1. Introduction
For decades, with the continuous development of science, technology, and industry, the performance requirements of various materials have become higher and higher. Much research has been performed in the area of benzoxazine, a high-performance thermosetting resin. During the curing process, benzoxazine (BOZ) does not emit small molecules and it does not shrink during the polymerization process [
1,
2,
3]. It has a highly crosslinked three-dimensional network, high dimensional stability, good mechanical properties, excellent thermal stability [
4,
5,
6], and low water absorption [
7,
8]. It is an irreplaceable material in the polymer industry and is widely used in navigation, aviation, aerospace, composite materials, and other fields [
9,
10,
11].
Polybenzoxazine (PBZ) resins are insoluble after cross-linking. At present, the main method to treat thermosetting resin materials is landfill, which pollutes the environment. In addition, the use of incineration, pyrolysis, and other methods consumes a lot of energy and brings secondary pollution. These methods are only simple treatments and do not solve the problem of material recovery at the root. Therefore, it is necessary to improve the recycling of materials through molecular structure design. Recyclable benzoxazine resins are mainly divided into two categories. One is the synthesis of self-repairing resin, and reversible dynamic covalent bonds can be broken and recombined by external excitation. In nature, many organisms have self-healing behaviors, and scientists are also developing self-healing materials. In the absence of external healing agents, polymer materials can self-heal physical damage caused by environmental or mechanical factors through dynamic reversible crosslinking, including covalent reactions, such as the Diels–Alder reaction [
12], transesterification reaction [
13], and disulfide bonding [
14], and noncovalent interactions, such as hydrogen bonding [
15]. For example, Rucigaj and his colleagues introduced network flexibility and fluidity into the copolymer system by selecting aliphatic molecules of stearylamine and jeffamine, and designed and crosslinked two benzoxazines based on bisphenol acids. They formed smart polymers with shape memory and self-healing abilities [
16]. Polybenzoxazine showed satisfactory recycling, reshaping, and self-healing properties in a study by Salendra and his colleagues because of the strong hydrogen bonding interactions and cleavage reformation of the S–S bond in vitrimers [
17]. Eco-friendly sustainable poly (benzoxazine-co-urethane) designed by Sriharshitha and his colleagues had excellent self-healing abilities due to the interaction of supramolecular hydrogen bonds, which could extend shelf life and reliability in the form of self-healing coatings and composite materials [
18].
The other way is that degradable structures are introduced into the molecular structure of benzoxazine, which enables the cross-linked polybenzoxazine resins to break at degradable structures under specified chemical environments and permit polybenzoxazine resins available for further recycling after usage. Many scholars have introduced unstable bonds such as acetal bonds, ester bonds, Schiff base bonds, disulfide bonds, hexahydrotriazine structures, borate bonds, and other dynamic chemical bonds into thermosetting resins so that they can be degraded and recycled effectively. These unstable bonds will break under certain conditions, such as heat, light, and specific pHs, which will degrade and recycle thermosetting resin [
19,
20,
21,
22,
23,
24,
25,
26,
27,
28]. Liu and his colleagues designed a degradable Schiff base benzoxazine thermosetting material with a high glass transition temperature [
29]. Cured benzoxazine can be chemically degraded under acidic conditions, and it undergoes controlled degradation when temperature, acidity, and solvent change. Adjaoud and his colleagues designed a high-glass-transition-temperature (
Tg) and degradable isosorbide-based polybenzoxazine vitrimer [
30]. The material was highly stable in pH-neutral water, even at 80 °C for 60 days, but due to the structure of isosorbide, obvious degradation was observed under acidic or alkaline conditions.
A remarkable feature of benzoxazine-based polymers is that they have low system shrinkage or even no volume shrinkage during curing. However, several shortcomings are also associated with benzoxazine resins including brittleness and a low molecular weight of prepolymer, which makes it difficult to process into films. Because of the rich design flexibility of the molecular structure, it opens up a broad opportunity for further improving the properties [
31]. Researchers have developed a new structure of benzoxazine by using the flexible molecular design of BOZ. That is, the main chain of a synthetic monomer or its copolymer contains benzoxazine rings, which is called main-chain benzoxazine (MCBP). Main-chain benzoxazine prepolymers tend to cross-link to obtain excellent strength and flexibility, and the material after heating and curing is still a thermosetting polymer, which has good application prospects, for example, being used in electronic packaging, printed circuit boards, aviation, and film materials. Liu et al. synthesized a series of bio-based fluorine-free main-chain benzoxazine precursors by a Mannich condensation reaction. The newly developed bio-based main-chain polybenzoxazine resin showed good thermal stability and low surface free energy, which implies great application potential as a high-performance resin matrix [
32]. By knowing the molecular weight distribution of the prepared mixture of the polybenzoxazine precursor, its influence on the thermal, mechanical, and viscoelastic properties of the resin during processing and polymerization could be investigated. Ručigaj et al. found that the mixture of precursors of main-chain polybenzoxazine with higher average molecular weight and wider molecular weight distribution showed faster curing and higher crosslinking density of cured resin, thus producing main-chain polybenzoxazine with improved properties [
33]. Li et al. synthesized several polybenzoxazines with different molecular structures from cardanol. The mechanical properties test showed that the impact strength of the double-capped cardanol backbone PBZ was 243% higher than that of traditional PBZ [
34]. The crosslinking density, thermal stability, carbon residue rate, and molecular diversity were enhanced after the main chain polybenzoxazine was terminated.
Many thermosetting resin raw materials come from non-renewable resources such as bisphenol A, which has adverse effects on the environment and the human endocrine system. Considering the limited reserves of fossil resources and environmental benefits, people have begun to look for bio-based materials with low prices and high environmental benefits, such as lignin, cellulose, vanillin, and other bio-based resources that have been used to prepare thermosetting resins. Erythritol ((R, S)-1,2,3,4-butanetriol) is a filling sweetener in sugar alcohol, which has no carcinogenicity and no blood sugar reaction. Martin et al. found that erythritol had no metabolism and almost no irritation in the digestive system by measuring the labeled erythritol in vitro. Most erythritol is absorbed into the blood through the small intestine and excreted through urine, and a small amount of unabsorbed erythritol, which is not easy to ferment, will enter the colon [
35]. Erythritol can also be used as a component and additive in polymer reactions to produce resin, polyether, and polyester. Yuan et al. synthesized a biodegradable epoxy monomer containing acetal structure [
36], which was derived from lignin derivative vanillin, bio-based polyol erythritol, and biogenic epichlorohydrin. When the acetal structure is alkaline and neutral, it is stable even in a wet-heat aging test, but it can be completely degradable under acidic conditions, and the degradation rate is accelerated with an increase in the polarity of organic solvents.
