1. Introduction
Textiles, as a common product made of fiber materials, have been extensively utilized in all aspects of our daily lives and industries. However, due to the general tendency of textile fiber materials to burn and cause fires, it can result in damage to property and even human life. Therefore, the application expansion of textiles is highly limited. For decades, tremendous efforts have been made to enhance the flame retardancy of textiles by incorporating flame retardants into the fiber matrix or directly modifying the surface of textiles.
Surface modification is the most commonly used technique thanks to its simplicity and ease of operation for both synthetic and natural fabrics [
1]. A wide variety of flame retardants are used to endow textiles with good flame retardancy, mainly involving inorganic or organic flame retardants. The common inorganic flame retardants, mainly inorganic phosphorus-containing, boron-containing, zinc-containing, iron-containing, and carbon-based materials, and the frequently used organic flame-retardants such as halogenated, phosphorus-containing, nitrogen-containing, and silicone-containing flame retardants [
2,
3,
4]. As early-stage commercial flame-retardants, halogenated compounds perform outstanding functions by releasing halogen radicals to eliminate reactive radicals during combustion [
2]. Unfortunately, it has been abandoned in its actual application since its severe toxicity to the environment and human safety [
5]. In response to this challenge, halogen-free flame retardants have emerged, and phosphorus-containing flame retardants stand out. The high flame retardancy is due to the promotion of the substrate to form a char layer, which isolates the transfer of heat and combustible gases, thus preventing further combustion of the substrate [
2,
3]. In addition, the silicone-containing flame retardants usually form a vitrified layer on the polymer surface during combustion, which effectively hinders the transfer of oxygen, heat, and mass and reduces the flammability of the polymer [
6,
7]. In contrast, nitrogen-containing flame retardants release noncombustible gases to dilute combustible gases such as oxygen during combustion, and the flame retardant effect is relatively poor but friendly to the environment [
8]. To effectively improve the flame retardant efficiency, the combination of different elements with flame retardant properties can provide synergistic flame retardant effects and impart additional thermal stability and mechanical properties to the composites. In general, the study of synergistic flame retardancy has gradually become the emphasis of flame retardancy research in recent years [
9].
Since the outbreak of COVID-19 in 2019, its prevalence has had a terribly negative impact on human health and caused enormous panic in the public. Against this background, the demand for medical protective equipment and anti-bacterial textiles is on the rise. As we all know, textiles have been protecting humans from external environmental harm for a long time. However, some textiles can also serve as breeding grounds or carriers for bacteria and viruses due to their hygroscopicity [
10], which may lead to the risk of inflammation, disease, and even death in the human body. Therefore, the antibacterial treatment of some fabrics has become extremely urgent and has received widespread attention from scholars. Notably, the surface treatment of fabrics with antibacterial agents is the most commonly used strategy, which is similar to the flame retardant surface treatment of fabrics. In addition, antibacterial agents are mainly divided into three categories: inorganic antibacterial agents (metal and oxide nanoparticles (NPs), carbon-based antibacterial materials along with their composites), organic antibacterial agents (quaternary ammonium salts, guanidine, halogenated amines, phenols, etc.), and natural antibacterial agents (chitin, CS), and then the antibacterial or bactericidal effect of the antibacterial substances is mainly exerted by either directly contacting the bacterial surface or releasing the antibacterial moiety onto the substrate [
11,
12]. With the emergence of inorganic and organic antibacterial agents, the industrial application of antibacterial products continues to deepen, and they are playing an irreplaceable role in the functional textile industry.
With the improvement of living conditions and the development of science and technology, the demand for multifunctional textiles in the market has grown in recent years. In particular, there is a large demand for flame retardant and antibacterial textiles in areas such as household products and medical protection. In order to develop novel functional fabrics with both flame-retardant and antibacterial properties, the relevant specific functional reagents and treatment methods have attracted considerable attention. Flame retardant and antibacterial dual-functional fabrics are usually achieved via a two-step or one-step method. The two-step method generally achieves the superposition of dual functions by introducing flame retardant and bacterial inhibitors in steps, which has the advantages of simplicity and wide applicability. However, there is a problem that some flame retardants or antibacterial agents may interact with each other, thus causing functional antagonism that is not conducive to simultaneously imparting excellent flame retardant or antibacterial properties to a fabric when the functional coating is applied in a stack. One-step methods normally treat fabrics by using synthetic agents with both flame retardant and antibacterial functions. However, the design of such multifunctional compounds is often difficult, and the synthesis process is complex. Therefore, it is a tremendous challenge to find the best processes and functional materials in the current direction of multifunctional textile research.
It is worth nothing that the specific finishing techniques have a pivotal influence on the processing efficiency and overall performance of the fabric. Then the traditional after-finishing technology mainly involves impregnation, padding, spraying, and pad-dry-cure techniques. These methods often do not require additional physical and chemical reactions but instead directly use functional solutions to treat the surface of fabrics, which have the advantages of being simple, effective, and easy to operate, but have the drawback of poor durability. In recent years, some promising environmentally friendly strategies have gradually attracted attention, such as LBL deposition, which usually uses deionized water as the solvent and positive and negative electrolytes as functional treatment agents [
13]. Similarly, the sol-gel process is another environmentally friendly strategy that is favorable for the construction of functional surfaces for textile fibers by depositing thin organic-inorganic hybrid sol-gel films. In addition, this method has been selected as a simple and effective method to form a multifunctional protective coating since two or more siloxane precursors with different organic functions could be applied simultaneously [
14,
15]. Compared with the aforementioned techniques, chemical grafting modification exhibits unprecedented durability due to the stronger chemical bond linkage between functional agents and substrates [
16]. Specifically, flame-retardant and antibacterial coatings are composed of inorganic nanomaterials, metal ions, or metal oxides. In situ modification technology has been extensively used for the construction of these coatings.
There is no systematic summary of research on flame-retardant antibacterial fabrics, especially the accompanying finishing techniques. This paper reviews the latest research results about the flame-retardant and antibacterial functional finishing of textiles over the past decade. Furthermore, it explores the nascent finishing agents as well as the adaptable post-finishing technique. Simultaneously, this review also discusses the advantages, disadvantages, and application scope of these techniques and briefly introduces the development of green environmental technologies.
3. Recent Advances in Multifunctional Textiles with Flame Retardant and Antibacterial Properties
The functional finishing of traditional textiles is mainly divided into two strategies. One is to mix the functional agents with textile raw materials and prepare functional textiles after the spinning process. This method has the advantage of good washing durability, but the cost is relatively high and usually has a significant influence on the mechanical properties of textile fibers. The other method is to perform surface modification treatments on textiles. Currently, surface modification technology is commonly used for the fabrication of functional fabrics such as flame retardant, antibacterial, and hydrophobic fabrics, which is a simple, convenient, and efficient way to endow traditional fibers/fabrics with specific functions. In the process of functional finishing, such as flame retardancy and bacteriostasis, the finishing techniques have a great influence on the final performance of the fabrics. Generally speaking, the finishing technology for fibers/fabrics mainly includes traditional finishing methods such as dip-coating [
20] and spraying techniques [
15] and some novel finishing strategies, including chemical grafting modification [
21,
22], layer-by-layer self-assembly [
23,
24], sol gel [
25,
26], and in situ deposition [
27].
Ulteriorly, the traditional finishing technology of fabrics mainly includes the traditional impregnation method, pad-dry-cure method, coating method, and spray method. These methods have low finishing costs but unsatisfactory washability, and long-term use of flame-retardant effects will be affected by water, light, and other conditions. Presently, various surface-modifying technologies, such as the sol-gel method, nanoparticle adsorption, layer-by-layer self-assembly method, plasma treatment, and the graft copolymerization modification method, have been utilized for preparing flame-retardant, anti-bacterial, hydrophobic, UV-resistant, self-cleaning, multi-functional textiles on the basis of synergistic flame-retardant technology.
