Abstract
C. berlandieri ssp. berlandieri (C. berlandieri) is one of the most common members of the group of plants known as quelites, which are dark leafy greens widely consumed in Mexico. This study aimed to evaluate the impact of two drying procedures (oven drying and freeze-drying/lyophilization) on the polyphenolic composition, antioxidant capacity, and proximal chemical analysis of C. berlandieri leaves and inflorescences (raw or boiled). The results indicated that the raw freeze-dried samples had higher amounts (p < 0.05) of total phenolic compounds, total flavonoids, and antioxidant capacity, mainly in the inflorescence. The oven-dried samples showed an increased concentration of polyphenols after boiling, while the lyophilized samples showed a slightly decreased concentration. The drying process was observed to have little impact on the proximal chemical composition. Quantification by UPLC-DAD-ESI-QToF/MS identified up to 23 individual phenolic compounds, with freeze-dried samples showing higher amounts of individual compounds compared with oven-dried. Procyanidin B2 was found exclusively in the inflorescences. The inflorescences have a higher content of phenolic compounds and greater antioxidant capacity than the leaves. Regardless of the drying process, the leaves and inflorescences of C. berlandieri contain an interesting variety of phenolic compounds that may have beneficial effects on health.
1. Introduction
Humankind has relied on plants as a food source since its earliest origins. Modern science has set itself the task of analyzing plants’ nutritional composition and identifying their bioactive compounds. Compounds of interest are phenolic compounds, considered natural antioxidants with the potential to prevent the risk of non-communicable diseases such as cancer, type 2 diabetes, and cardiovascular diseases, among other conditions [1]. By identifying the bioactive compounds of edible plants, scientists can improve dietary recommendations and identify the plants that are the best sources of these components. One such plant is the quelites group, comprising more than 500 native Mexican plants whose leaves, tender stalks, and inflorescences have been used as a food source in Mesoamerica since pre-Hispanic times. These include Amaranthus spp., (amaranth), Portulaca oleracea (purslane, known in Mexico as verdolaga), Cnidoscolus aconitifolius (tree spinach, or chaya), Chenopodium berlandieri ssp. nuttaliiae (huauzontle), and Chenopodium berlandieri ssp. Berlandieri (quelite cenizo) [2,3].
The last of these, quelite cenizo, is one of the most widely consumed plants in Mexico, and it is used extensively in several typical dishes and preparations [4]. Moreover, its leaves and inflorescences presumably exhibit antibacterial, chemopreventive, hepatoprotective, anti-inflammatory, and antioxidant effects, based on analyses of other Chenopodium species [2]. The leaves and inflorescences can be consumed raw, as decoctions (boiling process), fried or dried, or as spices to improve the flavor, color, or texture characteristics of the food preparations in which they are used [5].
It is well known that the biological effects of phenolic compounds depend largely on their chemical stability and the manner in which the plant that contains them is processed. Environmental effects, such as ultraviolet radiation, temperature, and oxygen, must be considered, as well as the intrinsic characteristics of the product, including water activity or processing conditions, such as the type of drying and heat treatments used in culinary or industrial processes [6]. Drying a plant sample lengthens its shelf life and can preserve the quality and quantity of the components of the sample, and in the food industry, drying is an essential method for inhibiting microbial growth [7]. However, some types of drying can affect nutritional compounds due to the enzymatic and non-enzymatic effects that occur during the drying process [8]. Prevalent drying methods include convection drying with hot air and freeze-drying [7]. Various studies have shown that hot-air drying has a negative effect on nutritional components because it degrades the proteins found in plant samples. Freeze-drying, in contrast, removes moisture from samples through sublimation, but it is an expensive process due to its high energy consumption and in some samples it has been observed that the freezing process can modify the properties of fiber in foods [9]. Most quelites can be dried to extend their shelf life by reducing enzymatic and non-enzymatic processes. Different drying methods may be used: sun drying, oven drying, and lyophilization. As it is known that ultraviolet radiation can degrade phenolic compounds and other components with antioxidant capacity, this method is not currently recommended, but it is useful to compare how the content of phenolic compounds is affected by two other methods: oven drying and freeze drying. Likewise, it is important to document how the culinary heat treatment used in the preparation of the plant influences the content of phenolic compounds in edible botanical structures, such as leaves and inflorescence [8]. Finally, in C. berlandieri, the leaves are consumed more often than the inflorescences, so it would be useful to determine which botanical structure holds the greater quantity of phenolic compounds. This research aimed to assess the impact of two drying methods (oven drying and lyophilization) on the proximal chemical analysis, phenolic profile, and antioxidant capacity of raw and boiled leaves and inflorescences of C. berlandieri.
2. Results and Discussion
2.1. Impact of Drying Method on the Proximal Composition of C. berlandieri Leaves and Inflorescences
Table 1 shows the proximal chemical composition of the leaves and inflorescences of C. berlandieri processed by oven drying and lyophilization. No significant differences (p > 0.05) were found in the moisture or protein content of the samples analyzed. The leaves had higher ash content (p < 0.05) than the inflorescences, but inflorescences contained a higher amount of total carbohydrates (p < 0.05).

Table 1.
Proximate chemical analysis of raw leaves and inflorescences of C. berlandieri by dry matter weight.
