Enhancing Flame Retardancy and Smoke Suppression in Epoxy Resin Composites with Sulfur–Phosphorous Reactive Flame Retardant
Abstract
:1. Introduction
2. Results and Discussion
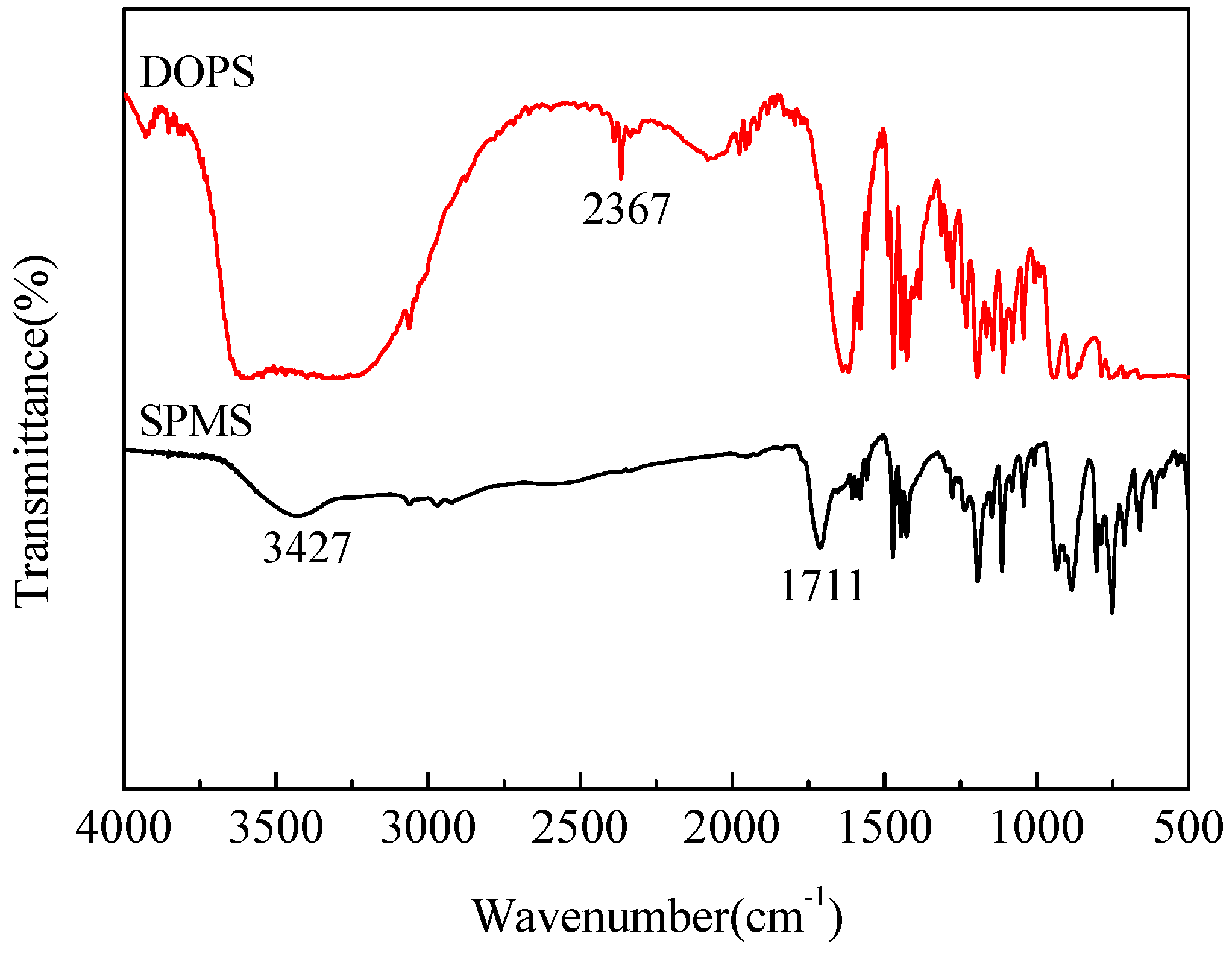
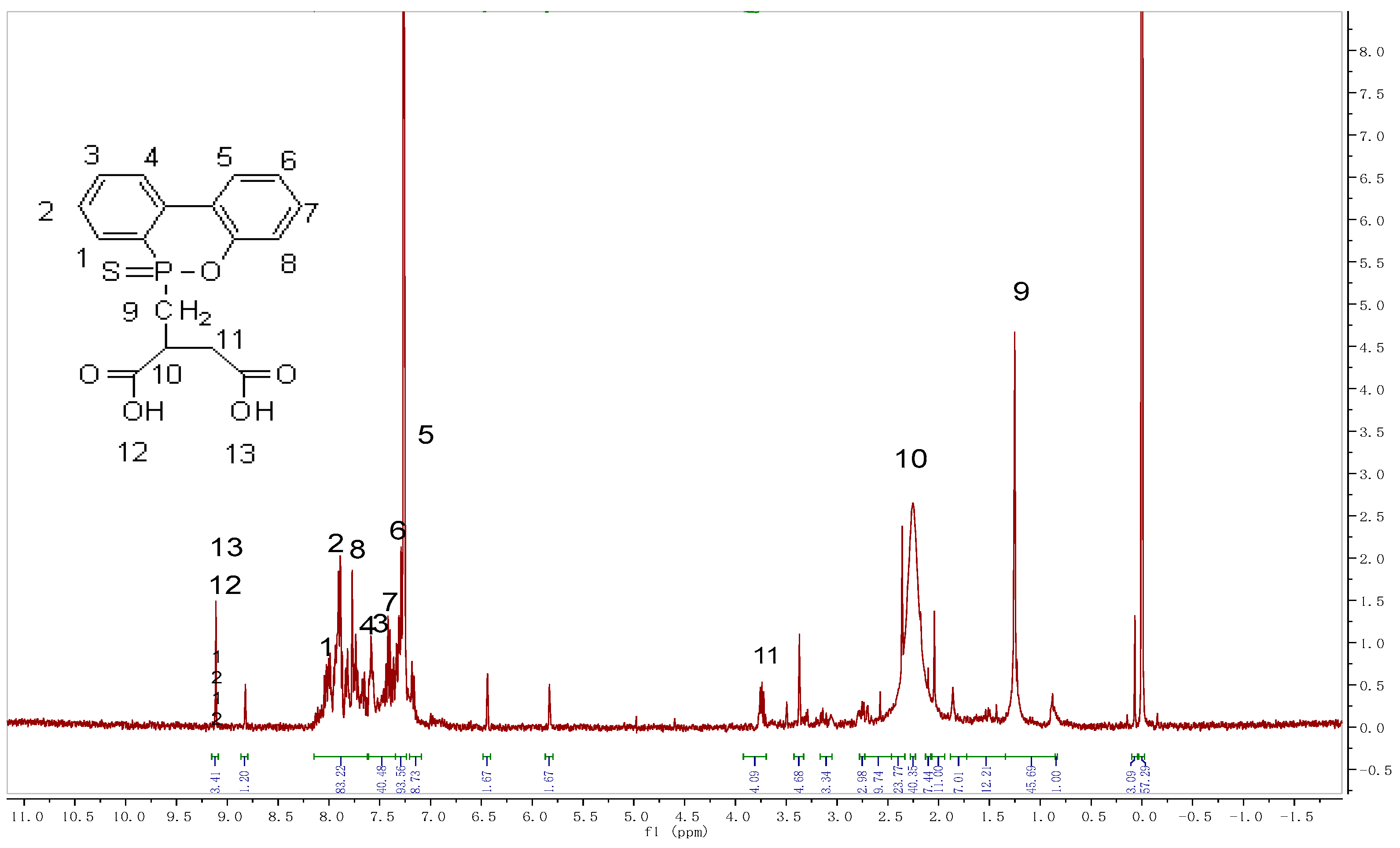
2.1. Characterization of the SPMS
2.2. Combustion Properties of the Cured Epoxy Resins
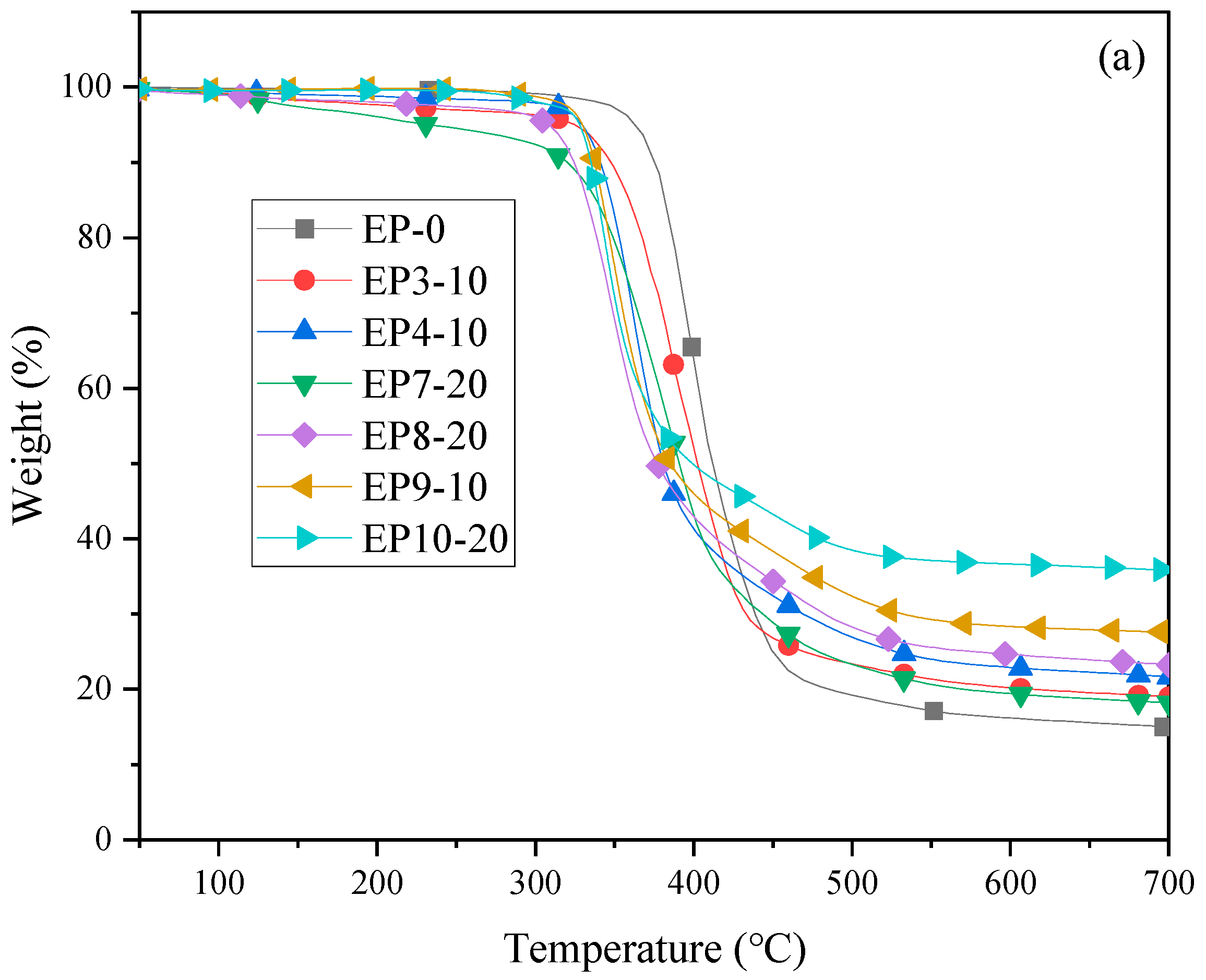
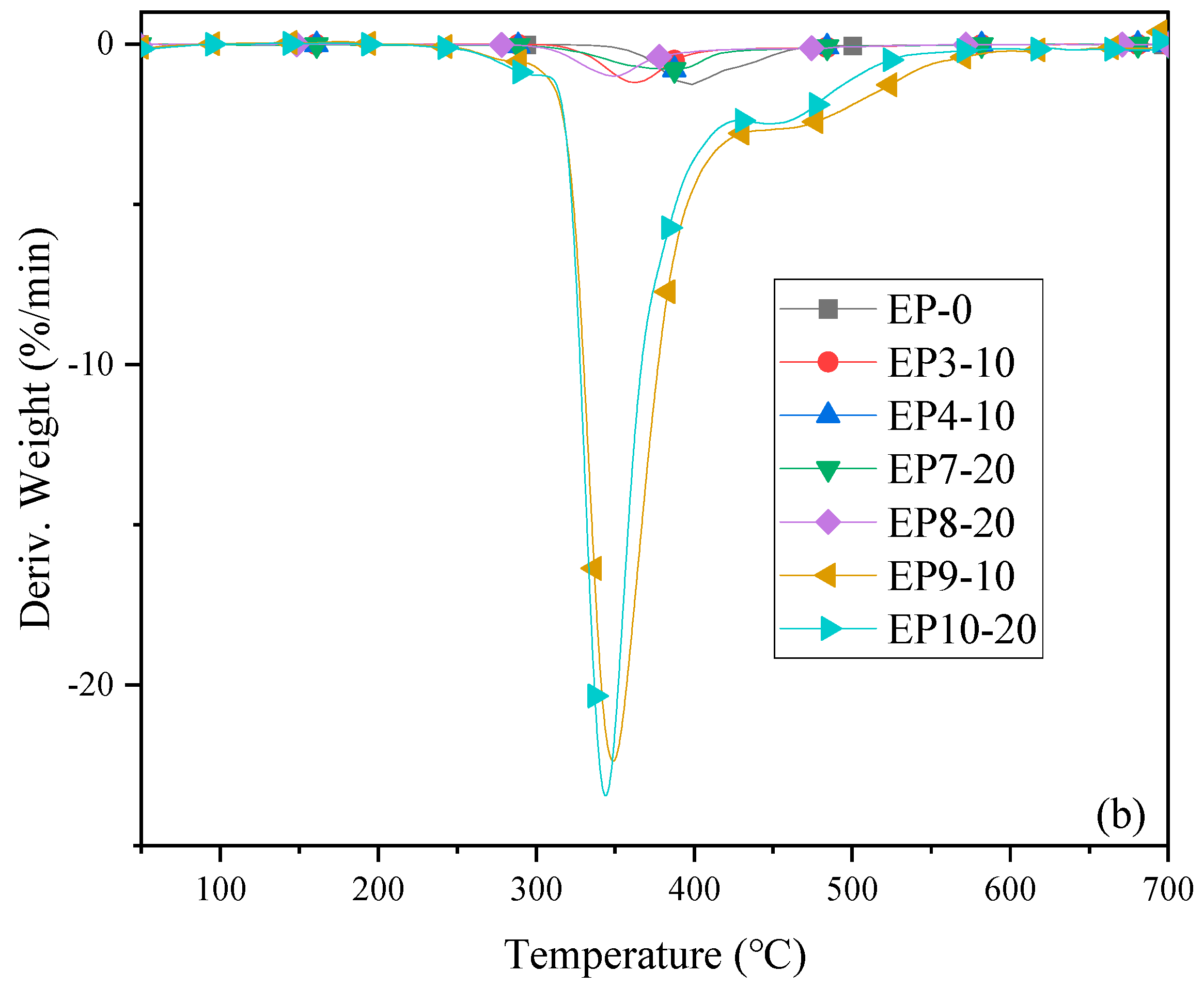
2.3. Thermal Properties of Cured Epoxy Resins
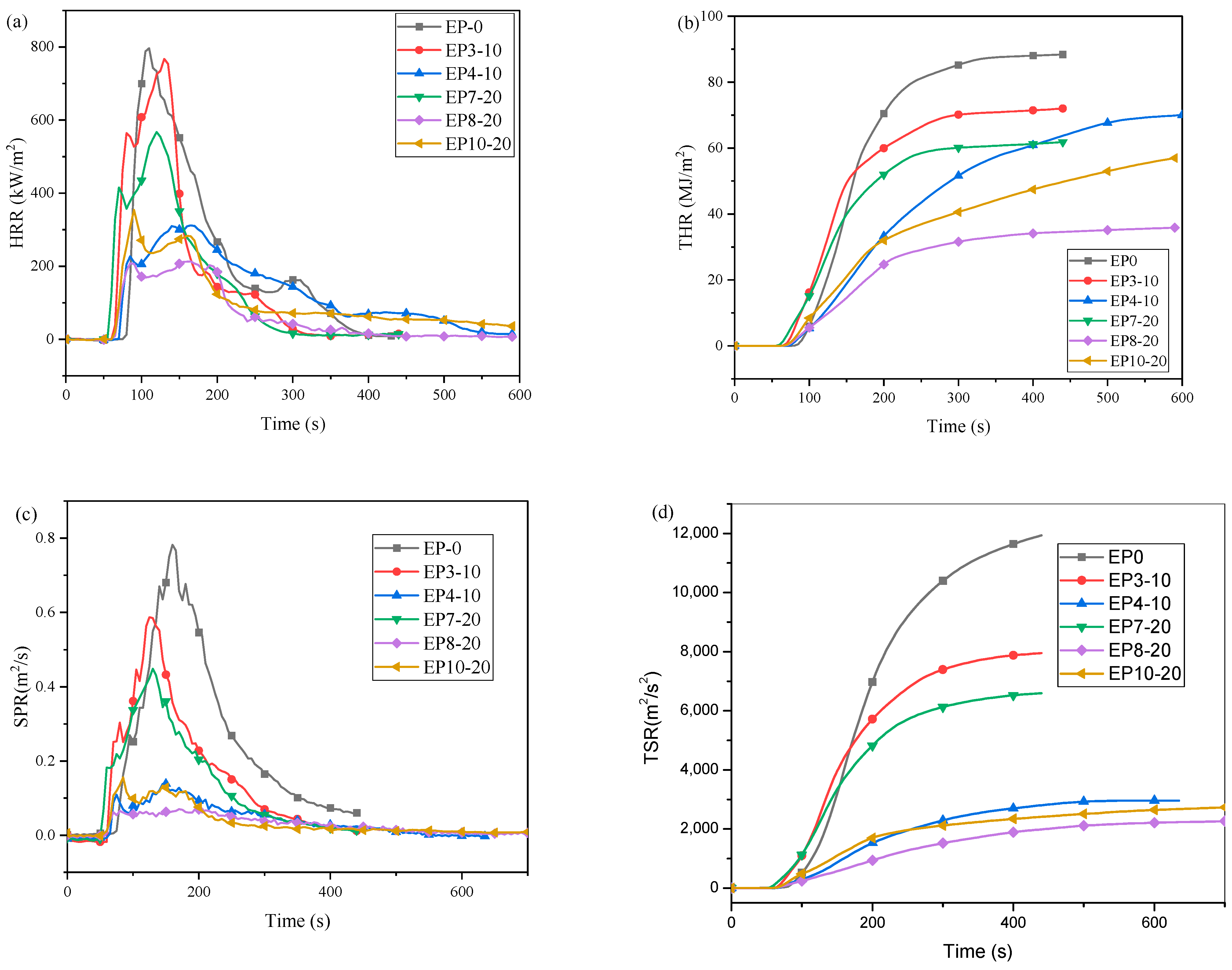
2.4. Fire Hazard Analysis
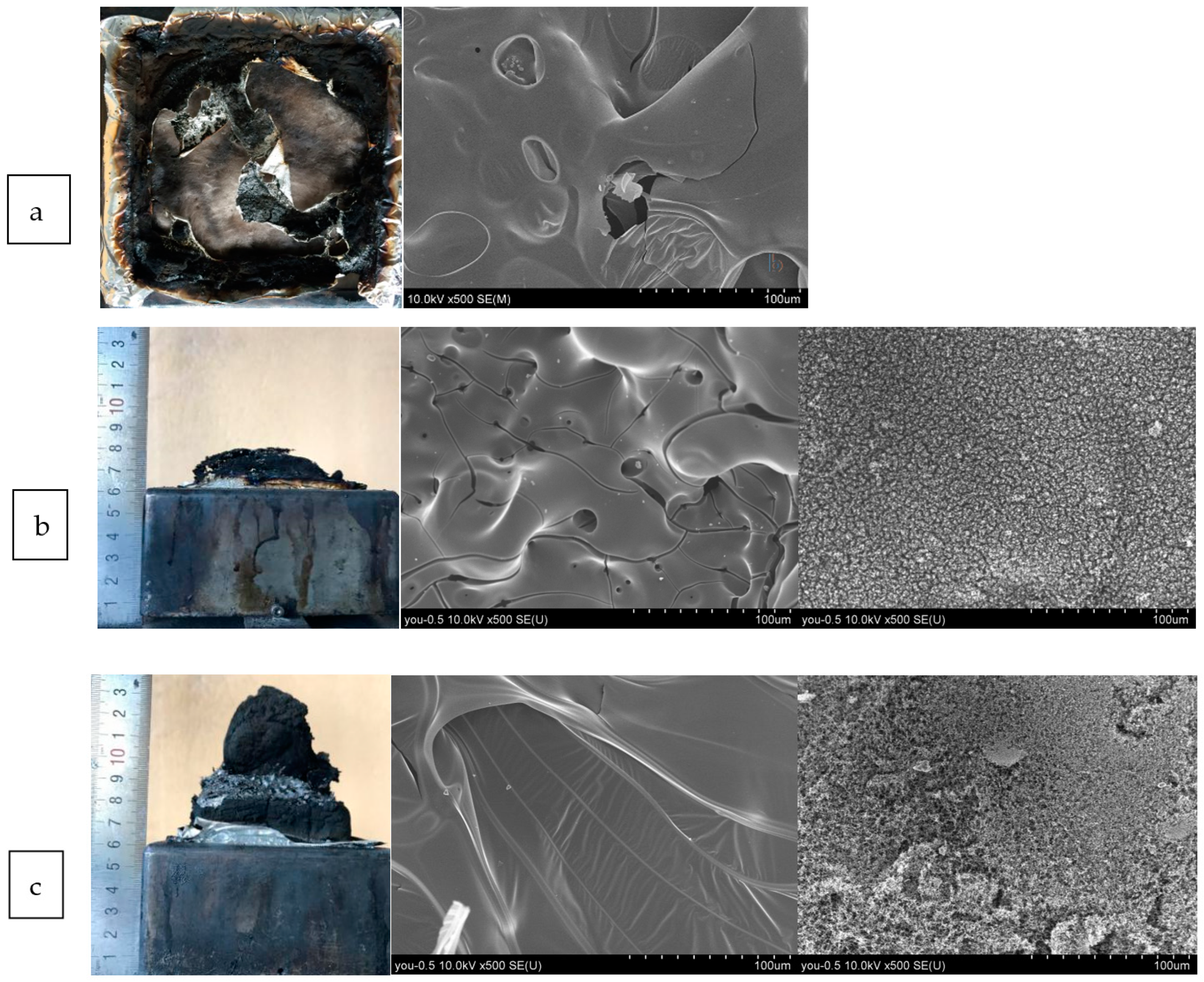
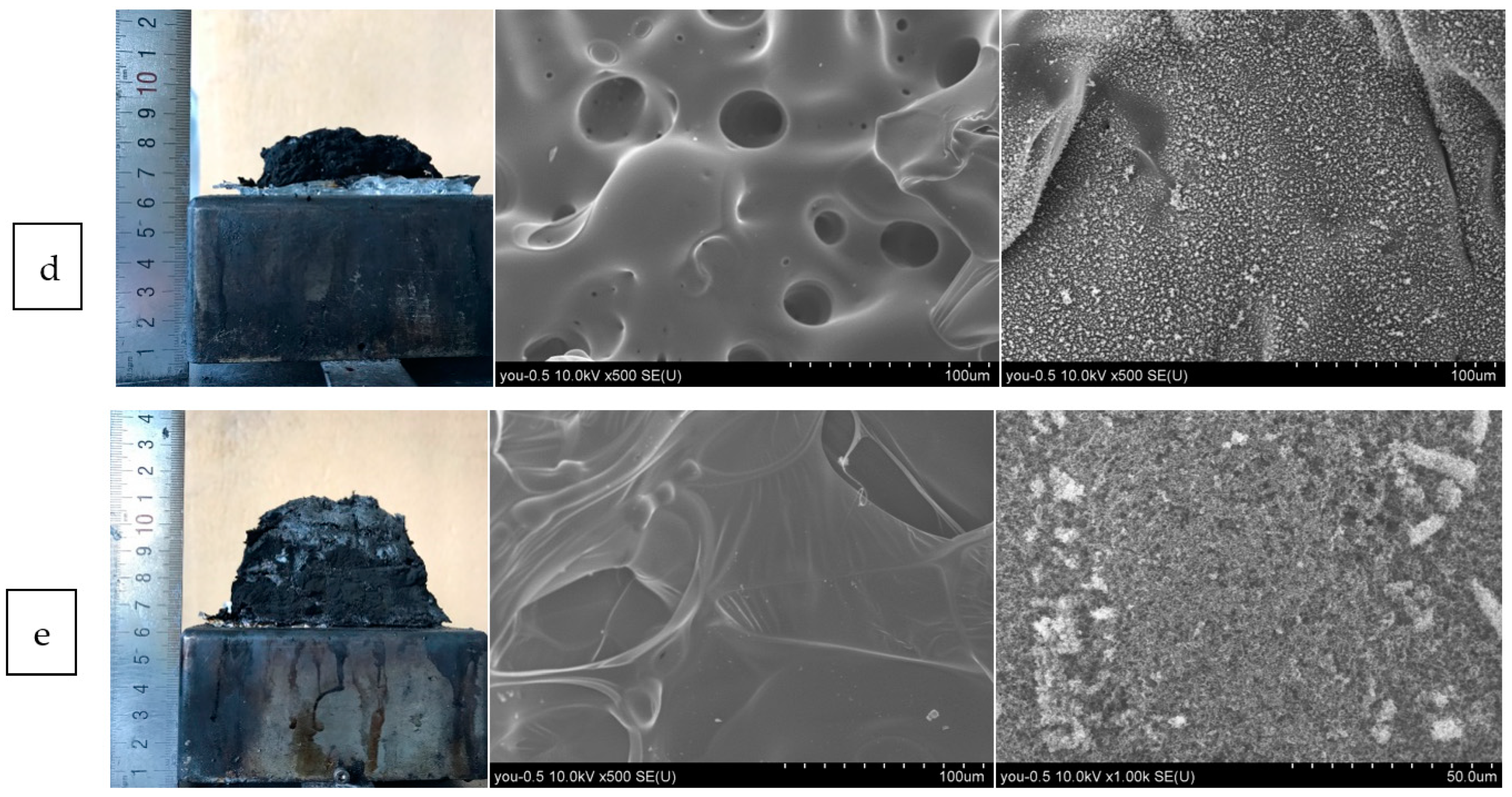
2.5. Residual Char Analysis
2.6. Mechanical Properties
3. Materials and Methods
3.1. Materials
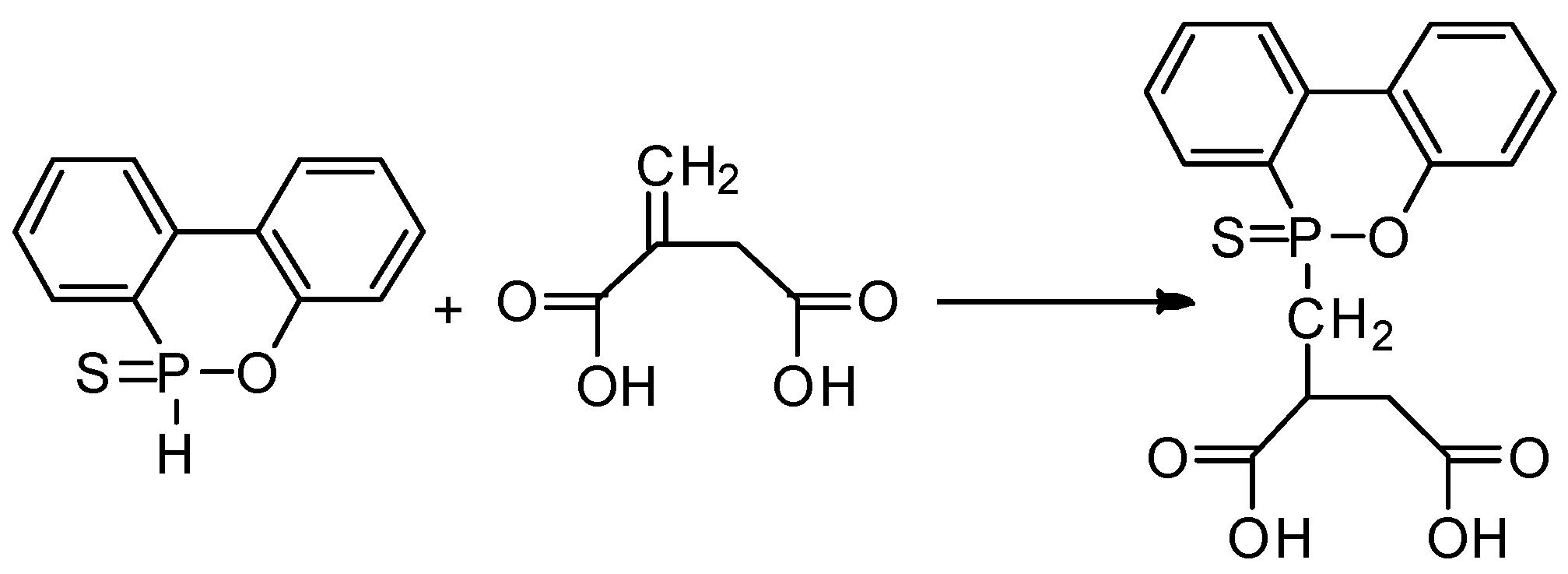
3.2. Synthesis of SPMS
3.3. Preparation of the EP/SPMS-APP Hybrid
3.4. Characterization
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Huo, S.Q.; Song, P.A.; Yu, B.; Ran, S.Y.; Chevali, V.S.; Liu, L.; Fang, Z.P.; Wang, H. Phosphorus-containing flame retardant epoxy thermosets: Recent advances and future perspectives. Prog. Polym. Sci. 2021, 114, 101366. [Google Scholar] [CrossRef]
