Abstract
In the search for new bioactive compounds, a methodology based on combining two molecules with biological properties into a new hybrid molecule was used to design and synthesize of a series of ten indole derivatives bearing imidazole, benzothiazole-2-thione, or benzoxazole-2-thione moieties at the C-3 position. The compounds were spectroscopically characterized and tested for their antioxidant, antibacterial, and fungicidal activities. The crystal structures were determined for five of them. Comparison of the closely related structures containing either benzothiazole-2-thione or benzoxazole-2-thione clearly shows that the replacement of -S- and -O- ring atoms modify molecular conformation in the crystal, changes intermolecular interactions, and has a severe impact on biological activity. The results indicate that indole-imidazole derivatives with alkyl substituent exhibit an excellent cytoprotective effect against AAPH-induced oxidative hemolysis and act as effective ferrous ion chelating agents. The indole-imidazole compound with chlorine atoms inhibited the growth of fungal strains: Coriolus versicolor (Cv), Poria placenta (Pp), Coniophora puteana (Cp), and Gloeophyllum trabeum (Gt). The indole-imidazole derivatives showed the highest antibacterial activity, for which the largest growth-inhibition zones were noted in M. luteus and P. fluorescens cultures. The obtained results may be helpful in the development of selective indole derivatives as effective antioxidants and/or antimicrobial agents.
1. Introduction
The synthesis of compounds based on nitrogen-, oxygen-, and sulfur-containing heterocyclic scaffolds has become increasingly important in recent years, particularly in medical chemistry [1]. Among nitrogen-heterocyclic compounds, indole derivatives deserve special attention due to their broad spectrum of biological activity. Synthetic indoles are promising therapeutic agents and have been found to exhibit antiviral, antibacterial, anti-inflammatory, and antidepressant activities [2,3,4,5]. Many of them have been approved as drugs, such as indomethacin, pindolol, and indapamide [6]. The indole structure is also present in the indole-3-carbinol, an important antitumor agent [7].
The indole moiety is widely distributed in nature. Naturally derived indole compounds include, among others, the amino acid tryptophan, tryptamine, serotonin, and melatonin [8]. Additionally, tryptophan is a precursor of several indole alkaloids, including reserpine, vincristine, yohimbine, and gramine [9,10,11,12,13].
One of the most important biological activities of indole derivatives is their antioxidant potential. The antioxidant properties of melatonin are probably due to the presence of the C-3 ethylamido side chain [14]. Also, synthetic indoles, bearing substitutes at the C-3 positions, are promising antioxidant agents [14,15,16,17]. Our previous study showed that gramine substituted by the pyrrolidinedithiocarbamate moiety has significant antioxidant properties [18]. Moreover, selected C-3 substituted indole-uracils [19] and indole-triazole [20] conjugates were able to inhibit AAPH-induced oxidative hemolysis effectively.
Another important class of nitrogen-containing heterocycles is imidazoles. The imidazole ring system is found in the amino acid histidine, hormone histamine, and purine [21]. Imidazole derivatives have antibacterial, anticancer, antitubercular, antifungal, and analgesic properties [22,23].
Benzothiazole and its related heterocycle benzoxazole are other versatile scaffolds in organic chemistry [24,25]. Their derivatives possess various biological activities, including anti-inflammatory, antitubercular, antifungal, and anti-diabetic properties [25,26,27,28]. Recently, Karaca et al. showed that some benzothiazole derivatives have a promising potential for the treatment Alzheimer’s and Parkinson’s diseases [29].
One of the methods for designing novel drug candidates is to combine two molecules with biological properties into a new hybrid with better affinity compared to the individual parent compounds [30].
As part of our ongoing research into the design of novel bioactive C-3 substituted indole derivatives and considering the importance of heterocyclic skeletons for developing bioactive compounds, we synthesized and characterized indole-imidazole, indole- benzothiazole-2-thione, or indole-benzoxazole-2-thione hybrids in an attempt to design effective antioxidant agents. Structural heterocyclic scaffolds used for synthesizing the target compounds are shown in Figure 1.
Figure 1.
Organic chemistry scaffolds.
The synthesized hybrids were evaluated for their ability to complex ferrous ions and inhibitory effect on 2,2′-azobis- (2-amidinopropane hydrochloride) (AAPH)–induced hemolysis of human erythrocytes.
In addition, the compounds obtained were screened for possible antifungal and antibacterial activities.
2. Results and Discussion
2.1. Synthesis and Spectroscopic Characterization of C-3 Substituted Indole Derivatives
To obtain C-3 substituted indole derivatives, gramine was used as a substrate with a good leaving group leading to a vinylogous intermediate [A]. The reaction of [A] with appropriate imidazole compounds (imidazole, 2-methylimidazole, 2-ethylimidazole, 2-isopropylimidazole, 2-ethyl-4-methylimidazole, 2,5-dichloroimidazole, 2-phenylimidazole, 2-benzimidazole), 2-mercaptobenzothiazole or 2-mercaptobenzoxazole gives compounds 2–11 (Figure 2).
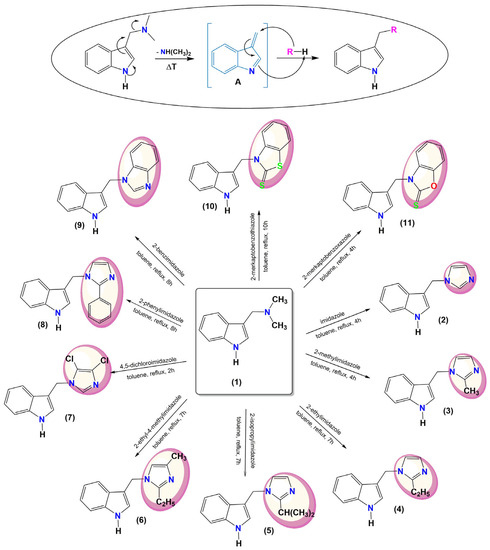
Figure 2.
Synthesis of indole derivatives with imidazole, benzothiazole–2–-thione and benzoxazole–2–thione moieties.
Indole-imidazole compounds are known in the literature [31,32,33,34,35,36,37,38,39], but some proposed syntheses are multi-step or require special reaction conditions [36,37,38]. Our one-pot synthesis method is based on a compound of natural origin, is simple, and does not need column chromatography.
The structures of all obtained compounds were determined by analyzing their 1H and 13C NMR, FT-IR, and EI-MS spectra. The spectroscopic data of literature-known compounds 2 and 9 agreed with those found in the references [18,38]. 13C NMR spectra of compounds 2–11 showed signals in the range of 108–136 ppm corresponding to indole moiety and near 180 ppm indicating the presence of a thiocarbonyl group (compounds 10 and 11). Characteristic signals at about 118–152 ppm (compounds 2–9) were assigned to the imidazole ring and at 110–146 ppm to benzimidazole, benzothiazole-2-thione, and benzoxazole-2-thione moiety (compounds 9–11). Additionally, signals of alkyl (methyl, ethyl, and isopropyl) groups for compounds 3–6 were observed in the 12–25 ppm range. The 1H NMR spectra of compounds 2–11 showed characteristic hydrogen singlets near 11 ppm that were assigned to the NH protons of gramine moiety and signals in the range 6.68–7.69 ppm for protons of the indole fragment. The protons of the C(10)H2 group gave signals near 5.00 ppm, and protons of the imidazole ring at 6.69–7.37 ppm. The protons of the alkyl groups (compounds 3–6) gave signals at 1.95–2.33, 1.12–2.69, and 1.15–3.29 ppm for methyl, ethyl, and isopropyl group, respectively. Characteristic signals of benzimidazole, benzothiazole-2-thione, and benzoxazole-2-thione protons are at 7.15–7.83 ppm. The NMR spectra (1H and 13C) of the investigated compounds are provided in the Supplementary part (Figures S1–S9). The FT-IR spectra of all compounds in KBr tablets showed the characteristic band at 3550–3300 cm−1 derived from the NH of the indole ring and characteristic indole ring absorption at 650–540 cm−1. Moreover, in the spectra of 10 and 11, there are narrow absorption bands from the S=C bond at 1266 and 1285 cm−1, respectively. The aromatic character of compounds was confirmed by the presence of absorption bands at 1625–1575 cm−1 and 1525–1450 cm−1. In the EI-MS spectra of almost all compounds (except 8), molecular ions were observed, and their relative abundance was 4–74%. For all new derivatives, the ion with an intensity of 100% is at m/z = 130 (C9H8N+). The NMR (1H and 13C), EI-MS, and FT-IR spectra of the investigated compounds are provided in the Supplementary Materials (Figures S1–S27).
2.2. X-Ray Analysis
Single crystals suitable for X-ray diffraction were obtained for six derivatives (4, 5, 8, 9, 10, 11). Since the crystal structure of 9 has already been determined [38], we report here the results of a single crystal X-ray analysis for the other five compounds.
