Thermal and Electrochemical Properties of Ionic Liquids Bearing Allyl Group with Sulfonate-Based Anions—Application Potential in Epoxy Resin Curing Process
Abstract
1. Introduction
2. Results and Discussion
2.1. Differential Scanning Calorimetry Analysis of Allyl-Based Ionic Liquids
2.2. Thermogravimetric Analysis of Allyl-Based Ionic Liquids
2.3. Electrochemical Properties of Allyl-Based Ionic Liquids
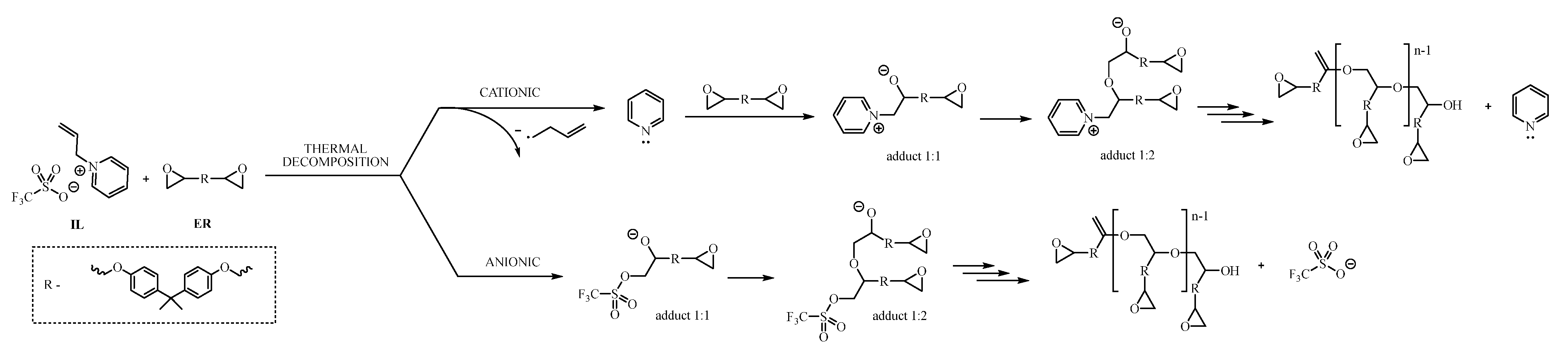
2.4. Curing Properties of Allyl-Based Ionic Liquids
3. Materials and Methods
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Earle, M.J.; Seddon, K.R. Ionic Liquids. Green Solvents for the Future. Pure Appl. Chem. 2000, 72, 1391–1398. [Google Scholar] [CrossRef]
- Wineinger, H.B.; Kelly, A.; Shamshina, J.L.; Rogers, R.D. Farmed Jumbo Shrimp Molts: An Ionic Liquid Strategy to Increase Chitin Yield per Animal While Controlling Molecular Weight. Green Chem. 2020, 22, 6001–6007. [Google Scholar] [CrossRef]
- Hallett, J.P.; Welton, T. Room-Temperature Ionic Liquids: Solvents for Synthesis and Catalysis. 2. Chem. Rev. 2011, 111, 3508–3576. [Google Scholar] [CrossRef]
- Quintana, A.A.; Sztapka, A.M.; Ebinuma, V.D.C.S.; Agatemor, C. Enabling Sustainable Chemistry with Ionic Liquids and Deep Eutectic Solvents: A Fad or the Future? Angew. Chem. Int. Ed. 2022, 61, e202205609. [Google Scholar] [CrossRef]
- Chiappe, C.; Pieraccini, D. Ionic Liquids: Solvent Properties and Organic Reactivity. J. Phys. Org. Chem. 2005, 18, 275–297. [Google Scholar] [CrossRef]
- Philippi, F.; Welton, T. Targeted Modifications in Ionic Liquids—From Understanding to Design. Phys. Chem. Chem. Phys. 2021, 23, 6993–7021. [Google Scholar] [CrossRef] [PubMed]
- Greer, A.J.; Jacquemin, J.; Hardacre, C. Industrial Applications of Ionic Liquids. Molecules 2020, 25, 5207. [Google Scholar] [CrossRef]
- Zajac, A.; Szpecht, A.; Szymanska, A.; Zielinski, D.; Stolarska, O.; Smiglak, M.; Maciejewski, H. Synthesis and Characterization of Nitrogen-Based Ionic Liquids Bearing Allyl Groups and Examples of Their Application. New J. Chem. 2020, 44, 12274–12288. [Google Scholar] [CrossRef]
- Zajac, A.; Szpecht, A.; Zielinski, D.; Rola, K.; Hoppe, J.; Komorowska, K.; Smiglak, M. Synthesis and Characterization of Potentially Polymerizable Amine-Derived Ionic Liquids Bearing 4-Vinylbenzyl Group. J. Mol. Liq. 2019, 283, 427–439. [Google Scholar] [CrossRef]
