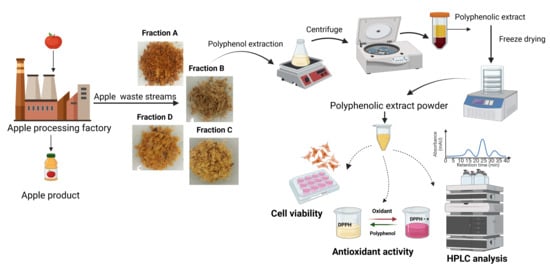
Chemical Composition, Antioxidant Activity and Cytocompatibility of Polyphenolic Compounds Extracted from Food Industry Apple Waste: Potential in Biomedical Application
Abstract
1. Introduction
2. Results and Discussion
2.1. Physicochemical Determination of the Apple Pomace Fractions
2.2. Total Polyphenolic Compounds
2.2.1. Ethanol Extraction
| Type of AP | Cultivar | Extraction Method | TPC (mg GAE/g db) | Ref |
|---|---|---|---|---|
| Peel, pulp, core | Gold Milenium, Papierowka | Ethanol:water (80:100, v/v), sample/solvent (1:25 w/v), three steps extraction with solvent, 60 °C, 1.5 h | Gold Milenium: Peel = 0.17 Pulp = 0.04 Core = 0.09 Papierowka: Peel = 0.43 Pulp = 0.07 Core = 0.19 | [30] |
