Triterpenes and Pheophorbides from Camellia ptilosperma and Their Cytotoxicity, Photocytotoxicity, and Photodynamic Antibacterial Activity
Abstract
1. Introduction
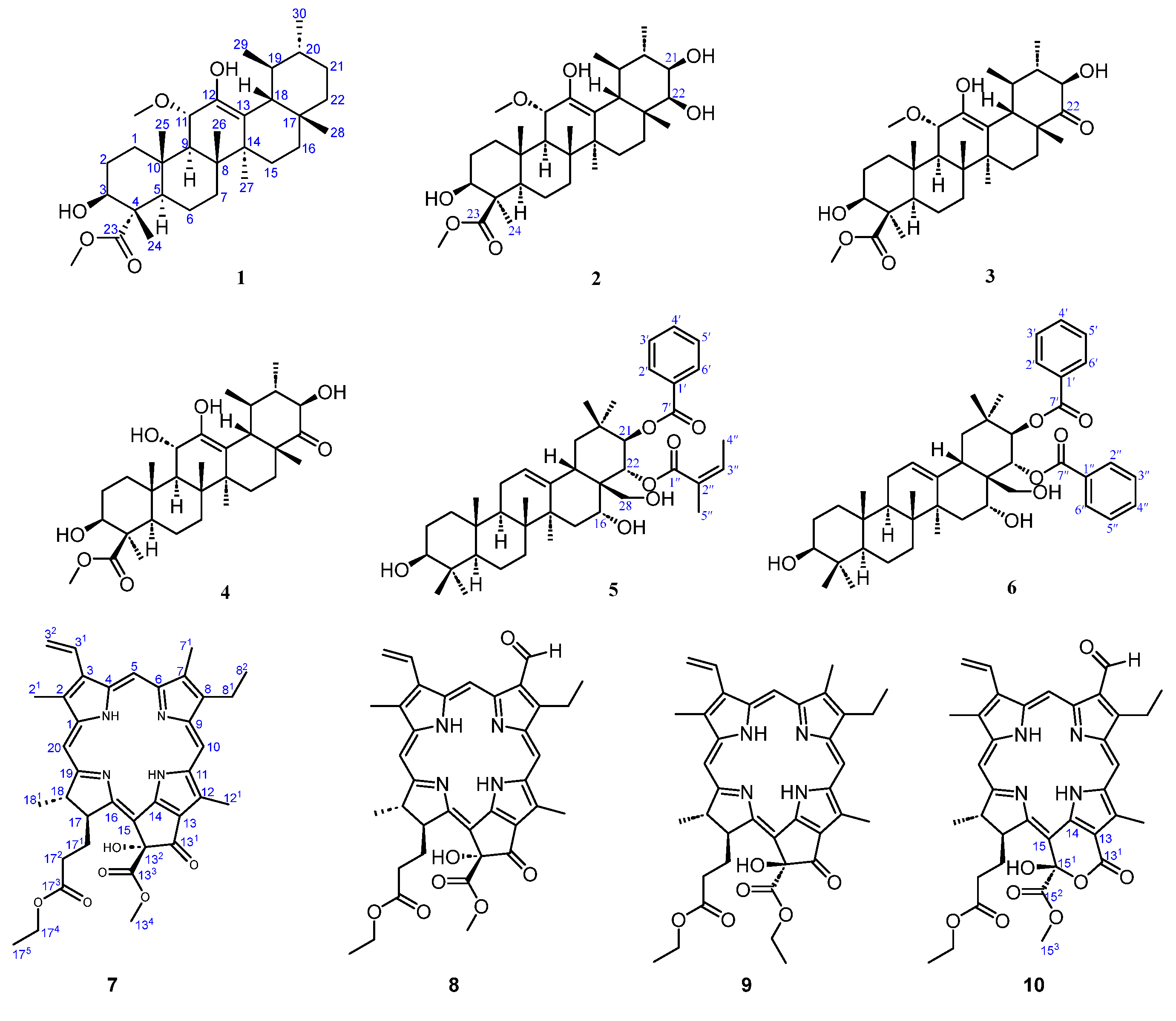
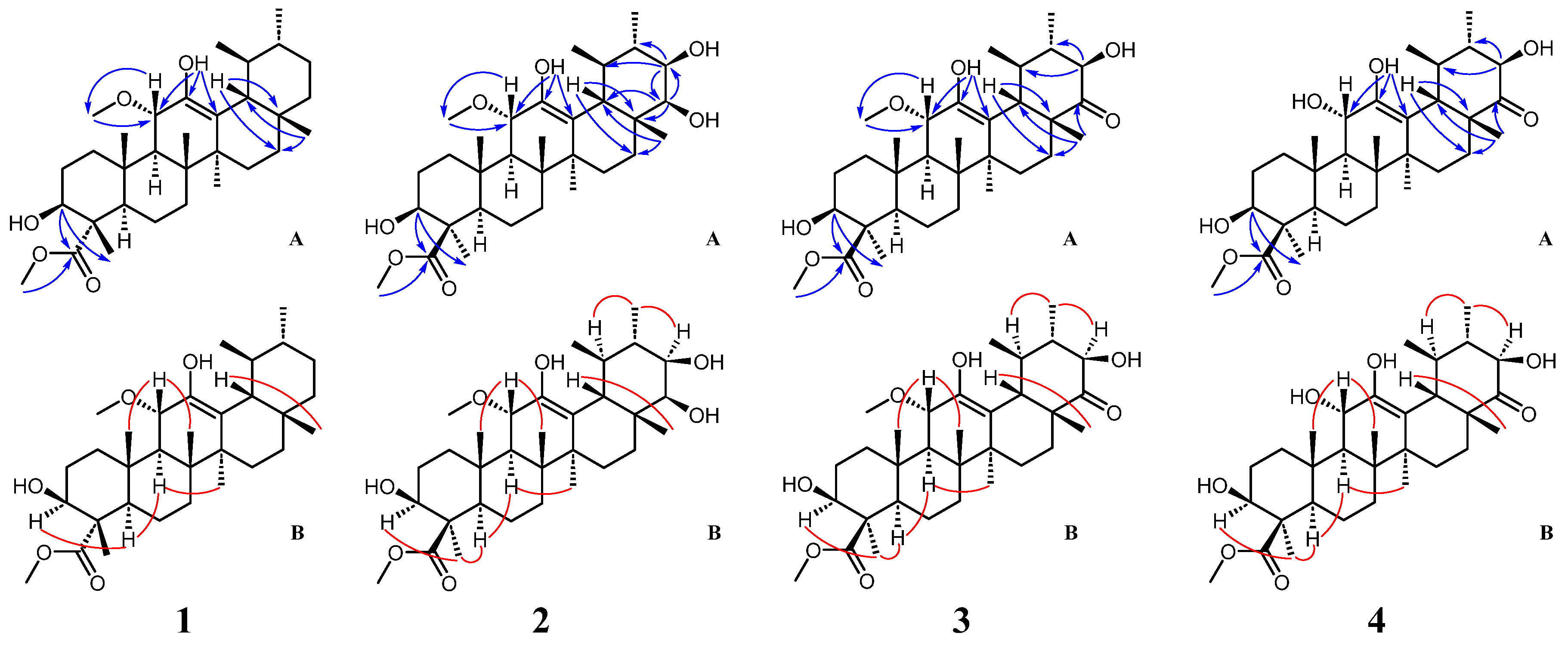
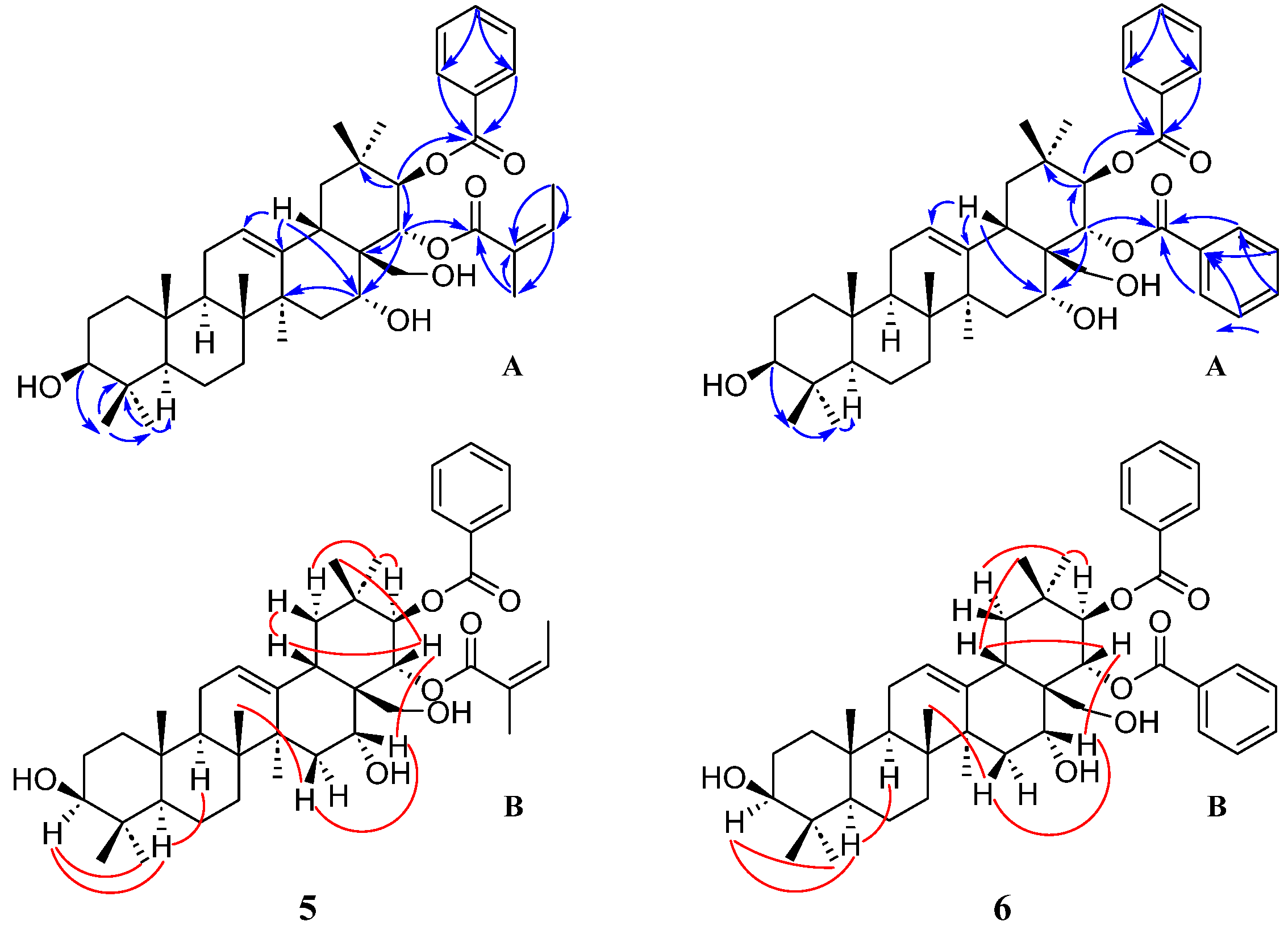
2. Results and Discussion
3. Experimental
3.1. General Experimental Procedures
3.2. Plant Material
3.3. Extraction and Isolation
3.3.1. Compound 1
3.3.2. Compound 2
3.3.3. Compound 3
3.3.4. Compound 4
3.3.5. Compound 5
3.3.6. Compound 6
3.3.7. Compound 7
3.3.8. Compound 8
3.3.9. Compound 9
3.3.10. Compound 10
3.4. Biological Assay
3.4.1. Cytotoxicity Assays
3.4.2. Photocytotoxicity Assay
3.4.3. Photodynamic Antibacterial Activity Assay
3.5. Statistical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Liang, S.Y. World list of yellow camellia plants. Guangxi Forest. Sci. 2007, 142, 221–223. [Google Scholar]
- Ning, E.C.; Qin, X.M.; Yang, H.H. Research on a processing technique of camellia oral liquid. Sci. Technol. Food Ind. 2006, 27, 121–122. [Google Scholar]
- Wei, L.; Ning, E.C.; Liu, Z.X.; Yang, J.W.; Tang, N.; Chen, Z.L. Development of Camellia nitidissima instant tea. J. Anhui Agric. Sci. 2011, 39, 238–240. [Google Scholar]
- Liu, B.C.; Chen, J.Y.; Zhang, W.J.; Huang, Y.Z.; Zhao, Y.Q. Biochemical components evaluation and optimal harvest period of the leaves of Camellia Sect. Chrysantha. Chin. J. Trop. Crops 2021, 42, 159–167. [Google Scholar]
- Song, L.X.; Wang, X.S.; Zheng, X.Q.; Huang, D.J. Polyphenolic antioxidant profiles of yellow camellia. Food Chem. 2011, 129, 351–357. [Google Scholar] [CrossRef]
- Wei, J.B.; Li, X.; Song, H.; Liang, Y.H.; Pan, Y.Z.; Ruan, J.X.; Qin, X.; Chen, Y.X.; Nong, C.L.; Su, Z.H. Characterization and determination of antioxidant components in the leaves of Camellia chrysantha (Hu) Tuyama based on composition-activity relationship approach. J. Food Drug Anal. 2015, 23, 40–48. [Google Scholar] [CrossRef]
- Oku, H.; Ogawa, Y.; Iwaoka, E.; Yamaguchi, Y.; Kagota, S.; Kazumasa, S.; Kunitomo, M.; Ishiguro, K. Preventive effects of the extract of kinka-cha, a folk tea, on a rat model of metabolic syndrome. J. Nat. Med. 2011, 65, 610–616. [Google Scholar] [CrossRef]
- Zhang, H.L.; Wu, Q.X.; Wei, X.; Qin, X.M. Pancreatic lipase and cholesterol esterase inhibitory effect of Camellia nitidissima Chi flower extracts in vitro and in vivo. Food Biosci. 2020, 37, 100682. [Google Scholar] [CrossRef]
- Chen, J.H.; Wu, X.H.; Zhou, Y.; He, J.H. Camellia nitidissima Chi leaf as pancreatic lipase inhibitors: Inhibition potentials and mechanism. J. Food Biochem. 2021, 45, e13837. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.X.; Liu, H.Y.; Wang, Z.N.; Qi, J.; Yuan, S.T.; Zhang, W.J.; Chen, H.J.; Finley, J.W.; Gu, L.W.; Jia, A.Q. Phytochemicals from Camellia nitidissima Chi inhibited the formation of advanced glycation end-products by scavenging methylglyoxal. Food Chem. 2016, 205, 204–211. [Google Scholar] [CrossRef]
