Quantitation of Lupinus spp. Quinolizidine Alkaloids by qNMR and Accelerated Debittering with a Resin-Based Protocol
Abstract
1. Introduction
2. Results
2.1. 1H-NMR Peak Assignment
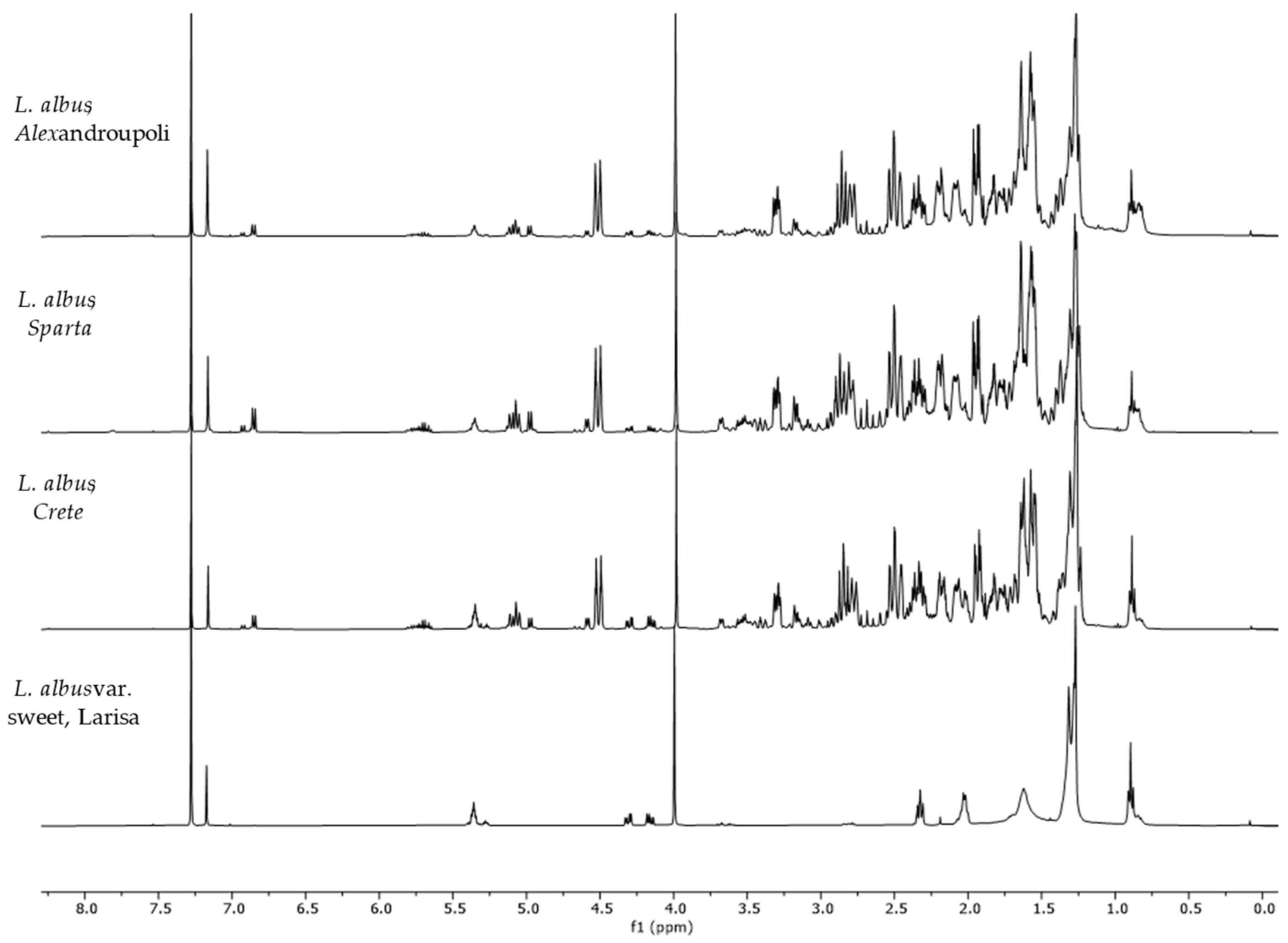
2.2. Differences among Species and Origins
2.3. Differences among Plant Parts
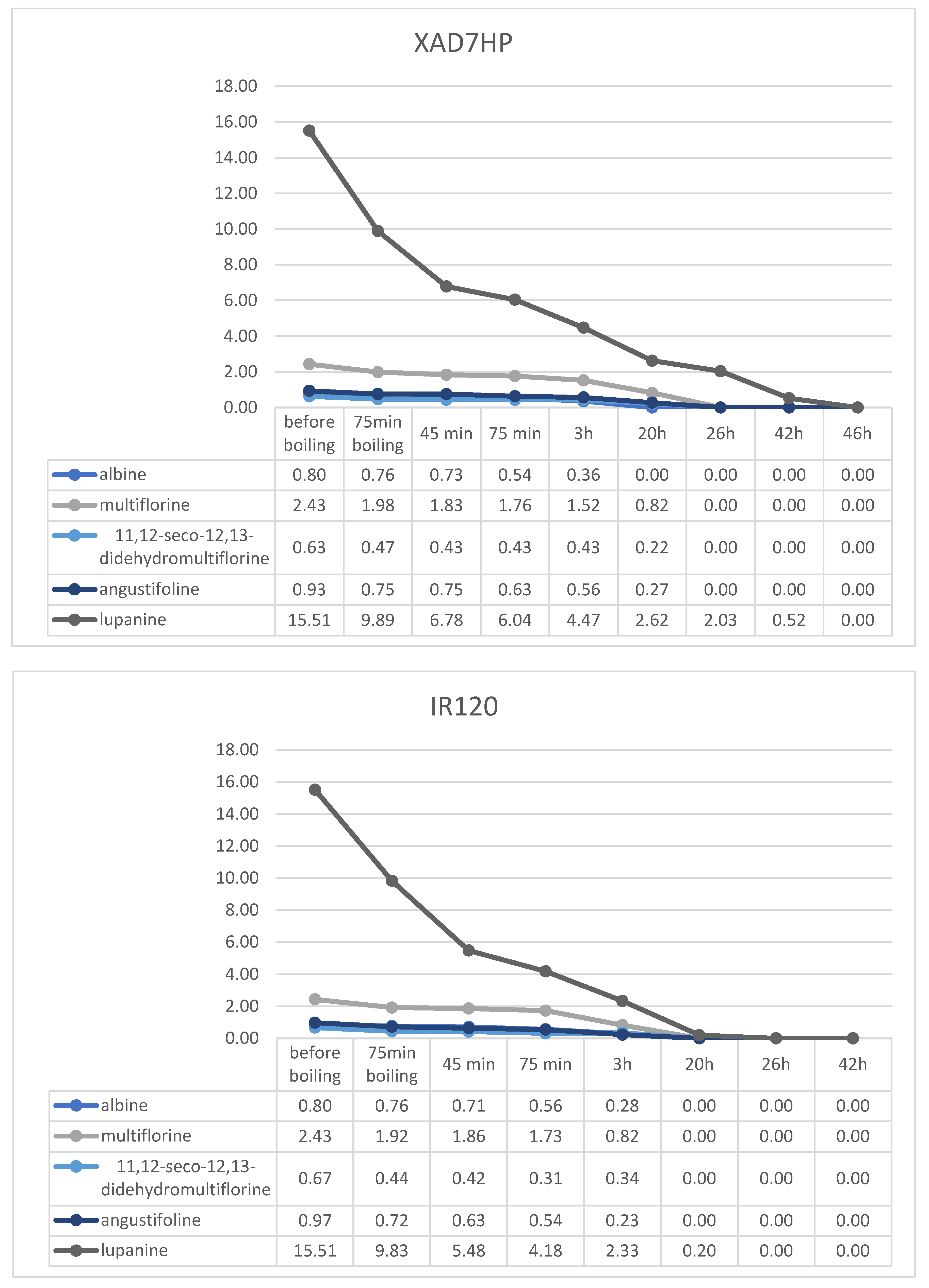
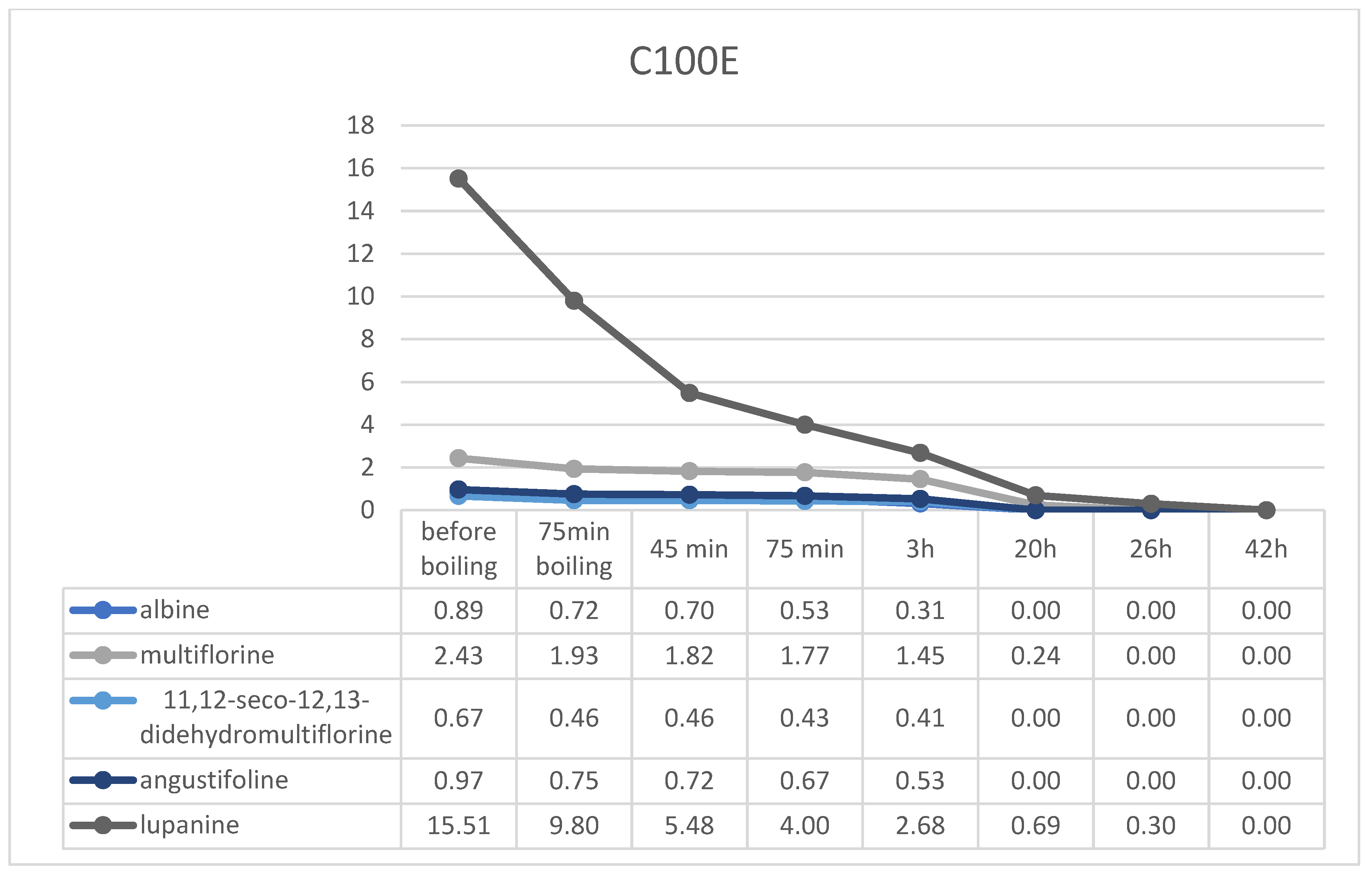
2.4. Resin Debittering Process
2.5. QAs Recovery from Resins
3. Discussion
4. Materials and Methods
4.1. General
4.2. Plant Material and Processed Products
4.3. Quinolizidine Alkaloids Extraction and Chemical Analysis of Plant Material
4.4. 1H-NMR Quantitation
- nis = 0.0005 g/MWsyr = 0.00000274 mol
- nQA = moles of each QA
- IQA = integration ratio between the proton signal of syringaldehyde (IS) and the corresponding proton of the studied analyte
- Iis = integration of internal standard
- MWQA = molecular weight of the studied analyte
- mQA = mass in g of each alkaloid
- a: Coefficient depending on the integration of each peak, integrating one scale of the double peak, and integrating the entire single peak or double with a small range of Hz
- a = 1, for a single peak which integrates for a proton or for a double peak which integrates for two protons when integrating only one scale of the double peak, a = 2, for a double peak which integrates for one proton when integrating only one scale of the double peak, as in the case of multiflorine and 11,12-seco-12,13-didehydromultiflorine
4.5. GC-MS Analysis
4.6. Isolation of Studied Bioactive Quinolizidine Alkaloids
4.7. Resin Debittering Process
Precision
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Käss, E.; Wink, M. Molecular Phylogeny and Phylogeography of Lupinus (Leguminosae) Inferred from Nucleotide Sequences of the Rbc L Gene and ITS 1 + 2 Regions of rDNA. Plant Syst. Evol. 1997, 208, 139–167. [Google Scholar] [CrossRef]
- Wolko, B.; Clements, J.C.; Naganowska, B.; Nelson, M.N.; Yang, H. Lupinus. In Wild Crop Relatives: Genomic and Breeding Resources; Kole, C., Ed.; Springer: Berlin/Heidelberg, Germany, 2011; pp. 153–206. ISBN 978-3-642-14386-1. [Google Scholar]
