Systematic Screening of the Chemical Constituents of Lanqin Oral Liquid by Ultra-High-Performance Liquid Chromatography Combined with Fourier Transform Ion Cyclotron Resonance Mass Spectrometry
Abstract
:1. Introduction
2. Results and Discussion
2.1. Chemical Profiling of Lanqin Oral Liquid
2.1.1. Characterization and Identification of Flavonoids
2.1.2. Characterization and Identification of Alkaloids
2.1.3. Characterization and Identification of Terpenoids
2.1.4. Characterization and Identification of Organic Acids
2.1.5. Characterization and Identification of Phenylpropanoids
3. Materials and Methods
3.1. Chemicals and Reagents
3.2. Sample Preparation
3.3. Liquid Chromatography
3.4. Mass Spectrometry
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Shen, X.; Zhao, Z.Y.; Wang, H.; Guo, Z.H.; Hu, B.X.; Zhang, G. Elucidation of the Anti-Inflammatory Mechanisms of Bupleuri and Scutellariae Radix Using System Pharmacological Analyses. Mediat. Inflamm. 2017, 2017, 3709874. [Google Scholar] [CrossRef]
- Li, C.R.; Lin, G.; Zuo, Z. Pharmacological effects and pharmacokinetics properties of Radix scutellariae and its bioactive flavones. Biopharm. Drug Dispos. 2011, 32, 427–445. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Zhu, Z.P.; Gao, T.H.; Chen, Y.; Yang, Q.S.; Fu, C.M.; Zhu, Y.N.; Wang, F.; Liao, W. Isatidis Radix and Isatidis Folium: A systematic review on ethnopharmacology, phytochemistry and pharmacology. J. Ethnopharmacol. 2022, 283, 114648. [Google Scholar] [CrossRef]
- Tian, J.Z.; Qin, S.S.; Han, J.Y.; Meng, J.; Liang, A.H. A review of the ethnopharmacology, phytochemistry, pharmacology and toxicology of Fructus gardeniae (Zhi-zi). J. Ethnopharmacol. 2022, 289, 114984. [Google Scholar] [CrossRef]
- Li, N.; Gao, A.; Gong, J.; Cao, M.Y.; Jia, X.; Zhang, X.Z.; Wang, M.; Fu, Z.H.; Ni, S.F. Overview of Pharmacological Research of Sterculia lychnophora Hance. J. Anhui Agri. Sci. 2011, 39, 9609–9610. [Google Scholar]
- Sun, Y.; Lenon, G.B.; Yang, A.W.H. Phellodendri Cortex: A Phytochemical, Pharmacological, and Pharmacokinetic Review. Evid.-Based Complement. Alternat. Med. 2019, 2019, 7621929. [Google Scholar] [CrossRef] [PubMed]
- Zhang, F.X.; Li, Z.T.; Li, M.; Yuan, Y.L.L.; Cui, S.S.; Chen, J.X.; Li, R.M. An integrated strategy for profiling the chemical components of Scutellariae Radix and their exogenous substances in rats by ultra-high-performance liquid chromatography/quadrupole time-of-flight mass spectrometry. Rapid Commun. Mass. Spectrom. 2020, 34, e8823. [Google Scholar] [CrossRef]
- Li, R.Y.; Zhu, Y.R.; Ma, M.Y.; Lei, M.; Zhao, Y.C.; Liu, T.; Yu, M.; Zhao, Y.L.; Yu, Z.G. Characterization of chemical constituents in Shuanghuanglian oral dosage forms by ultra-high performance liquid chromatography coupled with time-of-flight mass spectrometry. J. Sep. Sci. 2022, 45, 1020–1030. [Google Scholar] [CrossRef]
- Wang, H.M.; Hu, W.D.; Wang, H.D.; Wang, Y.F.; Yang, W.Z. Comprehensive multicomponent characterization and fingerprinting analysis of Lanqin Oral Liquid by ultra-high-performance liquid chromatography coupled with ion mobility-quadrupole time-of-flight mass spectrometry. J. Sep. Sci. 2021, 44, 4111–4122. [Google Scholar] [CrossRef]
- Fan, Y.L.; Liu, R.Z.; Tan, Q.; Zhao, H.L.; Song, M.; Wang, R.; Li, P.; Yang, H. A database-guided integrated strategy for comprehensive chemical profiling of traditional Chinese medicine. J. Chromatogr. A 2022, 1674, 463145. [Google Scholar] [CrossRef]
- Nikolaev, E.N.; Kostyukevich, Y.I.; Vladimirov, G.N. Fourier transform ion cyclotron resonance (FT ICR) mass spectrometry: Theory and simulations. Mass. Spectrom. Rev. 2016, 35, 219–258. [Google Scholar] [CrossRef] [PubMed]
- Frahm, J.L.; Howard, B.E.; Heber, S.; Muddiman, D.C. Accessible proteomics space and its implications for peak capacity for zero-, one- and two-dimensional separations coupled with FT-ICR and TOF mass spectrometry. J. Mass. Spectrom. 2006, 41, 281–288. [Google Scholar] [CrossRef] [PubMed]
- Liu, T.; Li, R.Y.; Cui, Y.; Yu, Z.G.; Zhao, Y.L. Metabonomic analysis of plasma biochemical changes in pyrexia rats after treatment with Gegenqinlian decoction, aspirin and itraconazole by UHPLC-FT-ICR-MS. J. Pharm. Anal. 2020, 10, 581–587. [Google Scholar] [CrossRef] [PubMed]
- Liu, T.; Cui, Y.; Tian, X.M.; Li, S.H.; Han, F.; Ji, B.; Zhao, Y.L.; Yu, Z.G. Detection of chemical constituents in Gegenqinlian decoction by ultra-high performance liquid chromatography coupled with Fourier transform ion cyclotron resonance mass spectrometry. Anal. Methods 2017, 9, 5890–5902. [Google Scholar] [CrossRef]
- Beszterda, M.; Frański, R. Detection of flavone C-glycosides in the extracts from the bark of Prunus avium L. and Prunus cerasus L. Eur. J. Mass. Spectrom. 2020, 26, 369–375. [Google Scholar] [CrossRef]
- Shuai, E.; Shang, Z.C.; Qin, S.H.; Li, K.L.; Liu, Y.N.; Wu, J.L.; Yan, F.; Cai, W. A Systematic Method for the Identification of Aporphine Alkaloid Constituents in Sabia schumanniana Diels Using UHPLC-Q-Exactive Orbitrap/Mass Spectrometry. Molecules 2022, 27, 7643. [Google Scholar]
- Wang, X.Q.; Chang, J.; Zhang, Q.; Lin, L.N.; Shao, P.; Li, Q. The mechanism of Isatidis Radix in the prevention of influenza and COVID-19 by HPLC-Q-TOF-MS combined with network pharmacology. Acta Pharm. Sin. 2022, 57, 3173–3185. [Google Scholar]
- Zhou, W.; Shan, J.J.; Meng, M.X. A two-step ultra-high-performance liquid chromatography-quadrupole/time of flight mass spectrometry with mass defect filtering method for rapid identification of analogues from known components of different chemical structure types in Fructus gardeniae-Fructus forsythiae herb pair extract and in rat’s blood. J. Chromatogr. A 2018, 1563, 99–123. [Google Scholar]
- Wang, L.; Liu, S.; Zhang, X.J.; Xing, J.P.; Liu, Z.Q.; Song, F.R. A strategy for identification and structural characterization of compounds from Gardenia jasminoides by integrating macroporous resin column chromatography and liquid chromatography-tandem mass spectrometry combined with ion-mobility spectrometry. J. Chromatogr. A 2016, 1452, 47–57. [Google Scholar] [CrossRef]
- Feng, W.; Dong, Q.J.; Liu, M.Y.; Li, S.; Liu, T.; Wang, X.G.; Niu, L.Y. Screening and identification of multiple constituents and their metabolites of Zhi-zi-chi decoction in rat urine and bile by ultra-high-performance liquid chromatography quadrupole time-of-flight mass spectrometry. Biomed. Chromatogr. 2017, 31, e3978. [Google Scholar] [CrossRef]
- Fu, Z.W.; Li, Z.X.; Hu, P.; Feng, Q.; Xue, R.; Hu, Y.Y.; Huang, C.G. A practical method for the rapid detection and structural characterization of major constituents from traditional Chinese medical formulas: Analysis of multiple constituents in Yinchenhao Decoction. Anal. Methods 2015, 7, 4678–4690. [Google Scholar] [CrossRef]
- Ren, W.; Li, Y.; Zuo, R.; Wang, H.J.; Si, N.; Zhao, H.Y.; Han, L.Y.; Yang, J.; Bian, B.L. Species-related difference between limonin and obacunone among five liver microsomes and zebrafish using ultra-high-performance liquid chromatography coupled with a LTQ-Orbitrap mass spectrometer. Rapid Commun. Mass. Spectrom. 2014, 28, 2292–2300. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.N.; Zhao, M.; Ou, Y.F.; Zeng, B.W.; Lou, X.Y.; Wang, M.; Zhao, C.J. Metabolic profile of esculin in rats by ultra high performance liquid chromatography combined with Fourier transform ion cyclotron resonance mass spectrometry. J. Chromatogr. B Analyt. Technol. Biomed. Life Sci. 2016, 1020, 120–128. [Google Scholar] [CrossRef] [PubMed]
- Hofmann, T.; Nebehaj, E.; Albert, L. The high-performance liquid chromatography/multistage electrospray mass spectrometric investigation and extraction optimization of beech (Fagus sylvatica L.) bark polyphenols. J. Chromatogr. A 2015, 1393, 96–105. [Google Scholar] [CrossRef]
- Wang, H.Y.; Sun, H.; Zhang, A.H.; Li, Y.; Wang, L.H.; Shi, H.; Dizou, X.N.L.; Wang, X.J. Rapid identification and comparative analysis of the chemical constituents and metabolites of Phellodendri amurensis cortex and Zhibai dihuang pill by ultra-performance liquid chromatography with quadrupole TOF-MS. J. Sep. Sci. 2013, 36, 3874–3882. [Google Scholar] [CrossRef]
- Liu, X.W.; Zhang, F.; Gao, S.H.; Jiang, B.; Chen, W.S. Metabolite profiling of Zi-Shen pill in rat biological specimens by UPLC-Q-TOF/MS. Chin. J. Nat. Med. 2015, 13, 145–160. [Google Scholar] [CrossRef]
- Li, R.R.; Cui, Y.F.; Zheng, X.F.; Qin, X.M.; Cao, J.J.; Li, Z.Y. Characterization of chemical components in the Guanxinning injection by liquid chromatography-mass spectrometry. J. Mass. Spectrom. 2020, 55, e4662. [Google Scholar] [CrossRef]
- Zhao, H.L.; Liu, R.Z.; Ding, M.; Fan, Y.L.; Lin, B.B.; Lu, H.W.; Wen, X.D.; Li, P.; Yang, H. Determination of 44 major components and chemical profiling of saccharide in Chinese medicinal formula Lanqin Oral Liquid. Phytochem. Anal. 2023, 34, 560–570. [Google Scholar] [CrossRef]
- Wang, L.P.; Ma, Q. Clinical benefits and pharmacology of scutellarin: A comprehensive review. Pharmacol. Ther. 2018, 190, 105–127. [Google Scholar] [CrossRef]
- Liao, H.; Ye, J.; Gao, L.; Liu, Y. The main bioactive compounds of Scutellaria baicalensis Georgi. for alleviation of inflammatory cytokines: A comprehensive review. Biomed. Pharmacother. 2021, 133, 110917. [Google Scholar] [CrossRef]
- Tsai, C.C.; Lin, M.T.; Wang, J.J.; Liao, J.F.; Huang, W.T. The antipyretic effects of baicalin in lipopolysaccharide-evoked fever in rabbits. Neuropharmacology 2006, 51, 709–717. [Google Scholar] [CrossRef]