- He, H.; Luebke, D.; Nulwala, H.; Matyjaszewski, K. Synthesis of Poly(Ionic Liquid)s by Atom Transfer Radical Polymerization with Ppm of Cu Catalyst. Macromolecules 2014, 47, 6601–6609. [Google Scholar] [CrossRef]
- Nakabayashi, K.; Sato, Y.; Isawa, Y.; Lo, C.-T.; Mori, H. Ionic Conductivity and Assembled Structures of Imidazolium Salt-Based Block Copolymers with Thermoresponsive Segments. Polymers 2017, 9, 616. [Google Scholar] [CrossRef] [PubMed]
- Maksym, P.; Tarnacka, M.; Bielas, R.; Hachuła, B.; Zajac, A.; Szpecht, A.; Smiglak, M.; Kaminski, K.; Paluch, M. Structure-Property Relationships of Tailored Imidazolium- and Pyrrolidinium-Based Poly(Ionic Liquid)s. Solid-like vs. Gel-like Systems. Polymer 2020, 192, 122262. [Google Scholar] [CrossRef]
- Green, O.; Grubjesic, S.; Lee, S.; Firestone, M.A. The Design of Polymeric Ionic Liquids for the Preparation of Functional Materials. Polym. Rev. 2009, 49, 339–360. [Google Scholar] [CrossRef]
- Zulfiqar, S.; Ilyas Sarwar, M.; Mecerreyes, D. Polymeric Ionic Liquids for CO2 Capture and Separation: Potential, Progress and Challenges. Polym. Chem. 2015, 6, 6435–6451. [Google Scholar] [CrossRef]
- Wieszczycka, K.; Filipowiak, K.; Wojciechowska, I.; Aksamitowski, P. Novel Ionic Liquid-Modified Polymers for Highly Effective Adsorption of Heavy Metals Ions. Sep. Purif. Technol. 2020, 236, 116313. [Google Scholar] [CrossRef]
- Mecerreyes, D. Polymeric Ionic Liquids: Broadening the Properties and Applications of Polyelectrolytes. Prog. Polym. Sci. 2011, 36, 1629–1648. [Google Scholar] [CrossRef]
- Lewandowska, A.; Gajewski, P.; Szcześniak, K.; Fojud, Z.; Robakowska, M.; Skrzypczak, A.; Voelkel, A.; Marcinkowska, A. Thiol–Ene Ionogels Based on Polymerizable Imidazolium Ionic Liquids. Polym. Chem. 2022, 13, 3154–3170. [Google Scholar] [CrossRef]
- Seki, S.; Kobayashi, T.; Kobayashi, Y.; Takei, K.; Miyashiro, H.; Hayamizu, K.; Tsuzuki, S.; Mitsugi, T.; Umebayashi, Y. Effects of Cation and Anion on Physical Properties of Room-Temperature Ionic Liquids. J. Mol. Liq. 2010, 152, 9–13. [Google Scholar] [CrossRef]
- Lemus, J.; Neves, C.M.S.S.; Marques, C.F.C.; Freire, M.G.; Coutinho, J.A.P.; Palomar, J. Composition and Structural Effects on the Adsorption of Ionic Liquids onto Activated Carbon. Environ. Sci. Process. Impacts 2013, 15, 1752–1759. [Google Scholar] [CrossRef]
