Nanoparticles as Drug Delivery Systems for the Targeted Treatment of Atherosclerosis
Abstract
1. Introduction
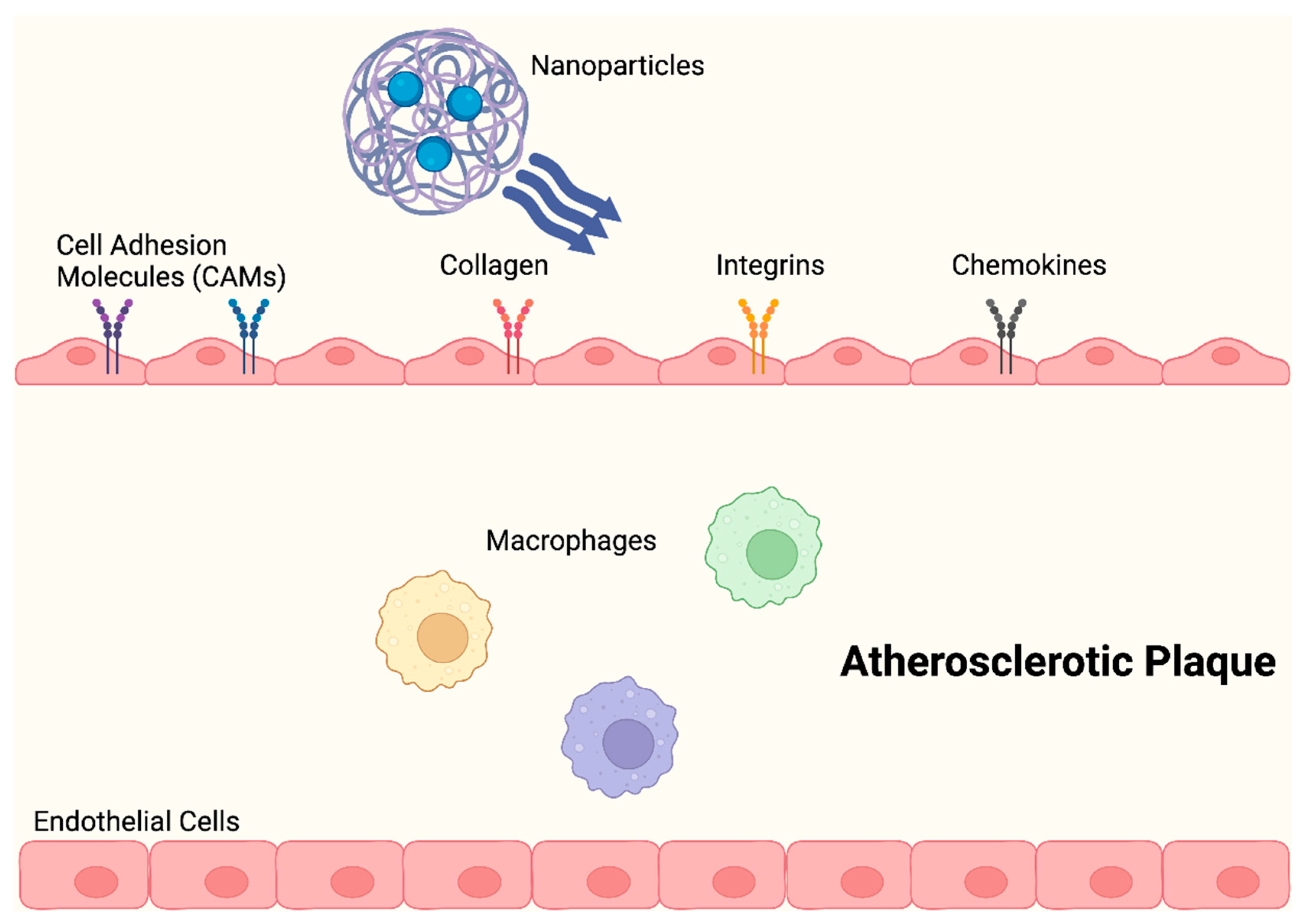
1.1. Atherosclerosis
1.2. Nanotheranostics
1.3. Nanoparticles in the Detection and Treatment of Coronary Artery Diseases
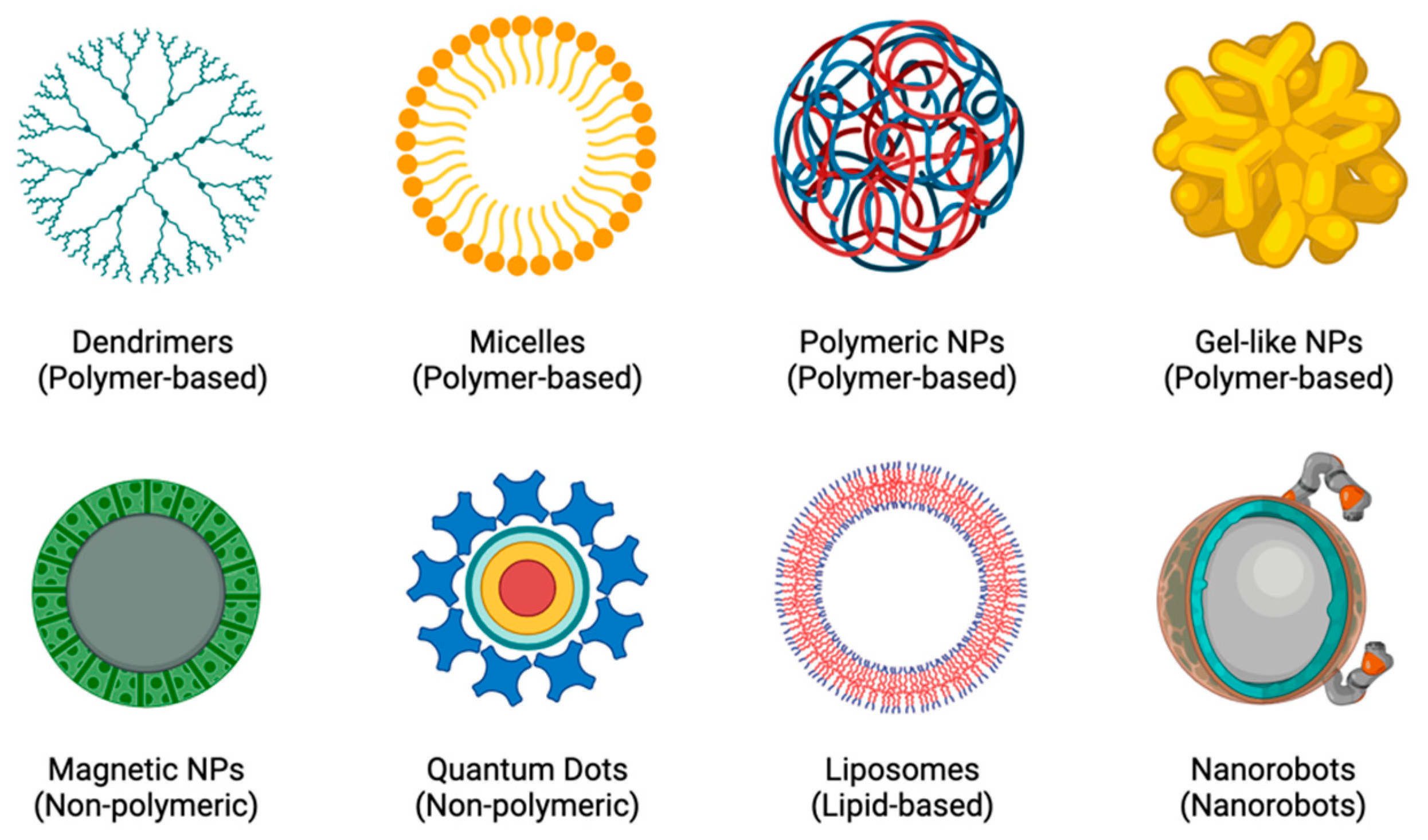
2. Nanoparticles for Diagnosis and Treatment of Atherosclerosis
2.1. Nanoparticle Drug Delivery Systems in Atherosclerosis
2.1.1. Liposomes for Drug Delivery
2.1.2. Dendrimers for Drug Delivery
2.1.3. Micelles for Drug Delivery
2.1.4. Polymeric Nanoparticles for Drug Delivery
2.1.5. Gel-like Nanoparticles for Drug Delivery
2.1.6. Magnetic Nanoparticles for Drug Delivery
2.1.7. Quantum Dots for Drug Delivery
2.1.8. Nanorobots for Drug Delivery
3. Limitations of Current Nanotheranostic Platforms in Atherosclerosis and Future Work
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| ADA | adamantane |
| AFM | atomic force microscope |
| apoB | apolipoprotein B |
| apoE | apolipoprotein E |
| ATO | arsenic trioxide |
| BBB | blood–brain barrier |
| β-CD | β-cyclodextrin |
| CAD | coronary artery disease |
| CCL | cationic lipoparticles |
| CD-MP | macrophages with β-CD decorations |
| ceMRI | contrast-enhanced MRI |
| Cur | curcumin |
| DDS | drug delivery systems |
| DES | drug-eluting stents |
| DXM | dexamethasone |
| DXM-liposomes | dexamethasone-liposomes |
| ECs | endothelial cells |
| ESS | endothelial shear stress |
| f-DXM | free DXM |
| Fe3O4 | iron MNP made of nanocrystalline magnetite |
| FITC | fluorescent marker fluorescein isothiocyanate |
| G0 | zero generation |
| Gd | gadolinium adamantane |
| GNS | gold nanospheres |
| GQDs | graphene quantum dots |
| HDL | high-density lipoproteins |
| HLA | hyaluronic acid |
| HMG | 3-hydroxy-3-methylglutaryl |
| IL-6 | interleukin-6 |
| IL-1β | interleukin-1β |
| ISR | in-stent restenosis |
| IV | intravenous |
| LCA | left coronary artery |
| MCP-1 | monocyte chemoattractant protein-1 |
| MDT | magnetic drug targeting |
| MI | myocardial infarction |
| miRNAs | microRNAs |
| MNPs | magnetic nanoparticles |
| MP-QT-NP | macrophage-liposome conjugate |
| MPs | microparticles |
| MRI | magnetic resonance imaging |
| NPs | nanoparticles |
| Ox-bCD | ROS-responsive β-cyclodextrin |
| PAMAM | polyamidoamine |
| PBS | phosphate-buffered saline |
| PCI | percutaneous coronary intervention |
| PDT | photodynamic therapy |
| PEG | polyethene glycol |
| PEG-PPS | PEG and poly(propylene sulphide) |
| PET | positron-emission tomography |
| PLGA | poly lactic-co-glycolic acid |
| PLN | platelet-like NPs |
| PLP | proteolipid protein |
| POBA | plain old balloon angioplasty procedures |
| PTX3 | pentraxin 3 |
| PVA | polyvinyl alcohol |
| QT | quercetin |
| QT-NP | quercetin-loaded liposome |
| RES | reticuloendothelial system |
| ROS | reactive oxygen species |
| SAMS | self-assembled monolayers |
| SPIOs | superparamagnetic iron oxides |
| SPIONs | superparamagnetic iron oxide NPs |
| SPR | surface plasmon resonance |
| SV MC | simvastatin-loaded micelles |
| USPIO | ultrasuperparamagnetic iron oxides |
| VCAM-1 | vascular cell adhesion molecule 1 |
| VEGF | vascular endothelial growth factor |
| VSMC | vascular smooth muscle cell |
| US FDA | United States Food and Drug Administration |
| ZnPc | zinc phthalocyanine |
| γFe2O3 | maghemite |
References
- Negre-Salvayre, A.; Guerby, P.; Gayral, S.; Laffargue, M.; Salvayre, R. Role of reactive oxygen species in atherosclerosis: Lessons from murine genetic models. Free Radic. Biol. Med. 2020, 149, 8–22. [Google Scholar] [CrossRef] [PubMed]
- Jacobin-Valat, M.J.; Laroche-Traineau, J.; Larivière, M.; Mornet, S.; Sanchez, S.; Biran, M.; Lebaron, C.; Boudon, J.; Lacomme, S.; Cérutti, M.; et al. Nanoparticles functionalised with an anti-platelet human antibody for in vivo detection of atherosclerotic plaque by magnetic resonance imaging. Nanomedicine 2015, 11, 927–937. [Google Scholar] [CrossRef] [PubMed]
- WHO. Cardiovascular Diseases (CVDs); WHO: Geneva, Switzerland, 2021. [Google Scholar]
- Tabas, I.; Williams, K.J.; Borén, J. Subendothelial lipoprotein retention as the initiating process in atherosclerosis: Update and therapeutic implications. Circulation 2007, 116, 1832–1844. [Google Scholar] [CrossRef] [PubMed]
- Glass, C.K.; Witztum, J.L. Atherosclerosis. The road ahead. Cell 2001, 104, 503–516. [Google Scholar] [CrossRef] [PubMed]
- Mestas, J.; Ley, K. Monocyte-endothelial cell interactions in the development of atherosclerosis. Trends Cardiovasc. Med. 2008, 18, 228–232. [Google Scholar] [CrossRef] [PubMed]
- Moore, K.J.; Tabas, I. Macrophages in the pathogenesis of atherosclerosis. Cell 2011, 145, 341–355. [Google Scholar] [CrossRef] [PubMed]
- Que, Y.; Shu, X.; Wang, L.; Hu, P.; Wang, S.; Xiong, R.; Liu, J.; Chen, H.; Tong, X. Inactivation of cysteine 674 in the SERCA2 accelerates experimental aortic aneurysm. J. Mol. Cell Cardiol. 2020, 139, 213–224. [Google Scholar] [CrossRef] [PubMed]
- Kasikara, C.; Doran, A.C.; Cai, B.; Tabas, I. The role of non-resolving inflammation in atherosclerosis. J. Clin. Investig. 2018, 128, 2713–2723. [Google Scholar] [CrossRef] [PubMed]
- Doran, A.C.; Yurdagul, A., Jr.; Tabas, I. Efferocytosis in health and disease. Nat. Rev. Immunol. 2020, 20, 254–267. [Google Scholar] [CrossRef]
- Bentzon, J.F.; Otsuka, F.; Virmani, R.; Falk, E. Mechanisms of plaque formation and rupture. Circ. Res. 2014, 114, 1852–1866. [Google Scholar] [CrossRef]
- Virani, S.S.; Alonso, A.; Aparicio, H.J.; Benjamin, E.J.; Bittencourt, M.S.; Callaway, C.W.; Carson, A.P.; Chamberlain, A.M.; Cheng, S.; Delling, F.N.; et al. Heart Disease and Stroke Statistics-2021 Update: A Report From the American Heart Association. Circulation 2021, 143, e254–e743. [Google Scholar] [CrossRef] [PubMed]