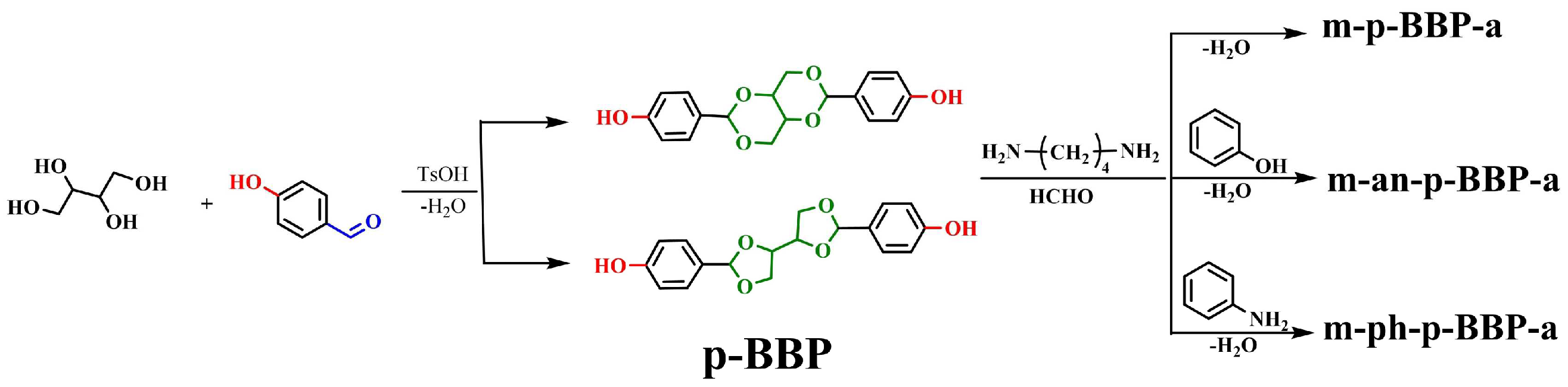
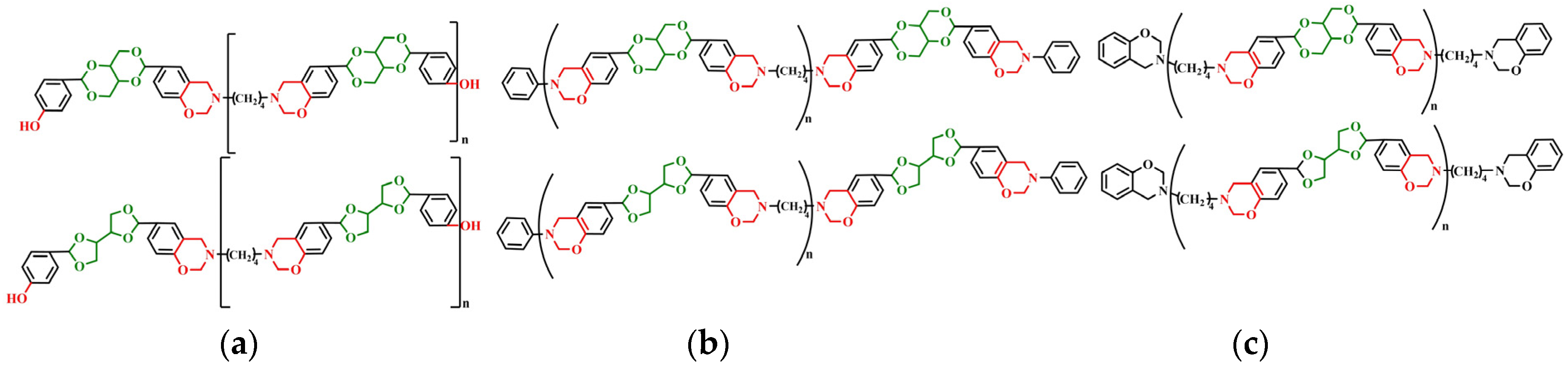
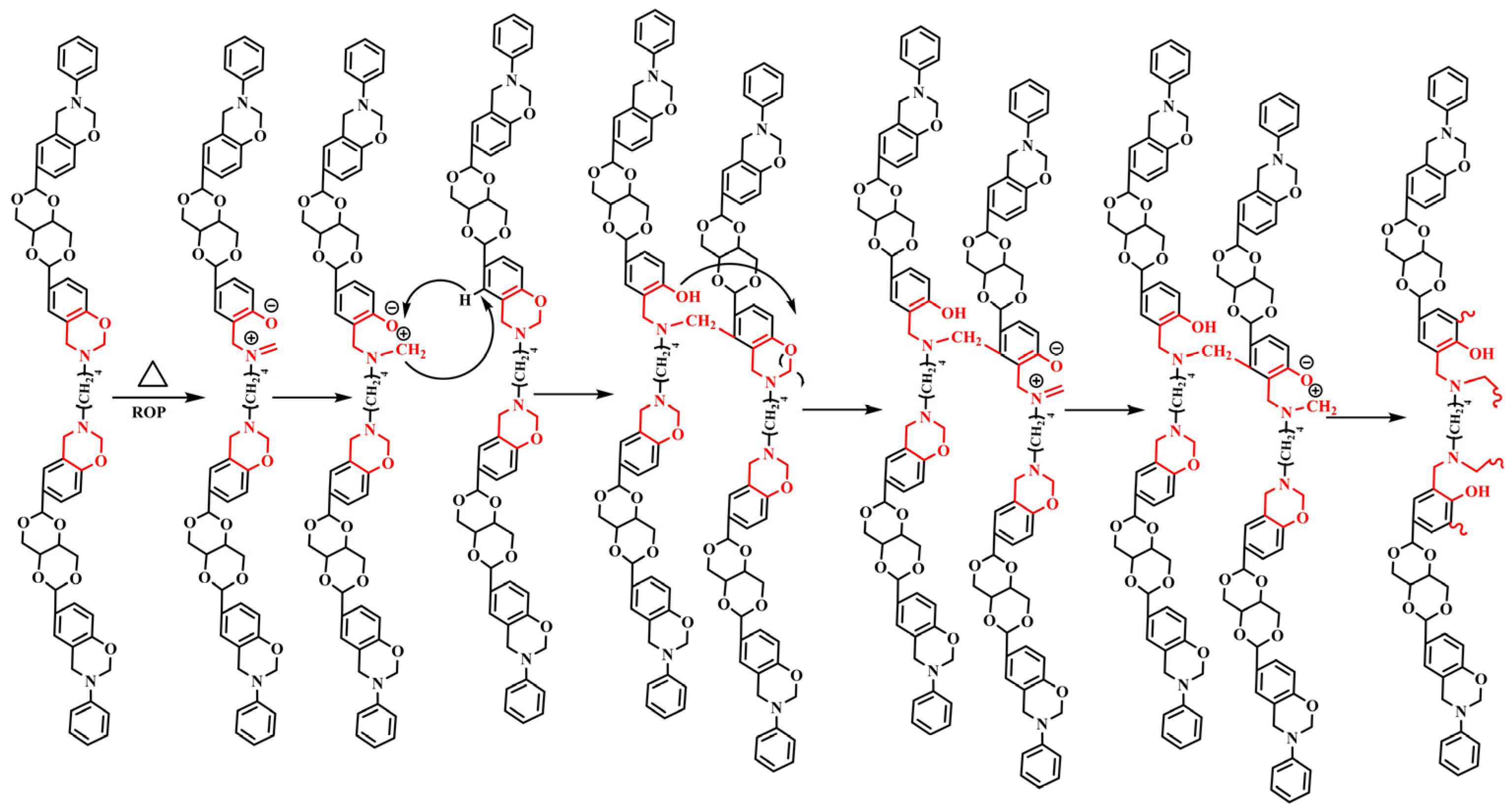
In this work, the acetal compound p-BBP was synthesized from the bio-based raw material erythritol. Erythritol widely exists in nature and is listed in the Generally Recognized as Safe list by the Food and Drug Administration, which is widely used for baked foods. The acetal structure is stable in alkaline and neutral conditions, and it will be hydrolyzed in an acidic solution. Then, with the acetal compound and butanediamine and paraformaldehyde as raw materials, benzoxazine-based resins with different structures of erythritol main chain (m-p-BBP-a, m-an-p-BBP-a, and m-ph-p-BBP-a) (
Figure 1) were synthesized by aniline or phenol capping (
Scheme 1). The polybenzoxazine resins were studied by several measuring methods. The results showed that polybenzoxazine resins were chemically degradable and could be degraded in an acidic solution, which is beneficial for recycling waste and saving resources, and has good environmental benefits.