- Szpecht, A.; Zajac, A.; Zielinski, D.; Maciejewski, H.; Smiglak, M. Versatile Method for the Simultaneous Synthesis of Two Ionic Liquids, Otherwise Difficult to Obtain, with High Atom Economy. ChemistryOpen 2019, 8, 972–983. [Google Scholar] [CrossRef]
- Aparicio, S.; Atilhan, M.; Karadas, F. Thermophysical Properties of Pure Ionic Liquids: Review of Present Situation. Ind. Eng. Chem. Res. 2010, 49, 9580–9595. [Google Scholar] [CrossRef]
- Tiago, G.A.O.; Matias, I.A.S.; Ribeiro, A.P.C.; Martins, L.M.D.R.S. Application of Ionic Liquids in Electrochemistry—Recent Advances. Molecules 2020, 25, 5812. [Google Scholar] [CrossRef] [PubMed]
- Lockett, V.; Gustafson, J.; Ray, W.J.; Salah, Y. Energy Storage Device, Ink for an Electrode of an Energy Storage Device and Method of Manufacturing an Energy Storage Device. TW Patent TWI618291B, 11 March 2018. [Google Scholar]
- Shi, C.; Ho, C.C.; Mackenzie, J.D. Ionic Liquid Gel for Electrolyte, Method of and Ink for Making the Same, and Printed Batteries Including Such Ionic Liquid Gels and/or Electrolytes. Eur. Pat. EP3226339B1, 14 July 2021. [Google Scholar]
- Mackenzie, J.D.; Ho, C.; Yogeeswaran, K.; Roberts, G.; SHI, C. Electrochemical Cells and Metal Salt-Based Electrolytes. U.S. Patent US10530011B1, 7 January 2020. [Google Scholar]
- Xiong, W.; Yin, Z.; Zhang, X.; Tu, Z.; Hu, X.; Wu, Y. Ionic Liquids Endowed with Novel Hybrid Anions for Supercapacitors. ACS Omega 2022, 7, 26368–26374. [Google Scholar] [CrossRef] [PubMed]
- Livi, S.; Baudoux, J.; Gérard, J.-F.; Duchet-Rumeau, J. Ionic Liquids: A Versatile Platform for the Design of a Multifunctional Epoxy Networks 2.0 Generation. Prog. Polym. Sci. 2022, 132, 101581. [Google Scholar] [CrossRef]
- Lv, G.; Jensen, E.; Shen, C.; Yang, K.; Evans, C.M.; Cahill, D.G. Effect of Amine Hardener Molecular Structure on the Thermal Conductivity of Epoxy Resins. ACS Appl. Polym. Mater. 2021, 3, 259–267. [Google Scholar] [CrossRef]
- Rahmathullah, M.A.M.; Jeyarajasingam, A.; Merritt, B.; VanLandingham, M.; McKnight, S.H.; Palmese, G.R. Room Temperature Ionic Liquids as Thermally Latent Initiators for Polymerization of Epoxy Resins. Macromolecules 2009, 42, 3219–3221. [Google Scholar] [CrossRef]
