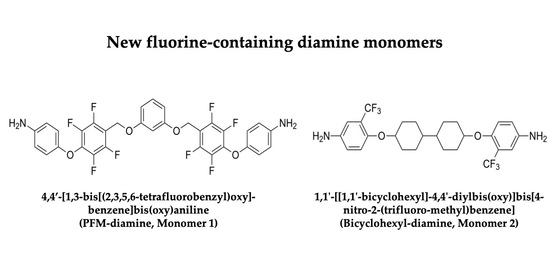
New Fluorine-Containing Diamine Monomers for Potentially Improved Polyimides
Abstract
:1. Introduction
2. Results and Discussion
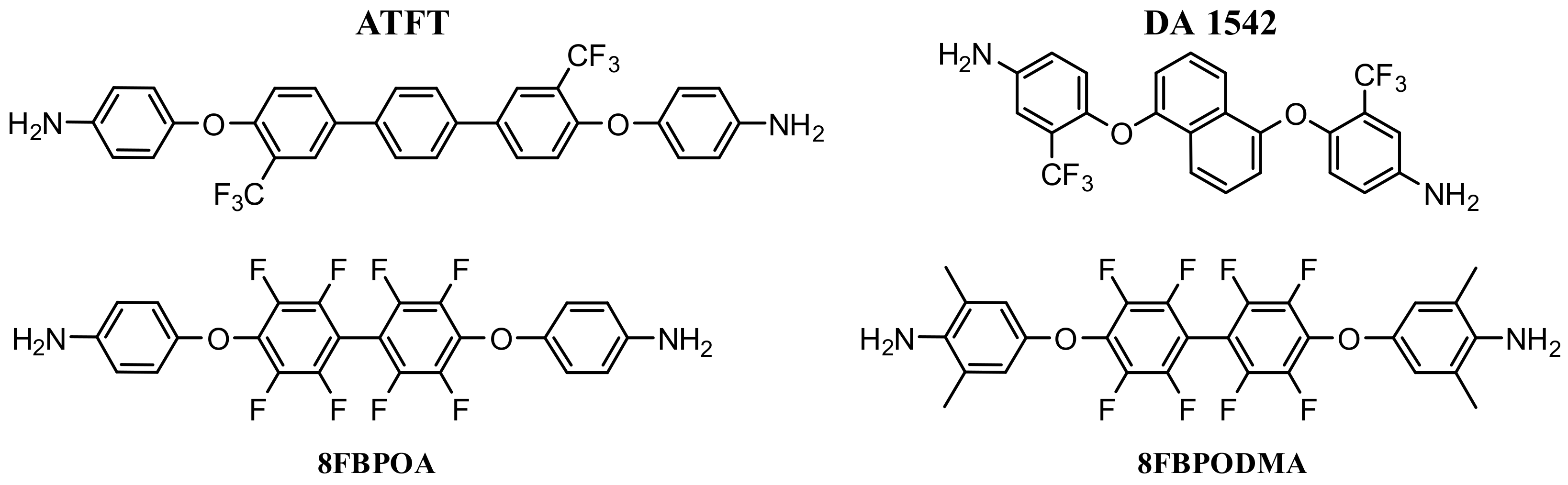
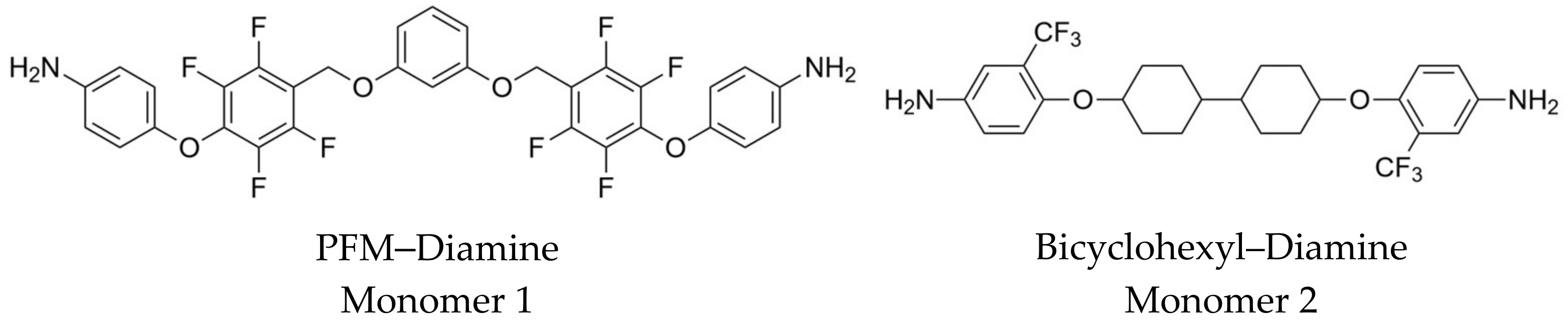
2.1. Design of New Diamine Monomers (Monomers 1 and 2)
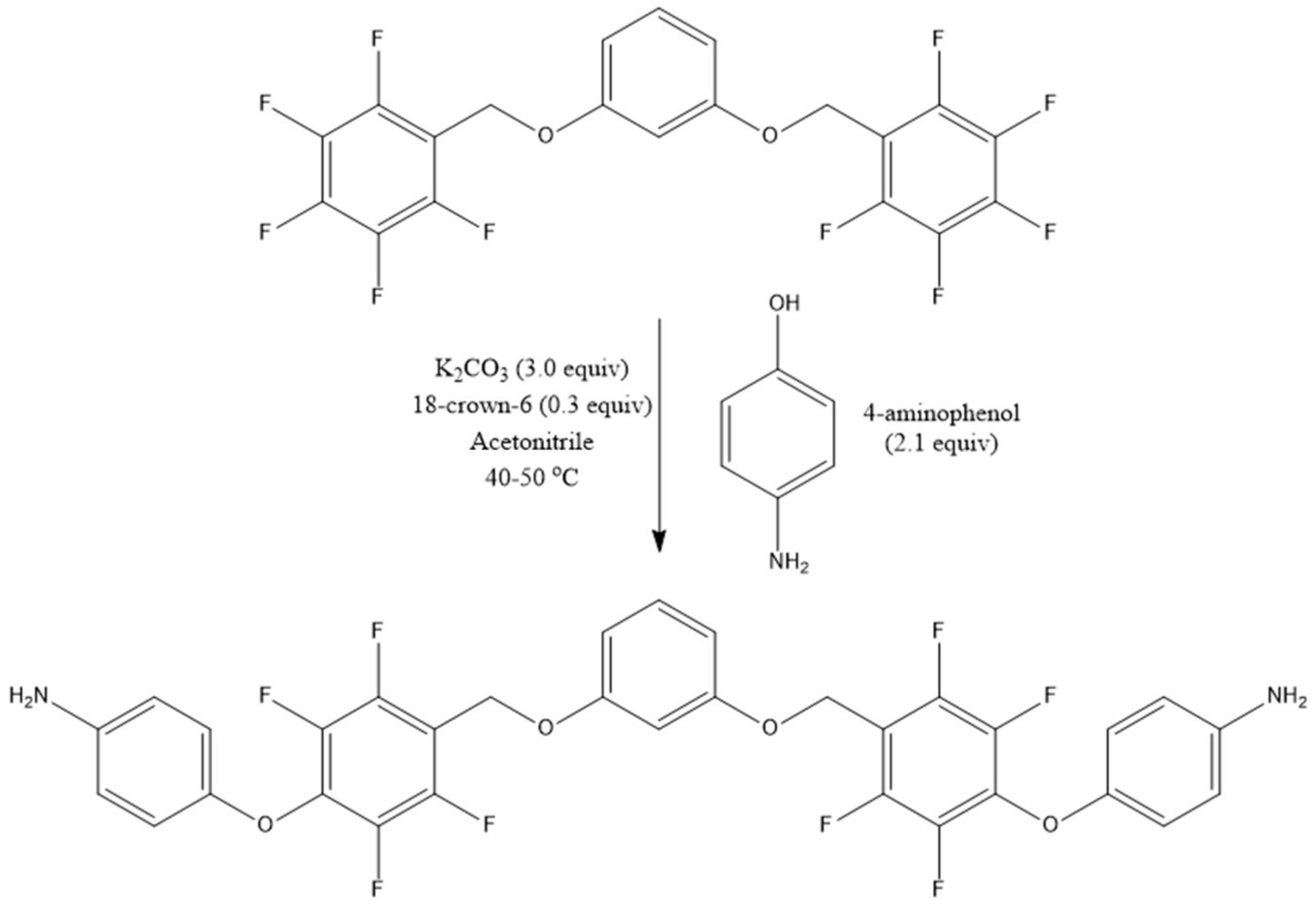