3. Materials and Methods
3.1. Materials
All the reagents were of analytical grade and used without further purification. Erythritol (98%), phenol, sodium bicarbonate (NaHCO
3), and
p-toluenesulfonic acid (TSOH) were obtained from Shanghai Maclean Biochemical Technology Co., Ltd. (Shanghai, China);
p-hydroxybenzaldehyde (98%) was purchased from Shanghai yuanye Bio-Technology Co., Ltd. (Shanghai, China); N,N-Dimethylformamide (DMF) was purchased from Beijing Chemical Factory (Beijing, China). Aniline and paraformaldehyde were purchased from Fuchen Chemical Reagent Co., Ltd. (Tianjin, China); 1,4-butanediamine (99%) was purchased from Acros Organics Co., Ltd. (Geel, Belgium). In addition, p-BBP was synthesized according to the route in
Scheme 1 [
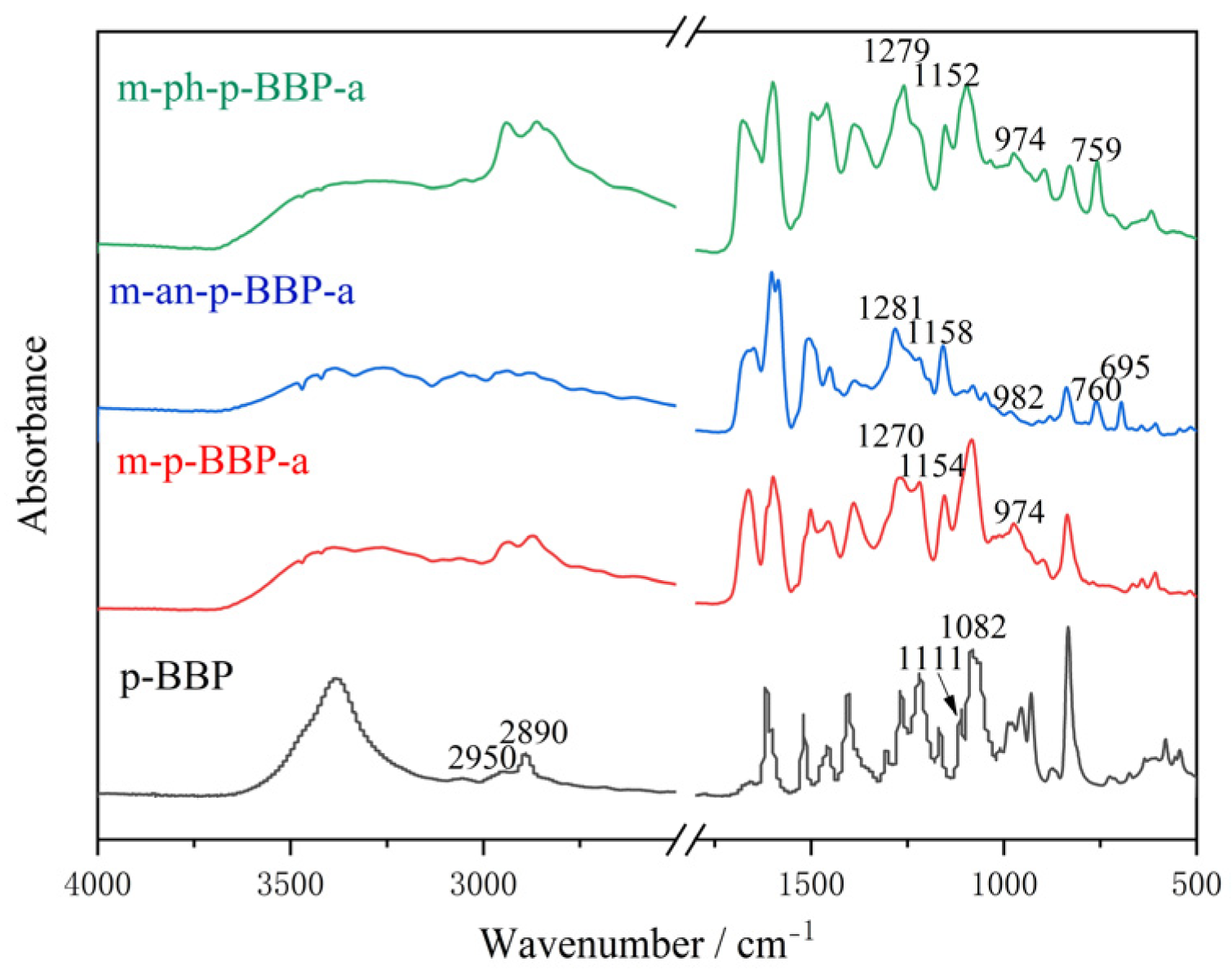
35]. FT-IR spectrum (KBr) (
Figure S1), cm
−1: 3381 (-phenolic hydroxyl -OH), 2950, 2890 (vibration peak -CH
2), 1111 (absorption peaks tertiary carbon C-O), 1082 (stretching vibration peak C-O-C).
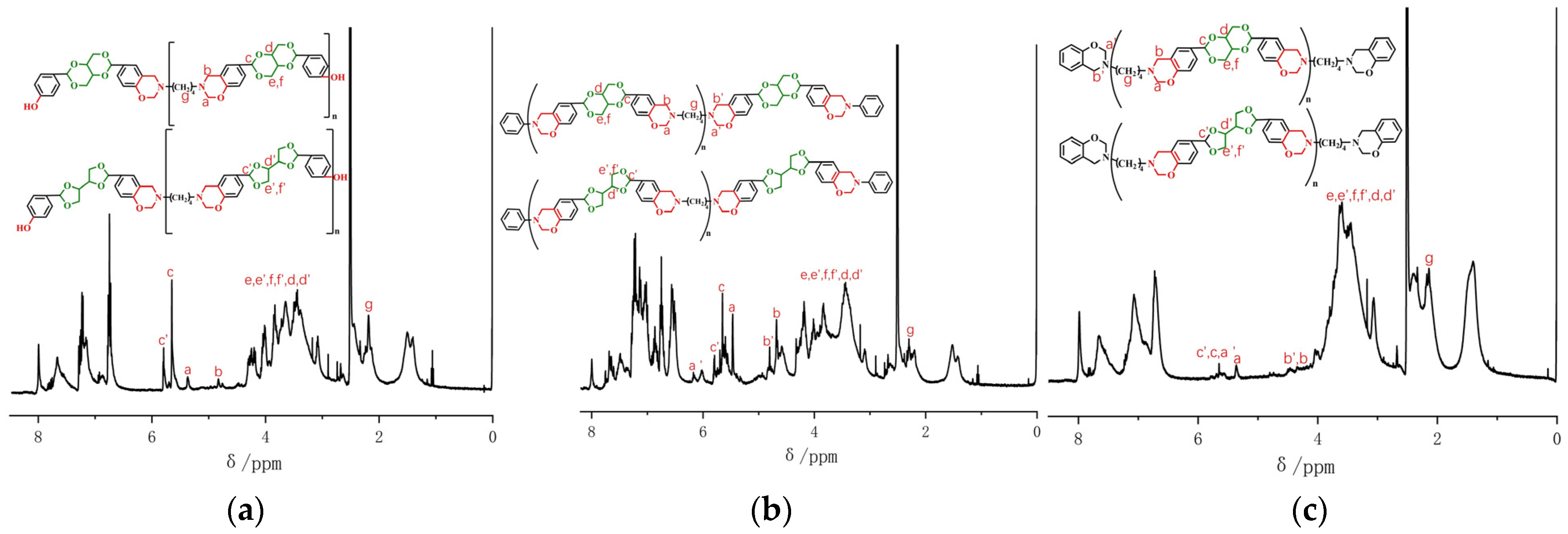
1H NMR spectrum (
Figure S2), ppm: 9.49 (phenolic hydroxyl), 7.27–6.76 (benzene ring), 5.80 (tertiary carbon C-H in five-membered acetal ring), 5.64 (tertiary carbon C-H in six-membered acetal ring), 4.31, 4.21, 4.06, and 4.02 (O-CH
2-C in two kinds of acetal rings), 3.86 and 3.83 (O-CH-C in two kinds of acetal rings).
13C NMR spectrum (
Figure S3), ppm: 115.36–158.68 (benzene ring), 105.05 and 103.93 (tertiary carbon C-H), 76.52 and 74.39 (acetal rings O-CH), 68.81 and 67.62 (acetal rings O-CH
2). In
Figure S4, the mass spectrum of p-BBP shows that the theoretical molecular weight was 330 g mol
−1 and the measured [M]
+ was 330 g·mol
−1.
3.2. Synthesis of m-p-BBP-a
A total of 1.65 g (0.005 mol) of p-BBP, 0.44 g (0.005 mol) of butanediamine, 0.60 g (0.02 mol) of paraformaldehyde, and 20 mL of N,N-dimethylformamide (DMF) was added to a 50 mL three-necked flask, the reaction temperature was set at 115 °C, and it was reacted for 24 h. After the reaction, the solvent DMF was removed from the obtained product by a rotary evaporator and then the reaction product was washed with 0.5 M sodium bicarbonate solution, then filtered, washed with deionized water until neutral, filtered, and dried at 50 °C to obtain the final product, namely, m-p-BBP-a, which was a light yellow powder (yield: 55.8%). FT-IR spectrum (KBr), cm−1: 1389 (-CH2- absorption vibration peak), 1501 (benzene ring C-C), 974, 1154, and 1270 (oxazine rings Ar-O-C), 1083 (tertiary carbon C-O), 3386 (–OH stretching vibration peak). 1H NMR spectrum, ppm: 5.38 (CH2 in N-CH2-O structure), 4.83 (CH2 in N-CH2-Ar), 6.77–7.96 (benzene ring), 3.05–4.26 (acetal spiro ring).
3.3. Synthesis of m-an-p-BBP-a
A total of 6.60 g (0.02 mol) of p-BBP, 0.88 g (0.01 mol) of butanediamine, 1.86 g (0.02 mol) of aniline, 2.40 g (0.08 mol) of paraformaldehyde, and 50 mL DMF was added to a 250 mL three-necked flask, and the reaction steps were the same as those for m-p-BBP-a. The prepared benzoxazine was the erythritol bis-p-hydroxybenzaldehyde-butanediamine benzoxazine-based resin terminated by aniline, which is called m-an-p-BBP-a. It is a burnt yellow powder (yield: 61.7%). FT-IR spectrum (KBr), cm−1: 1387 (-CH2- absorption vibration peak), 1505 (benzene ring C-C), 982, 1158, and 1281 (oxazine rings Ar-O-C), 760 and 695 (monosubstituted benzene rings). 1H NMR spectrum, ppm: 5.47 (CH2 in N-CH2-O), 4.80 (CH2 in N-CH2-Ar), 6.55–7.70 (benzene ring), 3.18–4.30 (acetal spiro ring).
