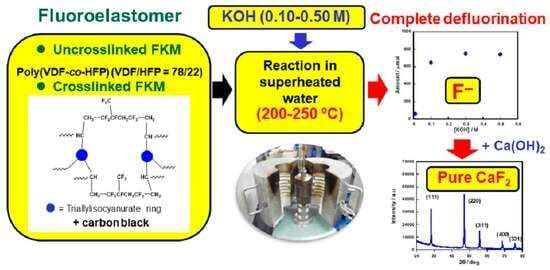
Efficient Mineralization of Fluoroelastomers Using Superheated Water in the Presence of Potassium Hydroxide
Abstract
:1. Introduction
2. Results and Discussion
2.1. Decomposition of Uncrosslinked FKM
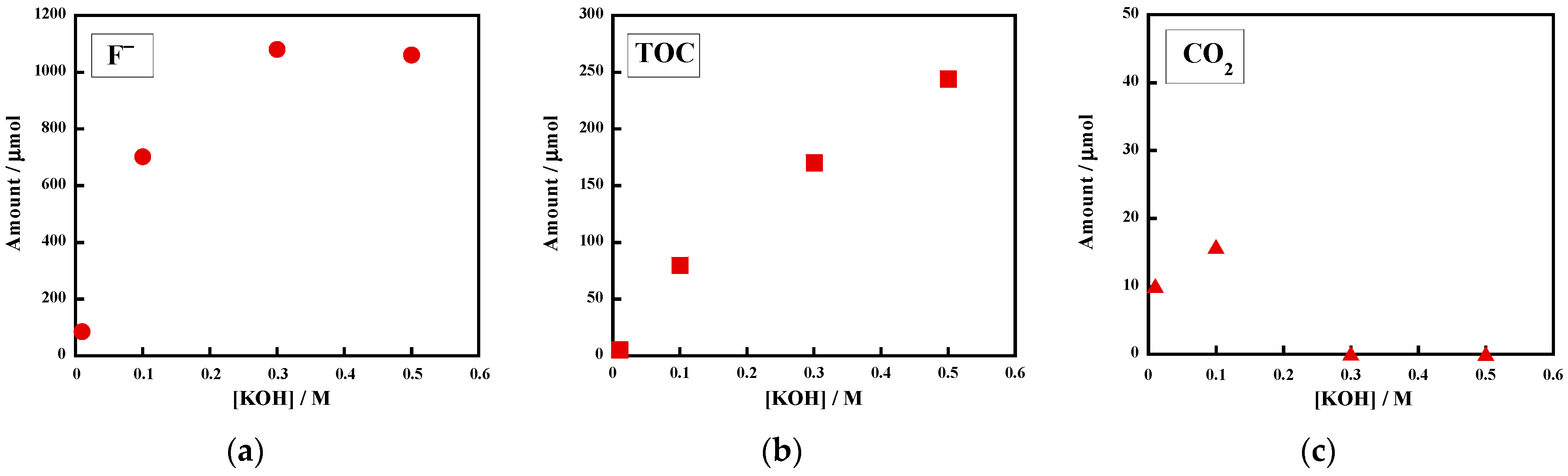
2.1.1. Effect of potassium hydroxide (KOH)
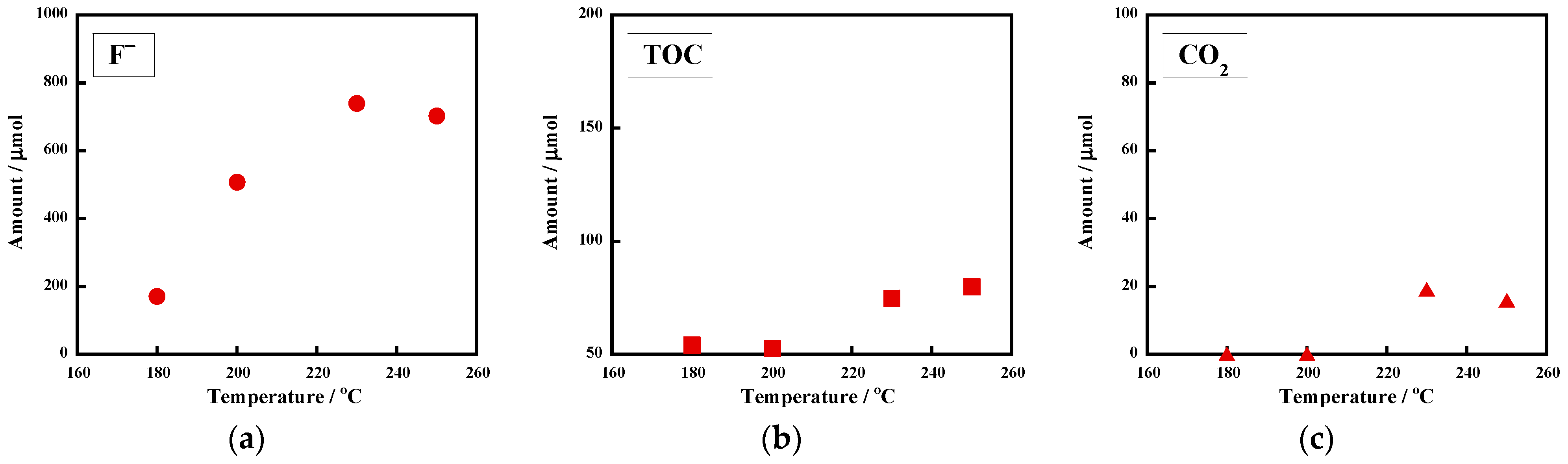
2.1.2. Mineralization at Lower Temperatures
2.1.3. Proposed Reaction Mechanism
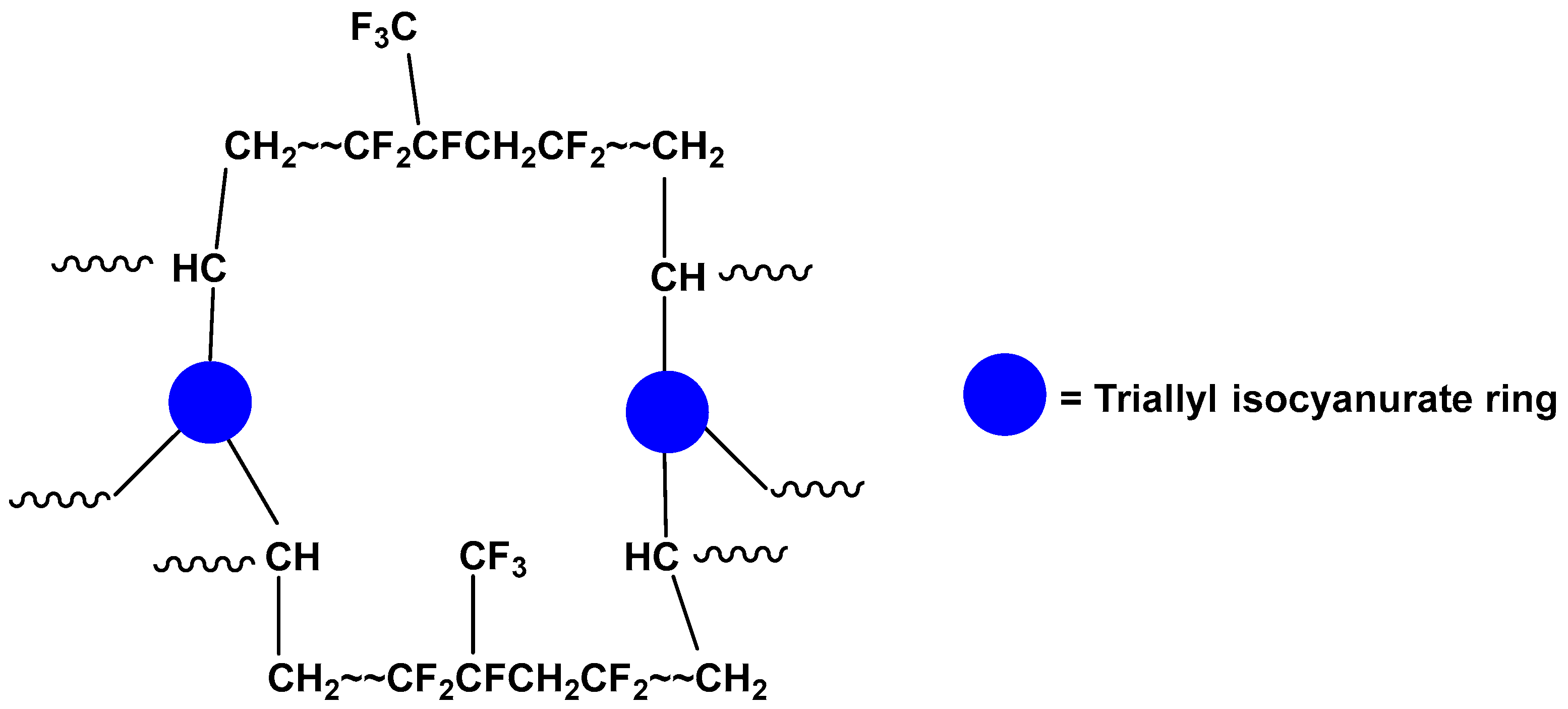
2.2. Decomposition of Crosslinked FKM
2.2.1. Effect of KOH
2.2.2. Complete Fluorine Mineralization at 200 °C
2.3. Synthesis of CaF2
3. Materials and Methods
3.1. Materials
3.2. Superheated Water Reactions
3.3. Analytical Methods
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Drobny, J.G. Technology of Fluoropolymers, 2nd ed.; CRC Press: Boca Raton, FL, USA, 2009. [Google Scholar]
