Advancements in Flame-Retardant Systems for Rigid Polyurethane Foam
Abstract
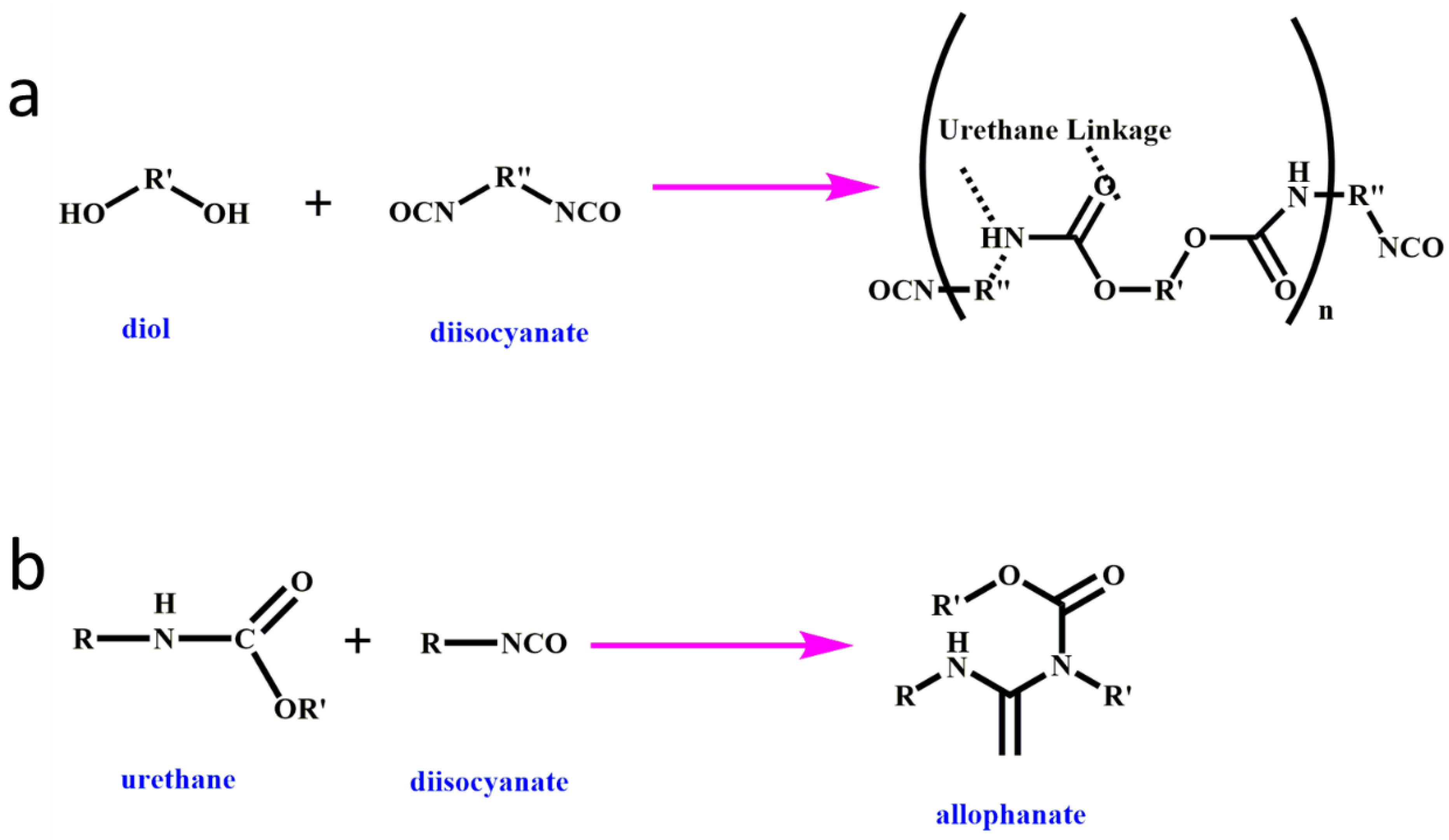
1. Introduction
| Flame-Retardant Type | Composite | Remarks | Ref. |
|---|---|---|---|
| Reactive flame retardants | RPUF/AMPO (polyol-bis(hydroxymethyl)-N, N-bis(2-hydroxyethyl)aminomethylphosphine oxide) |
| [39] (1982) |
| RPUF/GEP (glycerol/ethanol phosphate) |
| [40] (2015) | |
| RPUF/PPGE (phenylphosphoryl glycol ether oligomer) |
| [41] (2019) | |
| Additive flame retardants | RPUF/pEG-P(MA) (pulverized expandable graphite (pEG)-poly(methyl methacrylate-acrylic acid) copolymer) |
| [42] (2011) |
| RPUF/MATMP (melamine amino trimethylene phosphate) |
| [43] (2017) | |
| RPUF/MFAPP (melamine–formaldehyde resin-microencapsulated ammonium polyphosphate) |
| [44] (2020) | |
| Flame-retardant coating | RPUF/alginate/clay aerogel |
| [45] (2016) |
| RPUF/poly(VS-co-HEA) (copolymerization of hydroxyethyl acrylate (HEA) and sodium vinylsulfonate (VS)) |
| [2] (2021) |
2. Reactive-Type Flame Retardants
2.1. Incorporation of Phosphorus-Containing Groups
2.2. Incorporation of Nitrogen-Containing Groups
2.3. Incorporation of Sulfur-Containing Groups
3. Additive-Type Flame Retardants
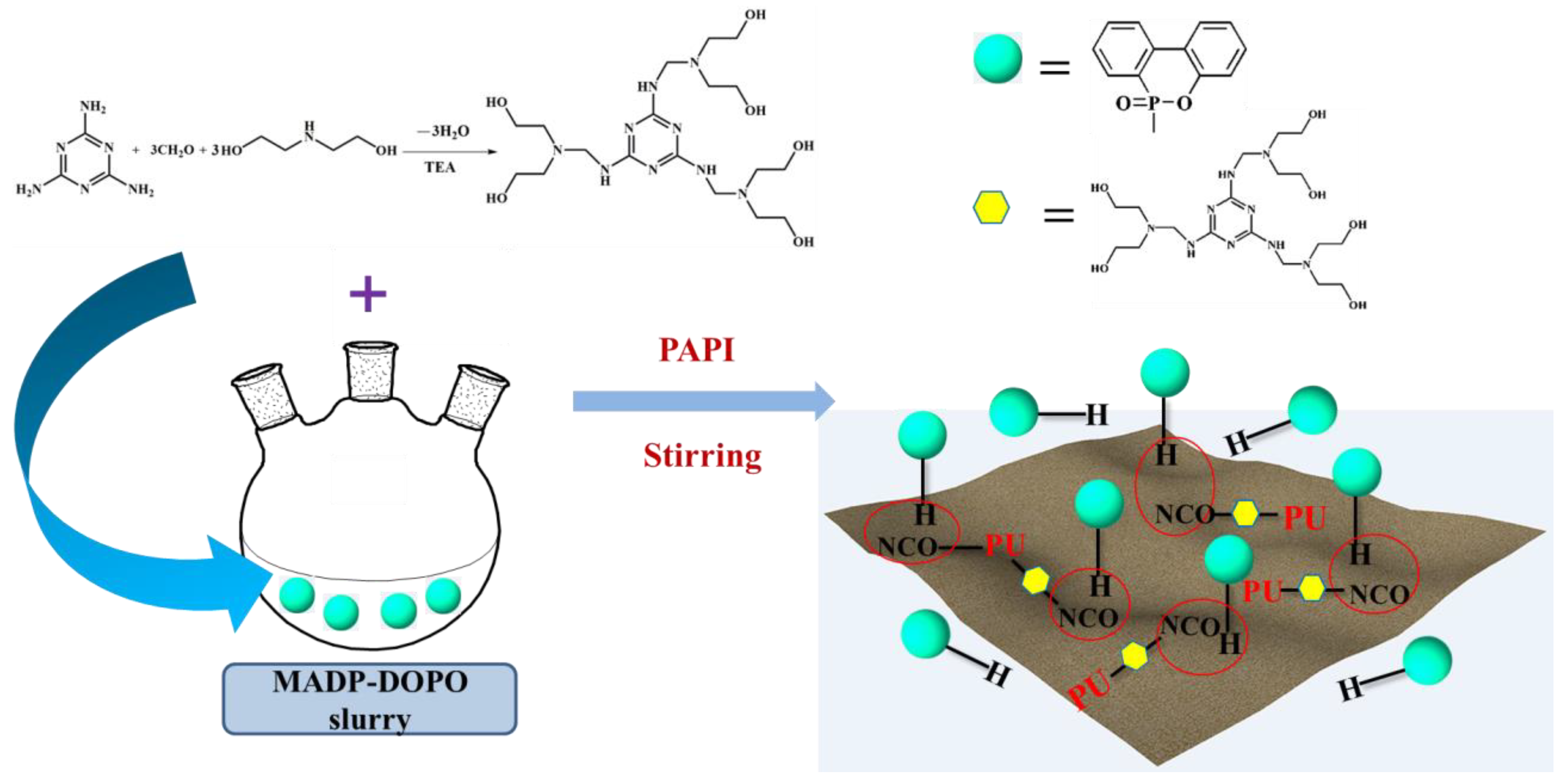
3.1. Addition of Phosphorous-Containing Flame Retardants
3.2. Addition of Phosphorus–Nitrogen-Based Flame Retardants
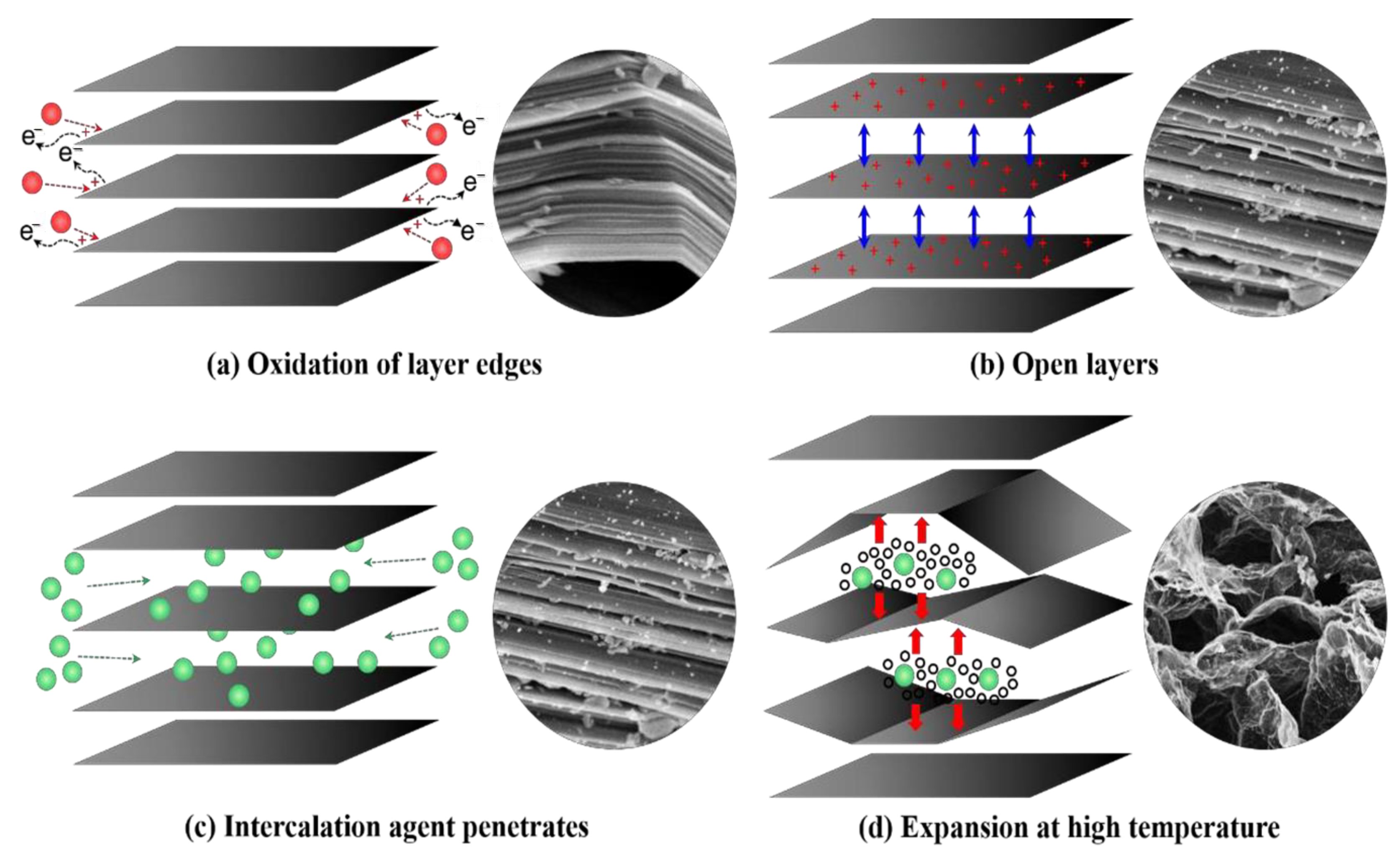
3.3. Addition of Expandable Graphite and Derivatives
3.4. Addition of Nanoclay and Other Nanoparticles
3.5. Addition of Phase-Change Materials
4. Flame-Retardant Coatings
5. Concluding Remarks and Future Aspects
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Chattopadhyay, D.K.; Webster, D.C. Thermal stability and flame retardancy of polyurethanes. Prog. Polym. Sci. 2009, 34, 1068–1133. [Google Scholar] [CrossRef]
- Ma, Z.; Liu, X.; Xu, X.; Liu, L.; Yu, B.; Maluk, C.; Huang, G.; Wang, H.; Song, P. Bioinspired, highly adhesive, nanostructured polymeric coatings for superhydrophobic fire-extinguishing thermal insulation foam. ACS Nano 2021, 15, 11667–11680. [Google Scholar] [CrossRef]
- Tao, J.; Yang, F.; Wu, T.; Shi, J.; Zhao, H.-B.; Rao, W. Thermal insulation, flame retardancy, smoke suppression, and reinforcement of rigid polyurethane foam enabled by incorporating a P/Cu-hybrid silica aerogel. Chem. Eng. J. 2023, 461, 142061. [Google Scholar] [CrossRef]
- Zhang, X.; Sun, S.; Liu, B.; Wang, Z.; Xie, H. Synergistic effect of combining amino trimethylphosphonate calcium and expandable graphite on flame retardant and thermal stability of rigid polyurethane foam. Int. J. Polym. Anal. Charact. 2022, 27, 302–315. [Google Scholar] [CrossRef]
- Singh, H.; Jain, A.; Sharma, T. Effect of phosphorus-nitrogen additives on fire retardancy of rigid polyurethane foams. J. Appl. Polym. Sci. 2008, 109, 2718–2728. [Google Scholar] [CrossRef]
- Septevani, A.A.; Evans, D.A.; Annamalai, P.K.; Martin, D.J. The use of cellulose nanocrystals to enhance the thermal insulation properties and sustainability of rigid polyurethane foam. Ind. Crops Prod. 2017, 107, 114–121. [Google Scholar] [CrossRef]
- Acuña, P.; Lin, X.; Calvo, M.S.; Shao, Z.; Pérez, N.; Villafañe, F.; Rodríguez-Pérez, M.Á.; Wang, D.-Y. Synergistic effect of expandable graphite and phenylphosphonic-aniline salt on flame retardancy of rigid polyurethane foam. Polym. Degrad. Stab. 2020, 179, 109274. [Google Scholar] [CrossRef]
- Zhu, M.; Ma, Z.; Liu, L.; Zhang, J.; Huo, S.; Song, P. Recent advances in fire-retardant rigid polyurethane foam. J. Mater. Sci. Technol. 2022, 112, 315–328. [Google Scholar] [CrossRef]