There was no clear trend in the impact of the drying process on chemical composition, but both types of lyophilized inflorescences (BLI and RLI) displayed higher amounts of lipids and dietary fiber than the other groups. The protein values obtained are higher than those indicated by Santiago-Saenz et al. [10] in samples of the same species (3.45%), and even in other species such as Amaranthus hybridus (1.81%) and Portulaca oleracea (3.65%), but it should be noted that the values of that study are reported for fresh matter. Considering the protein values reported for the leaves of the quelite cenizo (26.2%) [11], protein loss could be attributed to the drying process [12]. Drying method can positively or negatively affect the nutritional value of foods; in most cases, freeze- drying is better than oven drying for ensuring that plant samples retain their content of nutrients and bioactive compounds [13]. Although the scientific literature mentions that freeze-drying is a gentle dehydration method, it has been observed that, in some foods, it can cause certain changes in their physicochemical properties, even more than oven drying [14]. In a study carried out by Li et al. [15], freeze-drying of Vocia faba Linn (beans) removed more protein content than oven-drying, although the authors noted that the result may have been influenced by the drying time as well as the pre-freezing of the sample at a temperature of −80 °C. In a study by Oliveira-Alves et al. [12], in contrast, a decrease in the amount of protein was observed after oven drying samples of Salicornia ramosissima (sea asparagus); the authors mention a possible denaturation of the protein at a temperature of 70 °C for 72 h. However, other studies with different plant samples—including the present study—do not report significant differences [16].
Still, even when dried, C. berlandieri leaves and inflorescences show higher protein content than conventional edible leaves, such as cabbage (12.8%) and lettuce (14%) [17]. Differences in dietary fiber content could be attributed to chemical changes linked to polysaccharide reordering, which would increase the dietary fiber content of raw oven-dried leaves (ROL). However, the amount found in raw lyophilized inflorescences (RLI) could be comparable to the fiber content found in lyophilized and dried cauliflower (20.6 and 19.1–19.8%, respectively) [18], where the authors found that the degree of esterification of pectic polysaccharides experienced a significant heat-induced dietary fiber change. Dietary fiber is composed of water-soluble polymers (pectins, gums) and insoluble polymers (cellulose, hemicellulose, lignin) [19]. Some studies have found that heating foods can cause a breakdown of the cell matrix of the fiber plant sample [7]. Heat drying can cause the hydrolyzation of structural pectin or protopectin, transforming them into soluble pectins, thus increasing the amount of soluble fiber. However, this type of drying can also cause the degradation of hemicellulose and cellulose, transforming them into simple carbohydrates by decreasing the amount of insoluble fiber [20]. Freeze-drying can modify some fiber components (insoluble pectins) by activating the enzyme pectin-methyl-esterase, causing de-esterification of the fiber components and generating digestible carbohydrates [9]. It has been observed that the type of drying, whether hot or cold, can increase or decrease the amount of fiber in different plant samples [19], as observed in the present study. This is particularly true, for the purposes of our study, for ash content, where obtained values are higher than other quelites [10,21], even when dried [17].
2.2. Impact of the Drying Process on the Phytochemical Composition and Antioxidant Capacity of Raw and Boiled Leaves and Inflorescences of C. berlandieri
Inflorescences showed the highest total phenolic compounds (TPC), total flavonoids (TF), and the highest antioxidant capacity quantified by the DPPH, ABTS, and FRAP (Table 2) methods.

Table 2.
Effect of drying on the total phenolic composition and antioxidant capacity of raw and boiled leaves and inflorescences of C. berlandieri ssp. berlandieri.
As observed, boiling reduced TPC and FT, but resulted in higher FRAP in freeze-dried leaves than other treatments. In both types of plant material, freeze-drying contributed to preserving TPC and TF as well as antioxidant capacity. It has been reported that freeze-drying preserves the polyphenolic composition of food matrices and prevents their oxidation due to the combined effect of vacuum and low temperature [22]. There are no studies on the impact of drying methods on the phytochemical composition of the leaves and inflorescences of C. berlandieri. The TPC and TF contents obtained in this study are lower than those reported for the leaves of other quelites, but the inflorescences of C. berlandieri showed higher TPC values than several other Amaranthus spp. flowers [23] and values similar to those reported for flowers of common mullein (Verbascum thapsus) [24]. The highest values of phenolic compounds in boiled and oven-dried leaves are similar to the values reported by Godínez-Santillan et al. [25] in C. aconitifolius leaves previously dried at 40 °C and boiled for 10 min. Other studies, such as Fauziah et al. [26], also observed an increase in the amount of phenolic compounds and the antioxidant capacity of samples boiled for 5, 10 and 15 min. It has been observed that the thermal treatment of different plant foods causes an increase or decrease in the amount of phenolic compounds depending on the type of plant matter and the time invested in the process [27]. Coupled with this thermal effect, oven drying may cause a greater extraction of the polyphenolic compounds that are attached to the cell wall and subcellular compartments; compounds which likely contain more hydroxyl groups, increasing hydrogen donation [25].
Regarding antioxidant capacity, heat treatment did not improve radical scavenging, as analyzed by DPPH, FRAP, and ABTS assays. The freeze-dried raw vegetable samples presented greater radical scavenging activity, but a decrease in antioxidant capacity was observed after heat treatment, except in the FRAP assay. This may be due to an intermediate oxidation state of the polyphenolic compounds produced by the increasing number of reducing sugars or some products that may be generated by the Maillard reaction during the thermal processing of foods, resulting in a lower amount of polyphenols and an increase in antioxidant capacity [28]. Furthermore, it should be recognized that the vegetable matrix Is complex and contains different bioactive compounds that may respond with greater antioxidant capacity. In the antioxidant capacity tests, the results may also be influenced by different reaction mechanisms with the antioxidant compounds of the plant samples [23,29]. This phenomenon has been described for other quelites such as Portulaca oleracea, Amaranthus hypochondriacus, and Amaranthus caudatus [10,23,30]. However, heat treatment is essential for inactivating enzymes such as polyphenol oxidase and several peroxidases [27].