- Eastwood, R.; Drummond, C.; Schifino-Wittmann, M.; Hughes, C. Diversity and Evolutionary History of Lupins–Insights from New Phylogenies. Int. Lupin Assoc. 2008, 14–18, 346–354. [Google Scholar]
- Smith, C.P. Species Lupinorum; California Botanical Society: Berkeley, CA, USA, 1948. [Google Scholar]
- Wink, M.; Meißner, C.; Witte, L. Patterns of Quinolizidine Alkaloids in 56 Species of the Genus Lupinus. Phytochemistry 1995, 38, 139–153. [Google Scholar] [CrossRef]
- Gresta, F.; Wink, M.; Prins, U.; Abberton, M.; Capraro, J.; Scarafoni, A.; Hill, G. Lupins in European Cropping Systems; CABI: Wallingford, UK, 2017; p. 21. [Google Scholar]
- Reinhard, H.; Rupp, H.; Sager, F.; Streule, M.; Zoller, O. Quinolizidine Alkaloids and Phomopsins in Lupin Seeds and Lupin Containing Food. J. Chromatogr. A 2006, 1112, 353–360. [Google Scholar] [CrossRef] [PubMed]
- Plitmann, U. Evolutionary History of the Old World Lupines. Taxon 1981, 30, 430–437. [Google Scholar] [CrossRef]
- Cowling, W.; Buirchell, B.; Tapia, M. Lupin. Lupinus L. Promoting the Conservation and Use of Underutilized and Neglected Crops; Institute of Plant Genetics and Crop Plant Research, Gatersleben, and International Plant Genetic Resources Institute: Rome, Italy, 1998; ISBN 978-92-9043-372-9. [Google Scholar]
- Khan, M.K.; Karnpanit, W.; Nasar-Abbas, S.M.; Huma, Z.; Jayasena, V. Phytochemical Composition and Bioactivities of Lupin: A Review. Int. J. Food Sci. Technol. 2015, 50, 2004–2012. [Google Scholar] [CrossRef]
- Przybylak, J.K.; Ciesiołka, D.; Wysocka, W.; García-López, P.M.; Ruiz-López, M.A.; Wysocki, W.; Gulewicz, K. Alkaloid Profiles of Mexican Wild Lupin and an Effect of Alkaloid Preparation from Lupinus Exaltatus Seeds on Growth and Yield of Paprika (Capsicum annuum L.). Ind. Crops Prod. 2005, 21, 1–7. [Google Scholar] [CrossRef]
- Boschin, G.; Resta, D. Alkaloids Derived from Lysine: Quinolizidine (a Focus on Lupin Alkaloids). In Natural Products; Ramawat, K.G., Mérillon, J.-M., Eds.; Springer: Berlin/Heidelberg, Germany, 2013; pp. 381–403. ISBN 978-3-642-22143-9. [Google Scholar]
- Cely-Veloza, W.; Quiroga, D.; Coy-Barrera, E. Quinolizidine-Based Variations and Antifungal Activity of Eight Lupinus Species Grown under Greenhouse Conditions. Molecules 2022, 27, 305. [Google Scholar] [CrossRef]