- Bułdak, Ł. Cardiovascular Diseases-A Focus on Atherosclerosis, Its Prophylaxis, Complications and Recent Advancements in Therapies. Int. J. Mol. Sci. 2022, 23, 4695. [Google Scholar] [CrossRef] [PubMed]
- Frostegård, J. Immunity, atherosclerosis and cardiovascular disease. BMC Med. 2013, 11, 117. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.K.; Rahimi, M.; Filgueira, C.S. Nanotechnology applications for cardiovascular disease treatment: Current and future perspectives. Nanomed. Nanotechnol. Biol. Med. 2021, 34, 102387. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.; Schilperoort, M.; Cao, Y.; Shi, J.; Tabas, I.; Tao, W. Macrophage-targeted nanomedicine for the diagnosis and treatment of atherosclerosis. Nat. Rev. Cardiol. 2022, 19, 228–249. [Google Scholar] [CrossRef] [PubMed]
- Najahi-Missaoui, W.; Arnold, R.D.; Cummings, B.S. Safe Nanoparticles: Are We There Yet? Int. J. Mol. Sci. 2020, 22, 385. [Google Scholar] [CrossRef]
- Farokhzad, O.C.; Langer, R. Nanomedicine: Developing smarter therapeutic and diagnostic modalities. Adv. Drug Deliv. Rev. 2006, 58, 1456–1459. [Google Scholar] [CrossRef]
- Mitragotri, S.; Anderson, D.G.; Chen, X.; Chow, E.K.; Ho, D.; Kabanov, A.V.; Karp, J.M.; Kataoka, K.; Mirkin, C.A.; Petrosko, S.H.; et al. Accelerating the Translation of Nanomaterials in Biomedicine. ACS Nano 2015, 9, 6644–6654. [Google Scholar] [CrossRef]
- Hoshyar, N.; Gray, S.; Han, H.; Bao, G. The effect of nanoparticle size on in vivo pharmacokinetics and cellular interaction. Nanomedicine 2016, 11, 673–692. [Google Scholar] [CrossRef]
- Madamsetty, V.S.; Mukherjee, A.; Mukherjee, S. Recent Trends of the Bio-Inspired Nanoparticles in Cancer Theranostics. Front. Pharmacol. 2019, 10, 1264. [Google Scholar] [CrossRef]
- Kulkarni, S.A.; Feng, S.S. Effects of particle size and surface modification on cellular uptake and biodistribution of polymeric nanoparticles for drug delivery. Pharm. Res. 2013, 30, 2512–2522. [Google Scholar] [CrossRef]
- Elias, D.R.; Poloukhtine, A.; Popik, V.; Tsourkas, A. Effect of ligand density, receptor density, and nanoparticle size on cell targeting. Nanomedicine 2013, 9, 194–201. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.; Glackin, C.A.; Horwitz, M.A.; Zink, J.I. Nanomachines and Other Caps on Mesoporous Silica Nanoparticles for Drug Delivery. Acc. Chem. Res. 2019, 52, 1531–1542. [Google Scholar] [CrossRef]
- Vaidyanathan, K.; Gopalakrishnan, S. Nanomedicine in the Diagnosis and Treatment of Atherosclerosis-A Systematic Review. Cardiovasc. Hematol. Disord. Drug Targets 2017, 17, 119–131. [Google Scholar] [CrossRef] [PubMed]
- Karagkiozaki, V.; Logothetidis, S.; Pappa, A.-M. Nanomedicine for Atherosclerosis: Molecular Imaging and Treatment. J. Biomed. Nanotechnol. 2015, 11, 191–210. [Google Scholar] [CrossRef]
- Matoba, T.; Koga, J.-i.; Nakano, K.; Egashira, K.; Tsutsui, H. Nanoparticle-mediated drug delivery system for atherosclerotic cardiovascular disease. J. Cardiol. 2017, 70, 206–211. [Google Scholar] [CrossRef]
- Palekar, R.U.; Jallouk, A.P.; Lanza, G.M.; Pan, H.; Wickline, S.A. Molecular imaging of atherosclerosis with nanoparticle-based fluorinated MRI contrast agents. Nanomedicine 2015, 10, 1817–1832. [Google Scholar] [CrossRef]
- Weissleder, R.; Elizondo, G.; Wittenberg, J.; Rabito, C.A.; Bengele, H.H.; Josephson, L. Ultrasmall superparamagnetic iron oxide: Characterization of a new class of contrast agents for MR imaging. Radiology 1990, 175, 489–493. [Google Scholar] [CrossRef]
- Lakshmanan, R.; Maulik, N. Development of next generation cardiovascular therapeutics through bio-assisted nanotechnology. J. Biomed. Mater. Res. B Appl. Biomater. 2018, 106, 2072–2083. [Google Scholar] [CrossRef] [PubMed]
- Fan, C.; Joshi, J.; Li, F.; Xu, B.; Khan, M.; Yang, J.; Zhu, W. Nanoparticle-Mediated Drug Delivery for Treatment of Ischemic Heart Disease. Front. Bioeng. Biotechnol. 2020, 8, 687. [Google Scholar] [CrossRef]
- Bejarano, J.; Navarro-Marquez, M.; Morales-Zavala, F.; Morales, J.O.; Garcia-Carvajal, I.; Araya-Fuentes, E.; Flores, Y.; Verdejo, H.E.; Castro, P.F.; Lavandero, S.; et al. Nanoparticles for diagnosis and therapy of atherosclerosis and myocardial infarction: Evolution toward prospective theranostic approaches. Theranostics 2018, 8, 4710–4732. [Google Scholar] [CrossRef]
- Wu, Y.; Vazquez-Prada, K.X.; Liu, Y.; Whittaker, A.K.; Zhang, R.; Ta, H.T. Recent Advances in the Development of Theranostic Nanoparticles for Cardiovascular Diseases. Nanotheranostics 2021, 5, 499–514. [Google Scholar] [CrossRef]
- Rhee, J.W.; Wu, J.C. Advances in nanotechnology for the management of coronary artery disease. Trends Cardiovasc. Med. 2013, 23, 39–45. [Google Scholar] [CrossRef] [PubMed]
- Brito, L.; Amiji, M. Nanoparticulate carriers for the treatment of coronary restenosis. Int. J. Nanomed. 2007, 2, 143–161. [Google Scholar]
- Gundogan, B.; Tan, A.; Farhatnia, Y.; Alavijeh, M.S.; Cui, Z.; Seifalian, A.M. Bioabsorbable stent quo vadis: A case for nano-theranostics. Theranostics 2014, 4, 514–533. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Pala, R.; Pattnaik, S.; Busi, S.; Nauli, S.M. Nanomaterials as Novel Cardiovascular Theranostics. Pharmaceutics 2021, 13, 348. [Google Scholar] [CrossRef] [PubMed]
- Beldman, T.J.; Senders, M.L.; Alaarg, A.; Pérez-Medina, C.; Tang, J.; Zhao, Y.; Fay, F.; Deichmöller, J.; Born, B.; Desclos, E.; et al. Hyaluronan Nanoparticles Selectively Target Plaque-Associated Macrophages and Improve Plaque Stability in Atherosclerosis. ACS Nano 2017, 11, 5785–5799. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Zhang, X.; Millican, R.; Creutzmann, J.E.; Martin, S.; Jun, H.W. High density lipoprotein mimicking nanoparticles for atherosclerosis. Nano Converg. 2020, 7, 6. [Google Scholar] [CrossRef]