Individual phenolic compounds (Table 3) were identified by their exact molecular mass, by which 23 compounds were found (Table S1). Figure 1 shows the normalized abundance of each compound in the C. berlandieri samples, based on the amounts displayed in Table 3. Flavanols, flavanones, and flavonols were more abundant in the inflorescences than in the leaves, particularly quercetin-rhamnosyl-rhamnosyl-hexoside, (iso)-rhamnetin hexoside, and quercetin, coinciding with the higher amount of TF in inflorescences than in leaves, presented in Table 3. However, naringin, quercetin-rhamnosyl-rhamnosyl-hexoside, quercetin rutinoside, (iso)-rhamnetin hexoside, and dihydroxybenzoic acid hexoside were more abundant in the leaves. Quercetin rutinoside has been reported as one of the main flavanols of C. berlandieri and C. ambrosioides species [10,31], whereas kaempferol has not been previously reported for C. berlandieri. Procyanidin B2 was found, but only in the inflorescences. There are some current studies of the genus Chenopodium spp. that show it has hepatoprotective, antioxidant, antitumor, antibacterial, chemopreventive, and anticancer biological properties [2], probably due to its bioactive compounds.

Table 3.
Phenolic compounds from raw and boiled oven-dried and lyophilized C. berlandieri ssp. berlandieri leaves and inflorescences, quantified by UPLC-DAD-ESI-QTOF-MS (µg of each phenolic compound/g of lyophilized extract).
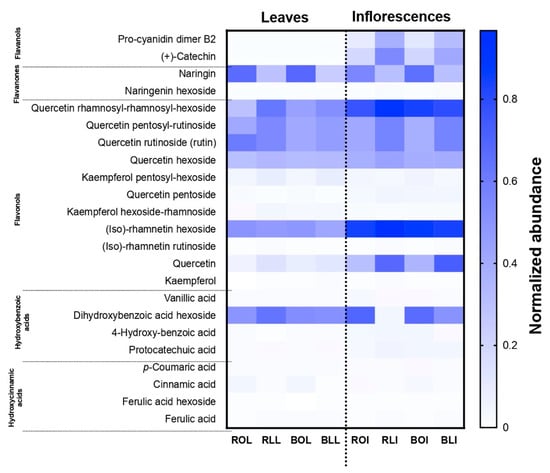
Figure 1.
Normalized abundance of individual phenolic compounds of raw and boiled C. berlandieri leaves and inflorescences after processing by oven drying and lyophilization.
The samples of raw freeze-dried leaf and inflorescences (RLL and RLI) presented higher amounts of individual phenols than the raw oven-dried raw samples of either leaves or inflorescence (ROL and ROI). Similarly, as mentioned above, after boiling, the oven-dried leaves and oven-dried inflorescence samples showed an increase in the quantity of phenolic compounds, while the lyophilized leaves and lyophilized inflorescences showed a decrease.
The results are the average normalized abundance of three independent experiments in triplicate. A min–max normalization was conducted as: (sample–min)/(max–min). BLI: Boiled lyophilized inflorescences; RLI: Raw lyophilized inflorescences; BOI: Boiled oven-dried inflorescences; ROI: Raw oven-dried inflorescences; BLL: Boiled lyophilized leaves; RLL: Raw lyophilized leaves; BOL: Boiled oven-dried leaves; ROL: Raw oven-dried leaves.
Coinciding with the results presented in Table 2. (+)-Catechin, kaempferol, and ferulic acid were found in the RLL but not in the ROL. However, after boiling the boiled oven-dried leaves (BOL), these compounds appeared. This may be due to a release of low-molecular-weight phenolic compounds that are bound to the plant fiber and were probably not released during oven drying [32]. In general, boiling favored the increase in phenolic compounds in the oven-dried samples and caused a slight decrease in the amount of some compounds in the freeze-dried samples, as shown in Table 3.
2.3. Principal Component Analysis (PCA) of Oven-Drying and Lyophilization Clustering
Figure 2 shows the PCA analysis of the variables and phytochemicals evaluated in raw and boiled C. berlandieri leaves (Figure 2A) and inflorescences (Figure 2B), clustered by drying process.
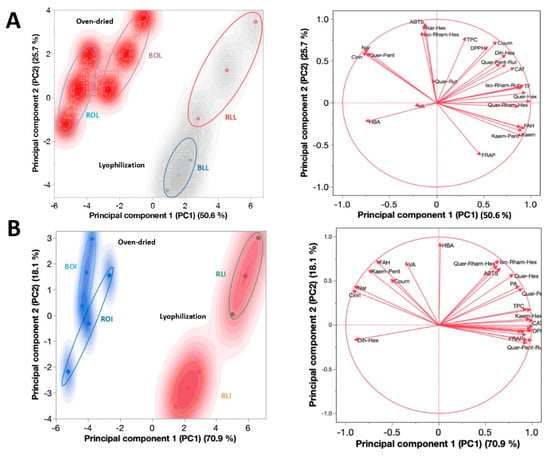
Figure 2.
Principal component analysis (PCA) of C. berlandieri (A) leaves and (B) inflorescences, clustered by drying process. BLI: Boiled lyophilized inflorescences; RLI: Raw lyophilized inflorescences; BOI: Boiled oven-dried inflorescences; ROI: Raw oven-dried inflorescences; BLL: Boiled lyophilized leaves; RLL: Raw lyophilized leaves; BOL: Boiled oven-dried leaves; ROL: Raw oven-dried leaves. Blue and red areas indicate dots areas given by the cluster analysis.