3.4. Synthesis of m-ph-p-BBP-a
A total of 3.30 g (0.01 mol) of p-BBP, 0.88 g (0.01 mol) of butanediamine, 0.94 g (0.01 mol) of phenol, 1.20 g (0.04 mol) of paraformaldehyde, and 20 mL DMF was added to a 50 mL three-necked flask. The reaction steps were the same as those for m-p-BBP-a. M-ph-p-BBP-a, the erythritol bis-p-hydroxybenzaldehyde-butanediamine benzoxazine-based resin terminated by phenol, was obtained as a yellow powder (yield: 68.4%). FT-IR spectrum (KBr), cm−1: 1388 (-CH2- absorption vibration peak), 1498 (benzene ring C-C), 974, 1153, and 1260 (oxazine rings Ar-O-C), 3292 (–OH stretching vibration peak). 1H NMR spectrum, ppm: 5.43 (CH2 in N-CH2-O), 4.34 (CH2 in N-CH2-Ar), 6.70–7.99 (benzene ring), 3.24–4.02 (acetal spiro ring).
3.5. Preparation of Polybenzoxazine Resins (m-p-BBP-a, m-an-p-BBP-a, and m-ph-p-BBP-a)
The m-p-BBP-a, m-an-p-BBP-a, and m-ph-p-BBP-a were ground into powder, which was dissolved in tetrahydrofuran with 4 wt% of 4,4′-Bis(3-aminophenoxy)diphenyl sulfone, respectively. Ultrasonic in a numerical control ultrasonic cleaner, evenly mixing, drying, and grinding for later use. The curing was accomplished by undergoing a cure cycle of 110 °C for 2 h, 130 °C for 2 h, 150 °C for 2 h, 170 °C for 2 h, 190 °C for 2 h, 200 °C for 2 h, and 220 °C for 4 h.
3.6. Preparation of Thermal Oxidation Aging Samples
The polybenzoxazine resins were polished into standard samples (50 mm × 4 mm × 3 mm) and kept at 250 °C, 300 °C, 325 °C, 350 °C, and 375 °C for 8 h, respectively, weighed and this was repeated 3 times, and the results were averaged.
3.7. Preparation of Wet-Heat Resistance Samples
The polybenzoxazine resins were polished into standard samples (50 mm × 4 mm × 3 mm), kept in ultrapure water at 100 °C for 120 h, weighed every 12 h, and this was repeated 3 times, and the results were averaged.
3.8. Degradation Properties of Polybenzoxazine Resins
The polybenzoxazine resins were cut into small pieces of the same size, and each small piece was similar in mass. They were then placed in environments with different acid concentrations, acid types, organic solvents, temperatures, and degradation times, and then cooled, filtered, dried, and weighed. They were tested 3 times and the average value was taken.
The calculation formula of degradation degree C is shown in Equation (7):
C is the degree of degradation (%); W1 is the initial cured product mass (mg); W2 is the mass of insoluble residue after degradation (mg).
The calculation formula of the degradation rate average value is shown in Equation (8):
v is the degradation rate average value (mg·h−1·mL−1); t is degradation time (h); V is the volume of the solution (mL).
3.8.1. Different Acid Concentrations
The mass of each piece was about 20 mg; DMF: water: hydrochloric acid = 5:2:4 (v/v/v). The concentrations of hydrochloric acid were 0.1 mol/L, 0.5 mol/L, and 1 mol/L and the acid concentrations of the mixed solutions were 0.036 mol/L, 0.182 mol/L, and 0.364 mol/L. They were degraded at 85 °C for 6 h. Because DMF has good solubility in a benzoxazine monomer, DMF was used as a solvent to prepare mixed solutions by changing the acid solution.
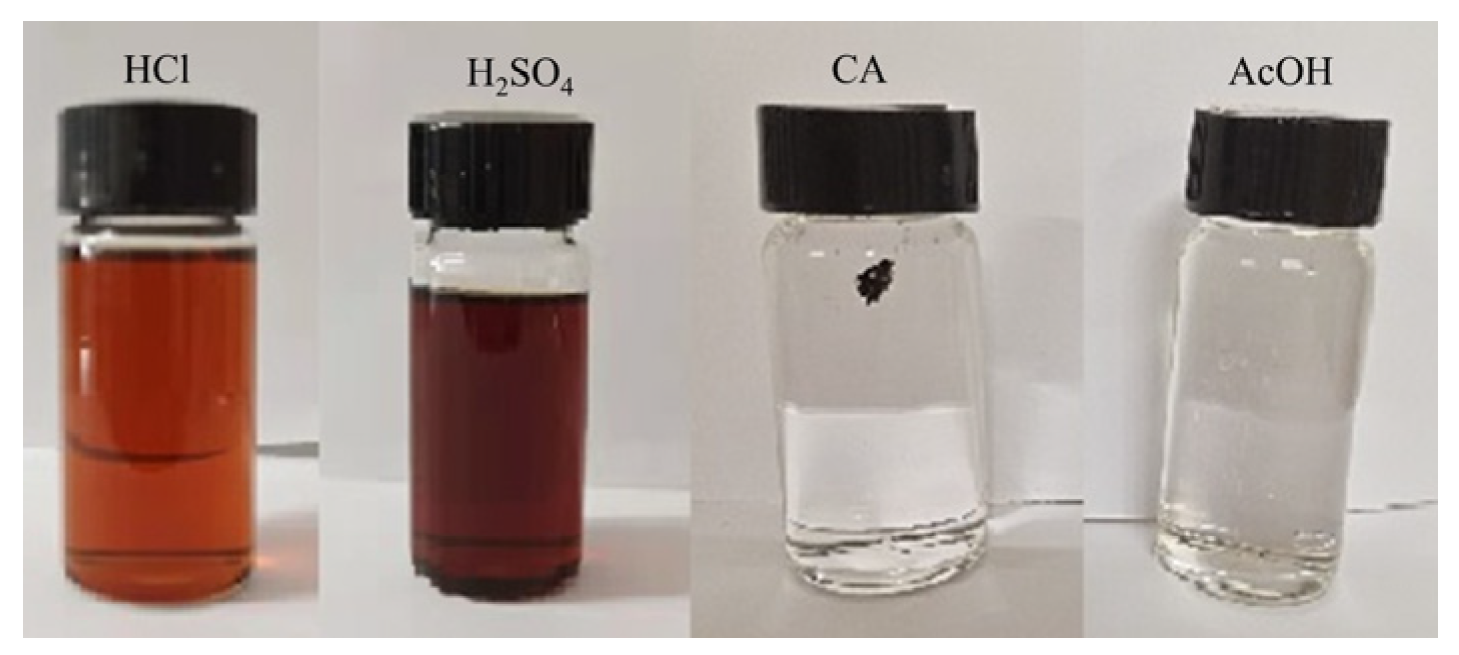
3.8.2. Different Acid Types
The mass of each piece was about 5 mg; DMF: water: 1 mol/L different acid solution = 5:2:4 (v/v/v), and the acids were HCl, H2SO4, anhydrous citric acid CA, and AcOH acetate. The acid concentrations of the mixed solutions were 0.364 mol/L, 0.727 mol/L, 1.091 mol/L, and 0.364 mol/L, which were degraded for 96 h at 55 °C.
