Bioavailability of Phenolic Compounds in Californian-Style Table Olives with Tunisian Aqueous Olive Leaf Extracts
Abstract
1. Introduction
2. Results and Discussion
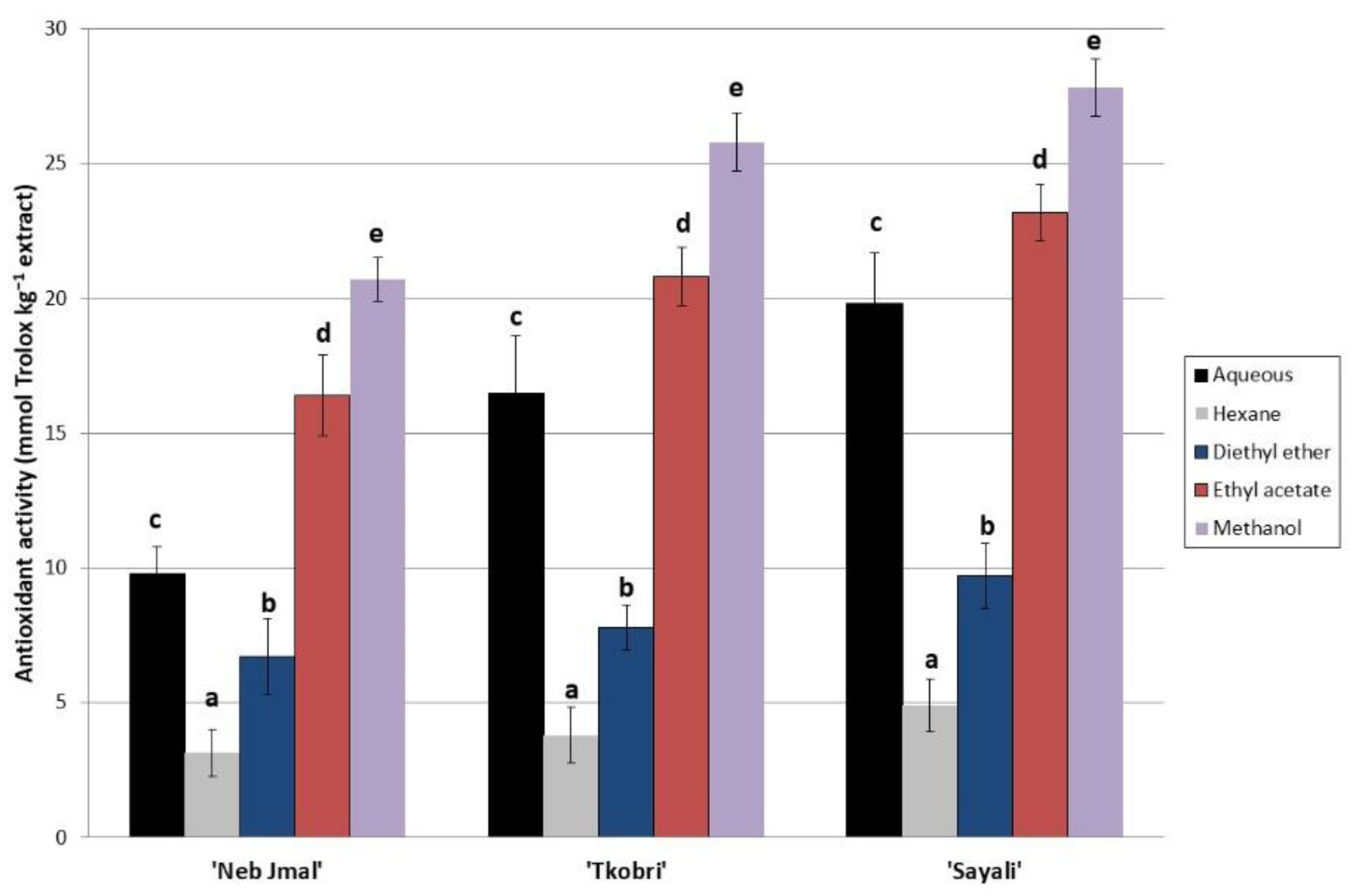
2.1. Phenolic Compounds in OLE
2.2. Phenolic Compounds Effect of ‘Sayali’ Aqueous Extract after In Vitro Gastrointestinal Evaluation
2.3. Influence of Aqueous Extracts in Californian-Style Black Olives
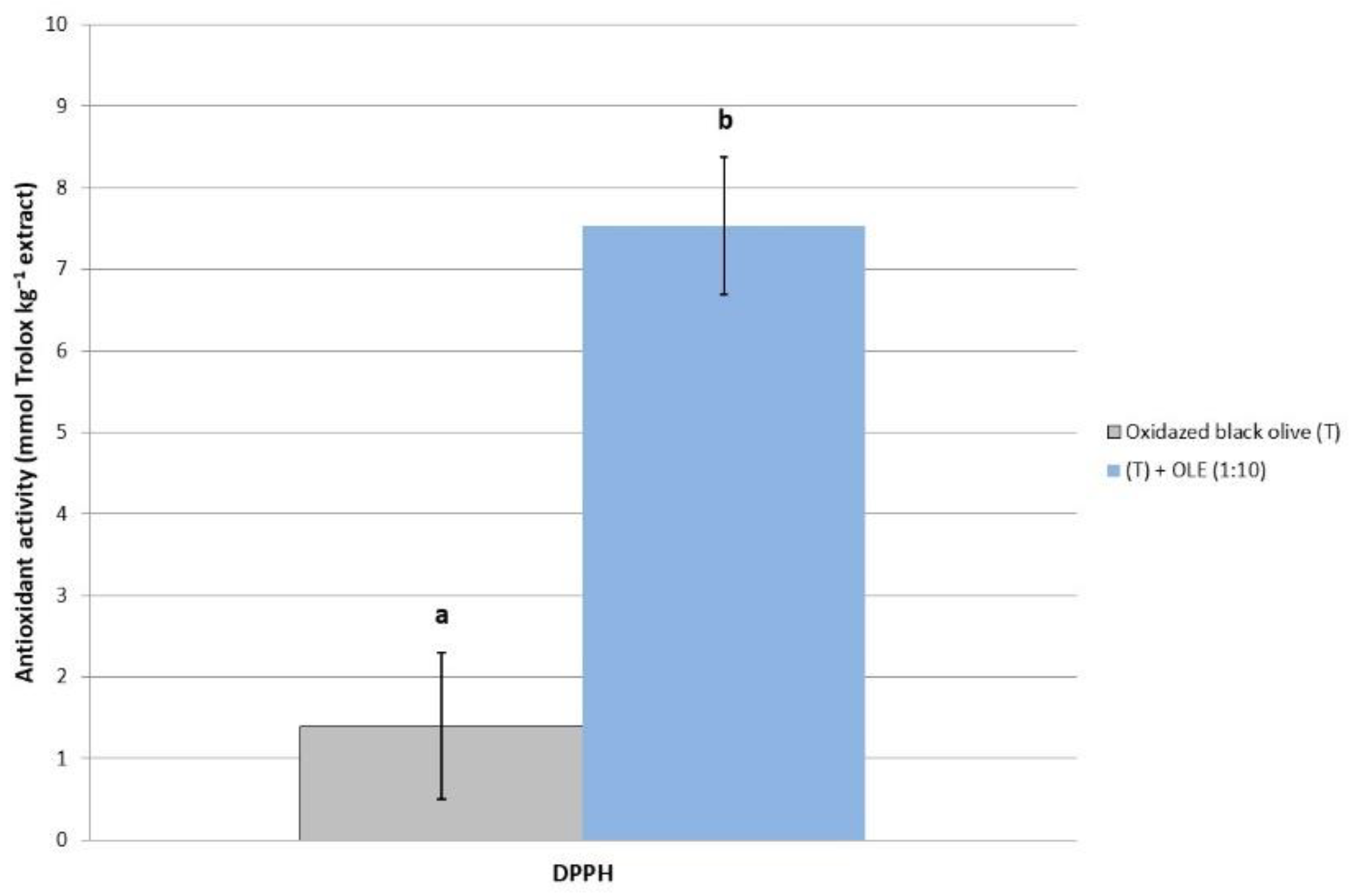
2.3.1. Phenol and Antioxidant Activity of ‘Hojiblanca’ Table Olives after OLE Addition
2.3.2. Gastrointestinal Activity of Californian-Style ‘Hojiblanca’ Table Olives after OLE Addition
3. Materials and Methods
3.1. Reagents and Standards
3.2. Plant Material
3.3. Extraction of Bioactive Compounds
3.4. Californian-Style Black Olives’ Elaboration Process
3.5. HPLC Analysis of Phenolic Compounds
3.6. Antioxidant Activity
3.7. Simulated Gastrointestinal Digestion
3.8. Statistical Software
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Krichene, D.; Allalout, A.; Baccouri, B.; Fregapane, G.Q.; Salvador, M.D.; Zarrouk, M. Territorial investigation based on the chemical composition of ‘Chemlali’ virgin olive oils. Asian J. Biochem. 2009, 4, 1–12. [Google Scholar] [CrossRef]
- Scognamiglio, M.; D’Abrosca, B.; Pacifico, S.; Fiumano, V.; De Luca, P.F.; Monaco, P.; Fiorentino, A. Polyphenol characterization and antioxidant evaluation of Olea europaea varieties cultivated in Cilento National Park (Italy). Food Res. Int. 2012, 46, 294–303. [Google Scholar] [CrossRef]
- Boudhrioua, N.; Bahloul, N.; Slimen, I.B.; Kechaou, N. Comparison on the total phenol contents and the color of fresh and infrared dried olive leaves. Ind. Crops Prod. 2009, 29, 412–419. [Google Scholar] [CrossRef]
- Bilgin, M.; Şahin, S. Effects of geographical origin and extraction methods on total phenolic yield of olive tree (Olea europaea) leaves. J. Taiwan Inst. Chem. Eng. 2013, 44, 8–12. [Google Scholar] [CrossRef]
- Mechi, D.; Fernández, A.; Baccouri, B.; Abaza, L.; Martín-Vertedor, D. Addition of ‘Chetoui’ olive leaf extract to reduce acrylamide in Californian-style black olive. Food Biosci. 2022, 50, 102080. [Google Scholar] [CrossRef]
- Baccouri, B.; Mechi, D.; Rajhi, I.; Martín-Vertedor, D. Tunisian Wild Olive Leaves: Phenolic compounds and antioxidant activity as an important step toward their valorization. Food Anal. Methods 2022, 1–9. [Google Scholar] [CrossRef]
- Park, J.-H.; Jung, J.H.; Yang, J.Y.; Kim, H.S. Olive leaf down-regulates the oxidative stress and immune dysregulation in streptozotocin-induced diabetic mice. Nutr. Res. 2013, 33, 942–951. [Google Scholar] [CrossRef]
- Čabarkapa, A.; Dekanski, D.; Živković, L.; Milanović-Čabarkapa, M.; Bajić, V.; Topalović, D. Unexpected effect of dry olive leaf extract on the level of DNA damage in lymphocytes of lead intoxicated workers, before and after CaNa2 EDTA chelation therapy. Food Chem. Toxicol. 2017, 106, 616–623. [Google Scholar] [CrossRef]