3.1. The Traditional Finishing Techniques
3.1.1. The Spray Method
The spraying method is one of the traditional flame-retardant finishing technologies. The finishing agents were dissolved into a certain solvent and then introduced onto the surface of fabric by simple spraying, which easily forms a thin functional coating on fabric surfaces [
28]. For instance, Attia et al. developed the novel nanocomposites (DPHM-Ag NP) based on diphosphate malonate as organic phosphates and silver NPs, and then the nanocomposites coatings were sprayed on the surface of polyester (PS) and cotton-polyester (CB) blend fabrics. The treated fabric meets the standards of high-class flame-retardant textiles with a 0 mm/min rate of burning. Furthermore, the antibacterial properties were enhanced with the clear bacterial inhibition zone reaching 4.48 mm for
Staphylococcus aureus (
S. aureus) [
29].
3.1.2. The Dip-Coating Method
Dip-coating is a finishing method that involves immersing fabrics in a functional agent solution and is accompanied by a drying treatment [
30]. This method is simple to operate; the crosslinking reaction between the fabric and the flame retardant is weak during the process of finishing, and most of the flame retardant is just attached to the surface of the fabric, so the durability of the flame-retardant fabric is generally poor. To achieve the flame-retardant antibacterial properties of textiles, a novel agent (tetramethylcyclosiloxyl-piperazin) tetra guanidine (GNCTSi) was designed and successfully applied to form a functional coating on the surface of cotton fabrics. The treated cotton fabrics have enhanced properties, with LOI reaching 30.1% and char length remaining at 6.5 cm after burning. To a certain extent, the coating improves washing durability and thus has less impact on the flame retardancy of cotton fabrics. Furthermore, it also exhibits improved antibacterial effects with inhibition zones of 2.5 mm and 2.3 mm against
Escherichia coli (
E. coli) and
S. aureus, respectively [
31]. In addition, Atousa et al. prepared a suspension with ZrO
2 NPs along with cetyltrimethylammonium bromide (CTAB), maleic acid (MA), sodium hypophosphite (SHP), and urea by using an impregnation bath. MA was used as a cross-linking agent, while SHP acted as the catalyst to stabilize NPs on the fabric surface and prevent fabric from creasing. In the end, the test results showed improved flame-retardant properties, antibacterial activities, and self-cleaning properties of the treated cotton fabrics [
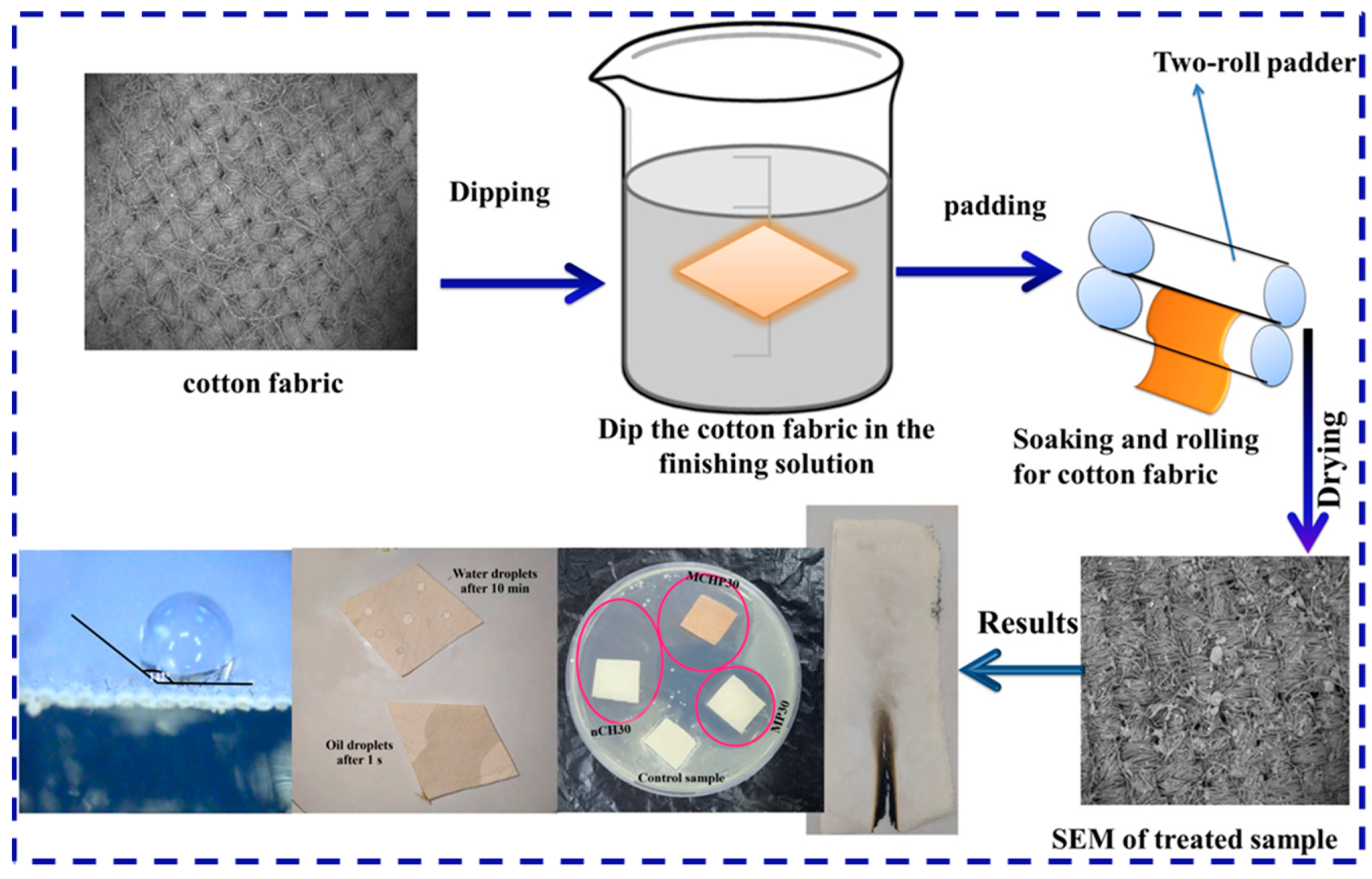
32]. In order to enhance the coating’s durability, some polymeric coatings, such as polyvinyl alcohol (PVA) and polyurethane (PU), were used as binder for flame retardant components. In the work of Ghada et al., nano chitosan (n CS), melamine phosphate (MP), and melamine salt of CS phosphate (MCSP) were prepared and then mixed with PVA to construct PVA/MCSP and PVA/n CS/MP coatings on cotton fabrics by the dip-coating method (as shown in
Figure 1). The PVA/MCSP30 coating displayed the optimum flame resistance with self-extinguished behavior and a very high LOI of 58.2%, while the LOI for the original fabric was only 17.2%. In addition, it also exhibited good coating durability as well as better antibacterial properties for both
E. coli (inhibition zone diameter of 27.6 mm) and
S. aureus (inhibition zone diameter of 30.5 mm) [
33]. Similarly, the condensed tannin extract from Dioscorea cirrhosa tubers was also used as the functional agent for silk fabric, and the treated fabric has good antibacterial properties and flame retardancy [
34].