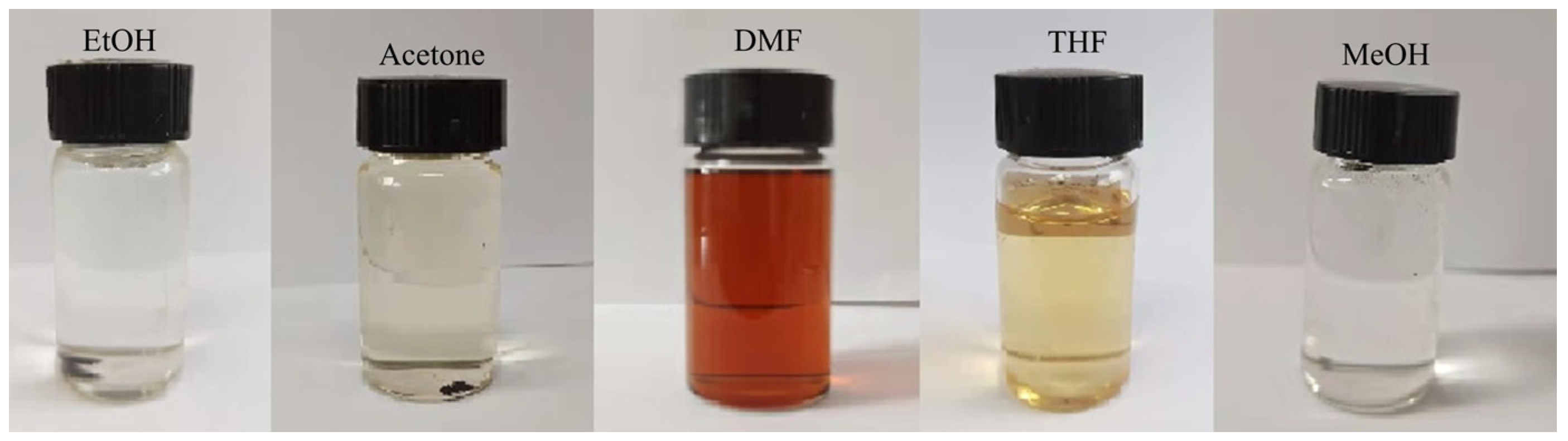
3.8.3. Different Organic Solvents
The mass of each piece was about 10 mg. The organic solvent: water: 1 mol/L hydrochloric acid = 5:2:4 (v/v/v). The organic solvents were ethanol (EtOH), acetone (CP), N,N-dimethylformamide (DMF), tetrahydrofuran (THF), and methanol (MeOH), and the acid concentration of the mixed solutions was 0.364 mol/L. The solutions were degraded for 120 h at 55 °C.
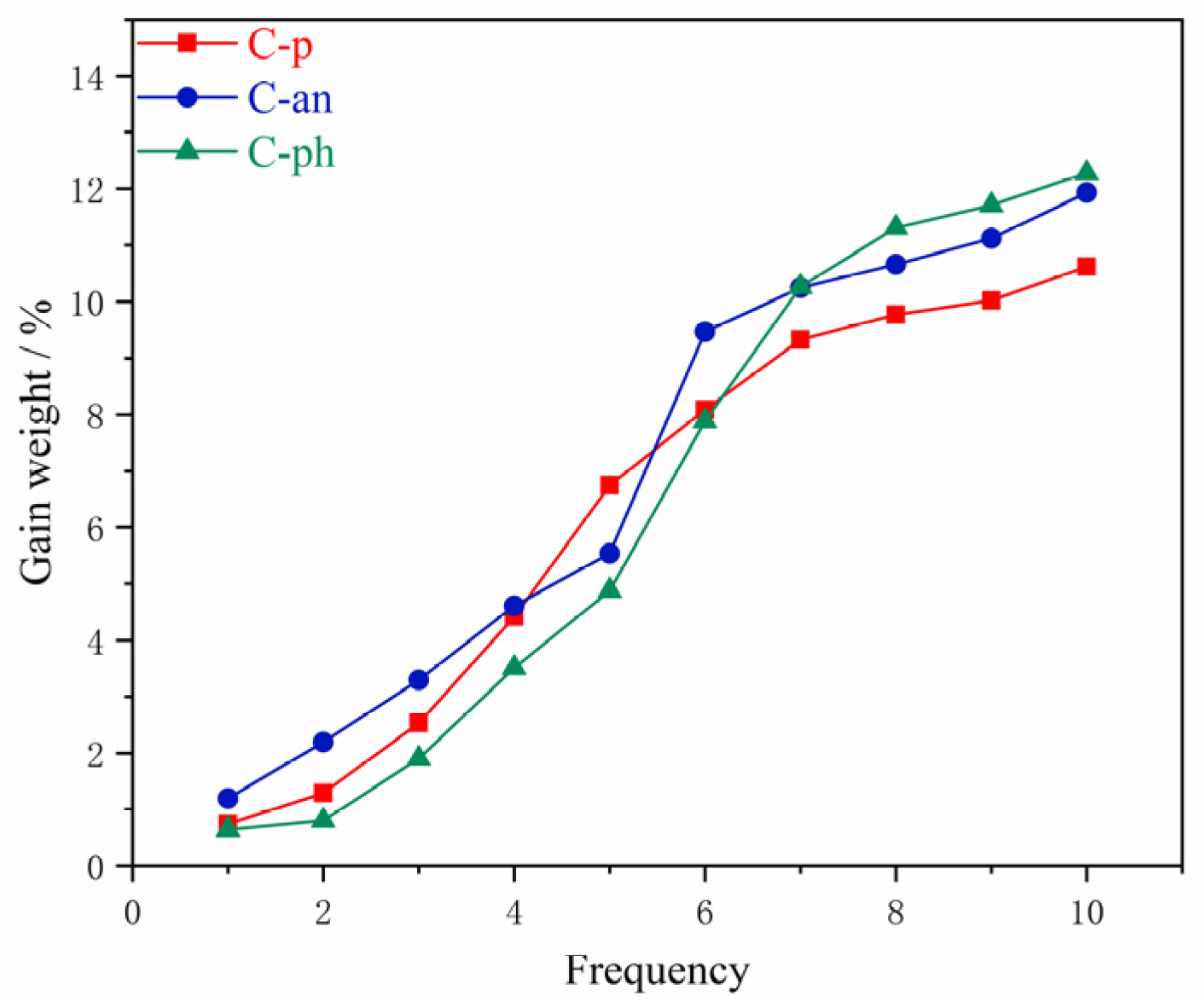
3.8.4. Different Polybenzoxazine Resins
The mass of each piece was about 10 mg; DMF: water: 1mol/L hydrochloric acid = 5:2:4 (v/v/v). The acid concentrations of the mixed solutions were 0.364 mol/L.
3.9. Characterization
Nuclear magnetic resonance (NMR) was performed on a Bruker ADVANCE 400 MHz nuclear magnetic resonance spectrometer using DMSO-d6 as a solvent.
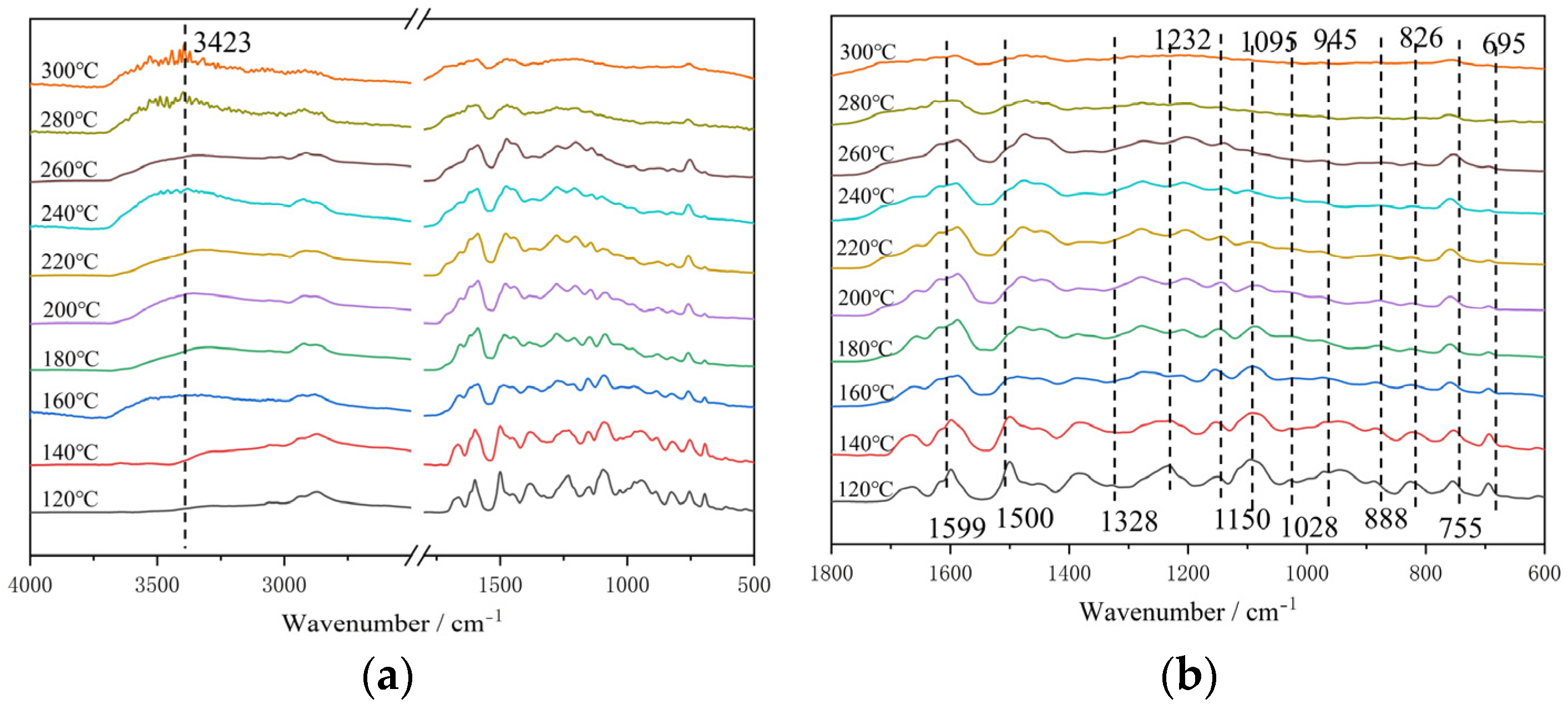
Fourier-transform infrared (FT-IR) spectra were recorded with Nicolet-IS5 using KBr pellets.