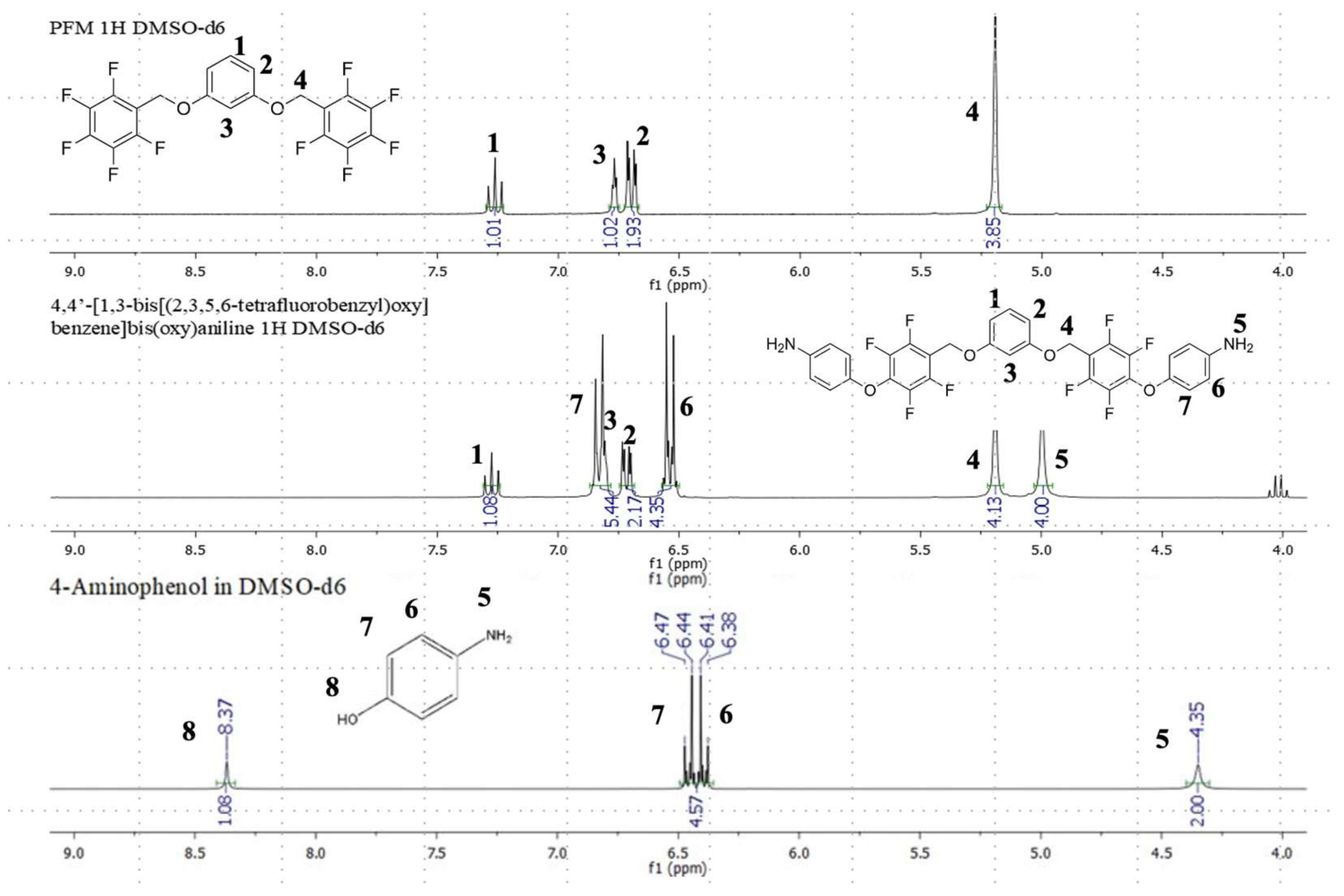
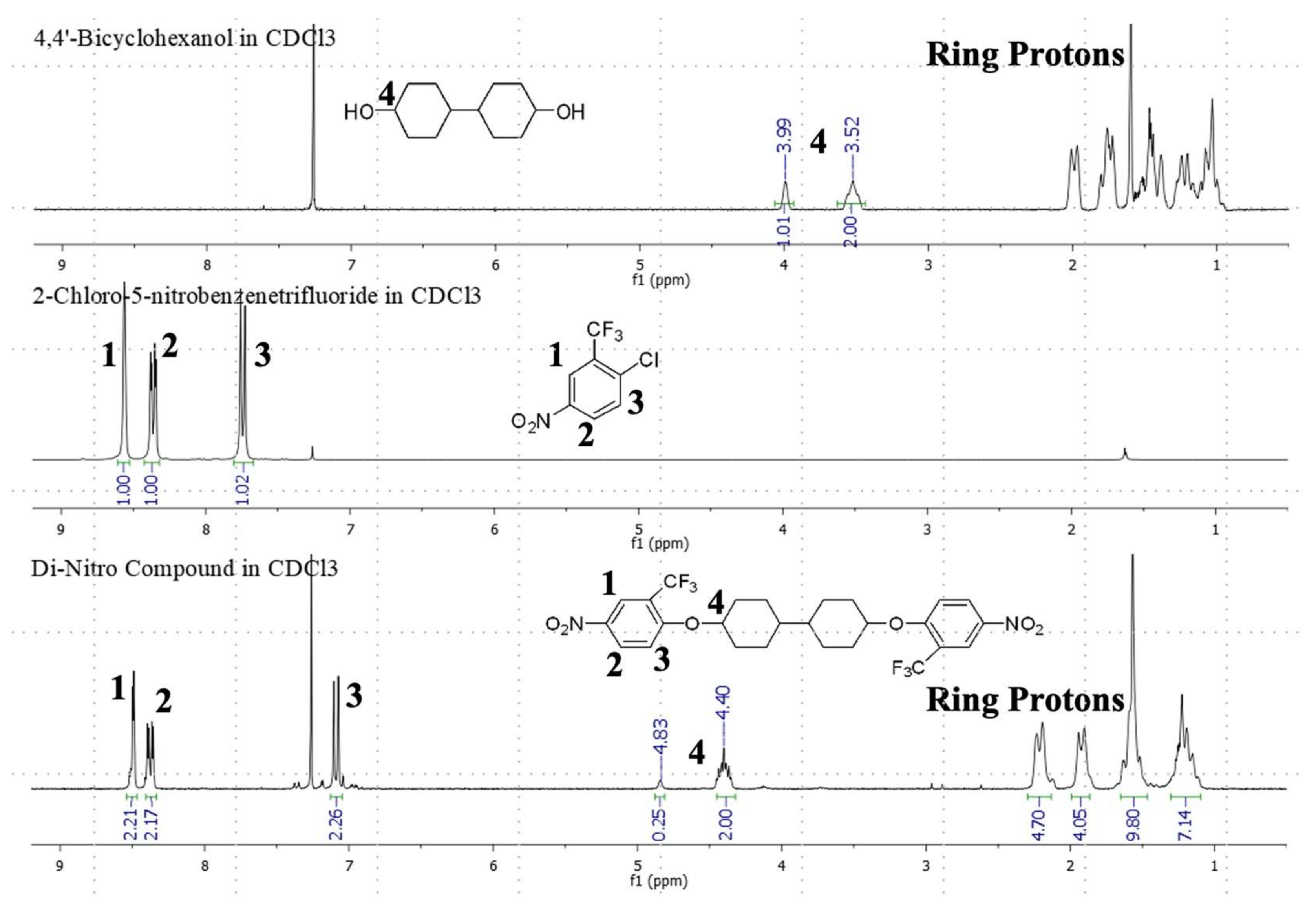
2.2. Preparation and Multinuclear NMR Spectroscopic Characterization of the New Diamine Monomers (Monomers 1 and 2)
2.3. Further Characterization of the PFM–Diamine Monomer (Monomer 1), the Bicyclohexyl–Dinitro Compound, and the