- Baldino, L.; Della Porta, G.; Osseo, L.S.; Reverchon, E.; Adami, R. Concentrated oleuropein powder from olive leaves using alcoholic extraction and supercritical CO2 assisted extraction. J. Supercrit. Fluids 2018, 133, 65–69. [Google Scholar] [CrossRef]
- Khemakhem, I.; Abdelhedi, O.; Trigui, I.; Ayadi, M.A.; Bouaziz, M. Structural, antioxidant and antibacterial activities of polysaccharides extracted from olive leaves. Int. J. Biol. Macromol. 2018, 106, 425–432. [Google Scholar] [CrossRef]
- Baccouri, B.; Rajhi, I. Potential Antioxidant Activity of Terpenes. In Terpenes and Terpenoids—Recent Advances; IntechOpen: London, UK, 2021; ISBN 9781838819163. [Google Scholar]
- Galanakis, C.M. Olive Mill Waste—Recent Advances for Sustainable Management; Academic Press: London, UK, 2017. [Google Scholar]
- Abaza, L.; Taamalli, A.; Nsir, H.; Zarrouk, M. Olive Tree (Olea europeae L.) Leaves: Importance and advances in the analysis of phenolic compounds. Antioxidants 2015, 4, 682–698. [Google Scholar] [CrossRef] [PubMed]
- Baccouri, B.; Rajhi, I.; Theresa, S.; Mohamed, S.N.; Willenberg, I. The potential of wild olive leaves (Olea europaea L. subsp. oleaster) addition as a functional additive in olive oil production: The effects on bioactive and nutraceutical compounds using LC–ESI–QTOF/MS. Eur. Food Res. Technol. 2022, 248, 2809–2823. [Google Scholar] [CrossRef] [PubMed]
- Akowuah, G.A.; IsmailI, Z.; Norhayati, I.; Sadikunet, A. The effects of different extraction solvents of varying polarities. Food Chem. 2005, 93, 311–331. [Google Scholar] [CrossRef]
- Ben Mansour, R.; Ksouri, W.M.; Cluzet, S.; Krisa, S.; Richard, T.; Ksouri, R. Assessment of antioxidant activity and neuroprotective capacity on PC12 cell line of Frankenia thymifolia and related phenolic LC-MS/MS identification. Evid. Based Complement. Alternat. Med. 2016, 2016, 2843463. [Google Scholar] [CrossRef]
- Fu, Z.-F.; Tu, Z.-C.; Zhang, L.; Wang, H.; Wen, Q.-H.; Huang, T. Antioxidant activities and polyphenols of sweet potato (Ipomoea batatas L.) leaves extracted with solvents of various polarities. Food Biosc. 2016, 15, 11–18. [Google Scholar] [CrossRef]
- Abaza, L.; Talorete, T.P.N.; Yamada, P.; Kurita, Y.; Zarrouk, M.; Isoda, H. Induction of growth inhibition and differentiation of human leukemia hl-60 cells by a Tunisian gerboui olive leaf extract. Biosci. Biotechnol. Biochem. 2007, 71, 1306–1312. [Google Scholar] [CrossRef]
- Abaza, L.; Taamalli, A.; Arráez-Román, D.; Segura-Carretero, A.; Fernández-Gutierrérez, A.; Zarrouk, M. Changes in phenolic composition in olive tree parts according to development stage. Food Res. Int. 2017, 100, 454–461. [Google Scholar] [CrossRef]
