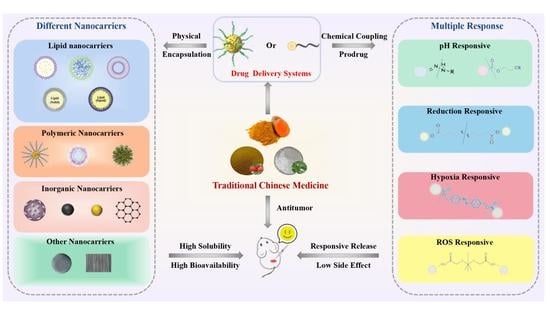
Smart Targeted Delivery Systems for Enhancing Antitumor Therapy of Active Ingredients in Traditional Chinese Medicine
Abstract
1. Introduction
2. Methodology
3. Nano-Delivery Systems Loaded with Antitumor Active Ingredients of TCM
3.1. Lipid Nano-Delivery System
3.1.1. Liposomes
3.1.2. Solid Lipid Nanoparticles (SLNs)
3.1.3. Nanostructured Lipid Carrier (NLCs)
3.1.4. Microemulsion and Self-Micro Emulsion Drug-Delivery System
3.2. Polymeric Nano-Delivery Systems
3.2.1. Polymer Micelles
3.2.2. Polymer Nanoparticles
- (1)
- Achieve responsive drug release, thereby increasing its therapeutic effect [58,59]. For example, He et al. [80] synthesized a polymer nanoparticle self-assembled by photoactivated metal polymer polymerization (Ru/PTX), where the photosensitizer Ru complex and PTX can attach to the polymer network and be delivered simultaneously to the tumor site through EPR effects. Singlet oxygen (1O2) produced by the photosensitizer after red light irradiation further triggers the release of PTX to achieve the combination of photodynamic and chemotherapy therapy. The results showed that the light-triggered cascaded drug release polymer nanoplatform could effectively reduce the non-specific release of drugs, and the tumor growth inhibition rate reached approximately 65%. In an in vivo antitumor study, 4T1 tumor-bearing mice could induce the photolysis of Poly (Ru/PTX) after local illumination, promote the release of PTX, and exert an anti-tumor effect, which is a feasible on-demand drug delivery strategy.
- (2)
- Polylactic acid, polyglycolic acid, and their copolymer poly (propylene glycol glycolic acid) (PLGA) are widely used due to their stability and controlled drug release. Snima et al. [81] prepared silymarin-supported PLGA nanoparticles (SNPs) by emulsifying solvent volatilization. The nanoparticle not only has good serum stability and blood compatibility, but also has good loading (the encapsulation rate is 60%) and drug-release ability. The results showed that the drug release was slow and continuous under physiological conditions, and the release rate reached 78% at 120 h. In vitro experiments demonstrated the potential of this nanoparticle in the treatment of prostate cancer. Flow results showed that SNPs could induce 63.6% apoptosis of PC-3 cells and inhibit tumor cell migration. Although PLGA-based nanoparticles show strong therapeutic effects in in vitro studies, high doses of organic materials during delivery in vivo may increase toxicity, and nanoparticles are easily recognized and cleared by the immune system. The delivery efficiency of solid tumors is greatly reduced [82]. Therefore, in order to improve the delivery effect of nanoparticles in vivo, Song et al. [83] developed a PH-sensitive bionic drug-delivery system (FRCS NPs) covered by erythrocyte membranes. The nanoparticle has an exquisite core–shell structure, and the core nanoparticle is formed from a natural polymer, sodium carboxymethyl cellulose, and stearic acid, which are self-assembled. Sodium carboxymethylcellulose is highly sensitive to the weakly acidic pH of the tumor microenvironment and promotes the responsive release of PTX. The nanoparticles coated with DSPE-polyethylene glycol (PEG) -FA-modified erythrocyte membrane can extend the cycle time in vivo and enhance the tumor-targeting ability. By observing the biological distribution of FRCS NPs in vivo, it was found that the fluorescence signal was strongest in tumors, while there was almost no fluorescence signal in organs such as the liver. Compared with free PTX, xenograft HepG2 tumors were effectively suppressed after caudal vein injection of FRCS NPs, and the inhibition rate reached 48.6%. In addition, FRCS NPs also have good safety in vivo, essentially have no effect on the liver function of mice, and can also reduce PTX-induced renal toxicity.
- (3)
- Polymeric nanoparticles can be easily modified to develop various functional nanoparticles. For instance, in order to enhance the antitumor effect, researchers investigated the use of dongle-in (ORI) poly (D, L-lactic acid) (PLA) nanoparticles [84]. Additionally, PLA nanoparticles were modified by arginine-glycine-aspartate peptide (RGD) to enhance H22 cells targeting. After dosing in tumor-bearing mice, RGD-modified RGD-ORI-PLA nanoparticles exhibited higher antitumor activity compared to non-targeted ORI-PLA-NPs.
- (4)
- Polymer nanoparticles can achieve dual-drug codelivery with drugs with different anticancer mechanisms. Due to the complexity of tumor formation, the chemotherapy effect of a single drug may be limited, so the simultaneous delivery of more than two drugs targeting different anti-cancer pathways is important to improve anti-cancer efficacy and reduce side effects. For example, Dox is a commonly used chemotherapy drug for the treatment of advanced liver cancer, which can cause DNA damage and promote apoptosis. CUR, as a common anti-tumor active component of TCM, not only has anti-angiogenesis activity but also has high safety. However, the physicochemical properties and pharmacokinetics of these two drugs are different, and it is difficult to achieve combined administration. Therefore, Zhang et al. [85] prepared a pH-sensitive polymer-based D-A-tophenol polyethylene glycol 1000 block poly (B-aminoester) (TPGS-PAE) self-assembled nanoparticle (D + C)/NPs for co-delivery of Dox and Cur, which can quickly release drugs into the acidic environment of cancer cells to achieve two-drug synergistic therapy. In vitro studies showed that the cytotoxicity of dual-drug co-delivery micelles was better than that of free single-drug micelles and showed stronger pro-apoptotic activity (an apoptosis rate of 76.2%). In addition, the total amount of Akt, mTOR, Erk, and FAK in D + C/NP-treated cells did not change, which proved that D + C/NPs could achieve an anti-angiogenesis effect by inhibiting the pathway induced by VEGF and is a dual-drug co-delivery platform with good application prospects. In the process of co-delivery, enhancing the targeting of nanoparticles is helpful to achieve accurate delivery of dual drugs. Kim et al. coupled the integrin ring (arginine-glycine-aspartic acid-phenylalanine-lysine) (cRGDfK) with active targeting and sulfonyl cyanide 5.5 (Cy5.5) with PEG-PLGA, respectively. Functionalized polymer nanoparticles based on polyethylene glycol -PLGA were formed via self-assembly for the targeted co-delivery of CUR and PTX to breast cancer [86]. The existence of cRGDfK significantly enhanced the uptake of nanoparticles by tumor cells, and CLSM images showed that the dual-drug co-delivery system had stronger red fluorescence in 4T1 cells, with a fluorescence intensity 1.83-fold that of passively targeted NPs. In vivo imaging showed that the fluorescence intensity of Cy5.5-cRGDfK-NPs/PTX + CUR was the highest and obviously concentrated at the tumor site, which also indicated that cRGDfK had a high affinity for integrins on the surface of 4T1 cells. Of note, CUR as a P-gp inhibitor combined with PTX can reverse the resistance of breast cancer to PTX and enhance the therapeutic effect. Compared with free PTX and CUR, the tumor volume of mice treated with dual-drug co-delivery nanoparticles for 18 days was only 400 mm3. Overall, the Cy5.5-cRGDfK-NPs/PTX + CUR showed good therapeutic potential for breast cancer.
3.2.3. Dendrimers
3.2.4. Biopolymer-Based Nanocarriers
- (1)
- Chitosan, a natural biopolymer, has been developed for the encapsulated delivery of traditional Chinese medicine active ingredients such as curcumin [94] and trans-resveratrol [95] due to its better biodegradability and targeting. Xu et al. [96] designed a polymer chain with active targeting, pH response, and imaging capability to form multi-functional polymer CS-BT-HBS-CB micelles via self-assembly for in vivo delivery of paclitaxel. Structurally, HBS with an aggregation-induced emission (AIE) effect is helpful to monitor micellar carrier delivery. CLSM images show that the delivery system can be effectively internalized by MCF-7 cells, and the yellow fluorescence in the cells is gradually enhanced with the increase in incubation time. This may be the result of targeted delivery mediated by biotin (BT) in the polymer chain. In addition, the breakdown of the benzoate imide bond at weakly acidic pH triggers a responsive drug release, with 80.8% of PTX released in vitro at pH 5.0 and only 33.3% at pH 7.4. This responsive drug release property not only reduces the toxic side effects of PTX, but also increases the anti-tumor effect of the polymer delivery system, with a tumor inhibition rate of 66.9%. In general, this biopolymer-based delivery system designed based on chitosan has good biocompatibility and superior anti-tumor efficacy, showing broad application prospects.
- (2)
- The hydrogels of biopolymers have a cross-linked polymer network that provides space for hydrophilic polymer chains to accommodate aqueous biofluids with good biocompatibility. Some of these hydrogels undergo phase changes when stimulated by the external environment; at the same time, the drug is released in a controlled manner with the change in its physical properties [97,98]. For example, curcumin can already be encapsulated in nanogels based on folic acid and casein for the treatment of skin cancer. Priya et al. [99] fabricated CUR-loaded nanogels (NGs) using a layer-by-layer technique (LbL) and modified NGs with folic acid (FA) and casein for drug delivery in skin cancer. Quantification of cell viability by MTT assay and light microscopy images showed that NG-scanned CURs were delivered directly to tumor cells, and the nanogels showed enhanced targeting ability to tumor cells and exhibited superior cytotoxicity in tumor cells due to folic acid receptor-mediated endocytosis, improving efficacy and reducing drug side effects.