Bicyclohexyl–Diamine Monomer (Monomer 2), including the Crystal Structure of the Bicyclohexyl–Diamine Monomer (Monomer 2)
2.4. Future Directions, including Initial Preparations of Poly(amic Acids) and Polyimides from Monomers 1 and 2
3. Materials and Methods
3.1. Materials
3.2. Instrumental Methods
3.3. Preparation of 4,4′-[1,3-bis[(2,3,5,6-tetrafluorobenzyl)oxy]benzene]bis(oxy)aniline (PFM–Diamine, Monomer 1)
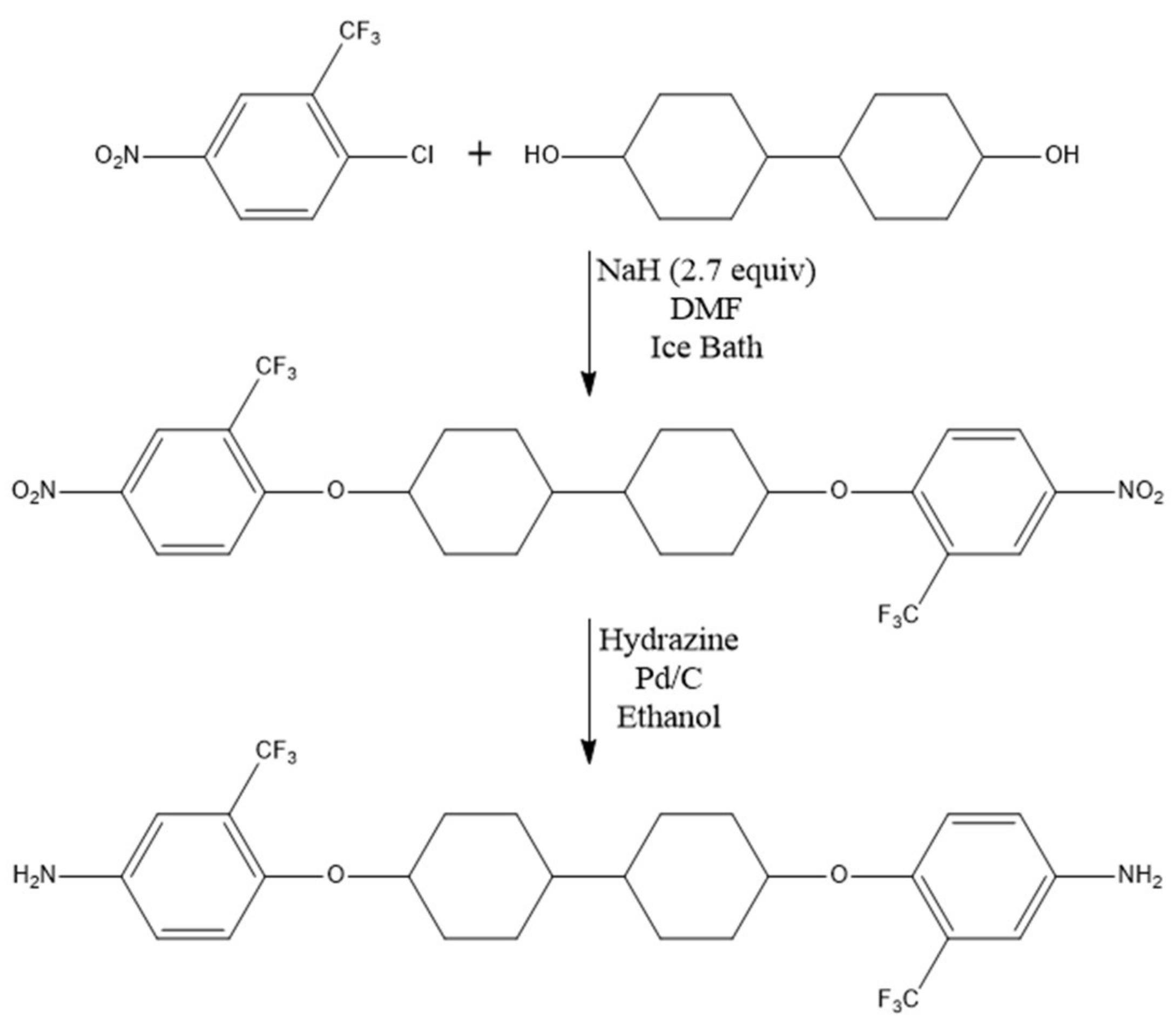
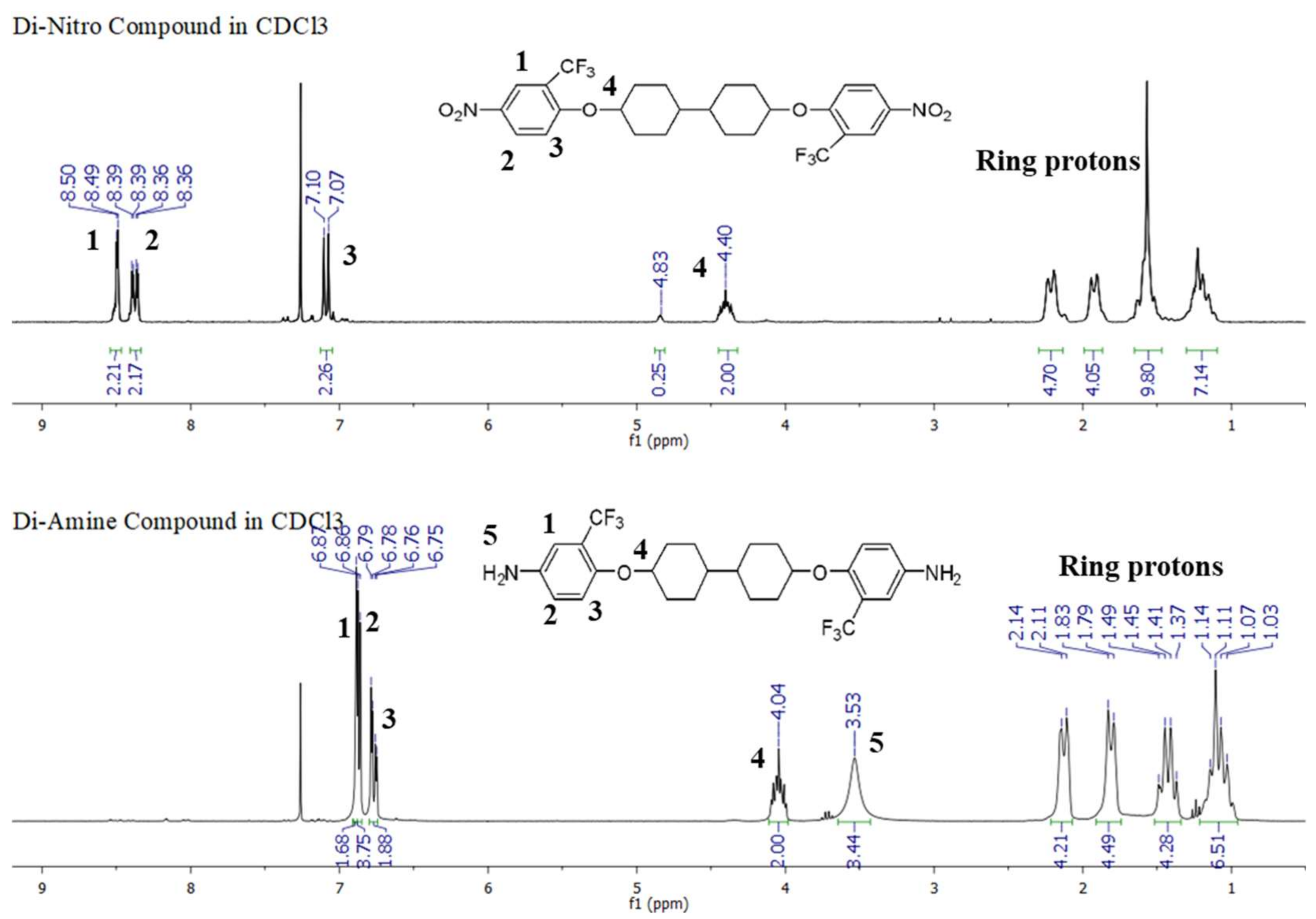
3.4. Preparation of 1,1′-[[1,1′-bicyclohexyl]-4,4′-diylbis(oxy)]bis[4-nitro-2-(trifluoromethyl)benzene]
3.5. Preparation of 4,4′-[[1,1′-bicyclohexyl]-4,4′-diylbis(oxy)]bis[2-(trifluoromethyl)aniline] (Bicyclohexyl–Diamine, Monomer 2)
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Glass Transition Temperatures. Available online: https://www.arsdcollege.ac.in/wp-content/uploads/2020/04/BScH-Chemistry-Semester-VI-Sec-B-Glass-Transition-Temperature-converted.pdf (accessed on 3 October 2020).