Two components explained >80% of the variance (Table 4) in both the leaves and inflorescences. Moreover, a differential clustering was observed for the leaves and inflorescences depending on the drying process: ABTS and phenolics such as naringin, cinnamic acid, quercetin pentoxide, and ferulic acid hexoside were the components most affected by oven drying. In contrast, TPC, DPPH, p-coumaric acid, dihydroxybenzoic acid hexoside, quercetin-hexoside, and protocatechuic acid were the most affected by lyophilization. Based on the loading of each variable on each component (Table S2), it was shown that quercetin hexoside, naringenin hexoside, TPC, and DPPH were the most influential variables in principal component 1 (PC1). In contrast, ABTS, naringenin hexoside, vanillic acid, (Iso)-rhamnetin-hexoside, and ferulic acid hexoside were the most influential variables in PC2. The close relationship between the drying process and polyphenolic composition is explained by variations in the abundance of phenolics from dried plant material, which is critical considering that quelites are sold in both fresh and dried forms [5]. During food drying, the generation and accumulation of different bioactive compounds that could have antagonistic or synergistic effects upon each other, or upon other constituents of the sample occurs; these chemical interactions are still under investigation [33].

Table 4.
Percentages and cumulative percentages of principal component analysis (PCA) analysis from C. berlandieri ssp. berlandieri (A) leaves and (B) inflorescences.
The analysis of the phenolic compound content and the antioxidant capacity of both botanical structures (leaves and inflorescences) suggests that it may be useful to include inflorescence in the human diet as a food or ingredient in different food dishes. In fact, the inflorescence of some quelites is the main edible product, such is the case of Chenopodium berlandieri ssp. nuttaliiae (huauzontle), which has been rarely studied.
3. Materials and Methods
3.1. Chemical Reagents
Ethanol, absolute methanol, sodium carbonate, aluminum trichloride, sodium hydroxide, sodium nitrate, potassium persulfate, hydrochloric acid, sodium acetate, glacial acetic acid, ferric chloride hexahydrate, gallic acid, catechin, 6-hydroxy-2,5,7,8-tetramethylchroman-2-carboxylic acid (Trolox), 2,2-diphenyl-1-picrylhydrazyl radical (DPPH), 2,4,6-tris (2-pyridyl)-s-triazine (TPTZ), 2,2′-azino-bis(3-ethylbenzothiazoline-6-sulfonic acid) (ABTS), Folin–Ciocalteu reagent, and phenolic HPLC-grade standards (p-coumaric acid, hydroxybenzoic acid, hesperidin, quercetin rutinoside, and rutin) were purchased from Sigma-Aldrich (St. Louis, MO, USA).
3.2. Plant Material and Study Design
Different samples of Chenopodium berlandieri were collected during August–September 2021 in La Barreta (Queretaro, Mexico), a town located at Lat. 20°49′47.6″ N and Lon. 100°30′11.9″ West and 2150 m above sea level. Plants were identified and registered (code: 00006282) by a specialist from the Jerzy Rzedowski Herbarium of the Universidad Autonoma de Queretaro. The study used a 23 factorial design according to the following variables: (1) the botanical part analyzed: leaves or inflorescences; (2) the type of drying: oven-drying or lyophilization; (3) whether the samples were left raw or were boiled before the corresponding analyses. This resulted in 8 groups of samples: boiled lyophilized inflorescences (BLI); raw lyophilized inflorescences (RLI); boiled oven-dried inflorescences (BOI); raw oven-dried inflorescences (ROI); boiled lyophilized leaves (BLL); raw lyophilized leaves (RLL); boiled oven-dried leaves (BOL); and raw oven-dried leaves (ROL).
3.3. Drying Process
Immediately after collection, the samples were manually cleaned, separating the leaves and inflorescences. The samples were divided into two equal parts to be dried in an oven or by lyophilization. For the oven-drying procedure, the samples (leaves and inflorescences) were placed in a forced-ventilation oven (FX 1375, Shel Lab, Cornelius, OR, USA) at 40 °C for 48 h. For the lyophilization procedure, the samples were frozen at −80 °C for 24 h, and then placed in a freeze dryer (Scientz-10N, Ningbo Scientz Biotechnology Co., Zhejiang, China) at −60 °C and 1 Pa. Once dried, samples were ground in an electric blender (Thomas Model 4 Wiley Mill®, Thomas Scientific, Swedesboro, NJ, USA) and sieved through a 500 µm mesh. The resulting powders were then placed in sealed bags and stored at −80 °C for further analysis.
3.4. Proximate Chemical Analysis
For the raw leaves and inflorescences, analysis was performed according to AOAC (Association of Analytical Communities) procedures [34], as follows: quantification of ash (method 942.05), protein (method 920.87), lipids (920.39), moisture (method 925.10), lipids (method 920.39), and dietary fiber (method 962.09). Total carbohydrates were calculated by difference, according to equation [12]. Determinations were performed in triplicate in each analysis and the results were expressed as a percentage by weight of dry matter.
Total carbohydrates (%) = 100 − % (moisture + fiber + fat + ash + protein)
3.5. Extraction, Identification, and Quantification of Phenolic Compounds
3.5.1. Methanolic Extraction of Raw and Boiled Samples
The dried samples (oven dried and freeze dried) were separated into two groups, the first group was left as is (raw) and the second part was boiled, as follows. The sample was mixed with distilled water (5 g in 100 mL), heated at 100 °C for 5 min, and then cooled to room temperature. The amount lost from the initial 100 mL of distilled water was volumetrically completed. Subsequently, it was topped up to 400 mL with absolute methanol to obtain a proportion of 80:20 v/v. Subsequently, a hydroalcoholic extraction was carried out as described in Figure 3. The raw samples were mixed directly in a proportion of 5 g of plant sample to 500 mL of the hydroalcoholic dilution (80:20 v/v, again, absolute methanol to water). All extractions were left for 16 h under constant stirring (100 rpm) at room temperature (25 ± 1 °C) protected from light. Then extracts were filtered through Whatman No. 40 paper and rota-evaporated (R-200, Büchi, Essen, Germany) at 40 °C and 100 mm Hg pressure. The resulting solution was lyophilized as described above. The powdered extracts were packed in sealed bags, protected from light, and stored at −80 °C for further analysis.