3.3. Metallic Nanocarrier
3.4. Miscellaneous Approaches
4. Covalently Combined Pro-Drug-Delivery Systems
4.1. pH-Responsive Pro-Drug-Delivery Systems
4.2. Reduction-Responsive Pro-Drug-Delivery Systems
4.3. Hypoxia Responsive Pro-Drug-Delivery Systems
4.4. Reactive Oxygen Responsive Pro-Drug-Delivery Systems
4.5. Multi-Response Pro-Drug-Delivery Systems
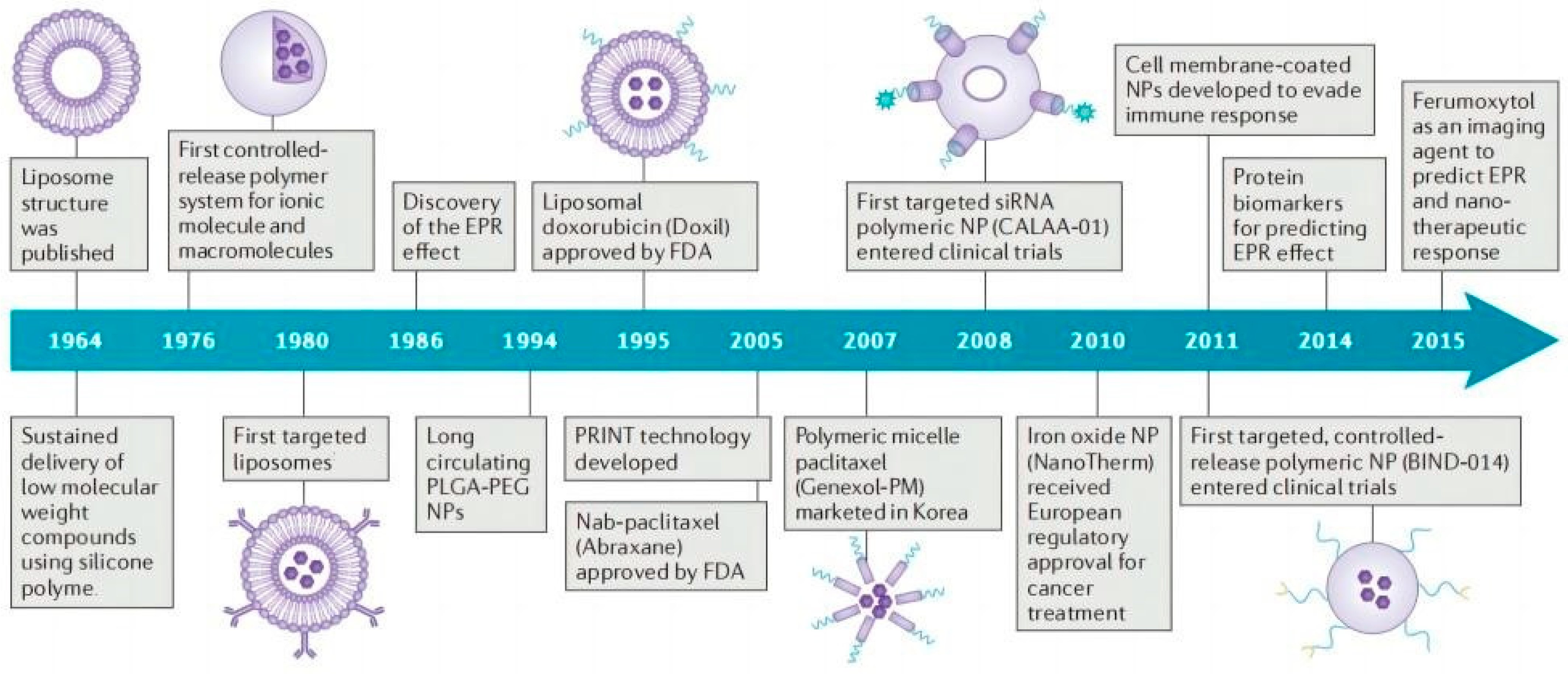
5. The Clinical Studies Based on Nanocarriers for TCM Applications
6. Limitations of Nanocarriers for TCM Applications and the Potential Toxicity
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Bray, F.; Ferlay, J.; Soerjomataram, I.; Siegel, R.L.; Torre, L.A.; Jemal, A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2018, 68, 394–424. [Google Scholar] [CrossRef] [PubMed]
- Slamon, D.J.; Leyland-Jones, B.; Fau-Shak, S.; Shak, S.; Fau-Fuchs, H.; Fuchs, H.; Fau-Paton, V.; Paton, V.; Fau-Bajamonde, A.; Bajamonde, A. Use of chemotherapy plus a monoclonal antibody against HER2 for metastatic breast cancer that overexpresses HER2. N. Engl. J. Med. 2001, 344, 783–792. [Google Scholar] [CrossRef]
- Van Cutsem, E.; Köhne, C.H.; Hitre, E.; Zaluski, J.; Chang Chien, C.R.; Makhson, A.; D’Haens, G.; Pintér, T.; Lim, R.; Rougier, P.; et al. Cetuximab and chemotherapy as initial treatment for metastatic colorectal cancer. N. Engl. J. Med. 2009, 360, 1408–1417. [Google Scholar] [CrossRef] [PubMed]
- Normile, D. The new face of traditional Chinese medicine. Science 2003, 299, 188–190. [Google Scholar] [CrossRef]
- Yang, Z.; Liao, X.; Lu, Y.; Xu, Q.; Tang, B.; Chen, X.; Yu, Y. Add-On Therapy with Traditional Chinese Medicine Improves Outcomes and Reduces Adverse Events in Hepatocellular Carcinoma: A Meta-Analysis of Randomized Controlled Trials. Evid. Based Complement. Altern. Med. 2017, 2017, 3428253. [Google Scholar] [CrossRef] [PubMed]
- Kalepu, S.; Nekkanti, V. Improved delivery of poorly soluble compounds using nanoparticle technology: A review. Drug Deliv. Transl. Res. 2016, 6, 319–332. [Google Scholar] [CrossRef]
- Ramalingam, P.; Ko, Y.T. Improved oral delivery of resveratrol from N-trimethyl chitosan-g-palmitic acid surface-modified solid lipid nanoparticles. Colloids Surf. B Biointerfaces 2016, 139, 52–61. [Google Scholar] [CrossRef]
- Sun, R.; Dai, J.; Ling, M.; Yu, L.; Yu, Z.; Tang, L. Delivery of triptolide: A combination of traditional Chinese medicine and nanomedicine. J. Nanobiotechnol. 2022, 20, 194. [Google Scholar] [CrossRef]
- Qiao, L.; Han, M.; Gao, S.; Shao, X.; Wang, X.; Sun, L.; Fu, X.; Wei, Q. Research progress on nanotechnology for delivery of active ingredients from traditional Chinese medicines. J. Mater. Chem. B 2020, 8, 6333–6351. [Google Scholar] [CrossRef]
- Liu, S.-H.; Chen, P.-S.; Huang, C.-C.; Hung, Y.-T.; Lee, M.-Y.; Lin, W.-H.; Lin, Y.-C.; Lee, A.Y.-L. Unlocking the Mystery of the Therapeutic Effects of Chinese Medicine on Cancer. Front. Pharmacol. 2021, 11, 601785. [Google Scholar] [CrossRef]
- Ahmed, K.S.; Hussein, S.A.; Ali, A.H.; Korma, S.A.; Lipeng, Q.; Jinghua, C. Liposome: Composition, characterisation, preparation, and recent innovation in clinical applications. J. Drug Target. 2019, 27, 742–761. [Google Scholar] [CrossRef]
- Shi, J.; Kantoff, P.W.; Wooster, R.; Farokhzad, O.C. Cancer nanomedicine: Progress, challenges and opportunities. Nat. Rev. Cancer 2017, 17, 20–37. [Google Scholar] [CrossRef]
- Zheng, Y.; Wang, Y.; Xia, M.; Gao, Y.; Zhang, L.; Song, Y.; Zhang, C. The combination of nanotechnology and traditional Chinese medicine (TCM) inspires the modernization of TCM: Review on nanotechnology in TCM-based drug delivery systems. Drug Deliv. Transl. Res. 2021, 12, 1306–1325. [Google Scholar] [CrossRef] [PubMed]
- Duan, X.; Xiao, J.; Yin, Q.; Zhang, Z.; Yu, H.; Mao, S.; Li, Y. Smart pH-sensitive and temporal-controlled polymeric micelles for effective combination therapy of doxorubicin and disulfiram. ACS Nano 2013, 7, 5858–5869. [Google Scholar] [CrossRef]
- Li, C.; Wang, J.; Wang, Y.; Gao, H.; Wei, G.; Huang, Y.; Jin, Y. Recent progress in drug delivery. Acta Pharm. Sin. B 2019, 9, 1145–1162. [Google Scholar] [CrossRef]
- Behravan, N.; Zahedipour, F.; Jaafari, M.R.; Johnston, T.P.; Sahebkar, A. Lipid-based nanoparticulate delivery systems for HER2-positive breast cancer immunotherapy. Life Sci. 2022, 291, 120294. [Google Scholar] [CrossRef]
- Vieira, I.R.S.; Conte-Junior, C.A. Nano-delivery systems for food bioactive compounds in cancer: Prevention, therapy, and clinical applications. Crit. Rev. Food Sci. Nutr. 2022, 8, 1–26. [Google Scholar] [CrossRef]
- Lu, Y.; Pan, X.; Nie, Q.; Zhou, Z.; Dai, X.; Liu, O. Administration methods of lipid-based nanoparticle delivery systems for cancer treatment. Biomater. Sci. 2023, 11, 3800–3812. [Google Scholar] [CrossRef] [PubMed]
- You, J.; Li, X.; de Cui, F.; Du, Y.-Z.; Yuan, H.; Hu, F.Q. Folate-conjugated polymer micelles for active targeting to cancer cells: Preparation, in vitro evaluation of targeting ability and cytotoxicity. Nanotechnology 2008, 19, 045102. [Google Scholar] [CrossRef] [PubMed]
- Sezgin, Z.; Yuksel, N.; Baykara, T. Investigation of pluronic and PEG-PE micelles as carriers of meso-tetraphenyl porphine for oral administration. Int. J. Pharm. 2007, 332, 161–167. [Google Scholar] [CrossRef]
- Zheng, S.; Chang, S.; Lu, J.; Chen, Z.; Xie, L.; Nie, Y.; Gu, Z. Characterization of 9-Nitrocamptothecin Liposomes: Anticancer Properties and Mechanisms on Hepatocellular Carcinoma In Vitro and In Vivo. PLoS ONE 2011, 6, e21064. [Google Scholar] [CrossRef] [PubMed]
- Lin, J.; Wang, X.; Wu, Q.; Dai, J.; Guan, H.; Cao, W.; Wang, Y. Development of Salvianolic acid B-Tanshinone II A-Glycyrrhetinic acid compound liposomes: Formulation optimization and its effects on proliferation of hepatic stellate cells. Int. J. Pharm. 2014, 462, 11–18. [Google Scholar] [CrossRef] [PubMed]
- Tang, W.-H.; Szeitz, A.; Kulkarni, J.; Cullis, P.; Li, S.-D. Systemic study of solvent-assisted active loading of gambogic acid into liposomes and its formulation optimization for improved delivery. Biomaterials 2018, 166, 13–26. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Yu, J.; Li, D.; Zhao, L.; Sun, B.; Wang, J.; Wang, Z.; Zhou, S.; Wang, M.; Yang, Y.; et al. Paclitaxel derivative-based liposomal nanoplatform for potentiated chemo-immunotherapy. J. Control. Release 2021, 341, 812–827. [Google Scholar] [CrossRef]
- Chen, H.; Wu, J.; Sun, M.; Guo, C.; Yu, A.; Cao, F.; Zhao, L.; Tan, Q.; Zhai, G. N-trimethyl chitosan chloride-coated liposomes for the oral delivery of curcumin. J. Liposome Res. 2011, 22, 100–109. [Google Scholar] [CrossRef]
- Li, J.; Chen, J.; Cai, B.-C.; Yang, T. Preparation, characterization and tissue distribution of brucine stealth liposomes with different lipid composition. Pharm. Dev. Technol. 2013, 18, 772–778. [Google Scholar] [CrossRef]