Elemental analyses were performed on a Vario EL cube.
Matrix-assisted laser desorption/ionization-time-of-flight (MALDI-TOF) mass spectrometry was conducted on a Bruker Autoflex III time-of-flight mass spectrometer; matrix: α-cyano-4-hydroxycinnamic acid (CCA), ionizing reagent: NaCl, KCl.
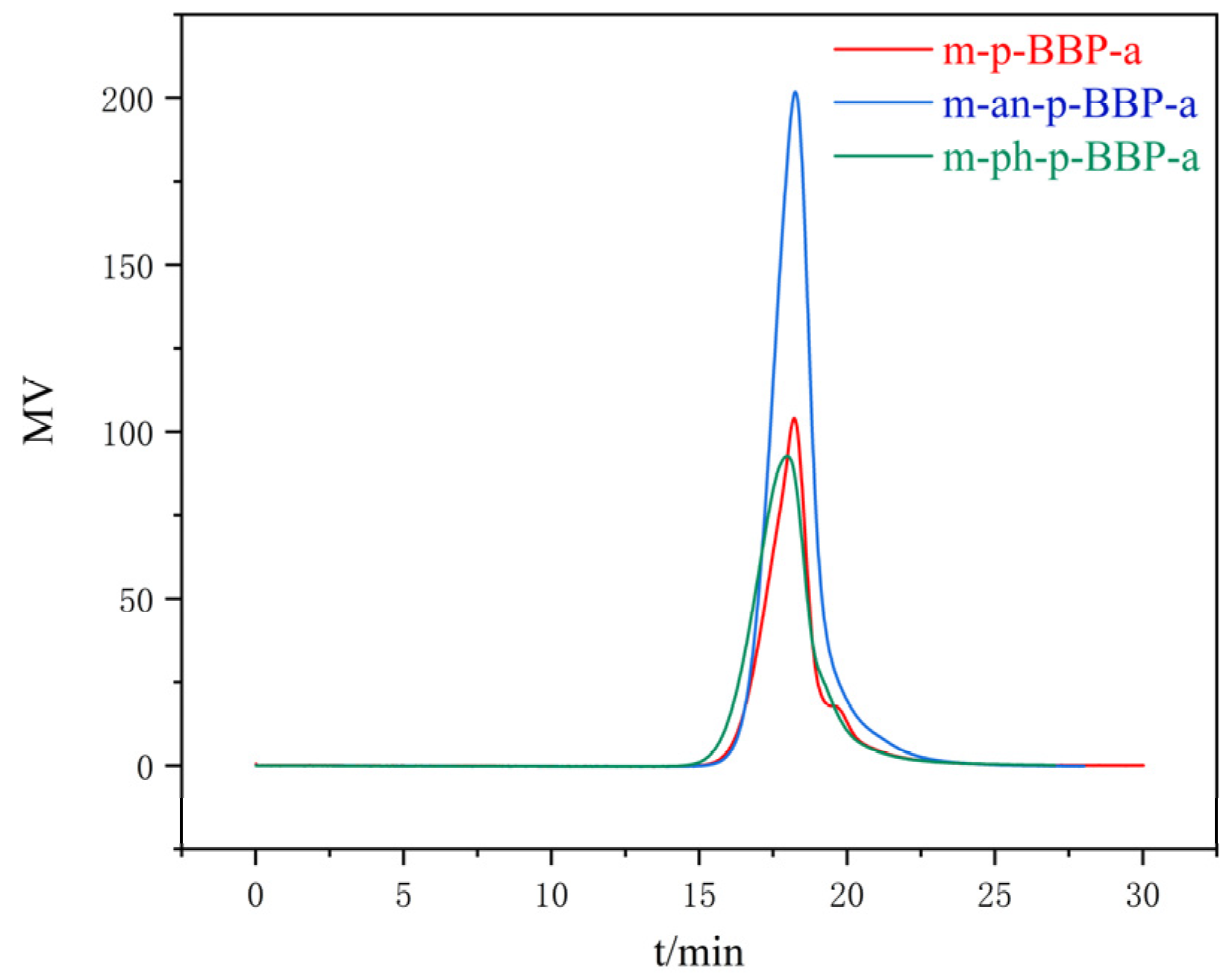
Gel permeation chromatography (GPC) was performed on PL-GPC-220; column temperature: 30 °C, eluant: DMF.
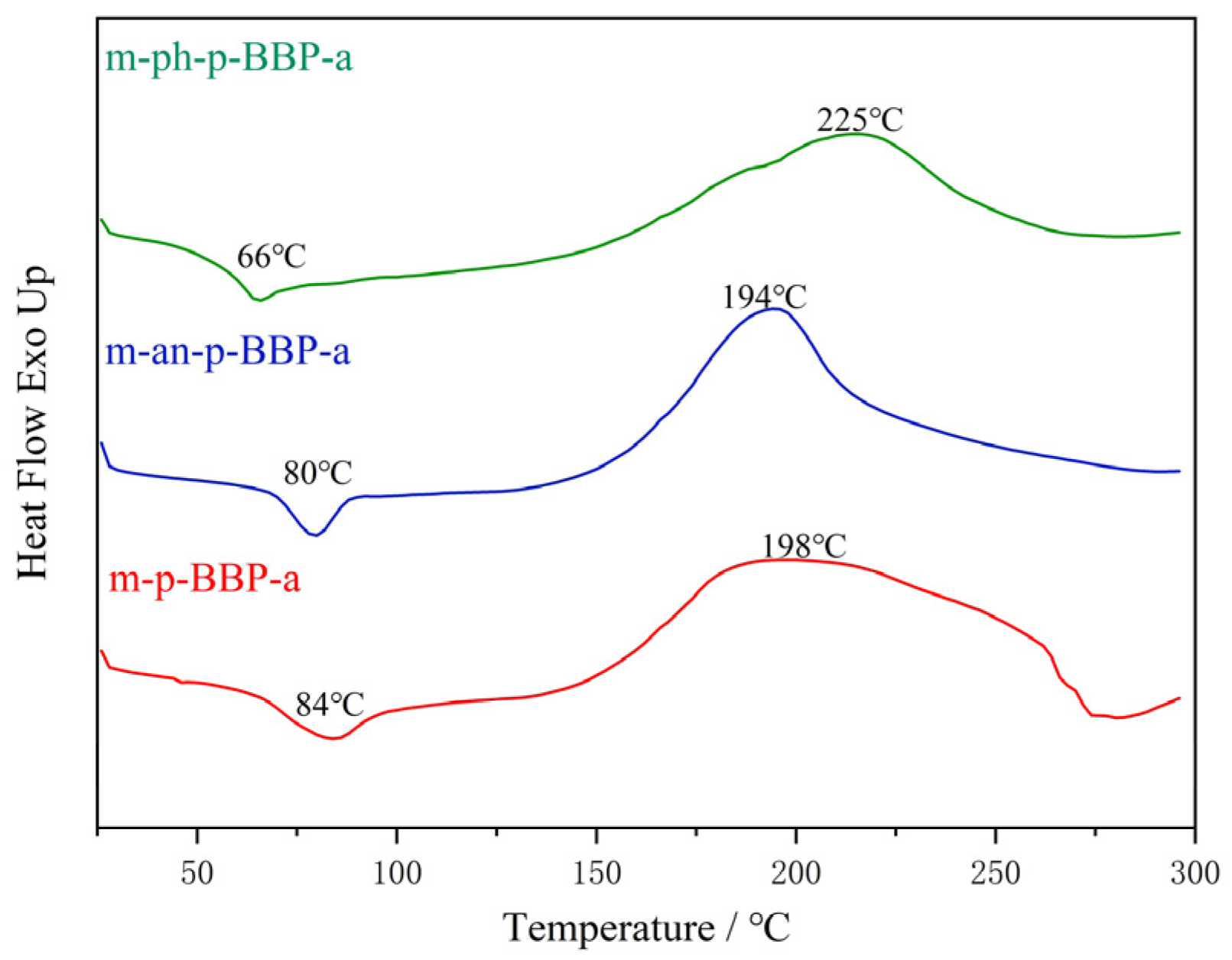
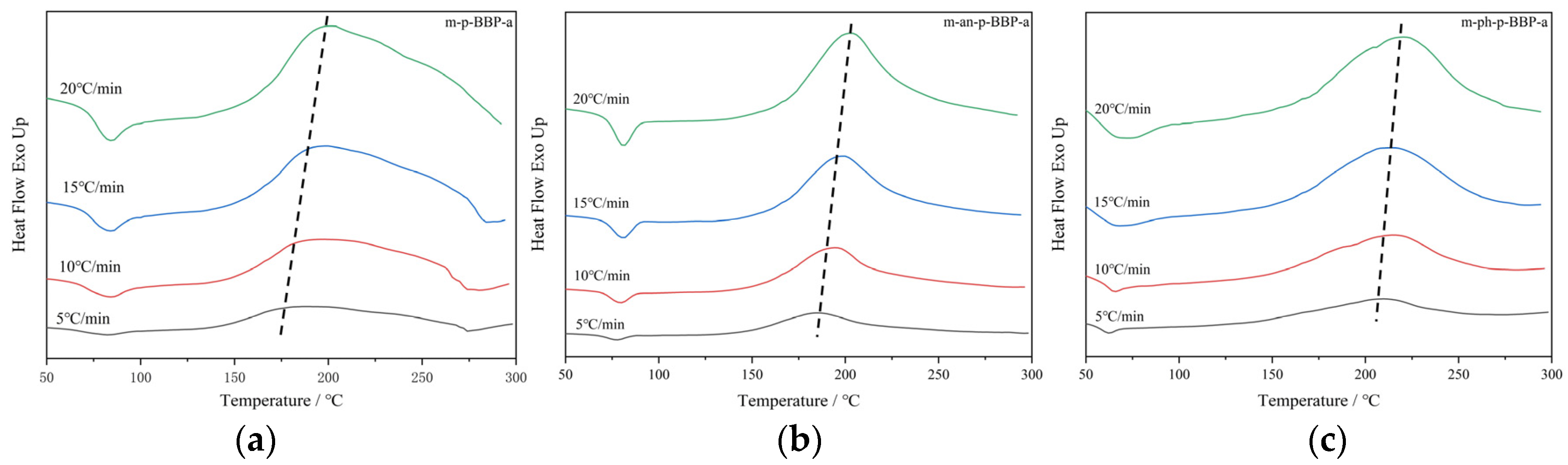