- Jamsaz, A.; Goharshadi, E.K. Graphene-based flame-retardant polyurethane: A critical review. Polym. Bull. 2023, 80, 11633–11669. [Google Scholar] [CrossRef]
- Gao, T.Y.; Wang, F.D.; Xu, Y.; Wei, C.X.; Zhu, S.E.; Yang, W.; Lu, H.D. Luteolin-based epoxy resin with exceptional heat resistance, mechanical and flame retardant properties. Chem. Eng. J. 2022, 428, 131173. [Google Scholar] [CrossRef]
- Liu, B.-W.; Zhao, H.-B.; Wang, Y.-Z. Advanced Flame-Retardant Methods for Polymeric Materials. Adv. Mater. 2022, 34, 2107905. [Google Scholar] [CrossRef]
- Zhang, J.H.; Mi, X.Q.; Chen, S.Y.; Xu, Z.J.; Zhang, D.H.; Miao, M.H.; Wang, J.S. A bio-based hyperbranched flame retardant for epoxy resins. Chem. Eng. J. 2020, 381, 122719. [Google Scholar] [CrossRef]
- Liu, X.D.; Zheng, X.T.; Dong, Y.Q.; He, L.X.; Chen, F.; Bai, W.B.; Lin, Y.C.; Jian, R.K. A novel nitrogen-rich phosphinic amide towards flame-retardant, smoke suppression and mechanically strengthened epoxy resins. Polym. Degrad. Stab. 2022, 196, 109840. [Google Scholar] [CrossRef]
- Wen, Y.; Cheng, Z.; Li, W.; Li, Z.; Liao, D.; Hu, X.; Pan, N.; Wang, D.; Hull, T.R. A novel oligomer containing DOPO and ferrocene groups: Synthesis, characterization, and its application in fire retardant epoxy resin. Polym. Degrad. Stab. 2018, 156, 111–124. [Google Scholar] [CrossRef]