- Chen, J.; Yan, G.J.; Hu, R.R.; Gu, Q.W.; Chen, M.L.; Gu, W.; Cai, B.C. Improved pharmacokinetics and reduced toxicity of brucine after encapsulation into stealth liposomes: Role of phosphatidylcholine. Int. J. Nanomed. 2012, 7, 3567–3577. [Google Scholar] [CrossRef]
- Xin, X.; Liu, W.; Zhang, Z.-A.; Han, Y.; Qi, L.-L.; Zhang, Y.-Y.; Zhang, X.-T.; Duan, H.-X.; Chen, L.-Q.; Jin, M.-J.; et al. Efficient Anti-Glioma Therapy through the Brain-Targeted RVG15-Modified Liposomes Loading Paclitaxel-Cholesterol Complex. Int. J. Nanomed. 2021, 16, 5755–5776. [Google Scholar] [CrossRef]
- Wang, W.; Sun, H.; Gong, Y.; Liu, X.; Liu, X.; Wang, M.; Li, S.; Li, J.; Zhu, L.; Meng, H. Ratiometric co-delivery of hydroxychloroquine and calculated low-dose paclitaxel efficiently suppresses tumor growth in hepatocellular carcinoma mouse models in vivo. Nano Today 2022, 44, 101446. [Google Scholar] [CrossRef]
- Li, R.T.; Zhu, Y.D.; Li, W.Y.; Hou, Y.K.; Zou, Y.M.; Zhao, Y.H.; Zou, Q.; Zhang, W.H.; Chen, J.X. Synergistic photothermal-photodynamic-chemotherapy toward breast cancer based on a liposome-coated core-shell AuNS@NMOFs nanocomposite encapsulated with gambogic acid. J. Nanobiotechnol. 2022, 20, 212. [Google Scholar] [CrossRef]
- Zhu, Y.; Wang, A.; Zhang, S.; Kim, J.; Xia, J.; Zhang, F.; Wang, D.; Wang, Q.; Wang, J. Paclitaxel-loaded ginsenoside Rg3 liposomes for drug-resistant cancer therapy by dual targeting of the tumor microenvironment and cancer cells. J. Adv. Res. 2023, 49, 159–173. [Google Scholar] [CrossRef] [PubMed]
- Dang, W.; Guo, P.; Song, X.; Zhang, Y.; Li, N.; Yu, C.; Xing, B.; Liu, R.; Jia, X.; Zhang, Q.; et al. Nuclear Targeted Peptide Combined with Gambogic Acid for Synergistic Treatment of Breast Cancer. Front. Chem. 2021, 9, 821426. [Google Scholar] [CrossRef] [PubMed]
- Wei, D.; Yang, H.; Zhang, Y.; Zhang, X.; Wang, J.; Wu, X.; Chang, J. Nano-traditional Chinese medicine: A promising strategy and its recent advances. J. Mater. Chem. B 2022, 10, 2973–2994. [Google Scholar] [CrossRef]
- Widjaya, A.S.; Liu, Y.; Yang, Y.; Yin, W.; Liang, J.; Jiang, Y. Tumor-permeable smart liposomes by modulating the tumor microenvironment to improve the chemotherapy. J. Control. Release 2022, 344, 62–79. [Google Scholar] [CrossRef]
- Sang, R.; Stratton, B.; Engel, A.; Deng, W. Liposome technologies towards colorectal cancer therapeutics. Acta Biomater. 2021, 127, 24–40. [Google Scholar] [CrossRef] [PubMed]
- Olusanya, T.O.B.; Haj Ahmad, R.R.; Ibegbu, D.M.; Smith, J.R.; Elkordy, A.A. Liposomal drug delivery systems and anticancer drugs. Molecules 2018, 23, 907. [Google Scholar] [CrossRef] [PubMed]
- Kang, M.H.; Yoon, H.Y.; Choi, Y.W. RIPL peptide as a novel cell-penetrating and homing peptide: Design, characterization, and application to liposomal nanocarriers for hepsin-specific intracellular drug delivery. In Nanostructures for Cancer Therapy; Elsevier: Amsterdam, The Netherlands, 2017; pp. 129–157. [Google Scholar] [CrossRef]
- Irache, J.M.; Esparza, I.; Gamazo, C.; Agüeros, M.; Espuelas, S. Nanomedicine: Novel approaches in human and veterinary therapeutics. Vet. Parasitol. 2011, 180, 47–71. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Chen, T.; Xu, H.; Ren, B.; Cheng, X.; Qi, R.; Liu, H.; Wang, Y.; Yan, L.; Chen, S.; et al. Curcumin-Loaded Solid Lipid Nanoparticles Enhanced Anticancer Efficiency in Breast Cancer. Molecules 2018, 23, 1578. [Google Scholar] [CrossRef]
- Jang, D.-J.; Moon, C.; Oh, E. Improved tumor targeting and antitumor activity of camptothecin loaded solid lipid nanoparticles by preinjection of blank solid lipid nanoparticles. Biomed. Pharmacother. 2016, 80, 162–172. [Google Scholar] [CrossRef]
- Nasirizadeh, S.; Jaafari, M.R.; Iranshahi, M.; Golmohammadzadeh, S.; Mahmoudi, A.; Ansari, L.; Mosallaei, N.; Malaekeh-Nikouei, B. The effect of efflux pump inhibitors on in vitro and in vivo efficacy of solid lipid nanoparticles containing SN38. J. Drug Deliv. Sci. Technol. 2020, 60, 101969. [Google Scholar] [CrossRef]
- Pi, C.; Zhao, W.; Zeng, M.; Yuan, J.; Shen, H.; Li, K.; Su, Z.; Liu, Z.; Wen, J.; Song, X.; et al. Anti-lung cancer effect of paclitaxel solid lipid nanoparticles delivery system with curcumin as co-loading partner in vitro and in vivo. Drug Deliv. 2022, 29, 1878–1891. [Google Scholar] [CrossRef] [PubMed]
- German-Cortés, J.; Vilar-Hernández, M.; Rafael, D.; Abasolo, I.; Andrade, F. Solid Lipid Nanoparticles: Multitasking Nano-Carriers for Cancer Treatment. Pharmaceutics 2023, 15, 831. [Google Scholar] [CrossRef] [PubMed]
- Sivadasan, D.; Ramakrishnan, K.; Mahendran, J.; Ranganathan, H.; Karuppaiah, A.; Rahman, H. Solid Lipid Nanoparticles: Applications and Prospects in Cancer Treatment. Int. J. Mol. Sci. 2023, 24, 6199. [Google Scholar] [CrossRef]
- Xu, W.; Lee, M.-K. Development and evaluation of lipid nanoparticles for paclitaxel delivery: A comparison between solid lipid nanoparticles and nanostructured lipid carriers. J. Pharm. Investig. 2015, 45, 675–680. [Google Scholar] [CrossRef]
- Battaglia, L.; Gallarate, M. Lipid nanoparticles: State of the art, new preparation methods and challenges in drug delivery. Expert Opin. Drug Deliv. 2012, 9, 497–508. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Feng, N. Nanocarriers for the delivery of active ingredients and fractions extracted from natural products used in traditional Chinese medicine (TCM). Adv. Colloid Interface Sci. 2015, 221, 60–76. [Google Scholar] [CrossRef] [PubMed]
- Marathe, S.; Shadambikar, G.; Mehraj, T.; Sulochana, S.P.; Dudhipala, N.; Majumdar, S. Development of α-Tocopherol Succinate-Based Nanostructured Lipid Carriers for Delivery of Paclitaxel. Pharmaceutics 2022, 14, 1034. [Google Scholar] [CrossRef]
- Chen, Y.; Yuan, L.; Congyan, L.; Zhang, Z.; Zhou, L.; Qu, D. Antitumor activity of tripterine via cell-penetrating peptide-coated nanostructured lipid carriers in a prostate cancer model. Int. J. Nanomed. 2013, 8, 4339–4350. [Google Scholar] [CrossRef]
- Akanda, M.; Getti, G.; Douroumis, D. In vivo evaluation of nanostructured lipid carrier systems (NLCs) in mice bearing prostate cancer tumours. Drug Deliv. Transl. Res. 2021, 13, 2083–2095. [Google Scholar] [CrossRef]
- Dolatabadi, S.; Karimi, M.; Nasirizadeh, S.; Hatamipour, M.; Golmohammadzadeh, S.; Jaafari, M.R. Preparation, characterization and in vivo pharmacokinetic evaluation of curcuminoids-loaded solid lipid nanoparticles (SLNs) and nanostructured lipid carriers (NLCs). J. Drug Deliv. Sci. Technol. 2021, 62, 102352. [Google Scholar] [CrossRef]