- Mog, B.; Asase, C.; Chaplin, A.; Gao, H.; Rajagopalan, S.; Maiseyeu, A. Nano-Antagonist Alleviates Inflammation and Allows for MRI of Atherosclerosis. Nanotheranostics 2019, 3, 342–355. [Google Scholar] [CrossRef]
- Manduteanu, I.; Simionescu, M. Inflammation in atherosclerosis: A cause or a result of vascular disorders? J. Cell Mol. Med. 2012, 16, 1978–1990. [Google Scholar] [CrossRef]
- Kamaly, N.; Fredman, G.; Fojas, J.J.; Subramanian, M.; Choi, W.I.; Zepeda, K.; Vilos, C.; Yu, M.; Gadde, S.; Wu, J.; et al. Targeted Interleukin-10 Nanotherapeutics Developed with a Microfluidic Chip Enhance Resolution of Inflammation in Advanced Atherosclerosis. ACS Nano 2016, 10, 5280–5292. [Google Scholar] [CrossRef] [PubMed]
- Linton, M.F.; Fazio, S. Macrophages, inflammation, and atherosclerosis. Int. J. Obes. Relat. Metab. Disord. 2003, 27, S35–S40. [Google Scholar] [CrossRef] [PubMed]
- Lessner, S.M.; Prado, H.L.; Waller, E.K.; Galis, Z.S. Atherosclerotic lesions grow through recruitment and proliferation of circulating monocytes in a murine model. Am. J. Pathol. 2002, 160, 2145–2155. [Google Scholar] [CrossRef] [PubMed]
- Hansson, G.K. Inflammatory mechanisms in atherosclerosis. J. Thromb. Haemost. 2009, 7, 328–331. [Google Scholar] [CrossRef] [PubMed]
- El-Sherbiny, I.M.; Elkholi, I.E.; Yacoub, M.H. Tissue plasminogen activator-based clot busting: Controlled delivery approaches. Glob. Cardiol. Sci. Pract. 2014, 2014, 46. [Google Scholar] [CrossRef] [PubMed]
- Carboni, E.; Tschudi, K.; Nam, J.; Lu, X.; Ma, A.W.K. Particle Margination and Its Implications on Intravenous Anticancer Drug Delivery. AAPS PharmSciTech 2014, 15, 762–771. [Google Scholar] [CrossRef] [PubMed]
- Müller, K.; Fedosov, D.A.; Gompper, G. Margination of micro- and nano-particles in blood flow and its effect on drug delivery. Sci. Rep. 2014, 4, 4871. [Google Scholar] [CrossRef] [PubMed]
- Nakano, K.; Egashira, K.; Masuda, S.; Funakoshi, K.; Zhao, G.; Kimura, S.; Matoba, T.; Sueishi, K.; Endo, Y.; Kawashima, Y.; et al. Formulation of nanoparticle-eluting stents by a cationic electrodeposition coating technology: Efficient nano-drug delivery via bioabsorbable polymeric nanoparticle-eluting stents in porcine coronary arteries. JACC Cardiovasc. Interv. 2009, 2, 277–283. [Google Scholar] [CrossRef] [PubMed]
- Uwatoku, T.; Shimokawa, H.; Abe, K.; Matsumoto, Y.; Hattori, T.; Oi, K.; Matsuda, T.; Kataoka, K.; Takeshita, A. Application of nanoparticle technology for the prevention of restenosis after balloon injury in rats. Circ. Res. 2003, 92, e62–e69. [Google Scholar] [CrossRef] [PubMed]
- Leuschner, F.; Dutta, P.; Gorbatov, R.; Novobrantseva, T.I.; Donahoe, J.S.; Courties, G.; Lee, K.M.; Kim, J.I.; Markmann, J.F.; Marinelli, B.; et al. Therapeutic siRNA silencing in inflammatory monocytes in mice. Nat. Biotechnol. 2011, 29, 1005–1010. [Google Scholar] [CrossRef]
- Duivenvoorden, R.; Tang, J.; Cormode, D.P.; Mieszawska, A.J.; Izquierdo-Garcia, D.; Ozcan, C.; Otten, M.J.; Zaidi, N.; Lobatto, M.E.; van Rijs, S.M.; et al. A statin-loaded reconstituted high-density lipoprotein nanoparticle inhibits atherosclerotic plaque inflammation. Nat. Commun. 2014, 5, 3065. [Google Scholar] [CrossRef] [PubMed]
- Sanchez-Gaytan, B.L.; Fay, F.; Lobatto, M.E.; Tang, J.; Ouimet, M.; Kim, Y.; van der Staay, S.E.; van Rijs, S.M.; Priem, B.; Zhang, L.; et al. HDL-mimetic PLGA nanoparticle to target atherosclerosis plaque macrophages. Bioconjug. Chem. 2015, 26, 443–451. [Google Scholar] [CrossRef] [PubMed]
- Cormode, D.P.; Skajaa, T.; van Schooneveld, M.M.; Koole, R.; Jarzyna, P.; Lobatto, M.E.; Calcagno, C.; Barazza, A.; Gordon, R.E.; Zanzonico, P.; et al. Nanocrystal core high-density lipoproteins: A multimodality contrast agent platform. Nano Lett. 2008, 8, 3715–3723. [Google Scholar] [CrossRef] [PubMed]
- Lewis, D.R.; Kamisoglu, K.; York, A.W.; Moghe, P.V. Polymer-based therapeutics: Nanoassemblies and nanoparticles for management of atherosclerosis. Wiley Interdiscip. Rev. Nanomed. Nanobiotechnol. 2011, 3, 400–420. [Google Scholar] [CrossRef] [PubMed]
- Patel, D.N.; Bailey, S.R. Nanotechnology in cardiovascular medicine. Catheter. Cardiovasc. Interv. 2007, 69, 643–654. [Google Scholar] [CrossRef] [PubMed]
- Anselmo, A.C.; Modery-Pawlowski, C.L.; Menegatti, S.; Kumar, S.; Vogus, D.R.; Tian, L.L.; Chen, M.; Squires, T.M.; Sen Gupta, A.; Mitragotri, S. Platelet-like nanoparticles: Mimicking shape, flexibility, and surface biology of platelets to target vascular injuries. ACS Nano 2014, 8, 11243–11253. [Google Scholar] [CrossRef]
- Serda, R.E. Mass Transport of Nanocarriers; Fang, X., Wu, L., Eds.; Pan Stanford Publishing: Singapore, 2013. [Google Scholar]
- Decuzzi, P.; Pasqualini, R.; Arap, W.; Ferrari, M. Intravascular Delivery of Particulate Systems: Does Geometry Really Matter? Pharm. Res. 2009, 26, 235–243. [Google Scholar] [CrossRef]
- Weissberg, P.L.; Bennett, M.R. Atherosclerosis—An inflammatory disease. N. Engl. J. Med. 1999, 340, 1928–1929. [Google Scholar] [PubMed]
- Boada, C.; Zinger, A.; Tsao, C.; Zhao, P.; Martinez, J.O.; Hartman, K.; Naoi, T.; Sukhoveshin, R.; Sushnitha, M.; Molinaro, R.; et al. Rapamycin-Loaded Biomimetic Nanoparticles Reverse Vascular Inflammation. Circ. Res. 2020, 126, 25–37. [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, K.; Li, T.; Maruf, A.; Qin, X.; Luo, L.; Zhong, Y.; Qiu, J.; McGinty, S.; Pontrelli, G.; et al. Macrophage membrane functionalized biomimetic nanoparticles for targeted anti-atherosclerosis applications. Theranostics 2021, 11, 164–180. [Google Scholar] [CrossRef]