- Deng, P.; Liu, Y.; Liu, Y.; Xu, C.; Wang, Q. Preparation of phosphorus-containing phenolic resin and its application in epoxy resin as a curing agent and flame retardant. Polym. Adv. Technol. 2018, 29, 1294–1302. [Google Scholar] [CrossRef]
- Luo, Y.; Xie, Y.H.; Jiang, H.; Chen, Y.; Zhang, L.; Sheng, X.X.; Xie, D.L.; Wu, H.; Mei, Y. Flame-retardant and form-stable phase change composites based on MXene with high thermostability and thermal conductivity for thermal energy storage. Chem. Eng. J. 2021, 420, 130466. [Google Scholar] [CrossRef]
- Vlad-Bubulac, T.; Hamciuc, C.; Serbezeanu, D.; Macsim, A.-M.; Lisa, G.; Anghel, I.; Preda, D.-M.; Kalvachev, Y.; Rîmbu, C.M. Simultaneous Enhancement of Flame Resistance and Antimicrobial Activity in Epoxy Nanocomposites Containing Phosphorus and Silver-Based Additives. Molecules 2023, 28, 5650. [Google Scholar] [CrossRef]
- Xia, W.J.; Wang, S.W.; Xu, T.; Jin, G.L. Flame retarding and smoke suppressing mechanisms of nano composite flame retardants on bitumen and bituminous mixture. Constr. Build. Mater. 2021, 266, 121203. [Google Scholar] [CrossRef]