- Lages, E.B.; Fernandes, R.S.; de Oliveira Silva, J.; de Souza, Â.M.; Cassali, G.D.; de Barros, A.L.B.; Ferreira, L.A.M. Co-delivery of doxorubicin, docosahexaenoic acid, and α-tocopherol succinate by nanostructured lipid carriers has a synergistic effect to enhance antitumor activity and reduce toxicity. Biomed. Pharmacother. 2020, 132, 110876. [Google Scholar] [CrossRef] [PubMed]
- Iqbal, A.; Shadab; Sahni, J.K.; Baboota, S.; Dang, S.; Ali, J. Nanostructured lipid carriers system: Recent advances in drug delivery. J. Drug Target. 2012, 20, 813–830. [Google Scholar] [CrossRef]
- Muller, R.H.; Shegokar, R.; Keck, C.M. 20 Years of Lipid Nanoparticles (SLN & NLC): Present State of Development & Industrial Applications. Curr. Drug Discov. Technol. 2011, 8, 207–227. [Google Scholar] [CrossRef] [PubMed]
- Rahdar, A.; Hajinezhad, M.R.; Sargazi, S.; Zaboli, M.; Barani, M.; Baino, F.; Bilal, M.; Sanchooli, E. Biochemical, Ameliorative and Cytotoxic Effects of Newly Synthesized Curcumin Microemulsions: Evidence from In Vitro and In Vivo Studies. Nanomaterials 2021, 11, 817. [Google Scholar] [CrossRef]
- Bolko, K.; Zvonar, A.; Gašperlin, M. Mixed lipid phase SMEDDS as an innovative approach to enhance resveratrol solubility. Drug Dev. Ind. Pharm. 2014, 40, 102–109. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Huang, P.; Hou, X.; Yan, F.; Jiang, Z.; Shi, J.; Xie, X.; Shen, J.; Fan, Q.; Wang, Z.; et al. Hybrid curcumin–phospholipid complex-near-infrared dye oral drug delivery system to inhibit lung metastasis of breast cancer. Int. J. Nanomed. 2019, 14, 3311–3330. [Google Scholar] [CrossRef]
- Theochari, I.; Goulielmaki, M.; Danino, D.; Papadimitriou, V.; Pintzas, A.; Xenakis, A. Drug nanocarriers for cancer chemotherapy based on microemulsions: The case of Vemurafenib analog PLX4720. Colloids Surf. B Biointerfaces 2017, 154, 350–356. [Google Scholar] [CrossRef]
- Fanun, M. Microemulsions as delivery systems. Curr. Opin. Colloid Interface Sci. 2012, 17, 306–313. [Google Scholar] [CrossRef]
- Farboudi, A.; Nouri, A.; Shirinzad, S.; Sojoudi, P.; Davaran, S.; Akrami, M.; Irani, M. Synthesis of magnetic gold coated poly (ε-caprolactonediol) based polyurethane/poly(N-isopropylacrylamide)-grafted-chitosan core-shell nanofibers for controlled release of paclitaxel and 5-FU. Int. J. Biol. Macromol. 2020, 150, 1130–1140. [Google Scholar] [CrossRef]
- Gao, C.; Zhang, L.; Xu, M.; Luo, Y.; Wang, B.; Kuang, M.; Liu, X.; Sun, M.; Guo, Y.; Teng, L.; et al. Pulmonary delivery of liposomes co-loaded with SN38 prodrug and curcumin for the treatment of lung cancer. Eur. J. Pharm. Biopharm. 2022, 179, 156–165. [Google Scholar] [CrossRef]
- Zahiri, M.; Taghdisi, S.M.; Abnous, K.; Ramezani, M.; Alibolandi, M. Fabrication of versatile targeted lipopolymersomes for improved camptothecin efficacy against colon adenocarcinoma in vitro and in vivo. Expert Opin. Drug Deliv. 2021, 18, 1309–1322. [Google Scholar] [CrossRef] [PubMed]
- Cheng, X.; Yu, P.; Zhou, X.; Zhu, J.; Han, Y.; Zhang, C.; Kong, L. Enhanced tumor homing of pathogen-mimicking liposomes driven by R848 stimulation: A new platform for synergistic oncology therapy. Acta Pharm. Sin. B 2022, 12, 924–938. [Google Scholar] [CrossRef] [PubMed]
- Xie, Y.; Ren, Z.; Chen, H.; Tang, H.; Zhu, M.; Lv, Z.; Bao, H.; Zhang, Y.; Liu, R.; Shen, Y.; et al. A novel estrogen-targeted PEGylated liposome co-delivery oxaliplatin and paclitaxel for the treatment of ovarian cancer. Biomed. Pharmacother. 2023, 160, 114304. [Google Scholar] [CrossRef] [PubMed]
- Dawoud, M. Chitosan coated solid lipid nanoparticles as promising carriers for docetaxel. J. Drug Deliv. Sci. Technol. 2021, 62, 102409. [Google Scholar] [CrossRef]
- Jagdale, S.; Narwade, M.; Sheikh, A.; Shadab; Salve, R.; Gajbhiye, V.; Kesharwani, P.; Gajbhiye, K.R. GLUT1 transporter-facilitated solid lipid nanoparticles loaded with anti-cancer therapeutics for ovarian cancer targeting. Int. J. Pharm. 2023, 637, 122894. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Zhou, M.; Xu, Y.; Peng, W.; Zhang, S.; Li, R.; Zhang, H.; Zhang, H.; Cheng, S.; Wang, Y.; et al. Resveratrol-Loaded TPGS-Resveratrol-Solid Lipid Nanoparticles for Multidrug-Resistant Therapy of Breast Cancer: In Vivo and In Vitro Study. Front. Bioeng. Biotechnol. 2021, 9, 762489. [Google Scholar] [CrossRef]
- Kim, C.H.; Lee, T.H.; Kim, B.D.; Kim, H.K.; Lyu, M.J.; Jung, H.M.; Goo, Y.T.; Kang, M.J.; Lee, S.; Choi, Y.; et al. Technology, Co-administration of tariquidar using functionalized nanostructured lipid carriers overcomes resistance to docetaxel in multidrug resistant MCF7/ADR cells. J. Drug Deliv. Sci. Technol. 2022, 71, 103323. [Google Scholar] [CrossRef]
- Rawal, S.; Bora, V.; Patel, B.; Patel, M. Surface-engineered nanostructured lipid carrier systems for synergistic combination oncotherapy of non-small cell lung cancer. Drug Deliv. Transl. Res. 2021, 11, 2030–2051. [Google Scholar] [CrossRef]
- de Moura, L.D.; Ribeiro, L.N.M.; de Carvalho, F.V.; Rodrigues da Silva, G.H.; Lima Fernandes, P.C.; Brunetto, S.Q.; Ramos, C.D.; Velloso, L.A.; de Araújo, D.R.; de Paula, E.; et al. Docetaxel and Lidocaine Co-Loaded (NLC-in-Hydrogel) Hybrid System Designed for the Treatment of Melanoma. Pharmaceutics 2021, 13, 1552. [Google Scholar] [CrossRef]
- Meher, J.G.; Dixit, S.; Singh, Y.; Pawar, V.K.; Konwar, R.; Saklani, R.; Chourasia, M.K. Paclitaxel-Loaded Colloidal Silica and TPGS-Based Solid Self-Emulsifying System Interferes Akt/mTOR Pathway in MDA-MB-231 and Demonstrates Anti-tumor Effect in Syngeneic Mammary Tumors. AAPS PharmSciTech 2020, 21, 313. [Google Scholar] [CrossRef]
- Fang, T.; Ye, Z.; Chen, X.; Wang, Y.; Wan, J.; Wang, H. Repurposing of camptothecin: An esterase-activatable prodrug delivered by a self-emulsifying formulation that improves efficacy in colorectal cancer. Int. J. Pharm. 2021, 599, 120399. [Google Scholar] [CrossRef] [PubMed]
- Campani, V.; Salaroglio, I.C.; Nele, V.; Kopecka, J.; Bernkop-Schnürch, A.; Riganti, C.; De Rosa, G. Targeted Self-Emulsifying Drug Delivery Systems to Restore Docetaxel Sensitivity in Resistant Tumors. Pharmaceutics 2022, 14, 292. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Zhao, L.; Chu, L.; Han, X.; Zhai, G. Preparation, optimization, characterization and cytotoxicity in vitro of Baicalin-loaded mixed micelles. J. Colloid Interface Sci. 2014, 434, 40–47. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Li, J.; He, X.; Zhang, B.; Liu, C.; Li, Q.; Zhu, Y.; Huang, W.; Zhang, W.; Qian, H.; et al. Histology and antitumor activity study of PTX-loaded micelle, a fluorescent drug delivery system prepared by PEG-TPP. Chin. Chem. Lett. 2019, 30, 1083–1088. [Google Scholar] [CrossRef]
- Liu, Y.; Chen, F.; Zhang, K.; Wang, Q.; Chen, Y.; Luo, X. pH-Responsive reversibly cross-linked micelles by phenol–yne click via curcumin as a drug delivery system in cancer chemotherapy. J. Mater. Chem. B 2019, 7, 3884–3893. [Google Scholar] [CrossRef]