- Muhammed Raji, A.; Hambali, H.U.; Khan, Z.I.; Binti Mohamad, Z.; Azman, H.; Ogabi, R. Emerging trends in flame retardancy of rigid polyurethane foam and its composites: A review. J. Cell. Plast. 2023, 59, 65–122. [Google Scholar] [CrossRef]
- Henry, C.; Gondaliya, A.; Thies, M.; Nejad, M. Studying the suitability of nineteen lignins as partial polyol replacement in rigid polyurethane/polyisocyanurate foam. Molecules 2022, 27, 2535. [Google Scholar] [CrossRef]
- Yang, R.; Wang, B.; Li, M.; Zhang, X.; Li, J. Preparation, characterization and thermal degradation behavior of rigid polyurethane foam using a malic acid based polyols. Ind. Crops Prod. 2019, 136, 121–128. [Google Scholar] [CrossRef]
- Srihanum, A.; Tuan Noor, M.T.; Devi, K.P.; Hoong, S.S.; Ain, N.H.; Mohd, N.S.; Nek Mat Din, N.S.M.; Kian, Y.S. Low density rigid polyurethane foam incorporated with renewable polyol as sustainable thermal insulation material. J. Cell. Plast. 2022, 58, 485–503. [Google Scholar] [CrossRef]
- Bayer, O.; Siefken, W.; Rinke, H.; Orthner, L.; Schild, H. A process for the production of polyurethanes and polyureas. Ger. Pat. DRP 1937, 728981, 13. [Google Scholar]
- Ma, C.; Qiu, S.; Xiao, Y.; Zhang, K.; Zheng, Y.; Xing, W.; Hu, Y. Fabrication of fire safe rigid polyurethane foam with reduced release of CO and NOx and excellent physical properties by combining phosphine oxide-containing hyperbranched polyol and expandable graphite. Chem. Eng. J. 2022, 431, 133347. [Google Scholar] [CrossRef]
- Xu, B.; Zhao, S.; Shan, H.; Qian, L.; Wang, J.; Xin, F. Effect of two boron compounds on smoke-suppression and flame-retardant properties for rigid polyurethane foams. Polym. Int. 2022, 71, 1210–1219. [Google Scholar] [CrossRef]
- Günther, M.; Lorenzetti, A.; Schartel, B. From Cells to Residues: Flame-retarded rigid polyurethane foams. Combust. Sci. Technol. 2020, 192, 2209–2237. [Google Scholar] [CrossRef]
- Günther, M.; Lorenzetti, A.; Schartel, B. Fire phenomena of rigid polyurethane foams. Polymers 2018, 10, 1166. [Google Scholar] [CrossRef]
- Chan, Y.Y.; Schartel, B. It Takes Two to Tango: Synergistic Expandable Graphite-Phosphorus Flame Retardant Combinations in Polyurethane Foams. Polymers 2022, 14, 2562. [Google Scholar] [CrossRef]
- Yang, Y.; Shen, H.; Luo, Y.; Zhang, R.; Sun, J.; Liu, X.; Zong, Z.; Tang, G. Rigid polyurethane foam composites based on iron tailing: Thermal stability, flame retardancy and fire toxicity. Cell. Polym. 2022, 41, 189–207. [Google Scholar] [CrossRef]
- Jia, P.; Ma, C.; Lu, J.; Yang, W.; Jiang, X.; Jiang, G.; Yin, Z.; Qiu, Y.; Qian, L.; Yu, X. Design of copper salt@ graphene nanohybrids to accomplish excellent resilience and superior fire safety for flexible polyurethane foam. J. Colloid Interface Sci. 2022, 606, 1205–1218. [Google Scholar] [CrossRef] [PubMed]
- Akar, A.; Değirmenci, B.; Köken, N. Fire-retardant and smoke-suppressant rigid polyurethane foam composites. Pigm. Resin Technol. 2023, 52, 237–245. [Google Scholar] [CrossRef]
- Agrawal, A.; Kaur, R. Effect of nano filler on the flammability of bio-based RPUF. Integr. Ferroelectr. 2019, 202, 20–28. [Google Scholar] [CrossRef]
- Son, M.-H.; Kim, Y.; Jo, Y.-H.; Kwon, M. Assessment of chemical asphyxia caused by toxic gases generated from rigid polyurethane foam (RPUF) fires. Forensic Sci. Int. 2021, 328, 111011. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Tawiah, B.; Shi, Y.; Cai, S.; Rao, X.; Liu, C.; Yang, Y.; Yang, F.; Yu, B.; Liang, Y. Highly effective flame-retardant rigid polyurethane foams: Fabrication and applications in inhibition of coal combustion. Polymers 2019, 11, 1776. [Google Scholar] [CrossRef] [PubMed]