- Zhang, H.L.; Wu, Q.X.; Qin, X.M. Camellia nitidissima Chi flower extracts inhibit α-amylase and α-glucosidase: In vitro by analysis of optimization of addition methods, inhibitory kinetics and mechanisms. Process Biochem. 2019, 86, 177–185. [Google Scholar] [CrossRef]
- Yang, R.; Guan, Y.; Zhou, J.W.; Sun, B.; Wang, Z.N.; Chen, H.J.; He, Z.C.; Jia, A.Q. Phytochemicals from Camellia nitidissima Chi flowers reduce the pyocyanin production and motility of Pseudomonas aeruginosa PAO1. Front. Microbiol. 2017, 8, 2640. [Google Scholar] [CrossRef]
- Lin, J.N.; Lin, H.Y.; Yang, N.S.; Li, Y.H.; Lee, M.R.; Chuang, C.H.; Ho, C.T.; Kuo, S.C.; Way, T.D. Chemical constituents and anticancer activity of yellow camellias against MDA-MB-231 human breast cancer cells. J. Agric. Food Chem. 2013, 61, 9638–9644. [Google Scholar] [CrossRef] [PubMed]
- Ma, S.Y.; Ge, L.; Li, Y.X.; Liao, N.Y.; Xie, J.Z.; Yang, K.D. Ursane-type triterpenes with a phenylpropanoid unit from Camellia ptilosperma and evaluation of their cytotoxic activities. J. Nat. Prod. 2023, 86, 1793–1800. [Google Scholar] [CrossRef] [PubMed]
- Dai, L.; Li, J.L.; Liang, X.Q.; Li, L.; Feng, Y.; Liu, H.Z.; Wei, W.E.; Ning, S.F.; Zhang, L.T. Flowers of Camellia nitidissima cause growth inhibition, cell-cycle dysregulation and apoptosis in a human esophageal squamous cell carcinoma cell line. Mol. Med. Rep. 2016, 14, 1117–1122. [Google Scholar] [CrossRef][Green Version]
- Xu, Y.N.; Ma, S.Y.; Han, X.F.; Su, L.; Ge, L.; Chen, Q.H.; Mo, Q.F.; Yang, K.D. Triterpenes and saponins from leaves of Camellia nitidissima, and cytotoxic activities against Bel-7402 and SMMC-7721 human liver cancer cells. Rec. Nat. Prod. 2022, 16, 550–558. [Google Scholar]
- He, D.Y.; Wang, X.T.; Zhang, P.; Luo, X.X.; Li, X.Y.; Wang, L.L.; Li, S.Y.; Xu, Y.P. Evaluation of the anxiolytic and antidepressant activities of the aqueous extract from Camellia euphlebia Merr. ex Sealy in mice. J. Evid.-Based Complement. Altern. Med. 2015, 2015, 618409. [Google Scholar]
- Tsoi, B.; Gao, C.; Yan, S.Y.; Du, Q.H.; Yu, H.; Li, P.; Deng, J.G.; Shen, J.G. Camellia nitidissima Chi extract promotes adult hippocampal neurogenesis and attenuates chronic corticosterone-induced depressive behaviours through regulating Akt/GSK3β/CREB signaling pathway. J. Funct. Foods 2022, 95, 105199. [Google Scholar] [CrossRef]
- Yang, Z.; Chen, C.H.; Zhang, Y.Y.; Yang, Y.; Zhang, P.; Bao, G.H. Chemical composition and antibacterial activity of 12 medicinal plant ethyl acetate extracts using LC-MS feature-based molecular networking. Phytochem. Anal. 2022, 33, 473–489. [Google Scholar] [CrossRef]
- Weinhold, A.; Doll, S.; Liu, M.; Schedl, A.; Poschl, Y.; Xu, X.L.; Neumann, S.; Dam, N.M. Tree species richness differentially affects the chemical composition of leaves, roots and root exudates in four subtropical tree species. J. Ecol. 2022, 110, 97–116. [Google Scholar] [CrossRef]