- Brunmair, B.; Lehner, Z.; Stadlbauer, K.; Adorjan, I.; Frobel, K.; Scherer, T.; Luger, A.; Bauer, L.; Fürnsinn, C. 55P0110, a Novel Synthetic Compound Developed from a Plant Derived Backbone Structure, Shows Promising Anti-Hyperglycaemic Activity in Mice. PLoS ONE 2015, 10, e0126847. [Google Scholar] [CrossRef]
- Kubo, H.; Kobayashi, J.; Higashiyama, K.; Kamei, J.; Fujii, Y.; Ohmiya, S. The Hypoglycemic Effect of (7R*,9aS*)-7-Phenyl-Octahydroquinolizin-2-One in Mice. Biol. Pharm. Bull. 2000, 23, 1114–1117. [Google Scholar] [CrossRef][Green Version]
- Kubo, H.; Inoue, M.; Kamei, J.; Higashiyama, K. Hypoglycemic Effects of Multiflorine Derivatives in Normal Mice. Biol. Pharm. Bull. 2006, 29, 2046–2050. [Google Scholar] [CrossRef] [PubMed]
- Lehner, Z.; Stadlbauer, K.; Brunmair, B.; Adorjan, I.; Genov, M.; Kautzky-Willer, A.; Scherer, T.; Scheinin, M.; Bauer, L.; Fürnsinn, C. Evidence That the Multiflorine-derived Substituted Quinazolidine 55P0251 Augments Insulin Secretion and Lowers Blood Glucose via Antagonism at α 2 -adrenoceptors in Mice. Diabetes Obes. Metab. 2020, 22, 290–302. [Google Scholar] [CrossRef] [PubMed]
- Villalpando-Vargas, F.; Medina-Ceja, L. Sparteine as an Anticonvulsant Drug: Evidence and Possible Mechanism of Action. Seizure 2016, 39, 49–55. [Google Scholar] [CrossRef] [PubMed]
- Wiedemann, M.; Gurrola-Díaz, C.; Vargas-Guerrero, B.; Wink, M.; García-López, P.; Düfer, M. Lupanine Improves Glucose Homeostasis by Influencing KATP Channels and Insulin Gene Expression. Molecules 2015, 20, 19085–19100. [Google Scholar] [CrossRef] [PubMed]
- Schrenk, D.; Bodin, L.; Chipman, J.K.; del Mazo, J.; Grasl-Kraupp, B.; Hogstrand, C.; Hoogenboom, L.; Leblanc, J.-C.; Nebbia, C.S.; Nielsen, E.; et al. Scientific Opinion on the Risks for Animal and Human Health Related to the Presence of Quinolizidine Alkaloids in Feed and Food, in Particular in Lupins and Lupin-Derived Products. EFSA J. 2019, 17, e05860. [Google Scholar] [CrossRef] [PubMed]
- Ofori, S.; Lu, C.; Olasupo, O.O.; Dennis, B.B.; Fairbairn, N.; Devereaux, P.J.; Mbuagbaw, L. Cytisine for Smoking Cessation: A Systematic Review and Meta-Analysis. Drug Alcohol Depend. 2023, 251, 110936. [Google Scholar] [CrossRef] [PubMed]