- Ching, Y.; Chuah, C.; Ching, K.; Abdullah, L.; Rahman, A. Applications of thermoplastic-based blends. In Recent Developments in Polymer Macro, Micro and Nano Blends; Woodhead Publishing: Cambridge, UK, 2017; pp. 111–129. [Google Scholar]
- Lubczak, J.; Lubczak, R. Thermally resistant polyurethane foams with reduced flammability. J. Cell. Plast. 2018, 54, 561–576. [Google Scholar] [CrossRef]
- Akdogan, E.; Erdem, M.; Ureyen, M.E.; Kaya, M. Rigid polyurethane foams with halogen-free flame retardants: Thermal insulation, mechanical, and flame retardant properties. J. Appl. Polym. Sci. 2020, 137, 47611. [Google Scholar] [CrossRef]
- Çalışkan, E.; Çanak, T.Ç.; Karahasanoğlu, M.; Serhatlı, I.E. Synthesis and characterization of phosphorus-based flame retardant containing rigid polyurethane foam. J. Therm. Anal. Calorim. 2022, 147, 4119–4129. [Google Scholar] [CrossRef]
- Tsuyumoto, I. Flame-retardant coatings for rigid polyurethane foam based on mixtures of polysaccharides and polyborate. J. Coat. Technol. Res. 2021, 18, 155–162. [Google Scholar] [CrossRef]
- Chen, Y.; Li, L.; Qi, X.; Qian, L. The pyrolysis behaviors of phosphorus-containing organosilicon compound modified APP with different polyether segments and their flame retardant mechanism in polyurethane foam. Compos. Part B Eng. 2019, 173, 106784. [Google Scholar] [CrossRef]
- Acosta, A.P.; Otoni, C.G.; Missio, A.L.; Amico, S.C.; Delucis, R.d.A. Rigid Polyurethane Biofoams Filled with Chemically Compatible Fruit Peels. Polymers 2022, 14, 4526. [Google Scholar] [CrossRef]
- Wang, C.; Wu, Y.; Li, Y.; Shao, Q.; Yan, X.; Han, C.; Wang, Z.; Liu, Z.; Guo, Z. Flame-retardant rigid polyurethane foam with a phosphorus-nitrogen single intumescent flame retardant. Polym. Adv. Technol. 2018, 29, 668–676. [Google Scholar] [CrossRef]
- Wang, J.; Xu, B.; Wang, X.; Liu, Y. A phosphorous-based bi-functional flame retardant for rigid polyurethane foam. Polym. Degrad. Stab. 2021, 186, 109516. [Google Scholar] [CrossRef]
- Han, S.; Zhu, X.; Chen, F.; Chen, S.; Liu, H. Flame-retardant system for rigid polyurethane foams based on diethyl bis (2-hydroxyethyl) aminomethylphosphonate and in-situ exfoliated clay. Polym. Degrad. Stab. 2020, 177, 109178. [Google Scholar] [CrossRef]
- Agrawal, A.; Kaur, R.; Singh Walia, R. Flame retardancy of ceramic-based rigid polyurethane foam composites. J. Appl. Polym. Sci. 2019, 136, 48250. [Google Scholar] [CrossRef]
- Zhu, H.; Xu, S. Preparation of flame-retardant rigid polyurethane foams by combining modified melamine–formaldehyde resin and phosphorus flame retardants. ACS Omega 2020, 5, 9658–9667. [Google Scholar] [CrossRef]
- Li, Y.; Tian, H.; Zhang, J.; Zou, W.; Wang, H.; Du, Z.; Zhang, C. Fabrication and properties of rigid polyurethane nanocomposite foams with functional isocyanate modified graphene oxide. Polym. Compos. 2020, 41, 5126–5134. [Google Scholar] [CrossRef]
- Wang, X.; Kalali, E.N.; Xing, W.; Wang, D.-Y. CO2 induced synthesis of Zn-Al layered double hydroxide nanostructures towards efficiently reducing fire hazards of polymeric materials. Nano Adv. 2018, 3, 12–17. [Google Scholar] [CrossRef][Green Version]
- Sivriev, H.; Borissov, G.; Zabski, L.; Walczyk, W.; Jedlinski, Z. Synthesis and studies of phosphorus-containing polyurethane foams based on tetrakis (hydroxymethyl) phosphonium chloride derivatives. J. Appl. Polym. Sci. 1982, 27, 4137–4147. [Google Scholar] [CrossRef]