- Hu, L.L.; Wang, J.; Wu, N.; Zhao, X.G.; Cai, D.H. Utilizing network pharmacology and experimental validation to investigate the underlying mechanism of phellodendrine on inflammation. PeerJ 2022, 10, e13852. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.H.; Zheng, J.; Zhang, N.; Li, C.D. Berberine improves airway inflammation and inhibits NF-κB signaling pathway in an ovalbumin-induced rat model of asthma. J. Asthma 2016, 53, 999–1005. [Google Scholar] [CrossRef] [PubMed]
- Zhao, G.H.; Li, T.; Qu, X.Y.; Zhang, N.N.; Lu, M.; Wang, J. Optimization of ultrasound-assisted extraction of indigo and indirubin from Isatis indigotica Fort. and their antioxidant capacities. Food Sci. Biotechnol. 2017, 26, 1313–1323. [Google Scholar] [CrossRef] [PubMed]
- Fu, H.; Zhu, H. Geniposidic acid protects lipopolysaccharide-induced acute lung injury via the TLR4/MyD88 signaling pathway in vitro and in vivo. Immunopharmacol. Immunotoxicol. 2022, 44, 984–992. [Google Scholar] [CrossRef]
- Yuan, J.W.; Zhang, J.H.; Cao, J.; Wang, G.X.; Bai, H.S. Geniposide alleviates traumatic brain injury in rats via anti-inflammatory effect and MAPK/NF-kB inhibition. Cell Mol. Neurobiol. 2020, 40, 511–520. [Google Scholar] [CrossRef]
- Wang, J.; Chen, L.; Liang, Z.; Li, Y.; Yuan, F.; Liu, J.; Tian, Y.; Hao, Z.; Zhou, F.; Liu, X.; et al. Genipin inhibits LPS-induced inflammatory response in BV2 microglial cells. Neurochem. Res. 2017, 42, 2769–2776. [Google Scholar] [CrossRef]
- Xie, X.X.; Zhang, M.Y.; Sun, L.; Wang, T.; Zhu, Z.Y.; Shu, R.N.; Wu, F.C.; Li, Z.Z. Crocin-I Protects Against High-Fat Diet-Induced Obesity via Modulation of Gut Microbiota and Intestinal Inflammation in Mice. Front. Pharmacol. 2022, 13, 894089. [Google Scholar] [CrossRef]
- Li, J.; Deng, S.H.; Li, J.; Li, L.; Zhang, F.; Zou, Y.; Wu, D.M.; Xu, Y. Obacunone alleviates ferroptosis during lipopolysaccharide-induced acute lung injury by upregulating Nrf2-dependent antioxidant responses. Cell Mol. Biol. Lett. 2022, 27, 29. [Google Scholar] [CrossRef]
- Sorgi, C.A.; de Campos Chaves Lamarque, G.; Verri, M.P.; Nelson-Filho, P.; Faccioli, L.H.; Paula-Silva, F.W.G. Multifaceted effect of caffeic acid against Streptococcus mutans infection: Microbicidal and immunomodulatory agent in macrophages. Arch. Microbiol. 2021, 203, 2979–2987. [Google Scholar] [CrossRef]
- Wang, L.; Pan, X.Q.; Jiang, L.S.; Chu, Y.; Gao, S.; Jiang, X.Y.; Zhang, Y.H.; Chen, Y.; Luo, S.J.; Peng, C. The Biological Activity Mechanism of Chlorogenic Acid and Its Applications in Food Industry: A Review. Front. Nutr. 2022, 9, 943911. [Google Scholar] [CrossRef] [PubMed]
- Li, C.X.; Li, J.C.; Lai, J.; Liu, Y. The pharmacological and pharmacokinetic properties of esculin: A comprehensive review. Phytother. Res. 2022, 36, 2434–2448. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.Z.; Ma, J.C.; Deng, P.; Ren, F.C.; Li, N. Chemical Constituents of Thesium chinense Turcz and Their In Vitro Antioxidant, Anti-Inflammatory and Cytotoxic Activities. Molecules 2023, 28, 2685. [Google Scholar] [CrossRef]
- Li, J.; Zhou, B.X.; Li, C.F.; Chen, Q.Y.; Wang, Y.T.; Li, Z.T.; Chen, T.T.; Yang, C.G.; Jiang, Z.H.; Zhong, N.S.; et al. Lariciresinol-4-O-β-D-glucopyranoside from the root of Isatis indigotica inhibits influenza A virus-induced pro-inflammatory response. J. Ethnopharmacol. 2015, 174, 379–386. [Google Scholar] [CrossRef] [PubMed]
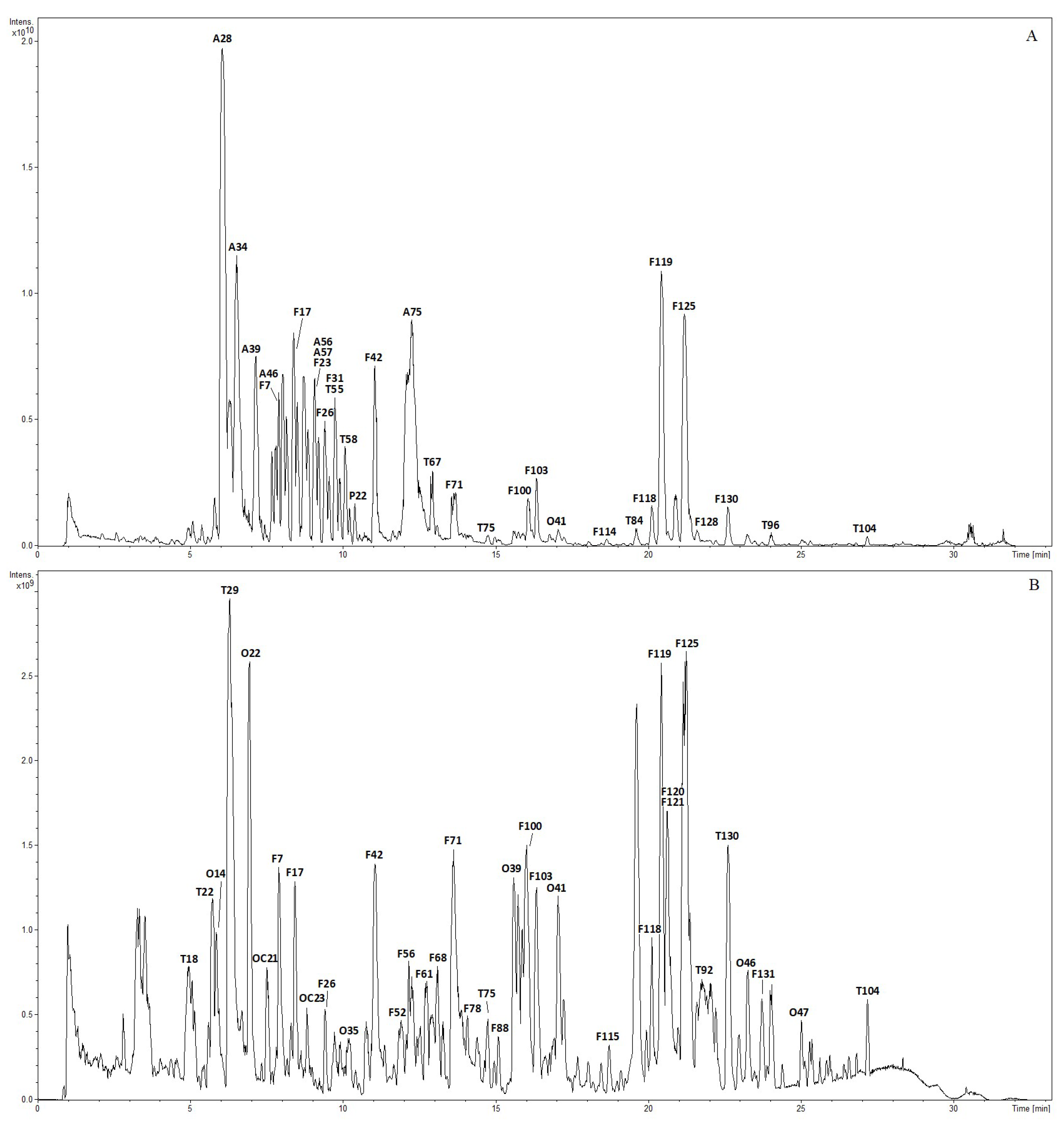
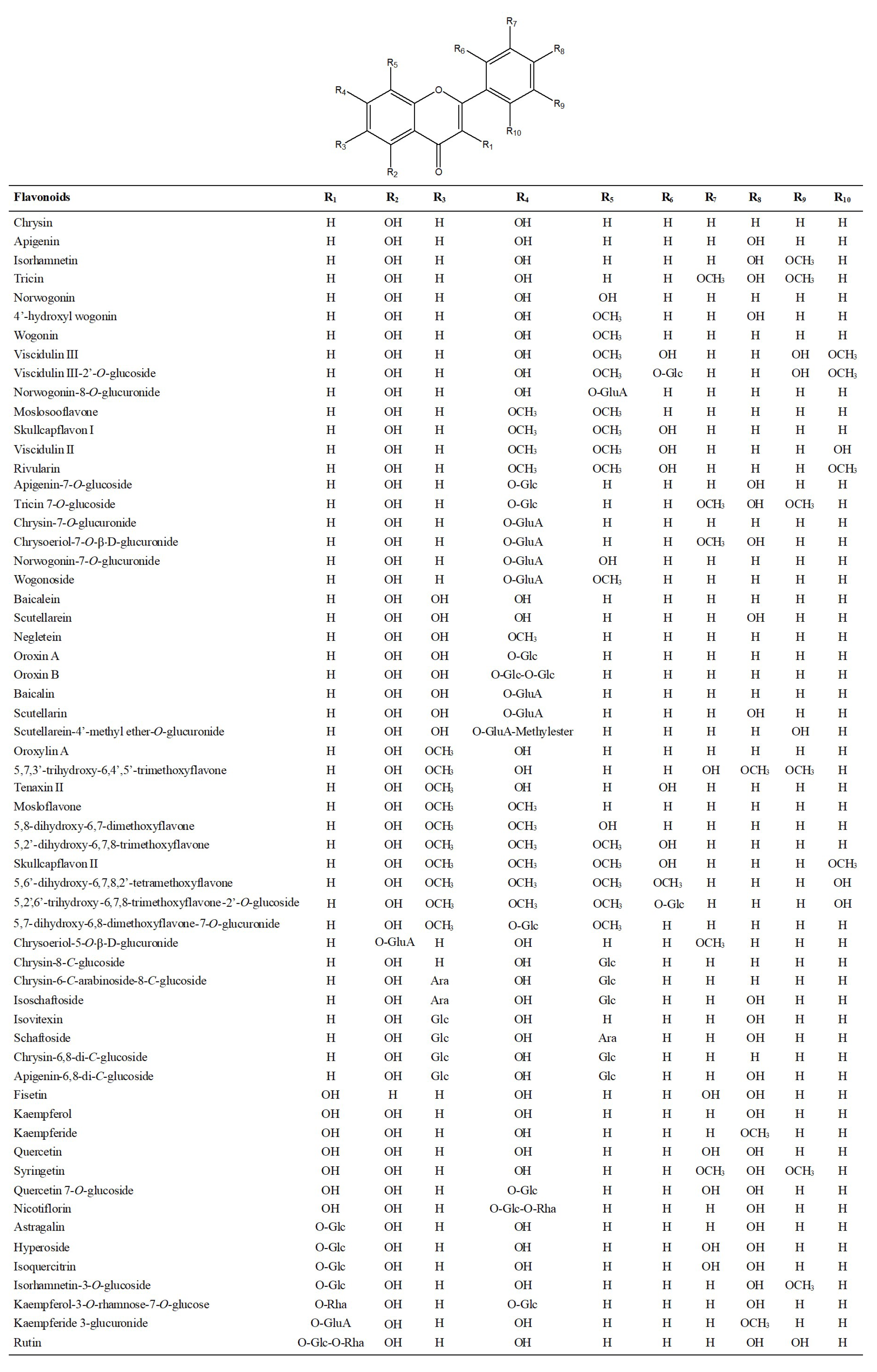

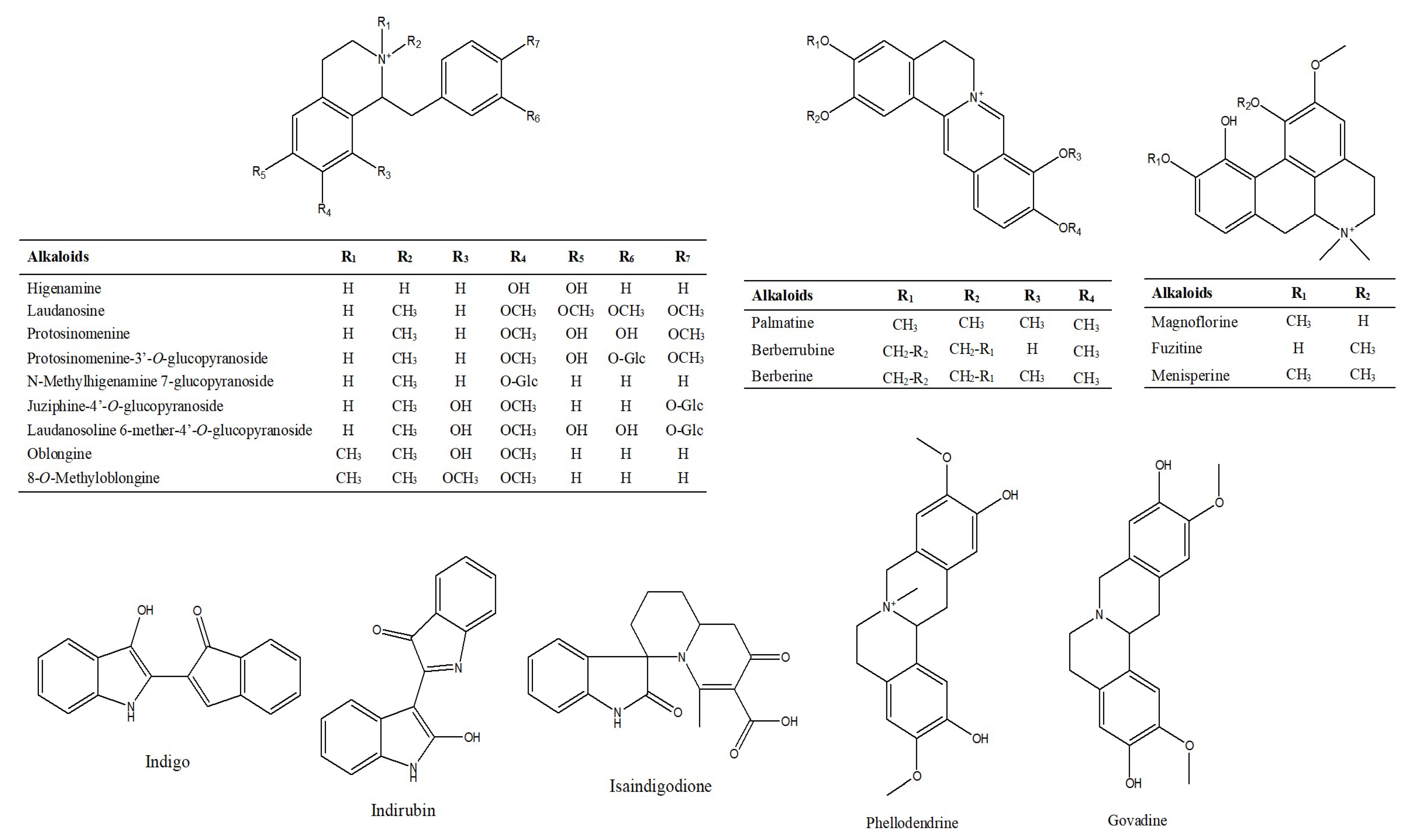
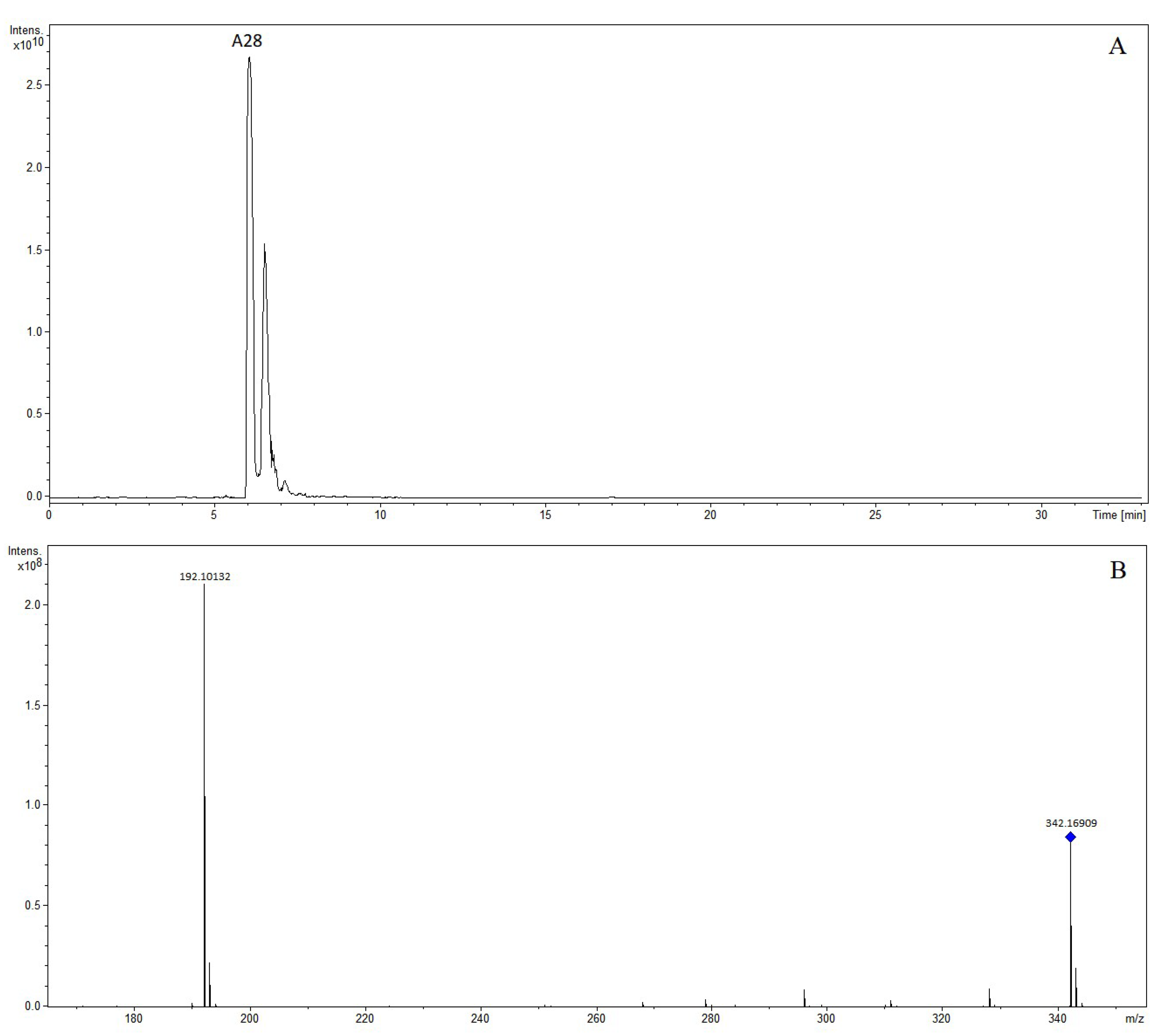
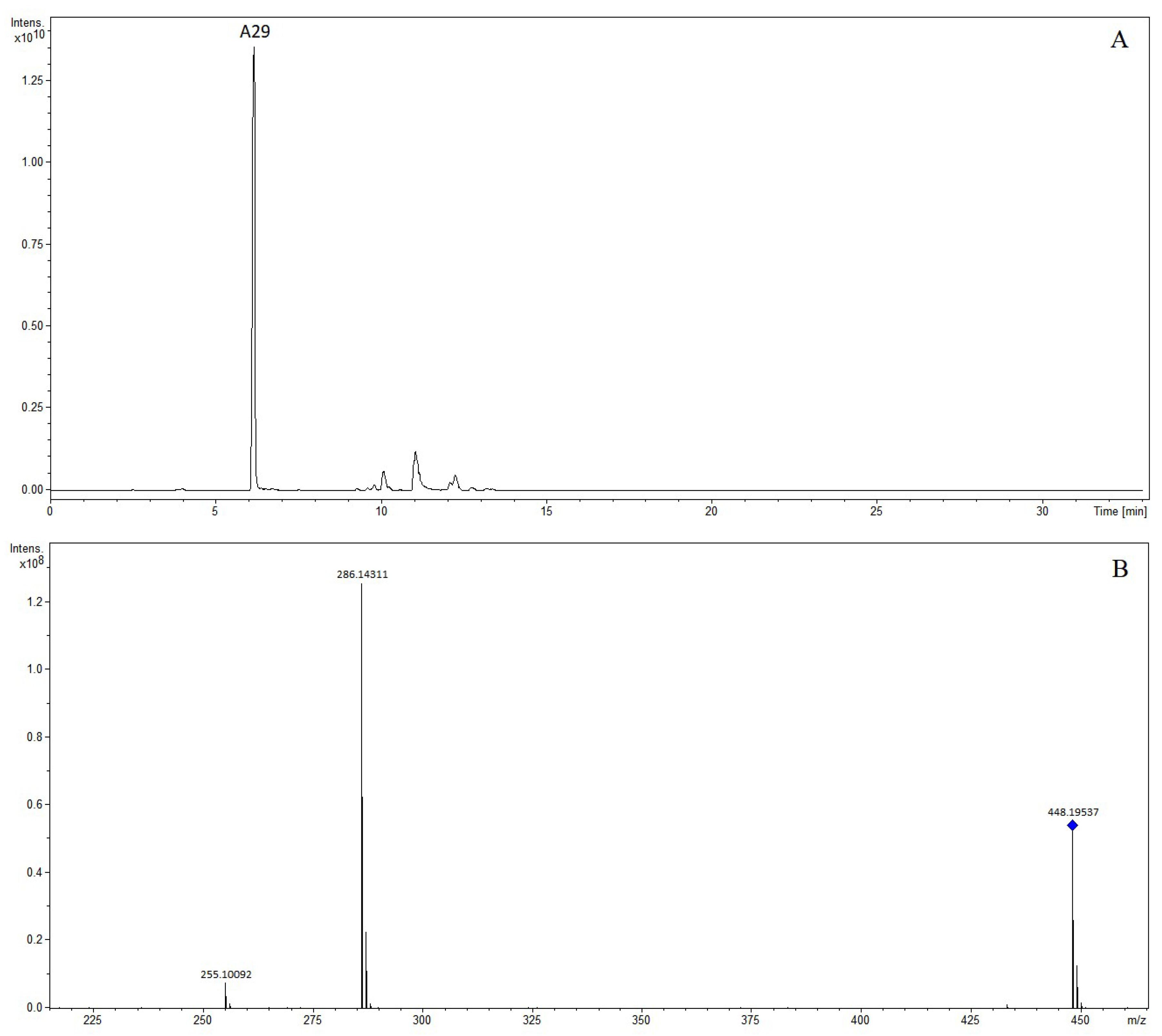
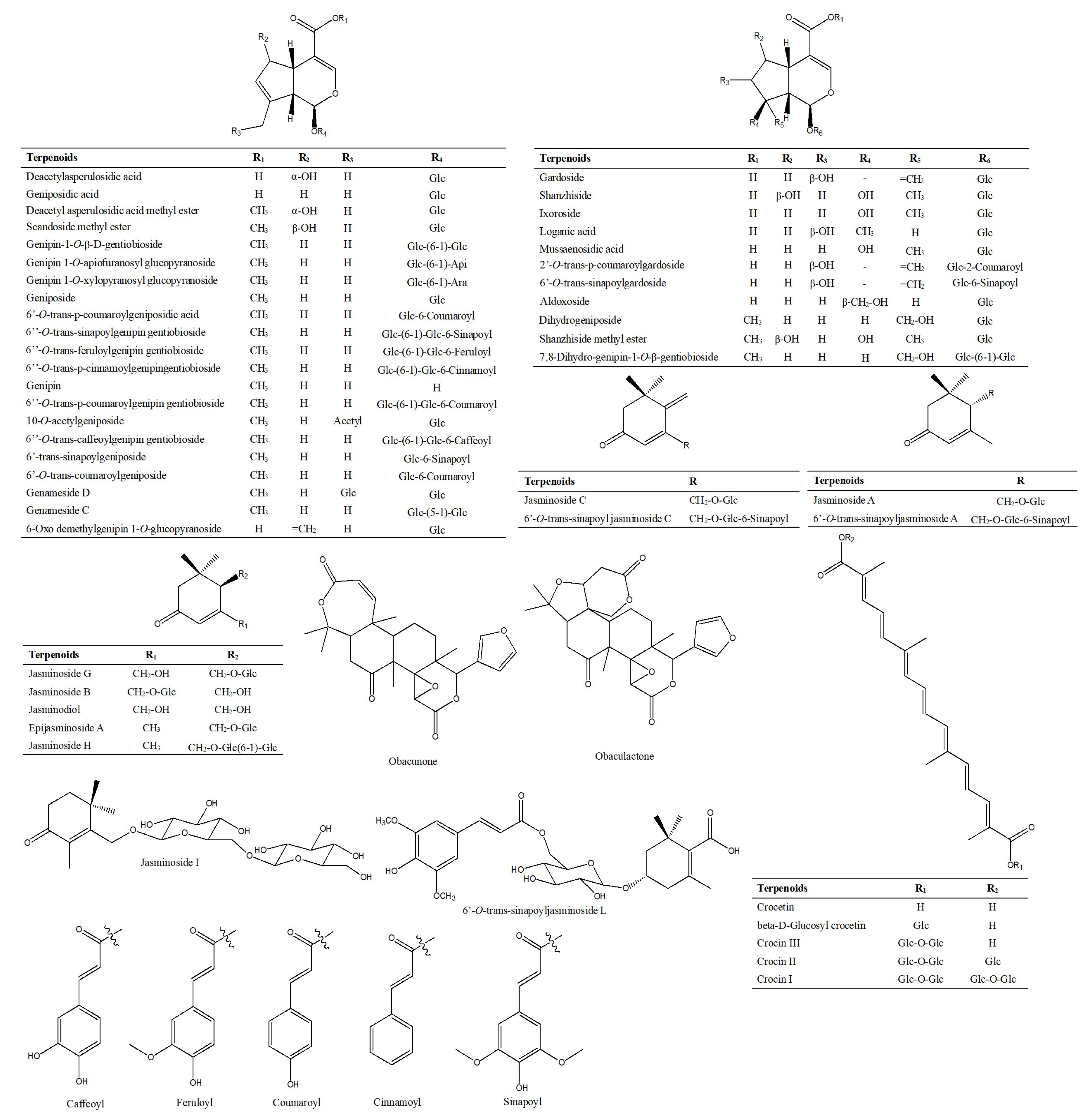
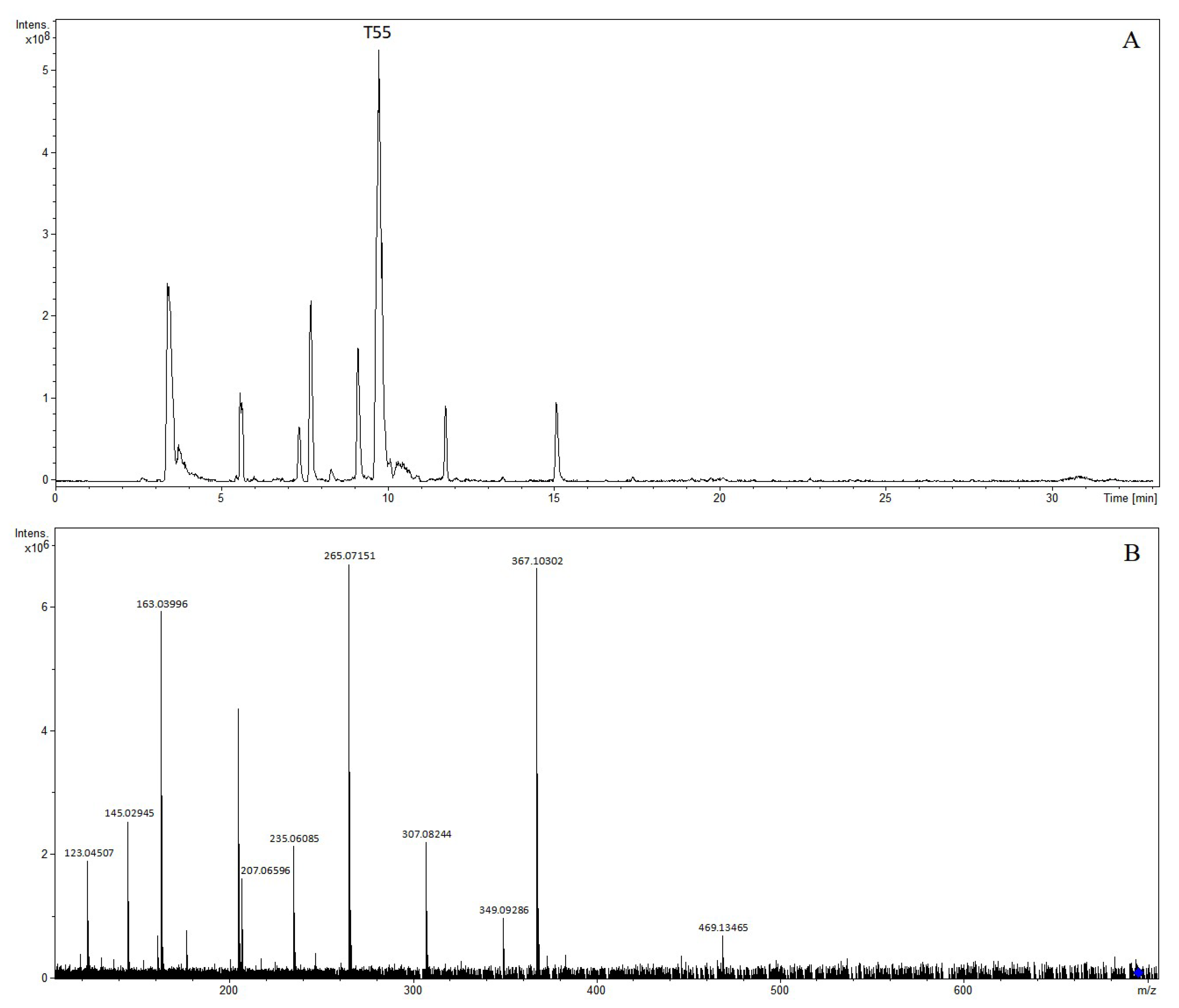
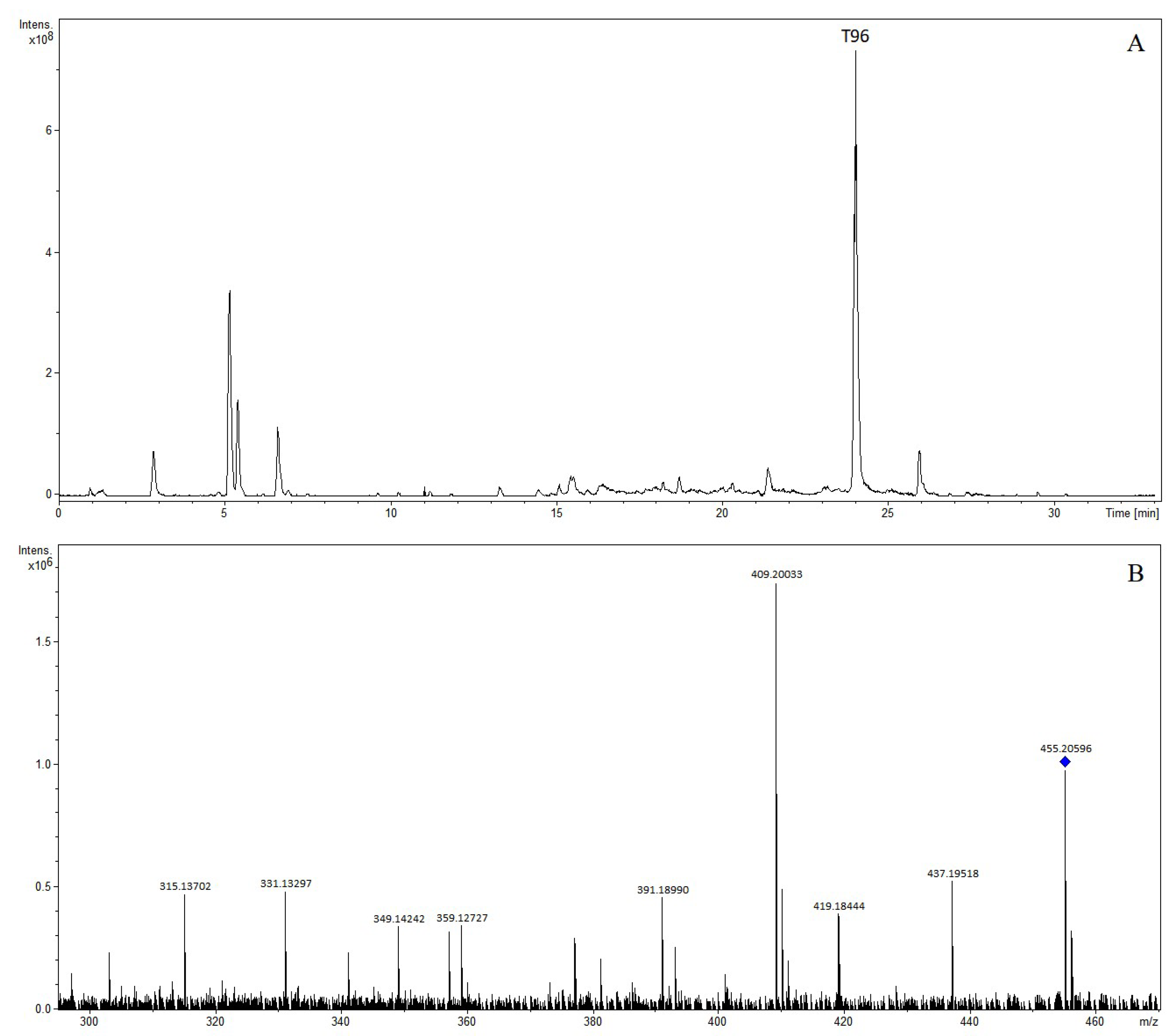
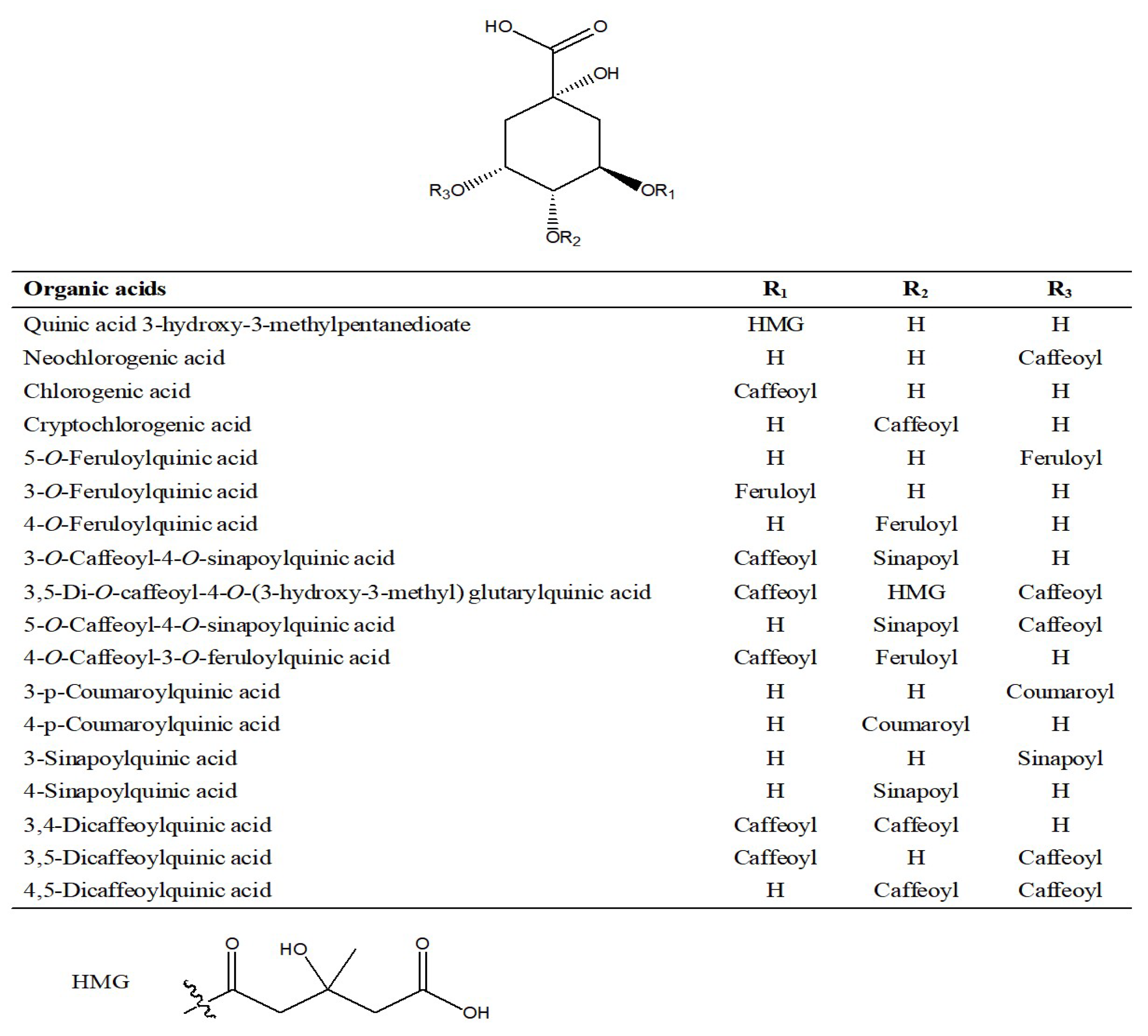
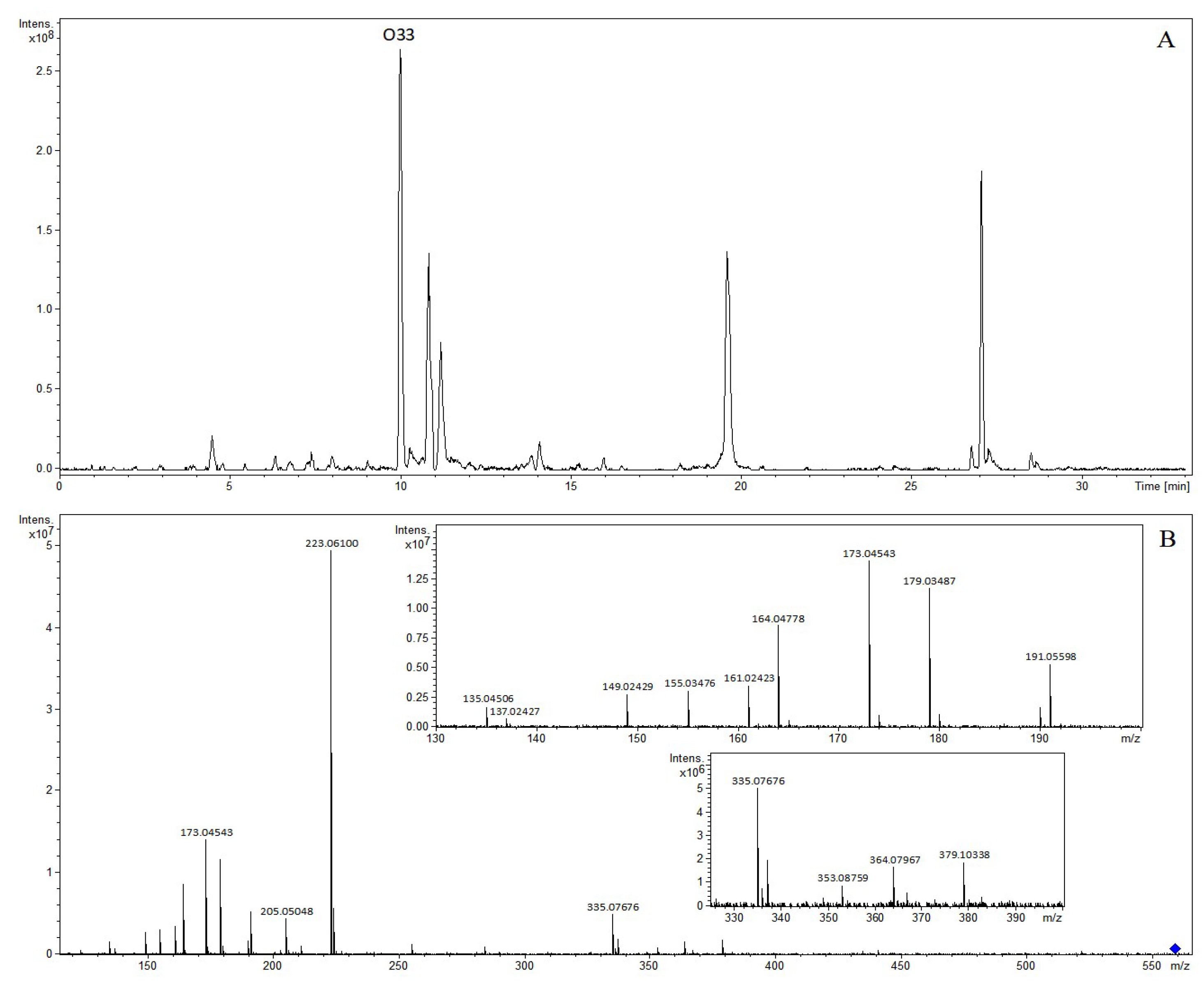


| No. | RT (min) | Identification | [M-H]+ | [M-H]− | Product Ion (m/z) | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Observed | Calculated | Error (ppm) | Formula | Observed | Calculated | Error (ppm) | Formula | ||||
| F1 | 6.02 | Apigenin-6,8-di-C-glucoside | - | - | - | - | 593.15122 | 593.15119 | −0.04 | C27H29O15 | (−): 503, 473, 383, 353 |
| F2 | 6.96 | Schaftoside | 565.15541 | 565.15518 | −0.41 | C26H29O14 | 563.14072 | 563.14063 | −0.16 | C26H27O14 | (−): 545, 515, 503, 485, 473, 455, 443, 437, 425, 413, 407, 395, 383, 365, 353, 337, 335, 325, 323, 311, 297 |
| F3 | 7.16 | Isoschaftoside | 565.15754 | 565.15518 | −4.17 | C26H29O14 | 563.14032 | 563.14063 | 0.55 | C26H27O14 | (−): 545, 515, 503, 485, 473, 455, 443, 437, 425, 413, 407, 395, 383, 365, 353, 337, 335, 325, 323, 311, 297 |
| F4 | 7.19 | Viscidulin III-2′-O-glucoside isomer | 509.13073 | 509.12897 | −3.46 | C23H25O13 | 507.11404 | 507.11441 | 0.73 | C23H23O13 | (+): 347 |
| F5 | 7.34 | Chrysin-6,8-di-C-glucoside | 579.17156 | 579.17083 | −1.25 | C27H31O14 | 577.15585 | 577.15628 | 0.75 | C27H29O14 | (−): 487, 457, 409, 397, 379, 367, 349, 337, 321, 319, 309, 295, 281, 267 |
| F6 | 7.82 | Rutin | 611.16025 | 611.16066 | 0.67 | C27H31O16 | 609.14542 | 609.14611 | 1.13 | C27H29O16 | (+): 465, 303 |
| F7 | 7.90 | Chrysin 6-C-glucoside 8-C-arabinoside | 549.15924 | 549.16027 | 1.87 | C26H29O13 | 547.14467 | 547.14571 | 1.92 | C26H27O13 | (+): 531, 513, 495, 483, 477, 465, 459, 447, 441, 429, 423, 417, 411, 405, 399, 393, 387, 375, 363, 357, 351, 345, 339, 333, 321, 309, 291, 279 |
| F8 | 7.95 | Isovitexin | 433.11379 | 433.11292 | −1.99 | C21H21O10 | - | - | - | - | (+): 415, 397, 379, 367, 361, 351, 349, 337, 323, 313, 309, 295, 283 |
| F9 | 8.09 | Tetrahydroxyflavanone | 289.07117 | 289.07066 | −1.77 | C15H13O6 | 287.05575 | 287.05611 | 1.26 | C15H11O6 | (+): 153 |
| F10 | 8.10 | Isoquercitrin | 465.10341 | 465.10275 | −1.41 | C21H21O12 | 463.08779 | 463.08820 | 0.88 | C21H19O12 | (+): 303 |
| F11 | 8.15 | Kaempferol-3-O-rhamnose-7-O-glucose | - | - | - | - | 593.15122 | 593.15119 | −0.04 | C27H29O15 | (−): 285 |
| F12 | 8.22 | Scutellarin * | 463.08755 | 463.08710 | −0.96 | C21H19O12 | 461.07229 | 461.07255 | 0.56 | C21H17O12 | (−): 285 |
| F13 | 8.24 | Hyperin | 465.10326 | 465.10275 | −1.10 | C21H21O12 | 463.08787 | 463.08820 | 0.71 | C21H19O12 | (+): 303 |
| F14 | 8.26 | 2′,3′,4′,5,7-pentahydroxyflavone | 303.04997 | 303.04993 | −0.12 | C15H11O7 | 301.03517 | 301.03538 | 0.70 | C15H9O7 | (+): 153 |
| F15 | 8.34 | Viscidulin III-2′-O-glucoside | 509.12956 | 509.12897 | −1.17 | C23H25O13 | 507.11389 | 507.11441 | 1.04 | C23H23O13 | (+): 347 |
| F16 | 8.37 | Luteolin-7-O-rutinoside | - | - | - | - | 593.14991 | 593.15119 | 2.17 | C27H29O15 | (−): 285 |
| F17 | 8.43 | Chrysin-6-C-arabinoside-8-C-glucoside | 549.15943 | 549.16027 | 1.52 | C26H29O13 | 547.14480 | 547.14571 | 1.68 | C26H27O13 | (−): 487,457, 427, 409, 397, 391, 379, 373, 367, 363, 349, 337, 333, 331, 321, 319, 309, 307, 295, 293, 281 |
| F18 | 8.56 | Tetrahydroxyflavanone | 289.07094 | 289.07066 | −0.95 | C15H13O6 | 287.05535 | 287.05611 | 2.65 | C15H11O6 | (+): 153 |
| F19 | 8.57 | Quercetin 7-O-glucoside | 465.10325 | 465.10275 | −1.07 | C21H21O12 | 463.08776 | 463.08820 | 0.94 | C21H19O12 | (−): 301, 151 |
| F20 | 8.62 | Chrysin-6-C-arabinoside-8-C-glucoside isomer | 549.16023 | 549.16027 | 0.07 | C26H29O13 | 547.14503 | 547.14571 | 1.25 | C26H27O13 | (−): 487,457, 427, 409, 397, 391, 379, 373, 367, 363, 349, 337, 333, 331, 321, 319, 309, 307, 295, 293, 281 |
| F21 | 8.71 | Nicotiflorin | 595.16729 | 595.16575 | −2.60 | C27H31O15 | 593.15093 | 593.15119 | 0.45 | C27H29O15 | (+): 287 |
| F22 | 8.92 | Kaempferol-3-O-rhamnose-7-O-glucose isomer | 595.16675 | 595.16575 | −1.69 | C27H31O15 | 593.15039 | 593.15119 | 1.35 | C27H29O15 | (−): 285 |
| F23 | 9.04 | 5,6′-dihydroxy-6,7-dimethoxyflavone-2′-O-glucoside | 493.13457 | 493.13405 | −1.06 | C23H25O12 | 491.11905 | 491.11950 | 0.91 | C23H23O12 | (−): 329 |
| F24 | 9.16 | Quercitrin | 449.10890 | 449.10784 | −2.37 | C21H21O11 | 447.09288 | 447.09329 | 0.91 | C21H19O11 | (−): 301 |
| F25 | 9.16 | Oroxin B | 595.16702 | 595.16575 | −2.14 | C27H31O15 | 593.15081 | 593.15119 | 0.65 | C27H29O15 | (−): 269 |
| F26 | 9.40 | Chrysin 8-C-glucoside | 417.11859 | 417.11801 | −1.40 | C21H21O9 | 415.10314 | 415.10346 | 0.77 | C21H19O9 | (+): 399, 381, 363, 351, 339, 335, 327, 321, 307, 297, 279, 267 |
| F27 | 9.43 | Apigenin-7-O-glucoside | 433.11325 | 433.11292 | −0.75 | C21H21O10 | 431.09772 | 431.09837 | 1.51 | C21H19H10 | (+): 271 |
| F28 | 9.49 | Linarin | - | - | - | - | 591.17133 | 591.17193 | 1.02 | C28H31O14 | (−): 283 |
| F29 | 9.55 | Isorhamnetin-3-O-glucoside | 479.11935 | 479.11840 | −1.98 | C22H23O12 | 477.10352 | 477.10385 | 0.70 | C22H21O12 | (+): 317 |
| F30 | 9.58 | Hesperidin | - | - | - | - | 609.18261 | 609.18249 | −0.19 | C28H33O15 | (−): 301 |
| F31 | 9.72 | 5,2′,6′-dihydroxy-6,7,8-trimethoxyflavone-2′-O-glucoside | 523.14492 | 523.14462 | −0.59 | C24H27O13 | 521.12952 | 521.13006 | 1.04 | C24H25O13 | (−): 359, 344, 329 |
| F32 | 9.84 | Kaempferide 3-glucuronide | 477.10324 | 477.10275 | −1.02 | C22H21O12 | 475.08784 | 475.08820 | 0.76 | C22H19O12 | (−): 299, 284 |
| F33 | 9.86 | Astragalin | 449.10851 | 449.10784 | −1.50 | C21H21O11 | 447.09287 | 447.09329 | 0.93 | C21H19O11 | (+): 287 |
| F34 | 9.95 | Viscidulin I | 303.05004 | 303.04993 | −0.37 | C15H11O7 | 301.03513 | 301.03538 | 0.81 | C15H9O7 | (−): 151 |
| F35 | 10.01 | Chrysoeriol-5-O-β-D-glucuronide | 477.10317 | 477.10275 | −0.88 | C22H21O12 | 475.08768 | 475.08820 | 1.09 | C22H19O12 | (−): 299 |