- Teng, F.; Li, W.X.; Zhong, W.Y. Flame-responsive aryl ether nitrile structure towards multiple fire hazards suppression of thermoplastic polyester. J. Hazard. Mater. 2021, 403, 123714. [Google Scholar]
- Li, L.; Li, S.; Wang, H.; Zhu, Z.; Yin, X.; Mao, J. Low flammability and smoke epoxy resins with a novel DOPO based imidazolone derivative. Polym. Adv. Technol. 2021, 32, 294–303. [Google Scholar] [CrossRef]
- Wang, Z.Z.; Gao, X.; Li, W.F. Epoxy resin/cyanate ester composites containing DOPO and wollastonite with simultaneously improved flame retardancy and thermal resistance. High. Perform. Polym. 2020, 32, 710–718. [Google Scholar] [CrossRef]
- Jian, R.K.; Ai, Y.F.; Xia, L.; Zhao, L.J.; Zhao, H.B. Single component phosphamide-based intumescent flame retardant with potential reactivity towards low flammability and smoke epoxy resins. J. Hazard. Mater. 2019, 371, 529–539. [Google Scholar] [CrossRef]
- Qi, Z.; Zhang, W.C.; He, X.D.; Yang, R.J. High-efficiency flame retardency of epoxy resin composites with perfect T-8 caged phosphorus containing polyhedral oligomeric silsesquioxanes (P-POSSs). Compos. Sci. Technol. 2016, 127, 8–19. [Google Scholar] [CrossRef]