The structures of the molecules, as seen in the crystals of compounds 4 (two symmetry independent molecules), 5, 8, 10, and 11, are shown in Figure 3. The main skeleton can be described as consisting of two methylene bridged subunits, each containing aromatic rings which are inclined with respect to each other at angles varying from 66.40(4) to 84.20(3)°. One of the fragments is always a planar indole moiety, while the other is either 2-substituted imidazole (4, 5, 8) or a planar benzothiazole-2-thione (10) or benzoxazole-2-thione (11). In molecules 4, 5, and 8, the indole part forms an angle of 62.7(2), 63.6(2), 65.6(1), and 61.4(1)° with a plane containing C-C and C-N bonds to the methylene group (called the methylene plane), while the imidazole fragment is inclined to this plane at only 10.3(1), 13.2(1), 31.3(2) and 28.0(2)°.
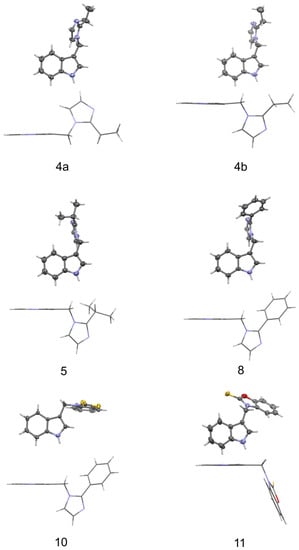
Figure 3.
Molecular conformation as present in crystals of compounds 4 (two independent molecules 4a and 4b), 5, 8, 10, and 11 in two representations (i.e., viewed perpendicular (above) and parallel (below) to the indole plane). Thermal ellipsoids are drawn at the 30% probability level.
Moreover, a comparison of two independent molecules in crystal of 4 (4a and 4b shown in Figure 3) shows that the two molecules are conformationally enantiomeric. This relationship can also be seen in the values of the torsion angles φ1 and φ2 measured along the C-C-C-N and C-C-N-C methylene bonds that are listed in Table 1. As one can see from this table, both torsion angles in a molecule always have the same sign, which is the condition for a propeller conformation. Due to the fact that the crystals are predominantly centrosymmetric (with the exception of 10, which crystallizes in Sohncke space group P212121) both types of propellers are uniformly distributed in the investigated crystal structures.

Table 1.
Torsion angles (◦) describing rotation around the methylene C-C and C-N bonds in molecules present in crystals.
The molecular conformation markedly changes with the replacement of imidazole fragments by either benzothiazole-2-thione (10) or benzoxazole-2-thione (11). While in the imidazole derivatives (4, 5, and 8), the imidazole ring lies almost in the methylene plane, and the indole fragment is significantly out of this plane, in 10, on the contrary, the indole ring is close to the methylene plane while the benzothiazole-2-thione moiety is out of this plane, the corresponding angles being 13.4(3) and 78.2(1)°. Such conformation is particularly well suited for the formation of an intramolecular hydrogen bond from the methylene C-H donor to the S=C acceptor. The C···S and H···S distances are 3.140(3) and 2.69 Å, and the C-H···S angle amounts to 109°. In 11 both rings are nearly perpendicular to the methylene plane and form angles of 89.7(1) and 80.8(1)° with this plane. This conformation is exceptional for the investigated series and is less favorable for the formation of C-H···S intramolecular hydrogen bond. As follows from the above, the molecules of 10 and 11 differ in conformation not only with respect to the other molecules in the series studied but also between each other (although the chemical difference between them arises solely from the chalcogen exchange). The observed significant alteration of the molecular conformation in the solid state caused by the S/O replacement has far reaching consequences with respect to intermolecular interactions. While in the crystals of 10 the donor N-H group is engaged in intermolecular N-H···π interactions to the benzene part of the indole ring (Table 2), in the crystals of 11 we observe N-H···S=C hydrogen bonds joining the molecules along c-direction (Table 2) and columnar stacking interactions between benzoxazole-2-thione fragments, related by the two-fold screw axis along b-direction (Figure 4). The distance between the centroids of the two fragments in a stack amounts to 3.658 Å, while the distance between their planes is 3.603 Å (symmetry code –x + 1, y + 1/2, −z + 1/2). The planes are nearly parallel, the interplanar angle being 10° and the displacement of the rings in a stack is only 0.399 Å.

Table 2.
Hydrogen bond parameters.
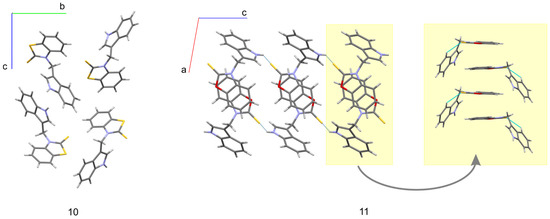
Figure 4.
Effect of chalcogen exchange on the solid-state structures of 10 (view along [100]) and 11 (view along [010]).
As expected, indole/imidazole derivatives 4, 5, and 8 are mainly engaged in N-H···N hydrogen bonds connecting the molecules into infinite chains (Table 2).
2.3. Antioxidant Properties
2.3.1. Hemolytic Activity
The biocompatibility of the new compounds is one of the main parameters determining their potential biomedical application. One of the main tests to determine the toxicity of compounds is their hemolytic activity. Hence, erythrocytes were used as model cells to test the biocompatibility of compounds 2–11 [40].
The hemolytic activity of all derivatives was evaluated in vitro using human red blood cells (RBC). As shown in Figure 5, the hemolytic activity of the tested compounds (0.1 mg/mL) depends on their chemical structure (compare Figure 5 with Figure 2). The hemolytic activity of indole derivatives with electron-donating substituents at the imidazole ring (3–6) was below 5%. This means that 3–6 are biocompatible compounds without the cell membrane-disrupting activity and are good candidates for further evaluation. The hemolytic activity of compound 3 (3.6%) with a methyl substituent at the imidazole ring is comparable to that of derivative 2 with unsubstituted imidazole (3.3%).
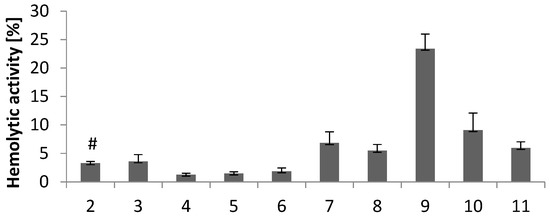
Figure 5.
Hemolytic activity of compounds 2–11 at a concentration of 0.1 mg/mL. Results (n = 7) are presented as the mean value ± standard deviation. # Data published in [18].
The electron-withdrawing substituents increase the hemolytic activity of compounds in the order of 9 > 10 > 7 > 11 > 8. The hemolytic activity of compounds 7, 8, 10, and 11 ranges from 5.5% to 9.1%. Compound 9 with a benzimidazole moiety has the highest hemolytic activity (23%). According to the above results, compounds 7–11 are not biocompatibile at the concentration tested.
2.3.2. Cytoprotective Activity against Oxidative Stress
Oxidative stress is an imbalance between the production of reactive oxygen species (ROS) and the efficiency of the antioxidant system. Overproduction of ROS are associated with cancer, cardiovascular, neurodegenerative, and autoimmune diseases [41]. A high levels of ROS can lead to lipid peroxidation, protein aggregation, and nucleic acid damage.
Exogenous antioxidants have received much attention in recent years, as they may play an important role in preventing oxidative damage in cells. Indole derivatives are known for their antioxidant properties, including protection of cells from ROS detrimental effects [18,19,20]. The high reactivity of indole antioxidants is probably due to the electron-rich aromatic ring system, which enables them to act as electron donors for the formation of cationic radicals or by the addition of electrophilic radicals at the C-3 position of the indole [16].
As shown in Figure 6, the cytoprotective activity of new derivatives at a concentration of 0.1 mg/mL strongly depends on the substituent present at the C-3 position of the indole ring.
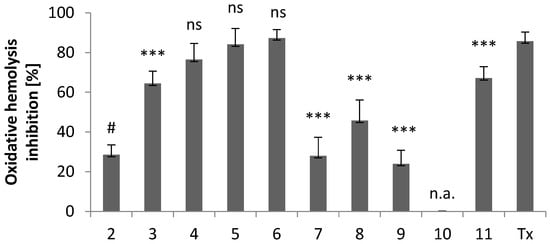
Figure 6.
Cytoprotective activity of compounds 2–11 and the standard antioxidant Trolox against oxidative hemolysis induced by free radicals generated from AAPH. The results (n = 7) are presented as the mean value ± standard deviation (*** p < 0.001) in comparison with the standard antioxidant Trolox. Non statistically significant difference (p > 0.05) is indicated as ns. Inactive compounds are indicated as n.a. # Data published in [18].
In compounds 3–8, the electron-withdrawing or electron-donating properties of the substituent present in the imidazole ring are of great importance. For derivatives with electron-donating groups, the cytoprotective activity increases in the order of substituents: methyl and ethyl (6) > isopropyl (5) > ethyl (4) > methyl (3). Compound 7 with electron-withdrawing substituents exhibits cytoprotective activity similar to the unsubstituted imidazole derivative (2). A similar cytoprotective activity is observed for compound 9. A slightly higher value of cytoprotective activity is observed for the derivative with a phenyl ring (8).