- Song, Y.; Huang, Z.; Liu, X.; Pang, Z.; Chen, J.; Yang, H.; Zhang, N.; Cao, Z.; Liu, M.; Cao, J.; et al. Platelet membrane-coated nanoparticle-mediated targeting delivery of Rapamycin blocks atherosclerotic plaque development and stabilizes plaque in apolipoprotein E-deficient (ApoE−/−) mice. Nanomedicine 2019, 15, 13–24. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Wang, Z.; Zhang, Y.; Cao, F.; Dong, K.; Ren, J.; Qu, X. Erythrocyte Membrane Cloaked Metal-Organic Framework Nanoparticle as Biomimetic Nanoreactor for Starvation-Activated Colon Cancer Therapy. ACS Nano 2018, 12, 10201–10211. [Google Scholar] [CrossRef] [PubMed]
- Gao, M.; Liang, C.; Song, X.; Chen, Q.; Jin, Q.; Wang, C.; Liu, Z. Erythrocyte-Membrane-Enveloped Perfluorocarbon as Nanoscale Artificial Red Blood Cells to Relieve Tumor Hypoxia and Enhance Cancer Radiotherapy. Adv. Mater. 2017, 29, 1701429. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Zhang, K.; Qin, X.; Li, T.; Qiu, J.; Yin, T.; Huang, J.; McGinty, S.; Pontrelli, G.; Ren, J.; et al. Biomimetic Nanotherapies: Red Blood Cell Based Core-Shell Structured Nanocomplexes for Atherosclerosis Management. Adv. Sci. 2019, 6, 1900172. [Google Scholar] [CrossRef] [PubMed]
- Betala, J.; Bae, S.; Langan, E.M., 3rd; LaBerge, M.; Lee, J.S. Combinatorial therapy of sirolimus and heparin by nanocarrier inhibits restenosis after balloon angioplasty ex vivo. Nanomedicine 2020, 15, 1205–1220. [Google Scholar] [CrossRef] [PubMed]
- Stefanini, G.G.; Holmes, D.R., Jr. Drug-eluting coronary-artery stents. N. Engl. J. Med. 2013, 368, 254–265. [Google Scholar] [CrossRef] [PubMed]
- Stone, G.W.; Moses, J.W.; Ellis, S.G.; Schofer, J.; Dawkins, K.D.; Morice, M.C.; Colombo, A.; Schampaert, E.; Grube, E.; Kirtane, A.J.; et al. Safety and efficacy of sirolimus- and paclitaxel-eluting coronary stents. N. Engl. J. Med. 2007, 356, 998–1008. [Google Scholar] [CrossRef]
- Wang, B.; Chen, G.; Urabe, G.; Xie, R.; Wang, Y.; Shi, X.; Guo, L.W.; Gong, S.; Kent, K.C. A paradigm of endothelium-protective and stent-free anti-restenotic therapy using biomimetic nanoclusters. Biomaterials 2018, 178, 293–301. [Google Scholar] [CrossRef] [PubMed]
- Feng, S.; Hu, Y.; Peng, S.; Han, S.; Tao, H.; Zhang, Q.; Xu, X.; Zhang, J.; Hu, H. Nanoparticles responsive to the inflammatory microenvironment for targeted treatment of arterial restenosis. Biomaterials 2016, 105, 167–184. [Google Scholar] [CrossRef] [PubMed]
- Dou, Y.; Chen, Y.; Zhang, X.; Xu, X.; Guo, J.; Zhang, D.; Wang, R.; Li, X.; Zhang, J. Non-proinflammatory and responsive nanoplatforms for targeted treatment of atherosclerosis. Biomaterials 2017, 143, 93–108. [Google Scholar] [CrossRef]
- Zhu, X.; Xie, H.; Liang, X.; Li, X.; Duan, J.; Chen, Y.; Yang, Z.; Liu, C.; Wang, C.; Zhang, H.; et al. Bilayered Nanoparticles with Sequential Release of VEGF Gene and Paclitaxel for Restenosis Inhibition in Atherosclerosis. ACS Appl. Mater. Interfaces 2017, 9, 27522–27532. [Google Scholar] [CrossRef] [PubMed]
- Hu, Q.; Fang, Z.; Ge, J.; Li, H. Nanotechnology for cardiovascular diseases. Innovation 2022, 3, 100214. [Google Scholar] [CrossRef] [PubMed]
- Kheirolomoom, A.; Kim, C.W.; Seo, J.W.; Kumar, S.; Son, D.J.; Gagnon, M.K.; Ingham, E.S.; Ferrara, K.W.; Jo, H. Multifunctional Nanoparticles Facilitate Molecular Targeting and miRNA Delivery to Inhibit Atherosclerosis in ApoE−/− Mice. ACS Nano 2015, 9, 8885–8897. [Google Scholar] [CrossRef] [PubMed]
- Sun, D.T.; Simmons, R.; Huo, D.; Pang, B.; Zhao, X.; Kim, D.C.W.; Jo, P.H.; Xia, P.Y. Targeted Delivery of Anti-miR-712 by VCAM1-Binding Au Nanospheres for Atherosclerosis Therapy. CHEMNANOMAT 2016, 2, 400–406. [Google Scholar] [CrossRef]
- Wong, W.W.; Dimitroulakos, J.; Minden, M.D.; Penn, L.Z. HMG-CoA reductase inhibitors and the malignant cell: The statin family of drugs as triggers of tumor-specific apoptosis. Leukemia 2002, 16, 508–519. [Google Scholar] [CrossRef] [PubMed]
- Yin, R.X.; Yang, D.Z.; Wu, J.Z. Nanoparticle drug- and gene-eluting stents for the prevention and treatment of coro-nary restenosis. Theranostics 2014, 4, 175–200. [Google Scholar] [CrossRef] [PubMed]
- Karimi, M.; Zare, H.; Bakhshian Nik, A.; Yazdani, N.; Hamrang, M.; Mohamed, E.; Sahandi Zangabad, P.; Moosavi Basri, S.M.; Bakhtiari, L.; Hamblin, M.R. Nanotechnology in diagnosis and treatment of coronary artery disease. Nanomedicine 2016, 11, 513–530. [Google Scholar] [CrossRef] [PubMed]
- Torchilin, V.P. Recent advances with liposomes as pharmaceutical carriers. Nat. Rev. Drug Discov. 2005, 4, 145–160. [Google Scholar] [CrossRef]
- Gupta, A.S. Nanomedicine approaches in vascular disease: A review. Nanomedicine 2011, 7, 763–779. [Google Scholar] [CrossRef]
- Kiaie, N.; Gorabi, A.M.; Penson, P.E.; Watts, G.; Johnston, T.P.; Banach, M.; Sahebkar, A. A new approach to the diagnosis and treatment of atherosclerosis: The era of the liposome. Drug Discov. Today 2020, 25, 58–72. [Google Scholar] [CrossRef]
- Lecommandoux, S.; Sandre, O.; Chécot, F.; Perzynski, R. Smart hybrid magnetic self-assembled micelles and hollow capsules. Prog. Solid State Chem. 2006, 34, 171–179. [Google Scholar] [CrossRef]
- Nasongkla, N.; Bey, E.; Ren, J.; Ai, H.; Khemtong, C.; Guthi, J.S.; Chin, S.F.; Sherry, A.D.; Boothman, D.A.; Gao, J. Multifunctional polymeric micelles as cancer-targeted, MRI-ultrasensitive drug delivery systems. Nano Lett. 2006, 6, 2427–2430. [Google Scholar] [CrossRef]
- Srinivasan, R.; Marchant, R.E.; Gupta, A.S. In vitro and in vivo platelet targeting by cyclic RGD-modified liposomes. J. Biomed. Mater. Res. 2010, 93A, 1004–1015. [Google Scholar] [CrossRef]
- Gutman, D.; Golomb, G. Liposomal alendronate for the treatment of restenosis. J. Control. Release 2012, 161, 619–627. [Google Scholar] [CrossRef]
- Gao, C.; Liu, C.; Chen, Q.; Wang, Y.; Kwong, C.H.T.; Wang, Q.; Xie, B.; Lee, S.M.Y.; Wang, R. Cyclodextrin-mediated conjugation of macrophage and liposomes for treatment of atherosclerosis. J. Control. Release 2022, 349, 2–15. [Google Scholar] [CrossRef]