- Qu, Q.; Wang, Y.; Zhang, L.; Zhang, X.; Zhou, S. A Nanoplatform with Precise Control over Release of Cargo for Enhanced Cancer Therapy. Small 2016, 12, 1378–1390. [Google Scholar] [CrossRef]
- Tang, L.; Liu, X.X.; Yang, X.D.; Tan, S.; Zou, Z.W. A compound formulation of EGF-modified paclitaxel micelles and EGF-modified emodin micelles enhance the therapeutic effect of ovarian cancer. J. Liposome Res. 2023, 33, 89–101. [Google Scholar] [CrossRef]
- Jin, G.-W.; Rejinold, N.S.; Choy, J.-H. Multifunctional Polymeric Micelles for Cancer Therapy. Polymers 2022, 14, 4839. [Google Scholar] [CrossRef]
- He, M.; He, G.; Wang, P.; Jiang, S.; Jiao, Z.; Xi, D.; Miao, P.; Leng, X.; Wei, Z.; Li, Y.; et al. A Sequential Dual-Model Strategy Based on Photoactivatable Metallopolymer for On-Demand Release of Photosensitizers and Anticancer Drugs. Adv. Sci. 2021, 8, 2103334. [Google Scholar] [CrossRef]
- Snima, K.S.; Arunkumar, P.; Jayakumar, R.; Lakshmanan, V.-K. Silymarin Encapsulated Poly(D, L-lactic-co-glycolic acid) Nanoparticles: A Prospective Candidate for Prostate Cancer Therapy. J. Biomed. Nanotechnol. 2014, 10, 559–570. [Google Scholar] [CrossRef]
- Mitchell, M.J.; Billingsley, M.M.; Haley, R.M.; Wechsler, M.E.; Peppas, N.A.; Langer, R. Engineering precision nanoparticles for drug delivery. Nat. Rev. Drug Discov. 2021, 20, 101–124. [Google Scholar] [CrossRef] [PubMed]
- Song, M.; Dong, S.; An, X.; Zhang, W.; Shen, N.; Li, Y.; Guo, C.; Liu, C.; Li, X.; Chen, S. Erythrocyte-biomimetic nanosystems to improve antitumor effects of paclitaxel on epithelial cancers. J. Control. Release 2022, 345, 744–754. [Google Scholar] [CrossRef] [PubMed]
- Feng, N.-P.; Xu, J.; Zhao, J.-H.; Liu, Y.; Zhang, Y.-T. RGD-modified poly(D,L-lactic acid) nanoparticles enhance tumor targeting of oridonin. Int. J. Nanomed. 2012, 7, 211–219. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Li, J.; Shi, Z.; Yang, Y.; Xie, X.; Lee, S.M.; Wang, Y.; Leong, K.W.; Chen, M. pH-sensitive polymeric nanoparticles for co-delivery of doxorubicin and curcumin to treat cancer via enhanced pro-apoptotic and anti-angiogenic activities. Acta Biomater. 2017, 58, 349–364. [Google Scholar] [CrossRef]
- Kim, K.R.; You, S.J.; Kim, H.J.; Yang, D.H.; Chun, H.J.; Lee, D.; Khang, G. Theranostic potential of biodegradable polymeric nanoparticles with paclitaxel and curcumin against breast carcinoma. Biomater. Sci. 2021, 9, 3750–3761. [Google Scholar] [CrossRef] [PubMed]
- Kang, S.; Yoon, T.W.; Kim, G.Y.; Kang, B. Review of Conjugated Polymer Nanoparticles: From Formulation to Applications. ACS Appl. Nano Mater. 2022, 5, 17436–17460. [Google Scholar] [CrossRef]
- Oliveira, J.M.; Salgado, A.J.; Sousa, N.; Mano, J.F.; Reis, R.L. Dendrimers and derivatives as a potential therapeutic tool in regenerative medicine strategies—A review. Prog. Polym. Sci. 2010, 35, 1163–1194. [Google Scholar] [CrossRef]
- Wang, L.; Xu, X.; Zhang, Y.; Zhang, Y.; Zhu, Y.; Shi, J.; Sun, Y.; Huang, Q. Encapsulation of curcumin within poly(amidoamine) dendrimers for delivery to cancer cells. J. Mater. Sci. Mater. Med. 2013, 24, 2137–2144. [Google Scholar] [CrossRef]
- Nikzamir, M.; Hanifehpour, Y.; Akbarzadeh, A.; Panahi, Y. Applications of Dendrimers in Nanomedicine and Drug Delivery: A Review. J. Inorg. Organomet. Polym. Mater. 2021, 31, 2246–2261. [Google Scholar] [CrossRef]
- Abderrezak, A.; Bourassa, P.; Mandeville, J.-S.; Sedaghat-Herati, R.; Tajmir-Riahi, H.-A. Dendrimers Bind Antioxidant Polyphenols and cisPlatin Drug. PLoS ONE 2012, 7, e33102. [Google Scholar] [CrossRef]
- Neffe, A.T.; Wischke, C.; Racheva, M.; Lendlein, A. Progress in biopolymer-based biomaterials and their application in controlled drug delivery. Expert Rev. Med. Devices 2013, 10, 813–833. [Google Scholar] [CrossRef]
- Shelke, N.B.; James, R.; Laurencin, C.T.; Kumbar, S.G. Polysaccharide biomaterials for drug delivery and regenerative engineering. Polym. Adv. Technol. 2014, 25, 448–460. [Google Scholar] [CrossRef]
- Akhtar, F.; Rizvi, M.M.A.; Kar, S.K. Oral delivery of curcumin bound to chitosan nanoparticles cured Plasmodium yoelii infected mice. Biotechnol. Adv. 2012, 30, 310–320. [Google Scholar] [CrossRef] [PubMed]
- Yao, Q.; Gan, L.; Hou, S.; Guo, X.; Yan, J.; Song, Q.; Gou, X. Development and biodistribution of trans-resveratrol loaded chitosan nanoparticles with free amino groups. Lat. Am. J. Pharm. 2012, 31, 1038–1042. [Google Scholar]
- Xu, Y.; Liang, N.; Liu, J.; Gong, X.; Yan, P.; Sun, S. Design and fabrication of chitosan-based AIE active micelles for bioimaging and intelligent delivery of paclitaxel. Carbohydr. Polym. 2022, 290, 119509. [Google Scholar] [CrossRef]
- Cirillo, G.; Hampel, S.; Spizzirri, U.G.; Parisi, O.I.; Picci, N.; Iemma, F. Carbon Nanotubes Hybrid Hydrogels in Drug Delivery: A Perspective Review. BioMed Res. Int. 2014, 2014, 825017. [Google Scholar] [CrossRef] [PubMed]
- Islam, A.; Riaz, M.; Yasin, T. Structural and viscoelastic properties of chitosan-based hydrogel and its drug delivery application. Int. J. Biol. Macromol. 2013, 59, 119–124. [Google Scholar] [CrossRef]
- Priya, P.; Raj, R.M.; Vasanthakumar, V.; Raj, V. Curcumin-loaded layer-by-layer folic acid and casein coated carboxymethyl cellulose/casein nanogels for treatment of skin cancer. Arab. J. Chem. 2020, 13, 694–708. [Google Scholar] [CrossRef]
- Vigderman, L.; Zubarev, E.R. Therapeutic platforms based on gold nanoparticles and their covalent conjugates with drug molecules. Adv. Drug Deliv. Rev. 2013, 65, 663–676. [Google Scholar] [CrossRef]
- Ding, Y.; Xu, H.; Xu, C.; Tong, Z.; Wang, W.J. A Nanomedicine Fabricated from Gold Nanoparticles-Decorated Metal–Organic Framework for Cascade Chemo/Chemodynamic Cancer Therapy. Adv. Sci. 2020, 7, 2001060. [Google Scholar] [CrossRef]
- Elahi, N.; Kamali, M.; Baghersad, M.H. Recent biomedical applications of gold nanoparticles: A review. Talanta 2018, 184, 537–556. [Google Scholar] [CrossRef] [PubMed]
- Zheng, X.; Wu, Y.; Zuo, H.; Chen, W.; Wang, K. Metal Nanoparticles as Novel Agents for Lung Cancer Diagnosis and Therapy. Small 2023, 19, e2206624. [Google Scholar] [CrossRef] [PubMed]
- Li, N.; Wang, Z.; Zhang, Y.; Zhang, K.; Xie, J.; Liu, Y.; Li, W.; Feng, N. Curcumin-loaded redox-responsive mesoporous silica nanoparticles for targeted breast cancer therapy. Artif. Cells Nanomed. Biotechnol. 2018, 46, 921–935. [Google Scholar] [CrossRef]
- Gao, J.; Fan, K.; Jin, Y.; Zhao, L.; Wang, Q.; Tang, Y.; Xu, H.; Liu, Z.; Wang, S.; Lin, J.; et al. PEGylated lipid bilayer coated mesoporous silica nanoparticles co-delivery of paclitaxel and curcumin leads to increased tumor site drug accumulation and reduced tumor burden. Eur. J. Pharm. Sci. 2019, 140, 105070. [Google Scholar] [CrossRef] [PubMed]