- Berlinck, R.; Kossuga, M. Modern Alkaloids: Structure, Isolation, Synthesis and Biology. In Modern Alkaloids: Structure, Isolation, Synthesis and Biology; Wiley: Hoboken, NJ, USA, 2007; pp. 305–337. ISBN 978-3-527-62107-1. [Google Scholar]
- Aniszewski, T. Alkaloids Chemistry, Biology, Ecology, and Applications, 2nd ed.; Elsevier: Amsterdam, The Netherlands, 2015; ISBN 978-0-444-59433-4. [Google Scholar]
- Wysocka, W.; Brukwicki, T.; Włodarczak, J. Effect of Substituents and Structural Modification on Conformational Equilibrium in Bis-Quinolizidine System. J. Mol. Struct. 2012, 1017, 123–134. [Google Scholar] [CrossRef]
- Carvajal-Larenas, F.E.; Nout, M.J.R.; van Boekel, M.A.J.S.; Koziol, M.; Linnemann, A.R. Modelling of the Aqueous Debittering Process of Lupinus Mutabilis Sweet. LWT Food Sci. Technol. 2013, 53, 507–516. [Google Scholar] [CrossRef]
- Cortés-Avendaño, P.; Tarvainen, M.; Suomela, J.-P.; Glorio-Paulet, P.; Yang, B.; Repo-Carrasco-Valencia, R. Profile and Content of Residual Alkaloids in Ten Ecotypes of Lupinus Mutabilis Sweet after Aqueous Debittering Process. Plant Foods Hum. Nutr. 2020, 75, 184–191. [Google Scholar] [CrossRef]
- Yaver, E.; Bilgiçli, N. Effect of Ultrasound-Accelerated Debittering Method on Total Alkaloid and Total Carotenoid Content of Lupin Seeds (Lupinus albus L.) and Storage Stability of Thermally Treated Lupin Flours. Food Meas. 2023, 17, 3378–3389. [Google Scholar] [CrossRef]
- Magalhães, S.C.Q.; Fernandes, F.; Cabrita, A.R.J.; Fonseca, A.J.M.; Valentão, P.; Andrade, P.B. Alkaloids in the Valorization of European Lupinus Spp. Seeds Crop. Ind. Crops Prod. 2017, 95, 286–295. [Google Scholar] [CrossRef]
- Lee, H.-W.; Hwang, I.-M.; Lee, H.M.; Yang, J.-S.; Park, E.J.; Choi, J.W.; Seo, H.Y.; Kim, S.H. Validation and Determination of Quinolizidine Alkaloids (QAs) in Lupin Products by Gas Chromatography with Flame Ionization Detection (GC-FID). Anal. Lett. 2020, 53, 606–613. [Google Scholar] [CrossRef]