| F36 | 10.11 | Scutellarein-4′-methyl ether-O-glucuronide | 477.10272 | 477.10275 | 0.06 | C22H21O12 | 475.08773 | 475.08820 | 0.99 | C22H19O12 | (−): 443, 284, 173, 155 |
| F37 | 10.27 | Scutellarein | 287.05510 | 287.05501 | −0.29 | C15H11O6 | 285.04023 | 285.04046 | 0.81 | C15H9O6 | (+): 269, 241 |
| F38 | 10.27 | 2′,6′,7-trihydroxy-5-methoxyflavanone | 303.08649 | 303.08631 | −0.58 | C16H15O6 | 301.07155 | 301.07176 | 0.72 | C16H13O6 | (+): 167, 152, 123 |
| F39 | 10.60 | Syringetin | 347.07655 | 347.07614 | −1.17 | C17H15O8 | 345.06107 | 345.06159 | 1.50 | C17H13O8 | (+): 332 |
| F40 | 10.93 | Dihydrokaempferol | 289.07086 | 289.07066 | −0.67 | C15H13O6 | 287.05588 | 287.05611 | 0.82 | C15H11O6 | (+): 271, 153 |
| F41 | 10.94 | Trihyroxy-dimethoxy flavone-O-glucuronide | 507.11405 | 507.11332 | −1.44 | C23H23O13 | 505.09814 | 505.09876 | 1.23 | C23H21O13 | (+): 331 |
| F42 | 11.05 | Baicalin * | 447.09220 | 447.09219 | −0.03 | C21H19O11 | 445.07677 | 445.07763 | 1.94 | C21H17O11 | (−): 269 |
| F43 | 11.06 | Fisetin | 287.05504 | 287.05501 | −0.09 | C15H11O6 | - | - | - | - | (+): 269, 258, 241, 231, 223, 213 |
| F44 | 11.12 | Viscidulin III | 347.07619 | 347.07614 | −0.14 | C17H15O8 | 345.06120 | 345.06159 | 1.14 | C17H13O8 | (+): 332, 314, 286 |
| F45 | 11.20 | Oroxin A | 433.11331 | 433.11292 | −0.89 | C21H21O10 | 431.09794 | 431.09837 | 1.01 | C21H19O10 | (+): 271 |
| F46 | 11.24 | Chrysin 6-C-glucoside | 417.11836 | 417.11801 | −0.85 | C21H21O9 | 415.10304 | 415.10346 | 1.01 | C21H19O9 | (+): 381, 363, 351, 345, 335, 327, 333, 321, 307, 297, 293, 279, 267 |
| F47 | 11.33 | Tetrahydroxyflavanone | 289.07083 | 289.07066 | −0.56 | C15H13O6 | 287.05592 | 287.05611 | 0.68 | C15H11O6 | (+): 153 |
| F48 | 11.38 | Trihyroxy-dimethoxy flavone-O-glucuronide | 507.11429 | 507.11332 | −1.91 | C23H23O13 | 505.09829 | 505.09876 | 0.94 | C23H21O13 | (+): 331 |
| F49 | 11.56 | Tetrahydroxyflavanone | 289.07097 | 289.07066 | −1.05 | C15H13O6 | 287.05586 | 287.05611 | 0.89 | C15H11O6 | (+): 153 |
| F50 | 11.67 | Baicalin ethyl ester or isomer | 475.12476 | 475.12349 | −2.68 | C23H23O11 | 473.10838 | 473.10894 | 1.17 | C23H21O11 | (+): 271 |
| F51 | 11.78 | Chrysoeriol-7-O-β-D-glucuronide | 477.10404 | 477.10275 | −2.71 | C22H21O12 | 475.08786 | 475.08820 | 0.72 | C22H19O12 | (−): 299, 284 |
| F52 | 11.88 | 5,6-dihydroxyl flavanone-7-O-glucuronide | 449.10885 | 449.10784 | −2.25 | C21H21O11 | 447.09264 | 447.09329 | 1.44 | C21H19O11 | (−): 271 |
| F53 | 11.89 | Trihydroxyflavanone | 273.07602 | 273.07575 | −0.97 | C15H13O5 | 271.06090 | 271.06120 | 1.08 | C15H11O5 | (+): 153 |
| F54 | 12.08 | Oroxin A isomer | - | - | - | - | 431.09797 | 431.09837 | 0.92 | C21H19O10 | (−): 269 |
| F55 | 12.08 | Oroxylin A 7-O-β-D-glucoside | 447.13030 | 447.12857 | −3.85 | C22H23O10 | 445.11344 | 445.11402 | 1.31 | C22H21O10 | (−): 268 |
| F56 | 12.16 | Dihydroxyl flavanone-O-glucuronide | 449.10921 | 449.10784 | −3.06 | C21H21O11 | 447.09263 | 447.09329 | 1.46 | C21H19O11 | (−): 271 |
| F57 | 12.26 | Norwogonin-7-O-glucuronide | 447.09270 | 447.09219 | −1.14 | C21H19O11 | 445.07679 | 445.07763 | 1.90 | C21H17O11 | (−): 269 |
| F58 | 12.38 | 2′-hydroxyformononetin | 285.07651 | 285.07575 | −2.66 | C16H13O5 | 283.06083 | 283.06120 | 1.29 | C16H11O5 | (+): 270 |
| F59 | 12.42 | Fisetin isomer | 287.05580 | 287.05501 | −2.74 | C15H11O6 | 285.04020 | 285.04046 | 0.91 | C15H9O6 | (+): 241 |
| F60 | 12.50 | Hydroxyl oroxylin-A-7-O-glucuronide | 477.10448 | 477.10275 | −3.62 | C22H21O12 | 475.08756 | 475.08820 | 1.35 | C22H19O12 | (−): 299, 284 |
| F61 | 12.72 | Norwogonin-8-O-glucuronide isomer | 447.09344 | 447.09219 | −2.81 | C21H19O11 | 445.07693 | 445.07763 | 1.57 | C21H17O11 | (−): 269 |
| F62 | 12.74 | Dihydroxyflavone-O-glucopyranoside | 417.11905 | 417.11801 | −2.50 | C21H21O9 | 415.10311 | 415.10346 | 0.83 | C21H19O9 | (+): 255 |
| F63 | 12.75 | Kaempferin | 433.11420 | 433.11292 | −2.94 | C21H21O10 | 431.09791 | 431.09837 | 1.06 | C21H19O10 | (+): 271 |
| F64 | 12.79 | Tricin 7-O-glucoside | - | - | - | - | 491.11895 | 491.11950 | 1.11 | C23H23O12 | (−): 329, 313 |
| F65 | 12.82 | Quercetin | - | - | - | - | 301.03524 | 301.03538 | 0.45 | C15H9O7 | (−): 151 |
| F66 | 12.97 | Oroxylin A-7-O-glucuronide | 461.10755 | 461.10784 | 0.62 | C22H21O11 | 459.09257 | 459.09329 | 1.55 | C22H19O11 | (+): 285, 270 |
| F67 | 13.00 | Chrysin-7-O-glucuronide | 431.09825 | 431.09727 | −2.26 | C21H19O10 | 429.08215 | 429.08272 | 1.34 | C21H17O10 | (−): 253 |
| F68 | 13.08 | Hydroxyl wogonoside | 477.10320 | 477.10275 | −0.94 | C22H21O12 | 475.08742 | 475.08820 | 1.65 | C22H19O12 | (−): 299 |
| F69 | 13.35 | Norwogonin-8-O-glucuronide | 447.09296 | 447.09219 | −1.73 | C21H19O11 | 445.07720 | 445.07763 | 0.97 | C21H17O11 | (−): 269 |
| F70 | 13.48 | Oroxin A isomer | 433.11342 | 433.11292 | −1.15 | C21H21O10 | 431.09809 | 431.09837 | 0.64 | C21H19H10 | (+): 271 |
| F71 | 13.62 | Wogonoside * | 461.10765 | 461.10784 | 0.40 | C22H21O11 | 459.09255 | 459.09329 | 1.60 | C22H19O11 | (−): 283, 268 |
| F72 | 13.84 | Dihydrooroxylin A | 287.09159 | 287.09140 | −0.65 | C16H15O5 | 285.07658 | 285.07685 | 0.94 | C16H13O5 | - |
| F73 | 13.85 | (2S)-5-hydroxy-6-methoxyflavanone 7-O-β-D-glucuronide | 463.12401 | 463.12349 | −1.12 | C22H23O11 | 461.10839 | 461.10894 | 1.18 | C22H21O11 | (−): 285, 270 |
| F74 | 13.91 | Trihydroxy-methoxyflavanone | 303.08658 | 303.08631 | −0.89 | C16H15O6 | 301.07153 | 301.07176 | 0.78 | C16H13O6 | (+): 147, 135 |
| F75 | 13.94 | Oroxin A isomer | 433.11357 | 433.11292 | −1.49 | C21H21O10 | 431.09808 | 431.09837 | 0.66 | C21H19H10 | (+): 271 |
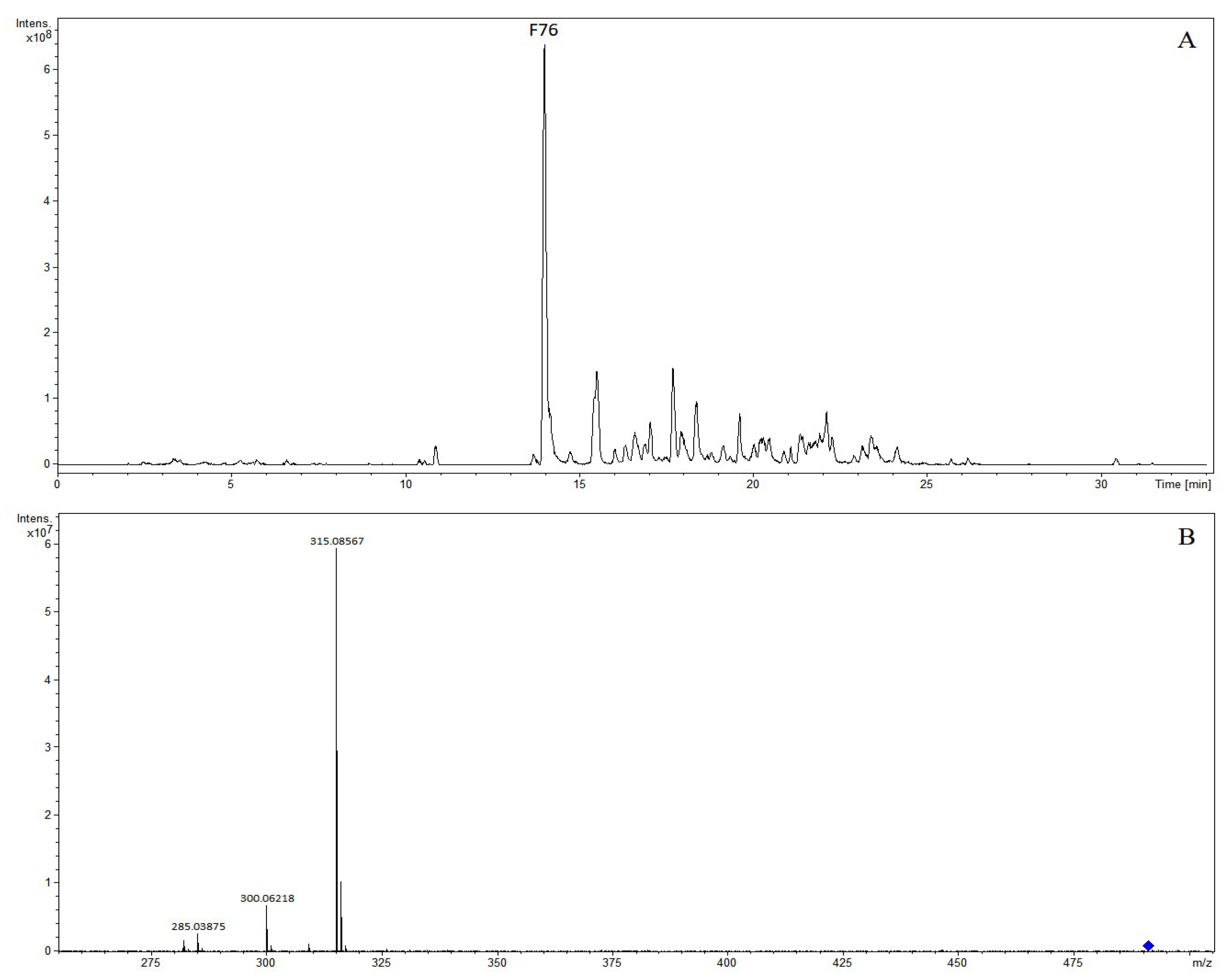
| F76 | 13.97 | 5,7-dihydroxy-6,8-dimethoxyflavone-7-O-glucuronide | 491.11836 | 491.11840 | 0.09 | C23H23O12 | 489.10335 | 489.10385 | 1.03 | C23H21O12 | (+): 315, 300, 285 |
| F77 | 14.08 | Viscidulin II isomer | 331.08144 | 331.08123 | −0.63 | C17H15O7 | 329.06652 | 329.06668 | 0.48 | C17H13O7 | (+): 316, 301 |
| F78 | 14.08 | (2S)-5-hydroxy-6-methoxyflavanone 7-O-β-D-glucuronide isomer | 463.12409 | 463.12349 | −1.30 | C22H23O11 | 461.10851 | 461.10894 | 0.93 | C22H21O11 | (−): 285, 270 |
| F79 | 14.10 | Baicalin ethyl ester or isomer | 475.12392 | 475.12349 | −0.92 | C23H23O11 | 473.10881 | 473.10894 | 0.26 | C23H21O11 | (+): 271 |
| F80 | 14.20 | Skullcapflavone II isomer | 375.10761 | 375.10744 | −0.45 | C19H19O8 | 373.09286 | 373.09289 | 0.08 | C19H17O8 | (+): 360, 345, 327 |
| F81 | 14.50 | Mosloflavone isomer | 299.09137 | 299.09140 | 0.10 | C17H15O5 | 297.07684 | 297.07685 | 0.02 | C17H13O5 | (+): 238 |
| F82 | 14.58 | Epimedoside C or isomer | 517.17064 | 517.17044 | −0.39 | C26H29O11 | 515.15542 | 515.15589 | 0.90 | C26H27O11 | (+): 355 |
| F83 | 14.68 | Chrysosplenin or isomer | 537.16057 | 537.16027 | −0.57 | C25H29O13 | - | - | - | - | (+): 375, 345 |
| F84 | 14.80 | Baicalin ethyl ester or isomer | 475.12353 | 475.12349 | −0.09 | C23H23O11 | 473.10828 | 473.10894 | 1.38 | C23H21O11 | (+): 271 |
| F85 | 14.82 | Phellamurin | 519.18583 | 519.18609 | 0.50 | C26H31O11 | 563.17651 | 563.17701 | 0.89 a | C27H31O13 | (−): 517 |
| F86 | 14.88 | Trihydroxy-methoxyflavanone | 303.08625 | 303.08631 | 0.21 | C16H15O6 | 301.07157 | 301.07176 | 0.64 | C16H13O6 | (+): 147, 135 |
| F87 | 14.95 | Alpinetin | 271.09657 | 271.09649 | −0.32 | C16H15O4 | - | - | - | - | (+): 167 |
| F88 | 15.06 | Tenaxin II | 301.07046 | 301.07066 | 0.69 | C16H13O6 | 299.05588 | 299.05611 | 0.77 | C16H11O6 | (−): 284 |
| F89 | 15.14 | Apigenin * | 271.05992 | 271.06010 | 0.65 | C15H11O5 | 269.04529 | 269.04555 | 0.96 | C15H9O5 | (−): 237, 225, 201, 175, 151, 149 |
| F90 | 15.14 | 5,2′,5′-trihydroxy-6,7,8-trimethoxyflavone or isomer | 361.09173 | 361.09179 | 0.17 | C18H17O8 | 359.07692 | 359.07724 | 0.90 | C18H15O8 | (+): 346, 331, 328, 313 |
| F91 | 15.15 | Kaempferol isomer | 287.05503 | 287.05501 | −0.06 | C15H11O6 | 285.04027 | 285.04046 | 0.67 | C15H9O6 | (−): 227, 211 |
| F92 | 15.23 | 5,2′,6′-dihydroxy-6,7,8-trimethoxyflavone-2′-O-glucoside isomer | 523.14464 | 523.14462 | −0.05 | C24H27O13 | 521.12962 | 521.13006 | 0.86 | C24H25O13 | (+): 361 |
| F93 | 15.31 | Viscidulin II isomer | 331.08119 | 331.08123 | 0.12 | C17H15O7 | 329.06642 | 329.06668 | 0.78 | C17H13O7 | (+): 316, 301 |
| F94 | 15.47 | Tectorigenin | 301.07062 | 301.07066 | 0.14 | C16H13O6 | 299.05586 | 299.05611 | 0.86 | C16H11O6 | (+): 286 |
| F95 | 15.52 | Kaempferol | 287.05507 | 287.05501 | −0.18 | C15H11O6 | 285.04023 | 285.04046 | 0.80 | C15H9O6 | (+): 269, 258, 241, 231, 213 |
| F96 | 15.73 | Norwogonin | 271.06005 | 271.06010 | 0.18 | C15H11O5 | 269.04531 | 269.04555 | 0.89 | C15H9O5 | (+): 253, 225 |
| F97 | 15.78 | Viscidulin II isomer | 331.08125 | 331.08123 | −0.07 | C17H15O7 | 329.06661 | 329.06668 | 0.21 | C17H13O7 | (+): 316 |
| F98 | 15.85 | Baicalin ethyl ester or isomer | 475.12347 | 475.12349 | 0.05 | C23H23O11 | 473.10934 | 473.10894 | −0.86 | C23H21O11 | (+): 271 |
| F99 | 16.00 | Trihydroxyflavanone | 273.07572 | 273.07575 | 0.12 | C15H13O5 | 271.06100 | 271.06120 | 0.74 | C15H11O5 | (+): 153 |
| F100 | 16.07 | 4′-hydroxyl wogonin | 301.07044 | 301.07066 | 0.75 | C16H13O6 | 299.05577 | 299.05611 | 1.15 | C16H11O6 | (+): 286 |
| F101 | 16.10 | 5,2′,5′-trihydroxy-6,7,8-trimethoxyflavone or isomer | 361.09207 | 361.09179 | −0.77 | C18H17O8 | 359.07696 | 359.07724 | 0.78 | C18H15O8 | (+): 346, 331, 328, 313 |
