Norcantharidin-Encapsulated C60-Modified Nanomicelles: A Potential Approach to Mitigate Cytotoxicity in Renal Cells and Simultaneously Enhance Anti-Tumor Activity in Hepatocellular Carcinoma Cells
Abstract
:1. Introduction
2. Results
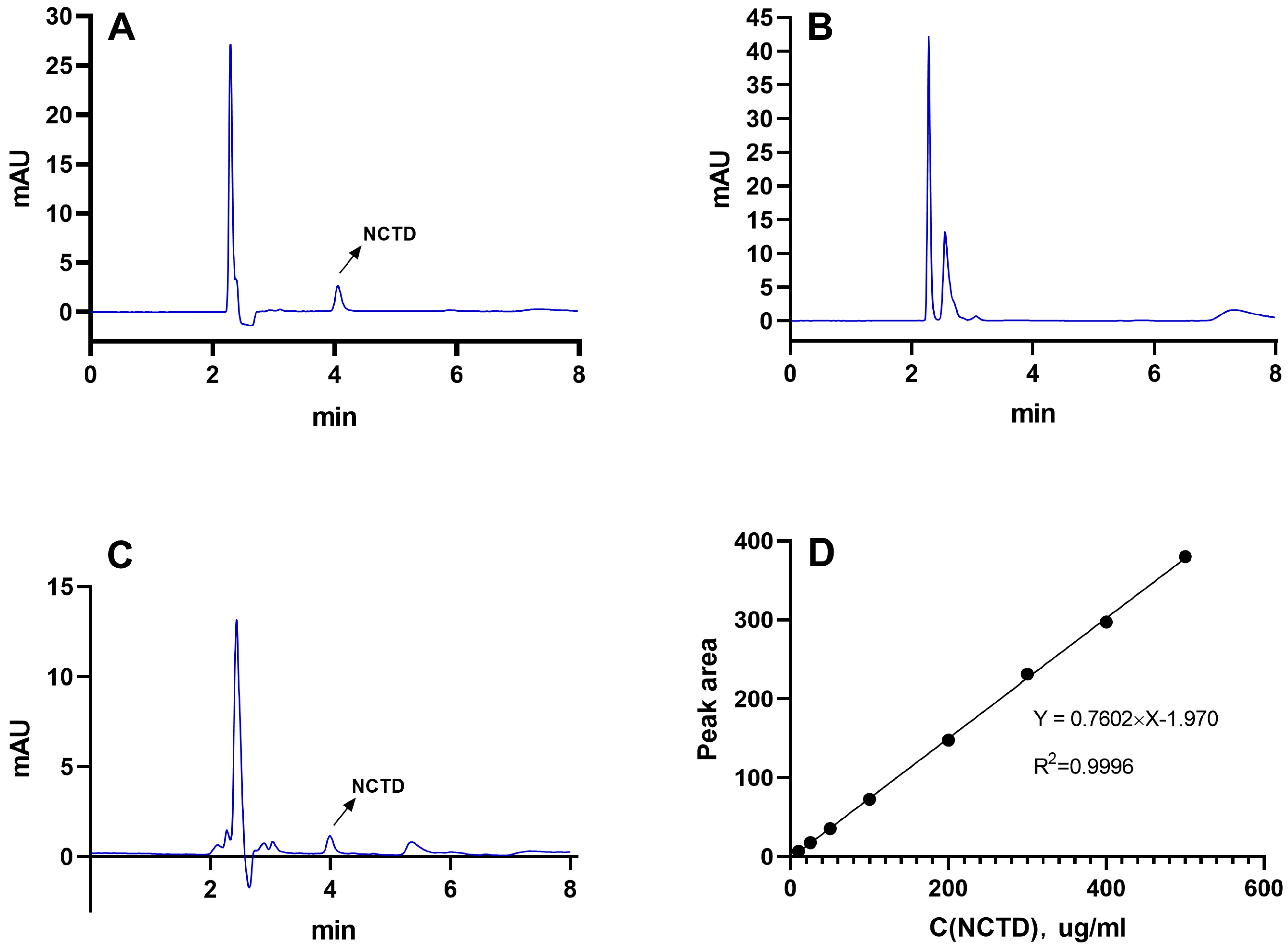
2.1. Determination of NCTD by HPLC
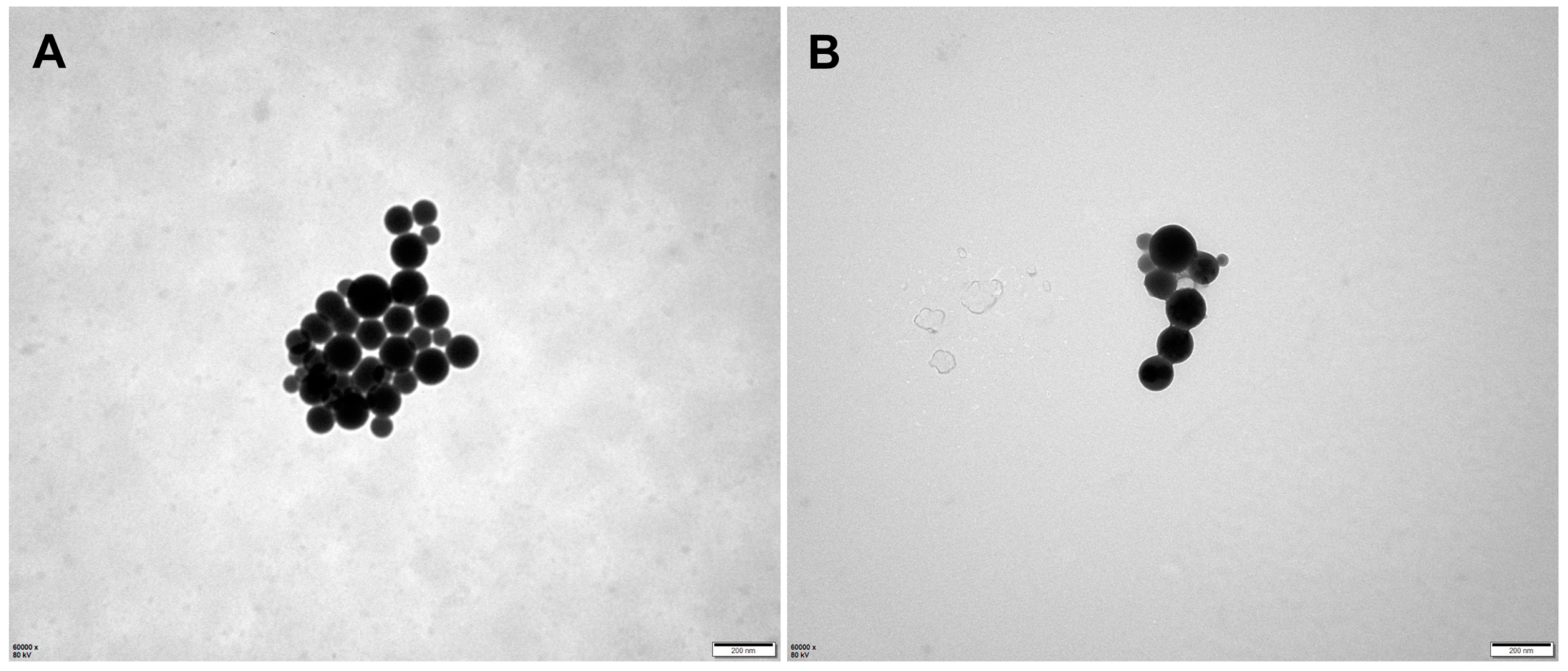
2.2. Preparation and Characterization of Micelles
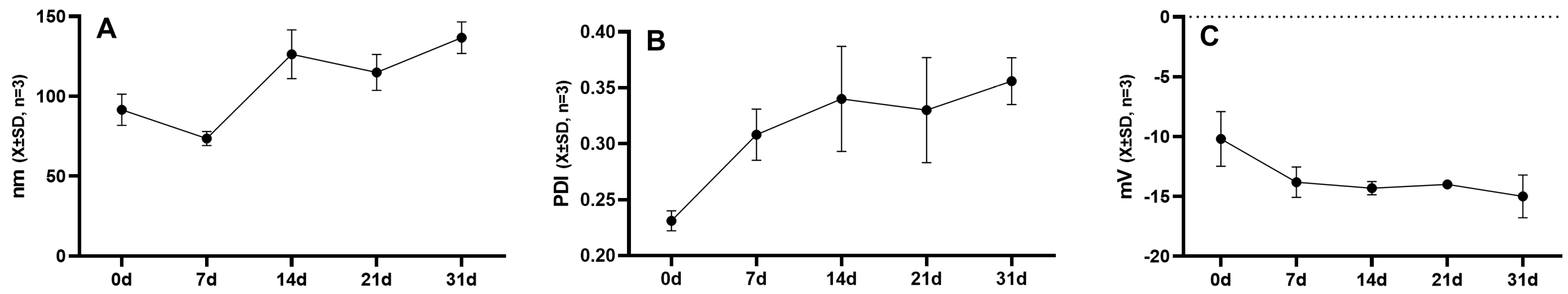
2.3. Stability of Micellar Solution
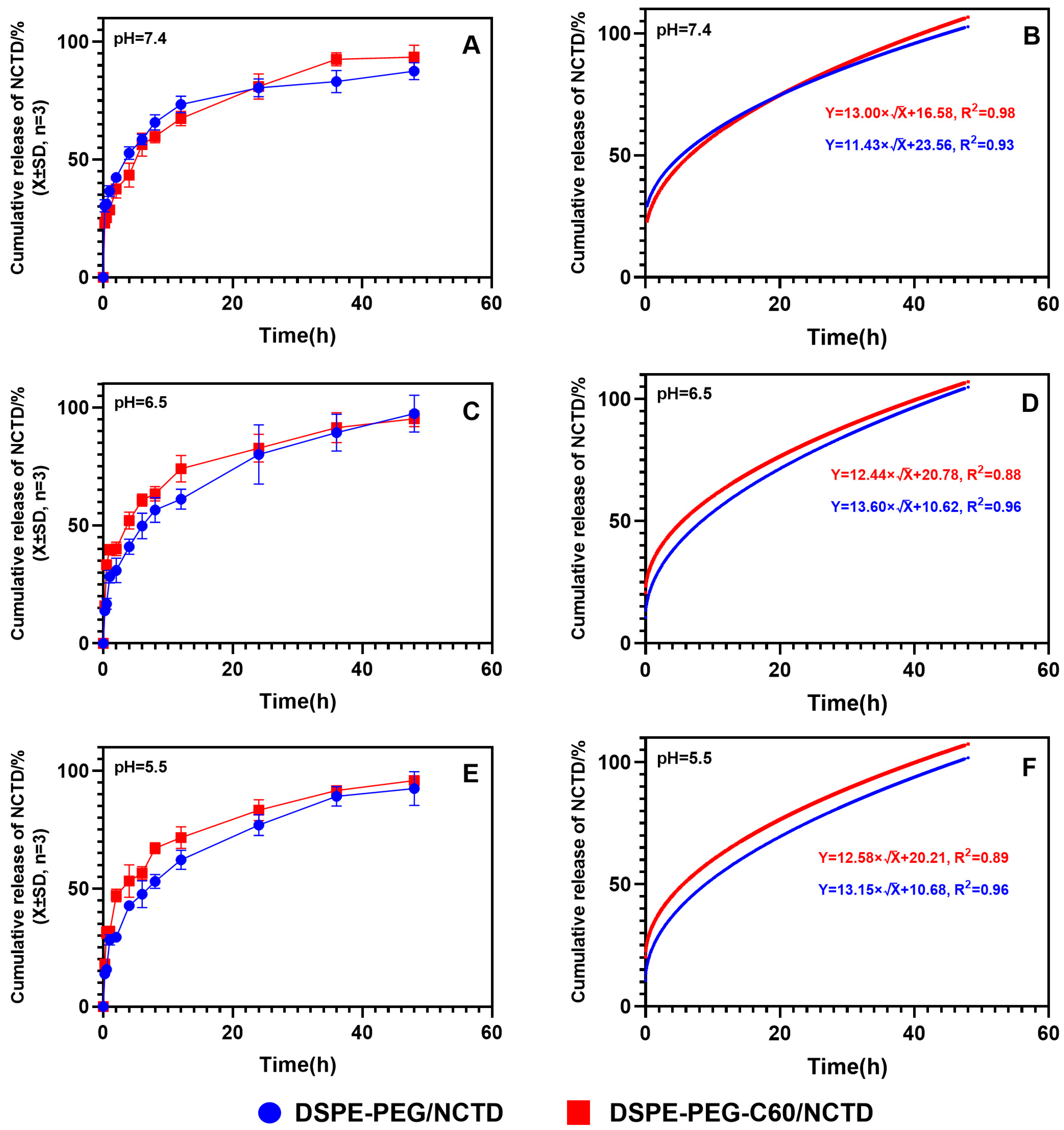
2.4. Drug Release Assay
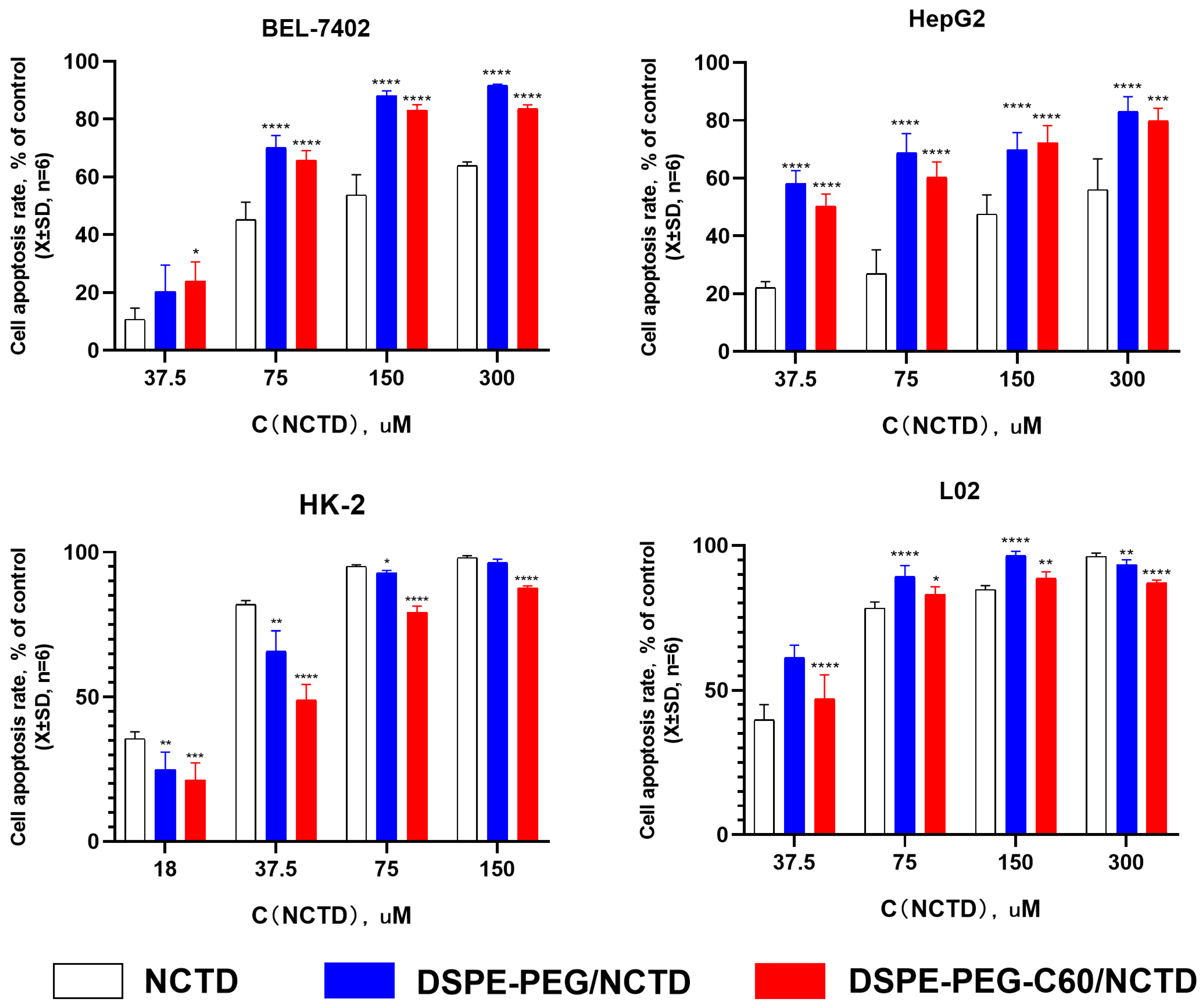
2.5. In Vitro Cytotoxicity Examination
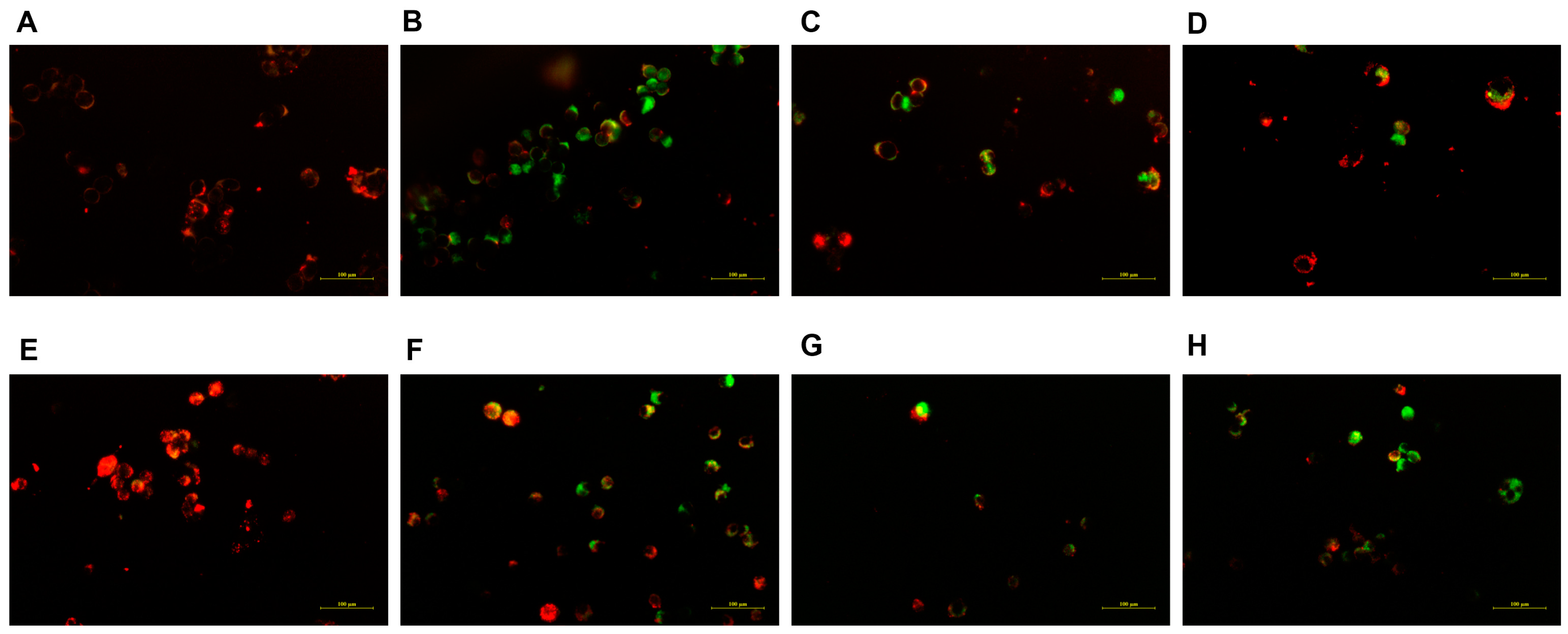
2.6. Cell Apoptosis Assay Using Acridine Orange/Ethidium Bromide (AO/EB) Staining
2.7. Mitochondrial Membrane Potential Assay
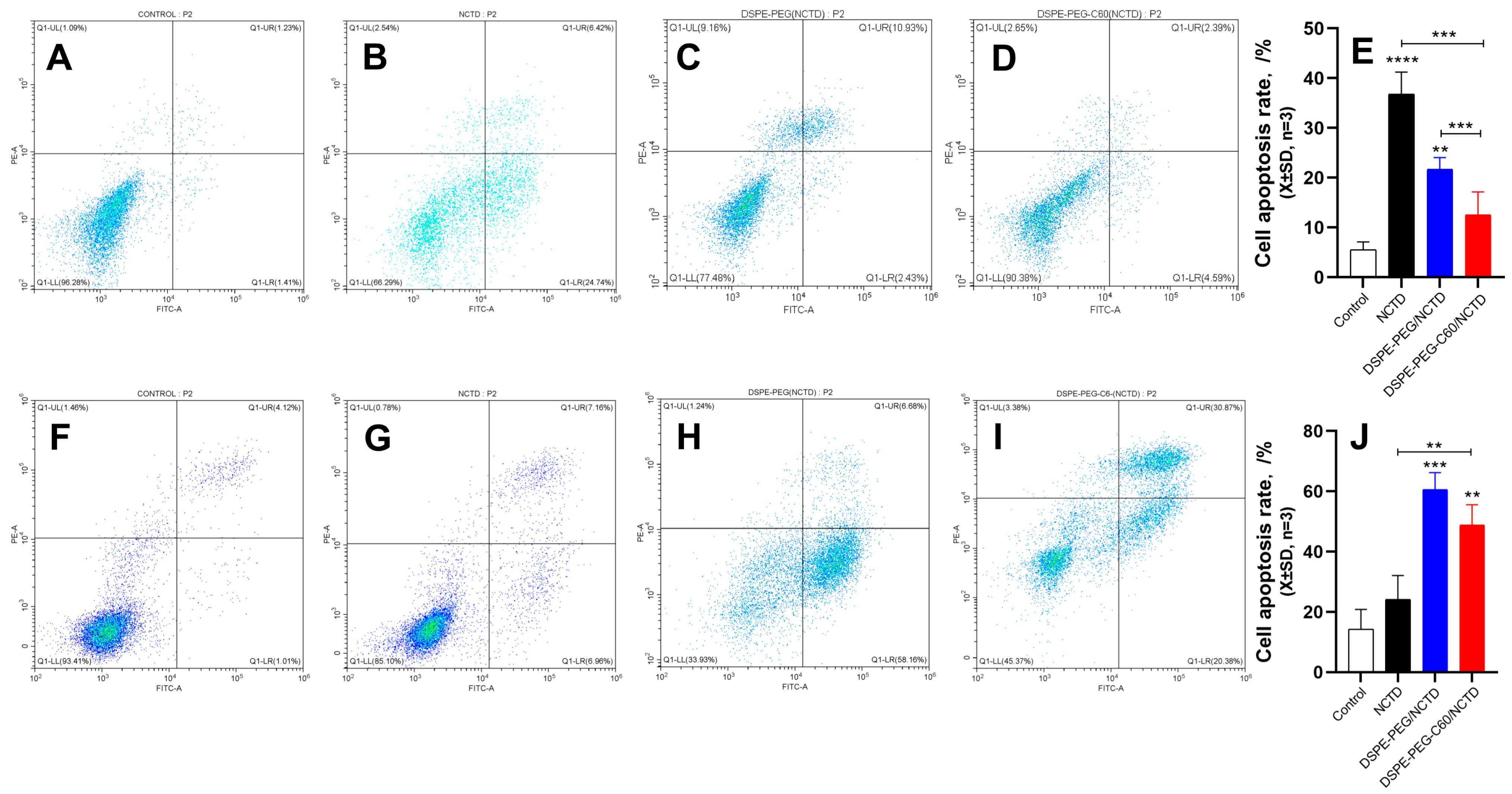
2.8. Cell Apoptosis by Flow Cytometry
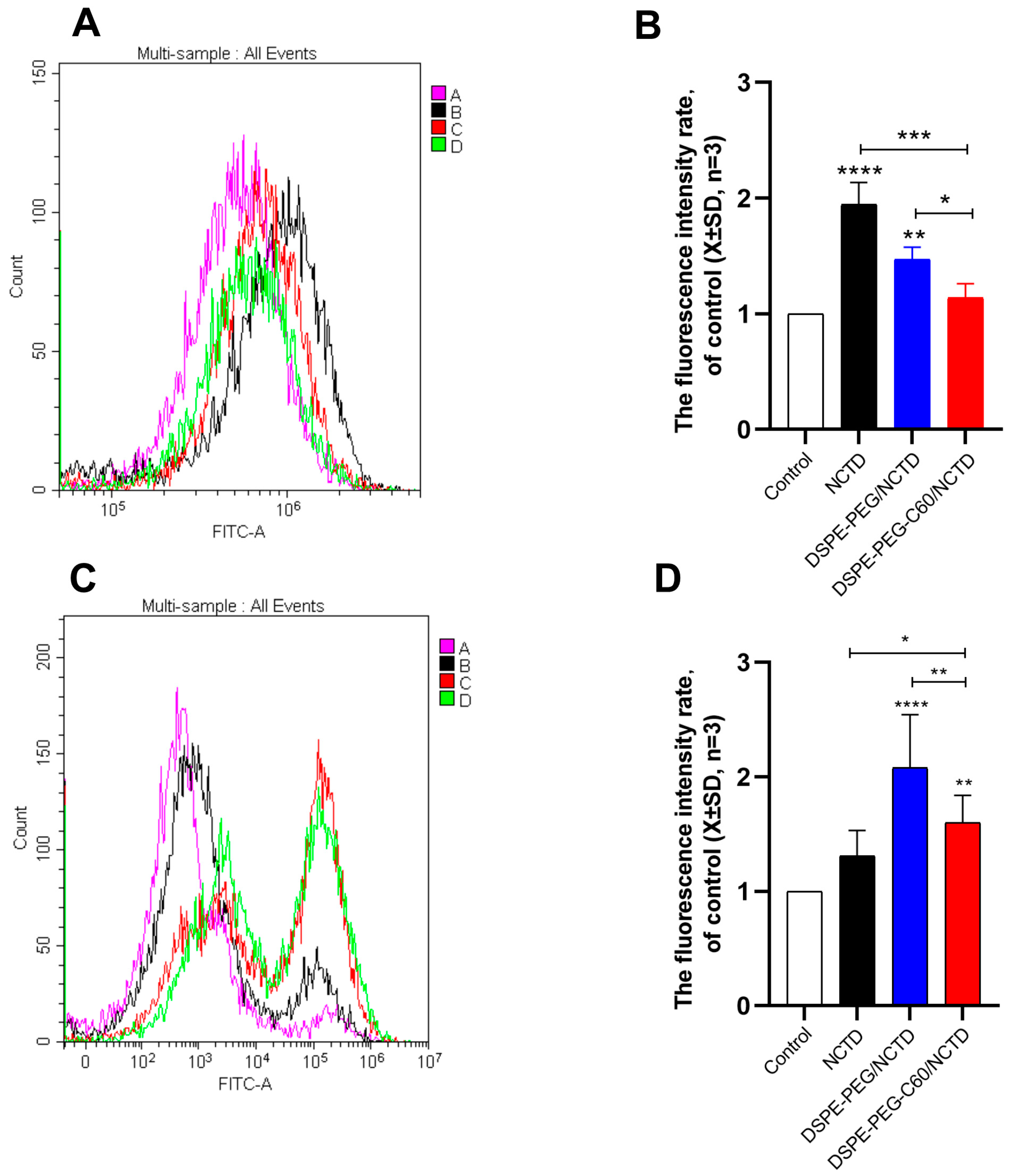
2.9. Intracellular ROS Level Evaluation
3. Discussion
4. Materials and Methods
4.1. Materials
4.2. Determination of NCTD Content
4.3. Synthesis of Fullerenol-Grafted Distearoyl Phosphatidylethanolamine—Polyethylene Glycol (DSPE-PEG-C60)
4.4. Preparation of Micelles
4.5. Characterization of Micelles
4.5.1. Morphology
4.5.2. Determination of Entrapment Efficiency
4.5.3. Stability Evaluation
4.5.4. In Vitro Drug Release Assay
4.6. Cell Lines and Cell Culture
4.6.1. Cell Viability Assay
4.6.2. Acridine Orange/Ethidium Bromide (AO/EB) Staining
4.6.3. JC-1 Staining
4.6.4. Apoptosis by Flow Cytometry
4.6.5. Intracellular ROS Detection
4.7. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| NCTD | Norcantharidin |
| CTD | Cantharidin |
| ROS | Reactive oxygen species |
| LD50 | Median lethal dose |
| DOX | Doxorubicin hydrochloride |