- Martín-Vertedor, D.; Schaide, T.; Boselli, E.; Martínez, M.; García-Parra, J.; Pérez-Nevado, F. Effect of high hydrostatic pressure in the storage of Spanish-style table olive fermented with olive leaf extract and Saccharomyces cerevisiae. Molecules 2022, 27, 2028. [Google Scholar] [CrossRef]
- Martín-Vertedor, D.; Fernández, A.; Mesías, M.; Martínez, M.; Martín-Tornero, E. Identification of mitigation strategies to reduce acrylamide levels during the production of black olives. J. Food Compost. Anal. 2021, 102, 104009. [Google Scholar] [CrossRef]
- Martín-Tornero, E.; Sánchez, R.; Lozano, J.; Martínez, M.; Arroyo, P.; Martín-Vertedor, D. Characterization of polyphenol and volatile fractions of Californian-style black olives and innovative application of e-nose for acrylamide determination. Foods 2021, 10, 2973. [Google Scholar] [CrossRef]
- Bouayed, J.; Deußer, H.; Hoffmann, L.; Bohn, T. Bioaccessible and dialysable polyphenols in selected apple varieties following in vitro digestion vs. their native patterns. Food Chem. 2012, 131, 1466–1472. [Google Scholar] [CrossRef]
- Bermúdez-Soto, M.-J.; Tomás-Barberán, F.-A.; García-Conesa, M.-T. Stability of polyphenols in chokeberry (Aronia melanocarpa) subjected to in vitro gastric and pancreatic digestion. Food Chem. 2007, 102, 865–874. [Google Scholar] [CrossRef]
- Taamalli, A.; Feriani, A.; Lozano-Sanchez, J.; Ghazouani, L.; El Mufti, A.; Allagui, M.S.; Segura-Carretero, A.; Mhamdi, R.; Arráez-Roman, D. potential hepatoprotective activity of super critical carbon dioxide olive leaf extracts against ccl4-induced liver damage. Foods 2020, 9, 804. [Google Scholar] [CrossRef]
- Hong, Y.H.; Song, C.; Shin, K.K.; Choi, E.; Hwang, S.H.; Jang, Y.J.; Taamalli, A.; Yum, J.; Kim, J.H.; Kim, E.; et al. Tunisian Olea europaea L. leaf extract suppresses Freund’s complete adjuvant-induced rheumatoid arthritis and lipopolysaccharide-induced inflammatory responses. J. Ethnopharmacol. 2021, 268, 113602. [Google Scholar] [CrossRef] [PubMed]
- Chaouche, T.M.; Haddouchi, F.; Atik-Bekara, F.; Ksouri, R.; Azzi, R.; Boucherit, Z. Antioxidant, haemolytic activities and HPLC–DAD–ESI–MSn characterization of phenolic compounds from root bark of Juniperus oxycedrus subsp. oxycedrus. Ind. Crops Prod. 2015, 64, 182–187. [Google Scholar] [CrossRef]
- Karker, M.; Falleh, H.; Msaada, K.; Smaoui, A.; Abdelly, C.; Legault, J.; Ksouri, R. Antioxidant, anti-inflammatory and anticancer activities of the medicinal halophyte Reaumuria vermiculata. EXCLI J. 2016, 15, 297–307. [Google Scholar]
- Medini, F.; Bourgou, S.; Lalancette, K.; Snoussi, M.; Mkadmini, K.; Coté, I.; Abdelly, C.; Legault, J.; Ksouri, R. Phytochemical analysis, antioxidant, anti-inflammatory, and anticancer activities of the halophyte Limonium densiflorum extracts on human cell lines and murine macrophages. S. Afr. J. Bot. 2015, 99, 158–164. [Google Scholar] [CrossRef]