| F102 | 16.16 | Viscidulin II | 331.08127 | 331.08123 | −0.13 | C17H15O7 | 329.06645 | 329.06668 | 0.69 | C17H13O7 | (+): 316 |
| F103 | 16.32 | Baicalein * | 271.05990 | 271.06010 | 0.72 | C15H11O5 | 269.04525 | 269.04555 | 1.11 | C15H9O5 | (+): 253, 225 |
| F104 | 16.35 | Fisetin isomer | 287.05543 | 287.05501 | −1.45 | C15H11O6 | 285.04034 | 285.04046 | 0.42 | C15H9O6 | (+): 241 |
| F105 | 16.36 | Skullcapflavone II isomer | 375.10807 | 375.10744 | −1.66 | C19H19O8 | 373.09286 | 373.09289 | 0.08 | C19H17O8 | (+): 360, 345, 327 |
| F106 | 16.40 | Kaempferide isomer | 301.07060 | 301.07066 | 0.21 | C16H13O6 | 299.05586 | 299.05611 | 0.86 | C16H11O6 | (+): 286 |
| F107 | 16.40 | 5,2′,5′-trihydroxy-6,7,8-trimethoxyflavone or isomer | 361.09177 | 361.09179 | 0.07 | C18H17O8 | 359.07724 | 359.07724 | 0.00 | C18H15O8 | (+): 346, 331, 328, 313 |
| F108 | 16.68 | Trihydroxyflavanone | 273.07580 | 273.07575 | −0.19 | C15H13O5 | 271.06095 | 271.06120 | 0.90 | C15H11O5 | (+): 153 |
| F109 | 16.75 | Tricin | 331.08117 | 331.08123 | 0.19 | C17H15O7 | 329.06638 | 329.06668 | 0.92 | C17H13O7 | (−): 314, 299 |
| F110 | 17.14 | 5,8,2′-trihydroxy-7-methoxyflavone | 301.07071 | 301.07066 | −0.14 | C16H13O6 | 299.05608 | 299.05611 | 0.12 | C16H11O6 | (+): 286 |
| F111 | 17.23 | 5,8,2′-trihydroxy-6,7-dimethoxyflavone | 331.08112 | 331.08123 | 0.32 | C17H15O7 | 329.06661 | 329.06668 | 0.21 | C17H13O7 | (+): 316, 301 |
| F112 | 17.40 | Trihydroxy-methoxyflavanone | 303.08621 | 303.08631 | 0.36 | C16H15O6 | 301.07156 | 301.07176 | 0.68 | C16H13O6 | (+): 147, 135 |
| F113 | 18.46 | 5,7-dihydroxy-8,2′,3′,6-tetramethoxyflavone or isomer | 391.10200 | 391.10236 | 0.92 | C19H19O9 | 389.08755 | 389.08781 | 0.66 | C19H17O9 | (+): 361 |
| F114 | 18.63 | Trimethoxyflavone | 313.10681 | 313.10705 | 0.76 | C18H17O5 | - | - | - | - | (+): 298, 283 |
| F115 | 18.70 | Trihydroxy-dimethoxyflavone | 331.08108 | 331.08123 | 0.46 | C17H15O7 | 329.06646 | 329.06668 | 0.66 | C17H13O7 | (+): 316, 301 |
| F116 | 19.68 | Dihydrooroxylin A isomer | 287.09142 | 287.09140 | −0.07 | C16H15O5 | 285.07672 | 285.07685 | 0.46 | C16H13O5 | - |
| F117 | 19.71 | 5,7,3′-trihydroxy-6,4′,5′-trimethoxyflavone | 361.09172 | 361.09179 | 0.21 | C18H17O8 | 359.07710 | 359.07724 | 0.39 | C18H15O8 | (−): 344, 329, 314 |
| F118 | 20.12 | Rivularin | 345.09650 | 345.09688 | 1.10 | C18H17O7 | 343.08212 | 343.08233 | 0.59 | C18H15O7 | (−): 328, 313, 298, 285, 270 |
| F119 | 20.41 | Wogonin * | 285.07536 | 285.07575 | 1.35 | C16H13O5 | 283.06071 | 283.06120 | 1.72 | C16H11O5 | (+): 270 |
| F120 | 20.61 | Amentoflavone isomer | 539.09726 | 539.09727 | 0.02 | C30H19O10 | 537.08211 | 537.08272 | 1.14 | C30H17O10 | (−): 391, 373, 268, 245 |
| F121 | 20.63 | Chrysin | 255.06516 | 255.06519 | 0.11 | C15H11O4 | 253.05045 | 253.05063 | 0.73 | C15H9O4 | (−): 209 |
| F122 | 20.78 | Skullcapflavone II isomer | 375.10723 | 375.10744 | 0.58 | C19H19O8 | 373.09290 | 373.09289 | −0.03 | C19H17O8 | (+): 360, 345, 327 |
| F123 | 20.82 | Dihydrooroxylin A isomer | 287.09149 | 287.09140 | −0.30 | C16H15O5 | 285.07676 | 285.07685 | 0.30 | C16H13O5 | - |
| F124 | 20.88 | Skullcapflavone I | 315.08618 | 315.08631 | 0.44 | C17H15O6 | 313.07148 | 313.07176 | 0.89 | C17H13O6 | (−): 298, 283, 255, 239, 227, 211, 201, 199, 183, 173, 155 |
| F125 | 21.21 | Skullcapflavone II | 375.10743 | 375.10744 | 0.03 | C19H19O8 | 373.09252 | 373.09289 | 0.99 | C19H17O8 | (−): 358, 343, 328, 315, 300, 285, 272, 269, 257, 213 |
| F126 | 21.33 | Oroxylin A | 285.07557 | 285.07575 | 0.62 | C16H13O5 | 283.06108 | 283.06120 | 0.43 | C16H11O5 | (+): 270 |
| F127 | 21.53 | Dihydrooroxylin A isomer | 287.09137 | 287.09140 | 0.10 | C16H15O5 | 285.07677 | 285.07685 | 0.25 | C16H13O5 | - |
| F128 | 21.59 | 5,8-dihydroxy-6,7-dimethoxyflavone | 315.08612 | 315.08631 | 0.63 | C17H15O6 | 313.07150 | 313.07176 | 0.84 | C17H13O6 | (−): 298, 283, 255, 239, 227, 211, 183 |
| F129 | 21.67 | Amentoflavone isomer | 539.09716 | 539.09727 | 0.20 | C30H19O10 | 537.08265 | 537.08272 | 0.13 | C30H17O10 | - |
| F130 | 22.60 | 5,2′-dihydroxy-6,7,8-trimethoxyflavone | 345.09651 | 345.09688 | 1.08 | C18H17O7 | 343.08199 | 343.08233 | 0.98 | C18H15O7 | (−): 328, 313, 298, 295, 270 |
| F131 | 23.71 | 7-O-methylscutellarein-O-baicalein | 553.11268 | 553.11292 | 0.44 | C31H21O10 | 551.09789 | 551.09837 | 0.87 | C31H19O10 | (−): 281, 269 |
| F132 | 24.09 | Mosloflavone isomer | 299.09128 | 299.09140 | 0.40 | C17H15O5 | - | - | - | - | (+): 284 |
| F133 | 24.77 | Moslosooflavone | 299.09130 | 299.09140 | 0.32 | C17H15O5 | - | - | - | - | (+): 284, 266, 238 |
| F134 | 25.46 | Mosloflavone | 299.09133 | 299.09140 | 0.22 | C17H15O5 | - | - | - | - | (+): 284, 255 |
| No. | RT (min) | Identification | [M-H]+ | [M-H]− | Product Ion (m/z) | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Observed | Calculated | Error (ppm) | Formula | Observed | Calculated | Error (ppm) | Formula | ||||
| A1 | 1.62 | Candicine | 180.13838 | 180.13829 | −0.51 | C11H18NO+ | - | - | - | - | (+): 121 |
| A2 | 2.51 | N-methylhigenamine 7-glucopyranoside isomer | 448.19671 | 448.19659 | −0.25 | C23H30NO8 | - | - | - | - | (+): 286 |
| A3 | 3.43 | Juziphine isomer | 300.15938 | 300.15942 | 0.13 | C18H22NO3 | - | - | - | - | (+): 107 |
| A4 | 3.99 | N-methylhigenamine 7-glucopyranoside isomer | 448.19649 | 448.19659 | 0.23 | C23H30NO8 | - | - | - | - | (+): 286 |
| A5 | 4.05 | Laudanosoline 6-mether-4′-O-glucopyranoside isomer | 478.20728 | 478.20716 | −0.26 | C24H32NO9 | - | - | - | - | (+): 316 |
| A6 | 4.37 | Higenamine | 272.12795 | 272.12812 | 0.64 | C16H18NO3 | - | - | - | - | (+): 107 |
| A7 | 4.42 | Laudanosoline 6-mether-4′-O-glucopyranoside | 478.20735 | 478.20716 | −0.40 | C24H32NO9 | - | - | - | - | (+): 316 |
| A8 | 4.43 | Juziphine-4′-O-glucopyranoside | 462.21227 | 462.21224 | −0.06 | C24H32NO8 | - | - | - | - | (+): 300 |
| A9 | 4.57 | Govadine | 328.15405 | 328.15433 | 0.87 | C19H22NO4 | - | - | - | - | (+): 178 |
| A10 | 4.71 | Coclaurine isomer | 286.14372 | 286.14377 | 0.16 | C17H20NO3 | - | - | - | - | - |
| A11 | 5.07 | Isaindigodione isomer | 327.13367 | 327.13393 | 0.80 | C18H19N2O4 | - | - | - | - | (+): 201 |
| A12 | 5.16 | Protosinomenine-3′-O-glucopyranoside | 492.22311 | 492.22281 | −0.62 | C25H34NO9 | - | - | - | - | (+): 330, 299, 192 |
| A13 | 5.28 | Unknown | 328.15414 | 328.15433 | 0.58 | C19H22NO4 | - | - | - | - | (+): 192 |
| A14 | 5.34 | Corypalmine | 342.17025 | 342.16998 | −0.78 | C20H24NO4 | - | - | - | - | (+): 297 |
| A15 | 5.37 | Isaindigodione | 327.13345 | 327.13393 | 1.47 | C18H19N2O4 | - | - | - | - | (+): 201 |
| A16 | 5.41 | Lotusine | 314.17516 | 314.17507 | −0.29 | C19H24NO3 | - | - | - | - | (+): 269, 237 |
| A17 | 5.43 | lndole-3-acetonitrile-6-O-β-D-glucopyranoside | 335.12407 | 335.12376 | −0.92 | C16H19N2O6 | 379.11463 | 379.11469 | 0.16 a | C17H19N2O8 | (−): 333, 306, 144 |
| A18 | 5.46 | Protosinomenine isomer | 330.17006 | 330.16998 | −0.23 | C19H24NO4 | - | - | - | - | - |
| A19 | 5.51 | (E)-3-(3′,5′-dimethoxy-4′-hydroxy-benzylidene)-2-indolinone | 298.10770 | 298.10738 | −1.06 | C17H16NO4 | - | - | - | - | - |
| A20 | 5.57 | Protosinomenine-3′-O-glucopyranoside isomer | 492.22283 | 492.22281 | −0.04 | C25H34NO9 | - | - | - | - | (+): 300 |
| A21 | 5.63 | Indican | 296.11296 | 296.11286 | −0.33 | C14H18NO6 | 340.10358 | 340.10379 | 0.61 a | C15H18NO8 | (−): 294, 161, 132 |
| A22 | 5.65 | Unknown | 356.14910 | 356.14925 | 0.43 | C20H22NO5 | - | - | - | - | (+): 206 |
| A23 | 5.74 | 3,4-dihydro-[(4-hydroxyphenyl)methyl]-7-methoxy-2-methyl-8-isoquinolinol | 298.14366 | 298.14377 | 0.35 | C18H20NO3 | - | - | - | - | (+): 283, 282, 254 |
| A24 | 5.80 | Magnocurarine | 314.17486 | 314.17507 | 0.66 | C19H24NO3 | - | - | - | - | (+): 269, 237 |
| A25 | 5.81 | 2,3-dihydro-1H-pyrrolo[2,1-c][1,4]benzodiazepine-5,11(10H,11ah)-dione | 217.09734 | 217.09715 | −0.85 | C12H13N2O2 | - | - | - | - | (+): 144 |
| A26 | 5.85 | Juziphine-4′-O-glucopyranoside | 462.21275 | 462.21224 | −1.10 | C24H32NO8 | - | - | - | - | (+): 300 |
| A27 | 5.95 | Unknown | 356.14938 | 356.14925 | −0.36 | C20H22NO5 | - | - | - | - | (+): 206 |
| A28 | 6.05 | Phellodendrine | 342.16909 | 342.16998 | 2.62 | C20H24NO4 | - | - | - | - | (+): 192 |
| A29 | 6.15 | N-methylhigenamine 7-glucopyranoside | 448.19537 | 448.19659 | 2.72 | C23H30NO8 | 446.18187 | 446.18204 | 0.37 | C23H28NO8 | (+): 286, 255 |
| A30 | 6.20 | Anthranilic acid-7-O-β-D-glucopyranose ester | 300.10827 | 300.10778 | −1.64 | C13H18NO7 | - | - | - | - | (+): 282, 264, 246, 138 |
| A31 | 6.40 | Tembetarine | 344.18565 | 344.18563 | −0.03 | C20H26NO4 | - | - | - | - | (+): 175, 137 |
| A32 | 6.43 | 8-O-methyloblongine | 328.19083 | 328.19072 | −0.33 | C20H26NO3 | - | - | - | - | (+): 313, 283 |
| A33 | 6.46 | (1S)-1,2,3,4-tetrahydro-7-hydroxy-1-[(4-hydroxybenzyl)methyl]-2,2-dimethyl-8-O-isoquinolinyl-[3-hydroxy-3-methylglutaryl]-β-D-glucopyranoside | 592.23893 | 592.23885 | −0.13 | C29H38NO12 | 590.22428 | 590.22430 | 0.03 | C29H36NO12 | (+): 530, 490, 448, 286 |
| A34 | 6.52 | Magnoflorine | 342.16988 | 342.16998 | 0.31 | C20H24NO4 | - | - | - | - | (+): 297, 282, 265, 237 |
| A35 | 6.61 | Piperlonguminine | 274.14417 | 274.14377 | −1.46 | C16H20NO3 | - | - | - | - | (+): 256, 227 |
| A36 | 6.66 | Unknown | 312.12357 | 312.12303 | −1.72 | C18H18NO4 | - | - | - | - | (+): 297 |
| A37 | 6.87 | Protosinomenine | 330.17032 | 330.16998 | −1.01 | C19H24NO4 | - | - | - | - | (+): 192 |