- Ameduri, B.; Boutevin, B. Well-Architectured Fluoropolymers: Synthesis, Properties and Applications; Elsevier: Amsterdam, The Netherlands, 2004. [Google Scholar]
- Gardiner, J. Fluoropolymers: Origin, production, and industrial and commercial applications. Aust. J. Chem. 2015, 68, 13–22. [Google Scholar] [CrossRef]
- Dams, R.; Hintzer, K. Industrial aspect of fluorinated oligomers and polymers. In Fluorinated Polymers, Vol. 2, Applications; Ameduri, B., Sawada, H., Eds.; Royal Society of Chemistry: Cambridge, UK, 2017; pp. 3–31. [Google Scholar]
- Ameduri, B.; Fomin, S. (Eds.) Fascinating Fluoropolymers and Their Applications; Elsevier: Amsterdam, The Netherlands, 2020. [Google Scholar]
- Puts, G.J.; Course, P.; Ameduri, B.A. Polytetrafluoroethylene: Synthesis and characterization of the original extreme polymer. Chem. Rev. 2019, 119, 1763–1805. [Google Scholar] [CrossRef] [PubMed]
- Arcella, V.; Ferro, R. Fluorocarbon elastomers. In Modern Fluoropolymers: High Performance Polymers for Diverse Applications; Sheirs, J., Ed.; John Wiley & Sons: Chichester, UK, 1997. [Google Scholar]
- Moore, A.L. Fluoroelastomers Handbook: The Definitive User’s Guide and Data Book; William Andrew Publishing: Norwich, NY, USA, 2006. [Google Scholar]
- Xu, Z.; Zhang, Y.; Li, A.; Wang, J.; Wang, G.; He, Q. Research progress on compounding agent and mechanical test method of fluororubber. J. Appl. Polym. Sci. 2021, 138, e50913. [Google Scholar] [CrossRef]
- Daikin Industries, High Performance Fluoroelastomers DAI-EL. Available online: https://www.daikinchemicals.com/library/pb_common/images/products/dai-el-fluoroelastomers/ERC-1d.pdf (accessed on 7 July 2023).