- Chiboub, O.; Ktari, L.; Sifaoui, I.; López-Arencibia, A.; Reyes-Batlle, M.; Mejri, M.; Valladares, B.; Abderrabba, M.; Piñero, J.E.; Lorenzo-Morales, J. In vitro amoebicidal and antioxidant activities of some Tunisian seaweeds. Exp. Parasitol. 2017, 183, 76–80. [Google Scholar] [CrossRef]
- Dirar, A.I.; Alsaadi, D.H.M.; Wada, M.; Mohamed, M.A.; Watanabe, T.; Devkota, H.P. Effects of extraction solvents on total phenolic and flavonoid contents and biological activities of extracts from Sudanese medicinal plants. S. Afr. J. Bot. 2018, 120, 261–267. [Google Scholar]
- Delgado-Adámez, J.; Franco, M.N.; Ayuso, M.C.; Martín-Vertedor, D. Oxidative stability, phenolic compounds and antioxidant potential of a virgin olive oil enriched with natural bioactive compounds. J. Oleo Sci. 2014, 63, 55–65. [Google Scholar] [CrossRef]
- Mechi, D.; Pérez-Nevado, F.; Montero-Fernández, I.; Baccouri, B.; Abaza, L.; Martín-Vertedor, D. Evaluation of Tunisian olive leaf extracts to reduce the bioavailability of acrylamide in Californian-style black olives. Antioxidants 2023, 12, 117. [Google Scholar] [CrossRef]
- Charoenprasert, S.; Mitchell, A. Influence of California-style black ripe olive processing on the formation of acrylamide. J. Agric. Food Chem. 2014, 62, 8716–8721. [Google Scholar] [CrossRef] [PubMed]
- Martín-Vertedor, D.; Rodrigues, N.; Marx, Í.M.; Veloso, A.C.; Peres, A.M.; Pereira, J.A. Impact of thermal sterilization on the physicochemical-sensory characteristics of Californian-style black olives and its assessment using an electronic tongue. Food Control 2020, 117, 107369. [Google Scholar] [CrossRef]
- Tang, S.; Avena-Bustillos, R.J.; Lear, M.; Sedej, I.; Holstege, D.M.; Friedman, M.; McHugh, T.H.; Wang, S.C. Evaluation of thermal processing variables for reducing acrylamide in canned black ripe olives. J. Food Eng. 2016, 191, 124–130. [Google Scholar] [CrossRef]
- Martín-Vertedor, D.; Garrido, M.; Pariente, J.A.; Espino, J.; Delgado-Adámez, J. Bioavailability of bioactive molecules from olive leaf extracts and its functional value. Phytother. Res. 2016, 30, 1172–1179. [Google Scholar] [PubMed]
- Filgueira-Garro, I.; González-Ferrero, C.; Mendiola, D.; Marín-Arroyo, M.R. Effect of cultivar and drying methods on phenolic compounds and antioxidant capacity in olive (Olea europaea L.) leaves. AIMS Agric. Food 2022, 7, 250–264. [Google Scholar] [CrossRef]
- Rashed, S.A.; Saad, T.I.; El-Darier, S.M. Potential aptitude of four olive cultivars as anticancer and antioxidant agents: Oleuropein content. Rend. Lincei. Sci. Fis. E Nat. 2022, 33, 195–203. [Google Scholar] [CrossRef]