- Boschin, G.; Annicchiarico, P.; Resta, D.; D’Agostina, A.; Arnoldi, A. Quinolizidine Alkaloids in Seeds of Lupin Genotypes of Different Origins. J. Agric. Food Chem. 2008, 56, 3657–3663. [Google Scholar] [CrossRef] [PubMed]
- Resta, D.; Boschin, G.; D’Agostina, A.; Arnoldi, A. Evaluation of Total Quinolizidine Alkaloids Content in Lupin Flours, Lupin-Based Ingredients, and Foods. Mol. Nutr. Food Res. 2008, 52, 490–495. [Google Scholar] [CrossRef] [PubMed]
- Annicchiarico, P.; Manunza, P.; Arnoldi, A.; Boschin, G. Quality of Lupinus albus L. (White Lupin) Seed: Extent of Genotypic and Environmental Effects. J. Agric. Food Chem. 2014, 62, 6539–6545. [Google Scholar] [CrossRef] [PubMed]
- Romeo, F.; Fabroni, S.; Ballistreri, G.; Muccilli, S.; Spina, A.; Rapisarda, P. Characterization and Antimicrobial Activity of Alkaloid Extracts from Seeds of Different Genotypes of Lupinus spp. Sustainability 2018, 10, 788. [Google Scholar] [CrossRef]
- Chludil, H.D.; Vilariño, M.D.P.; Franco, M.L.; Leicach, S.R. Changes in Lupinus Albus and Lupinus Angustifolius Alkaloid Profiles in Response to Mechanical Damage. J. Agric. Food Chem. 2009, 57, 6107–6113. [Google Scholar] [CrossRef] [PubMed]
- Eugelio, F.; Palmieri, S.; Fanti, F.; Messuri, L.; Pepe, A.; Compagnone, D.; Sergi, M. Development of an HPLC-MS/MS Method for the Determination of Alkaloids in Lupins. Molecules 2023, 28, 1531. [Google Scholar] [CrossRef]
- Khedr, T.; Juhász, A.; Singh, K.B.; Foley, R.; Nye-Wood, M.G.; Colgrave, M.L. Development and Validation of a Rapid and Sensitive LC-MS/MS Approach for Alkaloid Testing in Different Lupinus Species. J. Food Compos. Anal. 2023, 121, 105391. [Google Scholar] [CrossRef]
- Hwang, I.M.; Lee, H.-W.; Lee, H.M.; Yang, J.-S.; Seo, H.Y.; Chung, Y.-J.; Kim, S.H. Rapid and Simultaneous Quantification of Five Quinolizidine Alkaloids in Lupinus angustifolius L. and Its Processed Foods by UPLC–MS/MS. ACS Omega 2020, 5, 20825–20830. [Google Scholar] [CrossRef]
- Święcick, W.; Czepiel, K.; Wilczura, P.; Barzyk, P.; Kaczmarek, Z.; Kroc, M. Chromatographic Fingerprinting of the Old World Lupins Seed Alkaloids: A Supplemental Tool in Species Discrimination. Plants 2019, 8, 548. [Google Scholar] [CrossRef] [PubMed]