- Jiang, Z.; Wang, Q.; Liu, L.; Zhang, Y.; Du, F.; Pang, A. Dual-Functionalized Imidazolium Ionic Liquids as Curing Agents for Epoxy Resins. Ind. Eng. Chem. Res. 2020, 59, 3024–3034. [Google Scholar] [CrossRef]
- Binks, F.C.; Cavalli, G.; Henningsen, M.; Howlin, B.J.; Hamerton, I. Examining the Kinetics of the Thermal Polymerisation Behaviour of Epoxy Resins Initiated with a Series of 1-Ethyl-3-Methylimidazolium Based Ionic Liquids. Thermochim. Acta 2018, 663, 19–26. [Google Scholar] [CrossRef]
- Nguyen, T.K.L.; Livi, S.; Pruvost, S.; Soares, B.G.; Duchet-Rumeau, J. Ionic Liquids as Reactive Additives for the Preparation and Modification of Epoxy Networks. J. Polym. Sci. Part A Polym. Chem. 2014, 52, 3463–3471. [Google Scholar] [CrossRef]
- Maka, H.; Spychaj, T.; Pilawka, R. Epoxy Resin/Ionic Liquid Systems: The Influence of Imidazolium Cation Size and Anion Type on Reactivity and Thermomechanical Properties. Ind. Eng. Chem. Res. 2012, 51, 5197–5206. [Google Scholar] [CrossRef]
- Carvalho, A.P.A.; Santos, D.F.; Soares, B.G. Epoxy/Imidazolium-based Ionic Liquid Systems: The Effect of the Hardener on the Curing Behavior, Thermal Stability, and Microwave Absorbing Properties. J. Appl. Polym. Sci. 2020, 137, 48326. [Google Scholar] [CrossRef]
- Nguyen, T.K.L.; Livi, S.; Soares, B.G.; Pruvost, S.; Duchet-Rumeau, J.; Gérard, J.-F. Ionic Liquids: A New Route for the Design of Epoxy Networks. ACS Sustain. Chem. Eng. 2016, 4, 481–490. [Google Scholar] [CrossRef]
- Xu, Y.-J.; Shi, X.-H.; Lu, J.-H.; Qi, M.; Guo, D.-M.; Chen, L.; Wang, Y.-Z. Novel Phosphorus-Containing Imidazolium as Hardener for Epoxy Resin Aiming at Controllable Latent Curing Behavior and Flame Retardancy. Compos. Part B Eng. 2020, 184, 107673. [Google Scholar] [CrossRef]
- Varshneya, A.K.; Mauro, J.C. Chapter 2—Fundamentals of the Glassy State. In Fundamentals of Inorganic Glasses, 3rd ed.; Varshneya, A.K., Mauro, J.C., Eds.; Elsevier: Amsterdam, The Netherlands, 2019; pp. 19–35. ISBN 978-0-12-816225-5. [Google Scholar]
- Gómez, E.; Calvar, N.; Domínguez, Á.; Gómez, E.; Calvar, N.; Domínguez, Á. Thermal Behaviour of Pure Ionic Liquids; IntechOpen: London, UK, 2015; ISBN 978-953-51-2122-0. [Google Scholar]
- Shukla, S.K.; Mikkola, J.-P. Melting Point of Ionic Liquids. In Encyclopedia of Ionic Liquids; Zhang, S., Ed.; Springer: Singapore, 2020; pp. 1–9. ISBN 978-981-10-6739-6. [Google Scholar]
