Scholar] [CrossRef]
- International Olive Council (IOC). Available online: https://www.internationaloliveoil.org/wp-content/uploads/2019/11/COI-T20-Doc.-15-REV-10-2018-Eng.pdf (accessed on 15 October 2022).
- Caporale, G.; Policastro, S.; Monteleone, E. Bitterness Enhancement Induced by Cut Grass Odorant (cis-3-hexen-1-ol) in a Model Olive Oil. Food Qual. Prefer. 2004, 15, 219–227. [Google Scholar] [CrossRef]
- Republic of Turkey Ministry of Agriculture and Forestry. Turkey Olive Varieties Catalog; Olive Research Institute: İzmir, Turkey, 2015; pp. 5–56. [Google Scholar]
- Republic of Turkey Ministry of Agriculture and Forestry. Available online: https://www.tarimorman.gov.tr/BUGEM/kumelenme/Belgeler/Budama/Zeytinde%20%C3%87e%C5%9Fit%20Tan%C4%B1lama.pdf (accessed on 28 January 2023).
- International Olive Council (IOC). Available online: https://www.internationaloliveoil.org/wp-content/uploads/2019/11/COI-OH-Doc.-1-2011-Eng.pdf (accessed on 27 October 2021).
- Turkish Official Gazette. Available online: https://www.resmigazete.gov.tr/eskiler/2014/11/20141120-21.htm (accessed on 20 October 2021).
- International Olive Council (IOC). Available online: https://www.internationaloliveoil.org/wp-content/uploads/2022/06/Doc.-No-29-REV-2_ENK.pdf (accessed on 15 December 2021).
- Rodrigues, N.; Casal, S.; Pinho, T.; Peres, A.M.; Bento, A.; Baptista, P.; Pereira, J.A. Ancient Olive Trees as a Source of Olive Oils Rich in Phenolic Compounds. Food Chem. 2019, 276, 231–239. [Google Scholar] [CrossRef]
- Capanoglu, E.; de Vos, R.C.H.; Hall, R.D.; Boyacioglu, D.; Beekwilder, J. Changes in polyphenol Content during Production of Grape Juice Concentrate. Food Chem. 2013, 139, 521–526. [Google Scholar] [CrossRef]
- Osei, J.B.D.; Amiri, A.; Wang, J.; Tavares, M.T.; Kiatkittipong, W.; Najdanovic-Visak, V. Recovery of oils and antioxidants from olive stones. Biomass Bioenergy 2022, 166, 106623. [Google Scholar] [CrossRef]
- Korkmaz, A.; Atasoy, A.F.; Hayaloglu, A.A. Changes in volatile Compounds, Sugars and Organic Acids of Different Spices of Peppers (Capsicum annuum L.) during Storage. Food Chem. 2020, 311, 125910. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).