| EPR effect | Enhanced permeability and retention effect |
| DSPE-PEG2000 | 1,2-Distearoyl-sn-glycero-3-phosphoethanolamine-N-methoxy-[poly(ethylene glycol)]; PEG MW = 2000 |
| C60(OH)22 | Fullerenol |
| FDA | Food and Drug Administration |
| DSPE-PEG-C60 | Fullerenol-Grafted 1,2-Distearoyl-sn-glycero-3-phosphoethanolamine-N-methoxy-[poly(ethylene glycol)] |
| CCK-8 | Cell Counting Kit-8 |
| FITC | 3′,6′-dihydroxy-5-isothiocyanato-3H-spiro[isobenzofuran-1,9′-xanthen]-3-one |
| PI | Propidium iodide |
| HPLC | High- performance liquid chromatography |
| DMSO | Dimethyl sulfoxide |
| TEM | Transmission electron microscopy |
| DLS | Dynamic light scattering |
| LC | Loading content |
| EE | Entrapment efficiency |
| SDS | Sodium dodecyl sulfate |
| PBS | Phosphate-buffered saline |
| DCFDA | 2′,7′-Dichlorodihydrofluorescein diacetate |
| IC50 | Half-maximal inhibitory concentration |
| AO and ER | Acridine orange and ethidium bromide |
References
- Zhou, J.; Ren, Y.; Tan, L.; Song, X.; Wang, M.; Li, Y.; Cao, Z.; Guo, C. Norcantharidin: Research advances in pharmaceutical activities and derivatives in recent years. Biomed. Pharmacother. 2020, 131, 110755. [Google Scholar] [CrossRef] [PubMed]
- Pan, M.S.; Cao, J.; Fan, Y.Z. Insight into norcantharidin, a small-molecule synthetic compound with potential multi-target anticancer activities. Chin. Med. 2020, 15, 55. [Google Scholar] [CrossRef]
- Hsieh, C.H.; Chao, K.S.; Liao, H.F.; Chen, Y.J. Norcantharidin, derivative of cantharidin, for cancer stem cells. Evid.-Based Complement. Altern. Med. 2013, 2013, 838651. [Google Scholar] [CrossRef] [PubMed]
- Singh, R.; Cheng, S.; Li, J.; Kumar, S.; Zeng, Q.; Zeng, Q. Norcantharidin combined with 2-deoxy-d-glucose suppresses the hepatocellular carcinoma cells proliferation and migration. 3 Biotech 2021, 11, 142. [Google Scholar] [CrossRef] [PubMed]