- Hou, X.Y.; Du, H.Z.; Yang, R.; Qi, J.; Huang, Y.; Feng, S.Y.; Wu, Y.; Lin, S.S.; Liu, Z.X.; Jia, A.Q.; et al. The antitumor activity screening of chemical constituents from Camellia nitidissima Chi. Int. J. Mol. Med. 2018, 41, 2793–2801. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.F.; Han, Y.Y.; Di, T.M.; Gao, L.P.; Xia, T. Triterpene saponins from tea seed pomace (Camellia oleifera Abel) and their cytotoxic activity on MCF-7 cells in vitro. Nat. Prod. Res. 2021, 35, 2730–2733. [Google Scholar] [CrossRef] [PubMed]
- Ji, M.Y.; Gong, X.; Li, X.; Wang, C.C.; Li, M.H. Advanced Research on the antioxidant activity and mechanism of polyphenols from Hippophae Species—A review. Molecules 2020, 25, 917. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.L.; Wang, Y.H.; Mai, Y.X.; Li, H.Y.; Wang, Z.; Xu, J.W.; He, X.J. Health benefits of the flavonoids from onion: Constituents and their pronounced antioxidant and anti-neuroinflammatory capacities. J. Agric. Food Chem. 2020, 68, 799–807. [Google Scholar] [CrossRef] [PubMed]
- Brighenti, V.; Iseppi, R.; Pinzi, L.; Mincuzzi, A.; Ippolito, A.; Messi, P.; Sanzani, S.M.; Rastelli, G.; Pellati, F. Antifungal activity and DNA topoisomerase inhibition of hydrolysable tannins from Punica granatum L. Int. J. Mol. Sci. 2021, 22, 4175. [Google Scholar] [CrossRef]
- Chai, W.M.; Wei, Q.M.; Deng, W.L.; Zheng, Y.L.; Chen, X.Y.; Huang, Q.; Chong, O.Y.; Peng, Y.Y. Anti-melanogenesis properties of condensed tannins from Vigna angularis seeds with potent antioxidant and DNA damage protection activities. Food Funct. 2019, 10, 99–111. [Google Scholar] [CrossRef]
- Magozwi, D.K.; Dinala, M.; Mokwana, N.; Siwe-Noundou, X.; Krause, R.W.M.; Sonopo, M.; McGaw, L.J.; Augustyn, W.A.; Tembu, V.J. Flavonoids from the genus Euphorbia: Isolation, structure, pharmacological activities and structure-activity relationships. Pharmaceuticals 2021, 14, 428. [Google Scholar] [CrossRef]
- Lee, D.; Jang, S.Y.; Kwon, S.; Lee, Y.; Park, E.; Koo, H. Optimized combination of photodynamic therapy and chemotherapy using gelatin nanoparticles containing tirapazamine and pheophorbide a. ACS Appl. Mater. Interfaces 2021, 13, 10812–10821. [Google Scholar] [CrossRef]
- Aksel, M.; Bozkurt-Girit, O.; Bilgin, M.D. Pheophorbide a-mediated sonodynamic, photodynamic and sonophotodynamic therapies against prostate cancer. Photodiagn. Photodyn. Ther. 2020, 31, 101909. [Google Scholar] [CrossRef]
- Karg, C.A.; Wang, P.Y.; Kluibenschedl, F.; Muller, T.; Allmendinger, L.; Vollmar, A.M.; Moser, S. Phylloxanthobilins are abundant linear tetrapyrroles from chlorophyll breakdown with activities against cancer cells. Eur. J. Org. Chem. 2020, 29, 4499–4509. [Google Scholar] [CrossRef]
- Gariboldi, M.B.; Marras, E.; Vaghi, I.; Margheritis, A.; Malacarne, M.C.; Caruso, E. Phototoxicity of two positive-charged diaryl porphyrins in multicellular tumor spheroids. J. Photochem. Photobiol. B 2021, 225, 112353. [Google Scholar] [CrossRef]
- Schneider, L.; Kalt, M.; Larocca, M.; Babu, V.; Spingler, B. Potent PBS/polysorbate-soluble transplatin-derived porphyrin-based photosensitizers for photodynamic therapy. Inorg. Chem. 2021, 60, 9416–9426. [Google Scholar] [CrossRef] [PubMed]