- Ganzera, M.; Krüger, A.; Wink, M. Determination of Quinolizidine Alkaloids in Different Lupinus Species by NACE Using UV and MS Detection. J. Pharm. Biomed. Anal. 2010, 53, 1231–1235. [Google Scholar] [CrossRef] [PubMed]
- Gremigni, P.; Wong, M.T.F.; Edwards, N.K.; Harris, D.; Hamblin, J. Potassium Nutrition Effects on Seed Alkaloid Concentrations, Yield and Mineral Content of Lupins (Lupinus angustifolius). Plant Soil 2001, 234, 131–142. [Google Scholar] [CrossRef]
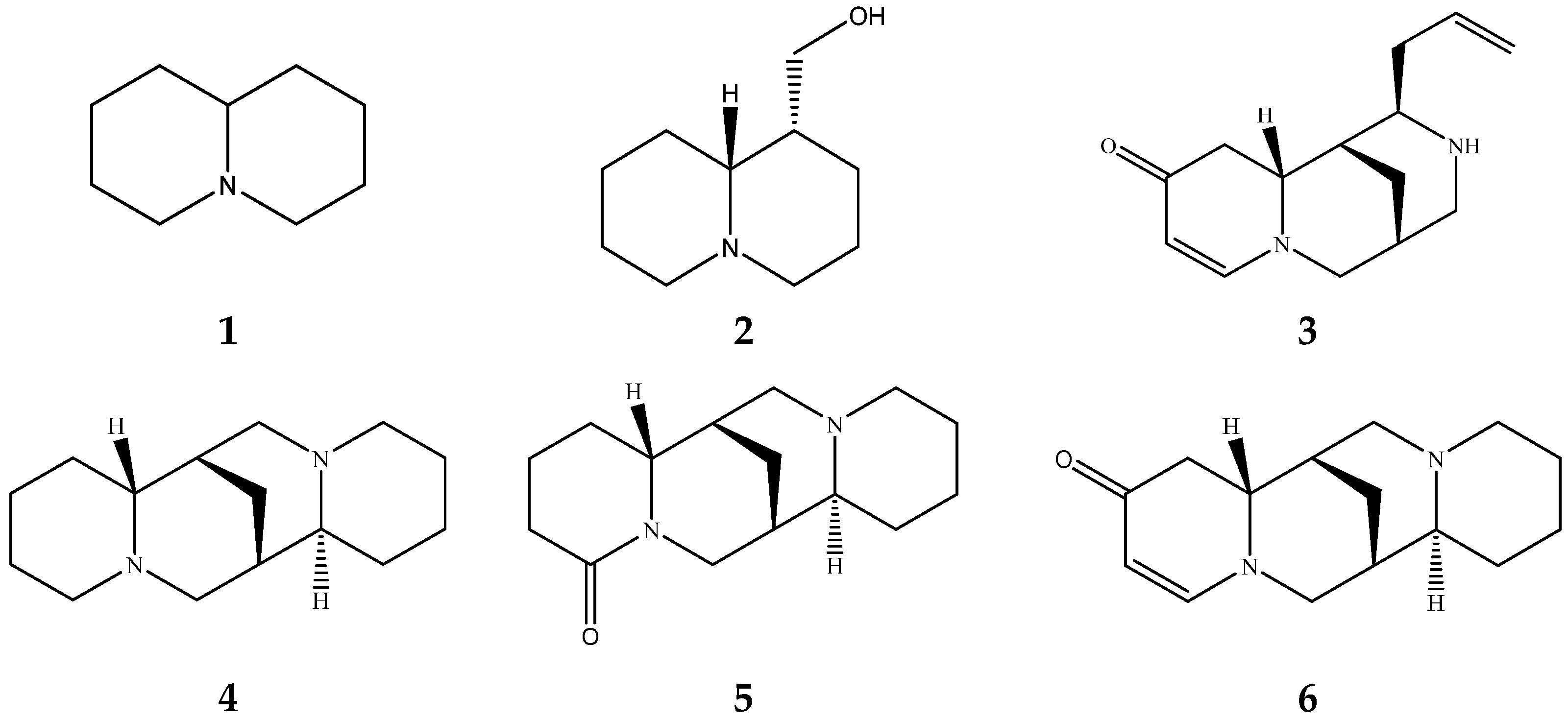



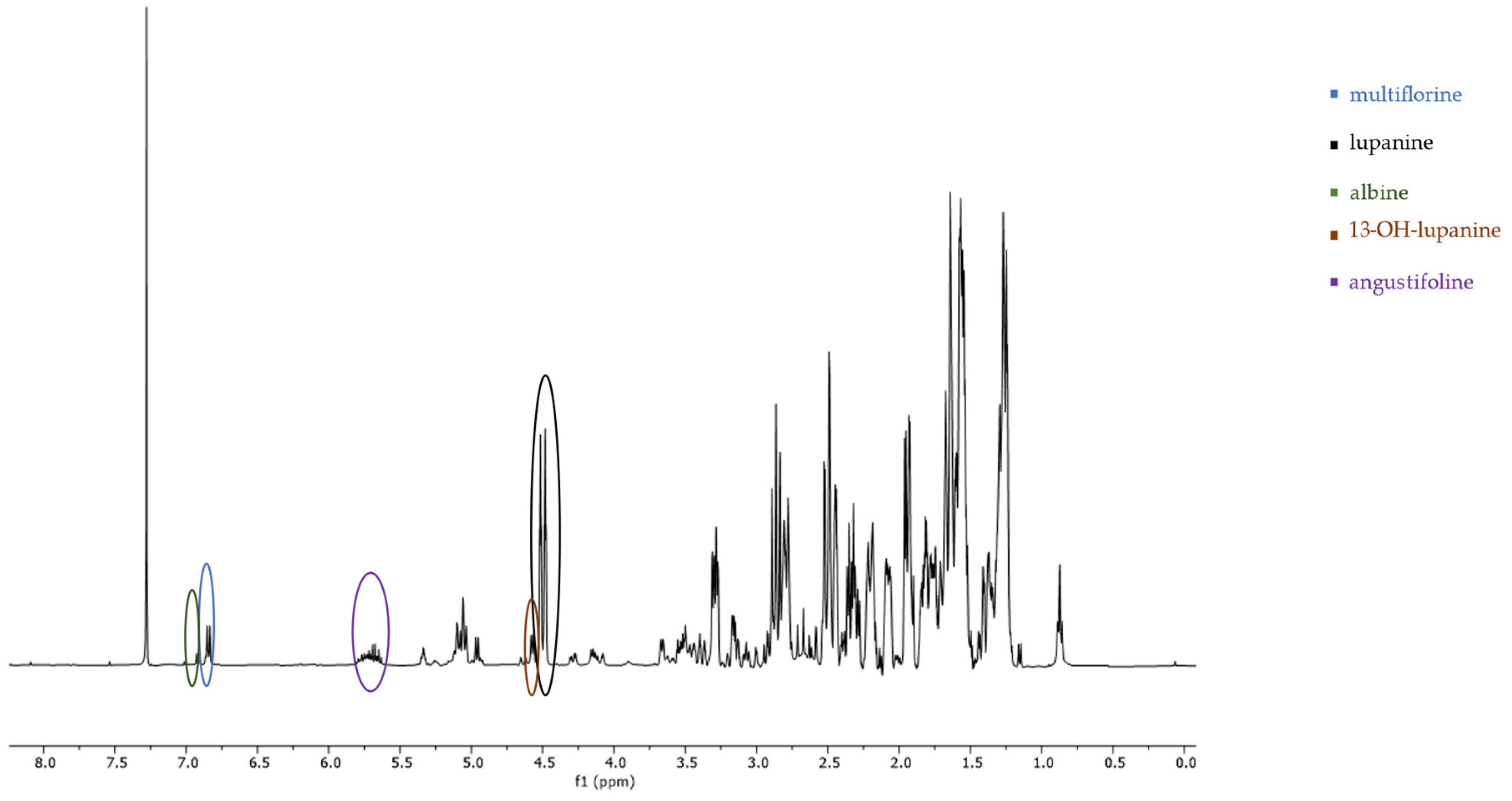
| Quinolizidine Alkaloid | Structure | Proton Signal | δ in ppm |
|---|---|---|---|
| Lupanine | 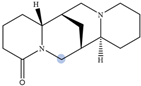 | H10 eq | 4.49 |
| 13-OH-lupanine |  | H10 eq | 4.58 |
| Sparteine | 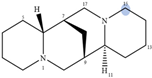 | H15 eq | 2.80 |
| Multiflorine | 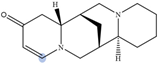 | H2 | 6.84 |
| 11,12-seco-12,13-didehydromultiflorine | 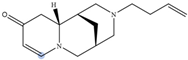 | H2 | 6.87 |
| Angustifoline | 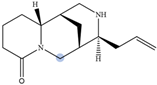 | H10 eq | 4.66 |
| Albine | 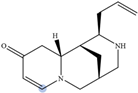 | H2 | 6.92 |
| Lupinine | 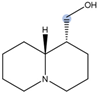 | H11 | 4.12 |
| Species (Origin) | Lupanine | Multiflorine | Albine | Angustifoline | ||||
|---|---|---|---|---|---|---|---|---|
| Mean | S.D. | Mean | S.D. | Mean | S.D. | Mean | S.D. | |
| L. pilosus, Mt. Athos | nd | nd | 6.83 | 0.09 | nd | 0.00 | nd | nd |
| L. pilosus, Crete | 5.81 | 0.12 | 2.33 | 0.06 | 0.15 | 0.01 | 0.13 | 0.01 |
| L. albus, Sparta | 22.55 | 0.36 | 2.49 | 0.05 | 1.31 | 0.09 | 0.74 | 0.03 |
| L. albus, Alexandroupoli | 12.17 | 0.22 | 1.16 | 0.06 | 0.37 | 0.02 | 0.30 | 0.02 |
| L. albus, Crete | 15.51 | 0.18 | 2.43 | 0.17 | 0.80 | 0.03 | 0.97 | 0.06 |
| L. albus var. sweet, Larisa | nd | nd | 0.16 | 0.01 | nd | 0.00 | nd | nd |
| L. angustifolius | 3.16 | 0.23 | nd | nd | nd | nd | 1.00 | 0.02 |
| L. mutabilis | 7.33 | 0.47 | nd | nd | nd | nd | nd | nd |
| L. elegans | 4.44 | 0.05 | nd | nd | nd | nd | nd | nd |
| L. luteus | nd | nd | nd | nd | nd | nd | nd | nd |
| L. perennis | 5.60 | 0.29 | nd | nd | nd | nd | 2.22 | 0.04 |
| L. nanus | 3.90 | 0.22 | nd | nd | nd | nd | nd | nd |