The antioxidant properties of compounds 2–9 are related to electrons delocalization in the imidazole and indole rings. The indole rings act as electron or hydrogen atom donors. When an electron is lost, a stabilized radical cation is formed. It is also possible to transfer a hydrogen atom from the antioxidant molecule to the radical and form a resonance-stabilized indolyl radical.
The results showed that the most important structural element affecting the cytoprotective activity of the compounds studied is the presence of alkyl substituents in the imidazole ring. The imidazole ring acts as the second electron-rich donor. The alkyl substituents contribute to the delocalization of radicals obtained and provide necessary stability to the system.
Particularly noteworthy is the comparison of the cytoprotective activity of derivatives 10 and 11. While compound 10, with benzothiazole-2-thione moiety, did not protect RBC from oxidative stress-induced hemolysis, derivative 11 with benzoxazole-2-thione scaffold showed significant cytoprotective activity. We are inclined to attribute this major difference in activity to the structural changes that take place upon replacement of -S- by –O- ring atom (vide supra). The changes involve both the molecular and supramolecular levels in the crystals, but may also reflect ligand/receptor interactions in the process of molecular recognition.
2.3.3. Chelating Activity
One measure of the compounds’ antioxidant properties is their capacity to chelate iron ions. Chelating compounds prevent the ability of the iron to catalyze the Haber-Wiess or Fenton-type reactions leading to hydroxyl radical formation. Depending on the conditions, biologically inactive ferric ions (Fe3+) can be reduced to active ferrous ions (Fe2+) and then oxidized back with the generation of ROS, leading to neurodegenerative diseases such as—Parkinson’s disease, Huntington’s disease, Alzheimer’s disease, and schizophrenia [42].
As shown in Figure 7, the ferrous chelating properties of the compounds studied are depend on their chemical structure (compare Figure 2 with Figure 7). Among the indole-imidazole derivatives (2–9), compounds 2–6 are the most effective as ferrous ion chelators, and their complexing ability is comparable to EDTA, used as a standard chelator. The chelating activity of compounds 3–6 is comparable to that of compound 2. The presence of an electron-donating alkyl substituent in the imidazole ring does not alter the chelating activity of the compounds.
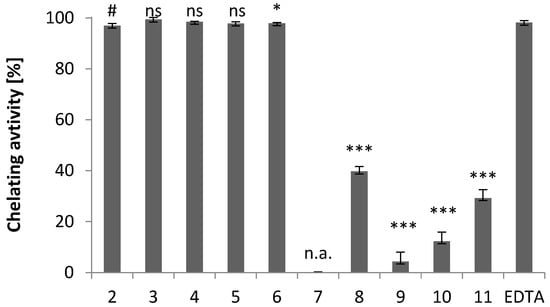
Figure 7.
Ferrous ion chelating activity of iron ions of compounds 2–11 and the standard chelating agent EDTA. The results (n = 3) are presented as the mean value ± standard deviation (* p < 0.05, *** p < 0.001) in comparison with EDTA. Non statistically significant difference (p > 0.05) is indicated as ns. Inactive compounds are indicated as n.a. # Data published in [18].
Compound 7 has no chelating activity, while the chelating activities of compounds 8 and 9 are 40% and 4%, respectively (Figure 7). This reduction in chelating activity can be attributed to the electron-withdrawing substituents, which reduce the electron density on the imidazole nitrogen atom and thus reduce the metal-ligand interaction.
The chelating activity values for compounds 10 and 11 are 12% and 29%, respectively. The lack of intermolecular hydrogen bonds to the thiolate sulfur atom in the crystals of 10 suggests that this atom is not readily available for interactions with the neighboring molecules, and thus also for metal-ligand interactions.
2.4. Antibacterial Study
The bacteria selected for this study (Bacillus subtilis, Micrococcus luteus, Escherichia coli, and Pseudomonas fluorescens) belong to the model species, due to their widespread occurrence in the natural environment, being a natural component of the human opportunistic microbiome [43,44,45].
Among all tested compounds, compound 2 showed the highest antimicrobial activity, for which the largest growth-inhibition zones were noted, particularly evident in the M. luteus (21 mm) and P. fluorescens (14.7 mm) cultures (Table 3). Compounds 4 and 8 also showed strong inhibitory effects on the tested bacteria, but the latter did not inhibit the growth of P. fluorescens. The weakest antagonists of the tested bacteria turned out to be two compounds—gramine (1) and their derivative 10. Gramine had a potent inhibitory effect only on the P. fluorescens strain, while it had the least effect on the growth of the population of other bacterial species. In contrast, derivative 10 only slightly inhibited the growth of M. luteus and E. coli.

Table 3.
Antibacterial activities of compounds 1–11.
2.5. Fungicidal Activity
Four fungal strains (Coriolus versicolor (Cv), Poria placenta (Pp), Coniophora puteana (Cp), and Gloeophyllum trabeum (Gt)) were used to study the antifungal activity of gramine (1) and their derivatives (2–11). These selected indoor, wood-destroying fungi contribute significantly to wood degradation [46] and are widely used in standard and custom mycological tests.
As shown in Table 4, the effective dose, ED100, for all tested compounds (i.e., no mycelial growth), occurs when 0.1% of the alkaloid is present in the agar medium. At a concentration of 0.01%, four derivatives (7, 8, 10, 11) show fungistatic activity. Among them, the indole-imidazole compound with chlorine atoms (7) demonstrated the strongest fungicidal effects against all tested fungal strains. Its 0.1% solution inhibited the growth of C. versicolor and G. trabeun by more than 90% and C. puteana and P. placenta by more than 70%. Derivative 10 with benzothiazole moiety showed a 71, 59, and 57% inhibition effect against the mycelia elongation of P. placenta, G. trabeun, and C. puteana, respectively. In contrast, benzoxazole derivative (11) inhibits only one strain of fungi P. placenta (64% inhibition). Compound 8 was the most potent against G. trabeun (53% inhibition). The other compounds tested were ineffective at this concentration.

Table 4.
Antifungal activity of compounds 1–11.
It is worth noting that of all compounds tested, three (7, 10, 11) showed fungicidal activity against C. puteana and P. placenta, which are highly resistant to currently used fungicides.
2.6. In Silico Study
The SwissADME website [47] was used to calculate the physicochemical and pharmacokinetic properties of the compounds and their drug-likeness. As shown in Table 5, all compounds investigated meet the criteria of Lipinski’s rule of five. The molecular mass (MW) is less than 500 g/mol, the partition coefficient (logP) is less than 5, and there are no more than 5 hydrogen bond donors (HBD) and 10 hydrogen bond acceptors (HBA) [48]. Lipophilicity is one of the descriptors of xenobiotics, that influences their biological properties, affecting the bioavailability, biodegradation, and toxicity of substances [49]. The alkyl substitution of the imidazole ring in compounds 3–6 improves their lipophilicity and facilitate incorporation into the cell membrane, making them excellent cytoprotective agents. LogP values of 2.20–2.84 for derivatives 3–6 appear ideal for passing the biological membrane. The partition coefficient calculated for this group of indole derivatives correlates well with their structural features—the longer the alkyl chain, the higher values of the partition coefficient. Of all compounds tested, derivative 10 is the most lipophilic (logP = 4.13). All derivatives analyzed have one hydrogen bond donor (HBD), and most have one hydrogen bond acceptor (HBA).

Table 5.
Physicochemical, pharmacokinetic and drug-likeness properties of indole derivatives 1–11.
The results showed that none of the synthesized compounds violated Veber’s rule, suggesting good oral bioavailability. It depends on the number of rotatable bonds (RTB) and the topological polar surface area (TPSA) [50]. The number of rotatable bonds influences, apart from bioavailability, the binding potency of compounds should be less than 10. As shown in Table 5, all analyzed derivatives have 2 or 3 RTB.
TPSA is a measure of the ability of a drug to pass cell membrane. A TPSA value of less than 140Å2 is characteristic of the passive transport of the molecule across cell membrane. Most of the indole derivatives obtained have TPSA values less than 34.
The pharmacokinetic properties of compounds involve various factors, among which GI (Gastrointestinal) absorption and BBB (Blood-Brain Barrier) permeation are important. The more a dose reaches the bloodstream after oral administration (mainly from the gastrointestinal tract), the more it can cross the BBB by passive diffusion [51]. All compounds investigated show high gastrointestinal absorption and can cross the BBB with the exception of 10. Although a derivative with benzothiazole-2-thione can be absorbed from the gastrointestinal tract, it cannot cross the BBB, likely due to its poor water solubility and high lipophilicity.
One of the main challenges of obtaining new potential drugs is the synthesis of a water-soluble product. A compound with higher water solubility is more bioavailable and requires a lower dose to reach a therapeutic plasma concentrations after oral administration [52]. Depending on the calculation method, the water solubility of compounds 2–9 is good or moderate (Table 6). As the most lipophilic, derivatives 10 and 11 are also the least soluble in water, showing moderate or poor solubility.