3.1.3. The Pad-Dry-Cure Method
The pad-dry-cure method treats the fabric by padding, drying, and curing it after it has absorbed enough functional solution [
35]. In these processes, high-temperature drying makes flame-retardant or antibacterial functional materials chemically cross-linked with fabrics to obtain durable flame retardancy. Ahmed et al. first fabricated blended fabrics with different compositions, weaving structures, grams per square meter, thicknesses, and thread densities. In the following step, a three-dimensional tetrakis (hydroxymethyl) phosphonium chloride (THPC)-urea polymer coating was synthesized and deposited onto the blended fabrics. The finished fabric showed excellent antibacterial properties (99.9%) and excellent flame retardancy (LOI~36.8%); additionally, it also possessed superior water repellence properties (151.5°) [
36]. Singh et al. prepared a thyme oil-embedded functional microcapsule via in situ synthesis of CS phosphate as the shell material, and then the microcapsules were introduced onto linen fabrics via the pad-dry method. The finished fabric presented excellent antibacterial properties (>98%), flame retardancy (LOI > 28), antioxidant activity (96%), mosquito repellency (100%), and an excellent fragrance. Moreover, the functional properties were durable after at least 20 washes [
37].
The pad-dry-cure method was also applied to deposit nanocomposite coatings on fabrics. The TiO
2 NPs were prepared by the sol-gel method using titanium tetraisopropoxide. The development of nano TiO
2 onto cotton fabric was accomplished when nano TiO
2 was coated onto cotton fabric by the traditional pad-dry-cure method in the presence of polycarboxylic acid [1,2,3,4-butane tetracarboxylic acid], SHP, and CS phosphate. The results confirmed that 1,2,3,4-butane tetracarboxylic acid, TiO
2, and CS phosphate are helpful in increasing the flame resistance and antibacterial properties of cotton fabrics [
38]. Similarly, Dhineshbabu et al. prepared colloidal methyl silicate and MgO nanoparticle-embedded methyl silicate solutions through the sol-gel method. Subsequently, cotton fabrics were separately modified with silica and MgO/methyl silicate composites via an optimized pad-dry-cure method. The MgO/methyl silicate composite-coated fabrics showed enhanced burning performance, significant water-repellent properties, and better antibacterial activity against
S. aureus and
E. coli than methyl silicate-coated and uncoated fabrics [
39]. In another work, an equimolar sol mixture of the precursors P, P-diphenyl-N-(3-(trimethoxysilyl)propyl) phosphinic amide (SiP) and 1H,1H,2H,2H-perfluorooctyltriethoxysilane (SiF) was employed to form a two-component sol-gel inorganic–organic hybrid coating on the fabric by the pad-dry-cure method. It leads to good flame retardancy and antibacterial properties, with an inhibition rate of 92.9% against
E. coli and 80.4% against
S. aureus [
40].
3.2. LBL Self-Assembly Technology
Whether in textile flame retardant finishing or other fields, LBL self-assembly technology has been widely used because of its simple operation, ease of control, and environmental friendliness [
41]. It is a simple and versatile technology for preparing multifunctional coatings. These coatings are formed by repeatedly depositing alternate layers of oppositely charged materials, which experience attraction and undergo self-regulation within individual layers by electrostatic action. LBL self-assembly can be uniformly coated on the surface of textiles to achieve flame retardant, antibacterial, and other multifunctional properties, which have been applied to PS, cotton, polyamide, and silk fabrics [
42]. At present, the commonly used positively charged compounds mainly include CS, polyetherimide (PEI), DL-arginine (DL), etc. The negatively charged electrolytes mainly include phytic acid (PA), sodium alginate (SA), ammonium polyphosphate (APP), and so on. Except for flame retardancy, some of these charged materials can also endow base materials with antibacterial properties, while others need to be combined with antibacterial materials to achieve multi-functionalization.
Herein, the bio-based materials PA and CS are often selected to impart flame retardant as well as antibacterial properties to fabrics through LBL technology. PA and its salts, such as ammonium phytate (AP), are generally composed of a large number of phosphoric acid groups and ammonium ions. They can catalyze the degradation of fabric substrate to form more char residues and release incombustible gases such as NH
3 or N
2 to dilute combustible gases during combustion. Therefore, the flame-retardant properties of fabrics in both condensed and gaseous phases are ultimately improved [
1,
43]. CS contains NH
2 groups that can generate -NH
3+ groups under acidic conditions, so it applies as a positive charge in LBL self-assembly. Moreover, the -NH
3+ group can destroy the cell wall or interfere with the normal physiological activities of cells, thus exerting both flame retardant and bacteriostatic effects [
44]. Liu et al. deposited CS and AP on cotton fabric to manufacture fully bio-based flame-retardant and antibacterial cotton fabrics using LBL technology. The modified textile with a low weight gain (8 wt%) performed perfect self-extinguishing behavior in the vertical burning test. Moreover, the CS/AP coating has an effective antimicrobial rate of 99.83% against E. coli, which mainly depends on the introduction of CS [
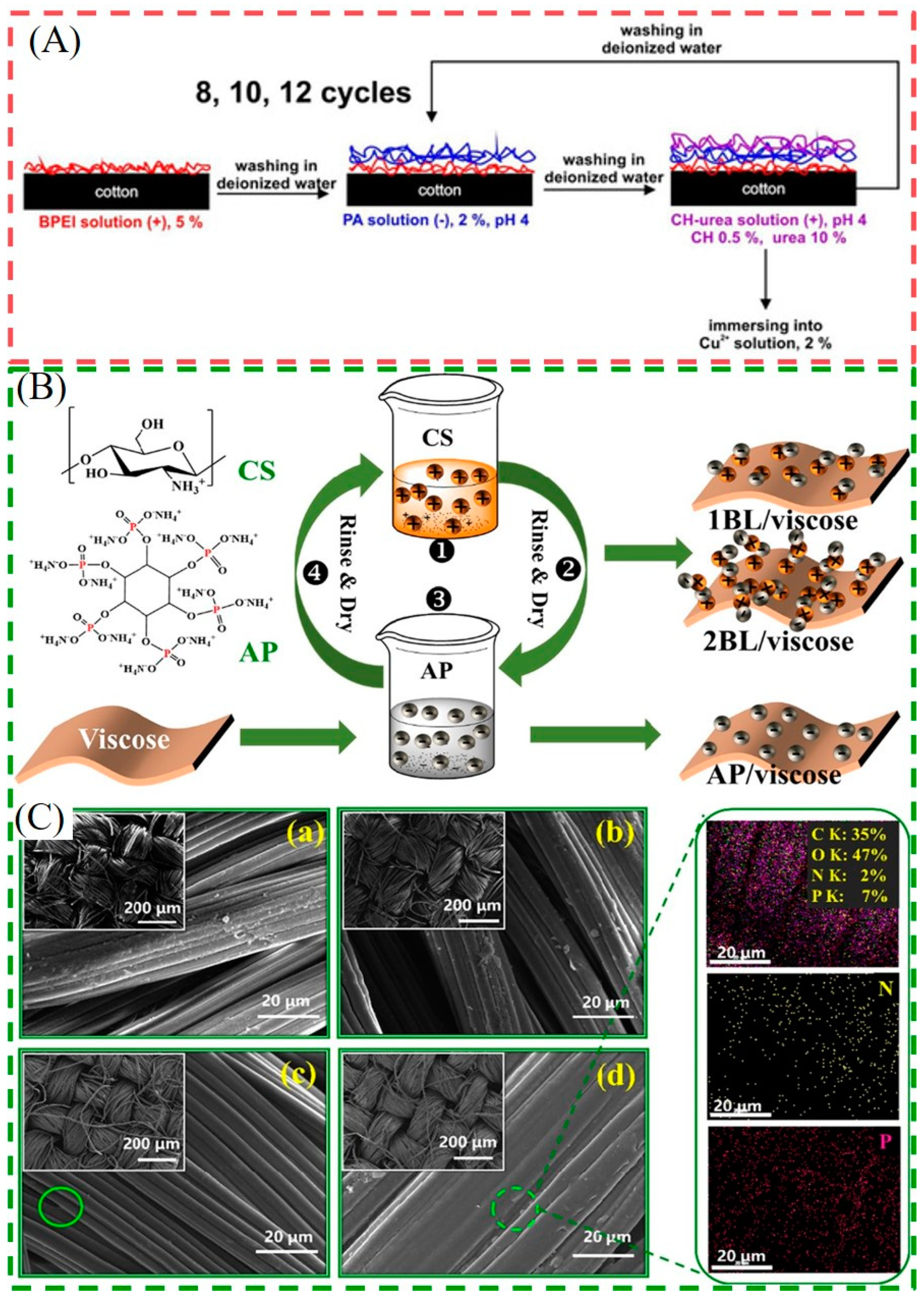
44]. In their other work, a fully bio-based CS/AP coating was also applied to a viscose fabric (as shown in
Figure 2B,C)). The 2BL/Viscose showed sharp improvements in thermal stability in the higher temperature zone, accompanied by a LOI of 29% and self-extinguishing behavior in the combustion test. Additionally, 2BL/Viscose possessed a high bacteriostatic function of 99.99% for both
E. coli and
S. aureus [
45]. However, the antibacterial ability of the system using PA/CS alone is limited and generally requires synergistic use with other antibacterial agents. In order to enhance the antimicrobial ability and improve the broad-spectrum antibacterial effect, it is necessary to introduce other antibacterial agents into the CS/AP coatings. For instance, Eva et al. soaked the LBL-treated samples in a 2% Cu
2+ solution after depositing PA and CS-urea on cotton. The VFT results showed that 12 BL of PA/CS-urea-Cu
2+ could stop the burning flame, and the PHRR and THR were reduced by 61% and 54%, respectively. Moreover, for the antibacterial test, 100% reduction of
S. aureus and Klebsiella pneumoniae can be achieved (as shown in
Figure 2A) [
13].