- Miyazaki, T.; Hasegawa, M. Highly Tough and Highly Transparent Soluble Polybenzoxazoles. High Perform. Polym. 2007, 19, 243–269. [Google Scholar] [CrossRef]
- Bogert, M.T.; Renshaw, R.R. 4-Amino-o-Phthalic Acid and Some of Its Derivatives. J. Am. Chem. Soc. 1908, 30, 1135–1144. [Google Scholar] [CrossRef]
- Edwards, W.M.; Robinson, I.M. Polyimides of Pyromellitic Acid. U.S. Patent 2,710,853, 14 June 1954. [Google Scholar]
- Edwards, W.M.; Robinson, I.M.; Squire, E.N. Preparation of Polypyromellitimides. U.S. Patent 2,867,609, 6 January 1959. [Google Scholar]
- Progar, D.J.; Clair, T.L.S. Flexible Backbone Aromatic Polyminde Adhesives. NASA Langley Res. Cent. Memo. 1988, 30, 100631. [Google Scholar]
- Gouzman, I.; Grossman, E.; Verker, R.; Atar, N.; Bolker, A.; Eliaz, N. Advances in Polyimide-Based Materials for Space Applications. Adv. Mater. 2019, 31, e1807738. [Google Scholar] [CrossRef] [PubMed]
- Sunaga, T.; Ishii, J.; Hasegawa, M. Polyimide, Polyamic Acid, Resin Composition and Substrate for Flexible Display. WO Patent 2016/013403, 28 January 2016. [Google Scholar]
- Radu, N.S.; Summers, J.D.; Auman, B.C.; Atkinson, W.; Li, W.; Ngai, C.K. Low-Color Polymers for Flexible Substrates in Electronic Devices. WO Patent 2019/032649, 14 February 2019. [Google Scholar]
- Lee, T.H.; Lee, B.K.; Park, J.S.; Park, J.; Kang, J.H.; Yoo, S.Y.; Park, I.; Kim, Y.-H.; Park, H.B. Surface Modification of Matrimid® 5218 Polyimide Membrane with Fluorine-Containing Diamines for Efficient Gas Separation. Membranes 2022, 12, 256, and references therein. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.-J.; Shee, N.K.; Kim, H.-J. Fabrication and properties of Sn(IV) porphyrin-linked porous organic polymer for environmental applications. RSC Adv. 2023, 13, 24077–24085. [Google Scholar] [CrossRef]
- Tapaswi, P.K.; Ha, C.S. Recent Trends on Transparent Colorless Polyimides with Balanced Thermal and Optical Properties: Design and Synthesis. Macromol. Chem. Phys. 2019, 220, 1800313. [Google Scholar] [CrossRef]
- Mathews, A.S.; Kim, I.; Ha, C.S. Fully Aliphatic Polyimides from Adamantane-Based Diamines for Enhanced Thermal Stability, Solubility, Transparency, and Low Dielectric Constant. J. Appl. Polym. Sci. 2006, 102, 3316–3326. [Google Scholar] [CrossRef]
- Ni, H.-J.; Liu, J.-G.; Wang, Z.-H.; Yang, S.-Y. A Review on Colorless and Optically Transparent Polyimide Films: Chemistry, Process and Engineering Applications. J. Ind. Eng. Chem. 2015, 28, 16–27. [Google Scholar] [CrossRef]
- Behniafar, H.; Sefid-Girandehi, N. Optical and Thermal Behavior of Novel Fluorinated Polyimides Capable of Preparing Colorless, Transparent and Flexible Films. J. Fluorine Chem. 2011, 132, 878–884. [Google Scholar] [CrossRef]
- Yi, L.; Huang, W.; Yan, D. Polyimides with Side Groups: Synthesis and Effects of Side Groups on Their Properties. J. Polym. Sci. Part A Polym. Chem. 2017, 55, 533–559. [Google Scholar] [CrossRef]
- Liu, B.; Hu, W.; Matsumoto, T.; Jiang, Z.; Ando, S. Synthesis and Characterization of Organosoluble Ditrifluoromethylated Aromatic Polyimides. J. Polym. Sci. Part A Polym. Chem. 2005, 43, 3018–3029. [Google Scholar] [CrossRef]
- Wang, C.S.; Yang, R.W. Synthesis and Properties of Fluorine-Containing Polyimides. J. Appl. Polym. Sci. 1997, 66, 609–617. [Google Scholar] [CrossRef]
- Park, J.K.; Lee, D.H.; Song, B.J.U.; Jae Buem, O.H.; Kim, H.K. Synthesis and Characterization of Novel Perfluorinated Polyimides with 3D-Controlled Structures for Photonic Applications. J. Polym. Sci. Part A Polym. Chem. 2006, 44, 1326–1342. [Google Scholar] [CrossRef]
- Yeo, H.; Goh, M.; Ku, B.-C.; You, N.-H. Synthesis and Characterization of Highly-Fluorinated Colorless Polyimides Derived from 4,4′-((Perfluoro-[1,1′-Biphenyl]-4,4′-Diyl)Bis(Oxy))Bis(2,6-Dimethylaniline) and Aromatic Dianhydrides. Polymer 2015, 76, 280–286. [Google Scholar] [CrossRef]
- Banerjee, S.; Madhra, M.K.; Salunke, A.K.; Maier, G. Synthesis and Properties of Fluorinated Polyimides. 1. Derived from Novel 4,4″-Bis(Aminophenoxy)-3,3″-Trifluoromethyl Terphenyl. J. Polym. Sci. Part A Polym. Chem. 2002, 40, 1016–1027. [Google Scholar] [CrossRef]
- Hager, C.J. Synthesis of Fluorinated Monomers and Polymers for Various Applications. Ph.D. Thesis, Clemson University, Clemson, SC, USA, 2022. [Google Scholar]