- Zhai, B.T.; Sun, J.; Shi, Y.J.; Zhang, X.F.; Zou, J.B.; Cheng, J.X.; Fan, Y.; Guo, D.Y.; Tian, H. Review targeted drug delivery systems for norcantharidin in cancer therapy. J. Nanobiotechnol. 2022, 20, 509. [Google Scholar] [CrossRef]
- Martínez-Razo, G.; Domínguez-López, M.L.; de la Rosa, J.M.; Fabila-Bustos, D.A.; Reyes-Maldonado, E.; Conde-Vázquez, E.; Vega-López, A. Norcantharidin toxicity profile: An in vivo murine study. Naunyn Schmiedebergs Arch. Pharmacol. 2023, 396, 99–108. [Google Scholar] [CrossRef]
- Chi, J.; Jiang, Z.; Chen, X.; Peng, Y.; Liu, W.; Han, B.; Han, B. Studies on anti-hepatocarcinoma effect, pharmacokinetics and tissue distribution of carboxymethyl chitosan based norcantharidin conjugates. Carbohydr. Polym. 2019, 226, 115297. [Google Scholar] [CrossRef]
- Andrade, E.B.; Martinez, A. Free radical scavenger properties of metal-fullerenes: C-60 and C-82 with Cu, Ag and Au (atoms and tetramers). Comput. Theor. Chem. 2017, 1115, 127–135. [Google Scholar] [CrossRef]
- Kazemzadeh, H.; Mozafari, M. Fullerene-based delivery systems. Drug Discov. Today 2019, 24, 898–905. [Google Scholar] [CrossRef]
- Kepinska, M.; Kizek, R.; Milnerowicz, H. Fullerene as a doxorubicin nanotransporter for targeted breast cancer therapy: Capillary electrophoresis analysis. Electrophoresis 2018, 39, 2370–2379. [Google Scholar] [CrossRef]
- Mashino, T. Development of Bio-active Fullerene Derivatives Suitable for Drug. Yakugaku Zasshi 2022, 142, 165–179. [Google Scholar] [CrossRef]
- Injac, R.; Boskovic, M.; Perse, M.; Koprivec-Furlan, E.; Cerar, A.; Djordjevic, A.; Strukelj, B. Acute doxorubicin nephrotoxicity in rats with malignant neoplasm can be successfully treated with fullerenol C60(OH)24 via suppression of oxidative stress. Pharmacol. Rep. 2008, 60, 742–749. [Google Scholar] [PubMed]