| L. polyphyllus | 6.82 | 0.37 | nd | nd | nd | nd | 0.78 | 0.03 |
| L. hartwegii | 4.57 | 0.17 | nd | nd | nd | nd | nd | nd |
| L. cruckshankii | 2.93 | 0.13 | nd | nd | nd | nd | nd | nd |
| Species (Origin) | 13-OH-Lupanine | 11,12-seco-12,13 Didehydromultiflorine | Lupinine | Sparteine | ||||
| Mean | S.D. | Mean | S.D. | Mean | S.D. | Mean | S.D. | |
| L. pilosus, Mt. Athos | nd | nd | nd | nd | 0.23 | 0.01 | nd | nd |
| L. pilosus, Crete | 0.16 | 0.01 | 0.16 | 0.01 | nd | nd | nd | nd |
| L. albus, Sparta | 2.75 | 0.07 | 0.55 | 0.02 | nd | nd | nd | nd |
| L. albus, Alexandroupoli | 0.86 | 0.05 | 0.28 | 0.01 | nd | nd | nd | nd |
| L. albus, Crete | 2.73 | 0.06 | 0.62 | 0.02 | nd | nd | nd | nd |
| L. albus var. sweet, Larisa | nd | nd | nd | nd | nd | nd | nd | nd |
| L. angustifolius | nd | nd | nd | nd | nd | nd | nd | nd |
| L. mutabilis | 0.23 | 0.01 | nd | nd | nd | nd | 6.92 | 0.22 |
| L. elegans | nd | nd | nd | nd | nd | nd | ov | nd |
| L. luteus | nd | nd | nd | nd | nd | nd | nd | nd |
| L. perennis | 0.53 | 0.04 | nd | nd | nd | nd | nd | nd |
| L. nanus | nd | nd | nd | nd | nd | nd | ov | nd |
| L. polyphyllus | 0.37 | 0.02 | nd | nd | nd | nd | nd | nd |
| L. hartwegii | nd | nd | nd | nd | nd | nd | ov | nd |
| L. cruckshankii | nd | nd | nd | nd | nd | nd | 2.43 | 0.18 |
| Plant Part | Lupanine | Multiflorine | Albine | |||
|---|---|---|---|---|---|---|
| Mean | S.D. | Mean | S.D. | Mean | S.D. | |
| flower | nd | 0.00 | 7.21 | 0.09 | nd | 0.00 |
| leaf | 0.12 | 0.01 | 3.59 | 0.11 | nd | 0.00 |
| pod | 3.56 | 0.12 | 6.12 | 0.11 | 0.13 | 0.01 |
| seeds | 5.81 | 0.12 | 2.33 | 0.06 | 0.15 | 0.01 |
| stem | 1.02 | 0.07 | nd | 0.00 | nd | 0.00 |
| Plant Part | Angustifoline | 13-OH-Lupanine | 11,12-seco-12,13 Didehydromultiflorine | |||
| Mean | S.D. | Mean | S.D. | Mean | S.D. | |
| flower | nd | 0.00 | nd | 0.00 | nd | 0.00 |
| leaf | nd | 0.00 | nd | 0.00 | nd | 0.00 |
| pod | 0.20 | 0.01 | 0.12 | 0.01 | 0.18 | 0.01 |
| seeds | 0.13 | 0.01 | 0.16 | 0.01 | 0.16 | 0.01 |
| stem | nd | 0.00 | nd | 0.00 | nd | 0.00 |
| Species | Origin | Series | Plant Part |
|---|---|---|---|
| L. albus | Crete | wild | seeds |
| L. albus | Alexandroupoli | wild | seeds |