Table 6.
Water solubility od derivatives 2–11 calculated in SwissADME. LogS in the table is the average value of logS calculated using three different methods. * Solubility class—logS scale: Insoluble < −10, Poorly < −6, Moderately < −4, Soluble < −2, Very < 0.
The results obtained suggest that the indole-imidazole derivatives 3–6 have good ADME parameters and can be considered as good candidates for the development of novel antioxidants.
3. Materials and Methods
3.1. Instrumentation and Chemicals
The melting points (mp) were obtained with a Büchi SMP-20 apparatus. 1H NMR and 13C NMR spectra were recorded on a Varian 300/400 spectrometer with DMSO-d6 as the solvent and TMS as the internal standard. Chemical shifts are reported in δ (parts per million) values. EI mass spectra were measured on Bruker 320MS/450GC mass spectrometer. FT-IR spectra were recorded on Nicolet iS 5 (KBr pellets). TLC analysis was used using Sigma-Aldrich silica gel 60 plates with a fluorescent indicator (254 nm) and visualized with UV. All chemicals or reagents used for syntheses were commercially available. In all reactions, anhydrous solvents were used.
3.2. Synthesis of Gramine Derivatives
Synthesis of compound 2 was already described in our previous paper [18].
A typical procedure for the synthesis of compounds 3–11.
A solution of gramine (1 mmol) and the appropriate nucleophilic compound (1 mmol) in 8–10 mL of toluene was heated under reflux for 2–10 h. After completion of the reaction, as indicated by TLC, the precipitate was filtered and crystallized from toluene.
- 3-((2-methyl-1H-imidazol-1-yl)methyl)-1H-indole (3)
Light yellow solid (180 mg, 85%); m.p. 173–174 °C; 1H NMR (400 MHz, DMSO-d6): δ 11.14 (s, 1H), 7.50 (d, J = 7.9 Hz, 1H), 7.39–7.37 (m, 2H), 7.12–7.07 (m, 2H), 6.99 (ddd, J = 8.0, 7.0, 1.0 Hz, 1H), 6.68 (d, J = 1.3 Hz, 1H), 5.21 (s, 2H), 2.33 (s, 3H); 13C NMR (101 MHz, DMSO-d6): δ 143.56, 136.32, 126.05, 125.85, 126.63, 121.41, 119.98, 118.92, 118.30, 111.67, 110.40, 40.99, 13.01; IR (KBr): 3550–3460, 3139–2776, 1524, 1455, 1427, 1360, 750; EI-MS (m/z, % int.): 211 (M+, 9), 130 (100). Analysis calculated for C13H13N3 (MW = 211.26): C 73.91, H 6.20, N 19.89; found: C 73.62, H 6.25, N 19.87%.
- 3-((2-ethyl-1H-imidazol-1-yl)methyl)-1H-indole (4)
White solid (225 mg, 89%); m.p. 183–186 °C; 1H NMR (400 MHz, DMSO-d6) δ 11.12 (s, 1H), 7.47 (d, J = 7.9 Hz, 1H), 7.37–7.41 (m, 2H), 7.14–7.08 (m, 2H), 6.98 (ddd, J = 8.0, 7.0, 1.1 Hz, 1H), 6.71 (d, J = 1.3 Hz, 1H), 5.22 (s, 2H), 2.69 (q, J = 7.5 Hz, 2H), 1.16 (t, J = 7.5 Hz, 3H); 13C NMR (101 MHz, DMSO-d6) δ 148.10, 136.31, 126.03, 125.81, 124.57, 121.38, 119.88, 118.89, 118.27, 111.65, 110.46, 40.74, 19.59, 12.01; IR (KBr): 3432, 3131–2612, 1455, 1422, 1379, 781; EI-MS (m/z, % int.): 225 (M+, 35), 130 (100). Analysis calculated for C14H15N3 (MW = 225.29): C 74.64, H 6.71, N 18.65; found: C 74.26, H 6.75, N 18.53%.
- 3-((2-isopropyl-1H-imidazol-1-yl)methyl)-1H-indole (5)
Light yellow solid (239 mg, 88%); m.p. 183–185 °C; 1H NMR (400 MHz, DMSO-d6) δ 11.12 (s, 1H), 7.45 (d, J = 8.0 Hz, 1H), 7.38 (dt, J = 8.2, 0.8 Hz, 1H), 7.32 (d, J = 2.5 Hz, 1H), 7.09 (ddd, J = 8.2, 7.1, 1.1 Hz, 1H), 7.02–6.98 (m, 2H), 6.71 (d, J = 1.2 Hz, 1H), 5.25 (s, 2H), 3.23 (hept, J = 6.7, 1H), 1.15 (d, J = 6.8, 6H); 13C NMR (101 MHz, DMSO-d6) δ 151.88, 136.30, 126.02, 125.77, 124.48, 121.38, 119.48, 118.88, 118.27, 111.65, 110.67, 40.68, 25.14, 21.98 (2x); IR (KBr): 3415, 3133–2872, 2613, 1875, 1488, 1432, 1379, 741; EI-MS (m/z, % int.): 239 (M+, 30), 130 (100). Analysis calculated for C15H17N3 (MW = 239.32): C 75.28, H 7.16, N 17.56; found: C 75.17, H 7.23, N 17.64%.
- 3-((2-ethyl-4-methyl-1H-imidazol-1-yl)methyl)-1H-indole (6)
Light yellow solid (239 mg, 69%); m.p. 186–189 °C; 1H NMR (400 MHz, DMSO-d6) δ 11.08 (s, 1H), 7.44 (d, J = 7.9 Hz, 1H), 7.33 (d, J = 2.4 Hz, 1H), 7.06 (ddd, 1H), 6.99–6.93 (m, 2H), 6.69 (d, J = 0.9 Hz, 1H), 5.09 (s, 2H), 2.66–2.60 (m, 2H), 1.95 (s, 3H), 1.10 (t, J = 7.5 Hz, 3H) 13C NMR (101 MHz, DMSO-d6) δ 147.30, 136.30, 133.93, 126.08, 124.54, 121.36, 118.89, 118.28, 115.91, 111.67, 110.65, 40.37, 19.53, 13.60, 12.15, 9.61; IR (KBr): 3422, 3136–2761, 1457, 1420, 1379, 777; EI-MS (m/z, % int.): 239 (M+, 75), 130 (100). Analysis calculated for C15H17N3 (MW = 239.32): C 75.28, H 7.16, N 17.56; found: C 75.19, H 7.18, N 17.62%.
- 3-((4,5-dichloro-1H-imidazol-1-yl)methyl)-1H-indole (7)
Dark pink oil (133 mg, 50%); 1H NMR (400 MHz, DMSO-d6) δ 11.22 (s, 1H), 7.97 (s, 1H), 7.62–7.57 (m, 1H), 7.48 (d, J = 2.6 Hz, 1H), 7.42–7.38 (m, 1H), 7.15–7.09 (m, 1H), 7.03 (ddd, J = 10.2, 5.6, 2.1 Hz, 1H), 5.34 (s, 2H).; 13C NMR (101 MHz, DMSO-d6) δ 136.20, 135.93, 128.88, 128.19, 125.71, 125.30, 121.58, 119.18, 117.99, 111.81, 108.34, 41.46. IR (KBr): 3411, 3057, 2927, 1456, 1252, 743 cm−1; EI-MS (m/z, % int.): 265 (M+, 3), 130 (100). Analysis calculated for C12H9N3Cl3 (MW = 266.13): C 54.16, H 3.41, N 15.79; found: C 54.21, H 3.50, N 15.83%.
- 3-((2-phenyl-2,5-dihydro-1H-imidazol-1-yl)methyl)-1H-indole (8)
Yellow solid (180 mg, 56%); m.p. 110–120 °C (with decomposition); 1H NMR (400 MHz, DMSO-d6) δ 11.09 (s, 1H), 7.72–7.64 (m, 2H), 7,54–7.39 (m, 3H), 7.35 (d, J = 8.1 Hz, 1H), 7.29–7.15 (m, 2H), 7.14 (d, J = 2.5 Hz, 1H), 7.13–7.03 (m, 1H), 6.99–6.86 (m, 2H), 5.42 (s, 2H); 13C NMR (101 MHz, DMSO-d6) δ 146.44, 136.19, 131.18, 128.59 (2×), 128.51 (2×), 128.39, 127.70, 125.81, 124.37, 121.85, 121.44, 118.95, 117.96, 111.68, 110.53, 42.34; IR (KBr): 3420, 3151–3059. 2982–2869, 1458, 1376, 1234 cm−1; EI-MS (m/z, % int.): 144 (M+, 17), 130 (100). Analysis calculated for C19H15N3 (MW = 273.33): C 78.52, H 6.22, N 15.26; found: C 78.48, H 6.19, N 15.31%.