- Heliawati, L.; Khatimah, H.; Hermawati, E.; Syah, Y.M. Four dammarane triterpenes and their inhibitory properties against eight receptor tyrosine kinases. Nat. Prod. Sci. 2020, 26, 345–350. [Google Scholar] [CrossRef]
- Farimani, M.M.; Abbas-Mohammadi, M. Two new polyhydroxylated triterpenoids from Salvia urmiensis and their cytotoxic activity. Fitoterapia 2016, 30, 2648–2654. [Google Scholar] [CrossRef]
- Gao, X.H.; Wang, X.Y.; Zhou, J.S.; Zhang, Y.; Liu, H.C.; Zhou, B.; Yue, J.M. Rearranged dichapetalin-type triterpenoids with cytotoxic activity from Dichapetalum gelonioides. Chin. J. Chem. 2022, 40, 2531–2538. [Google Scholar] [CrossRef]
- Lanzotti, V.; Termolino, P.; Dolci, M.; Curir, P. Paviosides A-H, eight new oleane type saponins from Aesculus pavia with cytotoxic activity. Bioorg. Med. Chem. 2012, 20, 3280–3286. [Google Scholar] [CrossRef]
- Barua, A.; Dutta, S.; Pal, S. Triterpenoids XXX. The structure of barringtogenol E. A new triterpenoid sapogenol from barringtonia acutangular. J. Indian Chem. Soc. 1967, 991, 937–940. [Google Scholar]
- Nakatani, Y.; Ourisson, G.; Beck, J. Chemistry and biochemistry of Chinese drugs. VII.: Cytostatic pheophytins from silkworm excreta, and derived photocytotoxic pheophorbides. Chem. Pharm. Bull. 1981, 29, 2261–2269. [Google Scholar] [CrossRef]
- Qi, J.; Shi, R.F.; Yu, J.M.; Li, Y.; Yuan, S.T.; Yang, J.Z.; Hu, J.M.; Jia, A.Q. Chemical constituents from leaves of Camellia nitidissima and their potential cytotoxicity on SGC7901 cells. Chin. Herb. Med. 2016, 8, 80–84. [Google Scholar] [CrossRef]
- Thao, N.T.P.; Hung, T.M.; Lee, M.K.; Kim, J.C.; Min, B.S.; Bae, K. Triterpenoids from Camellia japonica and their cytotoxic activity. Chem. Pharm. Bull. 2010, 58, 121–124. [Google Scholar] [CrossRef]
- Fan, L.M.; He, Y.F.; Xu, Y.J.; Li, P.H.; Zhang, J.S.; Zhao, J. Triterpenoid saponins in tea (Camellia sinensis) plants: Biosynthetic gene expression, content variations, chemical identification and cytotoxicity. Int. J. Food Sci. Nutr. 2021, 72, 308–323. [Google Scholar] [CrossRef] [PubMed]
- Zhou, H.; Wang, C.Z.; Ye, J.Z.; Chen, H.X. New triterpene saponins from the seed cake of Camellia Oleifera and their cytotoxic activity. Phytochem. Lett. 2014, 8, 46–51. [Google Scholar] [CrossRef]
- Lin, Y.Y.; Zhou, T.; Bai, R.R.; Xie, Y.Y. Porphyrins are able to generate monoclinic oxygen in the presence of light and oxygen, effectively killing tumour cells. J. Enzyme Inhib. Med. Chem. 2020, 35, 1080–1099. [Google Scholar] [CrossRef]
- Chavda, J.; Rajwar, A.; Bhatia, B.; Gupta, I. Synthesis of novel zinc porphyrins with bioisosteric replacement of sorafenib: Efficient theranostic agents for anti-cancer application. J. Inorg. Biochem. 2023, 22, 112384. [Google Scholar] [CrossRef] [PubMed]
- Pan, Z.H.; Fan, J.J.; Xie, Q.; Zhang, X.; Zhang, W.; Ren, Q.; Li, M.J.; Zheng, Q.S.; Lu, J.; Li, D. Novel sulfonamide porphyrin TBPoS-2OH used in photodynamic therapy for malignant melanoma. Biomed. Pharmacother. 2021, 133, 111042. [Google Scholar] [CrossRef] [PubMed]