- Wozniak, A.I.; Yegorov, A.S.; Ivanov, V.S.; Igumnov, S.M.; Tcarkova, K.V. Recent Progress in Synthesis of Fluorine Containing Monomers for Polyimides. J. Fluorine Chem. 2015, 180, 45–54. [Google Scholar] [CrossRef]
- Sokolov, A.N.; Goodfellow, B.; Grigg, R.D.; Spencer, L.P.; Kramer, J.W.; Devore, D.D.; Mukhopadhyay, S.; Trefonas, P., III. Quantum Dot Light Emitting Diodes. U.S. Patent 2019/0334106, 31 October 2019. [Google Scholar]
- You, N.H.; Yeo, H.U.; Goh, M.J.; Ku, B.C. Perfluorphenylene-Based Diamine for Polyimide Film with Lw Dielectric Constant and Low Refractivity; KIST: Daejon, Republic of Korea, 2019; KR101926667B1. [Google Scholar]
- Quast, M.J.; Argall, A.D.; Hager, C.J.; Mueller, A. Synthesis and Physical Properties of Highly Branched Perfluorinated Polymers from AB and AB 2 Monomers. J. Polym. Sci. Part A Polym. Chem. 2015, 53, 1880–1894. [Google Scholar] [CrossRef]
- Hager, C.; Quast, M.; Mueller, A. Thermal and mechanical properties of linear ABC polymers for application in proton exchange membranes. In Proceedings of the Abstracts of Papers, 251st ACS National Meeting & Exposition, San Diego, CA, USA, 13–17 March 2016. POLY-43. [Google Scholar]
- Quast, M.J.; Mueller, A. Hyperbranched Polyfluorinated Benzyl Ether Polymers: Mechanism, Kinetics, and Optimization. J. Polym. Sci. Part A Polym. Chem. 2014, 52, 985–994. [Google Scholar] [CrossRef]
- Mueller, A.; Kowalewski, T.; Wooley, K.L. Synthesis, Characterization, and Derivatization of Hyperbranched Polyfluorinated Polymers. Macromolecules 1998, 31, 776–786. [Google Scholar] [CrossRef]
- Baker, J.; Muir, M. The Meisenheimer Model for Predicting the Principal Site for Nucleophilic Substitution in Aromatic Perfluorocarbons—Generalization to Include Ring-Nitrogen Atoms and Non-Fluorine Ring Substituents. Can. J. Chem. 2010, 88, 588–597. [Google Scholar] [CrossRef]
- Buscemi, S.; Pace, A.; Calabrese, R. Fluorinated Heterocyclic Compounds. A Photochemical Synthesis. Tetrahedron 2001, 57, 5865–5871. [Google Scholar] [CrossRef]
- Ogikubo, J.; Worlinsky, J.L.; Fu, Y.J.; Brückner, C. A Two-Step, One-Pot Route to Swap the Pyrroline Moiety in Meso-Tetraaryldihydroxy-Chlorins with an O/N-Substituted Oxazoline. Tetrahedron Lett. 2013, 54, 1707–1710. [Google Scholar] [CrossRef]
- Fujii, S.; Maki, Y.; Kimoto, H. Nucleophilic Substitution of Pentafluorobenzenes with Imidazole. J. Fluor. Chem. 1989, 43, 131–144. [Google Scholar] [CrossRef]
- Shabalin, A.Y.; Adonin, N.Y.; Bardin, V.V. Substitution of Fluorine in M[C6F5BF3] with Organolithium Compounds: Distinctions between O- and N-Nucleophiles. Beilstein J. Org. Chem. 2017, 13, 703–713. [Google Scholar] [CrossRef] [PubMed]
- Li, P.H.; Wang, C.Y.; Li, G.; Jiang, J.M. Highly Organosoluble and Transparant Polyamides Containg Cyclohexane and Trifluoromethyl Moieties: Synthesis and Characterization. EXPRESS Polym. Lett. 2009, 11, 703–712. [Google Scholar] [CrossRef]
- Li, P.-H.; Wang, C.-Y.; Li, G.; Jiang, J.-M. Synthesis and characterization of novel polyamides derived from 1,4-bis((4-amino-2-(trifluoromethyl)phenoxy)methyl)cyclohexane and aromatic dicarboxylic acids. Polym. Bull. 2010, 64, 127–140. [Google Scholar] [CrossRef]
- Yang, Q.; Sheng, M.; Henkelis, J.J.; Tu, S.; Wiensch, E.; Zhang, H.; Zhang, Y.; Tucker, C.; Ejeh, D.E. Explosion Hazards of Sodium Hydride in Dimethyl Sulfoxide, N,N-Dimethylformamide, and N,N-Dimethylacetamide. Org. Process. Res. Dev. 2019, 23, 2210–2217. [Google Scholar] [CrossRef]
- Mo, X.; Wang, C.-Y.; Li, G.; Jiang, J.-M. High Optical Transparency and Low Dielectric Constant Polyimides Containing Trifluoromethyl and Cyclohexane Groups. J. Macromol. Sci. Part B Phys. 2012, 51, 1370–1383. [Google Scholar] [CrossRef]
- Hou, Y.; Chen, G.; Pei, X.; Fang, X. Synthesis and characterization of novel optically transparent and organosoluble polyimides based on diamines containing cyclohexane moiety. J. Polym. Res. 2012, 19, 9955. [Google Scholar] [CrossRef]
- Chen, C.; Wang, S.; Zhao, X.; Wang, D.; Zhou, H. Preparation of Diamine Monomer and Application in the Formation of Polyimide; Jilin University: Changchun, China, 2018; CN109053474. [Google Scholar]
- Chen, C.; Wang, S.; Zhao, X.; Wang, D.; Zhou, H. Flexible Diamine Monomer for Preparing Polyimide and Preparation Method Thereof; Jilin University: Changchun, China, 2018; CN108976135. [Google Scholar]
- Wilds, A.L.; Shunk, C.H.; Hoffman, C.H. Steroid Analogs Lacking Ring C. III. Synthesis of 4-(trans-4′-Hydrocyclohexyl)-cyclohexanone. J. Am. Chem. Soc. 1954, 76, 1733–1736. [Google Scholar] [CrossRef]