Figure 3.
Methodology of the drying and extraction process of C. berlandieri (A). Identification of the samples used in this study (B).
3.5.2. Spectrophotometric Determination of Total Phenolic Compounds (TPC) and Total Flavonoids (TF)
The total phenolic compounds (TPC) were quantified as reported by Singleton et al. [35] using the Folin–Ciocalteu reagent, and the results were expressed in mg gallic acid equivalents (GAE)/g sample. The total flavonoids (TF) were determined as described by Zhishen et al. [36], and the results were reported in mg of (+)-catechin equivalents/g sample.
3.5.3. Identification and Quantification of Individual Phenolic Compounds by UPLC-DAD-ESI-QToF/MS
Individual phenolic compounds were identified and quantified by ultra-high performance liquid chromatography (UPLC) coupled to diode array detection (DAD) with electrospray ionization (ESI) coupled to a quadrupole time-of-flight (QToF) mass spectrometry (MS) [37]. Before the injection (2 µL sample) into the equipment, 25 mg of lyophilized extract was resuspended in 500 µL UPLC-grade water, and the sample was separated on a BEH Acquity C18 column (2.1 × 100 mm, 1.7 µm granule size) (Waters Corp., Milford, MA, USA) at 35 °C. The separation was performed using two solvents: MS-grade water adjusted with 0.1% formic acid (A) and 100% MS-grade acetonitrile (B) using the gradient conditions (0.5 mL/min) as follows: 0% B (0 min), 15% B (2.5 min), 21% B (10 min), 90% B (12 min), 95% B (13 min), and 0% B (15 min). The absorbances were read at 214, 280, 320, and 360 nm. For the quantification of individual compounds, commercial HPLC-grade standards of procyanidin dimer B2, (+)-catechin, naringin, rutin, quercetin, kaempferol, vanillic acid, 4-hydroxybenzoic acid, protocatechuic acid, p-coumaric acid, cinnamic acid, and ferulic acid were used. The mass spectrometer was operated under the following conditions: capillary voltage: 2.0 kV; cone voltage: 40 eV; low-collision energy: 6 V; high-collision energy: 15–45 V; source temperature: 120 °C; cone gas flow: 50 L/h, desolvation gas (N2) flow: 800 L/h (450 °C). Data acquisition was carried out in negative ionization mode (ESI-) with a mass range 100–1200 Da. A leucine enkephalin solution (50 pg/mL) was used to lock mass correction (10 µL/min). Compounds were identified through the exact mass analysis of pseudomolecular ions (mass error < 5 ppm), isotope distribution, and fragmentation pattern. The results were expressed as µg of each compound/g lyophilized extract.
3.5.4. Antioxidant Capacity
Antioxidant capacity was determined by three different methods: the 1,1-diphenyl-2-picrylhydrazyl (DPPH) method reported by Brand-Williams et al. [38], the 2,2-azino-bis(3-ethylbenzothiazoline)-6-sulfonic acid (ABTS) method reported by Ozgen et al. [39], and the ferric reducing antioxidant power (FRAP) method reported by Benzie and Strain [40]. Results from the 3 methods were reported in µmol equivalents of Trolox/g sample. For all the assays, a Trolox standard curve was established (0–700 µM).
3.6. Statistical Analysis
The results were expressed as the mean ± SD of three independent experiments in triplicates. An analysis of variance (ANOVA) followed by post-hoc Tukey–Kramer’s test was conducted, establishing significance as p < 0.05. A Principal Component Analysis (PCA) was also carried out using a correlation matrix between the analyzed variables and the drying method (oven-dried or lyophilized), and all the analysis were performed in JMP v. 17 (SAS, Cary, NC, USA).
4. Conclusions
The study revealed that freeze-drying could better preserve the phenolic compounds and antioxidant capacity of C. berlandieri than oven drying. Despite the decrease in individual phenolic compounds in the freeze-dried samples after heat treatment, they present acceptable amounts of phenolic composition, mainly in the inflorescence. Several popular types of quelites are boiled before being consumed, which, as observed in the present study, results in some loss of phenolic compounds. Thus, plant products that are consumed dry and without any heat treatment may be an important source of phenolic compounds with potential antioxidant activity. In the present investigation, it was observed that the inflorescence is richer in phenolic compounds and antioxidant capacity than leaves. However, both plant samples show an interesting content of phenolic compounds that may have beneficial effects on health. Further in vitro and in vivo investigations should be performed to assess these biological effects.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/molecules28207235/s1, Table S1: Phenolic compounds from C. berlandieri leaves and inflorescences, identified by UPLC-DAD-ESI-QToF/MS, Table S2: Loading of each variable on each component from the PCA analysis for C. berlandieri ssp. berlandieri (A) leaves and (B) inflorescences.