- Xu, W.; Wang, G. Synthesis of polyhydric alcohol/ethanol phosphate flame retardant and its application in PU rigid foams. J. Appl. Polym. Sci. 2015, 132, 42298. [Google Scholar] [CrossRef]
- Wu, N.; Niu, F.; Lang, W.; Yu, J.; Fu, G. Synthesis of reactive phenylphosphoryl glycol ether oligomer and improved flame retardancy and mechanical property of modified rigid polyurethane foams. Mater. Des. 2019, 181, 107929. [Google Scholar] [CrossRef]
- Zhang, X.G.; Ge, L.L.; Zhang, W.Q.; Tang, J.H.; Ye, L.; Li, Z.M. Expandable graphite-methyl methacrylate-acrylic acid copolymer composite particles as a flame retardant of rigid polyurethane foam. J. Appl. Polym. Sci. 2011, 122, 932–941. [Google Scholar] [CrossRef]
- Liu, L.; Wang, Z.; Xu, X. Melamine amino trimethylene phosphate as a novel flame retardant for rigid polyurethane foams with improved flame retardant, mechanical and thermal properties. J. Appl. Polym. Sci. 2017, 134, 45234. [Google Scholar] [CrossRef]
- Tang, G.; Jiang, H.; Yang, Y.; Chen, D.; Liu, C.; Zhang, P.; Zhou, L.; Huang, X.; Zhang, H.; Liu, X. Preparation of melamine-formaldehyde resin-microencapsulated ammonium polyphosphate and its application in flame retardant rigid polyurethane foam composites. J. Polym. Res. 2020, 27, 375. [Google Scholar] [CrossRef]
- Chen, H.-B.; Shen, P.; Chen, M.-J.; Zhao, H.-B.; Schiraldi, D.A. Highly efficient flame retardant polyurethane foam with alginate/clay aerogel coating. ACS Appl. Mater. Interfaces 2016, 8, 32557–32564. [Google Scholar] [CrossRef]
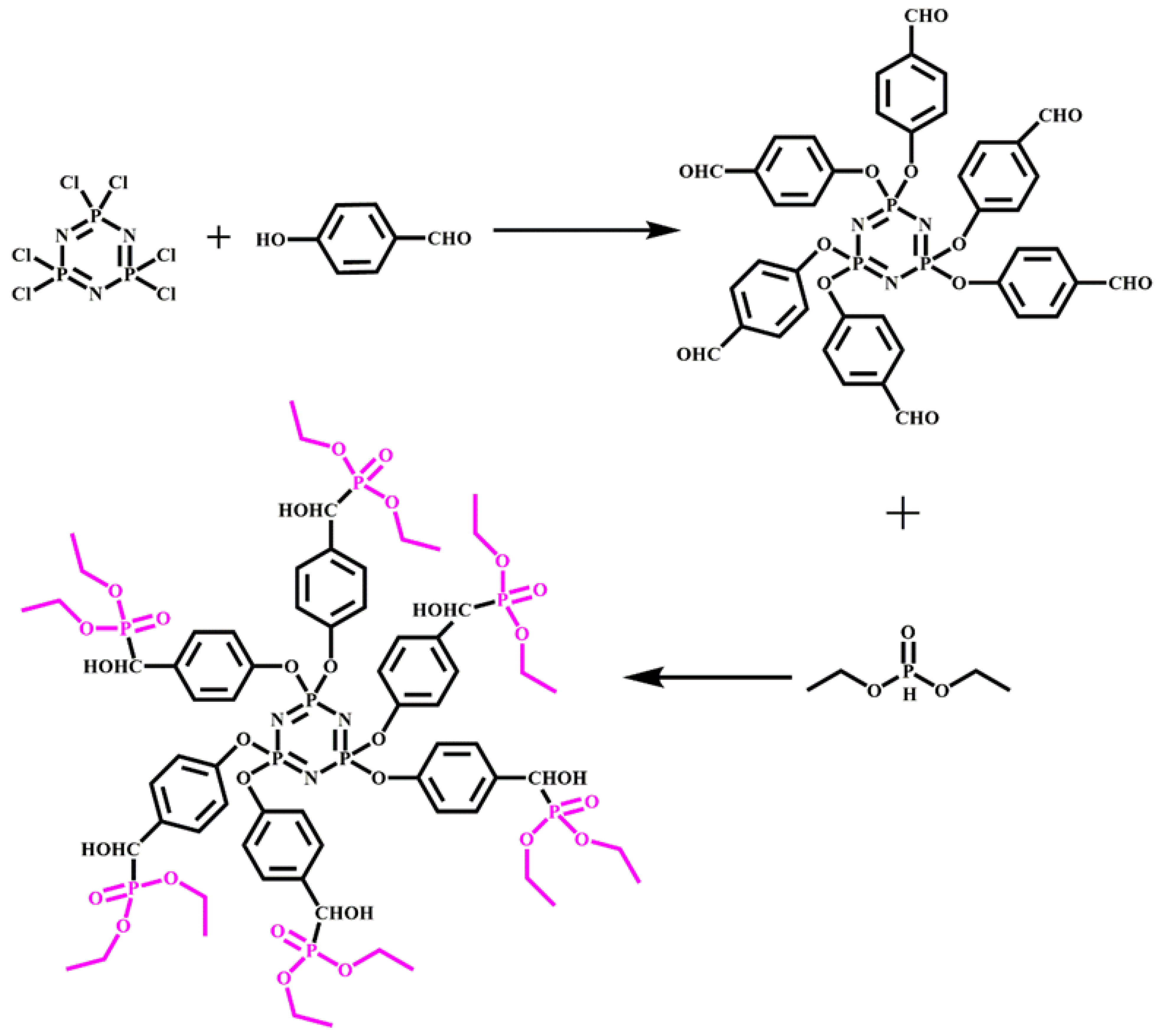
- Yang, R.; Wang, B.; Han, X.; Ma, B.; Li, J. Synthesis and characterization of flame retardant rigid polyurethane foam based on a reactive flame retardant containing phosphazene and cyclophosphonate. Polym. Degrad. Stab. 2017, 144, 62–69. [Google Scholar] [CrossRef]
- Yang, R.; Hu, W.; Xu, L.; Song, Y.; Li, J. Synthesis, mechanical properties and fire behaviors of rigid polyurethane foam with a reactive flame retardant containing phosphazene and phosphate. Polym. Degrad. Stab. 2015, 122, 102–109. [Google Scholar] [CrossRef]
- Qian, L.; Li, L.; Chen, Y.; Xu, B.; Qiu, Y. Quickly self-extinguishing flame retardant behavior of rigid polyurethane foams linked with phosphaphenanthrene groups. Compos. Part B Eng. 2019, 175, 107186. [Google Scholar] [CrossRef]
- Bhoyate, S.; Ionescu, M.; Kahol, P.; Gupta, R.K. Sustainable flame-retardant polyurethanes using renewable resources. Ind. Crops Prod. 2018, 123, 480–488. [Google Scholar] [CrossRef]
- Huang, X.; Wang, C.; Gao, J.; Zhou, Z.; Tang, G.; Wang, C. Research on two sides horizontal flame spread over rigid polyurethane with different flame retardants. J. Therm. Anal. Calorim. 2021, 146, 2141–2150. [Google Scholar] [CrossRef]
- Zhang, Q.; Chen, F.; Ma, L.; Zhou, X. Preparation and application of phosphorous-containing bio-polyols in polyurethane foams. J. Appl. Polym. Sci. 2014, 131, 40422. [Google Scholar] [CrossRef]
- Bhoyate, S.; Ionescu, M.; Kahol, P.; Chen, J.; Mishra, S.; Gupta, R.K. Highly flame-retardant polyurethane foam based on reactive phosphorus polyol and limonene-based polyol. J. Appl. Polym. Sci. 2018, 135, 46224. [Google Scholar] [CrossRef]
- Zhang, L.; Zhang, M.; Zhou, Y.; Hu, L. The study of mechanical behavior and flame retardancy of castor oil phosphate-based rigid polyurethane foam composites containing expanded graphite and triethyl phosphate. Polym. Degrad. Stab. 2013, 98, 2784–2794. [Google Scholar] [CrossRef]