Arginine, a kind of novel renewable material, has an alkaline cationic chemical group that possesses high antibacterial properties [
46]. In addition, non-combustible gases are released during combustion, acting as a gas-phase flame retardant [
47]. Recently, many researchers have proven that the combination of arginine and CS can have a good inhibitory effect on cell growth and hence exert an antibacterial function. Moreover, arginine-functionalized CS also exhibited good flame retardancy and smoke suppression [
48,
49,
50]. Except for CS, PA can also chelate with the ammonium ions of DL through hydrogen bonds, and they were deposited on CB fabric using LBL assembly. The finishing CT fabric with 20 bilayers showed enhanced fire safety and an efficient inhibition diameter of 4.0 mm against
S. aureus compared to the untreated CT fabric (0 mm) [
51]. In addition, bio-based riboflavin sodium phosphate (vitamin B2, VB2) is an anionic phosphorus-containing compound that has been extensively used in disease treatment. The negatively charged VB2 can cooperate with CS to be used as the LBL assembly agents for the modification of silk fabric, and the prepared colored silk fabric with 10 assembly bilayers exhibited great flame retardancy and antibacterial performance (>90% inhibition rate against both
S. aureus and
E. coli). The employment of LBL technology to prepare fully bio-based flame-retardant and antibacterial coatings follows the concept of environmentally friendly development and has been widely used for the functional modification of fabrics [
52].
Among numerous antibacterial agents, N-halamines have attracted extensive attention due to their broad-spectrum antibacterial activity, long-term efficacy, and renewability [
53,
54]. With the great demand for multifunctional materials, a novel nitrogen/silicon-containing N-halamine cationic polymer (PCQS) containing both flame-retardant and antibacterial components has been designed and used as the positive electrolyte, which then interacts with negatively charged PA molecules to coat cotton fabric. The cotton-PEI/(PCQS/PA)
30-Cl exhibited an increased LOI of 28.5% with a lower char length of 7.9 cm in the vertical flammability test. In addition, the treated fabric could inactivate 6.01 logs of
S. aureus and 6.00 logs of
E. coli within 1 min of contact time, demonstrating effective antimicrobial activity [
55].
3.3. Chemical Grafting Modification
Chemical grafting modification is a technique that introduces functional groups to fibers or fabrics by forming covalent bonds. Flame retardant and antibacterial functionalization can be realized via the chemical grafting technique, which generally shows higher durability than other finishing methods [
56]. As a typical scale inhibitor, diethylene triamine penta methylene phosphonic acid (DTPMPA) is rich in N and P elements and has become a potential flame retardant. In addition, the phosphonic acid groups in the structure of DTPMPA provide sufficient binding sites that can easily chelate with metal ions, such as silver ions. The lyocell fabric with flame-retardant properties (FR-lyocell) has been accomplished by grafting a novel flame-retardant ammonium salt of diethylene triamine penta methylene phosphonic acid (ADTPMPA). Subsequently, the FR-lyocell was further treated with Ag nanoparticles (Ag NPs) to develop antibacterial properties. The flame retardant and antibacterial lyocell fabric (FRAg-lyocell) exhibits unbelievable flame retardancy (LOI of 44.8%) and good washing durability (LOI has still been maintained at 31.3% after nearly 20 laundering cycles). Moreover, pHRR and THR values were suppressed effectively. At the same time, it also possesses excellent antimicrobial ability against both
S. aureus and
E. coli [
56]. Xu et al. synthesized a water-soluble N-halamine precursor based on s-triazine (TIAPC) by introducing iminodiacetic acid. After grafting modification with TIAPC, the treated cotton fabric was then chlorinated with NaClO solution and chelated with metal Al
3+ ions. Cotton-TIAPC-Cl-Al presented a high-efficacy and rapid bactericidal effect against
S. aureus and
E. coli, with 100% bacterial reduction in 1 min. In addition, the hydrophobic property of cotton-TIAPC-Cl-Al was greatly improved after chlorination. However, it cannot self-extinguish in the vertical burning test, implying limited flame retardancy [
57].