- Wilds, A.L.; Pearson, T.H.; Hoffman, C.H. Steroid Analogs Lacking Ring C. IV. 4-(cis-4′-Hydrocyclohexyl)-cyclohexanone. Configurations of the Bicyclohexyl-4,4′-diols. J. Am. Chem. Soc. 1954, 76, 1737–1740. [Google Scholar] [CrossRef]
- Inoki, D.; Furusato, S.; Kawabe, T.; Sajiki, H.; Ikawa, T.; Yamada, T.; Park, K. Method of producing diol compound having cyclohexane skeleton. JP Patent JP2023012993, 26 January 2023. [Google Scholar]
- Song, S.; Guo, W.; Zou, S.; Fu, Z.; Xu, J.; Fan, Z. Polyethylene containing aliphatic ring and aromatic ring defects in the main chain: Synthesis via ADMET and comparisons of thermal properties and crystalline structure. Polymer 2016, 107, 113–121. [Google Scholar] [CrossRef]
- Kenwright, A.M.; Sandford, G.; Tadeusiak, A.J.; Yufit, D.S.; Howard, J.A.K.; Kilickiran, P.; Nelles, G. Strategies for the synthesis of fluorinated liquid crystal derivatives from perbromofluoroaromatic systems. Tetrahedron 2010, 66, 9819–9827. [Google Scholar] [CrossRef]
- Ji, X.; Richter, W.; Fung, B.M.; van Der Helm, D.; Schadt, M. The Crystal and Molecular Structures of Two 4-Alkenyl-substituted Bicyclohexylnitriles. Liq. Cryst. 1991, 201, 29–40. [Google Scholar] [CrossRef]
- Chen, S.-C.; Zhu, Q.; Cao, Y.; Li, C.; Guo, Y.; Kong, L.; Che, J.; Guo, Z.; Chen, H.; Zhang, N.; et al. Dealkenylative Ni-Catalyzed Cross-Coupling Enabled by Tetrazine and Photoexcitation. J. Am. Chem. Soc. 2021, 143, 14046–14052. [Google Scholar] [CrossRef]
- Haldar, S.; Mandal, P.K.; Prathap, S.J.; Guru Row, T.N.; Haase, W. X-ray studies of the crystalline and nematic phases of 4′-(3,4,5-trifluorophenyl)-4-propylbicyclohexyl. Liq. Cryst. 2008, 35, 1307–1312. [Google Scholar] [CrossRef]
- Nath, A.; Gupta, S.; Mandal, P.; Paul, S.; Schenk, H. Structural analysis by X-ray diffraction of a non-polar alkenyl liquid crystalline compound. Liq. Cryst. 1996, 20, 765–770. [Google Scholar] [CrossRef]
- Light, M.; Giustiniano, F.; Whitby, R. CCDC 737127: Experimental Crystal Structure Determination; CCDC: Cambridge, UK, 2011. [Google Scholar] [CrossRef]
- Viswamitra, M.A.; Radhakrishnan, R.; Bandekar, J.; Desiraju, G.R. Evidence for O-H···C and N-H···C Hydrogen Bonding in Crystalline Alkynes, Alkenes, and Aromatics. J. Am. Chem. Soc. 1993, 115, 4868–4869, and references therein. [Google Scholar] [CrossRef]
- Mitchell, J.B.O.; Nandi, C.L.; Ali, S.; McDonald, I.K.; Thornton, J.M.; Price, S.L.; Singh, J. Amino/aromatic interactions. Nature 1993, 366, 413. [Google Scholar] [CrossRef]
- Rzepa, H. Henry Rzepa’s Blog: Chemistry with a Twist. How Does an Oh or NH Group Approach an Aromatic Ring to Hydrogen Bond with Its π-Face? Available online: https://www.ch.imperial.ac.uk/rzepa/blog/?p=16573 (accessed on 16 August 2023).
- Wehrli, F.W.; Wirthlin, T. Interpretation of Carbon-13 NMR Spectra; Heyden & Sons Inc.: Philadelphia, PA, USA, 1980; pp. 43–47. [Google Scholar]
- Bruker AXS Inc. Apex3; Bruker AXS Inc.: Madison, WI, USA, 2015. [Google Scholar]
- Sheldrick, G.M. SHELXT–Integrated space-group and crystal-structure determination. Acta Crystallogr. Sect. A Found. Adv. 2015, 71, 3–8. [Google Scholar] [CrossRef] [PubMed]
- Sheldrick, G.M. Crystal structure refinement with SHELXL. Acta Crystallogr. Sect. C Struct. Chem. 2015, 71, 3–8. [Google Scholar] [CrossRef] [PubMed]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hager, C.J.; McMillen, C.D.; Sachdeva, R.; Martin, A.W.; Thrasher, J.S. New Fluorine-Containing Diamine Monomers for Potentially Improved Polyimides. Molecules 2023, 28, 6855. https://doi.org/10.3390/molecules28196855
Hager CJ, McMillen CD, Sachdeva R, Martin AW, Thrasher JS. New Fluorine-Containing Diamine Monomers for Potentially Improved Polyimides. Molecules. 2023; 28(19):6855. https://doi.org/10.3390/molecules28196855
Chicago/Turabian StyleHager, Cassandra J., Colin D. McMillen, Rakesh Sachdeva, Arthur W. Martin, and Joseph S. Thrasher. 2023. "New Fluorine-Containing Diamine Monomers for Potentially Improved Polyimides" Molecules 28, no. 19: 6855. https://doi.org/10.3390/molecules28196855
APA StyleHager, C. J., McMillen, C. D., Sachdeva, R., Martin, A. W., & Thrasher, J. S. (2023). New Fluorine-Containing Diamine Monomers for Potentially Improved Polyimides. Molecules, 28(19), 6855. https://doi.org/10.3390/molecules28196855