- Covas, M.I. Bioactive effects of olive oil phenolic compounds in humans: Reduction of heart disease factors and oxidative damage. Inflammopharmacology 2008, 16, 216–218. [Google Scholar] [CrossRef] [PubMed]
- Lodolini, E.M.; Cabrera-Bañegil, M.; Fernández, A.; Delgado-Adámez, J.; Ramírez, R.; Martín-Vertedor, D. Monitoring of acrylamide and phenolic compounds in table olive after high hydrostatic pressure and cooking treatments. Food Chem. 2019, 286, 250–259. [Google Scholar] [CrossRef]
- Zbakh, H.; El Abbassi, A. Potential use of olive mill wastewater in the preparation of functional beverages: A review. J. Funct. Food. 2012, 4, 53–65. [Google Scholar] [CrossRef]
- Liu, R.H. Whole grain phytochemicals and health. J. Cereal Sci. 2007, 46, 207–219. [Google Scholar] [CrossRef]
- Adom, K.K.; Liu, R.H. Antioxidant activity of grains. J. Agric. Food Chem. 2002, 50, 6182–6187. [Google Scholar] [CrossRef] [PubMed]
- Selma, M.V.; Espin, J.C.; Tomas-Barberan, F.A. Interaction between phenolics and gut microbiota: Role in human health. J. Agric. Food Chem. 2009, 57, 6485–6501. [Google Scholar] [CrossRef] [PubMed]
- Fernández, A.; Talaverano, M.I.; Pérez-Nevado, F.; Boselli, E.; Cordeiro, A.M.; Martillanes, S.; Foligni, R.; Martín-Vertedor, D. Evaluation of phenolics and acrylamide and their bioavailability in high hydrostatic pressure treated and fried table olives. J. Food Process. Preserv. 2020, 44, e14384. [Google Scholar] [CrossRef]
- Andreasen, M.F.; Kroon, P.A.; Williamson, G.; Garcia-Conesa, M.T. Esterase activity able to hydrolyze dietary antioxidant hydroxycinnamates is distributed along the intestine of mammals. J. Agric. Food Chem. 2001, 49, 5679–5684. [Google Scholar] [CrossRef]
- Heleno, S.A.; Martins, A.; Queiroz, M.J.R.; Ferreira, I.C. Bioactivity of phenolic acids: Metabolites versus parent compounds: A review. Food Chem. 2015, 173, 501–513. [Google Scholar] [CrossRef]
- Cabrera-Bañegil, M.; Schaide, T.; Manzano, R.; Delgado-Adámez, J.; Durán-Merás, I.; Martín-Vertedor, D. Optimization and validation of a rapid liquid chromatography method for determination of the main polyphenolic compounds in table olives and in olive paste. Food Chem. 2016, 233, 164–173. [Google Scholar] [CrossRef]
- Espinal-Ruiz, M.; Restrepo-Sánchez, L.P.; Narváez-Cuenca, C.E.; McClements, D.J. Impact of pectin properties on lipid digestion under simulated gastrointestinal conditions: Comparison of citrus and banana passion fruit (Passifloratripartita var. mollissima) pectins. Food Hydrocoll. 2016, 52, 329–342. [Google Scholar] [CrossRef]
| Variety | E | Hydroxytyrosol | Tyrosol | PB1 | Epicatequin | Verbascoside | Quercetin-3-rutinoside | Luteolin-7-O-glucoside |
| ‘Sayali’ | 1 | 9278.0 ± 14.4 c | 258.8 ± 6.3 b | 120.5 ± 7.9 b | 115.3 ± 0.03 c | 782.7 ± 12.9 c | 709.4 ± 3.8 b | 2007.9 ± 7.3 b |