Author Contributions
Conceptualization, A.K.-G., H.V.-M., R.A.F.-M., S.H.G.-M. and J.L.C.-S.; investigation, Á.F.V.-M., I.L.-O., R.A.F.-M., L.H.-S. and S.H.G.-M.; methodology, Á.F.V.-M., A.K.-G., I.L.-O., I.F.P.-R., M.A.A.-L., O.R.-P., L.H.-S. and J.L.C.-S.; data curation, I.L.-O., M.A.A.-L., O.R.-P. and J.L.C.-S.; formal analysis, Á.F.V.-M., H.V.-M. and R.A.F.-M.; Project administration, Á.F.V.-M., M.A.A.-L. and J.L.C.-S.; resources, I.F.P.-R., M.A.A.-L., O.R.-P. and J.L.C.-S.; software, I.L.-O., I.F.P.-R. and R.A.F.-M.; supervision, A.K.-G., H.V.-M., I.F.P.-R., L.H.-S. and J.L.C.-S.; validation, A.K.-G., H.V.-M., M.A.A.-L. and I.F.P.-R.; visualization, I.L.-O., I.F.P.-R. and L.H.-S.; writing—original draft, Á.F.V.-M. and J.L.C.-S.; writing—review and editing, Á.F.V.-M., I.F.P.-R., S.H.G.-M. and J.L.C.-S. All authors have read and agreed to the published version of the manuscript.
Funding
The work of Angel F. Vargas-Madriz was supported by a PhD Scholarship from the Consejo Nacional de Humanidades, Ciencia y Tecnología (CONAHCyT-Mexico) [grant ID: 927742].
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data will be available upon reasonable request.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Zekrumah, M.; Begua, P.; Razak, A.; Wahab, J.; Moffo, N.; Ivane, A.; Oman, M.; Elrashied, H.; Zou, X.; Zhang, D. Role of dietary polyphenols in non-communicable chronic disease prevention, and interactions in food systems: An overview. Nutrition 2023, 112, 112034. [Google Scholar] [CrossRef] [PubMed]
- Santiago-Saenz, Y.O.; Hernández-Fuentes, A.D.; López-Palestina, C.U.; Garrido-Cauich, J.H.; Alatorre-Cruz, J.M.; Monroy-Torres, R.; Santiago-Saenz, Y.O.; Hernández-Fuentes, A.D.; López-Palestina, C.U.; Garrido-Cauich, J.H.; et al. Importancia Nutricional y Actividad Biológica de Los Compuestos Bioactivos de Quelites Consumidos En México. Rev. Chil. Nutr. 2019, 46, 593–605. [Google Scholar] [CrossRef]
- Vargas-Madriz, Á.F.; Luzardo-Ocampo, I.; Moreno-Celis, U.; Roldán-Padrón, O.; Chávez-Servín, J.L.; Vergara-Castañeda, H.A.; Martínez-Pacheco, M.; Mejía, C.; García-Gasca, T.; Kuri-García, A. Comparison of Phytochemical Composition and Untargeted Metabolomic Analysis of an Extract from Cnidoscolus Aconitifolius (Mill.) I. I. Johnst and Porophyllum Ruderale (Jacq.) Cass. and Biological Cytotoxic and Antiproliferative Activity in Vitro. Plants 2023, 12, 1987. [Google Scholar] [CrossRef]
- Pérez-Negrón, E.; Casas, A. Use, Extraction Rates and Spatial Availability of Plant Resources in the Tehuacán-Cuicatlán Valley, Mexico: The Case of Santiago Quiotepec, Oaxaca. J. Arid Environ. 2007, 70, 356–379. [Google Scholar] [CrossRef]
- Pascual-Mendoza, S.; Saynes-Vásquez, A.; Pérez-Herrera, A.; Meneses, M.E.; Coutiño-Hernández, D.; Sánchez-Medina, M.A. Nutritional Composition and Bioactive Compounds of Quelites Consumed by Indigenous Communities in the Municipality of Juquila Vijanos, Sierra Norte of Oaxaca, Mexico. Plant Foods Hum. Nutr. 2023, 78, 193–200. [Google Scholar] [CrossRef]
- Kumar, S.; Yadav, A.; Yadav, M.; Yadav, P. Effect of Climate Change on Phytochemical Diversity, Total Phenolic Content and in Vitro Antioxidant Activity of Aloe Vera (L.) Burm.F. BMC Res. Notes 2017, 10, 60. [Google Scholar] [CrossRef]
- Dadhaneeya, H.; Kesavan, R.K.; Inbaraj, B.S.; Sharma, M.; Kamma, S.; Nayak, P.K.; Sridhar, K. Impact of Different Drying Methods on the Phenolic Composition, In Vitro Antioxidant Activity, and Quality Attributes of Dragon Fruit Slices and Pulp. Foods 2023, 12, 1387. [Google Scholar] [CrossRef]
- Dong, Q.; He, D.; Ni, X.; Zhou, H.; Yang, H. Comparative Study on Phenolic Compounds, Triterpenoids, and Antioxidant Activity of Ganoderma Lucidum Affected by Different Drying Methods. J. Food Meas. Charact. 2019, 13, 3198–3205. [Google Scholar] [CrossRef]
- Garcia-Amezquita, L.E.; Tejada-Ortigoza, V.; Campanella, O.H.; Welti-Chanes, J. Influence of Drying Method on the Composition, Physicochemical Properties, and Prebiotic Potential of Dietary Fibre Concentrates from Fruit Peels. J. Food Qual. 2018, 2018, 9105237. [Google Scholar] [CrossRef]