- Cison PR Newswire, Global Fluoropolymers Markets Report 2022-2026-5G Communication Technology Boosts Demand for PTFE/Demands of Membrane Market Being Addressed by PVDF. Available online: https://www.prnewswire.com/news-releases/global-fluoropolymers-markets-report-2022-2026---5g-communication-technology-boosts-demand-for-ptfe--demands-of-membrane-market-being-addressed-by-pvdf-301600681.html (accessed on 7 July 2023).
- Aleksandrov, K.; Gehrmann, H.J.; Hauser, M.; Mätzing, H.; Pigeon, D.; Stapf, D.; Wexler, M. Waste incineration of polytetrafluoroethylene (PTFE) to evaluate potential formation of per- and poly-fluorinated alkyl substances (PFAS) in flue gas. Chemosphere 2019, 226, 898–906. [Google Scholar] [CrossRef]
- Conversio Market & Strategy GmbH, Fluoropolymer Waste in Europe 2020—End of Life (EOL) Analysis of Fluoropolymer Applications, Products and Associated Waste Streams. Available online: https://www.ft.dk/samling/20222/almdel/euu/spm/49/svar/1951975/2698345.pdf (accessed on 7 July 2023).
- Mineral Commodity Summaries 2023; U.S. Geological Survey: Reston, VA, USA, 2023; pp. 70–71. Available online: https://pubs.usgs.gov/periodicals/mcs2023/mcs2023.pdf (accessed on 7 July 2023).
- Ameduri, B.; Hori, H. Recycling and the end of life assessment of fluoropolymers: Recent developments, challenges and future trends. Chem. Soc. Rev. 2023, 52, 4208–4247. [Google Scholar] [CrossRef]
- Brunner, G. Near critical and supercritical water. Part I. Hydrolytic and hydrothermal processes. J. Supercrit. Fluids 2009, 47, 373–381. [Google Scholar] [CrossRef]
- Furimsky, E. Hydroprocessing in aqueous phase. Ind. Eng. Chem. Res. 2013, 52, 17695–17713. [Google Scholar] [CrossRef]
- Knez, Z.; Hrncic, M.K.; Colnik, M.; Skerget, M. Chemicals and value added compounds from biomass using sub-and supercritical water. J. Supercrit. Fluids 2018, 133, 591–602. [Google Scholar] [CrossRef]
- Silva Thomsen, L.B.; Carvalho, P.N.; dos Passos, J.S.; Anastasakis, K.; Bester, K.; Biller, P. Hydrothermal liquefaction of sewage sludge; energy considerations and fate of micropollutants during pilot scale processing. Water Res. 2020, 183, 116101. [Google Scholar] [CrossRef]
- Hori, H.; Honma, R. Decomposition of fluoropolymers by their mineralization in subcritical water. In Opportunities of Fluoropolymers; Ameduri, B., Fomin, S., Eds.; Elsevier: Amsterdam, The Netherlands, 2020; pp. 303–331. [Google Scholar]
- Hamaura, J.; Hori, H.; Manseri, A.; Ameduri, B. Efficient fluoride recovery from poly(vinylidene fluoride), poly(vinylidene fluoride-co-hexafluoropropylene) copolymer and poly(ethylene-co-tetrafluoroethylene) copolymer using superheated water with alkaline reagent. Eur. Polym. J. 2023, 182, 111724. [Google Scholar] [CrossRef]
- Schuster, J.; Lutz, J.; Shaik, Y.P.; Yadavalli, V.R. Recycling of fluoro-carbon-elastomers—A review. Adv. Indust. Engin. Polym. Res. 2022, 5, 248–254. [Google Scholar] [CrossRef]
- 3M Dyneon, Fluoropolymers Up-Cycling. Closing the Loop. Available online: https://multimedia.3m.com/mws/media/907323O/up-cycling-fluoropolymers-brochure.pdf?fn=Up-Cycling_Brchure_EN.pdf#:~:text=In%20March%202015,%20Dyneon%20opened%20the%20world%E2%80%99s%20first,thereby%20having%20a%20positive%20impact%20on%20the%20environment (accessed on 26 September 2023).