- Thomas, A.P.; Babu, P.S.S.; Nair, S.A.; Ramakrishnan, S.; Ramaiah, D.; Chandrashekar, T.K.; Srinivasan, A.; Pillai, M.R. meso-Tetrakis(p-sulfonatophenyl)N-confused porphyrin tetrasodium salt: A potential sensitizer for photodynamic therapy. J. Med. Chem. 2012, 55, 5110–5520. [Google Scholar] [CrossRef]
- Hynek, J.; Koncosova, M.; Zelenka, J.; Krizova, I.; Ruml, T.; Kubat, P.; Demel, J.; Lang, K. Phosphinatophenylporphyrins tailored for high photodynamic efficacy. Org. Biomol. Chem. 2018, 16, 7274–7281. [Google Scholar] [CrossRef]
- Steinke, S.J.; Gupta, S.; Piechota, E.J.; Moore, C.E.; Kodanko, J.J.; Turro, C. Photocytotoxicity and photoinduced phosphine ligand exchange in a Ru(II) polypyridyl complex. Chem. Sci. 2022, 13, 1933–1945. [Google Scholar] [CrossRef]
- Upadhyay, A.; Gautam, S.; Ramu, V.; Kondaiah, P.; Chakravarty, A. Photocytotoxic cancer cell-targeting platinum(II) complexes of glucose-appended curcumin and biotinylated 1,10-phenanthroline. Dalton Trans. 2019, 48, 17556–17565. [Google Scholar] [CrossRef]
- Banaspati, A.; Ramu, V.; Raza, M.K.; Goswami, T.K. Copper(II) curcumin complexes for endoplasmic reticulum targeted photocytotoxicity. RSC Adv. 2022, 12, 30722–30733. [Google Scholar] [CrossRef]
- Ragasa, C.Y.; Cornelio, K.B. Triterpenes from Euphorbia hirta and their cytotoxicity. Chin. J. Nat. Med. 2013, 11, 528–533. [Google Scholar] [CrossRef]
- Kikuchi, T.; Uchiyama, E.; Ukiya, M.; Tabata, K.; Kimura, Y.; Suzuki, T.; Akihisa, T. Cytotoxic and apoptosis-inducing activities of triterpene acids from Poria cocos. J. Nat. Prod. 2011, 74, 137–144. [Google Scholar] [CrossRef] [PubMed]


| 1 | 2 | 3 | 4 | |||||
|---|---|---|---|---|---|---|---|---|
| No. | δH (J in Hz) | δC | δH (J in Hz) | δC | δH (J in Hz) | δC | δH (J in Hz) | δC |
| 1 | 1.32 m 2.27 dt-like (13.6, 3.4) | 38.6 | 1.21 m 2.34 dt-like (13.4, 3.5) | 39.8 | 1.22 m 2.38 dt-like (13.3, 3.3) | 39.9 | 1.22 m 2.40 dt-like (13.3, 3.5) | 41.8 |
| 2 | 1.60–1.71 m | 26.8 | 1.77 m 2.03 m | 28.3 | 1.78 m 2.04 m | 28.4 | 1.79 m 2.04 m | 28.2 |
| 3 | 4.00 dd (11.5, 5.1) | 75.3 | 3.11 dd (12.1, 4.3) | 77.9 | 3.11 ddd (11.9, 11.9, 4.4) | 77.9 | 3.11 td-like (11.7, 4.3) | 77.8 |
| 4 | 54.2 | 49.3 | 49.4 | 49.2 | ||||
| 5 | 1.55 m | 51.1 | 0.95 m | 56.7 | 0.97 m | 56.8 | 0.97 m | 56.6 |
| 6 | 1.01 m 1.55 m | 21.3 | 1.66 m 1.82 m | 19.9 | 1.67 m 1.84 m | 19.8 | 1.68 m 1.85 m | 19.9 |
| 7 | 1.31 m 1.53 m | 33.7 | 1.35–1.48 m | 34.2 | 1.40–1.49 m | 34.5 | 1.38–1.51 m | 34.2 |
| 8 | 43.1 | 42.7 | 42.5 | 42.4 | ||||
| 9 | 1.94 d (10.4) | 46.3 | 1.86 d (10.7) | 45.3 | 1.89 d (10.7) | 44.7 | 1.61 d (9.8) | 53.3 |
| 10 | 37.7 | 38.5 | 38.6 | 38.5 | ||||
| 11 | 4.26 d (10.4) | 76.4 | 4.23 d (10.5) | 76.5 | 4.27 d (10.7) | 76.3 | 4.16 br d (10.7) | 70.5 |
| 12 | 141.7 | 142.8 | 143.4 | 145.9 | ||||
| 13 | 118.3 | 116.6 | 114.5 | 112.3 | ||||
| 14 | 40.6 | 41.0 | 40.6 | 40.8 | ||||
| 15 | 0.98 br d (13.5) 1.77 td-like (13.5, 5.1) | 27.1 | 1.02 m 1.81 m | 26.4 | 1.13 m 1.79 m | 25.6 | 1.13 m 1.76 m | 25.3 |