- Acuña, P.; Zhang, J.; Yin, G.-Z.; Liu, X.-Q.; Wang, D.-Y. Bio-based rigid polyurethane foam from castor oil with excellent flame retardancy and high insulation capacity via cooperation with carbon-based materials. J. Mater. Sci. 2021, 56, 2684–2701. [Google Scholar] [CrossRef]
- Zhou, W.; Hao, S.-J.; Feng, G.-D.; Jia, P.-Y.; Ren, X.-L.; Zhang, M.; Zhou, Y.-H. Properties of rigid polyurethane foam modified by tung oil-based polyol and flame-retardant particles. Polymers 2020, 12, 119. [Google Scholar] [CrossRef]
- Luo, Y.; Miao, Z.; Sun, T.; Zou, H.; Liang, M.; Zhou, S.; Chen, Y. Preparation and mechanism study of intrinsic hard segment flame-retardant polyurethane foam. J. Appl. Polym. Sci. 2021, 138, 49920. [Google Scholar] [CrossRef]
- Yuan, Y.; Yang, H.; Yu, B.; Shi, Y.; Wang, W.; Song, L.; Hu, Y.; Zhang, Y. Phosphorus and nitrogen-containing polyols: Synergistic effect on the thermal property and flame retardancy of rigid polyurethane foam composites. Ind. Eng. Chem. Res. 2016, 55, 10813–10822. [Google Scholar] [CrossRef]
- Wang, S.-X.; Zhao, H.-B.; Rao, W.-H.; Huang, S.-C.; Wang, T.; Liao, W.; Wang, Y.-Z. Inherently flame-retardant rigid polyurethane foams with excellent thermal insulation and mechanical properties. Polymer 2018, 153, 616–625. [Google Scholar] [CrossRef]
- Zhang, Z.; Li, D.; Xu, M.; Li, B. Synthesis of a novel phosphorus and nitrogen-containing flame retardant and its application in rigid polyurethane foam with expandable graphite. Polym. Degrad. Stab. 2020, 173, 109077. [Google Scholar] [CrossRef]
- Vakili, M.; Nikje, M.M.A.; Hajibeygi, M. The effects of a phosphorus/nitrogen-containing diphenol on the flammability, thermal stability, and mechanical properties of rigid polyurethane foam. Colloid Polym. Sci. 2023, 1–12. [Google Scholar] [CrossRef]
- Zhou, W.; Jia, P.; Zhang, M.; Zhou, Y. Preparation and characterization of nitrogen-containing heterocyclic tung oil-based rigid polyurethane foam. Chem. Ind. For. Prod. 2019, 39, 53–58. [Google Scholar]
- Yuan, Y.; Ma, C.; Shi, Y.; Song, L.; Hu, Y.; Hu, W. Highly-efficient reinforcement and flame retardancy of rigid polyurethane foam with phosphorus-containing additive and nitrogen-containing compound. Mater. Chem. Phys. 2018, 211, 42–53. [Google Scholar] [CrossRef]
- Zhu, H.; Xu, S.-a. Synthesis and properties of rigid polyurethane foams synthesized from modified urea-formaldehyde resin. Constr. Build. Mater. 2019, 202, 718–726. [Google Scholar] [CrossRef]
- Lewin, M.; Weil, E.D. Mechanisms and modes of action in flame retardancy of polymers. Fire Retard. Mater. 2001, 1, 31–68. [Google Scholar]
- Levchik, S.V.; Weil, E.D. Overview of recent developments in the flame retardancy of polycarbonates. Polym. Int. 2005, 54, 981–998. [Google Scholar] [CrossRef]
- Jia, D.; Yang, J.; He, J.; Li, X.; Yang, R. Melamine-based polyol containing phosphonate and alkynyl groups and its application in rigid polyurethane foam. J. Mater. Sci. 2021, 56, 870–885. [Google Scholar] [CrossRef]
- Liu, Y.; He, J.; Yang, R. The synthesis of melamine-based polyether polyol and its effects on the flame retardancy and physical–mechanical property of rigid polyurethane foam. J. Mater. Sci. 2017, 52, 4700–4712. [Google Scholar] [CrossRef]
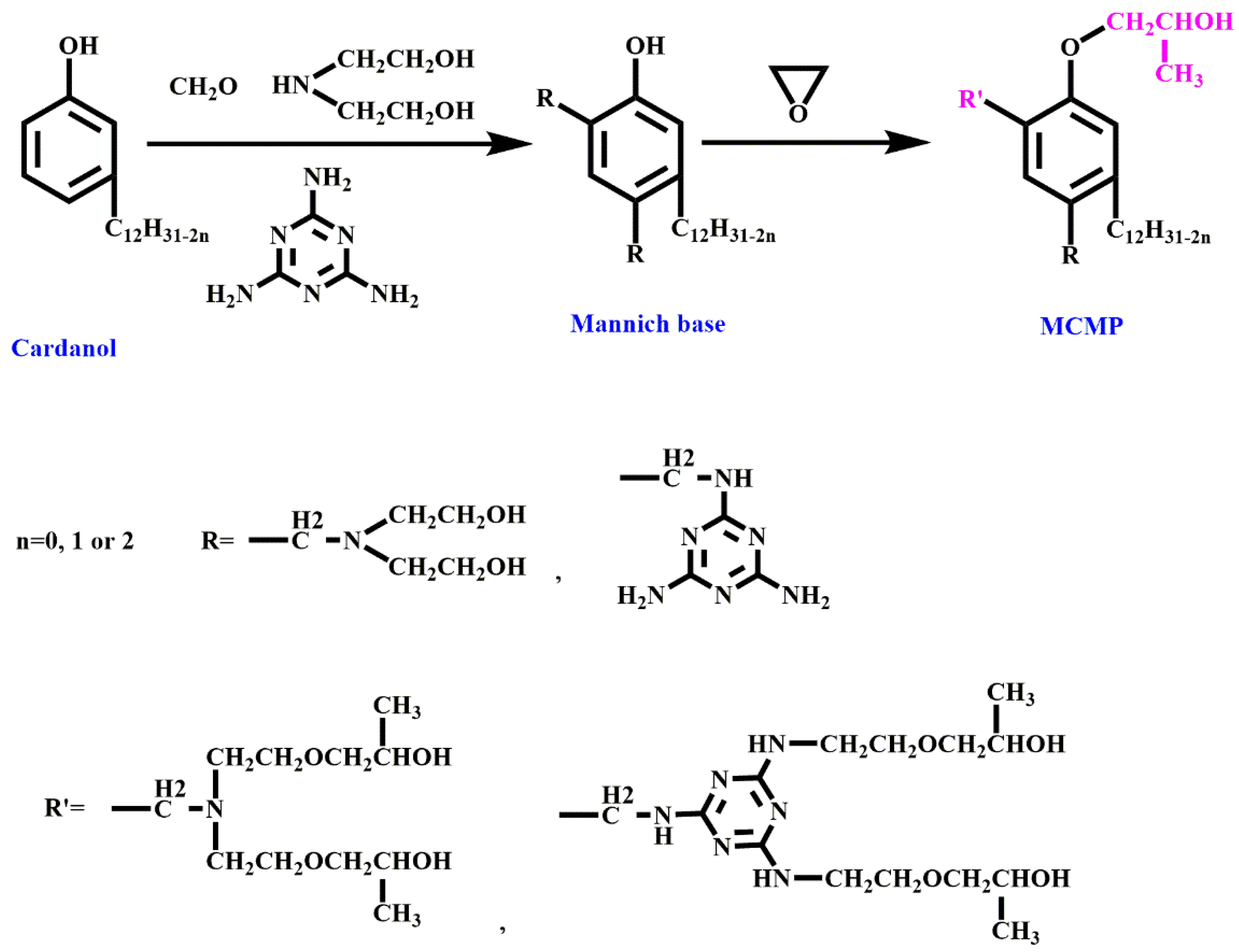
- Zhang, M.; Zhang, J.; Chen, S.; Zhou, Y. Synthesis and fire properties of rigid polyurethane foams made from a polyol derived from melamine and cardanol. Polym. Degrad. Stab. 2014, 110, 27–34. [Google Scholar] [CrossRef]