| Peel, pulp, core | Guoguang, Fuji, Wanglin, Golden Delicious | Aceton:water (80:100, v/v), sample/solvent (1:4 w/w), 5 min, chill temperature | * Peel = 1.19 ± 0.02–1.7 ± 0.02 * Pulp = 0.65 ± 0.05–1.02 ± 0.07 * Core = 0.9 ± 0.03–1.36 ± 0.12 | [32] |
| Seed | Golden Delicious, Red Delicious | Methanol:water (80:100, v/v), sample/solvent (1:10 w/v), room temperature, 30 min | Red delicious = 12.30 ± 0.96 Golden delicious = 7.17 ± 0.47 | [33] |
| Seed | Fuji Zhen Aztec, Granny Smith, Pink Lady, Super Chief, Jeromine | Methanol:water (80:100, v/v), sample/solvent (1:10 w/v), room temperature, 30 min | Fuji Zhen Aztec = 2.86 ± 0.02 Granny Smith = 3.58 ± 0.05 Pink Lady = 4.1 ± 0.1 Super Chief = 3.62 ± 0.03 Jeromine = 5.14 ± 0.05 | [31] |
| Whole fruit, peel | Red Delicious Starking, Golden Delicious, Granny Smith, Jona Gold, Royal Gala | Methanol: water (90:100, v/v), sample/solvent (2:1 w/v), chill temperature, 20 min, two steps extraction with solvent | * Peel = 1.56–4.00 * Whole fruit = 0.8–1.96 | [34] |