- 1-((1H-indol-3-yl)methyl)-1H-benzo[d]imidazole (9)
White solid (235 mg, 95%); m.p. 214–216 °C; 1H NMR (400 MHz, DMSO-d6): δ 11.14 (s, 1H), 8.45 (s, 1H), 7.70 (dt, J = 8.0, 1.0 Hz, 1H), 7.65–7.54 (m, 3H), 7.36 (dt, J = 8.1, 0.9 Hz, 1H), 7.25–7.10 (m, 2H), 7.07 (ddd, J = 8.1, 7.0, 1.2 Hz, 1H), 6.97 (ddd, J = 8.0, 7.1, 1.0 Hz, 1H), 5.61 (s, 2H); 13C NMR (101 MHz, DMSO-d6) δ 144.07, 143.62, 136.32, 133.71, 126.08, 125.33, 122.03, 121.40, 121.30, 119.34, 118.96, 118.25, 111.70, 110.89, 109.65, 39.89; IR (KBr): 3400–3000, 2927–2868, 1934, 1456, 1351, 1265, 740 cm−1; EI-MS (m/z, % int.): 247 (M+, 4), 130 (100). Analysis calculated for C16H13N3 (MW = 247.29): C 77.71, H 5.30, N 16.99; found: C 77.68, H 5.27, N 17.07%.
- 3-((1H-indol-3-yl)methyl)benzo[d]thiazole-2(3H)-thione (10)
Light yellow Solid (296 mg, 75%); m.p. 187–190 °C; 1H NMR (400 MHz, DMSO-d6): δ 11.17 (s, 1H), 7.84 (dd, J = 8.0, 1.1 Hz, 1H), 7.74 (d, J = 2.5 Hz, 1H), 7.68 (m, 2H), 7.59 (d, J = 2.5 Hz, 1H), 7.43–7.29 (m, 1H), 7.27–7.24 (m, 2H), 7.08 (m, 1H), 5.90 (s, 2H); 13C NMR (101 MHz, DMSO-d6) δ 188.32, 141.20, 136.20, 127.02, 126.51, 125.94, 125.86, 124.94, 121.81, 121.58, 119.11 (2X), 114.21, 111.78, 108.22, 42.22; IR (KBr): 3563, 3397, 3075, 3054, 1460, 1368, 747 cm−1; EI-MS (m/z, % int.): 296 (M+, 68), 130 (100). Analysis calculated for C16H12N2S2 (MW = 296.41): C 64.84, H 4.08, N 9.45, S 21.63; found: C 64.91, H 4.12, N 9.51, S 21.68%.
- 3-((1H-indol-3-yl)methyl)benzo[d]oxazole-2(3H)-thione (11)
Brown solid (280 mg, 75%); m.p. 157–159 °C; 1H NMR (400 MHz, DMSO-d6): δ 11.23 (s, 1H), 7.84 (d, J = 8.1 Hz, 1H), 7.73 (d, J = 2.5 Hz, 1H), 7.58–7.50 (m, 2H), 7.43–7.38 (m, 3H), 7.15–7.09 (m, 2H), 5.64 (s, 2H); 13C NMR (101 MHz, DMSO-d6) δ 179.04, 146.40, 136.23, 131.13, 126.55, 125.93, 124.93, 124.33, 121.57, 119.11, 118.94, 111.74, 111.37, 110.11, 107.50, 41.79; IR (KBr): 3407, 3111, 3058, 2942, 1490, 1423, 775 cm−1; EI-MS (m/z, % int.): 280 (M+, 63), 130 (100). Analysis calculated for C16H12N2SO (MW = 280.34): C 68.55, H 4.31, N 9.99, S 11.14; found: C 68.51, H 4.27, N 10.07, S 11.16%.
3.3. X-Ray Analysis
Single–crystal X-ray diffraction measurements were carried out with the monochromated CuKα radiation on a SuperNova diffractometer or MoKα radiation on an Xcalibur diffractometer. The data were collected and processed using the CrysAlisPro software [53]. The crystal structures were solved by direct methods with SHELXT [54] and refined by full-matrix least-squares calculations on F2 with SHELXL [55]. All non-H atoms were refined with anisotropic displacement parameters. Hydrogen atoms were placed at calculated positions based on the environment and perceived hybridization of the C atoms to which they are bonded (methyl C–H = 0.96 Å, methylene C–H = 0.97 Å, aromatic C-H = 0.93 Å and N-H = 0.86 Å) and refined as ‘riding’ on their carriers. During the refinement, isotropic displacement parameters for H-atoms were assigned 20% higher than the isotropic equivalent for the atom to which the H-atom was bonded. In crystals of 4 there were signs of disorder of the terminal ethyl groups. We have modelled the disorder for one of the two ethyl substituents by taking into account two alternative positions for the methylene group. During the refinement the occupancy ratios for the major and minor component were negatively coupled so the total occupancy remained equal to one. The component occupancy factors amount to 0.55 and 0.45, respectively. The final model is not fully satisfactory, namely the atomic displacement parameters for atoms constituting ethyl substituents are relatively high and the C-C bonds to these atoms appear to be shorter than expected. MERCURY [56] computer graphics programs were used to prepare drawings. The crystal data together with experimental and refinement details are collected in Table 7. CCDC 2223465-2223469 contains the supplementary crystallographic data for this paper (for structures 4, 5, 8, 10 and 11, respectively). These data can be obtained free of charge via http://www.ccdc.cam.ac.uk/conts/retrieving.html (or from the CCDC, 12 Union Road, Cambridge CB2 1EZ, UK; Fax: +44-1223-336033; E-mail: deposit@ccdc.cam.ac.uk.

Table 7.
Crystal data and structure refinement parameters for selected gramine derivatives.
3.4. Antioxidant Study
3.4.1. Ferrous Ions (Fe2+) Chelating Activity
Ferrous ions (Fe2+) chelating activity was evaluated by inhibition of the formation of Fe2+-ferrozine complex after incubation of the compounds tested with Fe2+. The Fe2+-chelating ability of compounds tested was determined by the absorbance of the ferrous ion-ferrozine complex at 562 nm. In brief, 0.1 mg/mL concentration of the compounds tested in 0.2 mL ethyl alcohol were added to a solution of 0.6 mM FeCl2 (0.05 mL). EDTA (ethylenediaminetetraacetic acid) was used as the standard EDTA chelating agent. The reaction was started by the addition of 5 mM ferrozine (0.05 mL) in ethyl alcohol and shaken vigorously immediately. The samples were stored for 10 min at room temperature (~22 °C). Following incubation, the absorbance (Abs) of the solutions was measured at 562 nm in a spectrophotometer. The percentage of inhibition of ferrozine—Fe2+ complex formation was calculated using the equation:
where Abs0 is the absorbance of the sample without the tested compound and Abs1 is the absorbance in the presence of the compound tested. Each sample was made in triplicate, and three independent experiments were performed.
Fe2+ chelating effect (%) = [1 − (Abs1/Abs0)] × 100%
3.4.2. Human Erythrocyte Preparation
All methods were carried out following relevant guidelines and regulations, and the Bioethics Committee approved all experimental protocols for Scientific Research at the Medical University of Poznań (agreement no. ZP/907/1002/18). Human RBC concentrates were purchased from Blood Bank in Poznań without any contact with blood donors.
The erythrocytes were washed three times (3000 rpm, 10 min, +4 °C) in 7.4 pH phosphate buffered saline (PBS: 137 mM NaCl, 2.7 mM KCl, 10 mM Na2HPO4, 1.76 mM KH2PO4) supplemented with 10 mM glucose. After washing, RBC were suspended in the PBS buffer at 1.65 × 109 cells/mL, stored at +4 °C, and used within 5 h.
3.4.3. Hemolysis Assay under the Compounds Tested
The cytotoxic activity of the compounds tested was determined by a standard hemolytic assay according to Mrówczyńska and Hägerstrand [57]. Briefly, RBC (1.65 × 108 cells/mL, ~1.5% hematocrit) were incubated in PBS buffer (7.4 pH) supplemented with 10 mM glucose and containing compounds tested (0.1 mg/mL) for 60 min at 37 °C in a shaking water bath. Samples with RBC incubated in PBS without compounds tested were taken as the control. Each sample was repeated three times, and the experiments were repeated 3 times with RBC from different donors. After incubation, the RBC suspensions were centrifuged (3000 rpm, 10 min), and the degree of hemolysis was estimated by measuring the absorbance (Ab) of the supernatant at 540 nm. The results were expressed as a percentage (%) of hemolysis which was determined using the following equation:
were positive control is Ab of supernatant of RBC in ice-cold H2O.