- Wang, P.; Yang, F.; Li, L.; Cai, Z. Flame retardancy and mechanical properties of epoxy thermosets modified with a novel DOPO-based oligomer. Polym. Degrad. Stab. 2016, 129, 156–167. [Google Scholar] [CrossRef]
- Liu, C.; Yang, D.; Sun, M.N.; Deng, G.J.; Jing, B.H.; Wang, K.; Shi, Y.Q.; Fu, L.B.; Feng, Y.Z.; Lv, Y.C.; et al. Phosphorous-Nitrogen flame retardants engineering MXene towards highly fire safe thermoplastic polyurethane. Compos. Commun. 2022, 29, 101055. [Google Scholar] [CrossRef]
- Xu, M.J.; Xu, G.R.; Leng, Y.; Li, B. Synthesis of a novel flame retardant based on cyclotriphosphazene and DOPO groups and its application in epoxy resins. Polym. Degrad. Stab. 2016, 123, 105–114. [Google Scholar] [CrossRef]
- Wang, H.; Li, S.; Zhu, Z.M.; Yin, X.Z.; Wang, L.X.; Weng, Y.X.; Wang, X.Y. A novel DOPO-based flame retardant containing benzimidazolone structure with high charring ability towards low flammability and smoke epoxy resins. Polym. Degrad. Stab. 2021, 183, 109426. [Google Scholar] [CrossRef]