- Dong, K.; Zhang, S.; Wang, J. Understanding the Hydrogen Bonds in Ionic Liquids and Their Roles in Properties and Reactions. Chem. Commun. 2016, 52, 6744–6764. [Google Scholar] [CrossRef]
- Domańska, U.; Żołek-Tryznowska, Z.; Królikowski, M. Thermodynamic Phase Behavior of Ionic Liquids. J. Chem. Eng. Data 2007, 52, 1872–1880. [Google Scholar] [CrossRef]
- Siedlecka, E.; Czerwicka, M.; Stolte, S.; Stepnowski, P. Stability of Ionic Liquids in Application Conditions. Curr. Org. Chem. 2011, 15, 1974–1991. [Google Scholar] [CrossRef]
- Crosthwaite, J.M.; Muldoon, M.J.; Dixon, J.K.; Anderson, J.L.; Brennecke, J.F. Phase Transition and Decomposition Temperatures, Heat Capacities and Viscosities of Pyridinium Ionic Liquids. J. Chem. Thermodyn. 2005, 37, 559–568. [Google Scholar] [CrossRef]
- Fredlake, C.P.; Crosthwaite, J.M.; Hert, D.G.; Aki, S.N.V.K.; Brennecke, J.F. Thermophysical Properties of Imidazolium-Based Ionic Liquids. J. Chem. Eng. Data 2004, 49, 954–964. [Google Scholar] [CrossRef]
- Pont, A.-L.; Marcilla, R.; De Meatza, I.; Grande, H.; Mecerreyes, D. Pyrrolidinium-Based Polymeric Ionic Liquids as Mechanically and Electrochemically Stable Polymer Electrolytes. J. Power Source. 2009, 188, 558–563. [Google Scholar] [CrossRef]
- Pitawela, N.R.; Shaw, S.K. Imidazolium Triflate Ionic Liquids’ Capacitance–Potential Relationships and Transport Properties Affected by Cation Chain Lengths. ACS Meas. Sci. Au 2021, 1, 117–130. [Google Scholar] [CrossRef]
- Coustan, L.; Bélanger, D. Electrochemical Activity of Platinum, Gold and Glassy Carbon Electrodes in Water-in-Salt Electrolyte. J. Electroanal. Chem. 2019, 854, 113538. [Google Scholar] [CrossRef]
- Sakaebe, H.; Matsumoto, H.; Tatsumi, K. Discharge–Charge Properties of Li/LiCoO2 Cell Using Room Temperature Ionic Liquids (RTILs) Based on Quaternary Ammonium Cation—Effect of the Structure. J. Power Source. 2005, 146, 693–697. [Google Scholar] [CrossRef]
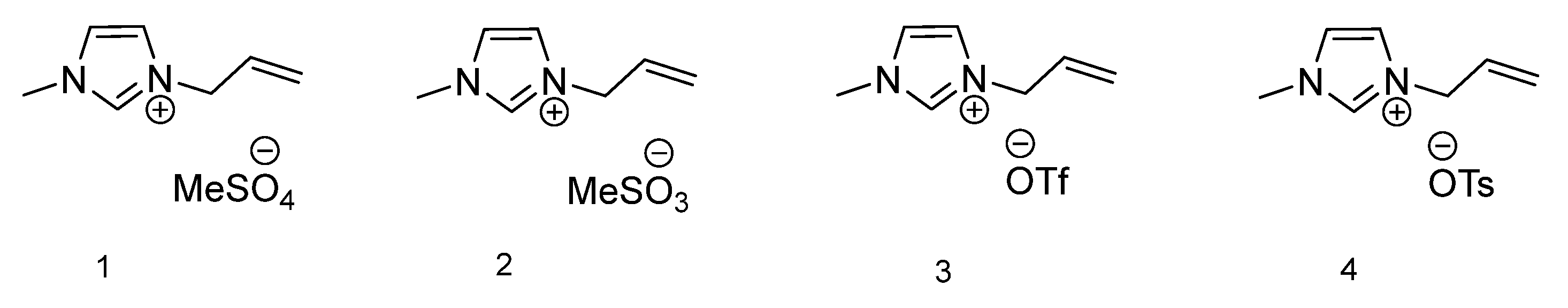
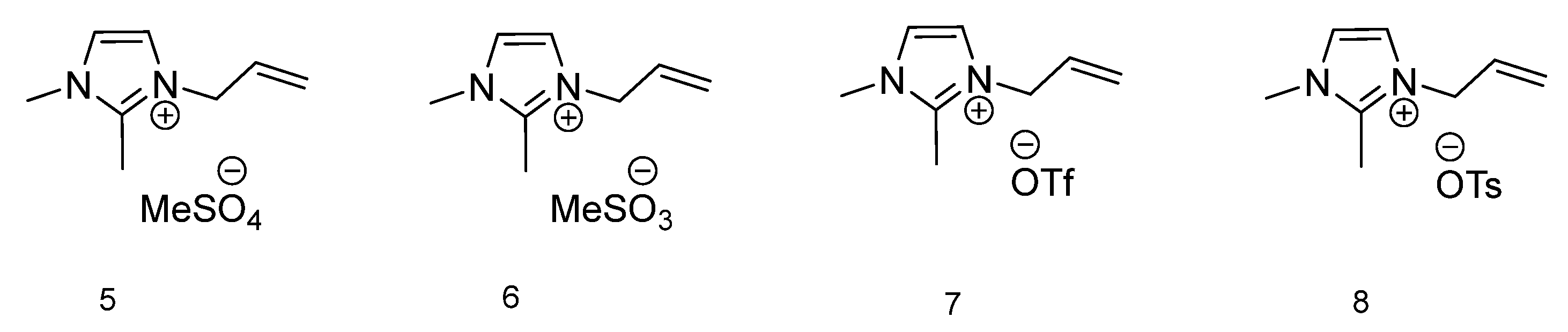


| IL Number | Tm (°C) | Tc (°C) | Tg (°C) |
|---|---|---|---|
| 1 | - | - | −54.55 |
| 2 | - | - | −39.87 |
| 3 | - | - | - |
| 4 | - | - | −37.41 |
| 5 | - | - | −63.26 |
| 6 | - | - | - |
| 7 | 51.20 | 29.71 | - |
| 8 | 126.06 | 47.72 | - |
| 9 | - | - | - |
| 10 | 73.06 | 1.75 | −57.19 |
| 11 | 1.34 | −15.46 | - |
| 12 | 96.90 | 4.16 | −33.70 |
| IL Number | Tm (°C) | Tc (°C) | Tg (°C) |