| 16 | 0.83 br d (13.5) 2.01 td-like (13.5, 4.8) | 27.5 | 1.02 m 1.81 m | 26.6 | 1.26 m 2.06 m | 28.0 | 1.26 m 2.05 m | 28.1 |
| 17 | 33.3 | 38.3 | 47.8 | 47.7 | ||||
| 18 | 2.24 dd (11.4, 1.3) | 47.6 | 2.62 br d (11.2) | 41.8 | 2.75 d (11.6) | 48.1 | 2.66 br d (11.6) | 47.7 |
| 19 | 1.37 m | 40.8 | 1.51 m | 39.1 | 1.95 m | 38.5 | 1.95 m | 38.4 |
| 20 | 1.03 m | 39.5 | 1.45 m | 39.1 | 1.29 m | 47.2 | 1.30 m | 47.3 |
| 21 | 1.26 m 1.41 m | 31.2 | 3.49 dd (10.5, 2.7) | 73.5 | 4.05 dd (11.6, 3.0) | 76.4 | 4.06 dd (11.6, 3.0) | 76.4 |
| 22 | 1.32–1.46 m | 41.6 | 3.40 d (2.7) | 78.7 | 215.3 | 215.2 | ||
| 23 | 178.1 | 1.42 s | 23.8 | 1.43 s | 23.8 | 1.44 s | 23.8 | |
| 24 | 1.18 s | 11.0 | 178.4 | 178.3 | 178.3 | |||
| 25 | 1.13 s | 16.6 | 0.95 s | 14.0 | 0.97 s | 14.0 | 0.98 s | 14.3 |
| 26 | 1.09 s | 18.0 | 1.12 s | 18.0 | 1.12 s | 18.1 | 1.10 s | 18.0 |
| 27 | 1.21 s | 24.0 | 1.19 s | 24.1 | 1.29 s | 23.6 | 1.28 s | 23.8 |
| 28 | 0.80 s | 28.5 | 0.96 s | 21.9 | 1.09 s | 20.1 | 1.09 s | 20.3 |
| 29 | 0.92 d (6.6) | 17.0 | 0.96 d (6.6) | 16.8 | 1.02 d (6.7) | 16.0 | 0.98 d (6.6) | 15.8 |
| 30 | 0.93 d (6.4) | 21.2 | 1.06 d (6.1) | 16.0 | 1.20 d (6.3) | 16.8 | 1.19 d (6.3) | 16.7 |
| 3-OH | 3.39 d (11.9) | 3.39 d (12.0) | ||||||
| 12-OH | 4.53 br s | 4.69 br s | 4.76 s | 4.88 br s | ||||
| 21-OH | 3.79 d (3.0) | 3.80 d (3.0) | ||||||
| 11-OCH3 | 3.18 s | 51.3 | 3.14 s | 51.1 | 3.14 s | 50.8 | ||
| 23-OCH3 | 3.73 s | 52.2 | ||||||
| 24-OCH3 | 3.69 s | 51.3 | 3.70 s | 51.3 | 3.71 s | 51.4 | ||
| 5 | 6 | |||
|---|---|---|---|---|
| No. | δH (J in Hz) | δC | δH (J in Hz) | δC |
| 1 | 1.00 m 1.65 m | 38.6 | 1.01 m 1.64 m | 38.6 |
| 2 | 1.54–1.66 m | 27.2 | 1.55–1.67 m | 27.1 |
| 3 | 3.24 dd (11.5, 4.3) | 78.9 | 3.23 dd (11.6, 4.4) | 79.0 |
| 4 | 38.8 | 38.8 | ||
| 5 | 0.76 br d (11.8) | 55.1 | 0.76 br d (10.9) | 55.2 |
| 6 | 1.40 m 1.56 m | 18.3 | 1.40 m 1.56 m | 18.3 |
| 7 | 1.30 m 1.57 m | 32.7 | 1.31 m 1.57 m | 32.7 |
| 8 | 41.0 | 41.0 | ||
| 9 | 1.64 m | 46.5 | 1.64 m | 46.5 |
| 10 | 36.9 | 36.9 | ||
| 11 | 1.88–1.96 m | 23.5 | 1.88–1.97 m | 23.5 |
| 12 | 5.48 t (3.3) | 124.8 | 5.50 t (3.3) | 125.1 |
| 13 | 140.6 | 140.7 | ||
| 14 | 39.7 | 39.7 | ||
| 15 | 1.37 m 1.70 m | 33.7 | 1.38 m 1.70 m | 33.7 |
| 16 | 3.97 br s | 69.9 | 4.02 br s | 69.8 |
| 17 | 47.7 | 47.7 | ||
| 18 | 2.77 dd (14.3, 4.2) | 39.2 | 2.82 dd (14.4, 4.7) | 39.2 |
| 19 | 1.32 m 2.62 dd (14.3, 13.2) | 46.4 | 1.32 m 2.66 dd (14.4, 13.9) | 46.4 |
| 20 | 36.1 | 36.1 | ||
| 21 | 6.01 d (10.2) | 78.7 | 6.19 d (10.2) | 78.6 |
| 22 | 5.55 d (10.2) | 73.1 | 5.66 d (10.2) | 74.5 |
| 23 | 1.00 s | 28.1 | 1.00 s | 28.1 |
| 24 | 0.79 s | 15.6 | 0.79 s | 15.6 |
| 25 | 0.94 s | 15.6 | 0.94 s | 15.6 |
| 26 | 0.91 s | 16.7 | 0.91 s | 16.7 |
| 27 | 1.47 s | 27.1 | 1.47 s | 27.1 |
| 28 | 2.93 d (11.5) 3.30 d (11.5) | 63.6 | 2.97 d (11.5) 3.32 d (11.5) | 63.6 |
| 29 | 0.96 s | 29.1 | 0.96 s | 29.1 |
| 30 | 1.18 s | 19.5 | 1.18 s | 19.5 |
| 1′ | 130.3 | 130.1 | ||