- Injac, R.; Perse, M.; Cerne, M.; Potocnik, N.; Radic, N.; Govedarica, B.; Djordjevic, A.; Cerar, A.; Strukelj, B. Protective effects of fullerenol C60(OH)24 against doxorubicin-induced cardiotoxicity and hepatotoxicity in rats with colorectal cancer. Biomaterials 2009, 30, 1184–1196. [Google Scholar] [CrossRef]
- Petrovic, D.; Seke, M.; Borovic, M.L.; Jovic, D.; Borisev, I.; Srdjenovic, B.; Rakocevic, Z.; Pavlovic, V.; Djordjevic, A. Hepatoprotective effect of fullerenol/doxorubicin nanocomposite in acute treatment of healthy rats. Exp. Mol. Pathol. 2018, 104, 199–211. [Google Scholar] [CrossRef] [PubMed]
- Tang, J.; Zhang, R.; Guo, M.; Shao, L.; Liu, Y.; Zhao, Y.; Zhang, S.; Wu, Y.; Chen, C. Nucleosome-inspired nanocarrier obtains encapsulation efficiency enhancement and side effects reduction in chemotherapy by using fullerenol assembled with doxorubicin. Biomaterials 2018, 167, 205–215. [Google Scholar] [CrossRef]
- Ding, M.; Li, M.; Zhang, E.M.; Yang, H.L. FULLEROL alleviates myocardial ischemia-reperfusion injury by reducing inflammation and oxidative stress in cardiomyocytes via activating the Nrf2/HO-1 signaling pathway. Eur. Rev. Med. Pharmacol. Sci. 2020, 24, 9665–9674. [Google Scholar] [CrossRef] [PubMed]
- Xu, B.; Ding, Z.; Hu, Y.; Zhang, T.; Shi, S.; Yu, G.; Qi, X. Preparation and Evaluation of the Cytoprotective Activity of Micelles with DSPE-PEG-C60 as a Carrier Against Doxorubicin-Induced Cytotoxicity. Front. Pharmacol. 2022, 13, 952800. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, B.; Biswas, S. Polymeric micelles in cancer therapy: State of the art. J. Control. Release 2021, 332, 127–147. [Google Scholar] [CrossRef]