|---|---|---|---|
| 1 [Cl] | - | - | −65.8 |
| 1 [NTf2] | −10 | - | - |
| 5 [Cl] | 116.6 | - | - |
| 5 [NTf2] | - | - | - |
| 9 [Cl] | 109.8 | - | - |
| 9 [NTf2] | −19.6 | - | - |
| IL Number | Tonset (°C) | Tonset5% (°C) | T1 (°C) |
|---|---|---|---|
| 1 | 208.08 | 179.38 | 309.92 |
| 2 | 328.11 | 300.80 | - |
| 3 | 234.97 | 191.80 | 390.48 |
| 4 | 330.50 | 316.45 | - |
| 5 | 290.83 | - | - |
| 6 | 341.81 | 316.81 | - |
| 7 | 225.33 | 174.71 | 404.68 |
| 8 | 337.03 | 315.11 | - |
| 9 | 276.02 | 240.97 | - |
| 10 | 255.20 | 222.44 | 307.54 |
| 11 | 234.36 | 202.22 | 351.58 |
| 12 | 288.30 | 264.25 | - |
| IL Number | Conductivity (mS/cm) | Electrochemical Stability Range (V) | |
|---|---|---|---|
| Pt | GC | ||
| 1 | 1.48 | 2.0 | 4.0 |
| 2 | 1.95 | 1.1 | 1.9 |
| 3 | 4.51 | 2.6 | 3.8 |
| 4 | 0.26 | 2.8 | 4.6 |
| 5 | 0.28 | 1.8 | 5.8 |
| 9 | 2.34 | 1.3 | 3.5 |
| 11 | 5.62 | 1.7 | 3.6 |
| IL Number | Tonset (°C) | Tmax (°C) | Tendset (°C) | ∆H (J/g) |
|---|---|---|---|---|
| 3 | 176.70 | 179.01 | 179.25 | 380.27 |
| 7 | 149.52 | 216.85 | 231.89 | 480.42 |
| 11 | 168.35 | 172.84 | 173.58 | 554.58 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Szpecht, A.; Zielinski, D.; Galinski, M.; Smiglak, M. Thermal and Electrochemical Properties of Ionic Liquids Bearing Allyl Group with Sulfonate-Based Anions—Application Potential in Epoxy Resin Curing Process. Molecules 2023, 28, 709. https://doi.org/10.3390/molecules28020709
Szpecht A, Zielinski D, Galinski M, Smiglak M. Thermal and Electrochemical Properties of Ionic Liquids Bearing Allyl Group with Sulfonate-Based Anions—Application Potential in Epoxy Resin Curing Process. Molecules. 2023; 28(2):709. https://doi.org/10.3390/molecules28020709
Chicago/Turabian StyleSzpecht, Andrea, Dawid Zielinski, Maciej Galinski, and Marcin Smiglak. 2023. "Thermal and Electrochemical Properties of Ionic Liquids Bearing Allyl Group with Sulfonate-Based Anions—Application Potential in Epoxy Resin Curing Process" Molecules 28, no. 2: 709. https://doi.org/10.3390/molecules28020709
APA StyleSzpecht, A., Zielinski, D., Galinski, M., & Smiglak, M. (2023). Thermal and Electrochemical Properties of Ionic Liquids Bearing Allyl Group with Sulfonate-Based Anions—Application Potential in Epoxy Resin Curing Process. Molecules, 28(2), 709. https://doi.org/10.3390/molecules28020709