- Manjusha, V.; Reshma, L.; Anirudhan, T. Mesoporous silica gated mixed micelle for the targeted co-delivery of doxorubicin and paclitaxel. J. Drug Deliv. Sci. Technol. 2023, 79, 104032. [Google Scholar] [CrossRef]
- Porrang, S.; Davaran, S.; Rahemi, N.; Allahyari, S.; Mostafavi, E. How Advancing are Mesoporous Silica Nanoparticles? A Comprehensive Review of the Literature. Int. J. Nanomed. 2022, 17, 1803–1827. [Google Scholar] [CrossRef]
- Yin, H.; Yan, Q.; Liu, Y.; Yang, L.; Liu, Y.; Luo, Y.; Chen, T.; Li, N.; Wu, M. Co-encapsulation of paclitaxel and 5-fluorouracil in folic acid-modified, lipid-encapsulated hollow mesoporous silica nanoparticles for synergistic breast cancer treatment. RSC Adv. 2022, 12, 32534–32551. [Google Scholar] [CrossRef]
- Qiu, Y.; Wu, C.; Jiang, J.; Hao, Y.; Zhao, Y.; Xu, J.; Yu, T.; Ji, P. Lipid-coated hollow mesoporous silica nanospheres for co-delivery of doxorubicin and paclitaxel: Preparation, sustained release, cellular uptake and pharmacokinetics. Mater. Sci. Eng. C 2017, 71, 835–843. [Google Scholar] [CrossRef]
- Olivieri, F.; Castaldo, R.; Cocca, M.; Gentile, G.; Lavorgna, M. Mesoporous silica nanoparticles as carriers of active agents for smart anticorrosive organic coatings: A critical review. Nanoscale 2021, 13, 9091–9111. [Google Scholar] [CrossRef]
- Li, Z.; Li, H.; Liu, L.; You, X.; Zhang, C.; Wang, Y. A pH-sensitive nanocarrier for co-delivery of doxorubicin and camptothecin to enhance chemotherapeutic efficacy and overcome multidrug resistance in vitro. RSC Adv. 2015, 5, 77097–77105. [Google Scholar] [CrossRef]
- Chen, B.; Cailian, W.; Zhang, H. Gambogic acid-loaded magnetic Fe3O4 nanoparticles inhibit Panc-1 pancreatic cancer cell proliferation and migration by inactivating transcription factor ETS1. Int. J. Nanomed. 2012, 2012, 781–787. [Google Scholar] [CrossRef] [PubMed]
- Peng, J.; Yin, Y.; Liang, H.; Lu, Y.; Zheng, H.; Wu, G.; Rao, S.; Chen, J.; Yan, F.; Hu, J. Tumor Microenvironment Responsive Pepper Mild Mottle Virus-Based Nanotubes for Targeted Delivery and Controlled Release of Paclitaxel. Front. Bioeng. Biotechnol. 2021, 9, 763661. [Google Scholar] [CrossRef] [PubMed]
- Ali, H.E.; Radwan, R.R. Synthesis, characterization and evaluation of resveratrol-loaded functionalized carbon nanotubes as a novel delivery system in radiation enteropathy. Eur. J. Pharm. Sci. 2021, 167, 106002. [Google Scholar] [CrossRef]
- Koh, B.; Park, S.B.; Yoon, E.; Yoo, H.M.; Lee, D.; Heo, J.N.; Ahn, S. α(V)β(3)-Targeted Delivery of Camptothecin-Encapsulated Carbon Nanotube-Cyclic RGD in 2D and 3D Cancer Cell Culture. J. Pharm. Sci. 2019, 108, 3704–3712. [Google Scholar] [CrossRef] [PubMed]
- Wan, X.; Zhong, H.; Pan, W.; Li, Y.; Chen, Y.; Li, N.; Tang, B. Programmed Release of Dihydroartemisinin for Synergistic Cancer Therapy Using a CaCO(3) Mineralized Metal-Organic Framework. Angew. Chem. Int. Ed. 2019, 58, 14134–14139. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Huang, X.; Pu, Y.; Yi, Y.; Zhang, T.; Wang, B. pH-sensitive and biocompatible quercetin-loaded GO-PEA-HA carrier improved antitumour efficiency and specificity. Artif. Cells Nanomed. Biotechnol. 2018, 46 (Suppl. S3), S28–S37. [Google Scholar] [CrossRef]
- Plamper, F.A.; Richtering, W. Functional Microgels and Microgel Systems. Accounts Chem. Res. 2017, 50, 131–140. [Google Scholar] [CrossRef]
- Kittel, Y.; Kuehne, A.J.C.; De Laporte, L. Translating Therapeutic Microgels into Clinical Applications. Adv. Healthc. Mater. 2021, 11, 2101989. [Google Scholar] [CrossRef]
- Chen, X.; Qian, H.; Qiao, H.; Dong, B.; Chen, E.; Huang, D.; Wang, T.; Chen, W. Tumor-Adhesive and pH-Degradable Microgels by Microfluidics and Photo-Cross-Linking for Efficient Antiangiogenesis and Enhanced Cancer Chemotherapy. Biomacromolecules 2020, 21, 1285–1294. [Google Scholar] [CrossRef]
- Ulker, D.; Barut, I.; Şener, E.; Bütün, V. Advanced liposome based PEGylated microgel as a novel release system for 5-fluorouracil against MCF-7 cancer cell. Eur. Polym. J. 2021, 146, 110270. [Google Scholar] [CrossRef]
- Li, F.; Pei, Z.; Chen, S.; Li, G.; Liu, M.; Ding, L.; Liu, J.; Qiu, F. Multifunctional nano-herb based on tumor microenvironment for enhanced tumor therapy of gambogic acid. Chin. Chem. Lett. 2023, 2023, 108752. [Google Scholar] [CrossRef]
- Ghosh, D.; Choudhury, S.T.; Ghosh, S.; Mandal, A.K.; Sarkar, S.; Ghosh, A.; Das Saha, K.; Das, N. Nanocapsulated curcumin: Oral chemopreventive formulation against diethylnitrosamine induced hepatocellular carcinoma in rat. Chem. Interactions 2012, 195, 206–214. [Google Scholar] [CrossRef] [PubMed]
- Luo, X.; Xu, G.; Song, H.; Yang, S.; Yan, S.; Jia, G.; Li, X. Biopharmaceutics, Promoted antitumor activities of acid-labile electrospun fibers loaded with hydroxycamptothecin via intratumoral implantation. Eur. J. Pharm. Biopharm. 2012, 82, 545–553. [Google Scholar] [CrossRef]
- Khandare, J.; Minko, T. Polymer–drug conjugates: Progress in polymeric prodrugs. Prog. Polym. Sci. 2006, 31, 359–397. [Google Scholar] [CrossRef]
- Zawilska, J.B.; Wojcieszak, J.; Olejniczak, A.B. Prodrugs: A challenge for the drug development. Pharmacol. Rep. 2013, 65, 1–14. [Google Scholar] [CrossRef]
- Zhou, W.; Jia, Y.; Liu, Y.; Chen, Y.; Zhao, P. Tumor Microenvironment-Based Stimuli-Responsive Nanoparticles for Controlled Release of Drugs in Cancer Therapy. Pharmaceutics 2022, 14, 2346. [Google Scholar] [CrossRef] [PubMed]
- Zhao, H.; Li, Y.; Shi, H.; Niu, M.; Li, D.; Zhang, Z.; Feng, Q.; Zhang, Y.; Wang, L. Prodrug nanoparticles potentiate tumor chemo-immunometabolic therapy by disturbing oxidative stress. J. Control. Release 2022, 352, 909–919. [Google Scholar] [CrossRef]
- Kedar, U.; Phutane, P.; Shidhaye, S.; Kadam, V. Advances in polymeric micelles for drug delivery and tumor targeting. Nanomed. Nanotechnol. Biol. Med. 2010, 6, 714–729. [Google Scholar] [CrossRef]
- Cukierman, E.; Khan, D.R. The benefits and challenges associated with the use of drug delivery systems in cancer therapy. Biochem. Pharmacol. 2010, 80, 762–770. [Google Scholar] [CrossRef]
- Du, H.; Zhao, S.; Wang, Y.; Wang, Z.; Chen, B.; Yan, Y.; Yin, Q.; Liu, D.; Wan, F.; Zhang, Q. pH/Cathepsin B Hierarchical-Responsive Nanoconjugates for Enhanced Tumor Penetration and Chemo-lmmunotherapy. Adv. Funct. Mater. 2020, 30, 2003757. [Google Scholar] [CrossRef]
- Danhier, F.; Feron, O.; Préat, V. To exploit the tumor microenvironment: Passive and active tumor targeting of nanocarriers for anti-cancer drug delivery. J. Control. Release 2010, 148, 135–146. [Google Scholar] [CrossRef]
- Mi, P.; Cabral, H.; Kataoka, K. Ligand-Installed Nanocarriers toward Precision Therapy. Adv. Mater. 2020, 32, e1902604. [Google Scholar] [CrossRef]