- Perumal, S.; Atchudan, R.; Lee, W. A Review of Polymeric Micelles and Their Applications. Polymers 2022, 14, 2510. [Google Scholar] [CrossRef]
- Gill, K.K.; Kaddoumi, A.; Nazzal, S. PEG-lipid micelles as drug carriers: Physiochemical attributes, formulation principles and biological implication. J. Drug Target. 2015, 23, 222–231. [Google Scholar] [CrossRef]
- Liang, H.; Ren, X.; Qian, J.; Zhang, X.; Meng, L.; Wang, X.; Li, L.; Fang, X.; Sha, X. Size-Shifting Micelle Nanoclusters Based on a Cross-Linked and pH-Sensitive Framework for Enhanced Tumor Targeting and Deep Penetration Features. ACS Appl. Mater. Interfaces 2016, 8, 10136–10146. [Google Scholar] [CrossRef] [PubMed]
- Mellor, R.D.; Uchegbu, I.F. Ultrasmall-in-Nano: Why Size Matters. Nanomaterials 2022, 12, 2476. [Google Scholar] [CrossRef] [PubMed]
- Yu, F.; Jiang, F.; Tang, X.; Wang, B. N-octyl-N-arginine-chitosan micelles for gambogic acid intravenous delivery: Characterization, cell uptake, pharmacokinetics, and biodistribution. Drug Dev. Ind. Pharm. 2018, 44, 615–623. [Google Scholar] [CrossRef] [PubMed]
- Song, Z.; Deng, P.; Teng, F.; Zhou, F.; Zhu, W.; Feng, R. Development on PEG-modified Poly (Amino Acid) Copolymeric Micelles for Delivery of Anticancer Drug. Anticancer Agents Med. Chem. 2017, 17, 784–801. [Google Scholar] [CrossRef]
- Al-Amili, M.; Jin, Z.; Wang, Z.; Guo, S. Self-Assembled Micelles of Amphiphilic PEGylated Drugs for Cancer Treatment. Curr. Drug Targets 2021, 22, 870–881. [Google Scholar] [CrossRef]
- Li, Y.; Zhang, T.; Liu, Q.; He, J. PEG-Derivatized Dual-Functional Nanomicelles for Improved Cancer Therapy. Front. Pharmacol. 2019, 10, 808. [Google Scholar] [CrossRef]