| 2 | 325.9 ± 14.3 a | 15.9 ± 1.4 a | 12.9 ± 2.4 a | 8.8 ± 2.6 a | 45.1 ± 1.8 a | 45.5 ± 2.4 a | 120.4 ± 10.4 a | |
| 3 | 3970.1 ± 33.85 b | 292.1 ± 0.8 c | 115.3 ± 5.6 b | 119.8 ± 2.6 c | 648.6 ± 14.7 b | 689.7 ± 6.5 b | 1983.6 ± 6.8 b | |
| 4 | 15,841.1 ± 46.0 d | 306.5 ± 2.9 c | 189.6 ± 9.9 c | 130.9 ± 2.6 d | 850.6 ± 11.0 d | 864.7 ± 8.9 c | 2213.5 ± 21.4 c | |
| 5 | 45,324.0 ± 119.1 e | 480.0 ± 1.3 d | 212.6 ± 10.2 d | 109.9 ± 5.8 b | 1236.8 ± 14.0 e | 1224.2 ± 24.8 d | 15,642.8 ± 444.8 d | |
| ‘Tkobri’ | 1 | 7589.6 ± 85.8 c | 168.6 ± 5.2 c | 104.7 ± 1.6 b | 42.7 ± 0.4 b | 655.8 ± 21.4 d | 437.7 ± 61.6 b | 1120.5 ± 5.3 c |
| 2 | 278.2 ± 5.9 a | 9.4 ± 2.6 a | 5.2 ± 2.3 a | 2.1 ± 4.3 a | 30.8 ± 3.2 a | 21.9 ± 2.1 a | 36.0 ± 10.2 a | |
| 3 | 2732.5 ± 12.5 b | 89.9 ± 6.5 b | 98.6 ± 2.3 b | 55.8 ± 2.3 c | 501.6 ± 12.6 b | 564.5 ± 12.5 c | 985.6 ± 9.8 b | |
| 4 | 9972.9 ± 135.2 d | 201.6 ± 8.4 d | 125.6 ± 6.5 c | 69.8 ± 5.4 d | 611.6 ± 21.8 c | 800.7 ± 24.6 d | 2576.9 ± 66.5 d | |
| 5 | 34,787.3 ± 492.2 e | 243.1 ± 10.7 e | 209.6 ± 2.5 d | 70.8 ± 6.7 d | 855.3 ± 24.6 e | 1285.6 ± 56.1 e | 8542.3 ± 627.5 e | |
| ‘Neb Jmel’ | 1 | 3559.9 ± 34.4 c | 108.5 ± 1.4 b | 102.6 ± 4.6 c | 17.0 ± 2.4 b | 355.4 ± 1.5 c | 331.2 ± 18.2 b | 629.9 ± 104.4 b |
| 2 | 187.5 ± 10.3 a | 5.4 ± 2.1 a | 5.1 ± 1.8 a | 0.9 ± 3.4 a | 18.8 ± 3.1 a | 11.6 ± 3.7 a | 31.5 ± 10.6 a | |
| 3 | 1721.0 ± 33.7 b | 98.6 ± 2.3 b | 70.6 ± 7.5 b | 20.9 ± 2.6 b | 301.5 ± 10.2 b | 405.8 ± 14.5 c | 897.6 ± 9.9 c | |
| 4 | 5385.8 ± 34.9 d | 180.6 ± 10.3 c | 125.6 ± 3.8 c | 35.6 ± 3.2 c | 501.6 ± 11.6 d | 689.7 ± 8.9 d | 1890.1 ± 118.1 d | |
| 5 | 16,265.6 ± 306.2 e | 201.3 ± 26.2 c | 207.9 ± 8.9 d | 55.6 ± 1.2 d | 756.9 ± 14.6 e | 991.6 ± 6.7 e | 10,642.7 ± 333.2 e | |
| Variety | E | Oleuropein | Quercetin | Gallic acid | Vanillic acid | Caffeic acid | p-Coumaric acid | Chlorogenic acid |
| ‘Sayali’ | 1 | 15,781.7 ± 84.7 b | 1120.8 ± 22.7 c | 2.7 ± 0.2 a | 1.0 ± 0.1 a | 311.3 ± 9.8 b | 1.5 ± 0.0 a | 60.4 ± 10.3 a |
| 2 | 849.1 ± 4.3 a | 156.0 ± 3.8 a | nq | nq | 18.6 ± 2.4 a | nq | nq | |
| 3 | 14,588.9 ± 71.6 b | 1079.2 ± 116.2 b | 3.0 ± 0.2 b | 1.3 ± 0.1 b | 365.4 ± 9.8 c | 1.9 ± 0.1 b | 74.8 ± 9.8 b | |
| 4 | 241,078.7 ± 105.0 c | 1194.8 ± 180.0 c | 5.0 ± 0.1 c | 2.6 ± 0.1 c | 453.5 ± 10.4 d | 3.0 ± 1.0 c | 80.5 ± 6.8 c | |
| 5 | 275,059.6 ± 69.8 d | 2980.6 ± 55.9 d | 6.4 ± 0.2 d | 3.3 ± 0.1 d | 532.9 ± 11.4 e | 3.8 ± 1.0 d | 117.7 ± 9.4 d | |
| ‘Tkobri’ | 1 | 5406.2 ± 245.5 b | 1100.6 ± 55.4 b | 1.8 ± 0.1 a | 0.3 ± 0.1 a | 201.3 ± 11.3 b | 1.0 ± 0.1 a | 32.4 ± 10.2 a |
| 2 | 230.3 ± 5.5 a | 75.0 ± 2.4 a | nq | nq | 11.1 ± 0.9 a | nq | nq | |
| 3 | 5287.9 ± 284.2 b | 1056.9 ± 48.9 b | 2.0 ± 0.1 b | 0.6 ± 0.1 b | 209.8 ± 10.4 b | 1.2 ± 0.1 b | 42.3 ± 9.6 a | |
| 4 | 243,177.8 ± 105.3 d | 1325.0 ± 139.3 c | 3.1 ± 0.1 c | 1.1 ± 0.1 c | 300.3 ± 9.9 c | 2.2 ± 0.1 c | 55.9 ± 7.8 b | |