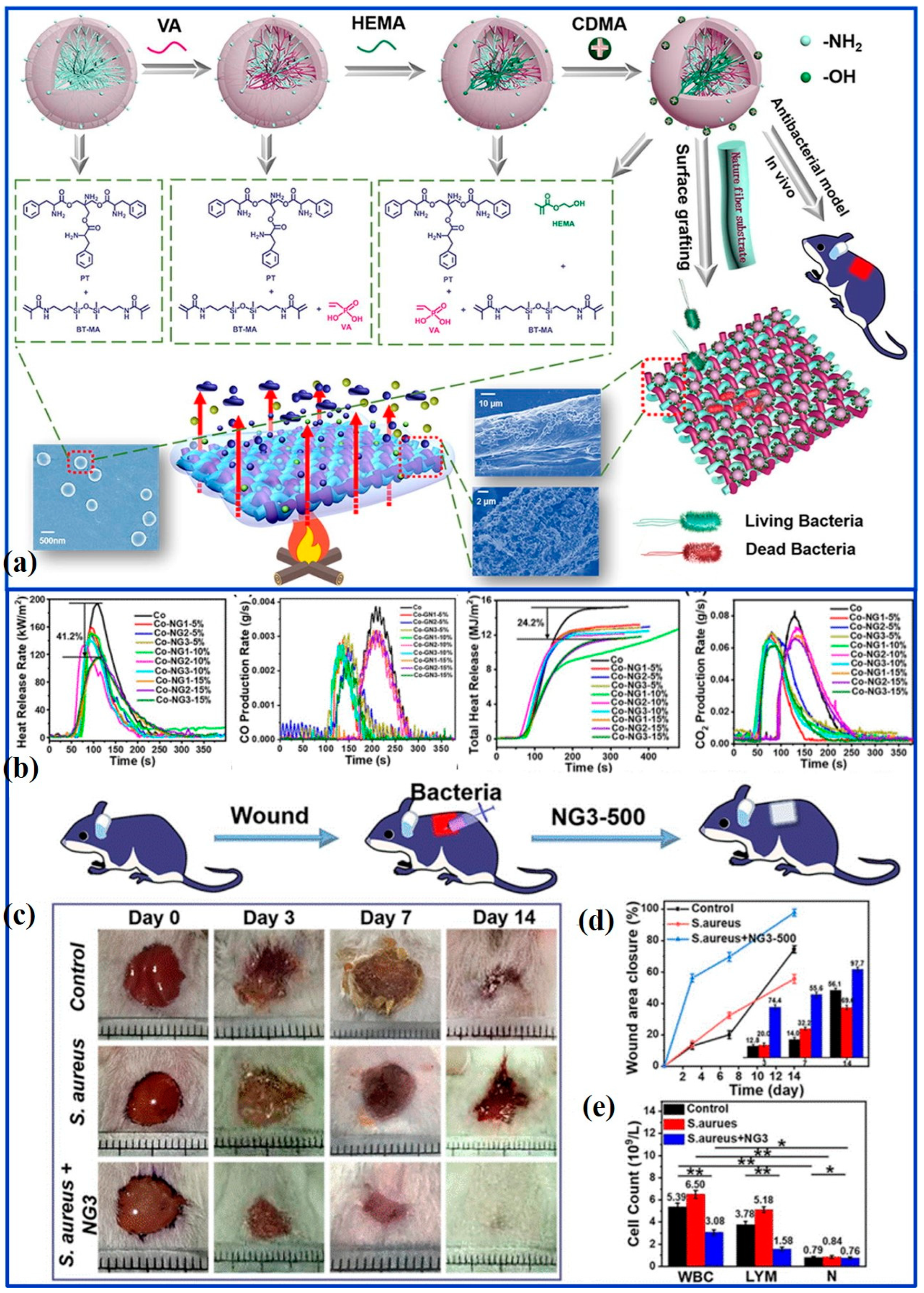
Recently, nanogels, with large specific surface areas and higher functional efficiency, have been widely exploited as antimicrobial materials [
58]. However, to date, there are very few reports in the literature on the use of nanogels for antimicrobial and flame retardant applications. In the work of Li et al., novel nanogels (NG3) were synthesized by Michael addition, which contain both the flame retardant elements of phosphorus, nitrogen, and silicon and the antibacterial component of N, N’-dimethyl-N-(3-(trimethoxysilyl) propyl) dodecane-1-chloroamine. By grafting on the cotton fibers and fabrics, the treated cotton fabric has self-extinguishing behavior, implying improved fire safety. Because NG3 easily destroys cell membranes and causes cell lysis, the grafted cotton fabrics can eliminate nearly 99% of bacteria for both
S. aureus and
E. coli. In addition, the NG3 with good biocompatibility and antibacterial properties plays a positive role in preventing wound infections, and anti-infection experiments of healing efficiency reaching 97.7% after 14 days’ treatment have confirmed it. More importantly, functional cotton fabrics modified with nanogels exhibit relatively low mechanical and comfort properties (
Figure 3) [
16]. In another work, a binary mixture of acrylonitrile and 4-vinyl pyridine under the condition of ceric ammonium nitrate as initiator was copolymerized and grafted on the cotton fabrics by chemical induction. Taking advantage of the flame-retardant properties of synergistic nitrogen and phosphorus elements, the modified cotton fabrics have self-extinguishing abilities with a slow propagation rate. Meanwhile, the treated samples possess excellent antibacterial properties, with 41~96% antibacterial activity [
59].
The guanidyl-based organic compounds can be used as antibacterial agents, which have the advantages of nontoxicity, high-temperature resistance, outstanding antibacterial effects, and long action periods. Commonly, the nitrogen-containing guanidyl group in the guanidyl-based antibacterial agent also exerts a better flame-retardant effect that can be combined with a phosphorus-containing group to form a phosphorus-nitrogen synergistic flame-retardant and antibacterial agent. Two novel and efficient antibacterial and flame-retardant guanidine-based compounds, N, N-di (ethyl phosphate) biguanide (DPG) and mono chlorotriazine triethyl phosphite guanidine (MCTPG), were successfully synthesized and then grafted onto cotton fabric by generating covalent bonds. The treated cotton fabric obtained good flame retardancy and antibacterial properties; the former DPG-treated cotton fabric obtained an LOI of 31.2%; and the antibacterial ratios of
S. aureus and
E. coli were 96.4% and 99.2%, respectively. The latter MCTPG-treated cotton fabric gained a LOI value of 31.2%, and the char length decreased to 8.5 cm. In addition, its inhibition zone base for
E. coli and
S. aureus reached 2.9 mm and 2.8 mm, respectively [
10,
60].
3.4. In Situ Deposition of Inorganic Metal Materials
In recent years, due to its excellent thermal and catalytic properties. Additionally, metal materials have excellent antibacterial properties with free radical capture abilities that have a wide application prospect in the functional fields of antibacterial and UV resistance. These metal materials were deposited on the surface of fabrics by direct reduction or synthesis [
61].
3.4.1. In Situ Deposition of Neat Metallic Oxide
The biomedical applications of NPs, especially metal oxides, have attracted great interest. ZnO NPs are the most famous type of NPs that inhibit the growth of Escherichia coli. Moreover, ZnO NPs with low cost, non-toxicity, and recyclability also acted as efficient photocatalysts. However, their easy deactivation and low acid resistance severely limit the development of ZnO NPs. To solve the problem, Bahare et al. synthesized ZnO@SiO2 NPs and coated them on the PET fabric by applying zinc acetate and sodium silicate as two precursors in an aqueous ammonia solution at 90 °C. The silica-supported ZnO improved these problems by limiting the size of the NPs (approximately 28.29 mm) and protecting them from acid solution corrosion. The treated samples have enhanced anti-dripping properties because of the inherent thermal resistance of Si. Furthermore, the treated PET fabrics eradicated almost 100% of
E. coli [
62]. However, some synthetic fibers, such as PS fibers, lack chemically active groups and have compact structures, which makes it difficult to absorb and carry functional reagents. In order to realize compatibility between these fiber products and functional components, the solvent crazing technique is applied to these fiber structures [
63].
3.4.2. In Situ Deposition Assisted by Polymer Coatings
Unfortunately, metal oxides often have insufficient adhesion to fabrics when used alone. Polymer coating is helpful to improve the adhesion of metal compounds to the surface of fabrics. Commonly, pyrrole and aniline are used to prepare polymer coatings on fabrics that could provide active sites for metallic or other inorganic materials. Mahmoud et al. produced a polypyrrole-silver composite (Ppy-Ag) coating on the cotton/PS substrate through vapor phase polymerization (VPP) and redox reactions [
64]. Polypyrrole can act as an effective stabilizer for Ag NP to solve the instability problem of treated fabrics after washing steps. The coated textile displayed an inhibition zone diameter of 25 mm and 28 mm for
E. coli and
S. aureus, respectively, verifying a supreme antibacterial property. It also had superior conductivity features with a low electrical resistance of 0.0218 kΩ. Furthermore, the treated fabrics show good washing fastness, implying improved stability of silver-containing coatings for textiles [
64]. Polyaniline (PANI), a popular conducting polymer, has been regarded as a flame retardant for polymers due to its better char-forming ability. Cai et al. fabricated a PANI @ TS-silk fabric electrode that exhibits good charging and discharging cycle stability and high area-specific capacitance. Furthermore, the treated fabric electrode has good flame retardance and excellent antibacterial properties (99%) [
65]. In another work, polyaniline was polymerized to form polyaniline chains on the surface of nanotubes in the presence of dispersed halloysite nanotubes. The polyaniline chains were decorated onto the fabric successfully due to the nanotubes aligned on the fabric’s surface. Compared with the untreated fabric, the burning rate of the coated fabric decreased by 72%, and the clear antibacterial inhibition zone was recorded at 6 mm. Furthermore, the tensile strength of coated textile fabrics was maintained due to the alignment of nanotubes on the surface of the fabrics [
66].