- Li, X.; Xiao, H.; Lin, C.; Sun, W.; Wu, T.; Wang, J.; Chen, B.; Chen, X.; Cheng, D. Synergistic effects of liposomes encapsulating atorvastatin calcium and curcumin and targeting dysfunctional endothelial cells in reducing atherosclerosis. Int. J. Nanomed. 2019, 14, 649–665. [Google Scholar] [CrossRef]
- Chono, S.; Tauchi, Y.; Deguchi, Y.; Morimoto, K. Efficient drug delivery to atherosclerotic lesions and the antiatherosclerotic effect by dexamethasone incorporated into liposomes in atherogenic mice. J. Drug Target. 2005, 13, 267–276. [Google Scholar] [CrossRef] [PubMed]
- Danenberg, H.D.; Fishbein, I.; Gao, J.; Mönkkönen, J.; Reich, R.; Gati, I.; Moerman, E.; Golomb, G. Macrophage depletion by clodronate-containing liposomes reduces neointimal formation after balloon injury in rats and rabbits. Circulation 2002, 106, 599–605. [Google Scholar] [CrossRef] [PubMed]
- Danenberg, H.D.; Golomb, G.; Groothuis, A.; Gao, J.; Epstein, H.; Swaminathan, R.V.; Seifert, P.; Edelman, E.R. Liposomal alendronate inhibits systemic innate immunity and reduces in-stent neointimal hyperplasia in rabbits. Circulation 2003, 108, 2798–2804. [Google Scholar] [CrossRef]
- Lobatto, M.E.; Fayad, Z.A.; Silvera, S.; Vucic, E.; Calcagno, C.; Mani, V.; Dickson, S.D.; Nicolay, K.; Banciu, M.; Schiffelers, R.M.; et al. Multimodal clinical imaging to longitudinally assess a nanomedical anti-inflammatory treatment in experimental atherosclerosis. Mol. Pharm. 2010, 7, 2020–2029. [Google Scholar] [CrossRef]
- Iyer, A.K.; Khaled, G.; Fang, J.; Maeda, H. Exploiting the enhanced permeability and retention effect for tumor targeting. Drug Discov. Today 2006, 11, 812–818. [Google Scholar] [CrossRef] [PubMed]
- Bark, D.L., Jr.; Ku, D.N. Wall shear over high degree stenoses pertinent to atherothrombosis. J. Biomech. 2010, 43, 2970–2977. [Google Scholar] [CrossRef] [PubMed]
- Yang, G.; Song, J.; Zhang, J. Biomimetic and bioresponsive nanotherapies for inflammatory vascular diseases. Nanomedicine 2020, 15, 1917–1921. [Google Scholar] [CrossRef] [PubMed]
- Shen, M.; Yao, S.; Li, S.; Wu, X.; Liu, S.; Yang, Q.; Du, J.; Wang, J.; Zheng, X.; Li, Y. A ROS and shear stress dual-sensitive bionic system with cross-linked dendrimers for atherosclerosis therapy. Nanoscale 2021, 13, 20013–20027. [Google Scholar] [CrossRef] [PubMed]
- Hawker, C.J.; Frechet, J.M.J. Preparation of polymers with controlled molecular architecture. A new convergent approach to dendritic macromolecules. J. Am. Chem. Soc. 1990, 112, 7638–7647. [Google Scholar] [CrossRef]
- Spyropoulos-Antonakakis, N.; Sarantopoulou, E.; Trohopoulos, P.N.; Stefi, A.L.; Kollia, Z.; Gavriil, V.E.; Bourkoula, A.; Petrou, P.S.; Kakabakos, S.; Semashko, V.V.; et al. Selective aggregation of PAMAM dendrimer nanocarriers and PAMAM/ZnPc nanodrugs on human atheromatous carotid tissues: A photodynamic therapy for atherosclerosis. Nanoscale Res. Lett. 2015, 10, 210. [Google Scholar] [CrossRef] [PubMed]
- Oddone, N.; Lecot, N.; Fernández, M.; Rodriguez-Haralambides, A.; Cabral, P.; Cerecetto, H.; Benech, J.C. In vitro and in vivo uptake studies of PAMAM G4.5 dendrimers in breast cancer. J. Nanobiotechnol. 2016, 14, 45. [Google Scholar] [CrossRef] [PubMed]
- Kharwade, R.; More, S.; Warokar, A.; Agrawal, P.; Mahajan, N. Starburst pamam dendrimers: Synthetic approaches, surface modifications, and biomedical applications. Arab. J. Chem. 2020, 13, 6009–6039. [Google Scholar] [CrossRef]
- Liu, H.; Wang, J.; Li, W.; Hu, J.; Wang, M.; Kang, Y. Cellular Uptake Behaviors of Rigidity-Tunable Dendrimers. Pharmaceutics 2018, 10, 99. [Google Scholar] [CrossRef]
- Haeri, A.; Sadeghian, S.; Rabbani, S.; Anvari, M.S.; Lavasanifar, A.; Amini, M.; Dadashzadeh, S. Sirolimus-loaded stealth colloidal systems attenuate neointimal hyperplasia after balloon injury: A comparison of phospholipid micelles and liposomes. Int. J. Pharm. 2013, 455, 320–330. [Google Scholar] [CrossRef]
- Mu, D.; Li, J.; Qi, Y.; Sun, X.; Liu, Y.; Shen, S.; Li, Y.; Xu, B.; Zhang, B. Hyaluronic acid-coated polymeric micelles with hydrogen peroxide scavenging to encapsulate statins for alleviating atherosclerosis. J. Nanobiotechnol. 2020, 18, 179. [Google Scholar] [CrossRef]
- Wu, T.; Chen, X.; Wang, Y.; Xiao, H.; Peng, Y.; Lin, L.; Xia, W.; Long, M.; Tao, J.; Shuai, X. Aortic plaque-targeted andrographolide delivery with oxidation-sensitive micelle effectively treats atherosclerosis via simultaneous ROS capture and anti-inflammation. Nanomedicine 2018, 14, 2215–2226. [Google Scholar] [CrossRef] [PubMed]
- Shen, M.; Li, H.; Yao, S.; Wu, X.; Liu, S.; Yang, Q.; Zhang, Y.; Du, J.; Qi, S.; Li, Y. Shear stress and ROS-responsive biomimetic micelles for atherosclerosis via ROS consumption. Mater. Sci. Eng. C Mater. Biol. Appl. 2021, 126, 112164. [Google Scholar] [CrossRef] [PubMed]
- Doshi, N.; Prabhakarpandian, B.; Rea-Ramsey, A.; Pant, K.; Sundaram, S.; Mitragotri, S. Flow and adhesion of drug carriers in blood vessels depend on their shape: A study using model synthetic microvascular networks. J. Control Release 2010, 146, 196–200. [Google Scholar] [CrossRef] [PubMed]
- Serruys, P.W.; Kutryk, M.J.; Ong, A.T. Coronary-artery stents. N. Engl. J. Med. 2006, 354, 483–495. [Google Scholar] [CrossRef]
- Mehran, R.; Dangas, G.D. Off-label use of drug-eluting stents: Assessing the risk. Nat. Clin. Pract. Cardiovasc. Med. 2007, 4, 594–595. [Google Scholar] [CrossRef]
- Ducrocq, G.; Serebruany, V.; Tanguay, J.F. Antiplatelet therapy in the era of drug-eluting stents: Current and future perspectives. Expert. Rev. Cardiovasc. Ther. 2007, 5, 939–953. [Google Scholar] [CrossRef]
- Windecker, S.; Meier, B. Late coronary stent thrombosis. Circulation 2007, 116, 1952–1965. [Google Scholar] [CrossRef]