| 5 | 203,808.5 ± 96.8 c | 1554.6 ± 23.5 d | 4.6 ± 0.1 d | 1.9 ± 0.1 d | 411.4 ± 9.9 d | 2.3 ± 0.1 c | 68.5 ± 8.6 c | |
| ‘Neb Jmel’ | 1 | 6272.4 ± 204.1 b | 805.9 ± 55.7 c | 1.1 ± 0.1 a | 0.6 ± 0.1 a | 200.5 ± 11.5 b | 0.8 ± 0.0 a | 29.8 ± 9.7 a |
| 2 | 253.6 ± 6.4 a | 50.3 ± 2.7 a | nq | nq | 12.0 ± 1.8 a | nq | nq | |
| 3 | 22,417.7 ± 434.4 c | 582.2 ± 40.6 b | 1.2 ± 0.1 a | 1.0 ± 0.1 bc | 227.8 ± 9.7 c | 1.0 ± 0.1 b | 25.9 ± 9.5 a | |
| 4 | 141,859.7 ± 105.6 d | 775.1 ± 96.3 c | 2.0 ± 0.1 b | 1.2 ± 0.1 c | 287.9 ± 8.6 d | 1.3 ± 0.1 c | 39.5 ± 10.3 b | |
| 5 | 186,837.0 ± 318.3 e | 1865.4 ± 20.8 d | 2.6 ± 0.1 c | 1.9 ± 0.1 d | 300.6 ± 10.6 e | 2.0 ± 0.2 d | 58.5 ± 11.2 c |
| Variety | Phases | Hydroxtyrosol | Tyrosol | PB1 | Epicatequin | Verbascoside | Quercetin-3-rutinoside | Luteolin-7-O-glucoside |
| ‘Sayali’ | Fresh | 9278.0 ± 14.4 f | 258.3 ± 6.2 e | 120.5 ± 7.9 f | 115.3 ± 2.5 f | 782.7 ± 12.9 e | 709.4 ± 3.8 d | 2007.9 ± 7.3 f |
| O | 8521.7 ± 15.6 e | 224.6 ± 6.8 d | 114.6 ± 4.6 e | 105.9 ± 5.6 e | 755.9 ± 15.4 d | 700.4 ± 5.5 d | 1975.4 ± 9.8 e | |
| G | 2674.2 ± 21.4 d | 102.6 ± 5.4 c | 75.6 ± 5.5 d | 69.8 ± 3.4 d | 150.8 ± 9.6 c | 168.9 ± 6.8 c | 855.7 ± 7.4 d | |
| SI | 1650.9 ± 13.6 c | 92.4 ± 6.8 b | 60.7 ± 9.8 c | 52.9 ± 7.1 c | 125.4 ± 5.8 b | 115.7 ± 4.8 b | 655.8 ± 5.8 c | |
| LI | 1006.7 ± 9.8 a | 69.7 ± 7.9 a | 40.9 ± 4.7 a | 35.7 ± 2.5 a | 95.6 ± 6.7 a | 90.7 ± 9.8 a | 489.7 ± 10.2 a | |
| C-LI | 1250.6 ± 10.7 b | 75.9 ± 6.8 a | 55.9 ± 2.6 b | 40.8 ± 6.1 b | 110.4 ± 8.2 a | 101.7 ± 7.9 a | 521.7 ± 7.1 b | |
| Variety | Phases | Oleuropein | Quercetin | Gallic acid | Vanillic acid | Caffeic acid | p-Coumaric acid | Chlorogenic acid |
| ‘Sayali’ | Fresh | 15,781.7 ± 84.7 f | 1120.8 ± 22.7 f | 2.7 ± 0.2 f | 1.0 ± 0.1 b | 311.3 ± 9.8 e | 1.5 ± 0.0 c | 60.4 ± 10.3 d |
| O | 12,358.7 ± 12.3 e | 1005.8 ± 5.9 e | 2.3 ± 0.1 e | 0.9 ± 0.1 b | 296.5 ± 8.5 d | 1.1 ± 0.1 b | 54.3 ± 8.7 c | |
| G | 5542.9 ± 20.4 d | 304.8 ± 6.4 d | 1.0 ± 0.0 d | 0.2 ± 0.1 a | 45.6 ± 5.4 c | 0.3 ± 0.1 a | 23.1 ± 8.6 b | |
| SI | 4987.6 ± 9.8 c | 255.9 ± 8.7 c | 0.9 ± 0.0 c | nq | 40.1 ± 2.6 b | nq | 18.3 ± 9.5 b | |
| LI | 2079.4 ± 11.7 a | 198.4 ± 11.6 a | 0.7 ± 0.0 a | nq | 34.2 ± 7.1 a | nq | 14.3 ± 4.8 a | |
| C-LI | 2555.7 ± 13.9 b | 225.7 ± 14.8 b | 1.0 ± 0.0 b | nq | 44.6 ± 2.7 c | nq | 19.9 ± 6.3 b |
| ‘Hojiblanca’ | ||
|---|---|---|
| Phenolic Profile (mg 100 g−1) | Oxidized Black Olive (T) | (T) + OLE (1:10) |
| Hydroxytyrosol | 464.3 ± 10.3 a | 2171.5 ± 30.3 b |
| Tyrosol | 25.4 ± 4.5 a | 82.1 ± 8.9 b |
| PB1 | 18.3 ± 4.3 a | 55.5 ± 4.3 b |
| Epicatechin | 2.7 ± 2.2 a | 42.7 ± 9.1 b |
| Verbascoside | 3.9 ± 0.2 a | 284.9 ± 10.4 b |
| Quercetin-3-rutinoside | 2.3 ± 1.2 a | 300.1 ± 12.0 b |