- Santiago-Saenz, Y.O.; Hernández-Fuentes, A.D.; Monroy-Torres, R.; Cariño-Cortés, R.; Jiménez-Alvarado, R. Physicochemical, Nutritional and Antioxidant Characterization of Three Vegetables (Amaranthus hybridus L., Chenopodium berlandieri L., Portulaca oleracea L.) as Potential Sources of Phytochemicals and Bioactive Compounds. J. Food Meas. Charact. 2018, 12, 2855–2864. [Google Scholar] [CrossRef]
- Román-Cortés, N.R.; García-Mateos, M.D.; Castillo-González, A.M.; Sahagún-Castellanos, J.; Jiménez-Arellanes, M.A. Características Nutricionales y Nutracéuticas de Hortalizas de Uso Ancestral En México. Rev. Fitotec. Mex. 2018, 41, 245–253. [Google Scholar] [CrossRef][Green Version]
- Oliveira-Alves, S.C.; Andrade, F.; Prazeres, I.; Silva, A.B.; Capelo, J.; Duarte, B.; Caçador, I.; Coelho, J.; Serra, A.T.; Bronze, M.R. Impact of Drying Processes on the Nutritional Composition, Volatile Profile, Phytochemical Content and Bioactivity of Salicornia Ramosissima j. Woods. Antioxidants 2021, 10, 1312. [Google Scholar] [CrossRef] [PubMed]
- Shonte, T.T.; Duodu, K.G.; de Kock, H.L. Effect of Drying Methods on Chemical Composition and Antioxidant Activity of Underutilized Stinging Nettle Leaves. Heliyon 2020, 6, e03938. [Google Scholar] [CrossRef] [PubMed]
- Radojčin, M.; Pavkov, I.; Kovačević, D.B.; Putnik, P.; Wiktor, A.; Stamenković, Z.; Kešelj, K.; Gere, A. Effect of Selected Drying Methods and Emerging Drying Intensification Technologies on the Quality of Dried Fruit: A Review. Processes 2021, 9, 132. [Google Scholar] [CrossRef]
- Li, S.; Liu, F.; Wu, M.; Li, Y.; Song, X.; Yin, J. Effects of Drying Treatments on Nutritional Compositions, Volatile Flavor Compounds, and Bioactive Substances of Broad Beans. Foods 2023, 12, 2160. [Google Scholar] [CrossRef] [PubMed]
- Raza, N.; Arshad, M.U.; Anjum, F.M.; Saeed, F.; Maan, A.A.; Bader Ul Ain, H. Impact of Drying Methods on Composition and Functional Properties of Date Powder Procured from Different Cultivars. Food Sci. Nutr. 2019, 7, 2345–2352. [Google Scholar] [CrossRef]
- Adeboye, A.O.; Bolaji, O.A.; Fasogbon, B.M.; Okunyemi, B.M. An Evaluation of the Impact of Drying on the Nutritional Composition, Functional Properties, and Sensory Characteristics of a Ready-to-Cook C. volubile Leaf Soup Powder. J. Culin. Sci. Technol. 2019, 18, 244–253. [Google Scholar] [CrossRef]
- Femenia, A.; Selvendran, R.R.; Ring, S.G.; Robertson, J.A. Effects of Heat Treatment and Dehydration on Properties of Cauliflower Fiber. J. Agric. Food Chem. 1999, 47, 728–732. [Google Scholar] [CrossRef]
- Borchani, C.; Besbes, S.; Masmoudi, M.; Bouaziz, M.A.; Blecker, C.; Attia, H. Influence of Oven-Drying Temperature on Physicochemical and Functional Properties of Date Fibre Concentrates. Food Bioprocess Technol. 2012, 5, 1541–1551. [Google Scholar] [CrossRef]
- Quispe-Fuentes, I.; Vega-Gálvez, A.; Aranda, M.; Poblete, J.; Pasten, A.; Bilbao-Sainz, C.; Wood, D.; McHugh, T.; Delporte, C. Effects of Drying Processes on Composition, Microstructure and Health Aspects from Maqui Berries. J. Food Sci. Technol. 2020, 57, 2241–2250. [Google Scholar] [CrossRef]
- Díaz-Batalla, L.; Hernández-Uribe, J.P.; Román-Gutiérrez, A.D.; Cariño-Cortés, R.; Castro-Rosas, J.; Téllez-Jurado, A.; Gómez-Aldapa, C.A. Chemical and Nutritional Characterization of Raw and Thermal-Treated Flours of Mesquite (Prosopis Laevigata) Pods and Their Residual Brans. CyTA—J. Food 2018, 16, 444–451. [Google Scholar] [CrossRef]
- De Ancos, B.; Sánchez-Moreno, C.; Zacarías, L.; Rodrigo, M.J.; Sáyago Ayerdí, S.; Blancas Benítez, F.J.; Domínguez Avila, J.A.; González-Aguilar, G.A. Effects of Two Different Drying Methods (Freeze-Drying and Hot Air-Drying) on the Phenolic and Carotenoid Profile of ‘Ataulfo’ Mango by-Products. J. Food Meas. Charact. 2018, 12, 2145–2157. [Google Scholar] [CrossRef]
- Li, H.; Deng, Z.; Liu, R.; Zhu, H.; Draves, J.; Marcone, M.; Sun, Y.; Tsao, R. Characterization of Phenolics, Betacyanins and Antioxidant Activities of the Seed, Leaf, Sprout, Flower and Stalk Extracts of Three Amaranthus Species. J. Food Compos. Anal. 2015, 37, 75–81. [Google Scholar] [CrossRef]
- Soto, K.M.; Luzardo-Ocampo, I.; López-Romero, J.M.; Mendoza, S.; Loarca-Piña, G.; Rivera-Muñoz, E.M.; Manzano-Ramírez, A. Gold Nanoparticles Synthesized with Common Mullein (Verbascum Thapsus) and Castor Bean (Ricinus Communis) Ethanolic Extracts Displayed Antiproliferative Effects and Induced Caspase 3 Activity in Human HT29 and SW480 Cancer Cells. Pharmaceutics 2022, 14, 2069. [Google Scholar] [CrossRef] [PubMed]