- Li, M.; Zhang, L.; Xuan, Y.; Zhi, D.; Wang, W.; Zhang, W.; Zhao, Y.; Zhang, S.; Zhang, S. pH-sensitive hyaluronic acid-targeted prodrug micelles constructed via a one-step reaction for enhanced chemotherapy. Int. J. Biol. Macromol. 2022, 206, 489–500. [Google Scholar] [CrossRef] [PubMed]
- Vander Heiden, M.G.; DeBerardinis, R.J. Understanding the Intersections between Metabolism and Cancer Biology. Cell 2017, 168, 657–669. [Google Scholar] [CrossRef] [PubMed]
- Guo, Q.; Li, X.; Zhou, W.; Chu, Y.; Chen, Q.; Zhang, Y.; Li, C.; Chen, H.; Liu, P.; Zhao, Z.; et al. Sequentially Triggered Bacterial Outer Membrane Vesicles for Macrophage Metabolism Modulation and Tumor Metastasis Suppression. ACS Nano 2021, 15, 13826–13838. [Google Scholar] [CrossRef]
- Mura, S.; Nicolas, J.; Couvreur, P. Stimuli-responsive nanocarriers for drug delivery. Nat. Mater. 2013, 12, 991–1003. [Google Scholar] [CrossRef]
- Yi, X.; Zeng, W.; Wang, C.; Chen, Y.; Zheng, L.; Zhu, X.; Ke, Y.; He, X.; Kuang, Y.; Huang, Q. A step-by-step multiple stimuli-responsive metal-phenolic network prodrug nanoparticles for chemotherapy. Nano Res. 2021, 15, 1205–1212. [Google Scholar] [CrossRef]
- Li, Y.; Pei, Q.; Cui, B.; Zhang, H.; Han, L.; Li, W.; Zhu, W.; Feng, X.; Xie, Z. A redox-responsive dihydroartemisinin dimeric nanoprodrug for enhanced antitumor activity. J. Nanobiotechnol. 2021, 19, 441. [Google Scholar] [CrossRef]
- Yang, Q.; Ma, X.; Xiao, Y.; Zhang, T.; Yang, L.; Yang, S.; Liang, M.; Wang, S.; Wu, Z.; Xu, Z.; et al. Engineering prodrug nanomicelles as pyroptosis inducer for codelivery of PI3K/mTOR and CDK inhibitors to enhance antitumor immunity. Acta Pharm. Sin. B 2022, 12, 3139–3155. [Google Scholar] [CrossRef]
- Ling, X.; Jiang, X.; Li, Y.; Han, W.; Rodriguez, M.; Xu, Z.; Lin, W. Sequential Treatment of Bioresponsive Nanoparticles Elicits Antiangiogenesis and Apoptosis and Synergizes with a CD40 Agonist for Antitumor Immunity. ACS Nano 2021, 15, 765–780. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Zhu, L.; Tang, L.; Xie, J.; Gao, Y.; Yu, C.; Shang, K.; Han, H.; Liu, C.; Lu, Y. Glutathione-Responsive Nanoparticles of Camptothecin Prodrug for Cancer Therapy. Adv. Sci. 2023, 10, e2205246. [Google Scholar] [CrossRef]
- Hao, Y.; Chen, Y.; He, X.; Han, R.; Yang, C.; Liu, T.; Yang, Y.; Liu, Q.; Qian, Z. RGD peptide modified platinum nanozyme Co-loaded glutathione-responsive prodrug nanoparticles for enhanced chemo-photodynamic bladder cancer therapy. Biomaterials 2023, 293, 121975. [Google Scholar] [CrossRef]
- Agostinis, P.; Berg, K.; Cengel, K.A.; Foster, T.H.; Golab, J. Photodynamic Therapy of Cancer. CA Cancer J. Clin. 2011, 61, 250–281. [Google Scholar] [CrossRef] [PubMed]
- Zhou, S.; Hu, X.; Xia, R.; Liu, S.; Pei, Q.; Chen, G.; Xie, Z.; Jing, X. A Paclitaxel Prodrug Activatable by Irradiation in a Hypoxic Microenvironment. Angew. Chem. Int. Ed. 2020, 59, 23198–23205. [Google Scholar] [CrossRef] [PubMed]
- Yang, D.-C.; Yang, X.-Z.; Luo, C.-M.; Wen, L.-F.; Liu, J.-Y.; Lin, Z. A promising strategy for synergistic cancer therapy by integrating a photosensitizer into a hypoxia-activated prodrug. Eur. J. Med. Chem. 2022, 243, 114749. [Google Scholar] [CrossRef]
- Zhuang, F.; Ma, Q.; Dong, C.; Xiang, H.; Shen, Y.; Sun, P.; Li, C.; Chen, Y.; Lu, B.; Chen, Y.; et al. Sequential Ultrasound-Triggered and Hypoxia-Sensitive Nanoprodrug for Cascade Amplification of Sonochemotherapy. ACS Nano 2022, 16, 5439–5453. [Google Scholar] [CrossRef] [PubMed]
- Hao, Y.; Chen, Y.; He, X.; Yu, Y.; Han, R.; Li, Y.; Yang, C.; Hu, D.; Qian, Z. Polymeric Nanoparticles with ROS-Responsive Prodrug and Platinum Nanozyme for Enhanced Chemophotodynamic Therapy of Colon Cancer. Adv. Sci. 2020, 7, 2001853. [Google Scholar] [CrossRef]
- Cao, L.; Tian, H.; Fang, M.; Xu, Z.; Tang, D.; Chen, J.; Yin, J.; Xiao, H.; Shang, K.; Han, H.; et al. Activating cGAS-STING pathway with ROS-responsive nanoparticles delivering a hybrid prodrug for enhanced chemo-immunotherapy. Biomaterials 2022, 290, 121856. [Google Scholar] [CrossRef]
- Li, Y.; Chen, M.; Yao, B.; Lu, X.; Zhang, X.; He, P.; Vasilatos, S.N.; Ren, X.; Bian, W.; Yao, C. Transferrin receptor-targeted redox/pH-sensitive podophyllotoxin prodrug micelles for multidrug-resistant breast cancer therapy. J. Mater. Chem. B 2019, 7, 5814–5824. [Google Scholar] [CrossRef]
- Wang, B.; Hu, W.; Yan, H.; Chen, G.; Zhang, Y.; Mao, J.; Wang, L. Lung cancer chemotherapy using nanoparticles: Enhanced target ability of redox-responsive and pH-sensitive cisplatin prodrug and paclitaxel. Biomed. Pharmacother. 2021, 136, 111249. [Google Scholar] [CrossRef]
- Yin, W.; Ke, W.; Lu, N.; Wang, Y.; Japir, A.A.-W.M.M.; Mohammed, F.; Wang, Y.; Pan, Y.; Ge, Z. Glutathione and Reactive Oxygen Species Dual-Responsive Block Copolymer Prodrugs for Boosting Tumor Site-Specific Drug Release and Enhanced Antitumor Efficacy. Biomacromolecules 2020, 21, 921–929. [Google Scholar] [CrossRef] [PubMed]
- Wang, N.; Liu, C.; Yao, W.; Zhou, H.; Yu, S.; Chen, H.; Qiao, W. A traceable, GSH/pH dual-responsive nanoparticles with spatiotemporally controlled multiple drugs release ability to enhance antitumor efficacy. Colloids Surf. B Biointerfaces 2021, 205, 111866. [Google Scholar] [CrossRef] [PubMed]
- Xiao, Y.; Zhang, T.; Ma, X.; Yang, Q.; Yang, L.; Yang, S.; Liang, M.; Xu, Z.; Sun, Z. Microenvironment-Responsive Prodrug-Induced Pyroptosis Boosts Cancer Immunotherapy. Adv. Sci. 2021, 8, e2101840. [Google Scholar] [CrossRef] [PubMed]
- Silverman, J.A.; Deitcher, S.R. Marqibo® (vincristine sulfate liposome injection) improves the pharmacokinetics and pharmacodynamics of vincristine. Cancer Chemother. Pharmacol. 2013, 71, 555–564. [Google Scholar] [CrossRef] [PubMed]
- De Luca, R.; Blasi, L.; Alù, M.; Gristina, V.; Cicero, G. Clinical efficacy of nab-paclitaxel in patients with metastatic pancreatic cancer. Drug Des. Dev. Ther. 2018, 12, 1769. [Google Scholar] [CrossRef]
- Neesse, A.; Michl, P.; Tuveson, D.A.; Ellenrieder, V. nab-Paclitaxel: Novel Clinical and Experimental Evidence in Pancreatic Cancer. Z. Gastroenterol. 2014, 52, 360–366. [Google Scholar] [CrossRef]
- Werner, M.E.; Cummings, N.D.; Sethi, M.; Wang, E.C.; Sukumar, R.; Moore, D.T.; Wang, A.Z. Preclinical Evaluation of Genexol-PM, a Nanoparticle Formulation of Paclitaxel, as a Novel Radiosensitizer for the Treatment of Non-Small Cell Lung Cancer. Int. J. Radiat. Oncol. 2013, 86, 463–468. [Google Scholar] [CrossRef]
- Lim, W.T.; Tan, E.H.; Toh, C.K.; Hee, S.W.; Leong, S.S.; Ang, P.C.S.; Wong, N.S.; Chowbay, B. Phase I pharmacokinetic study of a weekly liposomal paclitaxel formulation (Genexol®-PM) in patients with solid tumors. Ann. Oncol. 2010, 21, 382–388. [Google Scholar] [CrossRef]