| Luteolin-7-O-glucoside | 2.4 ± 1.3 a | 570.0 ± 45.6 b |
| Oleuropein | 110.2 ± 14.6 a | 3460.9 ± 75.6 b |
| Quercetin | nq | 295.7 ± 32.5 |
| Vanillic acid | 2.9 ± 0.3 ns | 2.9 ± 0.5 ns |
| Caffeic acid | nq | 124.9 ± 10.3 |
| p-Coumaric | 8.4 ± 0.7 ns | 6.9 ± 1.3 ns |
| Chlorogenic acid | nq | 25.2 ± 3.6 |
| Σ phenols | 640.8 ± 6.8 a | 7423.3 ± 15.6 b |
| ‘Hojiblanca’ | |||||
|---|---|---|---|---|---|
| Phenolic Profile (mg 100 g−1) | O | G | SI | LI | C-LI |
| Hydroxytyrosol | 1843.4 ± 45.6 e | 803.1 ± 98.7 d | 410.6 ± 56.5 c | 256.4 ± 20.7 a | 311.2 ± 30.7 b |
| Tyrosol | 70.3 ± 1.4 d | 30.1 ± 2.1 c | 13.4 ± 2.2 b | 8.9 ± 2.4 a | 10.7 ± 1.1 a |
| PB1 | 50.4 ± 3.5 d | 20.2 ± 1.5 c | 10.1 ± 2.2 b | 6.7 ± 2.3 a | 8.4 ± 2.1 a |
| Epicatechin | 38.8 ± 4.3 d | 16.1 ± 1.1 c | 7.2 ± 2.0 b | 4.8 ± 1.1 a | 5.8 ± 1.4 a |
| Verbascoside | 260.1 ± 21.4 d | 104.0 ± 2.1 c | 52.0 ± 2.5 b | 34.7 ± 2.3 a | 40.6 ± 2.2 a |
| Quercetin-3-rutinoside | 272.8 ± 13.3 d | 109.1 ± 3.0 c | 54.6 ± 3.4 b | 36.4 ± 3.1 a | 43.7 ± 3.3 a |
| Luteolin-7-O-glucoside | 500.3 ± 25.5 e | 200.1 ± 30.8 d | 100.1 ± 67.1 c | 66.7 ± 7.8 a | 80.0 ± 7.2 b |
| Oleuropein | 3146.3 ± 89.5 e | 1258.5 ± 90.1 d | 629.3 ± 95.2 c | 419.5 ± 25.2 a | 497.5 ± 45.2 b |
| Quercetin | 268.8 ± 14.5 d | 107.5 ± 5.1 c | 53.8 ± 6.8 b | 35.8 ± 5.8 a | 40.0 ± 5.1 a |
| Vanillic acid | 2.7 ± 0.3 b | 1.1 ± 0.2 a | nq | nq | nq |
| Caffeic acid | 113.5 ± 12.4 c | 45.4 ± 13.2 b | 22.7 ± 6.4 a | 15.1 ± 5.2 a | 18.2 ± 4.6 a |
| p-Coumaric | 6.2 ± 2.4 b | 2.5 ± 2.1 a | nq | nq | nq |
| Chlorogenic acid | 22.9 ± 3.3 c | 9.2 ± 1.3 b | 4.6 ± 1.4 a | 3.1 ± 0.6 a | 3.7 ± 0.7 a |
| Σ phenols | 6596.7 ± 18.6 e | 2706.9 ± 31.6 d | 1358.3 ± 37.2 c | 888.2 ± 41.1 a | 1059.7 ± 40.2 b |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mechi, D.; Baccouri, B.; Martín-Vertedor, D.; Abaza, L. Bioavailability of Phenolic Compounds in Californian-Style Table Olives with Tunisian Aqueous Olive Leaf Extracts. Molecules 2023, 28, 707. https://doi.org/10.3390/molecules28020707
Mechi D, Baccouri B, Martín-Vertedor D, Abaza L. Bioavailability of Phenolic Compounds in Californian-Style Table Olives with Tunisian Aqueous Olive Leaf Extracts. Molecules. 2023; 28(2):707. https://doi.org/10.3390/molecules28020707
Chicago/Turabian StyleMechi, Dalel, Bechir Baccouri, Daniel Martín-Vertedor, and Leila Abaza. 2023. "Bioavailability of Phenolic Compounds in Californian-Style Table Olives with Tunisian Aqueous Olive Leaf Extracts" Molecules 28, no. 2: 707. https://doi.org/10.3390/molecules28020707
APA StyleMechi, D., Baccouri, B., Martín-Vertedor, D., & Abaza, L. (2023). Bioavailability of Phenolic Compounds in Californian-Style Table Olives with Tunisian Aqueous Olive Leaf Extracts. Molecules, 28(2), 707. https://doi.org/10.3390/molecules28020707