- Li, X.; Yu, Z.; Zhang, L. Synthesis of a green reactive flame-retardant polyether polyol and its application. J. Appl. Polym. Sci. 2021, 138, 50154. [Google Scholar] [CrossRef]
- Gong, Q.; Qin, L.; Yang, L.; Liang, K.; Wang, N. Effect of flame retardants on mechanical and thermal properties of bio-based polyurethane rigid foams. RSC Adv. 2021, 11, 30860–30872. [Google Scholar] [CrossRef] [PubMed]
- Tang, G.; Liu, M.; Deng, D.; Zhao, R.; Liu, X.; Yang, Y.; Yang, S.; Liu, X. Phosphorus-containing soybean oil-derived polyols for flame-retardant and smoke-suppressant rigid polyurethane foams. Polym. Degrad. Stab. 2021, 191, 109701. [Google Scholar] [CrossRef]
- Yang, H.; Wang, X.; Song, L.; Yu, B.; Yuan, Y.; Hu, Y.; Yuen, R.K. Aluminum hypophosphite in combination with expandable graphite as a novel flame retardant system for rigid polyurethane foams. Polym. Adv. Technol. 2014, 25, 1034–1043. [Google Scholar] [CrossRef]
- Chaudhary, B.; Barry, R.; Cheung, Y.; Ho, T.; Guest, M.; Stobby, W. Halogenated Fire-Retardant Compositions and Foams and Fabricated Articles Therefrom. U.S. Patent Application No. 09/728, 8 August 2002. [Google Scholar]
- Chen, Y.; Bai, Z.; Xu, X.; Guo, J.; Chen, X.; Hsu, S.L.; Lu, Z.; Wu, H. Phosphonitrile decorating expandable graphite as a high-efficient flame retardant for rigid polyurethane foams. Polymer 2023, 283, 126268. [Google Scholar] [CrossRef]
- Chen, Y.; Li, L.; Wu, X. Construction of an efficient ternary flame retardant system for rigid polyurethane foam based on bi-phase flame retardant effect. Polym. Adv. Technol. 2020, 31, 3202–3210. [Google Scholar] [CrossRef]
- Yang, H.; Song, L.; Hu, Y.; Yuen, R.K. Diphase flame-retardant effect of ammonium polyphosphate and dimethyl methyl phosphonate on polyisocyanurate-polyurethane foam. Polym. Adv. Technol. 2018, 29, 2917–2925. [Google Scholar] [CrossRef]
- Xu, J.; Wu, Y.; Zhang, B.; Zhang, G. Synthesis and synergistic flame-retardant effects of rigid polyurethane foams used reactive DOPO-based polyols combination with expandable graphite. J. Appl. Polym. Sci. 2021, 138, 50223. [Google Scholar] [CrossRef]
- Zhang, M.; Luo, Z.; Zhang, J.; Chen, S.; Zhou, Y. Effects of a novel phosphorus–nitrogen flame retardant on rosin-based rigid polyurethane foams. Polym. Degrad. Stab. 2015, 120, 427–434. [Google Scholar] [CrossRef]
- Ranaweera, C.; Ionescu, M.; Bilic, N.; Wan, X.; Kahol, P.; Gupta, R.K. Biobased polyols using thiol-ene chemistry for rigid polyurethane foams with enhanced flame-retardant properties. J. Renew. Mater. 2017, 5, 1. [Google Scholar] [CrossRef]
- Wu, D.H.; Zhao, P.H.; Liu, Y.Q.; Liu, X.Y.; Wang, X.F. Halogen Free flame retardant rigid polyurethane foam with a novel phosphorus− nitrogen intumescent flame retardant. J. Appl. Polym. Sci. 2014, 131, 39581. [Google Scholar] [CrossRef]
- Xu, Q.; Zhai, H.; Wang, G. Mechanism of smoke suppression by melamine in rigid polyurethane foam. Fire Mater. 2015, 39, 271–282. [Google Scholar] [CrossRef]
- Chen, Y.; Luo, Y.; Guo, X.; Chen, L.; Xu, T.; Jia, D. Structure and flame-retardant actions of rigid polyurethane foams with expandable graphite. Polymers 2019, 11, 686. [Google Scholar] [CrossRef]
- Chen, Y.; Bai, Z.; Xu, X.; Guo, X.; Guo, J.; Chen, X.; Lu, Z.; Wu, H. Fabrication of a novel P-N flame retardant and its synergistic flame retardancy with expandable graphite on rigid polyurethane foam. J. Appl. Polym. Sci. 2023, 140, e54013. [Google Scholar] [CrossRef]
- Liu, M.; Shen, H.; Luo, Y.; Zhang, R.; Tao, Y.; Liu, X.; Zong, Z.; Tang, G. Rigid polyurethane foam compounds with excellent fire performance modified by a piperazine pyrophosphate/expandable graphite synergistic system. Fire Mater. 2023, 47, 925–937. [Google Scholar] [CrossRef]