| L. albus | Sparta | wild | seeds |
| L. albus var. sweet | Larisa | cultivated | seeds |
| L. pilosus | Rethymn, Crete | wild | flower, leaf, pod, steam, seeds |
| L. pilosus | Mt. Athos, Northeastern Greece | wild | seeds, leaf |
| L. angustifolius | England | cultivated | seeds |
| L. cruckshankii | England | ‘Sunrise,’ Paysons lupin, Paradox lupine | seeds |
| L. elegans | England | ‘Pink Fairy,’ Mexico lupin | seeds |
| L. hartwegii | England | ‘Avalune Red White,’ Dwarf lupin | seeds |
| L. mutabilis var. cruckshankii | England | ‘Javelin White’ | seeds |
| L. nanus | England | ‘Snow Pixie,’ Dwarf lupin, Sky lupin | seeds |
| L. perennis | Mexico | wild | seeds |
| L. polyphyllus | England | ‘Band of Nobles’ series, ‘Noble Maiden’ | seeds |
| L. luteus | England | cultivated | seeds |
| Resin Type | Resin Characteristics |
|---|---|
| Amberlite® XAD7HP, 20–60 mesh (St. Louis, MO, USA) | nonionic, aliphatic acrylic polymer |
| Amberlite® IRC120 H, hydrogen form (St. Louis, MO, USA) | strongly acidic cation exchange resin |
| Purolite® C100E, Ionic Form (Na+ form) (St. Louis, MO, USA) | Polystyrenic Gel, Strong Acid Cation Resin |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Madelou, N.A.; Melliou, E.; Magiatis, P. Quantitation of Lupinus spp. Quinolizidine Alkaloids by qNMR and Accelerated Debittering with a Resin-Based Protocol. Molecules 2024, 29, 582. https://doi.org/10.3390/molecules29030582
Madelou NA, Melliou E, Magiatis P. Quantitation of Lupinus spp. Quinolizidine Alkaloids by qNMR and Accelerated Debittering with a Resin-Based Protocol. Molecules. 2024; 29(3):582. https://doi.org/10.3390/molecules29030582
Chicago/Turabian StyleMadelou, Nikoleta Anna, Eleni Melliou, and Prokopios Magiatis. 2024. "Quantitation of Lupinus spp. Quinolizidine Alkaloids by qNMR and Accelerated Debittering with a Resin-Based Protocol" Molecules 29, no. 3: 582. https://doi.org/10.3390/molecules29030582
APA StyleMadelou, N. A., Melliou, E., & Magiatis, P. (2024). Quantitation of Lupinus spp. Quinolizidine Alkaloids by qNMR and Accelerated Debittering with a Resin-Based Protocol. Molecules, 29(3), 582. https://doi.org/10.3390/molecules29030582