- Hwang, D.; Ramsey, J.D.; Kabanov, A.V. Polymeric micelles for the delivery of poorly soluble drugs: From nanoformulation to clinical approval. Adv. Drug Deliv. Rev. 2020, 156, 80–118. [Google Scholar] [CrossRef]
- Lee, K.S.; Chung, H.C.; Im, S.A.; Park, Y.H.; Kim, C.S.; Kim, S.B.; Rha, S.Y.; Lee, M.Y.; Ro, J. Multicenter phase II trial of Genexol-PM, a Cremophor-free, polymeric micelle formulation of paclitaxel, in patients with metastatic breast cancer. Breast Cancer Res. Treat. 2008, 108, 241–250. [Google Scholar] [CrossRef]
- Kumbar, V.M.; Muddapur, U.; Bin Muhsinah, A.; Alshehri, S.A.; Alshahrani, M.M.; Almazni, I.A.; Kugaji, M.S.; Bhat, K.; Peram, M.R.; Mahnashi, M.H.; et al. Curcumin-Encapsulated Nanomicelles Improve Cellular Uptake and Cytotoxicity in Cisplatin-Resistant Human Oral Cancer Cells. J. Funct. Biomater. 2022, 13, 158. [Google Scholar] [CrossRef]
- Kulthe, S.S.; Choudhari, Y.M.; Inamdar, N.N.; Mourya, V. Polymeric micelles: Authoritative aspects for drug delivery. Des. Monomers Polym. 2012, 15, 465–521. [Google Scholar] [CrossRef]
- Li, W.; Wu, J.; Zhang, J.; Wang, J.; Xiang, D.; Luo, S.; Li, J.; Liu, X. Puerarin-loaded PEG-PE micelles with enhanced anti-apoptotic effect and better pharmacokinetic profile. Drug Deliv. 2018, 25, 827–837. [Google Scholar] [CrossRef] [PubMed]
- Demina, T.; Grozdova, I.; Krylova, O.; Zhirnov, A.; Istratov, V.; Frey, H.; Kautz, H.; Melik-Nubarov, N. Relationship between the structure of amphiphilic copolymers and their ability to disturb lipid bilayers. Biochemistry 2005, 44, 4042–4054. [Google Scholar] [CrossRef] [PubMed]
- Barron, A.R. [60]Fullerene-peptides: Bio-nano conjugates with structural and chemical diversity. J. Enzym. Inhib. Med. Chem. 2016, 31, 164–176. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.W.; Yang, J.; Barron, A.R.; Monteiro-Riviere, N.A. Endocytic mechanisms and toxicity of a functionalized fullerene in human cells. Toxicol. Lett. 2009, 191, 149–157. [Google Scholar] [CrossRef] [PubMed]