3.4.3. In Situ Deposition Assisted by Complexation Reaction
Many organic compound molecules (ions) containing unsaturated or active groups such as amino, carboxyl, and hydroxyl groups are prone to interact with metal ions, and organometallic complexes are usually obtained through certain coordination, complexation, and redox reactions. At present, this method is widely used in fabric functional finishing. Owing to the abundant nitrogen source, guanidine salts (e.g., guanidine carbonate, nitrate, and phosphate) exhibited intriguing flame retardancy. These compounds are able to improve the flame retardancy of the polymer matrix and promote the formation of the carbonized layer, which can act as a physical barrier. Guanazole, a low-cost compound with the chemical structure of 3,5-diamino-1,2,4-triazole, can coordinate with metal centers, so it has become an excellent flame retardant ligand. Hu and Wang et al. formed guanazole-zinc and guanazole-silver in aqueous solutions and then deposited them on cotton fabric surfaces by a dipping process. As expected, the cotton fabrics modified with guanazole-zinc and guanazole-silver exhibit outstanding flame retardancy with 29.5% and 27.5% LOI, respectively. Additionally, the samples possess self-extinguished behavior after removing the igniter during the vertical burning test and have reached the UL-94 V-0 level of flame retardant after the vertical burning test. In the micro-scale combustion calorimeter test, the HRR of guanazole-zinc and guanazole-silver modified cotton fabrics, respectively, reached 64.4% and 59.1%, while the THR of guanazole-zinc and guanazole-silver modified cotton fabrics reached 26.4% and 14.8%. More than this, the treated cotton fabrics also showed augmented antibacterial capacity against
S. aureus and
E. coli. Notably, the guanazole-silver-coated cotton fabrics also reflect the antifungal effect on Penicillium, Aspergillus niger, and Fusarium chlamydosporum [
67].
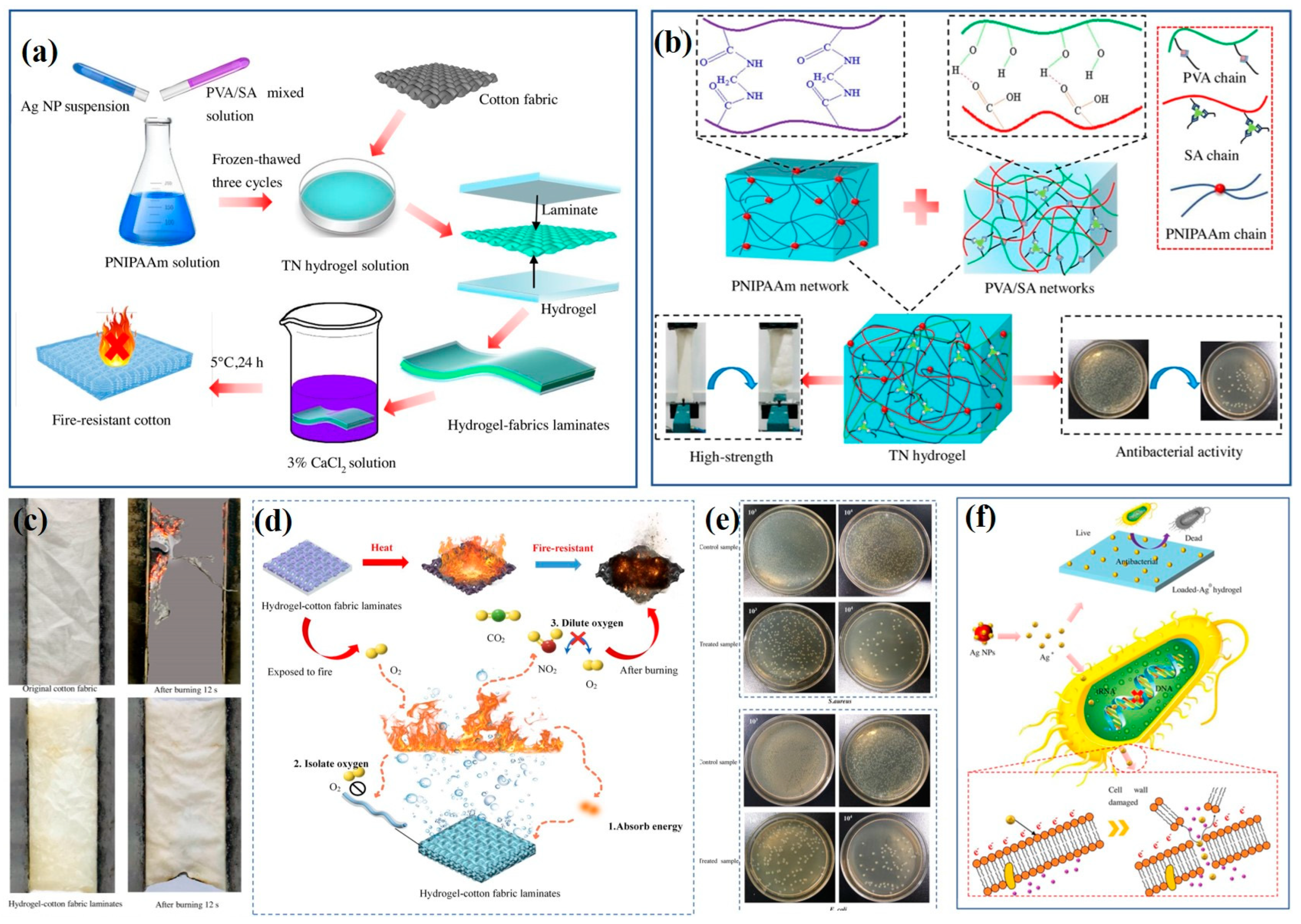
As we all know, water and fire are incompatible. However, it is difficult to directly apply water as a fire-resistant material due to its high mobility. Hydrogel polymers with water as the main component can be used as fire-retardant materials to form a fire-resistant layer and reduce water loss, thereby improving the flame-retardancy of the coated fabric. Therefore, in the work of Yu et al., a novel fire-preventing triple-network (TN) hydrogel composed of poly (N-isopropylacrylamide) (PNIPAAm)/SA/PVA was prepared and laminated on cotton fabric, which was then put through the ionic coordination crosslinking process in CaCl
2 solution to form a stable structure. During the process, Ag NPs were also embedded into the hydrogel. Compared to neat fabric, the hydrogel-fabric laminates were nearly undamaged after being exposed to fire for 12 s, which is attributed to energy absorption as the water in the hydrogel is heated and evaporates. At the same time, outstanding antibacterial functions (>96%) against
E. coli and
S. aureus were achieved. Moreover, the introduction of a hydrogel layer also improves the mechanical strength of fabrics. Thus, the results demonstrated that the TN hydrogel, as a fire-resistant polymer, has potential for life-saving (
Figure 4) [
68].