- Finn, A.V.; Nakazawa, G.; Joner, M.; Kolodgie, F.D.; Mont, E.K.; Gold, H.K.; Virmani, R. Vascular responses to drug eluting stents: Importance of delayed healing. Arterioscler. Thromb. Vasc. Biol. 2007, 27, 1500–1510. [Google Scholar] [CrossRef]
- Leal, B.H.; Velasco, B.; Cambón, A.; Pardo, A.; Fernandez-Vega, J.; Arellano, L.; Al-Modlej, A.; Mosquera, V.X.; Bouzas, A.; Prieto, G.; et al. Combined Therapeutics for Atherosclerosis Treatment Using Polymeric Nanovectors. Pharmaceutics 2022, 14, 258. [Google Scholar] [CrossRef]
- Chan, J.M.; Rhee, J.W.; Drum, C.L.; Bronson, R.T.; Golomb, G.; Langer, R.; Farokhzad, O.C. In vivo prevention of arterial restenosis with paclitaxel-encapsulated targeted lipid-polymeric nanoparticles. Proc. Natl. Acad. Sci. USA 2011, 108, 19347–19352. [Google Scholar] [CrossRef] [PubMed]
- Chan, J.M.; Zhang, L.; Tong, R.; Ghosh, D.; Gao, W.; Liao, G.; Yuet, K.P.; Gray, D.; Rhee, J.W.; Cheng, J.; et al. Spatiotemporal controlled delivery of nanoparticles to injured vasculature. Proc. Natl. Acad. Sci. USA 2010, 107, 2213–2218. [Google Scholar] [CrossRef] [PubMed]
- Chan, J.M.; Zhang, L.; Yuet, K.P.; Liao, G.; Rhee, J.W.; Langer, R.; Farokhzad, O.C. PLGA-lecithin-PEG core-shell nanoparticles for controlled drug delivery. Biomaterials 2009, 30, 1627–1634. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Chan, J.M.; Gu, F.X.; Rhee, J.W.; Wang, A.Z.; Radovic-Moreno, A.F.; Alexis, F.; Langer, R.; Farokhzad, O.C. Self-assembled lipid--polymer hybrid nanoparticles: A robust drug delivery platform. ACS Nano 2008, 2, 1696–1702. [Google Scholar] [CrossRef] [PubMed]
- James, S.K.; Stenestrand, U.; Lindbäck, J.; Carlsson, J.; Scherstén, F.; Nilsson, T.; Wallentin, L.; Lagerqvist, B. Long-term safety and efficacy of drug-eluting versus bare-metal stents in Sweden. N. Engl. J. Med. 2009, 360, 1933–1945. [Google Scholar] [CrossRef] [PubMed]
- Stone, G.W.; Lansky, A.J.; Pocock, S.J.; Gersh, B.J.; Dangas, G.; Wong, S.C.; Witzenbichler, B.; Guagliumi, G.; Peruga, J.Z.; Brodie, B.R.; et al. Paclitaxel-eluting stents versus bare-metal stents in acute myocardial infarction. N. Engl. J. Med. 2009, 360, 1946–1959. [Google Scholar] [CrossRef] [PubMed]
- Kukreja, N.; Onuma, Y.; Daemen, J.; Serruys, P.W. The future of drug-eluting stents. Pharmacol. Res. 2008, 57, 171–180. [Google Scholar] [CrossRef]
- Libby, P.; Theroux, P. Pathophysiology of coronary artery disease. Circulation 2005, 111, 3481–3488. [Google Scholar] [CrossRef]
- Gu, F.; Zhang, L.; Teply, B.A.; Mann, N.; Wang, A.; Radovic-Moreno, A.F.; Langer, R.; Farokhzad, O.C. Precise engineering of targeted nanoparticles by using self-assembled biointegrated block copolymers. Proc. Natl. Acad. Sci. USA 2008, 105, 2586–2591. [Google Scholar] [CrossRef]
- Nguyen, K.T.; Shukla, K.P.; Moctezuma, M.; Braden, A.R.; Zhou, J.; Hu, Z.; Tang, L. Studies of the cellular uptake of hydrogel nanospheres and microspheres by phagocytes, vascular endothelial cells, and smooth muscle cells. J. Biomed. Mater. Res. A 2009, 88, 1022–1030. [Google Scholar] [CrossRef]
- Reddy, M.K.; Vasir, J.K.; Sahoo, S.K.; Jain, T.K.; Yallapu, M.M.; Labhasetwar, V. Inhibition of apoptosis through localized delivery of rapamycin-loaded nanoparticles prevented neointimal hyperplasia and reendothelialized injured artery. Circ. Cardiovasc. Interv. 2008, 1, 209–216. [Google Scholar] [CrossRef] [PubMed]
- Manshadi, M.K.D.; Saadat, M.; Mohammadi, M.; Shamsi, M.; Dejam, M.; Kamali, R.; Sanati-Nezhad, A. Delivery of magnetic micro/nanoparticles and magnetic-based drug/cargo into arterial flow for targeted therapy. Drug Deliv. 2018, 25, 1963–1973. [Google Scholar] [CrossRef] [PubMed]
- Korin, N. Mechanoresponsive nanotherapeutic for localized drug delivery to flow obstructed blood vessels. Ther. Deliv. 2015, 6, 895–897. [Google Scholar] [CrossRef] [PubMed]
- Nurhidayah, D.; Maruf, A.; Zhang, X.; Liao, X.; Wu, W.; Wang, G. Advanced drug-delivery systems: Mechanoresponsive nanoplatforms applicable in atherosclerosis management. Future Med. 2019, 14, 3105–3122. [Google Scholar] [CrossRef] [PubMed]
- Chorny, M.; Fishbein, I.; Forbes, S.; Alferiev, I. Magnetic nanoparticles for targeted vascular delivery. IUBMB Life 2011, 63, 613–620. [Google Scholar] [CrossRef]
- Zohra, F.T.; Medved, M.; Lazareva, N.; Polyak, B. Functional behavior and gene expression of magnetic nanoparticle-loaded primary endothelial cells for targeting vascular stents. Nanomedicine 2015, 10, 1391–1406. [Google Scholar] [CrossRef] [PubMed]
- Sun, C.; Lee, J.S.H.; Zhang, M. Magnetic nanoparticles in MR imaging and drug delivery. Adv. Drug Deliv. Rev. 2008, 60, 1252–1265. [Google Scholar] [CrossRef] [PubMed]
- Gupta, A.K.; Gupta, M. Synthesis and surface engineering of iron oxide nanoparticles for biomedical applications. Biomaterials 2005, 26, 3995–4021. [Google Scholar] [CrossRef] [PubMed]
- Tartaj, P.; Morales, M.d.P.; Veintemillas-Verdaguer, S.; Gonzalez-Carreno, T.; Serna, C.J. The preparation of magnetic nanoparticles for applications in biomedicine. J. Phys. D Appl. Phys. 2003, 36, R182. [Google Scholar] [CrossRef]
- McNeil, S.E. Nanotechnology for the biologist. J. Leukoc. Biol. 2005, 78, 585–594. [Google Scholar] [CrossRef]
- Frullano, L.; Meade, T.J. Multimodal MRI contrast agents. J. Biol. Inorg. Chem. 2007, 12, 939–949. [Google Scholar] [CrossRef] [PubMed]
- Chouly, C.; Pouliquen, D.; Lucet, I.; Jeune, J.J.; Jallet, P. Development of superparamagnetic nanoparticles for MRI: Effect of particle size, charge and surface nature on biodistribution. J. Microencapsul. 1996, 13, 245–255. [Google Scholar] [CrossRef] [PubMed]
- Moghimi, S.M.; Hunter, A.C.; Murray, J.C. Long-circulating and target-specific nanoparticles: Theory to practice. Pharmacol. Rev. 2001, 53, 283–318. [Google Scholar] [PubMed]