- Ilinskaya, A.N.; Shah, A.; Enciso, A.E.; Chan, K.C.; Kaczmarczyk, J.A.; Blonder, J.; Simanek, E.E.; Dobrovolskaia, M.A. Nanoparticle physicochemical properties determine the activation of intracellular complement. Nanomed. Nanotechnol. Biol. Med. 2019, 17, 266–275. [Google Scholar] [CrossRef]
- Choi, K.Y.; Min, K.H.; Yoon, H.Y.; Kim, K.; Park, J.H.; Kwon, I.C.; Choi, K.; Jeong, S.Y. PEGylation of hyaluronic acid nanoparticles improves tumor targetability in vivo. Biomaterials 2011, 32, 1880–1889. [Google Scholar] [CrossRef]
- Kobayashi, H.; Choyke, P.L. Super enhanced permeability and retention (SUPR) effects in tumors following near infrared photoimmunotherapy. Nanoscale 2016, 8, 12504–12509. [Google Scholar] [CrossRef]
- Attia, M.F.; Anton, N.; Wallyn, J.; Omran, Z.; Vandamme, T.F. An overview of active and passive targeting strategies to improve the nanocarriers efficiency to tumour sites. J. Pharm. Pharmacol. 2019, 71, 1185–1198. [Google Scholar] [CrossRef]


| Batch | NCTD:DSPE-PEG-C60 (W/W) | Ultrasound Time | Ultrasound Power | Particle Size (nm) | PDI | Zeta Potential (mV) |
|---|---|---|---|---|---|---|
| 1 | 1:10 | 10 min | 200 W | 117.8 ± 1.041 | 0.375 ± 0.034 | −10.1 ± 0.49 |
| 2 | 1:15 | 10 min | 200 W | 91.57 ± 9.78 | 0.231 ± 0.01 | −13.8 ± 1.28 |
| 3 | 1:20 | 10 min | 200 W | 45.74 ± 3.35 | 0.439 ± 0.016 | −8.07 ± 1.87 |
| 4 | 1:15 | 5 min | 200 W | 42.15 ± 1.13 | 0.428 ± 0.08 | −9.25 ± 1.45 |
| 5 | 1:15 | 15 min | 200 W | 38.96 ± 5.37 | 0.295 ± 0.10 | −12.0 ± 3.96 |
| 6 | 1:15 | 15 min | 100 W | 42.23 ± 0.845 | 0.430 ± 0.01 | −9.73 ± 2.06 |
| 7 | 1:15 | 15 min | 300 W | 57.98 ± 2.35 | 0.260 ± 0.02 | −10.4 ± 1.22 |