| Peel | Rome Beauty | Aceton:water (80:100, v/v), sample/solvent (1:4 w/w), 5 min, chill temperature | * Fresh peel = 5.2 ± 0.14 * Air-dried blanched peel = 4.64 ± 0.27 * Freeze-dried blanched peel = 4.60 ± 0.42 | [35] |
| Peel, pulp, pulp + peel | Cortland, Idared, Rome Beauty, Golden Delicious | Aceton: water (80:100, v/v), sample/solvent (1:4 w/w), 5 min, chill temperature, two steps extraction with solvent | Peel = 3.09 ± 0.32–5.89 ± 0.83 Pulp = 0.76 ± 0.04–1.03 ± 0.12 pulp + peel = 1.19 ± 0.15–1.59 ± 0.15 | [28] |
| AP, industrial AP (Nectar) | Pinova, Reinders, Jonagold, Iduna, Braeburn | Methanol:water (80:100, v/v), sample/solvent (1:16 w/v) 150 min, room temperature | Pinova = 7.96 ± 0.37 Reinders = 8.67 ± 0.39 Jonagold = 8.53 ± 0.39 Iduna = 6.47 ± 0.31 Braeburn = 5.59 ± 0.25 Nectar = 4.22 ± 0.18 | [36] |
| AP | Golden Delicious | Methanol, ethanol, acetone, ethyl acetate, chloroform sample/solvent (1:5 w/v), 37 °C, 40 min | Methanol = 3.05 ± 0.82 Ethanol = 2.87 ± 0.75 Acetone = 2.15 ± 0.35 Ethyl acetate = 2.51 ± 0.42 chloroform = 1.62 ± 0.23 | [37] |
| Industrial AP Fraction A, Fraction B, Fraction C, Fraction D Industrial AP (Fraction D) | Reinette Hernaut, Cox, Topaz | Ethanol:water (50:50, v/v), sample/solvent (1:80 w/v), 20 min, 60 °C | Fraction A = 8.60 ± 0.26 Fraction B = 9.56 ± 0.22 Fraction C = 7.14 ± 0.29 Fraction D = 10.78 ± 0.94 | Present study |
| Subcritical water extraction, sample/solvent: (1:100 w/v), 75 min, 203.71 °C, mean sample particle size: 500 µm | Fraction D = 39.08 ± 1.10 | Present study |
2.2.2. Subcritical Water Extraction
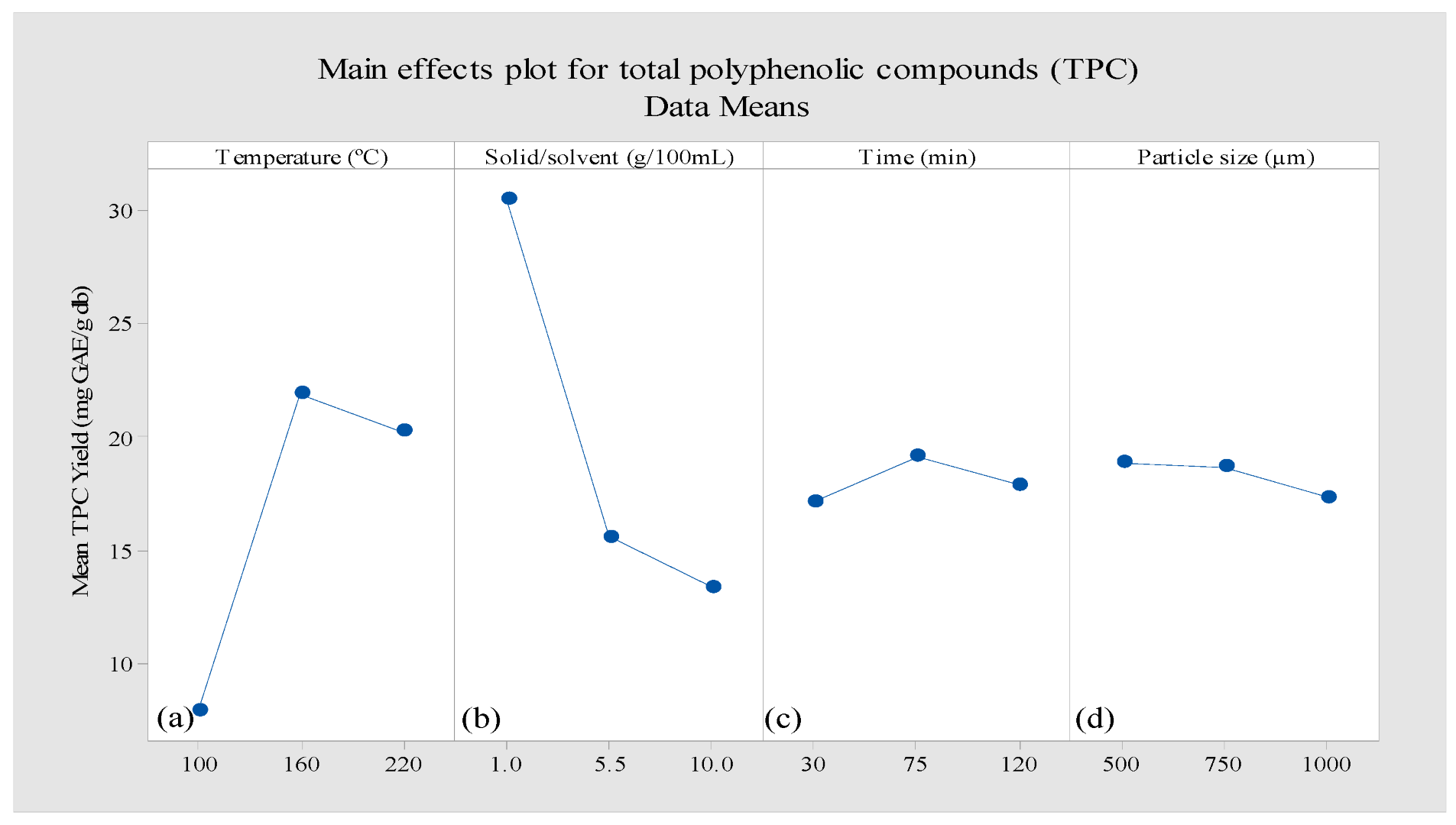
Independent Effects of Process Variables on TPC