- Chi, Z.Y.; Guo, Z.W.; Xu, Z.C.; Zhang, M.J.; Li, M.; Shang, L.; Ao, Y.H. A DOPO-based phosphorus-nitrogen flame retardant bio-based epoxy resin from diphenolic acid: Synthesis, flame-retardant behavior and mechanism. Polym. Degrad. Stab. 2020, 176, 109151. [Google Scholar] [CrossRef]
- Xiao, L.; Sun, D.; Niu, T.; Yao, Y. Syntheses and characterization of two novel 9,10-dihydro-9-oxa-10-phosphaphenanthrene-10-oxide-based flame retardants for epoxy resin. High. Perform. Polym. 2014, 26, 52–59. [Google Scholar] [CrossRef]
- Nabipour, H.; Wang, X.; Song, L.; Hu, Y. Intrinsically Anti-Flammable Apigenin-derived Epoxy Thermosets with High Glass Transition Temperature and Mechanical Strength. Chin. J. Polym. Sci. 2022, 40, 1259–1268. [Google Scholar] [CrossRef]
- Chen, S.M.; Lai, F.; Pei, L.; Wei, G.; Hai, F.; Yin, X.G.; Ban, D.M. Synthesis of Flame Retardant Based on Phosphaphenanthrene and Flame Retardancy Study of Epoxy Resin Modified by Intumescent Flame Retardant System Composed of Ammonium Polyphosphate. Acta Polym. Sin. 2017, 8, 1358–1365. [Google Scholar]
| Sample | Mass Fraction (%) | LOI (%) | UL-94 | ||
|---|---|---|---|---|---|
| EP + PDA | SPMS | APP | |||
| EP0 | 100 | 0.00 | 0.00 | 19.8 | N.R. |
| EP-5 | 95 | 0.00 | 5.00 | 21.4 | N.R. |
| EP1-5 | 95 | 5.00 | 0.00 | 21.1 | V-1 |