| PSD (nm) | Zeta (mV) | EE (%) | LC (%) | |
|---|---|---|---|---|
| DSPE-PEG/NCTD | 96.1 ± 8.01 | −12.0 ± 3.96 | 81.31 ± 0.6 | 5.08 ± 0.37 |
| DSPE-PEG-C60/NCTD | 91.57 ± 9.78 | −13.8 ± 1.28 | 96.54 ± 0.03 | 6.05 ± 0.02 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ding, Z.; Xu, B.; Zhang, H.; Wang, Z.; Sun, L.; Tang, M.; Ding, M.; Zhang, T.; Shi, S. Norcantharidin-Encapsulated C60-Modified Nanomicelles: A Potential Approach to Mitigate Cytotoxicity in Renal Cells and Simultaneously Enhance Anti-Tumor Activity in Hepatocellular Carcinoma Cells. Molecules 2023, 28, 7609. https://doi.org/10.3390/molecules28227609
Ding Z, Xu B, Zhang H, Wang Z, Sun L, Tang M, Ding M, Zhang T, Shi S. Norcantharidin-Encapsulated C60-Modified Nanomicelles: A Potential Approach to Mitigate Cytotoxicity in Renal Cells and Simultaneously Enhance Anti-Tumor Activity in Hepatocellular Carcinoma Cells. Molecules. 2023; 28(22):7609. https://doi.org/10.3390/molecules28227609
Chicago/Turabian StyleDing, Zhongpeng, Beihua Xu, Huimin Zhang, Zhenyu Wang, Luying Sun, Mengjie Tang, Meihong Ding, Ting Zhang, and Senlin Shi. 2023. "Norcantharidin-Encapsulated C60-Modified Nanomicelles: A Potential Approach to Mitigate Cytotoxicity in Renal Cells and Simultaneously Enhance Anti-Tumor Activity in Hepatocellular Carcinoma Cells" Molecules 28, no. 22: 7609. https://doi.org/10.3390/molecules28227609
APA StyleDing, Z., Xu, B., Zhang, H., Wang, Z., Sun, L., Tang, M., Ding, M., Zhang, T., & Shi, S. (2023). Norcantharidin-Encapsulated C60-Modified Nanomicelles: A Potential Approach to Mitigate Cytotoxicity in Renal Cells and Simultaneously Enhance Anti-Tumor Activity in Hepatocellular Carcinoma Cells. Molecules, 28(22), 7609. https://doi.org/10.3390/molecules28227609