- Dai, C.; Gu, C.; Liu, B.; Lyu, Y.; Yao, X.; He, H.; Fang, J.; Zhao, G. Preparation of low-temperature expandable graphite as a novel steam plugging agent in heavy oil reservoirs. J. Mol. Liq. 2019, 293, 111535. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, F.; Dong, Q.; Yuan, W.; Liu, P.; Ding, Y.; Zhang, S.; Yang, M.; Zheng, G. Expandable graphite encapsulated by magnesium hydroxide nanosheets as an intumescent flame retardant for rigid polyurethane foams. J. Appl. Polym. Sci. 2018, 135, 46749. [Google Scholar] [CrossRef]
- Akdogan, E.; Erdem, M.; Ureyen, M.E.; Kaya, M. Synergistic effects of expandable graphite and ammonium pentaborate octahydrate on the flame-retardant, thermal insulation, and mechanical properties of rigid polyurethane foam. Polym. Compos. 2020, 41, 1749–1762. [Google Scholar] [CrossRef]
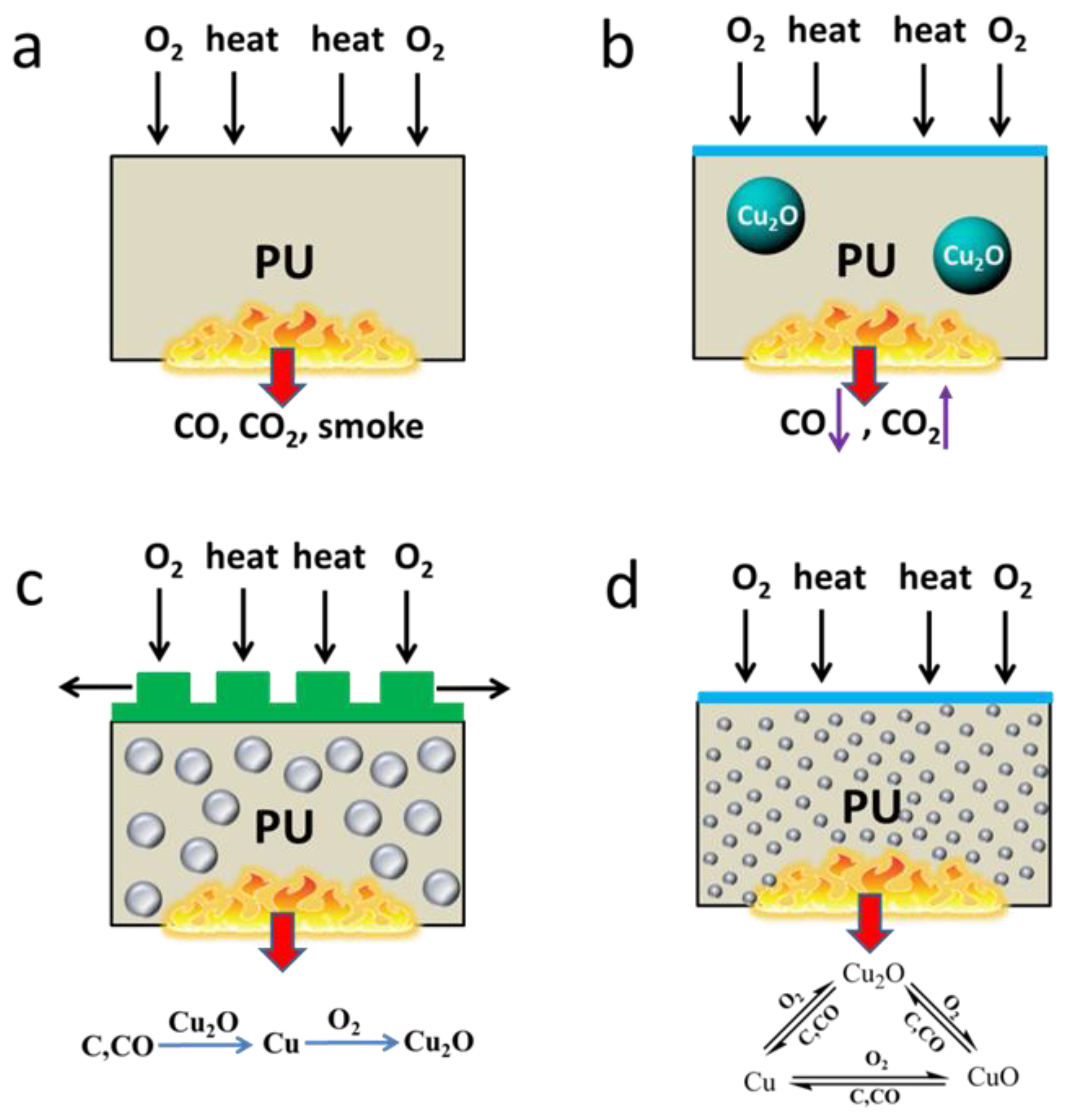
- Yuan, Y.; Wang, W.; Shi, Y.; Song, L.; Ma, C.; Hu, Y. The influence of highly dispersed Cu2O-anchored MoS2 hybrids on reducing smoke toxicity and fire hazards for rigid polyurethane foam. J. Hazard. Mater. 2020, 382, 121028. [Google Scholar] [CrossRef] [PubMed]
- Xu, W.; Wang, G.; Xu, J.; Liu, Y.; Chen, R.; Yan, H. Modification of diatomite with melamine coated zeolitic imidazolate framework-8 as an effective flame retardant to enhance flame retardancy and smoke suppression of rigid polyurethane foam. J. Hazard. Mater. 2019, 379, 120819. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Pan, H.; Shi, Y.; Yu, B.; Pan, Y.; Liew, K.M.; Song, L.; Hu, Y. Sandwichlike coating consisting of alternating montmorillonite and β-FeOOH for reducing the fire hazard of flexible polyurethane foam. ACS Sustain. Chem. Eng. 2015, 3, 3214–3223. [Google Scholar] [CrossRef]
- Agrawal, A.; Kaur, R.; Walia, R.S. Investigation on flammability of rigid polyurethane foam-mineral fillers composite. Fire Mater. 2019, 43, 917–927. [Google Scholar] [CrossRef]
- Darder, M.; Matos, C.R.S.; Aranda, P.; Gouveia, R.F.; Ruiz-Hitzky, E. Bionanocomposite foams based on the assembly of starch and alginate with sepiolite fibrous clay. Carbohydr. Polym. 2017, 157, 1933–1939. [Google Scholar] [CrossRef]
- Alis, A.; Majid, R.A.; Mohamad, Z. Morphologies and Thermal Properties of Palm-oil Based Rigid Polyurethane/Halloysite Nanocomposite Foams. CET J. Chem. Eng. Trans. 2019, 72, 415–420. [Google Scholar]
- Pang, X.Y.; Chang, R.; Weng, M.Q. Halogen-free flame retarded rigid polyurethane foam: The influence of titanium dioxide modified expandable graphite and ammonium polyphosphate on flame retardancy and thermal stability. Polym. Eng. Sci. 2018, 58, 2008–2018. [Google Scholar] [CrossRef]
- Salasinska, K.; Borucka, M.; Leszczyńska, M.; Zatorski, W.; Celiński, M.; Gajek, A.; Ryszkowska, J. Analysis of flammability and smoke emission of rigid polyurethane foams modified with nanoparticles and halogen-free fire retardants. J. Therm. Anal. Calorim. 2017, 130, 131–141. [Google Scholar] [CrossRef]
- Bian, X.C.; Tang, J.H.; Li, Z.M. Flame retardancy of whisker silicon oxide/rigid polyurethane foam composites with expandable graphite. J. Appl. Polym. Sci. 2008, 110, 3871–3879. [Google Scholar] [CrossRef]
- Yuan, Y.; Yu, B.; Shi, Y.; Ma, C.; Song, L.; Hu, W.; Hu, Y. Highly efficient catalysts for reducing toxic gases generation change with temperature of rigid polyurethane foam nanocomposites: A comparative investigation. Compos. Part A Appl. Sci. Manuf. 2018, 112, 142–154. [Google Scholar] [CrossRef]