- Godínez-Santillán, R.I.; Chávez-Servín, J.L.; García-Gasca, T.; Guzmán-Maldonado, S.H. Caracterización Fenólica y Capacidad Antioxidante de Extractos Alcohólicos de Hojas Crudas y Hervidas de Cnidoscolus Aconitifolius (Euphorbiaceae). Acta Bot. Mex. 2019, 126, 1–15. [Google Scholar] [CrossRef]
- Fauziah, I.; Prangdimurti, E.; Palupi, N. Effect of Boiling Time on the Stability of the Phenolic Compounds in Wedang Uwuh after Gastric Digestion. IOP Conf. Ser. Earth Environ. Sci. 2023, 1200, 012014. [Google Scholar] [CrossRef]
- Balunkeswar, N.; Rui Hai, L.; Juming, T. Effect of Processing on Phenolic Antioxidants of Effect of Processing on Phenolic Antioxidants of Fruits, Vegetables, and Grains—A Review. Crit. Rev. Food Sci. Nutr. ISSN 2015, 55, 887–918. [Google Scholar] [CrossRef]
- Nurkhoeriyati, T.; Kulig, B.; Sturm, B.; Hensel, O. The Effect of Pre-Drying Treatment and Drying Conditions on Quality and Energy Consumption of Hot Air-Dried Celeriac Slices: Optimisation. Foods 2021, 10, 1758. [Google Scholar] [CrossRef]
- Kraujalis, P.; Venskutonis, P.R.; Kraujalienė, V.; Pukalskas, A. Antioxidant Properties and Preliminary Evaluation of Phytochemical Composition of Different Anatomical Parts of Amaranth. Plant Foods Hum. Nutr. 2013, 68, 322–328. [Google Scholar] [CrossRef]
- Fukalova-Fukalova, T.; García-Martínez, M.D.; Raigón, M.D. Nutritional Composition, Bioactive Compounds, and Volatiles Profile Characterization of Two Edible Undervalued Plants: Portulaca oleracea L. and Porophyllum Ruderale (Jacq.) Cass. Plants 2022, 11, 377. [Google Scholar] [CrossRef]
- Jesus, R.S.; Piana, M.; Freitas, R.B.; Brum, T.F.; Alves, C.F.S.; Belke, B.V.; Mossmann, N.J.; Cruz, R.C.; Santos, R.C.V.; Dalmolin, T.V.; et al. In Vitro Antimicrobial and Antimycobacterial Activity and HPLC–DAD Screening of Phenolics from Chenopodium ambrosioides L. Braz. J. Microbiol. 2018, 49, 296. [Google Scholar] [CrossRef]
- Arias-Rico, J.; Macías-León, F.J.; Alanís-García, E.; Cruz-Cansino, N.D.; Jaramillo-Morales, O.A.; Barrera-Gálvez, R.; Ramírez-Moreno, E. Study of Edible Plants: Effects of Boiling on Nutritional, Antioxidant, and Physicochemical Properties. Foods 2020, 9, 599. [Google Scholar] [CrossRef] [PubMed]
- Youssef, K.M.; Mokhtar, S.M. Effect of Drying Methods on the Antioxidant Capacity, Color and Phytochemicals of Portulaca oleracea L. Leaves. J. Nutr. Food Sci. 2014, 4, 1–6. [Google Scholar] [CrossRef]
- AOAC. Official Methods of Analysis of AOAC International; Horwitz, W., Latimer, G.W., Eds.; Seventeen; AOAC International: Gaithersburg, VA, USA, 2002; Volume 15, ISBN 0-935584-77-3. [Google Scholar]
- Singleton, V.L.; Orthofer, R.; Lamuela-Raventós, R.M. Analysis of Total Phenols and Other Oxidation Substrates and Antioxidants by Means of Folin-Ciocalteu Reagent. Methods Enzymol. 1999, 299, 152–178. [Google Scholar] [CrossRef]
- Zhishen, J.; Mengcheng, T.; Jianming, W. The Determination of Flavonoid Contents in Mulberry and Their Scavenging Effects on Superoxide Radicals. Food Chem. 1999, 64, 555–559. [Google Scholar] [CrossRef]
- Reynoso-Camacho, R.; Rodríguez-Villanueva, L.D.; Sotelo-González, A.M.; Ramos-Gómez, M.; Pérez-Ramírez, I.F. Citrus Decoction By-Product Represents a Rich Source of Carotenoid, Phytosterol, Extractable and Non-Extractable Polyphenols. Food Chem. 2021, 350, 129239. [Google Scholar] [CrossRef]
- Brand-Williams, W.; Cuvelier, M.E.; Berset, C. Use of a Free Radical Method to Evaluate Antioxidant Activity; Elsevier: Amsterdam, The Netherlands, 1995; Volume 28. [Google Scholar]
- Ozgen, M.; Reese, R.N.; Tulio, A.Z.; Scheerens, J.C.; Miller, A.R. Modified 2,2-Azino-Bis-3-Ethylbenzothiazoline-6-Sulfonic Acid (ABTS) Method to Measure Antioxidant Capacity of Selected Small Fruits and Comparison to Ferric Reducing Antioxidant Power (FRAP) and 2,2′-Diphenyl-1-Picrylhydrazyl (DPPH) Methods. J. Agric. Food Chem. 2006, 54, 1151–1157. [Google Scholar] [CrossRef]
- Benzie, I.F.F.; Strain, J.J. The Ferric Reducing Ability of Plasma (FRAP) as a Measure of “Antioxidant Power”: The FRAP Assay. Anal. Biochem. 1996, 239, 70–76. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).