- Gref, R.; Lück, M.; Quellec, P.; Marchand, M.; Dellacherie, E.; Harnisch, S.; Blunk, T.; Müller, R.H. ‘Stealth’ corona-core nanoparticles surface modified by polyethylene glycol (PEG): Influences of the corona (PEG chain length and surface density) and of the core composition on phagocytic uptake and plasma protein adsorption. Colloids Surf. B Biointerfaces 2000, 18, 301–313. [Google Scholar] [CrossRef] [PubMed]
- Puig-de-Morales-Marinkovic, M.; Turner, K.T.; Butler, J.P.; Fredberg, J.J.; Suresh, S. Viscoelasticity of the human red blood cell. Am. J. Physiol. Cell Physiol. 2007, 293, C597–C605. [Google Scholar] [CrossRef] [PubMed]
- Suresh, S. Biomechanics and biophysics of cancer cells. Acta Biomater. 2007, 3, 413–438. [Google Scholar] [CrossRef] [PubMed]
- Cherry, E.M.; Eaton, J.K. A comprehensive model of magnetic particle motion during magnetic drug targeting. Int. J. Multiph. Flow 2014, 59, 173–185. [Google Scholar] [CrossRef]
- Haverkort, J.W.; Kenjeres, S.; Kleijn, C.R. Computational simulations of magnetic particle capture in arterial flows. Ann. Biomed. Eng. 2009, 37, 2436–2448. [Google Scholar] [CrossRef] [PubMed]
- Badfar, H.; Yekani Motlagh, S.; Sharifi, A. Numerical Simulation of Magnetic Drug Targeting to the Stenosis Vessel Using Fe3O4 Magnetic Nanoparticles Under the Effect of Magnetic Field of Wire. Cardiovasc. Eng. Technol. 2020, 11, 162–175. [Google Scholar] [CrossRef]
- Khashan, S.A.; Haik, Y. Numerical simulation of biomagnetic fluid downstream an eccentric stenotic orifice. Phys. Fluids 2006, 18, 113601. [Google Scholar] [CrossRef]
- Akbar, N.S. Metallic nanoparticles analysis for the blood flow in tapered stenosed arteries: Application in nanomedicines. Int. J. Biomath. 2016, 9, 1650002. [Google Scholar] [CrossRef]
- Nadeem, S.; Ijaz, S. Theoretical examination of nanoparticles as a drug carrier with slip effects on the wall of stenosed arteries. Int. J. Heat Mass Transf. 2016, 93, 1137–1149. [Google Scholar] [CrossRef]
- Bietenbeck, M.; Florian, A.; Faber, C.; Sechtem, U.; Yilmaz, A. Remote magnetic targeting of iron oxide nanoparticles for cardiovascular diagnosis and therapeutic drug delivery: Where are we now? Int. J. Nanomed. 2016, 11, 3191–3203. [Google Scholar] [CrossRef] [PubMed]
- Chatzizisis, Y.S.; Baker, A.B.; Sukhova, G.K.; Koskinas, K.C.; Papafaklis, M.I.; Beigel, R.; Jonas, M.; Coskun, A.U.; Stone, B.V.; Maynard, C.; et al. Augmented expression and activity of extracellular matrix-degrading enzymes in regions of low endothelial shear stress colocalize with coronary atheromata with thin fibrous caps in pigs. Circulation 2011, 123, 621–630. [Google Scholar] [CrossRef] [PubMed]
- Katsuki, S.; Matoba, T.; Nakashiro, S.; Sato, K.; Koga, J.; Nakano, K.; Nakano, Y.; Egusa, S.; Sunagawa, K.; Egashira, K. Nanoparticle-mediated delivery of pitavastatin inhibits atherosclerotic plaque destabilization/rupture in mice by regulating the recruitment of inflammatory monocytes. Circulation 2014, 129, 896–906. [Google Scholar] [CrossRef] [PubMed]
- Paul, A.; Shao, W.; Abbasi, S.; Shum-Tim, D.; Prakash, S. PAMAM dendrimer-baculovirus nanocomplex for microencapsulated adipose stem cell-gene therapy: In vitro and in vivo functional assessment. Mol. Pharm. 2012, 9, 2479–2488. [Google Scholar] [CrossRef] [PubMed]
- Liu, F.; Ding, N.; Huo, D.; Yang, G.; Wei, K.; Guan, G.; Li, Y.; Yang, J.; Wang, T.; Wang, Y.; et al. Surface-Engineered Monocyte Inhibits Atherosclerotic Plaque Destabilization via Graphene Quantum Dot-Mediated MicroRNA Delivery. Adv. Healthc. Mater. 2019, 8, 1900386. [Google Scholar] [CrossRef] [PubMed]
- Samal, S.K.; Soenen, S.; Puppi, D.; De Wael, K.; Pati, S.; De Smedt, S.; Braeckmans, K.; Dubruel, P. Bio-Nanohybrid Gelatin/Quantum Dots for Cellular Imaging and Biosensing Applications. Int. J. Mol. Sci. 2022, 23, 11867. [Google Scholar] [CrossRef] [PubMed]
- Nistor, M.T.; Rusu, A.G. Chapter 3—Nanorobots with Applications in Medicine. In Polymeric Nanomaterials in Nanotherapeutics; Vasile, C., Ed.; Elsevier: Amsterdam, The Netherlands, 2019; pp. 123–149. [Google Scholar] [CrossRef]
- Fredman, G.; Kamaly, N.; Spolitu, S.; Milton, J.; Ghorpade, D.; Chiasson, R.; Kuriakose, G.; Perretti, M.; Farokhzad, O.; Tabas, I. Targeted nanoparticles containing the proresolving peptide Ac2-26 protect against advanced atherosclerosis in hypercholesterolemic mice. Sci. Transl. Med. 2015, 7, 275ra220. [Google Scholar] [CrossRef]
- Lin, L.; Chen, L.; Yan, J.; Chen, P.; Du, J.; Zhu, J.; Yang, X.; Geng, B.; Li, L.; Zeng, W. Advances of nanoparticle-mediated diagnostic and theranostic strategies for atherosclerosis. Front. Bioeng. Biotechnol. 2023, 11, 1268428. [Google Scholar] [CrossRef]
- Duan, L.; Yang, L.; Jin, J.; Yang, F.; Liu, D.; Hu, K.; Wang, Q.; Yue, Y.; Gu, N. Micro/nano-bubble-assisted ultrasound to enhance the EPR effect and potential theranostic applications. Theranostics 2020, 10, 462–483. [Google Scholar] [CrossRef] [PubMed]


| Nanocarrier | Therapeutic Agent | Characteristics | Advantages | Disadvantages | Reference No. |
|---|---|---|---|---|---|
| Liposome | PEG-coated | Liposomes have a multi-layered structure that enable the use of a single liposomal formulation as DDS for drugs and contrast agents. | Liposomes are effective carriers for delivering genes, stem cells, as well as anti-inflammatory or antiangiogenic drugs, to the site of plaque formation. Liposomes reduce LDL cholesterol levels and have also been utilised in the development of vaccines targeting atherosclerotic mediators. | The flow of blood in the vessels exerts shear stress on the endothelial wall, which can lead to NPS being washed away from the targeted site and reduce the duration of interaction between NPs and their target within the plaque. There is an urgent need to address the large-scale production of targeted liposomes with various ligands attached to their surface. | [82] |