| 2′, 6′ | 7.98 d (7.7) | 129.5 | 7.91 d (7.8) | 129.7 |
| 3′, 5′ | 7.41 dd (7.7, 7.3) | 128.3 | 7.32 dd (7.8, 7.2) | 128.4 |
| 4′ | 7.53 t (7.3) | 132.8 | 7.46 t (7.2) | 132.3 |
| 7′ | 166.3 | 168.3 | ||
| 1″ | 169.3 | 129.0 | ||
| 2″ | 126.9 | 7.88 d (7.8) | 129.5 | |
| 3″ | 5.91 br q (7.2) | 139.7 | 7.33 dd (7.8, 7.2) | 128.2 |
| 4″ | 1.77 br d (7.2) | 15.6 | 7.42 t (7.2) | 132.0 |
| 5″ | 1.67 br s | 20.3 | 7.33 dd (7.8, 7.2) | 128.2 |
| 6″ | 7.88 d (7.8) | 129.5 | ||
| 7″ | 166.5 | |||
| No. | 7 | 8 | 9 | 10 |
|---|---|---|---|---|
| 21 | 3.42, s | 3.39, s | 3.43, s | 3.37, s |
| 31 | 7.99, dd (17.8, 11.5) | 7.99, dd (17.8, 11.6) | 8.03, dd (17.8, 11.6) | 7.90, dd (17.8, 11.6) |
| 32 | 6.18, dd (11.5, 1.2) 6.29, dd (17.8, 1.2) | 6.23, d, (11.6) 6.37, d, (17.8) | 6.20, dd (11.6, 1.3) 6.31, dd (17.8, 1.3) | 6.17, d (11.6) 6.35, d (17.8) |
| 5 | 9.42, s | 10.37, s | 9.50, s | 10.28, s |
| 71 | 3.23, s | 11.11, s | 3.28, s | 11.00, s |
| 81 | 3.68, q (7.7) | 4.01, m | 3.73, q (7.7) | 3.91, q (7.5) |
| 82 | 1.69, t (7.7) | 1.80, t (7.7) | 1.71, t (7.7) | 1.75, t (7.5) |
| 10 | 9.57, s | 9.64, s | 9.64, s | 9.65, s |
| 121 | 3.71, s | 3.69, s | 3.74, s | 3.83, s |
| 134 | 3.65, s | 3.66, s | 4.09, m 4.25, m | |
| 135 | 0.90, t (7.1) | |||
| 153 | 3.79, s | |||
| 17 | 4.69, br d (8.5) | 4.67, br dd (8.5, 2.2) | 4.17 br dd (9.2, 2.5) | 4.09, br d (9.2) |
| 171 | 2.12, m 2.27, m | 2.09, m 2.28, m | 2.27, m 2.94, m | 1.85, m 2.62, m |
| 172 | 2.04, m 2.44, m | 2.09, m 2.46, m | 2.25, m 2.52, m | 2.27, m 2.51, m |
| 174 | 4.02, m | 4.04, m | 4.10, q (7.1) | 3.95–4.09, m |
| 175 | 1.12, t (7.1) | 1.14, t (7.1) | 1.15, t (7.1) | 1.11, t (7.1) |
| 18 | 4.49, br q (7.3) | 4.49, br q (7.3) | 4.50, br q (7.3) | 4.45, br q (7.1) |
| 181 | 1.69, d (7.3) | 1.71, d (7.3) | 1.60, d (7.3) | 1.62, d (7.1) |
| 20 | 8.61, s | 8.59, s | 8.65, s | 8.64, s |
| 132-OH | 5.35, br s | 5.36, br s | 5.51, br s | |
| 151-OH | 6.34, br s | |||
| NH 1 | −1.73, 0.42 (br s) | −1.62, 0.44 (br s) | −1.84, 0.26 (br s) | −1.17, −0.71 (br s) |
| No. | 7 | 8 | 9 | 10 |
|---|---|---|---|---|
| 1 | 142.1 | 143.6 | 142.0 | 143.1 |
| 2 | 131.9 | 132.3 | 131.8 | 131.9 |
| 21 | 12.1 | 12.1 | 12.1 | 12.0 |
| 3 | 136.3 | 137.9 | 136.3 | 137.8 |
| 31 | 129.0 | 128.6 | 129.1 | 128.4 |
| 32 | 122.9 | 123.7 | 122.9 | 123.6 |
| 4 | 136.4 | 137.0 | 136.2 | 137.0 |
| 5 | 97.9 | 102.0 | 98.0 | 103.8 |
| 6 | 155.6 | 151.2 | 155.3 | 151.4 |
| 7 | 136.5 | 133.0 | 136.5 | 132.9 |
| 71 | 11.3 | 187.7 | 11.3 | 187.6 |
| 8 | 145.3 | 159.4 | 145.3 | 159.4 |
| 81 | 19.5 | 19.1 | 19.5 | 19.1 |
| 82 | 17.5 | 19.4 | 17.5 | 19.5 |
| 9 | 151.0 | 147.1 | 151.3 | 146.1 |
| 10 | 104.3 | 106.7 | 104.2 | 106.4 |
| 11 | 137.7 | 137.7 | 137.8 | 141.7 |
| 12 | 129.6 | 132.9 | 129.5 | 131.5 |
| 121 | 12.3 | 12.5 | 12.3 | 12.5 |
| 13 | 126.3 | 127.1 | 127.0 | 112.3 |
| 131 | 192.0 | 192.0 | 192.2 | 160.5 |
| 132 | 89.1 | 89.0 | 88.9 | |
| 133 | 173.5 | 173.1 | 172.3 | |
| 134 | 53.8 | 53.9 | 62.8 | |
| 135 | 14.0 | |||
| 14 | 150.2 | 151.1 | 150.0 | 136.7 |