Hemolysis (%) = (sample Ab/positive control Ab) × 100%
3.4.4. Inhibition of the Free-Radical-Induced Hemolysis
RBC (1.65 × 108 cells/mL, ~1.5% hematocrit) were incubated in PBS buffer (pH 7.4) supplemented with 10 mM glucose and containing compounds tested in the sublytic concentration (0.1 mg/mL) for 20 min at 37 °C in a shaking water bath. After pre-incubation, 2,2’-azobis(2-methylpropionamidine) dihydrochloride (AAPH) was added at the final concentration of 60 mM. Samples were incubated for the next 4 h at 37 °C in a shaking water bath. Erythrocytes incubated in PBS only and in the presence of AAPH, were taken as the negative and positive controls, respectively. After incubation, the erythrocyte suspensions were centrifuged (4000 rpm, 5 min, +4 °C), and the degree of hemolysis was determined by measuring the absorbance (Ab) of the supernatant at 540 nm in a spectrophotometer. The percentage of inhibition was calculated using the following equation:
where Absample is the absorbance value of supernatant obtained from samples incubated with compounds tested in the presence of AAPH, AbPBS is the absorbance of supernatant obtained from PBS control (samples without compounds tested) and AbAAPH is the absorbance of supernatant obtained from AAPH controls (without compounds tested). Each sample was made in triplicate, and the results are presented as a mean value ± SD (n = 9) value of three independent experiments with RBC from different donors.
Inhibition of hemolysis (%) = 100 − [(Absample − AbPBS/AbAAPH − AbPBS) × 100%]
3.4.5. Statistical Analysis
For antioxidant and cytoprotective properties, data were plotted as the mean value ± standard deviation (SD) of the results of three independent experiments, with every sample in triplicate (n = 9). A paired t-Student test was used to compare the derivatives activity with the activity of the standard antioxidants Trolox or EDTA, respectively. Statistical significance was defined as p < 0.05. Inactive compounds were indicated as na. Non statistically significant difference was indicated as n.s.
3.5. Antibacterial Activity Measurements
The antimicrobial properties of compounds were determined against selected bacteria: Micrococcus luteus, Bacillus subtilis, Escherichia coli and Pseudomonas fluorescens which came from the collection of Pure Cultures of the Facility of Microbiology of the Department of Soil Science and Microbiology of the Poznan University of Life Sciences.
The well-diffusion method was used to evaluate the antimicrobial properties of compounds. 6 mL each of liquidized broth medium was poured into sterile Petri dishes and allowed to solidify. After which, two sterile glass rings with a diameter of 0.5 cm were placed on the surface of each plate. Then 20 mL each of liquid broth medium containing suspensions of the tested bacterial strains at a density of 107 cells/cm3, obtained from 48-h cultures on broth slants, was introduced. After the medium solidified, the glass rings were removed with a pencil, obtaining two wells on each plate. 0.1 mL of compound dissolved in pure dimethyl sulphoxide was introduced into one well, and 0.1 mL of pure dimethyl sulphoxide was introduced into the other well, which was as a control. Each compound was tested in four replicates. The plates were incubated for 48 h in a thermostat at 27 °C for M. luteus, B. subtilis and P. fluorescens cultures, and the E. coli culture at 37 °C. At the end of the incubation, the diameters of growth inhibition of the tested strains were measured using calipers.
3.6. Antifungal Activity Measurements
Fungal strains: Coriolus versicolor (Cv), Poria placenta (Pp), Coniophora puteana (Cp) and Gloeophyllum trabeum (Gt) were used as a test organism for the experimental determination of the antifungal activity of 1–11. The fungus were obtained from the collection of the Department of Wood Chemical Technology, Faculty of Forestry and Wood Technology, Poznań, University of Life Sciences, Poland. The fungal growth rates were measured in 90 mm-diameter plastic dishes using the agar-plate method, described by Ważny and Thorton [58]. Five concentrations of gramine and its derivatives in the range of 0.1% to 0.00001% were studied. The compound was applied on the agar (2% agar and 5% malt-extract) prepared on the Petri dish and previously autoclaved. The combination of the compound and fungus was repeated three times. Given sample was centrally inoculated with a 5 mm diameter disc taken from the submargin of 5-day-old malt agar plates. The plates were incubated in darkness at 21 ± 2 °C and 70 ± 5% relative air humidity. The growth duration was determined by the total coverage of the reference plate. The radius of the area covered by the fungus compared with the reference agar plate was employed to calculate the effective doses, ED50, of preservative concentrations retarding the fungal growth rate by 50%.
3.7. In Silico Study
The physicochemical calculations were conducted using the SwissADME website: www.swissadme.ch (accessed on 3 October 2022).
4. Conclusions
Based on gramine, a compound of natural origin, we have developed an efficient and easy method to obtained indolylmethane derivatives, which are of significant interest due to their biological activities, especially antioxidant properties. Analysis of the structure-activity relationship showed that the newly synthesized compounds having indole fragment joined by the methylene group with alkyl-substituted imidazole moieties exhibit excellent cytoprotective activity and are effective chelators of ferrous ion.
Of particular interest are the results obtained for 10 and 11, the chemically closely related yet structurally significantly different compounds. The structural disparity of 10 and 11, observed at both molecular and supramolecular levels, is reflected in their cytoprotective activity, which is absent in 10 and relatively high in 11. The enhancement of the cytoprotective activity may have its origin in the ability of a molecule to adopt a conformation that maximizes the number and assortment of intermolecular interactions and therefore is particularly well suited for a wide variety of supramolecular interactions, such as ligand/receptor interactions.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/molecules28020708/s1, Figure S1: (a) 13C NMR spectrum (101 MHz, DMSO-d6): compound 3, (b) 1H NMR spectrum (400 MHz, DMSO-d6): compound 3; Figure S2: EI-MS spectrum: compound 3; Figure S3: IR spectrum: compound 3; Figure S4: (a) 13C NMR spectrum (101 MHz, DMSO-d6): compound 4, (b) 1H NMR spectrum (400 MHz, DMSO-d6): compound 4; Figure S5: EI-MS spectrum: compound 4; Figure S6: IR spectrum: compound 4; Figure S7: (a) 13H NMR spectrum (101 MHz, DMSO-d6): compound 5, (b) 1H NMR spectrum (400 MHz, DMSO-d6): compound 5; Figure S8: EI-MS spectrum: compound 5; Figure S9: IR spectrum: compound 5; Figure S10: (a) 13H NMR spectrum (101 MHz, DMSO-d6): compound 6, (b) 1H NMR spectrum (400 MHz, DMSO-d6): compound 6; Figure S11: EI-MS spectrum: compound 6; Figure S12: IR spectrum: compound 6; Figure S13: (a) 13H NMR spectrum (101 MHz, DMSO-d6): compound 7, (b) 1H NMR spectrum (400 MHz, DMSO-d6): compound 7; Figure S14: EI-MS spectrum: compound 7; Figure S15: IR spectrum: compound 7; Figure S16: (a) 13C NMR spectrum (101 MHz, DMSO-d6): compound 8, (b) 1H NMR spectrum (400 MHz, DMSO-d6):compound 8; Figure S17: EI-MS spectrum: compound 8; Figure S18: IR spectrum: compound 8; Figure S19: (a) 13C NMR spectrum (101 MHz, DMSO-d6): compound 9, (b) 1H NMR spectrum (400 MHz, DMSO-d6): compound 9; Figure S20: EI-MS spectrum: compound 9; Figure S21: IR spectrum: compound 9; Figure S22: (a) 13C NMR spectrum (101 MHz, DMSO-d6): compound 10, (b) 1H NMR spectrum (400 MHz, DMSO-d6):compound 10; Figure S23: EI-MS spectrum: compound 10; Figure S24: IR spectrum: compound 10; Figure S25: (a) 13C NMR spectrum (101 MHz, DMSO-d6): compound 11, (b) 1H NMR spectrum (400 MHz, DMSO-d6): compound 11; Figure S26: EI-MS spectrum: compound 11; Figure S27: IR spectrum: compound 11; Table S1: Hydrogen bond parameters; Table S2: Crystal data and structure refinement parameters for selected gramine derivatives.
Author Contributions
Conceptualization, B.J. and L.M.; methodology, B.J., L.M., B.W., J.S. and G.C.; validation, B.W. and U.R.; investigation B.J.; resources, B.J.; data curation, B.W.; writing—original draft preparation, K.B.; writing—review and editing, B.J., L.M. and U.R.; visualization, B.W. and K.B.; supervision, B.J.; project administration, K.B.; funding acquisition, B.J., L.M. and K.B. All authors have read and agreed to the published version of the manuscript.
Funding
This work was financially supported within the Research Subsidy at the Faculty of Chemistry of the Adam Mickiewicz University in Poznań, the Research Subsidy at the Faculty of Biology of the Adam Mickiewicz University in Poznań and by the ID-UB 054 grant number 054/12/SNŚ.0011.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
Sample Availability
Samples of the compounds are not available from the authors.