- Kosaka, Y.; Saeki, T.; Takano, T.; Aruga, T.; Yamashita, T.; Masuda, N.; Koibuchi, Y.; Osaki, A.; Watanabe, J.; Suzuki, R. Multicenter Randomized Open-Label Phase II Clinical Study Comparing Outcomes of NK105 and Paclitaxel in Advanced or Recurrent Breast Cancer. Int. J. Nanomed. 2022, 17, 4567–4578. [Google Scholar] [CrossRef]
- Gulati, S.; Choudhury, A.; Mohan, G.; Katiyar, R.; P, M.A.K.M.; Kumar, S.; Varma, R.S. Metal–organic frameworks (MOFs) as effectual diagnostic and therapeutic tools for cancer. J. Mater. Chem. B 2023, 11, 6782–6801. [Google Scholar] [CrossRef]
- Zhang, S.; Rong, F.; Guo, C.; Duan, F.; He, L.; Wang, M.; Zhang, Z.; Kang, M.; Du, M. Metal–organic frameworks (MOFs) based electrochemical biosensors for early cancer diagnosis in vitro. Coord. Chem. Rev. 2021, 439, 213948. [Google Scholar] [CrossRef]



| Carrier Type | Material | Drug | Tumor Type | Ref. |
|---|---|---|---|---|
| Liposome | Cholesterol (CHOL) Stearylamine Soy lecithin poly (ε-caprolactone) | PTX | Breast Cancer | [60] |
| PolyethyleneGlycol (PEG) CHOL Soy lecithin (SPC) | CUR SN38 | Lung Cancer | [61] | |
| Poly(ethyleneglycol)-poly(lacticacid) (PEG-PLA) Dipalmitoylphosphatidylcholine (DPPC) | CPT | Colon Cancer | [62] | |
| SPC CHOL Mycoplasma Membrane | Podophyllotoxin (POD) | Breast Cancer | [63] | |
| 1,2-distearoyl-sn-glycero-3-phosphoethanolamine-N-[methoxy(polyethylene glycol)-2000] (DSPE-mPEG2000) CHOL Egg yolk lecithin | Oxaliplatin PTX | Ovarian cancer | [64] | |
| SLNs | Chitosan Polyvinyl alcohol MTT(3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide) | Docetaxel | Melanoma Colorectal Cancer | [65] |
| Stearic acid SPC | PTX | Ovarian Cancer | [66] | |
| HSPC 1,2-distearoyl-sn-glycero-3-phosphoethanolamine-N-[methoxy(polyethylene glycol)-2000] (DSPE-PEG2000) | CUR PTX | Lung Cancer | [42] | |
| Alpha-tocopherol polyethylene glycol 1000 succinate (TPGS) Stearic acid EPC | Resveratrol (Res) | Breast Cancer | [67] | |
| NLCs | 1,2-Distearoyl-sn-glycero-3-phosphoethanolamine-N-[maleimide(polyethylene glycol2000)](DSPE-PEG2000-Mal) | Docetaxel (DTX) Tariquidar (TRQ) | Breast Cancer | [68] |
| Oleic Stearic acids | Curcumin (CRN) | Prostate Cancer | [50] | |
| Phospholipon PEG 4000 monostearate Octadecylamine | Doxorubicin (DT) Curcumin (CR) | Non-small Cell Lung Cancer | [69] | |
| Myristyl myristate (MM), Miglyol 812® (MG) | Doxorubicin (DTX) Lidocaine (LDC) | Melanoma | [70] | |
| Microemulsion and self-micro emulsion drug delivery system | Fumed colloidal silica Vitamin E a-Tocopherol polyethylene glycol succinate (TPGS) Gelucire® Capryol®90 | PTX | Breast Cancer | [71] |
| Polysorbate 80 | CPT | Colorectal Cancer | [72] | |
| Enoxaparin (Enox) PeceolTM (glyceryl monooleate) Cremophor EL (polyoxyl-35 castor oil) Labrafil M 1944 (oleoyl polyoxyl-6 glycerides) propylene glycol (PG) | DTX | Non-small Cell Lung Cancer | [73] |
| Nanocarriers | First Development | Advantages | Limitations | Improvement Methods | Clinical Application | Ref. |
|---|---|---|---|---|---|---|
| Liposome | 1960s | Biologically inert Biodegradable Biocompatible Low inherent toxic | Thermodynamically unstable systems Rapid clearance from the bloodstream Drug leakage | Modification of natural or synthetic polymeric molecules on the surface of liposomes Develop SLNs | Doxil® Myocet® Marqibo® LipoplatinTM EndoTAG-1 | [35,36,37] |
| Solid Lipid Nanoparticles | 1990s | Biocompatibility Slow water absorption Greater stability Low sensitivity to erosion | Temperature during Preparation affects stability | Drying of SLNs to powder form for storage Develop NLCs | Mucosolvan Retard Nanobase | [43,44] |
| Nanostructured lipid carriers | 1999/2000s | Increased drug-loading capacity Low toxicity More stable in storage Reduced drug leakage | Toxicity studies required for High concentrations of NLCs | Optimizing the physicochemical properties of drug and lipid components | _ | [53,54] |
| Microemulsion and self-micro emulsion drug delivery system | 1970s | Multiple routes of administration Simple preparation process Easy to industrialize Low viscosity | Examine its safety | Searching for efficient and low-toxicity emulsifiers and emulsifiers | _ | [58,59] |
| Polymer micelles | 1992s | Easy retouching Structurally stable Hydrophilic outer layer to avoid macrophage phagocytosis | Only insoluble drugs can be loaded | Optimized micelle structure | Genexol®PM NK105 Paclical®PM | [78,79] |
| Polymer nanoparticles | 1992s | Small size High specific surface area | Poisonous Particle aggregation | Optimizing the composition of polymer nanoparticles | _ | [87] |
| Dendrimers | 1985s | Load multiple types of drugs Increase drug solubility Commonly used for nucleic acid and small molecule delivery | Toxicity Non-degradability | Selection of biocompatible or biodegradable materials Surface modification of dendrimers | VivaGel | [90] |
| Gold nanoparticles | 1951s | Easy surface finishing High biosafety Electrochemical characterization | High preparation costs Easy to be oxidized | Modification of the structure of NPs | _ | [102,103] |
| Mesoporous silica nanoparticles | 1990s | Many holes Large surface area Easy to modify | Limited drug-carrying capacity Leakage of drugs Slower metabolism | Development of HMSNs Development of degradable MSNs Avoiding drug leakage by encapsulating MSNs with membranes or plugging pores | _ | [107,110,111] |
| Metal-Organic Frame | 1990s | Large specific surface area Strong adsorption performance Early cancer diagnosis in vitro | Poor stability Complex synthesis process Difficulties in industrial production | Improved synthesis of MOFs | HKUST-1 | [161,162] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kang, C.; Wang, J.; Li, R.; Gong, J.; Wang, K.; Wang, Y.; Wang, Z.; He, R.; Li, F. Smart Targeted Delivery Systems for Enhancing Antitumor Therapy of Active Ingredients in Traditional Chinese Medicine. Molecules 2023, 28, 5955. https://doi.org/10.3390/molecules28165955
Kang C, Wang J, Li R, Gong J, Wang K, Wang Y, Wang Z, He R, Li F. Smart Targeted Delivery Systems for Enhancing Antitumor Therapy of Active Ingredients in Traditional Chinese Medicine. Molecules. 2023; 28(16):5955. https://doi.org/10.3390/molecules28165955
Chicago/Turabian StyleKang, Chenglong, Jianwen Wang, Ruotong Li, Jianing Gong, Kuanrong Wang, Yuxin Wang, Zhenghua Wang, Ruzhe He, and Fengyun Li. 2023. "Smart Targeted Delivery Systems for Enhancing Antitumor Therapy of Active Ingredients in Traditional Chinese Medicine" Molecules 28, no. 16: 5955. https://doi.org/10.3390/molecules28165955
APA StyleKang, C., Wang, J., Li, R., Gong, J., Wang, K., Wang, Y., Wang, Z., He, R., & Li, F. (2023). Smart Targeted Delivery Systems for Enhancing Antitumor Therapy of Active Ingredients in Traditional Chinese Medicine. Molecules, 28(16), 5955. https://doi.org/10.3390/molecules28165955