Interaction Effects of Variation in the Process Variables on TPC
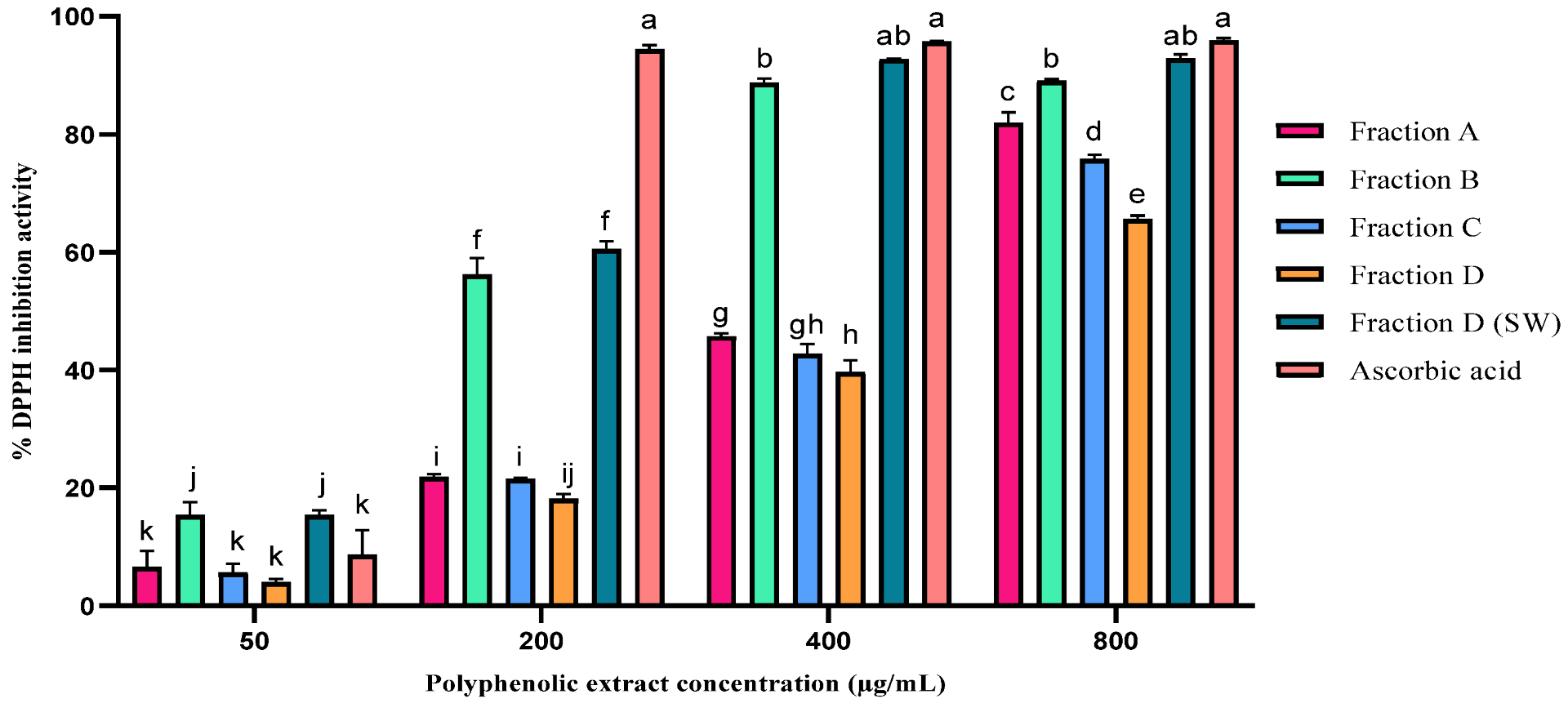
2.3. Antioxidant Activity of the Polyphenolic Extract
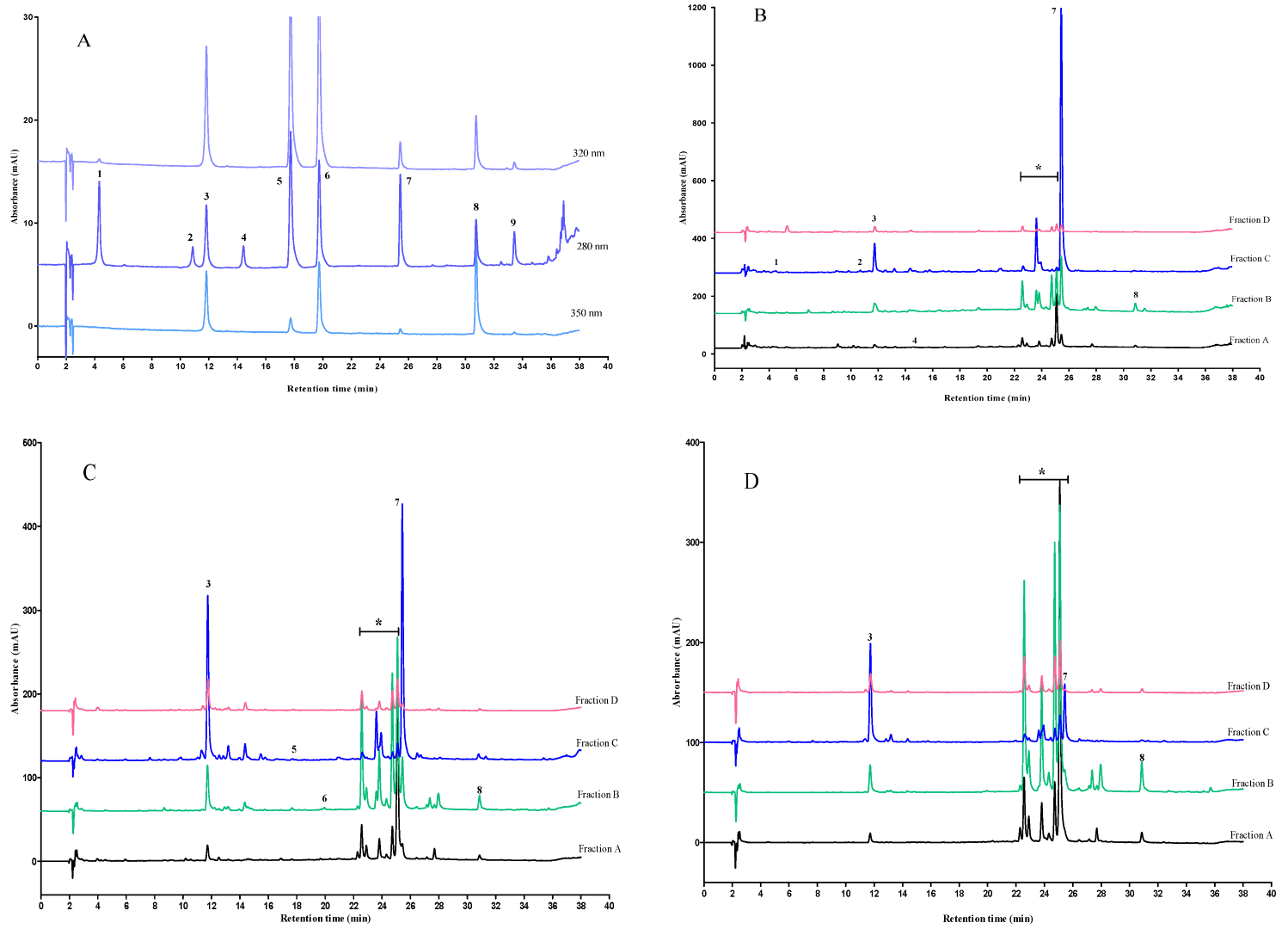
2.4. HPLC analysis of Polyphenolic Compounds
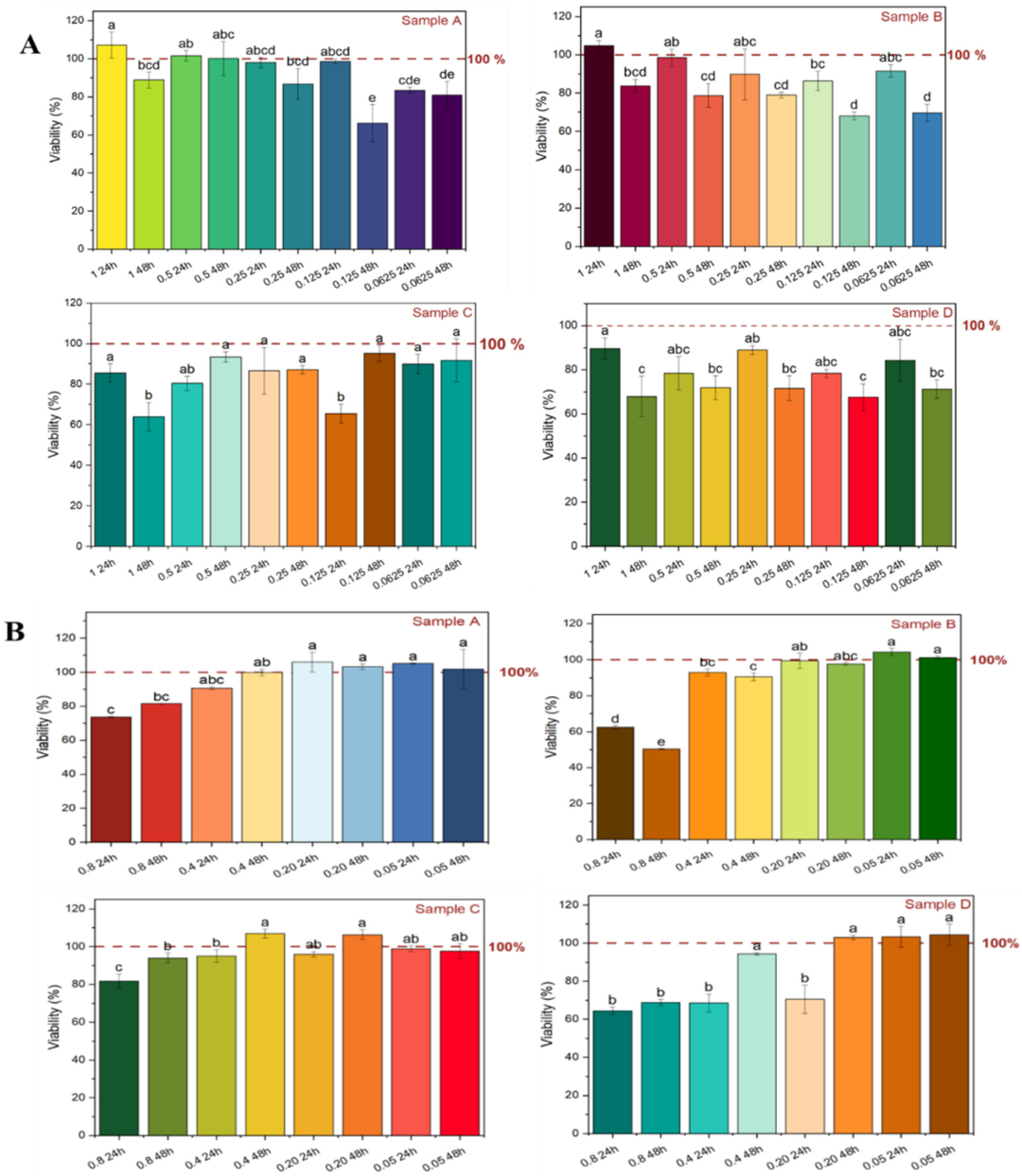
2.5. Cell Viability of the Polyphenolic Extract
3. Materials and Methods
3.1. Materials
3.2. Physicochemical Characterization
3.3. Extraction Procedure of Polyphenolic Compounds
3.3.1. Ethanol-Water Extraction of Polyphenolic Compounds
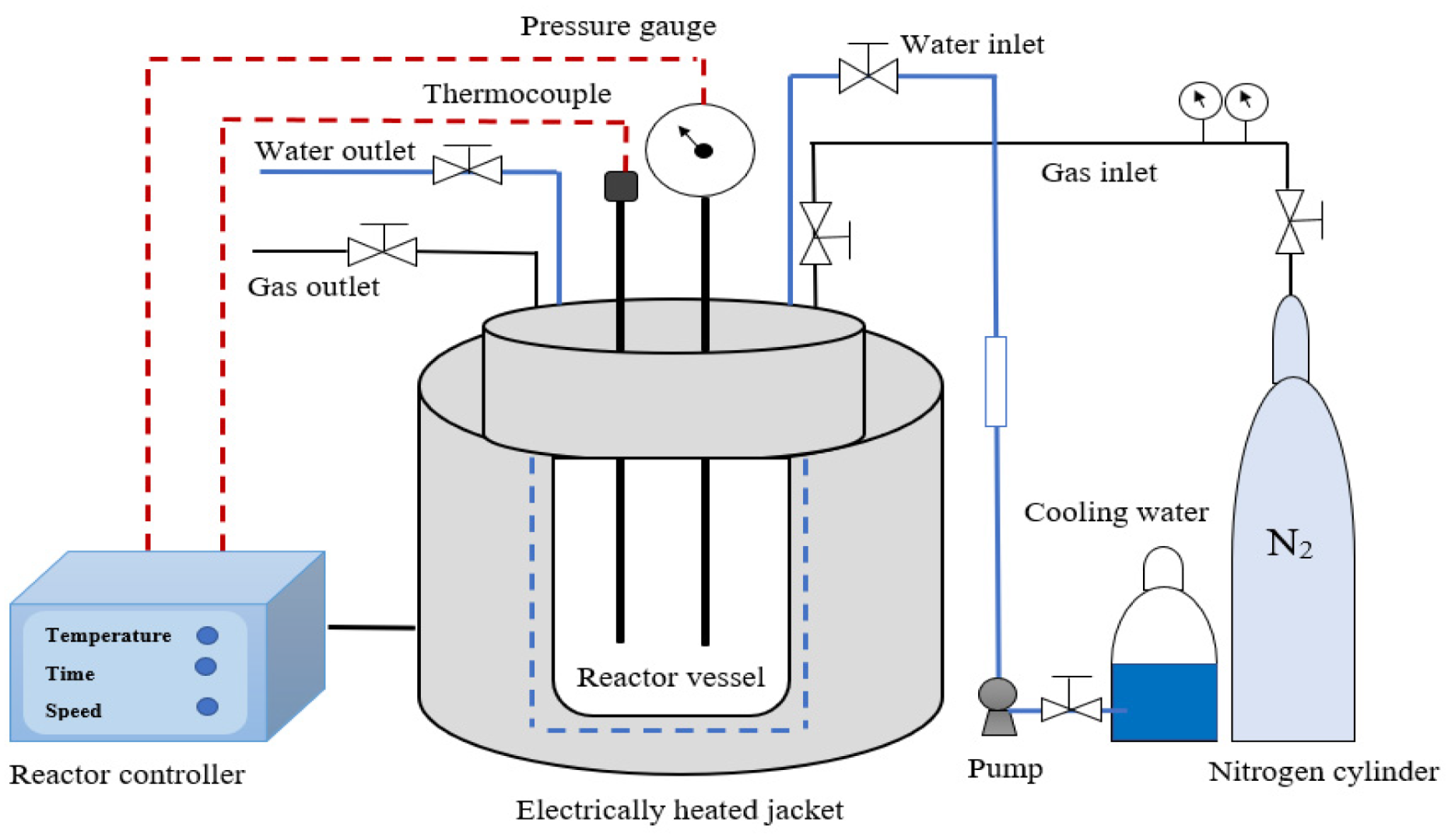
3.3.2. Subcritical Water Extraction of Polyphenolic Compounds
3.4. Measurement of Total Polyphenolic Content (TPC)
3.5. HPLC-DAD/UV Identification of Polyphenolic Compounds
3.6. Measurement of the Antioxidant Activity
3.7. In Vitro Cell Viability