| A38 | 7.11 | Fuzitine | 342.16969 | 342.16998 | 0.86 | C20H24NO4 | - | - | - | - | (+): 192 |
| A39 | 7.13 | Oblongine | 314.17491 | 314.17507 | 0.51 | C19H24NO3 | - | - | - | - | (+): 269, 237, 175, 143 |
| A40 | 7.19 | Laudanosine | 358.20120 | 358.20128 | 0.24 | C21H28NO4 | - | - | - | - | (+): 327, 189 |
| A41 | 7.29 | Deoxyvasicinone | 187.08643 | 187.08659 | 0.83 | C11H11N2O | - | - | - | - | (+): 146 |
| A42 | 7.35 | Unknown | 358.20108 | 358.20128 | 0.56 | C21H28NO4 | - | - | - | - | (+): 189 |
| A43 | 7.43 | Menisperine | 356.18596 | 356.18563 | −0.92 | C21H26NO4+ | - | - | - | - | (+): 311, 296, 279 |
| A44 | 7.74 | Tetrahydroberberine isomer | 340.15482 | 340.15433 | −1.41 | C20H22NO4 | - | - | - | - | - |
| A45 | 7.85 | Tembetarine isomer | 344.18582 | 344.18563 | −0.54 | C20H26NO4+ | - | - | - | - | (+): 137 |
| A46 | 7.85 | Methylcorydine | 356.18579 | 356.18563 | −0.43 | C21H26NO4+ | - | - | - | - | (+): 311, 279 |
| A47 | 7.93 | Pilocarpine isomer | 260.12826 | 260.12812 | −0.53 | C15H18NO3 | - | - | - | - | - |
| A48 | 8.09 | 8-O-methyloblongine isomer | 328.19121 | 328.19072 | −1.50 | C20H26NO3 | - | - | - | - | (+): 283 |
| A49 | 8.27 | Tetrahydroberberine isomer | 340.15452 | 340.15433 | −0.55 | C20H22NO4 | - | - | - | - | (+): 309, 192 |
| A50 | 8.28 | 1-Methoxyindole-3-acetamide | 205.09713 | 205.09715 | 0.11 | C11H13N2O2 | - | - | - | - | (+): 188 |
| A51 | 8.30 | Cryptopine | 370.16531 | 370.16490 | −1.11 | C21H24NO5 | - | - | - | - | - |
| A52 | 8.49 | Isaindigodione isomer | 327.13486 | 327.13393 | −2.83 | C18H19N2O4 | - | - | - | - | (+): 201 |
| A53 | 8.66 | 5,8,13,13a-tetrahydro-2,9,10,11-tetrahydro-3-methoxy-7-methy-6H-dibenz[a,g]quinoline | 324.12323 | 324.12303 | −0.60 | C19H18NO4 | - | - | - | - | (+): 294, 280 |
| A54 | 8.79 | Dasycarpamin | 304.15478 | 304.15433 | −1.46 | C17H22NO4 | - | - | - | - | - |
| A55 | 8.85 | Armepavine | 314.17539 | 314.17507 | −1.00 | C19H24NO3 | - | - | - | - | (+): 269, 237 |
| A56 | 9.02 | Rotundine | 356.18565 | 356.18563 | −0.03 | C21H26NO4 | - | - | - | - | (+): 192 |
| A57 | 9.03 | Platydesmine | 260.12836 | 260.12812 | −0.91 | C15H18NO3 | - | - | - | - | (+): 242 |
| A58 | 9.15 | Demethyleneberberine | 324.12337 | 324.12303 | −1.03 | C19H18NO4 | - | - | - | - | (+): 309, 308, 294, 280 |
| A59 | 9.70 | Indole-3-acetonitrile-2-S-β-D-glucopyranoside | - | - | - | - | 349.08596 | 349.08637 | 1.17 | C16H17N2O5S | (−): 187, 160 |
| A60 | 9.91 | Platydesmine isomer | 260.12817 | 260.12812 | −0.19 | C15H18NO3 | - | - | - | - | - |
| A61 | 10.00 | 11-hydroxypalmatine or 13-hydroxypalmatine | 368.14971 | 368.14925 | −1.25 | C21H22NO5 | - | - | - | - | (+): 352, 323, 322 |
| A62 | 10.00 | 1-hydroxyberberine | 352.11789 | 352.11795 | 0.17 | C20H18NO5 | - | - | - | - | (+): 308 |
| A63 | 10.10 | 3-(2′-carboxyphenyl)-4(3H)-quinazolinone | 267.07661 | 267.07642 | −0.73 | C15H11N2O3 | - | - | - | - | (+): 249 |
| A64 | 10.14 | N-methylcanadine isomer | 354.17009 | 354.16998 | −0.28 | C21H24NO4 | - | - | - | - | (+): 190 |
| A65 | 10.30 | Cryptopine isomer | 370.16510 | 370.16490 | −0.55 | C21H24NO5 | - | - | - | - | - |
| A66 | 10.69 | Indole-3-acetonitrile-4-methoxy-2-S-β-D-glucopyranoside or N-Methoxyindole-3-acetonitrile-2-S-β-D-glucopyranoside | 381.11206 | 381.11148 | −1.51 | C17H21N2O6S | 379.09657 | 379.09693 | 0.95 | C17H19N2O6S | (+): 219 |
| A67 | 11.10 | Berberrubine | 322.10757 | 322.10738 | −0.57 | C19H16NO4 | - | - | - | - | (+): 307 |
| A68 | 11.10 | Tetrahydrocorysamine | 338.13873 | 338.13868 | −0.13 | C20H20NO4 | - | - | - | - | (+): 323, 322, 308, 294 |
| A69 | 11.11 | N-methyltetrahydropalmatine | 370.20188 | 370.20128 | −1.60 | C22H28NO4 | - | - | - | - | (+): 206 |
| A70 | 11.21 | Tetrahydroberberine | 340.15458 | 340.15433 | −0.73 | C20H22NO4 | - | - | - | - | (+): 176 |
| A71 | 11.25 | 3-(2′-hydroxyphenyl)-4-(3H)-quinazolinone | 239.08162 | 239.08150 | −0.50 | C14H11N2O2 | - | - | - | - | (+): 221 |
| A72 | 11.26 | N-p-Coumaroyltyramine | 284.12834 | 284.12812 | −0.79 | C17H18NO3 | - | - | - | - | (+): 147 |
| A73 | 11.30 | N-methylcanadine | 354.17029 | 354.16998 | −0.87 | C21H24NO4 | - | - | - | - | (+): 190 |
| A74 | 11.97 | Palmatine * | 352.15457 | 352.15433 | −0.67 | C21H22NO4 | - | - | - | - | (+): 336, 308 |
| A75 | 12.25 | Berberine | 336.12277 | 336.12303 | 0.79 | C20H18NO4 | - | - | - | - | (+): 320, 292 |
| A76 | 12.90 | 9-hydroxy-10-methoxy-13-methyl-5,6-dihydro-[1,3]dioxolo[4,5-g]isoquinolino[3,2-a]isoquinolin-7-ium | 336.12296 | 336.12303 | 0.22 | C20H18NO4 | - | - | - | - | (+): 292 |
| A77 | 14.15 | Unknown | 486.19116 | 486.19111 | −0.09 | C29H28NO6 | - | - | - | - | (+): 336 |
| A78 | 14.38 | 8-oxopalmatine | 368.14933 | 368.14925 | −0.22 | C21H22NO5 | - | - | - | - | (+): 338 |
| A79 | 14.72 | Canthin-6-one | 221.07097 | 221.07094 | −0.13 | C14H9N2O | - | - | - | - | (+): 193, 167, 140 |
| A80 | 15.25 | Skimmianine | 260.09171 | 260.09173 | 0.10 | C14H14NO4 | - | - | - | - | (+): 227 |
| A81 | 15.69 | γ-fagarine | 230.08119 | 230.08117 | −0.07 | C13H12NO3 | - | - | - | - | (+): 215 |
| A82 | 17.21 | Dictamnine | 200.07066 | 200.07060 | −0.30 | C12H10NO2 | - | - | - | - | (+): 185 |
| A83 | 18.56 | 7-hydroxyrutaecarpine | 304.10797 | 304.10805 | 0.26 | C18H14N3O2 | - | - | - | - | - |
| A84 | 19.36 | 8-oxopalmatine | 368.14907 | 368.14925 | 0.49 | C21H22NO5 | - | - | - | - | (+): 338 |
| A85 | 21.56 | Oxyberberine | 352.11792 | 352.11795 | 0.07 | C20H18NO5 | - | - | - | - | (+): 337, 322, 294 |
| A86 | 21.69 | Indigo * | 263.08146 | 263.08150 | 0.18 | C16H11N2O2 | - | - | - | - | (+): 245, 235, 219 |
| A87 | 22.72 | N-methylflindersine | 242.11759 | 242.11756 | −0.15 | C15H16NO2 | - | - | - | - | - |
| A88 | 23.49 | Indirubin * | 263.08145 | 263.08150 | 0.21 | C16H11N2O2 | - | - | - | - | (+): 245, 235, 219 |
| No. | RT (min) | Identification | [M-H]+ | [M-H]− | Product Ion (m/z) | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Observed | Calculated | Error (ppm) | Formula | Observed | Calculated | Error (ppm) | Formula | ||||
| T1 | 1.75 | Gardoside | - | - | - | - | 373.11372 | 373.11402 | 0.80 | C16H21O10 | (−): 211, 193, 167, 149, 123 |
| T2 | 1.96 | Aldoxoside | 383.13136 | 383.13125 | −0.28 a | C16H24NaO9 | 405.13976 | 405.14024 | 1.17 b | C17H25O11 | (−): 359 |
| T3 | 1.98 | Deacetylasperulosidic acid | - | - | - | - | 389.10856 | 389.10894 | 0.96 | C16H21O11 | (−): 345, 227, 209, 183, 165 |
| T4 | 2.05 | Shanzhiside | 393.13904 | 393.13914 | 0.25 | C16H25O11 | 391.12397 | 391.12459 | 1.56 | C16H23O11 | (−): 229, 211, 185, 167, 149 |
| T5 | 2.34 | Geniposidic acid | 375.12859 | 375.12857 | −0.04 | C16H23O10 | 373.11374 | 373.11402 | 0.76 | C16H21O10 | (−): 211, 193, 167, 149, 123 |
| T6 | 2.43 | Ixoroside | 383.13112 | 383.13125 | 0.36 a | C16H24NaO9 | 405.13997 | 405.14024 | 0.65 b | C17H25O11 | (−): 359 |
| T7 | 2.54 | 6-oxo demethylgenipin 1-O-glucopyranoside | - | - | - | - | 387.09334 | 387.09329 | −0.14 | C16H19O11 | (−): 343 |
| T8 | 2.80 | Deacetyl asperulosidic acid methyl ester | 427.12108 | 427.12108 | 0.00 a | C17H24NaO11 | 449.12935 | 449.13006 | 1.58 b | C18H25O13 | (−): 241 |
| T9 | 3.07 | Deacetylasperulosidic acid isomer | 391.12351 | 391.12349 | −0.06 | C16H23O11 | 389.10898 | 389.10894 | −0.12 | C16H21O11 | (−): 345 |
| T10 | 3.21 | Gardenoside | 427.12095 | 427.12108 | 0.32 a | C17H24NaO11 | 449.12939 | 449.13006 | 1.50 b | C18H25O13 | (−): 241 |
| T11 | 3.49 | Jasminoside G | 347.17007 | 347.17004 | −0.09 | C16H27O8 | 345.15527 | 345.15549 | 0.63 | C16H25O8 | (−): 165 |
| T12 | 3.65 | Scandoside methyl ester | 427.12100 | 427.12108 | 0.19 a | C17H24NaO11 | 449.12977 | 449.13006 | 0.66 b | C18H25O13 | (−): 241 |
| T13 | 4.02 | Shanzhiside methyl ester isomer | 407.15474 | 407.15479 | 0.13 | C17H27O11 | 405.13998 | 405.14024 | 0.64 | C17H25O11 | (−): 361, 225 |
| T14 | 4.53 | Jasminoside B isomer | 347.16989 | 347.17004 | 0.44 | C16H27O8 | 391.16066 | 391.16097 | 0.81 b | C17H27O10 | (−): 165 |
| T15 | 4.56 | Galioside | 427.12080 | 427.12108 | 0.66 a | C17H24NaO11 | 449.13007 | 449.13006 | −0.01 b | C18H25O13 | (−): 241 |
| T16 | 4.66 | Loganic acid | - | - | - | - | 375.12974 | 375.12967 | −0.19 | C16H23O10 | (−): 213 |
| T17 | 4.89 | Jasminoside C | 329.15945 | 329.15948 | 0.09 | C16H25O7 | 327.14492 | 327.14493 | 0.01 | C16H23O7 | (−): 165 |
| T18 | 4.96 | Jasminoside B | 347.16974 | 347.17004 | 0.89 | C16H27O8 | 391.16044 | 391.16097 | 1.36 b | C17H27O10 | (−): 345, 179, 165, 161 |
| T19 | 5.31 | Shanzhiside methyl ester | - | - | - | - | 405.14012 | 405.14024 | 0.29 | C17H25O11 | (−): 243 |
| T20 | 5.32 | 7-epiloganin | - | - | - | 389.14535 | 389.14532 | −0.09 | C17H25O10 | (−): 181, 166 | |
| T21 | 5.65 | Mussaenosidic acid | - | - | - | - | 375.12956 | 375.12967 | 0.29 | C16H23O10 | (−): 191 |
| T22 | 5.70 | Genipin-1-O-β-D-gentiobioside | 551.19677 | 551.19705 | 0.51 | C23H35O15 | 595.18735 | 595.18797 | 1.04 b | C24H35O17 | (−): 549, 225, 207, 147, 123, 101 |
| T23 | 5.72 | 8-Epiapodantheroside | 389.14434 | 389.14422 | −0.31 | C17H25O10 | 387.12895 | 387.12967 | 1.86 | C17H23O10 | - |
| T24 | 6.00 | Genameside C | 551.19796 | 551.19705 | −1.66 | C23H35O15 | 595.18720 | 595.18797 | 1.30 b | C24H35O17 | (−): 549, 225, 123 |
| T25 | 6.06 | 7,8-dihydro-genipin-1-O-β-gentiobioside | - | - | - | - | 597.20326 | 597.20362 | 0.61 b | C24H37O17 | (−): 551, 389, 227 |
| T26 | 6.07 | Jasminoside I | 493.22826 | 493.22795 | −0.62 | C22H37O12 | 537.21830 | 537.21888 | 1.08 b | C23H37O14 | (−): 491, 167 |