Metal phenolic networks (MPNs), which consist of a variety of phenolic compounds and metals, have been promising candidates for the surface functionalization of substrates. For decades, naturally occurring compounds and their derivatives as eco-friendly antibacterial and flame-retardant agents for fabrics have attracted extensive attention from scholars. Researchers [
69,
70] have applied MPNs on the surface of silk fabrics, wherein Zhang et al. combined tannic acid (TA) with ferrous ions to form FR and antibacterial materials and applied them to silk fabrics that possess durable increasing FR with a 27.5% LOI value and almost no decrease even after 20 washes. In the vertical burning test, the treated sample shows a damaged length of only 11.2 cm but 30.0 cm of the original. The antibacterial activity significantly increased from 22% to 95% and maintained over 90% of its properties even after 20 washes [
69]. In another work, Cheng et al. extracted polyphenols under alkaline conditions to develop flame-retardant macromolecular polyphenols through oxidative polymerization. The silk was dyed with the aforementioned extracted natural dyes and post-mordanted with metallic salts. The results not only showed improved flame-retardant and antibacterial properties but also antioxidant behaviors, washing fastness, perspiration, and wet rubbing fastness [
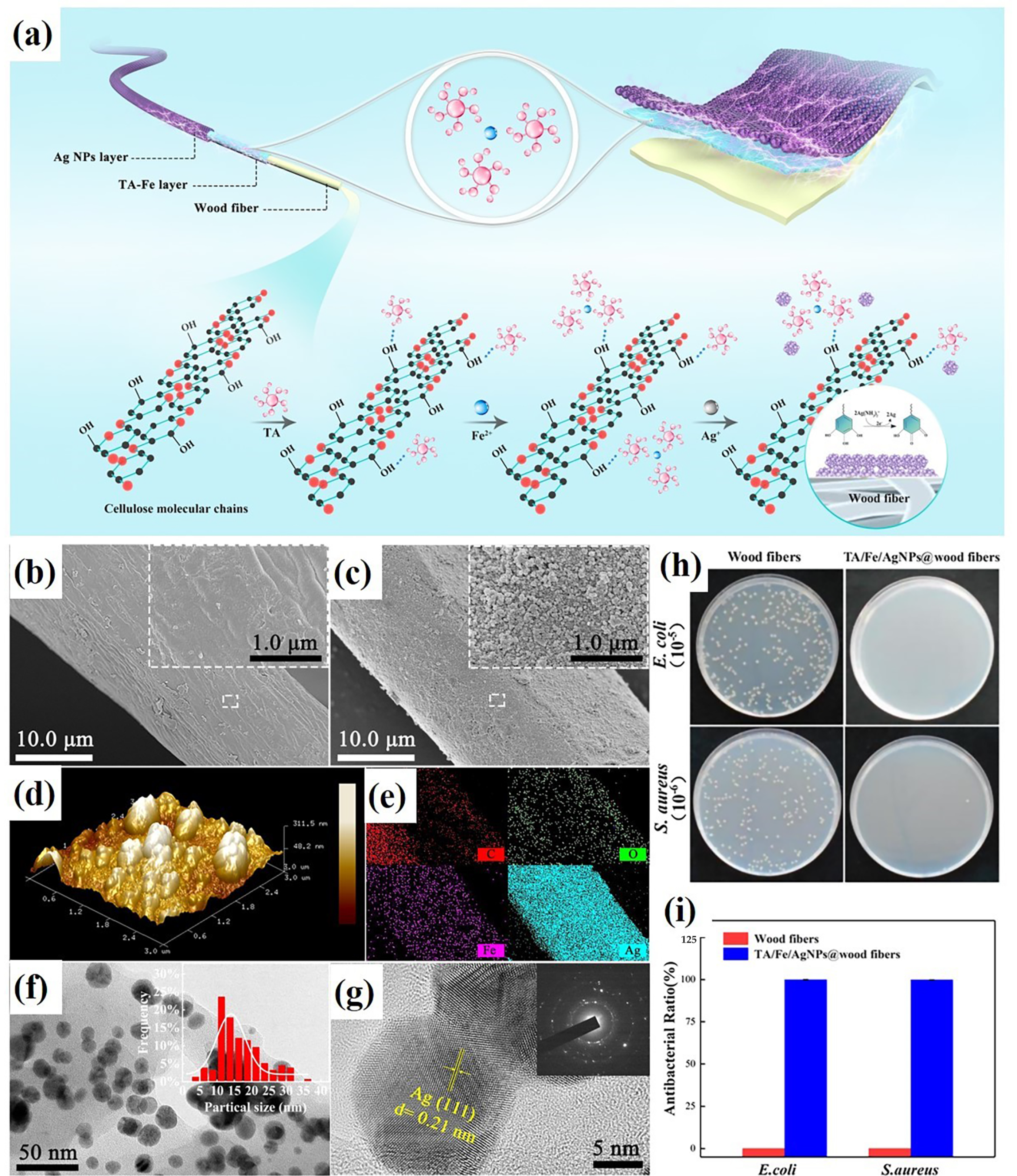
70]. The MPNs can be applied to wood fibers as well to solve the problem of limiting application caused by their poor flame retardancy and antibacterial behavior. In the study of Jiang et al., wood fibers were immersed in a single solution of TA and ferrous salt successively to form a TA-Fe-wood complex and then further modified with silver nanoparticles (Ag NPs) to structure an Ag NPs layer. The TA/Fe/Ag NPs@wood fibers were successfully prepared (as shown in
Figure 5). In the test of cone calorimetry, the TA/Fe/Ag NPs@wood fibers displayed enhanced flame retardancy, with the peak heat release rate and the peak smoke production rate reducing by 71.5% and 56.5%, respectively. Not only that, it also increases antibacterial activity for both
E. coli and
S. aureus. At the same time, the problem of the matrix being darkened by MPNs was solved [
71].
Apart from TA (
Figure 6a), some other flavonoids, including Catechin (
Figure 6b), Proanthocyandins (
Figure 6c), Rutin (
Figure 6d)), Quercetins (
Figure 6f), Baicalin (
Figure 6g), have attracted great attention in the fields of dyeing and functionalization of textiles simultaneously. Guo et al. applied grape seed proanthocyanidins (GSPs), which are a kind of recycled low-value byproduct rich in polyphenolic compounds, to the coloration of silk with a flame-retardant and antibacterial functionalized treatment. The dyed silk performs progressive flame retardancy due to the condensed phase flame retardancy mechanism of GSPs. In addition to enhancing the antibacterial properties effectively, washing, rubbing, perspiration, and light color fastness have also been improved to a certain extent [
72]. Three flavonoids (baicalin, quercetin, and rutin) have been utilized in silk fabrics under the action of two metal salts (ferrous sulfate and titanium sulfate) mordanting as well. The results of the vertical burning test indicate improved flame-retardancy (the detailed data are shown in
Table 1) and smoke suppression due to the good char formation ability of the silk fabrics in the process of combustion [
73].