References
- Al-Mulla, A. A Review: Biological Importance of Heterocyclic Compounds. Der Pharma Chem. 2017, 9, 141–147. [Google Scholar]
- Dorababu, A. Indole—A Promising Pharmacophore in Recent Antiviral Drug Discovery. RSC Med. Chem. 2020, 11, 1335–1353. [Google Scholar] [CrossRef]
- Dadashpour, S.; Emami, S. Indole in the Target-Based Design of Anticancer Agents: A Versatile Scaffold with Diverse Mechanisms. Eur. J. Med. Chem. 2018, 150, 9–29. [Google Scholar] [CrossRef] [PubMed]
- Song, F.; Li, Z.; Bian, Y.; Huo, X.; Fang, J.; Shao, L.; Zhou, M. Indole/Isatin-Containing Hybrids as Potential Antibacterial Agents. Arch. Pharm. 2020, 353, 2000143. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Li, H.; Liu, J.; Zhong, R.; Li, H.; Fang, S.; Liu, S.; Lin, S. Synthesis and Biological Evaluation of Indole-Based Peptidomimetics as Antibacterial Agents against Gram-Positive Bacteria. Eur. J. Med. Chem. 2021, 226, 113813. [Google Scholar] [CrossRef] [PubMed]
- Kumari, A.; Singh, R.K. Medicinal Chemistry of Indole Derivatives: Current to Future Therapeutic Prospectives. Bioorg. Chem. 2019, 89, 103021. [Google Scholar] [CrossRef]
- Singh, A.A.; Patil, M.P.; Kang, M.-J.; Niyonizigiye, I.; Kim, G.-D. Biomedical Application of Indole-3-Carbinol: A Mini-Review. Phytochem. Lett. 2021, 41, 49–54. [Google Scholar] [CrossRef]
- Slominski, A.; Semak, I.; Pisarchik, A.; Sweatman, T.; Szczesniewski, A.; Wortsman, J. Conversion of L-Tryptophan to Serotonin and Melatonin in Human Melanoma Cells. FEBS Lett. 2002, 511, 102–106. [Google Scholar] [CrossRef]
- Strawbridge, R.; Javed, R.R.; Cave, J.; Jauhar, S.; Young, A.H. The Effects of Reserpine on Depression: A Systematic Review. J. Psychopharmacol. 2022, 02698811221115762. [Google Scholar] [CrossRef]
- Li, G.; Hu, Y.; Li, D.; Zhang, Y.; Guo, H.; Li, Y.; Chen, F.; Xu, J. Vincristine-Induced Peripheral Neuropathy: A Mini-Review. NeuroToxicology 2020, 81, 161–171. [Google Scholar] [CrossRef]
- Moran-Santa Maria, M.M.; Baker, N.L.; McRae-Clark, A.L.; Prisciandaro, J.J.; Brady, K.T. Effects of yohimbine and drug cues on impulsivity and attention in cocaine-dependent men and women and sex-matched controls. Drug Alcohol Depend. 2016, 162, 56–63. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.-H.; Guo, Q.; Wang, H.-Y.; Li, Y.-H.; Khamis, M.Y.; Ma, L.-Y.; Wang, B.; Liu, H.-M. Gramine-Based Structure Optimization to Enhance Anti-Gastric Cancer Activity. Bioorg. Chem. 2021, 107, 104549. [Google Scholar] [CrossRef] [PubMed]
- Wei, Y.; Shi, L.; Wang, K.; Liu, M.; Yang, Q.; Yang, Z.; Ke, S. Discovery of Gramine Derivatives That Inhibit the Early Stage of EV71 Replication in Vitro. Molecules 2014, 19, 8949–8964. [Google Scholar] [CrossRef]
- Süzen, S. Antioxidant Activities of Synthetic Indole Derivatives and Possible Activity Mechanisms. In Bioactive Heterocycles V; Khan, M.T.H., Ed.; Springer: Berlin/Heidelberg, Germany, 2007; pp. 145–178. ISBN 978-3-540-73406-2. [Google Scholar]
- Kumar, J.; Kumar, N.; Sati, N.; Hota, P.K. Antioxidant Properties of Ethenyl Indole: DPPH Assay and TDDFT Studies. New J. Chem. 2020, 44, 8960–8970. [Google Scholar] [CrossRef]
- Silveira, C.C.; Mendes, S.R.; Soares, J.R.; Victoria, F.N.; Martinez, D.M.; Savegnago, L. Synthesis and Antioxidant Activity of New C-3 Sulfenyl Indoles. Tetrahedron Lett. 2013, 54, 4926–4929. [Google Scholar] [CrossRef]
- Kanwal; Khan, K.M.; Chigurupati, S.; Ali, F.; Younus, M.; Aldubayan, M.; Wadood, A.; Khan, H.; Taha, M.; Perveen, S. Indole-3-Acetamides: As Potential Antihyperglycemic and Antioxidant Agents; Synthesis, In Vitro α-Amylase Inhibitory Activity, Structure–Activity Relationship, and In Silico Studies. ACS Omega 2021, 6, 2264–2275. [Google Scholar] [CrossRef] [PubMed]
- Jasiewicz, B.; Kozanecka-Okupnik, W.; Przygodzki, M.; Warżajtis, B.; Rychlewska, U.; Pospieszny, T.; Mrówczyńska, L. Synthesis, Antioxidant and Cytoprotective Activity Evaluation of C-3 Substituted Indole Derivatives. Sci. Rep. 2021, 11, 15425. [Google Scholar] [CrossRef]
- Kozanecka-Okupnik, W.; Jasiewicz, B.; Pospieszny, T.; Jastrząb, R.; Skrobańska, M.; Mrówczyńska, L. Spectroscopy, Molecular Modeling and Anti-Oxidant Activity Studies on Novel Conjugates Containing Indole and Uracil Moiety. J. Mol. Struct. 2018, 1169, 130–137. [Google Scholar] [CrossRef]
- Kozanecka-Okupnik, W.; Sierakowska, A.; Berdzik, N.; Kowalczyk, I.; Mrówczyńska, L.; Jasiewicz, B. New Triazole-Bearing Gramine Derivatives—Synthesis, Structural Analysis and Protective Effect against Oxidative Haemolysis. Nat. Prod. Res. 2022, 36, 3413–3419. [Google Scholar] [CrossRef]
- Rosemeyer, H. The Chemodiversity of Purine as a Constituent of Natural Products. Chem. Biodivers. 2004, 1, 361–401. [Google Scholar] [CrossRef]
- Verma, A.; Joshi, S.; Singh, D. Imidazole: Having Versatile Biological Activities. J. Chem. 2013, 2013, 329412. [Google Scholar] [CrossRef]
- Alghamdi, S.S.; Suliman, R.S.; Almutairi, K.; Kahtani, K.; Aljatli, D. Imidazole as a Promising Medicinal Scaffold: Current Status and Future Direction. Drug Des. Devel. Ther. 2021, 15, 3289–3312. [Google Scholar] [CrossRef] [PubMed]
- Bozdag, M.; Supuran, C.T.; Esposito, D.; Angeli, A.; Carta, F.; Monti, S.M.; De Simone, G.; Alterio, V. 2-Mercaptobenzoxazoles: A Class of Carbonic Anhydrase Inhibitors with a Novel Binding Mode to the Enzyme Active Site. Chem. Commun. Camb. Engl. 2020, 56, 8297–8300. [Google Scholar] [CrossRef] [PubMed]
- Azam, M.A.; Suresh, B. Biological Activities of 2-Mercaptobenzothiazole Derivatives: A Review. Sci. Pharm. 2012, 80, 789–823. [Google Scholar] [CrossRef] [PubMed]
- Safak, C.; Simsek, R.; Erol, K.; Vural, K. Analgesic and Antiinflammatory Effects of Some 2-Mercaptobenzoxazole Derivatives. Die Pharm. 1996, 51, 180–182. [Google Scholar]
- Staniszewska, M.; Kuryk, Ł.; Gryciuk, A.; Kawalec, J.; Rogalska, M.; Baran, J.; Łukowska-Chojnacka, E.; Kowalkowska, A. In Vitro Anti-Candida Activity and Action Mode of Benzoxazole Derivatives. Molecules 2021, 26, 5008. [Google Scholar] [CrossRef] [PubMed]
- Mir, F.; Shafi, S.; Zaman, M.S.; Kalia, N.P.; Rajput, V.S.; Mulakayala, C.; Mulakayala, N.; Khan, I.A.; Alam, M.S. Sulfur Rich 2-Mercaptobenzothiazole and 1,2,3-Triazole Conjugates as Novel Antitubercular Agents. Eur. J. Med. Chem. 2014, 76, 274–283. [Google Scholar] [CrossRef]
- Karaca, Ş.; Osmaniye, D.; Sağlık, B.N.; Levent, S.; Ilgın, S.; Özkay, Y.; Karaburun, A.Ç.; Kaplancıklı, Z.A.; Gundogdu-Karaburun, N. Synthesis of Novel Benzothiazole Derivatives and Investigation of Their Enzyme Inhibitory Effects against Alzheimer’s Disease. RSC Adv. 2022, 12, 23626–23636. [Google Scholar] [CrossRef]