| Cross-linked dendrimer NPs | Simvastatin acid (SA) | A biomimetic DDS with dual responsiveness to ROS and shear stress for atherosclerotic treatment was developed, which involved loading SA into cross-linked dendrimer NPs (SA PAM). These NPs were then adsorbed onto the surface of RBCs to create SA PAM@RBCs. This novel DDS was designed to respond to both ROS and shear stress, providing a targeted and controlled drug delivery. | SA PAM exhibited the ability to detach from RBC surface when exposed to shear stress. The efficacy of SA PAM@RBCs was evaluated using both the FeCl3 and ApoE−/− models, with results showing superior therapeutic effects compared to free SA. In vivo studies demonstrated excellent safety of SA PAM@RBCs. | - | [96] |
| Micelles | Simvastatin (SV) | A DDS using SV-loaded micelles (SV MC)@RBCs, was developed with a dual responsiveness to ROS and shear stress. This system effectively releases the drug SV in the presence of ROS, offering targeted therapy while minimising the risk of bleeding associated with SV administration. The SV MC@RBCs DDS demonstrates remarkable therapeutic efficacy in the treatment of atherosclerosis, while maintaining excellent safety within the effective dosage range. | SV MC@RBCs effectively inhibit macrophage uptake and prevent systemic clearance, leading to enhanced drug retention. Controlled release of SV at specific sites is achieved through the stimuli-responsive nature of the system, triggered by ROS and high shear stress. SV MC contributes to the reduction of cellular oxidative stress, resulting in a synergistic therapeutic effect. | - | [105] |
| Polymeric PLGA NPs | miRNA-124a and statin atorvastatin (Ato) | Polymeric NPs were modified with an antibody capable of binding to vascular adhesion molecule-1 (VCAM1), which is overexpressed in an inflamed arterial endothelium, resulting in sustained release of the cargoes within the cells. Dual-loaded NPs demonstrated the superior prevention of LDL accumulation within macrophages and greater preservation of cellular morphology compared to the single-loaded NPs. | NPs loaded with Ato and miRNA exhibited non-toxicity to cells across a wide range of concentrations, allowing for a significant reduction in the levels of proinflammatory cytokines IL-6 and TNF-α, as well as ROS, in both LPS-activated macrophages and vessel endothelial cells. | - | [112] |
| Magnetic Fe3O4 NPs | Unspecified drug (numerical simulation) | A numerical simulation was conducted to study the MDT of Fe3O4 NPs coated with drugs to the stenosis region using a magnetic field generated by a wire. | Optimal MDT performance is achieved when the magnetic number is around 164, at which the positive effect of magnetophoresis is high, and the negative effect of vortex formation is low. | Vortices negatively impact the MDT process by causing the drug to diffuse outside the intended target tissue. | [141] |
| Graphene quantum dots (GQDs) | miRNA223 | A new gene delivery system utilising GQDs-miRNA is created through the surface engineering of monocytes. Treating macrophages with gene regulators to inhibit plaque formation proves to be an effective approach in reducing the risk of plaque rupture. | In vivo, the injection of engineered monocytes with modified cell function demonstrates the effective reduction of plaque inflammatory reactions and plaque formation. | The measurement of miR223 concentration or retention in atherosclerotic plaques was not performed in this study. Additional investigations are necessary to determine the appropriate time interval for intravenous administration to achieve sustained regulation. | [149] |
| Nanorobots | Collagen type IV particles | Nanomachines may be directly involved in the treatment process mechanically or chemically, since nanorobots can be used to locate atherosclerotic lesions in stenotic vessels. | Nanorobots can also come pre-loaded with a contrast or therapeutic agent to help them find the target area, prevent infection, and speed up the healing process of inflamed tissues. | The space available for the built-in energy source for efficient controllable propulsion and steering is extremely limited because of the small size of nanorobots. Due to the absence of a proven technology for producing nanorobotic systems, especially for biomedical applications, this domain is still only a dream. | [151] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pang, A.S.-R.; Dinesh, T.; Pang, N.Y.-L.; Dinesh, V.; Pang, K.Y.-L.; Yong, C.L.; Lee, S.J.J.; Yip, G.W.; Bay, B.H.; Srinivasan, D.K. Nanoparticles as Drug Delivery Systems for the Targeted Treatment of Atherosclerosis. Molecules 2024, 29, 2873. https://doi.org/10.3390/molecules29122873
Pang AS-R, Dinesh T, Pang NY-L, Dinesh V, Pang KY-L, Yong CL, Lee SJJ, Yip GW, Bay BH, Srinivasan DK. Nanoparticles as Drug Delivery Systems for the Targeted Treatment of Atherosclerosis. Molecules. 2024; 29(12):2873. https://doi.org/10.3390/molecules29122873
Chicago/Turabian StylePang, Alexander Shao-Rong, Tarini Dinesh, Natalie Yan-Lin Pang, Vishalli Dinesh, Kimberley Yun-Lin Pang, Cai Ling Yong, Shawn Jia Jun Lee, George W. Yip, Boon Huat Bay, and Dinesh Kumar Srinivasan. 2024. "Nanoparticles as Drug Delivery Systems for the Targeted Treatment of Atherosclerosis" Molecules 29, no. 12: 2873. https://doi.org/10.3390/molecules29122873
APA StylePang, A. S.-R., Dinesh, T., Pang, N. Y.-L., Dinesh, V., Pang, K. Y.-L., Yong, C. L., Lee, S. J. J., Yip, G. W., Bay, B. H., & Srinivasan, D. K. (2024). Nanoparticles as Drug Delivery Systems for the Targeted Treatment of Atherosclerosis. Molecules, 29(12), 2873. https://doi.org/10.3390/molecules29122873