- Yuan, Y.; Wang, W.; Xiao, Y.; Yuen, A.C.Y.; Mao, L.; Pan, H.; Yu, B.; Hu, Y. Surface modification of multi-scale cuprous oxide with tunable catalytic activity towards toxic fumes and smoke suppression of rigid polyurethane foam. Appl. Surf. Sci. 2021, 556, 149792. [Google Scholar] [CrossRef]
- Cheng, J.; Niu, S.; Kang, M.; Liu, Y.; Zhang, F.; Qu, W.; Guan, Y.; Li, S. The thermal behavior and flame retardant performance of phase change material microcapsules with modified carbon nanotubes. Energy 2022, 240, 122821. [Google Scholar] [CrossRef]
- Jiang, Y.; Yan, P.; Wang, Y.; Zhou, C.; Lei, J. Form-stable phase change materials with enhanced thermal stability and fire resistance via the incorporation of phosphorus and silicon. Mater. Des. 2018, 160, 763–771. [Google Scholar] [CrossRef]
- Niu, S.; Cheng, J.; Zhao, Y.; Kang, M.; Liu, Y. Preparation and characterization of multifunctional phase change material microcapsules with modified carbon nanotubes for improving the thermal comfort level of buildings. Constr. Build. Mater. 2022, 347, 128628. [Google Scholar] [CrossRef]
- Hou, L.; Li, H.; Liu, Y.; Niu, K.; Shi, Z.; Liang, L.; Yao, Z.; Liu, C.; Tian, D. Synergistic effect of silica aerogels and hollow glass microspheres on microstructure and thermal properties of rigid polyurethane foam. J. Non Cryst. Solids 2022, 592, 121753. [Google Scholar] [CrossRef]
- Huang, Y.; Zhou, J.; Sun, P.; Zhang, L.; Qian, X.; Jiang, S.; Shi, C. Green, tough and highly efficient flame-retardant rigid polyurethane foam enabled by double network hydrogel coatings. Soft Matter 2021, 17, 10555–10565. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Wang, X.; Wang, X.; Li, H.; Sun, J.; Sun, W.; Yao, Y.; Gu, X.; Zhang, S. Surface coated rigid polyurethane foam with durable flame retardancy and improved mechanical property. Chem. Eng. J. 2020, 385, 123755. [Google Scholar] [CrossRef]
- Huang, Y.; Jiang, S.; Liang, R.; Sun, P.; Hai, Y.; Zhang, L. Thermal-triggered insulating fireproof layers: A novel fire-extinguishing MXene composites coating. Chem. Eng. J. 2020, 391, 123621. [Google Scholar] [CrossRef]
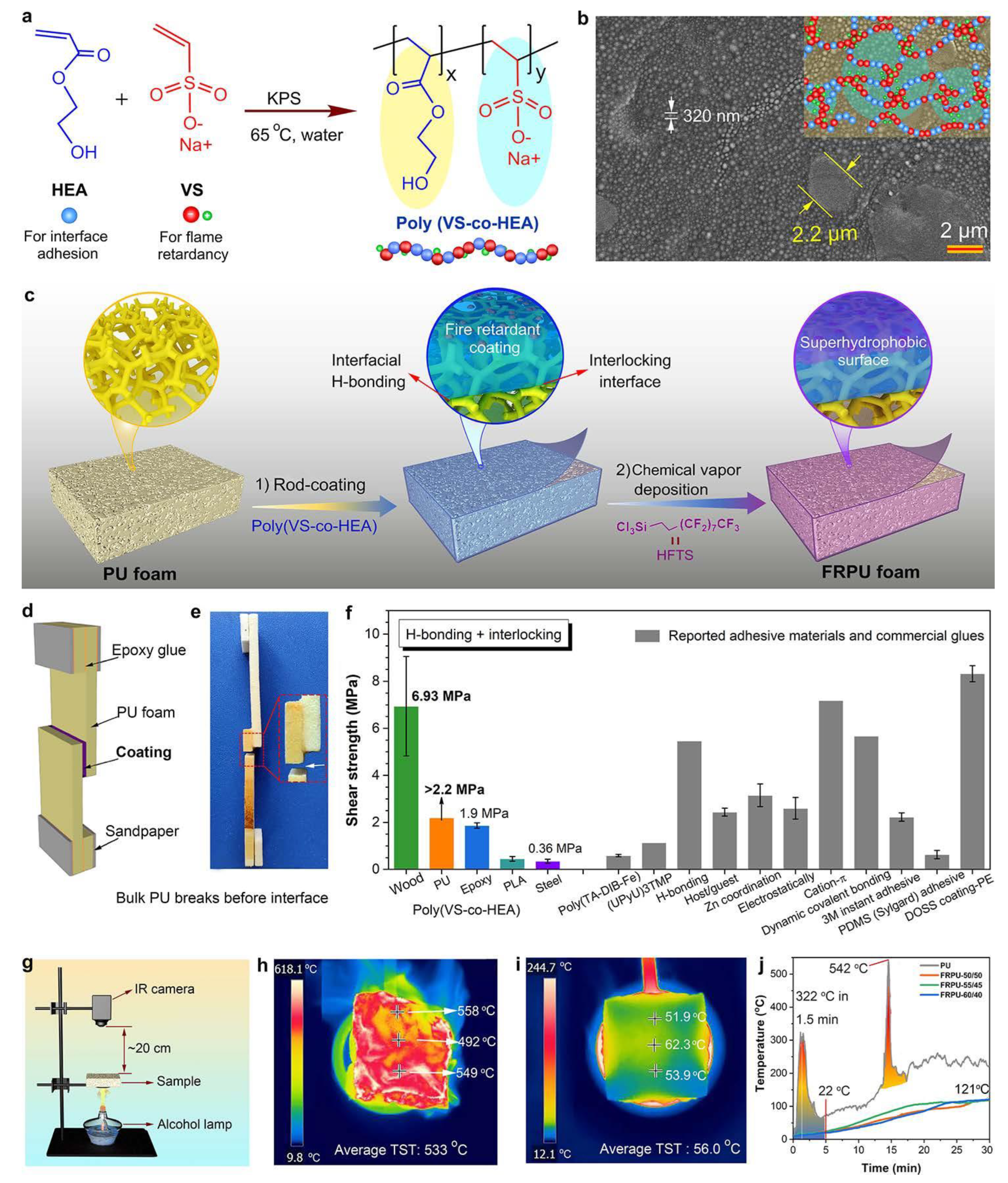
- Zhang, C.; Xie, H.; Du, Y.; Li, X.; Zhou, W.; Wu, T.; Qu, J. Shelter Forest Inspired Superhydrophobic Flame-Retardant Composite with Root-Soil Interlocked Micro/Nanostructure Enhanced Mechanical, Physical, and Chemical Durability. Adv. Funct. Mater. 2023, 33, 2213398. [Google Scholar] [CrossRef]
- Chen, Y.; Wang, W.; Qiu, Y.; Li, L.; Qian, L.; Xin, F. Terminal group effects of phosphazene-triazine bi-group flame retardant additives in flame retardant polylactic acid composites. Polym. Degrad. Stab. 2017, 140, 166–175. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yuan, Y.; Lin, W.; Xiao, Y.; Yu, B.; Wang, W. Advancements in Flame-Retardant Systems for Rigid Polyurethane Foam. Molecules 2023, 28, 7549. https://doi.org/10.3390/molecules28227549
Yuan Y, Lin W, Xiao Y, Yu B, Wang W. Advancements in Flame-Retardant Systems for Rigid Polyurethane Foam. Molecules. 2023; 28(22):7549. https://doi.org/10.3390/molecules28227549
Chicago/Turabian StyleYuan, Yao, Weiliang Lin, Yi Xiao, Bin Yu, and Wei Wang. 2023. "Advancements in Flame-Retardant Systems for Rigid Polyurethane Foam" Molecules 28, no. 22: 7549. https://doi.org/10.3390/molecules28227549
APA StyleYuan, Y., Lin, W., Xiao, Y., Yu, B., & Wang, W. (2023). Advancements in Flame-Retardant Systems for Rigid Polyurethane Foam. Molecules, 28(22), 7549. https://doi.org/10.3390/molecules28227549