3.5. Sol-Gel Method
The sol-gel technique involves hydrolysis and condensation reactions using siloxane or metal alkoxide as precursors. First, a sol system is formed through the hydrolysis process; subsequently, a micro-nanoscale organic or inorganic coating will be formed on the surface of the fabric through a condensation reaction [
74]. This method has the advantages of a simple process, mild reaction conditions, high efficiency, and good film-forming properties. It plays an important role in the functionalization of textiles, such as wrinkle resistance, dyeing, UV protection, antistatic, antibacterial, flame retardant, and hydrophobic properties. The durable antibacterial and flame-retardant cotton fabrics were developed via simultaneous hydrolytic condensation of N
3P
3[NH(CH
2)
3Si(OC
2H
5)
3]
6 and polymerization of dopamine (PDA) on cotton fabric. Ag NPs were then introduced onto fabrics via in situ reactions with PDA. Considerable flame retardancy can be observed for treated cotton fabric even at a low loading (7.2%) of the hybrid coating. In addition, the antibacterial activity of the treated fabric reached 99.99% for both
S. aureus and
E. coli. The modification showed excellent durability and nearly intact antimicrobial properties, and flame retardancy was maintained after 30 washing cycles [
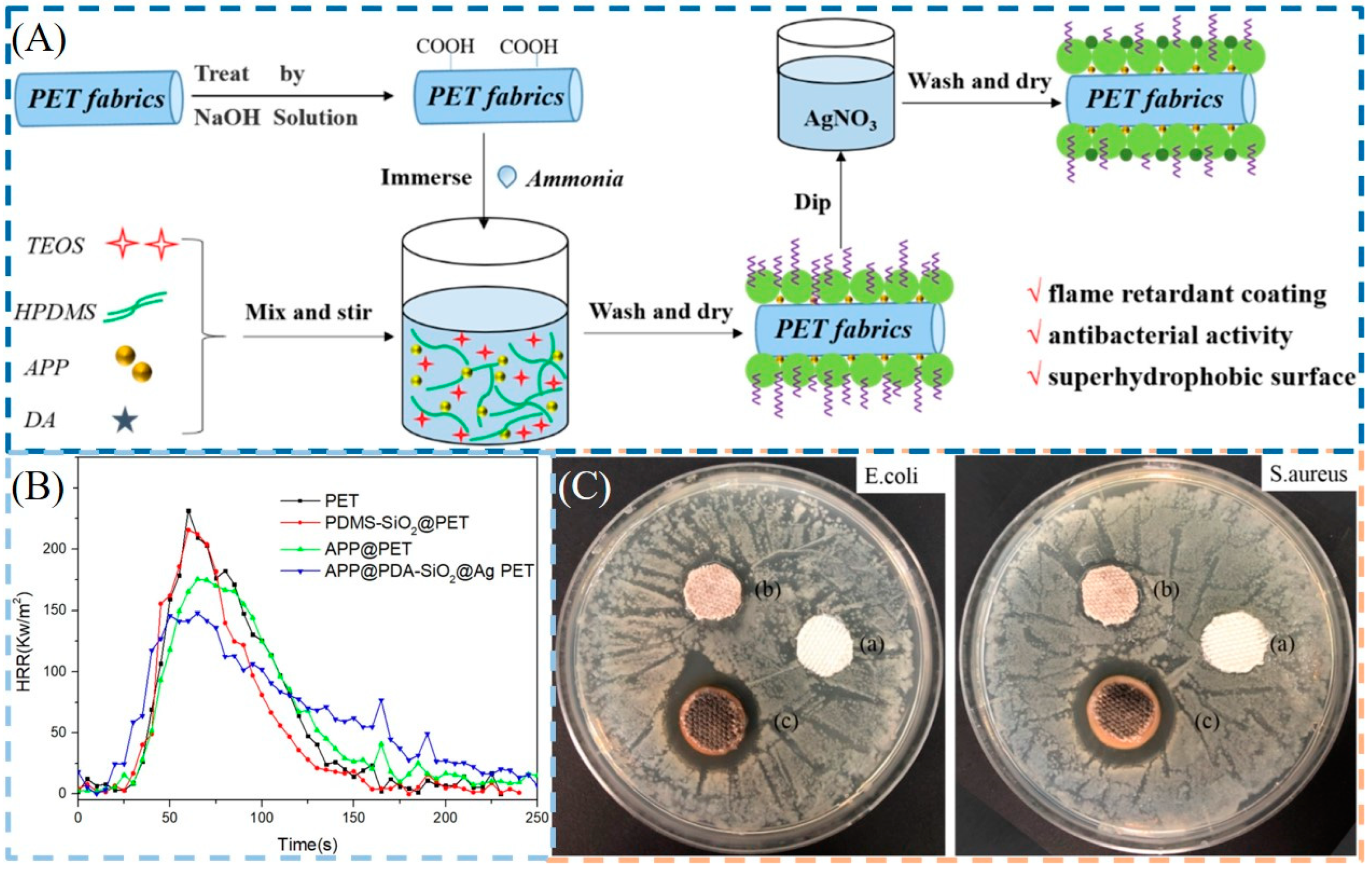
75]. In another work, a multifunctional composite coating (APP @ SiO
2-PDA @ Ag) composed of APP, PDA, PDMS-silica (PDMS-SiO
2), and Ag NPs was constructed on the surface of PET fabrics. The APP @ SiO
2-PDA @ Ag PET fabric showed an LOI of 29.0% and could self-extinguish in the VFT, and its PHRR and THR were 34% and 26% lower than those of the pure PET fabric. Notably, the multifunctional PET fabrics also exhibited excellent antibacterial activity against
E. coli and
S. aureus and superhydrophobicity (>150°). More importantly, the APP@SiO
2-PDA@Ag-coated PET fabrics still maintained good flame retardant and antibacterial performances after multiple washing cycles [
76] (
Figure 7).
The introduction of functionalized trialkoxysilane as the sol-gel agent has made significant progress in the chemical modification process of textiles, which is beneficial for producing unique surface properties. A three-component equimolar sol mixture of SiF, 3-(trimethoxysilyl)-propyldimethyloctadecyl ammonium chloride (SiQ), P, and SiP was constructed on cotton fabric by the sol-gel method. The treated fabrics simultaneously achieved flame-retardant, antibacterial (bacterial reduction of 100%), and water-repellent properties due to the thermal stability of SiP, the antibacterial properties of SiQ, and the hydrophobicity of SiF [
77]. Following this work, the same group further optimized the structure of the multifunctional coating to increase the washing speed of treated cotton fabrics. They applied the prepared Sto¨ber silica particles onto cotton fibers to form a particle-containing polysiloxane layer, which is based on tetraethyl orthosilicate, in the preparation work before the process of sol-gel. Eventually, the results showed enhanced washing fastness under the influence of the deposition of the silica particles. At the same time, it still exhibits excellent antibacterial activity, with R values of 81.6 and 100% for
E. coli and
S. aureus [
14].
4. Conclusions and Perspectives
This review summarizes the different treatments and adaptable finishing agents to obtain flame-retardant, antibacterial, multi-functional fabrics. Through the latest decade of research, it was found that the treatment methods not only affect processing efficiency but also have strong ties with the final functional properties and wearability of fabric. At the same time, the relevant functional materials were further introduced here, including their source, characteristics, and mechanism. Moreover, the advantages and disadvantages of these treatment methods and finishing agents were briefly introduced, which can provide a basic reference for relevant research in this field.
Although finishing methods and agents have been developing rapidly, many shortcomings remain and need to be solved. (1) Improving durability. The functional-coating textile products inescapably undergo friction and water-washing during daily applications. It will lead to bad results from a damaged coating and decrease or even eliminate the effect of the functional fabrics. Therefore, it is proposed that more research focus be laid on the durability of the coating. (2) Developing environmentally friendly but high-performance finishing agents. It is an eternal task for the whole of humanity to promote sustainable development. However, the effect of most green functional treatments is greatly limited. Therefore, it is integral to spend effort researching innocuous technology and chemicals while developing high-performance fabrics. (3) Increasing yields and realizing industrialization earlier. Plenty of research just stays in the laboratory stage, and it can hardly be applied in practice. Increasing production could promote the industrialization process, and realizing industrialization earlier will improve the quality of life and accelerate social progress. (4) It is well known that dual-functional fabrics can be realized by a two-step or one-step method. The two-step method may suffer from functional antagonism when flame retardants and antimicrobial agents are utilized simultaneously, which is detrimental to the construction of bifunctional coatings. In addition, the one-step method is important in the design and synthesis of multifunctional compounds. Therefore, to achieve the multi-functionalization of textiles, the best efforts are needed to find the optimal process and functional materials.
The above challenges will be gradually overcome with the continuous development of science and technology as well as the appearance of innovative technologies and materials. Owing to their powerful functionality and portability, multifunctional fabrics have been increasing in popularity in markets and will have good development prospects for a long time in the future.