| T27 | 6.17 | Asperulosidic acid | - | - | - | - | 431.11920 | 431.11950 | 0.68 | C18H23O12 | - |
| T28 | 6.18 | Genipin 1-O-apiofuranosyl glucopyranoside | - | - | - | - | 565.17704 | 565.17741 | 0.65 b | C23H33O16 | (−): 519, 225 |
| T29 | 6.28 | Geniposide | 411.12648 | 411.12617 | −0.76 a | C17H24NaO10 | 433.13452 | 433.13515 | 1.45 b | C18H25O12 | (−): 387, 225, 207, 123, 101 |
| T30 | 6.39 | Genameside D | 551.19796 | 551.19705 | −1.66 | C23H35O15 | 595.18773 | 595.18797 | 0.41 b | C24H35O17 | (−): 549, 387, 225 |
| T31 | 6.44 | Genipin 1-O-xylopyranosyl glucopyranoside | - | - | - | - | 565.17731 | 565.17741 | 0.17 b | C23H33O16 | (−): 519, 225, 207 |
| T32 | 6.60 | Dihydrogeniposide | - | - | - | - | 435.15050 | 435.15080 | 0.68 b | C18H27O12 | - |
| T33 | 6.69 | Jasminoside O | 463.21823 | 463.21739 | −1.81 | C21H35O11 | 461.20303 | 461.20284 | −0.42 | C21H33O11 | (+): 331, 169 |
| T34 | 6.81 | Epijasminoside A or Jasminoside A/E/K | 331.17512 | 331.17513 | 0.03 | C16H27O7 | 375.16572 | 375.16606 | 0.90 b | C17H27O9 | (+): 169 |
| T35 | 6.88 | Epijasminoside A or Jasminoside A/E/K | 331.17507 | 331.17513 | 0.17 | C16H27O7 | 375.16591 | 375.16606 | 0.39 b | C17H27O9 | (+): 169 |
| T36 | 7.07 | Epijasminoside A or Jasminoside A/E/K | 331.17569 | 331.17513 | −1.69 | C16H27O7 | 375.16573 | 375.16606 | 0.87 b | C17H27O9 | (+): 169 |
| T37 | 7.34 | Jasminoside C | 329.15980 | 329.15948 | −0.96 | C16H25O7 | 373.15019 | 373.15041 | 0.59 b | C17H25O9 | (−): 165 |
| T38 | 7.36 | Genipin | - | - | - | - | 225.07668 | 225.07685 | 0.75 | C11H13O5 | (−): 147 |
| T39 | 7.61 | Jasminodiol | - | - | - | - | 183.10241 | 183.10267 | 1.42 | C10H15O3 | (−): 139 |
| T40 | 7.66 | 6″-O-trans-p-coumaroylgenipin gentiobioside isomer | 697.23369 | 697.23383 | 0.20 | C32H41O17 | 695.21832 | 695.21927 | 1.37 | C32H39O17 | (−): 163 |
| T41 | 7.83 | 10-O-succinoylgeniposide isomer | - | - | - | - | 487.14544 | 487.14571 | 0.56 | C21H27O13 | (−): 207 |
| T42 | 8.23 | (1R,7R,8S,10R)-7,8,11-trihydroxyguai-4-en-3-one-8-β-D-glucopyranside | 431.22786 | 431.22756 | −0.70 | C21H35O9 | 429.21274 | 429.21301 | 0.62 | C21H33O9 | (+): 269 |
| T43 | 8.24 | 10-O-acetylgeniposide | 453.13736 | 453.13673 | −1.39 a | C19H26NaO11 | 475.14536 | 475.14571 | 0.75 b | C20H27O13 | (−): 209 |
| T44 | 8.37 | 2′-O-trans-p-coumaroylgardoside | - | - | - | - | 519.15007 | 519.15080 | 1.41 | C25H27O12 | (−): 163, 145, 123 |
| T45 | 8.59 | 6′-O-trans-sinapoylgardoside | 581.18719 | 581.18648 | −1.21 | C27H33O14 | 579.17142 | 579.17193 | 0.87 | C27H31O14 | (−): 325, 223, 205 |
| T46 | 8.67 | 6″-O-trans-caffeoylgenipin gentiobioside | - | - | - | - | 711.21306 | 711.21419 | 1.58 | C32H39O18 | (−): 179, 123 |
| T47 | 8.78 | 6′-O-trans-p-coumaroylgeniposidic acid | - | - | - | - | 519.15061 | 519.15080 | 0.37 | C25H27O12 | (−): 211, 163, 145, 123 |
| T48 | 8.88 | 10-O-succinoylgeniposide | - | - | - | - | 487.14528 | 487.14571 | 0.89 | C21H27O13 | (−): 207 |
| T49 | 8.95 | 10-(6-O-trans-sinapoylglucopyranosyl)-gardendiol | 567.20828 | 567.20722 | −1.87 | C27H35O13 | 565.19190 | 565.19266 | 1.35 | C27H33O13 | (−): 325, 295, 265, 223 |
| T50 | 8.99 | 6′-O-trans-sinapoylgardoside isomer | 581.18726 | 581.18648 | −1.34 | C27H33O14 | 579.17130 | 579.17193 | 1.08 | C27H31O14 | (−): 325, 223, 205 |
| T51 | 9.09 | Jasminoside R | 513.19610 | 513.19425 | −3.61 a | C22H34NaO12 | 535.20260 | 535.20323 | 1.18 b | C23H35O14 | - |
| T52 | 9.09 | 6″-O-trans-p-coumaroylgenipin gentiobioside isomer | - | - | - | - | 695.21812 | 695.21927 | 1.66 | C32H39O17 | (−): 469, 163 |
| T53 | 9.31 | 11-(6-O-trans-sinapoylglucopyranosyl)-gardendiol | 567.20835 | 567.20722 | −1.99 | C27H35O13 | 565.19236 | 565.19266 | 0.55 | C27H33O13 | (−): 265, 223 |
| T54 | 9.41 | 10-O-acetylgeniposide isomer | 453.13754 | 453.13673 | −1.78 a | C19H26NaO11 | 475.14530 | 475.14571 | 0.87 b | C20H27O13 | (−): 209 |
| T55 | 9.72 | 6″-O-trans-p-coumaroylgenipin gentiobioside | 697.23461 | 697.23383 | −1.12 | C32H41O17 | 695.21845 | 695.21927 | 1.18 | C32H39O17 | (−): 469, 367, 349, 307, 265, 235, 207, 163, 145, 123 |
| T56 | 9.75 | Jasminoside I/S/H | - | - | - | - | 537.21830 | 537.21888 | 1.08 b | C23H37O14 | (−): 491 |
| T57 | 9.91 | 6″-O-trans-sinapoylgenipin gentiobioside | - | - | - | - | 755.23881 | 755.24040 | 2.11 | C34H43O19 | (−): 427, 325, 265, 223, 205 |
| T58 | 10.05 | 6″-O-trans-feruloylgenipin gentiobioside | 727.24630 | 727.24439 | −2.63 | C33H43O18 | 725.22867 | 725.22984 | 1.61 | C33H41O18 | (−): 397, 295, 235, 225, 207, 193, 175, 123 |
| T59 | 10.33 | Jasminoside R | - | - | - | - | 489.19730 | 489.19775 | 0.92 | C22H33O12 | - |
| T60 | 10.34 | Crocin I | - | - | - | - | 975.37046 | 975.37148 | 1.04 | C44H63O24 | (−): 651 |
| T61 | 10.68 | 6′-trans-sinapoylgeniposide isomer | 617.18520 | 617.18408 | −1.82 a | C28H34NaO14 | 593.18700 | 593.18758 | 0.97 | C28H33O14 | (−): 367, 223 |
| T62 | 10.75 | 6′-O-trans-sinapoyljasminoside L | 553.22916 | 553.22795 | −2.19 | C27H37O12 | 551.21267 | 551.21340 | 1.32 | C27H35O12 | (−): 223 |
| T63 | 11.36 | 6′-trans-sinapoylgeniposide | 595.20406 | 595.20213 | −3.24 | C28H35O14 | 593.18666 | 593.18758 | 1.55 | C28H33O14 | (−): 367, 223, 205, 123 |
| T64 | 11.47 | 6′-O-trans-coumaroylgeniposide | 535.18261 | 535.18100 | −3.00 | C26H31O12 | 533.16592 | 533.16645 | 1.00 | C26H29O12 | (−): 307, 145 |
| T65 | 11.70 | Jasminoside I/S/H | - | - | - | - | 491.21363 | 491.21340 | −0.47 | C22H35O12 | - |
| T66 | 11.72 | 6″-O-trans-p-coumaroylgenipin gentiobioside isomer | - | - | - | - | 695.21805 | 695.21927 | 1.75 | C32H39O17 | (−): 207, 163 |
| T67 | 12.06 | Jasminoside I/S/H | - | - | - | - | 491.21248 | 491.21340 | 1.87 | C22H35O12 | - |
| T68 | 12.10 | Rehmapicrogenin or isomer | - | - | - | - | 183.10239 | 183.10267 | 1.50 | C10H15O3 | - |
| T69 | 12.42 | Rutaevin | 487.19808 | 487.19626 | −3.74 | C26H31O9 | 485.18117 | 485.18171 | 1.10 | C26H29O9 | (−): 467, 423 |
| T70 | 13.27 | 6″-O-trans-p-cinnamoylgenipin gentiobioside | 703.22355 | 703.22086 | −3.83 a | C32H40NaO16 | 725.22811 | 725.22984 | 2.38 b | C33H41O18 | (−): 679, 531, 355, 225, 207, 147, 123 |
| T71 | 13.97 | 6′-O-trans-sinapoyljasminoside A | 537.23366 | 537.23304 | −1.15 | C27H37O11 | 535.21809 | 535.21849 | 0.74 | C27H35O11 | (−): 223 |
| T72 | 14.44 | Obacunoic acid | 473.21640 | 473.21699 | 1.26 | C26H33O8 | 471.20217 | 471.20244 | 0.59 | C26H31O8 | (−): 325 |
| T73 | 14.47 | Crocin I acid | - | - | - | - | 1007.37486 | 1007.37656 | 1.69 | C48H63O23 | (−): 683 |
| T74 | 14.63 | 6′-trans-sinapoylgeniposide isomer | 617.18462 | 617.18408 | −0.89 a | C28H34NaO14 | 593.18691 | 593.18758 | 1.14 | C28H33O14 | (−): 367, 223, 205, 123 |
| T75 | 14.75 | 6′-O-trans-sinapoyjasminoside C | 535.21774 | 535.21739 | −0.65 | C27H35O11 | 533.20202 | 533.20284 | 1.53 | C27H33O11 | (−): 223, 205 |
| T76 | 15.40 | 12α-hydroxylimonin | 487.19607 | 487.19626 | 0.38 | C26H31O9 | - | - | - | - | - |
| T77 | 16.49 | Neocrocin E | - | - | - | - | 975.37036 | 975.37148 | 1.15 | C44H63O24 | (−): 651 |
| T78 | 16.86 | Crocin-Ⅲ or 13-cis-crocetin-8′-O-β-D-gentiobioside or isomer | 653.28032 | 653.28038 | 0.09 | C32H45O14 | 651.26523 | 651.26583 | 0.92 | C32H43O14 | (−): 327, 283, 239 |
| T79 | 17.91 | Crocin II | 837.31452 | 837.31515 | 0.75 a | C38H54NaO19 | 813.31723 | 813.31865 | 1.75 | C38H53O19 | (−): 651 |
| T80 | 17.93 | beta-D-glucosyl crocetin | 491.22716 | 491.22756 | 0.82 | C26H35O9 | 489.21276 | 489.21301 | 0.50 | C26H33O9 | (−): 327 |
| T81 | 18.35 | 6′-O-trans-sinapoyljasminoside A | - | - | - | - | 535.21815 | 535.21849 | 0.62 | C27H35O11 | (−): 223 |
| T82 | 18.37 | beta-D-glucosyl crocetin | 491.22719 | 491.22756 | 0.76 | C26H35O9 | 489.21277 | 489.21301 | 0.48 | C26H33O9 | (−): 327 |
| T83 | 19.14 | 6′-O-trans-sinapoyljasminoside A isomer | - | - | - | - | 535.21823 | 535.21849 | 0.49 | C27H35O11 | (−): 223 |
| T84 | 19.60 | Obaculactone | 471.20104 | 471.20134 | 0.64 | C26H31O8 | 469.18655 | 469.18679 | 0.51 | C26H29O8 | (+): 453, 427, 425, 409, 367, 339, 161 |
| T85 | 20.34 | Crocin II | 837.31579 | 837.31515 | −0.77 a | C38H54NaO19 | 813.31960 | 813.31865 | −1.17 | C38H53O19 | (−): 651 |
| T86 | 20.35 | Obacunoic acid | 473.21675 | 473.21699 | 0.52 | C26H33O8 | 471.20245 | 471.20244 | −0.02 | C26H31O8 | (−): 325, 307 |
| T87 | 20.70 | Crocin-Ⅲ or 13-cis-crocetin-8′-O-β-D-gentiobioside or isomer | 675.26198 | 675.26233 | 0.52 a | C32H44NaO14 | 651.26503 | 651.26583 | 1.22 | C32H43O14 | (−): 327, 283, 239 |
| T88 | 21.09 | Obacunoic acid isomer | 473.21690 | 473.21699 | 0.20 | C26H33O8 | 471.20262 | 471.20244 | −0.39 | C26H31O8 | (−): 325 |
| T89 | 21.19 | Neocrocin B | - | - | - | - | 987.35048 | 987.35035 | −0.14 | C48H59O22 | - |
| T90 | 21.38 | Crocin-Ⅲ or 13-cis-crocetin-8′-O-β-D-gentiobioside or isomer | 653.28052 | 653.28038 | −0.21 | C32H45O14 | 651.26515 | 651.26583 | 1.04 | C32H43O14 | (−): 327 |
| T91 | 21.95 | Dehydrolimonin | 469.18526 | 469.18569 | 0.93 | C26H29O8 | 513.17635 | 513.17662 | 0.53 b | C27H29O10 | (−): 467, 449 |
| T92 | 22.05 | Crocin-Ⅲ or 13-cis-crocetin-8′-O-β-D-gentiobioside or isomer | 653.27972 | 653.28038 | 1.02 | C32H45O14 | 651.26462 | 651.26583 | 1.86 | C32H43O14 | (−): 327 |
| T93 | 22.90 | beta-D-glucosylcrocetin | 491.22735 | 491.22756 | 0.43 | C26H35O9 | 489.21272 | 489.21301 | 0.58 | C26H33O9 | (−): 327 |
| T94 | 23.14 | beta-D-glucosylcrocetin | 491.22726 | 491.22756 | 0.61 | C26H35O9 | 489.21268 | 489.21301 | 0.68 | C26H33O9 | (−): 327 |