- Montaudo, G.; Puglisi, C.; Scamporrino, E.; Vitalini, D. Correlation of thermal degradation mechanisms: Polyacetylene and vinyl and vinylidene polymers. J. Polym Sci. Part A Polym. Chem. 1986, 24, 301–316. [Google Scholar] [CrossRef]
- Ross, G.J.; Watts, J.F.; Hill, M.P.; Morrissey, P. Surface modification of poly(vinylidene fluoride) by alkaline treatment 1. The degradation mechanism. Polymer 2000, 41, 1685–1696. [Google Scholar] [CrossRef]
- Choi, S.-S.; Kim, Y.-K. Analysis of pyrolysis products of poly(vinylidene fluoride-co-hexafluoropropylene) by pyrolysis-gas chromatography/mass spectrometry. J. Fluorine Chem. 2014, 165, 33–38. [Google Scholar] [CrossRef]
- Schmiegel, W.W. Crosslinking of elastomeric vinylidene fluoride copolymers with nucleophiles. Angew. Makromol. Chem. 1979, 76–77, 39–65. [Google Scholar] [CrossRef]
- Ferrari, A.C.; Robertson, J. Resonance Raman spectroscopy of disordered, amorphous, and diamondlike carbon. Phys. Rev. B 2001, 64, 075414. [Google Scholar] [CrossRef]
- Sheka, E.F.; Golubev, Y.A.; Popova, N.A. Graphene domain signature of Raman spectra of sp2 amorphous carbons. Nanomaterials 2020, 10, 2021. [Google Scholar] [CrossRef]
- Jawhari, T.; Roid, A.; Casado, J. Raman spectroscopic characterization of some commercially available carbon black materials. Carbon 1995, 33, 1561–1565. [Google Scholar] [CrossRef]