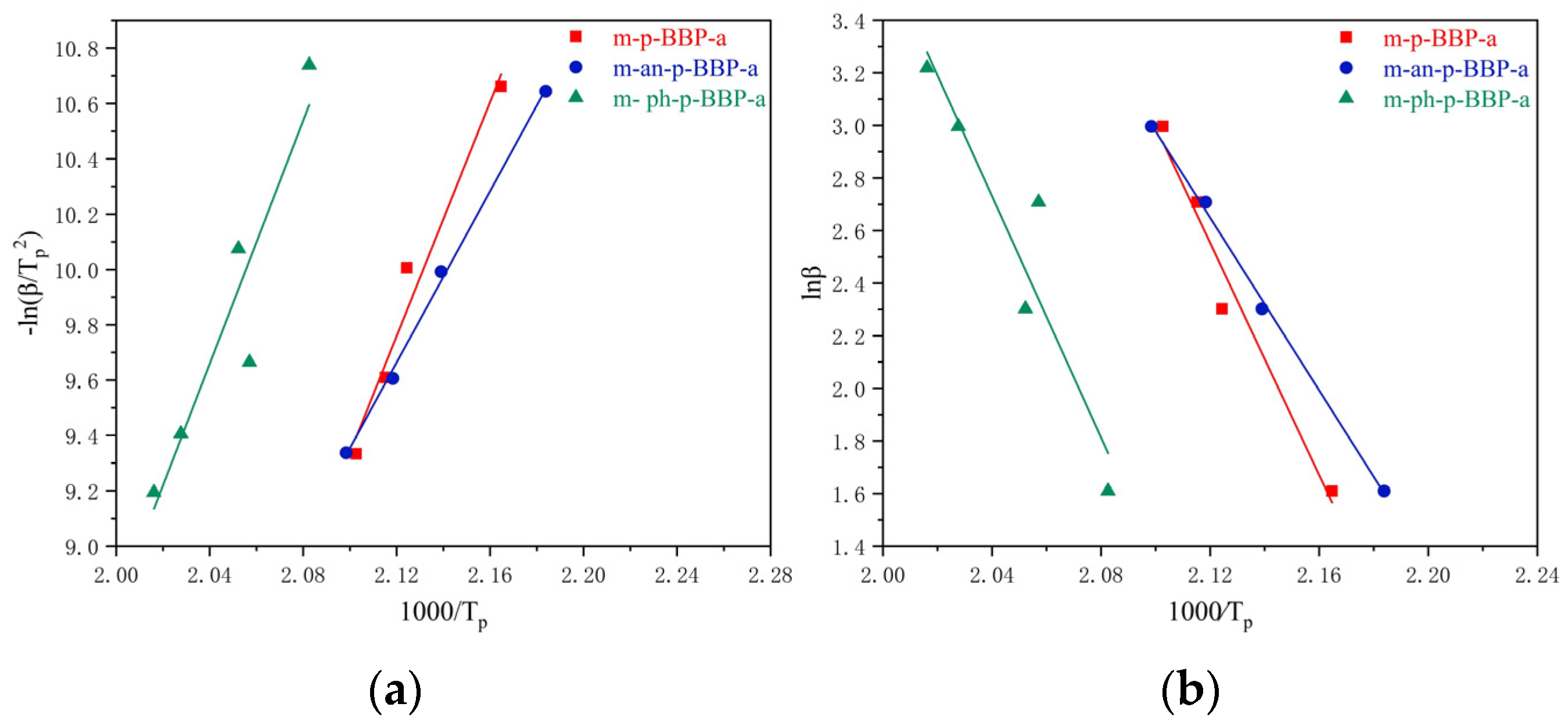
Differential scanning calorimetric (DSC) analysis was performed on Q100 at a heating rate of 10 °C/min under a nitrogen flow rate of 50 mL/min.
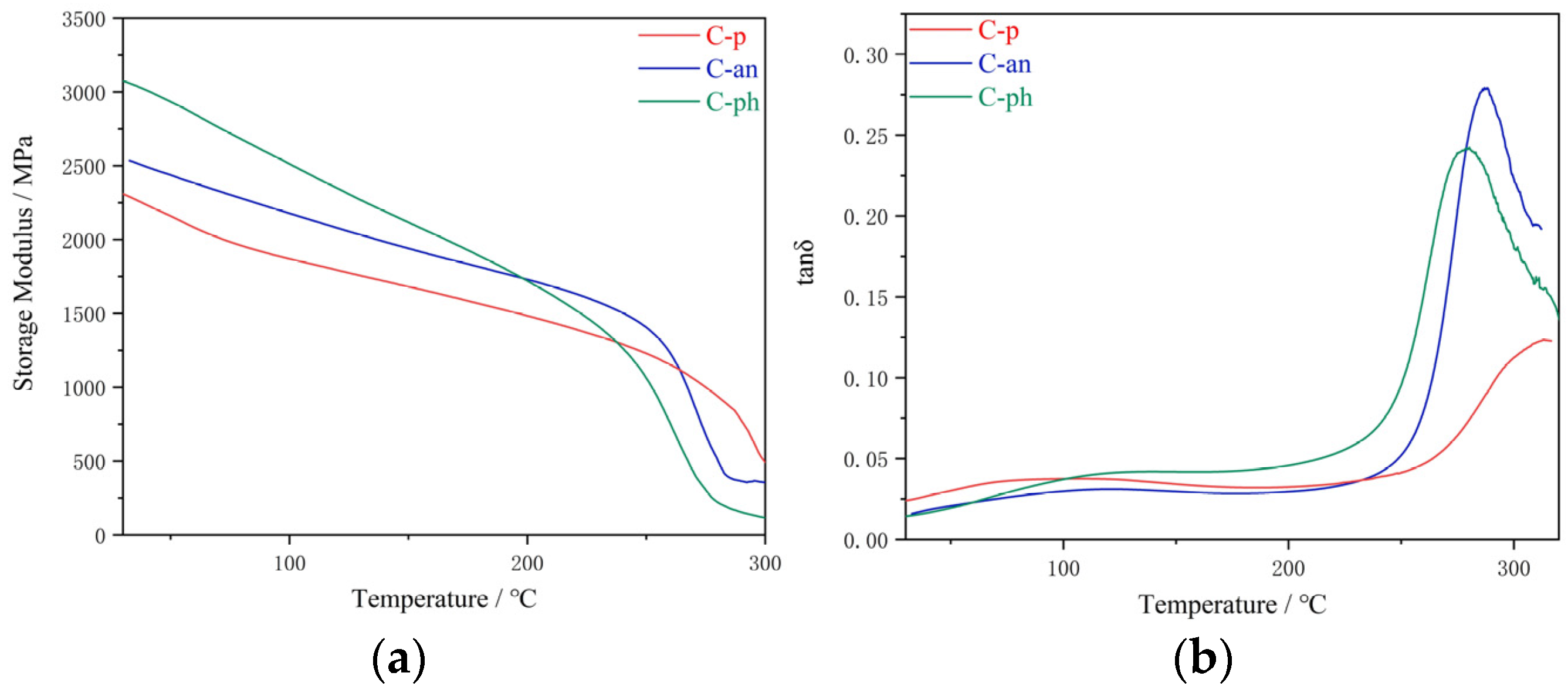
Dynamic mechanical thermal analysis (DMTA) was performed on a Q800 dynamic mechanical analyzer. The measurements were taken under nitrogen in the temperature range of approximately 40–400 °C. A temperature ramp of 5 °C/min was used to determine the storage modulus and damping factor (tan δ) of the material at a frequency of 1 Hz.