| T95 | 23.47 | beta-D-glucosylcrocetin | 491.22722 | 491.22756 | 0.69 | C26H35O9 | 489.21265 | 489.21301 | 0.72 | C26H33O9 | (−): 327 |
| T96 | 24.02 | Obacunone | 455.20596 | 455.20643 | 1.04 | C26H31O7 | 453.19169 | 453.19188 | 0.40 | C26H29O7 | (+): 437, 419, 409, 391, 359, 349, 331, 315 |
| T97 | 25.09 | Crocetin | 329.17453 | 329.17474 | 0.63 | C20H25O4 | 327.15993 | 327.16018 | 0.77 | C20H23O4 | (−): 283 |
| T98 | 25.36 | Erubigenin | 489.35728 | 489.35745 | 0.35 | C30H49O5 | 487.34244 | 487.34290 | 0.93 | C30H47O5 | (−): 469 |
| T99 | 25.68 | Dikamaliartanes A | 501.32111 | 501.32107 | −0.08 | C30H45O6 | 499.30640 | 499.30651 | 0.23 | C30H43O6 | - |
| T100 | 25.83 | Crocetin isomer | 329.17472 | 329.17474 | 0.04 | C20H25O4 | 327.15992 | 327.16018 | 0.81 | C20H23O4 | (−): 283 |
| T101 | 25.95 | Obacunone isomer | 455.20628 | 455.20643 | 0.33 | C26H31O7 | 453.19150 | 453.19188 | 0.83 | C26H29O7 | (+): 437, 411, 409, 359 |
| T102 | 25.96 | Gardenic acid B | 487.34171 | 487.34180 | 0.19 | C30H47O5 | 485.32690 | 485.32725 | 0.71 | C30H45O5 | - |
| T103 | 26.56 | Crocetin isomer | 329.17474 | 329.17474 | 0.00 | C20H25O4 | 327.15986 | 327.16018 | 0.99 | C20H23O4 | (−): 283 |
| T104 | 27.17 | Crocetin isomer | 329.17470 | 329.17474 | 0.10 | C20H25O4 | 327.15981 | 327.16018 | 1.14 | C20H23O4 | (−): 283 |
| T105 | 27.78 | 27-O-p-(E)-coumaroyloxyursolic acid | - | - | - | - | 617.38369 | 617.38476 | 1.73 | C39H54O6 | - |
| T106 | 29.02 | 9,19-cyclolanost-24-ene-3,23-dione | - | - | - | - | 469.33097 | 469.33233 | 2.91 | C30H45O4 | - |
| T107 | 29.52 | Quadrangularic acid E | - | - | - | - | 471.34652 | 471.34798 | 3.10 | C30H47O4 | - |
| T108 | 30.44 | Betulinic acid | - | - | - | - | 455.35162 | 455.35307 | 3.19 | C30H47O3 | (−): 407 |
| No. | RT (min) | Identification | [M-H]+ | [M-H]− | Product Ion (m/z) | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Observed | Calculated | Error (ppm) | Formula | Observed | Calculated | Error (ppm) | Formula | ||||
| O1 | 0.94 | Quinic acid | - | - | - | - | 191.05582 | 191.05611 | 1.55 | C7H11O6 | (−): 173, 127 |
| O2 | 1.27 | Succinic acid | - | - | - | - | 117.01917 | 117.01933 | 1.37 | C4H5O4 | - |
| O3 | 1.32 | Malic acid | - | - | - | - | 133.01404 | 133.01425 | 1.59 | C4H5O5 | (−): 115 |
| O4 | 1.69 | Quinic acid 3-hydroxy-3-methylpentanedioate | 337.11289 | 337.11292 | 0.10 | C13H21O10 | 335.09793 | 335.09837 | 1.31 | C13H19O10 | (−): 191 |
| O5 | 2.19 | Dihydroxybenzoic acid glucoside | - | - | - | - | 315.07186 | 315.07216 | 0.93 | C13H15O9 | (−): 153, 109 |
| O6 | 2.27 | Vanillic acid hexose | 331.10239 | 331.10236 | −0.09 | C14H19O9 | 329.08737 | 329.08781 | 1.32 | C14H17O9 | (−): 167 |
| O7 | 2.51 | 3-({3-[4-(β-glucopyranosyloxy)-3-methoxyphenyl]-2-propenoyl}oxy)-1,4,5-trihydroxycyclohexanecarboxylic acid | 531.17052 | 531.17083 | 0.58 | C23H31O14 | 529.15607 | 529.15628 | 0.40 | C23H29O14 | (−): 193, 191 |
| O8 | 2.59 | Dihydroxybenzoic acid glucoside isomer | 317.08701 | 317.08671 | −0.95 | C13H17O9 | 315.07189 | 315.07216 | 0.85 | C13H15O9 | (−): 153, 109 |
| O9 | 3.41 | Neochlorogenic acid | 355.10206 | 355.10236 | 0.83 | C16H19O9 | 353.08749 | 353.08781 | 0.91 | C16H17O9 | (−): 191, 179, 173, 135 |
| O10 | 3.81 | 4-O-caffeoyl-3-O-feruloylquinic acid | 531.17059 | 531.17083 | 0.45 | C23H31O14 | 529.15610 | 529.15628 | 0.34 | C23H29O14 | (−): 191, 179, 173 |
| O11 | 5.19 | 4-O-caffeoyl-3-O-feruloylquinic acid isomer | 531.17049 | 531.17083 | 0.64 | C23H31O14 | 529.15606 | 529.15628 | 0.41 | C23H29O14 | (−): 191, 179, 173 |
| O12 | 5.27 | 3-p-coumaroylquinic acid | 339.10747 | 339.10744 | −0.08 | C16H19O8 | 337.09277 | 337.09289 | 0.35 | C16H17O8 | (−): 191, 163 |
| O13 | 5.60 | Chlorogenic acid * | 355.10232 | 355.10236 | 0.11 | C16H19O9 | 353.08757 | 353.08781 | 0.67 | C16H17O9 | (−): 191, 179, 173, 135 |
| O14 | 5.83 | 5-O-feruloylquinic acid | 369.11802 | 369.11801 | −0.02 | C17H21O9 | 367.10306 | 367.10346 | 1.08 | C17H19O9 | (−): 193, 191, 173, 149, 134 |
| O15 | 5.85 | Cryptochlorogenic acid | 355.10257 | 355.10236 | −0.59 | C16H19O9 | 353.08759 | 353.08781 | 0.62 | C16H17O9 | (−): 191, 179, 173, 135 |
| O16 | 6.04 | 4-sinapoylquinic acid | - | - | - | - | 397.11376 | 397.11402 | 0.65 | C18H21O10 | (−): 223, 191, 169, 146 |
| O17 | 6.17 | Caffeic acid * | - | - | - | - | 179.03487 | 179.03498 | 0.64 | C9H7O4 | (−): 135 |
| O18 | 6.58 | 4-p-coumaroylquinic acid | 339.10753 | 339.10744 | −0.25 | C16H19O8 | 337.09281 | 337.09289 | 0.26 | C16H17O8 | (−): 191, 163 |
| O19 | 6.60 | 3,4-dicaffeoyl quinic acid isomer | 517.13521 | 517.13405 | −2.24 | C25H25O12 | 515.11895 | 515.11950 | 1.07 | C25H23O12 | (−): 353, 191, 179, 135 |
| O20 | 6.62 | 4-O-β-D-glucosyl-cis-p-coumaric acid | - | - | - | - | 325.09285 | 325.09289 | 0.13 | C15H17O8 | - |
| O21 | 6.67 | 2-hydroxy-1,4-phthalic acid | - | - | - | - | 181.01400 | 181.01425 | 1.39 | C8H5O5 | (−): 137 |
| O22 | 6.92 | 3-O-feruloylquinic acid | 369.11805 | 369.11801 | −0.12 | C17H21O9 | 367.10342 | 367.10346 | 0.10 | C17H19O9 | (−): 193, 191, 173, 134 |
| O23 | 7.06 | 3-Sinapoylquinic acid | 399.12895 | 399.12857 | −0.94 | C18H23O10 | 397.11398 | 397.11402 | 0.11 | C18H21O10 | (−): 223, 191, 169, 146 |
| O24 | 7.23 | Dicaffeoyl quinic acid-glucopyranoside | - | - | - | - | 677.17142 | 677.17232 | 1.33 | C31H33O17 | - |
| O25 | 7.57 | 4-O-feruloylquinic acid | 369.11813 | 369.11801 | −0.34 | C17H21O9 | 367.10337 | 367.10346 | 0.24 | C17H19O9 | (−): 193, 191, 173 |
| O26 | 7.75 | Dicaffeoyl quinic acid-glucopyranoside | - | - | - | - | 677.17156 | 677.17232 | 1.13 | C31H33O17 | (−): 515 |
| O27 | 8.05 | Dicaffeoyl quinic acid-glucopyranoside | - | - | - | - | 677.17136 | 677.17232 | 1.42 | C31H33O17 | (−): 515 |
| O28 | 8.14 | Ferulic acid | 195.06547 | 195.06519 | −1.46 | C10H11O4 | 193.05029 | 193.05063 | 1.79 | C10H9O4 | (−): 149 |
| O29 | 8.42 | Ferulic acid isomer | - | - | - | - | 193.05041 | 193.05063 | 1.16 | C10H9O4 | (−): 149 |
| O30 | 8.73 | 3,4-dicaffeoyl quinic acid | 517.13626 | 517.13405 | −4.27 | C25H25O12 | 515.11906 | 515.11950 | 0.86 | C25H23O12 | (−): 353, 191, 179, 135 |
| O31 | 9.13 | 3,5-dicaffeoyl quinic acid | 517.13648 | 517.13405 | −4.69 | C25H25O12 | 515.11893 | 515.11950 | 1.11 | C25H23O12 | (−): 353, 191, 179, 135 |
| O32 | 9.65 | 4,5-dicaffeoyl quinic acid | 517.13521 | 517.13405 | −2.24 | C25H25O12 | 515.11877 | 515.11950 | 1.42 | C25H23O12 | (−): 353, 191, 179, 135 |
| O33 | 9.99 | 3-O-caffeoyl-4-O-sinapoylquinic acid | 561.16097 | 561.16027 | −1.26 | C27H29O13 | 559.14509 | 559.14571 | 1.12 | C27H27O13 | (−): 379, 364, 353, 335, 223, 205, 191, 179, 173, 164, 161, 155, 149, 137, 135 |
| O34 | 10.02 | 4-p-coumaroylquinic acid isomer | 339.10758 | 339.10744 | −0.39 | C16H19O8 | - | - | - | - | - |
| O35 | 10.17 | 3,5-di-O-caffeoyl-4-O-(3-hydroxy-3-methyl) glutarylquinic acid | 661.17765 | 661.17631 | −2.02 | C31H33O16 | 659.16151 | 659.16176 | 0.38 | C31H31O16 | (−): 353, 191 |
| O36 | 10.82 | 5-O-caffeoyl-4-O-sinapoylquinic acid | 561.16144 | 561.16027 | −2.09 | C27H29O13 | 559.14515 | 559.14571 | 1.00 | C27H27O13 | (−): 379, 364, 353, 335, 223, 205, 191, 179, 173, 164, 161, 155, 149, 137, 135 |
| O37 | 11.17 | 5-O-caffeoyl-4-O-sinapoylquinic acid isomer | 561.16144 | 561.16027 | −2.09 | C27H29O13 | 559.14501 | 559.14571 | 1.26 | C27H27O13 | (−): 379, 364, 353, 335, 223, 205, 191, 179, 173, 164, 161, 155, 149, 137, 135 |
| O38 | 12.55 | Phellinsin A or isomer | 359.07730 | 359.07614 | −3.22 | C18H15O8 | 357.06125 | 357.06159 | 0.96 | C18H13O8 | (−): 339, 321, 313, 295 |
| O39 | 15.59 | 9,12-dihydroxy-13-oxooctadec-14-enoic acid | 329.23207 | 329.23225 | 0.55 | C18H33O5 | 327.21739 | 327.21770 | 0.94 | C18H31O5 | (−): 291, 273, 247, 239, 229, 211, 209, 203, 197, 193, 183, 171 |
| O40 | 15.87 | 9,12-dihydroxy-13-oxooctadec-14-enoic acid isomer | 329.23259 | 329.23225 | −1.03 | C18H33O5 | 327.21725 | 327.21770 | 1.37 | C18H31O5 | (−): 291, 273, 247, 239, 229, 211, 209, 203, 197, 193, 183, 171 |
| O41 | 17.04 | 9,12-dihydroxy-13-oxooctadecanoic acid | 353.22966 | 353.22984 | 0.51 a | C18H34NaO5 | 329.23302 | 329.23335 | 1.01 | C18H33O5 | (−): 311, 229, 211, 183, 171 |
| O42 | 18.71 | 9,12-dihydroxy-13-oxooctadecanoic acid isomer | 353.22964 | 353.22984 | 0.59 a | C18H34NaO5 | 329.23322 | 329.23335 | 0.40 | C18H33O5 | (−): 311, 229, 211 |
| O43 | 18.94 | 9,12-dihydroxy-13-oxooctadecanoic acid isomer | 353.22958 | 353.22984 | 0.75 a | C18H34NaO5 | 329.23317 | 329.23335 | 0.54 | C18H33O5 | (−): 311, 229, 211 |
| O44 | 19.64 | 9,12-dihydroxy-13-oxooctadecanoic acid isomer | 353.22977 | 353.22984 | 0.20 a | C18H34NaO5 | 329.23308 | 329.23335 | 0.80 | C18H33O5 | (−): 311, 229, 211 |
| O45 | 20.54 | 9,12-dihydroxy-13-oxooctadecanoic acid isomer | 353.22972 | 353.22984 | 0.37 a | C18H34NaO5 | 329.23337 | 329.23335 | −0.08 | C18H33O5 | (−): 311, 229, 211 |
| O46 | 23.27 | (9Z,12Z)-15,16-dihydroxyoctadeca-9,12-dienoic acid | 335.21901 | 335.21928 | 0.80 a | C18H32NaO4 | 311.22236 | 311.22278 | 1.36 | C18H31O4 | (−): 293, 275, 223, 205 |
| O47 | 25.00 | 9/12-Octadecanedioic acid or isomer | 337.23464 | 337.23493 | 0.87 a | C18H34NaO4 | 313.23799 | 313.23843 | 1.42 | C18H33O4 | (−): 295, 277 |
| O48 | 25.30 | 9/12-Octadecanedioic acid or isomer | 337.23471 | 337.23493 | 0.65 a | C18H34NaO4 | 313.23821 | 313.23843 | 0.71 | C18H33O4 | (−): 295 |
| O49 | 29.94 | γ-Linolenic acid | - | - | - | - | 277.21655 | 277.21730 | 2.73 | C18H29O2 | (−): 233 |