- Goto, M. Subcritical and supercritical fluid technology for recycling waste plastics. J. Jpn. Pet. Inst. 2016, 59, 254–258. [Google Scholar] [CrossRef]
- Vo Dong, P.A.; Azzaro-Pantel, C.; Cadene, A.-L. Economic and environmental assessment of recovery and disposal pathways for CFRP waste management. Resour. Conserv. Recycl. 2018, 133, 63–75. [Google Scholar] [CrossRef]
- Wang, R.; Xu, Z. Recycling of non-metallic fractions from waste electrical and electronic equipment (WEEE): A review. Waste Manag. 2014, 34, 1455–1469. [Google Scholar] [CrossRef] [PubMed]


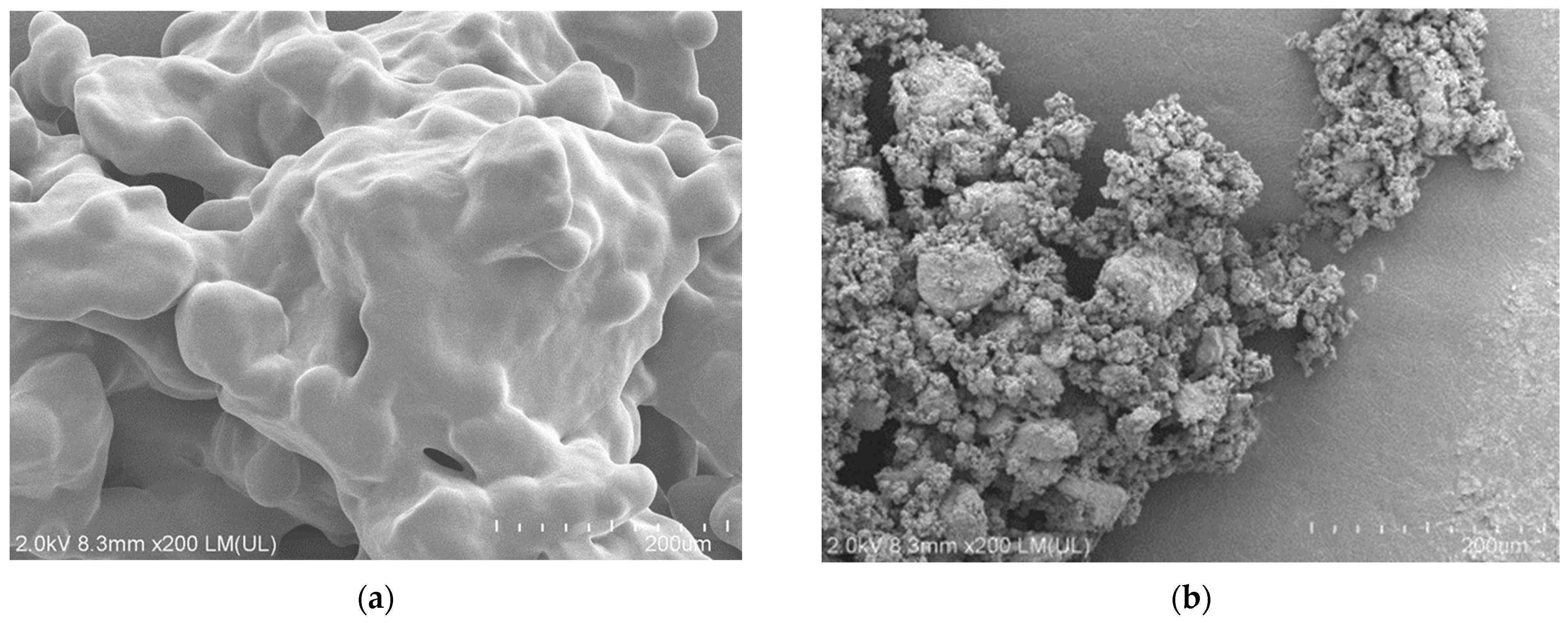
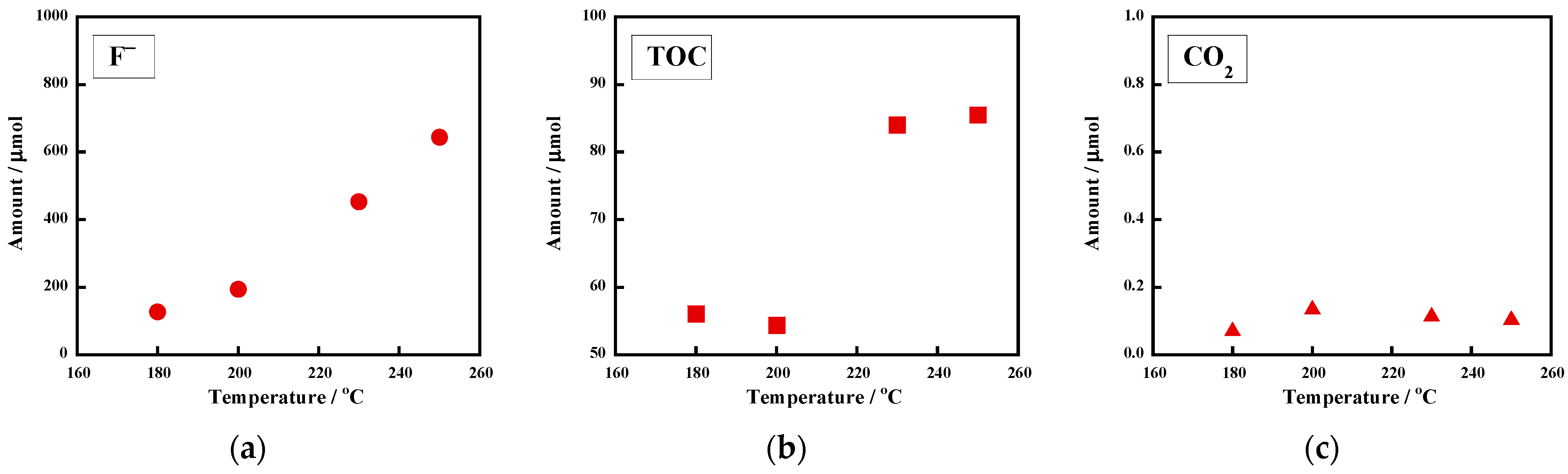

| Entry | Polymer [Initial Amount/mg] | KOH Conc. /M | Atmosphere | T/ °C | P/ MPa | Time /h | F−/μmol [Yield/%] | TOC/μmol [Ratio/%] | CO2/μmol [Yield/%] |
|---|---|---|---|---|---|---|---|---|---|
| 1 | uncrosslinked FKM [29.8] | 0 | argon | 250 | 4.4 | 6 | 0.9 [0] | <0.2 a [0] | 1.6 [0] |
| 2 b | PVDF [30.0] | 0 | argon | 250 | 4.4 | 6 | 1.4 [0] | 3.7 [0] | 2.1 [0] |
| 3 | uncrosslinked FKM [30.5] | 0.10 | argon | 250 | 4.6 | 6 | 702 [68] | 80 [10] | 15.9 [2] |
| 4 a | PVDF [30.4] | 0.125 | argon | 250 | 4.4 | 6 | 794 [83] | 62 [7] | 0.8 [0] |
| 5 | uncrosslinked FKM [30.5] | 0.50 | argon | 250 | 4.5 | 6 | 1060 [102] | 244 [31] | 0.08 [0] |
| 6 | uncrosslinked FKM [30.1] | 0.50 | O2 | 250 | 4.3 | 6 | 1040 [102] | 123 [15] | 1.8 [0] |