| EP2-5 | 95 | 1.67 | 3.33 | 20.8 | V-0 |
| EP3-10 | 90 | 10.0 | 0.00 | 21.5 | V-0 |
| EP4-10 | 90 | 3.33 | 6.67 | 21.7 | V-0 |
| EP5-15 | 85 | 15.0 | 0.00 | 24.7 | V-0 |
| EP6-15 | 85 | 5.00 | 10.0 | 26.4 | V-0 |
| EP7-20 | 80 | 20.0 | 0.00 | 25.0 | V-0 |
| EP8-20 | 80 | 6.67 | 13.33 | 27.3 | V-0 |
| EP9-10 | 90 | 0.00 | 10.0 | 26.8 | V-0 |
| EP10-20 | 80 | 0.00 | 20.0 | 34.5 | V-0 |
| Sample | Td (°C) | T50% (°C) | Tmax (°C) | Rmax (%/min) | R700 (%) |
|---|---|---|---|---|---|
| EP0 | 357 | 408 | 398 | 1.26 | 15 |
| EP3-10 | 324 | 401 | 382 | 0.97 | 19 |
| EP4-10 | 329 | 379 | 363 | 1.20 | 21 |
| EP7-20 | 233 | 389 | 387 | 0.80 | 18 |
| EP8-20 | 306 | 375 | 348 | 1.00 | 23 |
| EP9-10 | 330 | 385 | 349 | 22.37 | 27 |
| EP10-20 | 328 | 399 | 344 | 23.44 | 36 |
| Sample | TTI (s) | pHRR (kW·m−2) | pSPR (m2·s−1) | THR at 400 s (MJ·m−2) | TSR at 400 s (m2·m−2) | FPI (s·m2·kW−1) |
|---|---|---|---|---|---|---|
| EP0 | 70 | 933 | 0.77 | 88 | 11,640 | 0.075 |
| EP3-10 | 59 | 767 | 0.58 | 71 | 7880 | 0.076 |
| EP4-10 | 65 | 311 | 0.14 | 70 | 2695 | 0.20 |
| EP7-20 | 48 | 567 | 0.44 | 61 | 6529 | 0.084 |
| EP8-20 | 60 | 164 | 0.05 | 34 | 1891 | 0.36 |