| 15 | 107.6 | 107.4 | 107.8 | 102.0 |
| 151 | 100.5 | |||
| 152 | 170.6 | |||
| 153 | 54.3 | |||
| 16 | 161.9 | 164.7 | 162.4 | 169.0 |
| 17 | 50.2 | 50.4 | 51.8 | 53.9 |
| 171 | 30.2 | 30.0 | 31.1 | 31.3 |
| 172 | 31.2 | 31.3 | 31.6 | 32.2 |
| 173 | 173.0 | 172.9 | 173.6 | 173.1 |
| 174 | 60.4 | 60.5 | 60.5 | 60.5 |
| 175 | 14.1 | 14.1 | 14.1 | 14.1 |
| 18 | 50.8 | 50.8 | 50.4 | 50.2 |
| 181 | 22.7 | 22.7 | 22.8 | 22.2 |
| 19 | 172.8 | 174.6 | 172.4 | 173.3 |
| 20 | 93.4 | 93.7 | 93.6 | 94.1 |
| Compounds | 1 | 2 | 3 | 4 | 5 | 6 | Doxorubicin |
|---|---|---|---|---|---|---|---|
| Hela | >50 | >50 | >50 | >50 | >50 | >50 | 5.19 ± 0.26 |
| MCF-7 | >50 | 22.18 ± 8.95 | 30.93 ± 5.10 | 19.62 ± 2.16 | >50 | >50 | 12.03 ± 1.15 |
| BEL-7402 | 37.20 ± 5.46 | >50 | >50 | >50 | >50 | 20.04 ± 3.46 | 4.82 ± 0.76 |
| A549 | 14.08 ± 1.16 | >50 | >50 | 48.02 ± 12.14 | >50 | >50 | 8.05 ± 1.12 |
| HepG2 | 29.07 ± 5.69 | 2.57 ± 0.29 | >50 | 42.63 ± 3.97 | >50 | >50 | 2.49 ± 0.36 |
| MDA-MB231 | >50 | 27.05 ± 7.18 | 19.09 ± 0.75 | 11.31 ± 3.05 | 5.52 ± 0.13 | >50 | 7.96 ± 1.17 |
| Compounds | 7 | 8 | 9 | 10 |
|---|---|---|---|---|
| Hela | 18.19 ± 3.62 | 18.08 ± 2.48 | 59.26 ± 5.50 | 35.41 ± 7.62 |
| MCF-7 | 5.26 ± 0.71 | 46.26 ± 4.81 | 59.08 ± 3.09 | 76.84 ± 4.93 |
| BEL-7402 | 22.72 ± 4.98 | 51.04 ± 6.36 | 75.07 ± 9.73 | 7.68 ± 1.87 |
| A549 | 20.48 ± 2.16 | 76.43 ± 8.75 | 27.13 ± 6.08 | 39.31 ± 3.23 |
| HepG2 | 60.53 ± 9.40 | 12.65 ± 4.96 | 53.57 ± 10.28 | 3.77 ± 0.49 |
| MDA-MB231 | 25.92 ± 3.30 | 37.50 ± 5.09 | 22.06 ± 1.51 | 40.06 ± 8.33 |
| Compounds | 7 | 8 | 9 | 10 | Positive Control |
|---|---|---|---|---|---|
| S. aureus | 2.5 | 5.0 | 1.25 | 2.5 | 1.25 (ampicillin) |
| E. coli | 5.0 | 0.625 | 2.5 | 0.625 | 2.50 (ampicillin) |
| P. aeruginosa | >10 | >10 | 5.0 | >10 | 0.625 (ceftazidimea) |
| K. peneumoniae | >10 | >10 | >10 | >10 | 1.25 (ceftazidimea) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ma, S.; Weng, M.; Yang, T.; Ge, L.; Yang, K. Triterpenes and Pheophorbides from Camellia ptilosperma and Their Cytotoxicity, Photocytotoxicity, and Photodynamic Antibacterial Activity. Molecules 2023, 28, 7058. https://doi.org/10.3390/molecules28207058
Ma S, Weng M, Yang T, Ge L, Yang K. Triterpenes and Pheophorbides from Camellia ptilosperma and Their Cytotoxicity, Photocytotoxicity, and Photodynamic Antibacterial Activity. Molecules. 2023; 28(20):7058. https://doi.org/10.3390/molecules28207058
Chicago/Turabian StyleMa, Siyuan, Mengling Weng, Ting Yang, Li Ge, and Kedi Yang. 2023. "Triterpenes and Pheophorbides from Camellia ptilosperma and Their Cytotoxicity, Photocytotoxicity, and Photodynamic Antibacterial Activity" Molecules 28, no. 20: 7058. https://doi.org/10.3390/molecules28207058
APA StyleMa, S., Weng, M., Yang, T., Ge, L., & Yang, K. (2023). Triterpenes and Pheophorbides from Camellia ptilosperma and Their Cytotoxicity, Photocytotoxicity, and Photodynamic Antibacterial Activity. Molecules, 28(20), 7058. https://doi.org/10.3390/molecules28207058