| O50 | 30.69 | Linoleic acid | - | - | - | - | 279.23222 | 279.23295 | 2.62 | C18H31O2 | (−): 261 |
| O51 | 31.59 | Oleic acid | 283.26315 | 283.26316 | 0.02 | C18H35O2 | 281.24796 | 281.24860 | 2.30 | C18H33O2 | - |
| No. | RT (min) | Identification | [M-H]+ | [M-H]− | Product Ion (m/z) | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Observed | Calculated | Error (ppm) | Formula | Observed | Calculated | Error (ppm) | Formula | ||||
| OC1 | 0.90 | Arginine | 175.11906 | 175.11895 | −0.61 | C6H15N4O2 | 173.10429 | 173.10440 | 0.63 | C6H13N4O2 | (+): 158 |
| OC2 | 1.16 | Cytidine | 244.09273 | 244.09280 | 0.28 | C9H14N3O5 | - | - | - | - | (+): 112 |
| OC3 | 1.37 | Tyrosine | 182.08116 | 182.08117 | 0.07 | C9H12NO3 | - | - | - | - | (+): 165, 147, 136 |
| OC4 | 1.37 | Uridine | 245.07684 | 245.07681 | −0.11 | C9H13N2O6 | 243.06197 | 243.06226 | 1.21 | C9H11N2O6 | (−): 110 |
| OC5 | 1.39 | Adenosine | 268.10389 | 268.10403 | 0.53 | C10H14N5O4 | - | - | - | - | (+): 136 |
| OC6 | 1.41 | Guanosine | 284.09891 | 284.09894 | 0.11 | C10H14N5O5 | 282.08390 | 282.08439 | 1.74 | C10H12N5O5 | (−): 150, 133 |
| OC7 | 1.60 | Unknown | 363.16472 | 363.16496 | 0.66 | C16H27O9 | 361.15007 | 361.15041 | 0.94 | C16H25O9 | (−): 181, 137 |
| OC8 | 2.05 | Phenylalanine hexose | 328.13890 | 328.13908 | 0.55 | C15H22NO7 | 326.12431 | 326.12453 | 0.66 | C15H20NO7 | (−): 164 |
| OC9 | 2.11 | Phenylalanine | 166.08639 | 166.08626 | −0.82 | C9H12NO2 | - | - | - | - | (+): 149 |
| OC10 | 2.36 | Unknown | 363.16535 | 363.16496 | −1.08 | C16H27O9 | 361.15003 | 361.15041 | 1.05 | C16H25O9 | (−): 181, 137 |
| OC11 | 2.82 | 4-(2-hydroxyethyl)phenyl 6-O-apiofuranosyl-β-D-glucopyranoside | 433.17064 | 433.17044 | −0.47 | C19H29O11 | 477.16109 | 477.16136 | 0.57 c | C20H29O13 | (−): 431, 293 |
| OC12 | 2.88 | 1-O-syringoyl-β-D-glucopyranoside | 361.11286 | 361.11292 | 0.18 | C15H21O10 | 359.09821 | 359.09837 | 0.43 | C15H19O10 | (−): 197 |
| OC13 | 3.81 | Tryptophan | 205.09704 | 205.09715 | 0.57 | C11H13N2O2 | 203.08247 | 203.08260 | 0.67 | C11H11N2O2 | (+): 188, 146 |
| P1 | 4.40 | Esculin | 341.08642 | 341.08671 | 0.85 | C15H17O9 | 339.07211 | 339.07216 | 0.13 | C15H15O9 | (+): 179, 151, 133, 123 |
| OC14 | 4.80 | Osmanthuside H | - | - | - | - | 431.15579 | 431.15589 | 0.23 | C19H27O11 | (−): 191, 149 |
| P2 | 5.03 | Coniferin | 365.12059 | 365.12069 | 0.27 a | C16H22NaO8 | 387.12932 | 387.12967 | 0.90 c | C17H23O10 | (−): 341, 179 |
| OC15 | 5.13 | Darendoside A | 433.17064 | 433.17044 | −0.47 | C19H29O11 | 431.15566 | 431.15589 | 0.52 | C19H27O11 | (−): 299, 191, 149 |
| P3 | 5.18 | Liriodendrin isomer | - | - | - | - | 787.26642 | 787.26662 | 0.25 c | C35H47O20 | (−): 741 |
| OC16 | 5.39 | Darendoside A isomer | 433.17153 | 433.17044 | −2.52 | C19H29O11 | 431.15558 | 431.15589 | 0.71 | C19H27O11 | (−): 299, 191, 149 |
| P4 | 5.61 | Syringin | 390.17599 | 390.17586 | −0.34 b | C17H28NO9 | 417.13995 | 417.14024 | 0.68 c | C18H25O11 | (−): 209 |
| P5 | 6.09 | Esculetin * | - | - | - | - | 177.01901 | 177.01933 | 1.82 | C9H5O4 | (−): 149, 133 |
| P6 | 6.18 | Clemastanin B | - | - | - | - | 729.26007 | 729.26114 | 1.46 c | C33H45O18 | (−): 683, 521, 359, 329 |
| OC17 | 6.41 | Unknown | - | - | - | - | 563.16146 | 563.16176 | 0.52 c | C23H31O16 | (−): 517, 225 |
| P7 | 6.42 | Lariciresinol-4′-O-β-D-glucoside | - | - | - | - | 521.20303 | 521.20284 | −0.37 | C26H33O11 | (−): 359 |
| OC18 | 6.59 | Sinapyglucoside | 387.12897 | 387.12857 | −1.03 | C17H23O10 | 385.11395 | 385.11402 | 0.17 | C17H21O10 | (−): 205 |
| OC19 | 7.02 | 2-methyl-L-erythritol-4-O-(6-O-transsinapoyl)-β-D-glucopyranoside | 527.17422 | 527.17351 | −1.34 a | C22H32NaO13 | 503.17659 | 503.17701 | 0.85 | C22H31O13 | (−): 205 |
| P8 | 7.09 | Liriodendrin | - | - | - | - | 787.26595 | 787.26662 | 0.85 c | C35H47O20 | (−): 741 |
| P9 | 7.27 | 5,5′-dimethoxy lariciresinol-4′-O-glucoside isomer | 605.22181 | 605.22046 | −2.22 a | C28H38NaO13 | 581.22325 | 581.22396 | 1.23 | C28H37O13 | (−): 415 |
| P10 | 7.40 | (−)-Secoisolariciresinol 4-O-β-D-glucopyranoside | - | - | - | - | 523.21827 | 523.21849 | 0.41 | C26H35O11 | (−): 361 |
| OC20 | 7.43 | Darendroside B | - | - | - | - | 475.18169 | 475.18210 | 0.87 | C21H31O12 | (−): 329 |
| OC21 | 7.53 | 1-O-feruloyl heptopyranose | 409.11089 | 409.11052 | −0.91 a | C17H22NaO10 | 385.11362 | 385.11402 | 1.05 | C17H21O10 | (+): 387 |
| P11 | 7.97 | 3,3′,4-trihydroxy-4-methoxy-7,7-epoxylignan or isomer | 331.15427 | 331.15400 | −0.82 | C19H23O5 | 329.13885 | 329.13945 | 1.81 | C19H21O5 | (+): 316 |
| P12 | 7.97 | Lariciresinol-4-O-β-D-glucoside | - | - | - | - | 521.20218 | 521.20284 | 1.27 | C26H33O11 | (−): 359 |
| P13 | 8.06 | 5,5′-dimethoxy lariciresinol-4′-O-glucoside | - | - | - | - | 581.22346 | 581.22396 | 0.87 | C28H37O13 | (−): 419 |
| P14 | 8.15 | Staunoside C | - | - | - | - | 595.20287 | 595.20323 | 0.60 | C28H35O14 | - |
| P15 | 8.16 | Prinsepiol | - | - | - | - | 389.12383 | 389.12419 | 0.92 | C20H21O8 | (−): 374, 359, 175, 151, 136 |
| OC22 | 8.22 | Acteoside isomer | 647.19555 | 647.19464 | −1.40 a | C29H36NaO15 | 623.19736 | 623.19814 | 1.25 | C29H35O15 | (−): 461, 315, 179, 161 |
| P16 | 8.23 | Conicaoside | 575.21087 | 575.20990 | −1.69 a | C27H36NaO12 | 551.21302 | 551.21340 | 0.70 | C27H35O12 | - |
| OC23 | 8.82 | Acteoside | - | - | - | - | 623.19721 | 623.19814 | 1.49 | C29H35O15 | (−): 461, 315, 179, 161 |
| P17 | 8.87 | Isolariciresinol | 361.16492 | 361.16456 | −0.98 | C20H25O6 | 405.15502 | 405.15549 | 1.16 c | C21H25O8 | (−): 359 |
| P18 | 8.91 | Pinoresinol-4-O-glucoside | - | - | - | - | 519.18674 | 519.18719 | 0.86 | C26H31O11 | (−): 357, 342, 151, 136 |
| P19 | 9.08 | Aschantin | 401.16042 | 401.15948 | −2.34 | C22H25O7 | - | - | - | - | (−): 383 |
| P20 | 9.12 | Acanthoside B | 603.20697 | 603.20481 | −3.58 a | C28H36NaO13 | 579.20790 | 579.20831 | 0.72 | C28H35O13 | (−): 417, 402, 387, 181, 166 |
| P21 | 10.30 | 8-hydroxypinoresinol | 375.14347 | 375.14383 | 0.95 | C20H23O7 | 373.12925 | 373.12928 | 0.07 | C20H21O7 | (−): 313 |
| P22 | 10.38 | 8-hydroxysyringaresinol | 435.16542 | 435.16496 | −1.07 | C22H27O9 | 433.15003 | 433.15041 | 0.87 | C22H25O9 | (−): 403, 385, 373, 358, 343, 325 |
| P23 | 10.38 | Pinoresinol-4-O-glucoside isomer | - | - | - | - | 519.18688 | 519.18719 | 0.58 | C26H31O11 | (−): 357 |
| P24 | 10.41 | Secoisolariciresinol | - | - | - | - | 361.16543 | 361.16566 | 0.65 | C20H25O6 | (−): 346, 165 |
| P25 | 10.47 | Hydroxymedioresinol | - | - | - | - | 403.13921 | 403.13984 | 1.58 | C21H23O8 | (−): 343 |
| OC24 | 10.52 | Leucosceptoside A or isomer | 639.22994 | 639.22835 | −2.49 | C30H39O15 | 637.21278 | 637.21379 | 1.60 | C30H37O15 | (−): 461 |
| F40 | 10.60 | Syringetin | 347.07655 | 347.07614 | −1.17 | C17H15O8 | 345.06107 | 345.06159 | 1.50 | C17H13O8 | (+): 332 |
| P26 | 10.93 | Lariciresinol-9-O-β-D-glucopyranoside | 523.21844 | 523.21739 | −2.00 | C26H35O11 | 521.20242 | 521.20284 | 0.80 | C26H33O11 | (−): 506 |
| P27 | 11.24 | 3,3′,4-trihydroxy-4-methoxy-7,7-epoxylignan or isomer | 331.15428 | 331.15400 | −0.85 | C19H23O5 | 329.13936 | 329.13945 | 0.27 | C19H21O5 | - |
| P28 | 11.26 | Hydroxymedioresinol isomer | - | - | - | - | 403.13953 | 403.13984 | 0.77 | C21H23O8 | - |
| OC25 | 11.58 | Cistanoside D | - | - | - | - | 651.22826 | 651.22944 | 1.81 | C31H39O15 | (−): 505 |
| OC26 | 12.49 | Isomartynoside | - | - | - | - | 651.22861 | 651.22944 | 1.27 | C31H39O15 | (−): 505 |
| OC27 | 13.28 | Ethyl caffeate | - | - | - | - | 207.06606 | 207.06628 | 1.08 | C11H11O4 | (−): 179, 161, 135 |
| OC28 | 13.49 | Variolaric acid | 315.05025 | 315.04993 | −1.03 | C16H11O7 | 313.03517 | 313.03538 | 0.66 | C16H9O7 | (−): 269 |
| P29 | 14.09 | Unknown | 329.06581 | 329.06558 | −0.71 | C17H13O7 | 327.05071 | 327.05103 | 0.95 | C17H11O7 | (−): 299, 283, 265, 255, 237, 222, 209 |
| OC29 | 16.83 | Lariciresinol | 361.16492 | 361.16456 | −0.98 | C20H25O6 | 359.14979 | 359.15001 | 0.61 | C20H23O6 | (−): 329 |
| OC30 | 17.58 | Unknown | 729.14456 | 729.14501 | 0.62 | C37H29O16 | 727.12966 | 727.13046 | 1.10 | C37H27O16 | (+): 553, 283 |
| OC31 | 19.81 | Unknown | 729.14475 | 729.14501 | 0.35 | C37H29O16 | 727.12995 | 727.13046 | 0.70 | C37H27O16 | (+): 553, 283 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, T.; Lin, S. Systematic Screening of the Chemical Constituents of Lanqin Oral Liquid by Ultra-High-Performance Liquid Chromatography Combined with Fourier Transform Ion Cyclotron Resonance Mass Spectrometry. Molecules 2023, 28, 7053. https://doi.org/10.3390/molecules28207053
Liu T, Lin S. Systematic Screening of the Chemical Constituents of Lanqin Oral Liquid by Ultra-High-Performance Liquid Chromatography Combined with Fourier Transform Ion Cyclotron Resonance Mass Spectrometry. Molecules. 2023; 28(20):7053. https://doi.org/10.3390/molecules28207053
Chicago/Turabian StyleLiu, Ting, and Shu Lin. 2023. "Systematic Screening of the Chemical Constituents of Lanqin Oral Liquid by Ultra-High-Performance Liquid Chromatography Combined with Fourier Transform Ion Cyclotron Resonance Mass Spectrometry" Molecules 28, no. 20: 7053. https://doi.org/10.3390/molecules28207053
APA StyleLiu, T., & Lin, S. (2023). Systematic Screening of the Chemical Constituents of Lanqin Oral Liquid by Ultra-High-Performance Liquid Chromatography Combined with Fourier Transform Ion Cyclotron Resonance Mass Spectrometry. Molecules, 28(20), 7053. https://doi.org/10.3390/molecules28207053