- Decker, M. Introduction. In Design of Hybrid Molecules for Drug Development; Decker, M., Ed.; Elsevier: Amsterdam, The Netherlands, 2017; pp. 1–3. ISBN 978-0-08-101011-2. [Google Scholar]
- Singla, R.; Gupta, K.B.; Upadhyay, S.; Dhiman, M.; Jaitak, V. Design, Synthesis and Biological Evaluation of Novel Indole-Benzimidazole Hybrids Targeting Estrogen Receptor Alpha (ER-α). Eur. J. Med. Chem. 2018, 146, 206–219. [Google Scholar] [CrossRef]
- Wang, R.; Shi, H.-F.; Zhao, J.-F.; He, Y.-P.; Zhang, H.-B.; Liu, J.-P. Design, Synthesis and Aromatase Inhibitory Activities of Novel Indole-Imidazole Derivatives. Bioorg. Med. Chem. Lett. 2013, 23, 1760–1762. [Google Scholar] [CrossRef]
- Naureen, S.; Ijaz, F.; Nazeer, A.; Chaudhry, F.; Munawar, M.A.; Khan, M.A. Facile, Eco-Friendly, One-Pot Protocol for the Synthesis of Indole-Imidazole Derivatives Catalyzed by Amino Acids. Synth. Commun. 2017, 47, 1478–1484. [Google Scholar] [CrossRef]
- Hogendorf, A.S.; Hogendorf, A.; Popiołek-Barczyk, K.; Ciechanowska, A.; Mika, J.; Satała, G.; Walczak, M.; Latacz, G.; Handzlik, J.; Kieć-Kononowicz, K.; et al. Fluorinated Indole-Imidazole Conjugates: Selective Orally Bioavailable 5-HT7 Receptor Low-Basicity Agonists, Potential Neuropathic Painkillers. Eur. J. Med. Chem. 2019, 170, 261–275. [Google Scholar] [CrossRef] [PubMed]
- Naureen, S.; Chaudhry, F.; Munawar, M.A.; Ashraf, M.; Hamid, S.; Khan, M.A. Biological Evaluation of New Imidazole Derivatives Tethered with Indole Moiety as Potent α-Glucosidase Inhibitors. Bioorg. Chem. 2018, 76, 365–369. [Google Scholar] [CrossRef] [PubMed]
- James, D.A.; Koya, K.; Li, H.; Chen, S.; Xia, Z.; Ying, W.; Wu, Y.; Sun, L. Conjugated Indole-Imidazole Derivatives Displaying Cytotoxicity against Multidrug Resistant Cancer Cell Lines. Bioorg. Med. Chem. Lett. 2006, 16, 5164–5168. [Google Scholar] [CrossRef]
- Li, Z.-Z.; Tangadanchu, V.K.R.; Battini, N.; Bheemanaboina, R.R.Y.; Zang, Z.-L.; Zhang, S.-L.; Zhou, C.-H. Indole-Nitroimidazole Conjugates as Efficient Manipulators to Decrease the Genes Expression of Methicillin-Resistant Staphylococcus Aureus. Eur. J. Med. Chem. 2019, 179, 723–735. [Google Scholar] [CrossRef]
- Pillaiyar, T.; Uzair, M.; Ullah, S.; Schnakenburg, G.; Müller, C.E. Decarboxylative Coupling Reaction of 2-(1H-Indol-3-Yl)Acetic Acids with Indole, Azaindole, Benzimidazole and Indazole Derivatives. Adv. Synth. Catal. 2019, 361, 4286–4293. [Google Scholar] [CrossRef]
- Decods, G.; Wakselman, M. Condensations thermiques de la gramine avec les polyphénols, l’imidazole et ses derives. Comptes Rendus Hebd. Séances Académie Sci. 1968, 266, 1168–1170. [Google Scholar]
- Podsiedlik, M.; Markowicz-Piasecka, M.; Sikora, J. Erythrocytes as model cells for biocompatibility assessment, cytotoxicity screening of xenobiotics and drug delivery. Chem. Biol. Interact. 2020, 332, 109305. [Google Scholar] [CrossRef]
- Liguori, I.; Russo, G.; Curcio, F.; Bulli, G.; Aran, L.; Della-Morte, D.; Gargiulo, G.; Testa, G.; Cacciatore, F.; Bonaduce, D.; et al. Oxidative stress, aging, and diseases. Clin. Interv. Aging. 2018, 26, 757–772. [Google Scholar] [CrossRef]
- Gulcin, I.; Alwasel, S.H. Metal ions, metal chelators and metal chelating assay as antioxidant method. Processes 2022, 10, 132. [Google Scholar] [CrossRef]
- Zhu, M.; Zhu, Q.; Yang, Z.; Liang, Z. Clinical Characteristics of Patients with Micrococcus luteus Bloodstream Infection in a Chinese Tertiary-Care Hospital. Pol. J. Microbiol. 2021, 70, 321–326. [Google Scholar] [CrossRef] [PubMed]
- Necel, A.; Bloch, S.; Topka-Bielecka, G.; Janiszewska, A.; Łukasiak, A.; Nejman-Faleńczyk, B.; Węgrzyn, G. Synergistic Effects of Bacteriophage vB_Eco4-M7 and Selected Antibiotics on the Biofilm Formed by Shiga Toxin-Producing Escherichia coli. Antibiotics 2022, 25, 712. [Google Scholar] [CrossRef] [PubMed]
- Wołejko, E.; Wydro, U.; Jabłońska-Trypuć, A.; Butarewicz, A.; Łoboda, T. Pseudomonas fluoresces occurrence in soil after fertilization with sewage sludge. Ekon. I Sr. —Econ. Environ. 2018, 65, 195–204. [Google Scholar]
- Kozicki, M.; Wiejak, A.; Piasecki, M.; Abram, A. Identification of MVOCs Produced by Coniophora puteana and Poria placenta Growing on WPC Boards by Using Subtraction Mass Spectra. Int. J. Environ. Res. Public Health 2019, 16, 2499. [Google Scholar] [CrossRef]
- Daina, A.; Michielin, O.; Zoete, V. SwissADME: A Free Web Tool to Evaluate Pharmacokinetics, Drug-Likeness and Medicinal Chemistry Friendliness of Small Molecules. Sci. Rep. 2017, 7, 42717. [Google Scholar] [CrossRef] [PubMed]
- Lipinski, C.A. Lead- and Drug-like Compounds: The Rule-of-Five Revolution. Drug Discov. Today Technol. 2004, 1, 337–341. [Google Scholar] [CrossRef] [PubMed]
- Roleira, F.M.F.; Siquet, C.; Orrù, E.; Garrido, E.M.; Garrido, J.; Milhazes, N.; Podda, G.; Paiva-Martins, F.; Reis, S.; Carvalho, R.A.; et al. Lipophilic phenolic antioxidants: Correlation between antioxidant profile, partition coefficients and redox properties. Bioorg. Med. Chem. 2010, 18, 5816–5825. [Google Scholar] [CrossRef]
- Veber, D.F.; Johnson, S.R.; Cheng, H.Y.; Smith, B.R.; Ward, K.W.; Kopple, K.D. Molecular properties that influence the oral bioavailability of drug candidates. J. Med. Chem. 2002, 45, 2615–2623. [Google Scholar] [CrossRef]
- Daina, A.; Zoete, V.A. BOILED-Egg to Predict Gastrointestinal Absorption and Brain Penetration of Small Molecules. Chem. Med. Chem. 2016, 11, 1117–1121. [Google Scholar] [CrossRef]
- Savjani, K.T.; Gajjar, A.K.; Savjani, J.K. Drug Solubility: Importance and Enhancement Techniques. ISRN Pharm. 2012, 195727. [Google Scholar] [CrossRef]
- CrysAlisPro. Agilent Technologies, Version 1.171.37.33; Agilent Technologies, Ltd.: Yarnton, UK, 2014. [Google Scholar]
- Sheldrick, G.M. SHELXT—Integrated space-group and crystal-structure determination. Acta Crystallogr. Sect. A Found. Adv. 2015, 71, 3–8. [Google Scholar] [CrossRef] [PubMed]
- Sheldrick, G.M. Crystal structure refinement with SHELXL, Acta Crystallogr. Sect. C Struct. Chem. 2015, 71, 3–8. [Google Scholar] [CrossRef] [PubMed]
- Bruno, I.J.; Cole, J.C.; Edgington, P.R.; Kessler, M.; Macrae, C.F.; McCabe, P.; Pearson, J.; Taylor, R. New software for searching the Cambridge Structural Database and visualizing crystal structures. Acta Crystallogr. Sect. B Struct. Sci. 2002, 58, 389–397. [Google Scholar] [CrossRef] [PubMed]
- Mrówczyńska, L.; Hagerstrand, H. Platelet-activating factor interaction with the human erythrocyte membrane. J. Biochem. Mol. Toxicol. 2009, 23, 345–348. [Google Scholar] [CrossRef]
- Ważny, J.; Thornton, J.D. Comparative Laboratory Testing of Strains of the Dry Rot Fungus Serpula lacrymans (Schum. ex Fr.) S.F. Gray. II. The Action of Some Wood Preservatives in Agar Media. Holzforschung 1986, 40, 383–388. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).