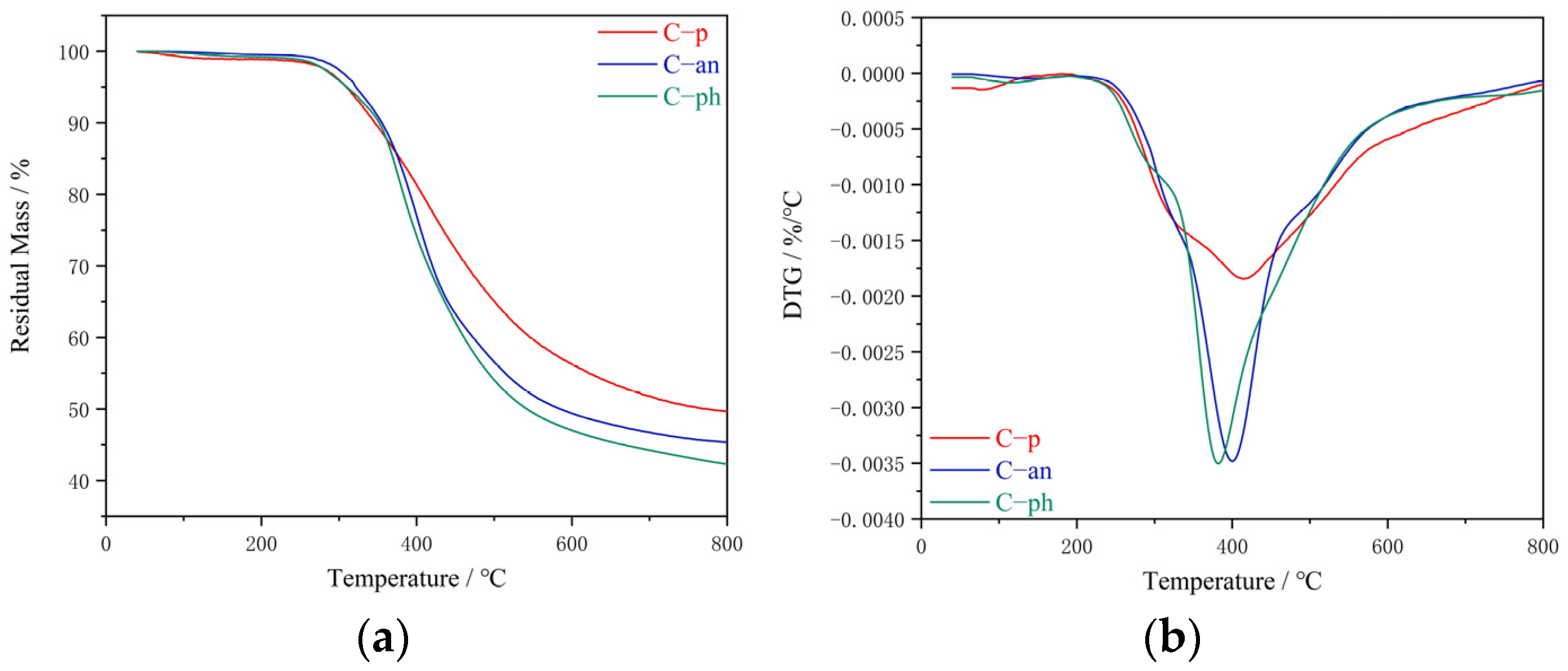
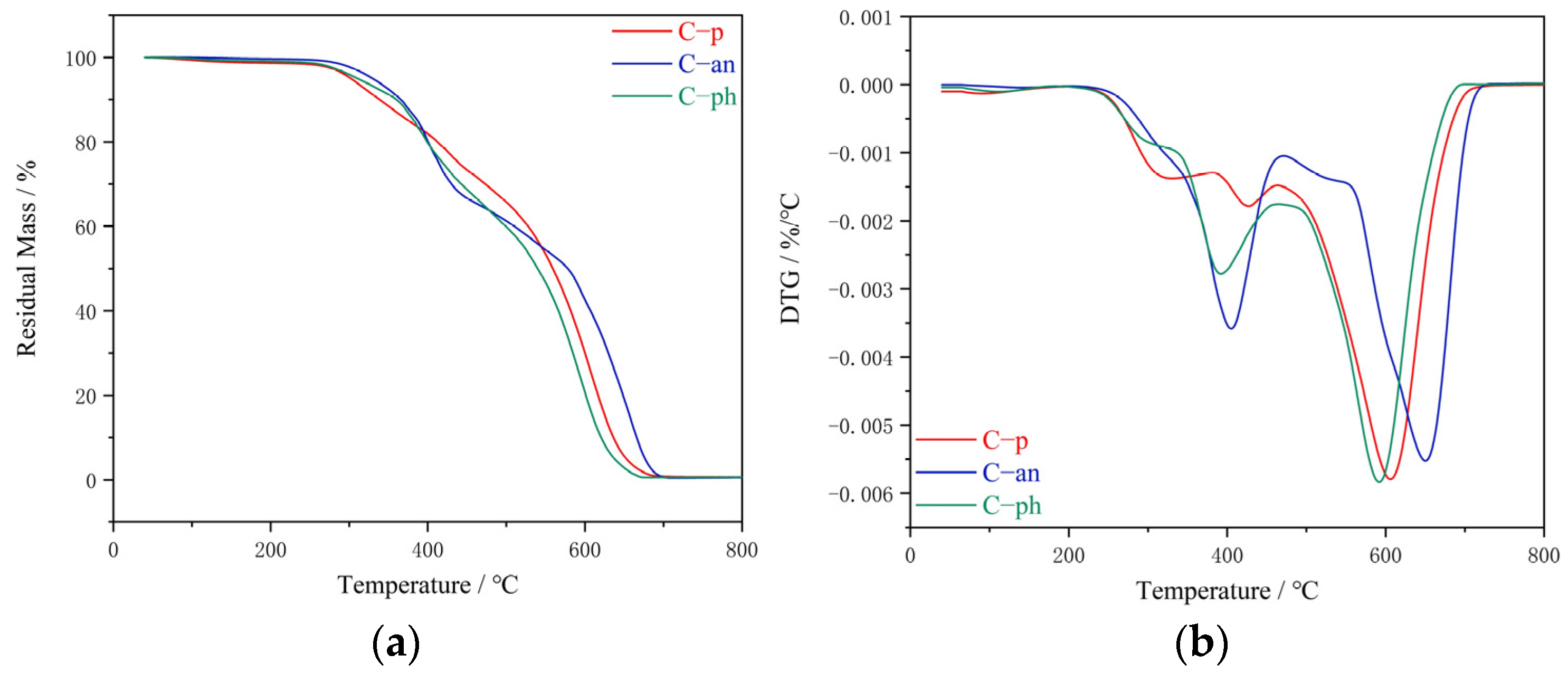
Thermogravimetric analysis (TGA) was performed on TGA Q500 at a heating rate of 10 °C/min under a nitrogen or air purge of 100 mL/min.