| 7 | uncrosslinked FKM [29.9 ± 0.1] | 0.20 | argon | 200 | 2.3 | 18 | 1008 ± 2 [100 ± 1] | 138 ± 18 [17 ± 3] | 0.2 [0] |
| 8 | crosslinked FKM [30.0] | 0 | argon | 250 | 4.5 | 6 | 8.8 [1] | <0.2 a [0] | 33.1 [3] |
| 9 | crosslinked FKM [30.5] | 0.50 | argon | 250 | 4.4 | 6 | 740 [101] | 337 [26] | 0.1 [0] |
| 10 | crosslinked FKM [30.5] | 0.20 | argon | 200 | 2.3 | 18 | 732 ± 3 [100] | 258 ± 12 [20 ± 1] | 0.1 [0] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hamaura, J.; Hori, H.; Fujishima, A.; Mukae, H. Efficient Mineralization of Fluoroelastomers Using Superheated Water in the Presence of Potassium Hydroxide. Molecules 2023, 28, 7057. https://doi.org/10.3390/molecules28207057
Hamaura J, Hori H, Fujishima A, Mukae H. Efficient Mineralization of Fluoroelastomers Using Superheated Water in the Presence of Potassium Hydroxide. Molecules. 2023; 28(20):7057. https://doi.org/10.3390/molecules28207057
Chicago/Turabian StyleHamaura, Jin, Hisao Hori, Ayane Fujishima, and Hirofumi Mukae. 2023. "Efficient Mineralization of Fluoroelastomers Using Superheated Water in the Presence of Potassium Hydroxide" Molecules 28, no. 20: 7057. https://doi.org/10.3390/molecules28207057
APA StyleHamaura, J., Hori, H., Fujishima, A., & Mukae, H. (2023). Efficient Mineralization of Fluoroelastomers Using Superheated Water in the Presence of Potassium Hydroxide. Molecules, 28(20), 7057. https://doi.org/10.3390/molecules28207057