| EP9-20 | 59 | 354 | 0.16 | 47 | 2341 | 0.167 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ma, X.; Kang, N.; Zhang, Y.; Min, Y.; Yang, J.; Ban, D.; Zhao, W. Enhancing Flame Retardancy and Smoke Suppression in Epoxy Resin Composites with Sulfur–Phosphorous Reactive Flame Retardant. Molecules 2024, 29, 227. https://doi.org/10.3390/molecules29010227
Ma X, Kang N, Zhang Y, Min Y, Yang J, Ban D, Zhao W. Enhancing Flame Retardancy and Smoke Suppression in Epoxy Resin Composites with Sulfur–Phosphorous Reactive Flame Retardant. Molecules. 2024; 29(1):227. https://doi.org/10.3390/molecules29010227
Chicago/Turabian StyleMa, Xulong, Ni Kang, Yonghang Zhang, Yang Min, Jianhua Yang, Daming Ban, and Wei Zhao. 2024. "Enhancing Flame Retardancy and Smoke Suppression in Epoxy Resin Composites with Sulfur–Phosphorous Reactive Flame Retardant" Molecules 29, no. 1: 227. https://doi.org/10.3390/molecules29010227
APA StyleMa, X., Kang, N., Zhang, Y., Min, Y., Yang, J., Ban, D., & Zhao, W. (2024). Enhancing Flame Retardancy and Smoke Suppression in Epoxy Resin Composites with Sulfur–Phosphorous Reactive Flame Retardant. Molecules, 29(1), 227. https://doi.org/10.3390/molecules29010227