3.8. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Van Dyk, J.S.; Gama, R.; Morrison, D.; Swart, S.; Pletschke, B.I. Food processing waste: Problems, current management and prospects for utilisation of the lignocellulose component through enzyme synergistic degradation. Renew. Sustain. Energy Rev. 2013, 26, 521–531. [Google Scholar] [CrossRef]
- Martau, G.A.; Teleky, B.E.; Ranga, F.; Pop, I.D.; Vodnar, D.C. Apple Pomace as a Sustainable Substrate in Sourdough Fermentation. Front. Microbiol. 2021, 12, 742020. [Google Scholar] [CrossRef] [PubMed]
- Okoro, O.V.; Amenaghawon, A.; Podstawczyk, D.; Alimoradi, H.; Khalili, M.R.; Anwar, M.; Milan, P.B.; Nie, L.; Shavandi, A. Fruit pomace-lignin as a sustainable biopolymer for biomedical applications. J. Clean. Prod. 2021, 328, 129498. [Google Scholar] [CrossRef]
- Okoro, O.V.; Shavandi, A. An assessment of the utilization of waste apple slurry in bio-succinic acid and bioenergy production. Int. J. Environ. Sci. Technol. 2021, 19, 1323–1334. [Google Scholar] [CrossRef]
- Okoro, O.V.; Nie, L.; Hobbi, P.; Shavandi, A. Valorization of Waste Apple Pomace for Production of Platform Biochemicals: A Multi-Objective Optimization Study. Waste Biomass Valor 2021, 12, 6887–6901. [Google Scholar] [CrossRef]
- Panzella, L.; Moccia, F.; Nasti, R.; Marzorati, S.; Verotta, L.; Napolitano, A. Bioactive Phenolic Compounds From Agri-Food Wastes: An Update on Green and Sustainable Extraction Methodologies. Front. Nutr. 2020, 7, 60. [Google Scholar] [CrossRef]
- Barreira, J.C.M.; Arraibi, A.A.; Ferreira, I.C.F.R. Bioactive and functional compounds in apple pomace from juice and cider manufacturing: Potential use in dermal formulations. Trends Food Sci. Technol. 2019, 90, 76–87. [Google Scholar] [CrossRef]
- Perussello, C.A.; Zhang, Z.; Marzocchella, A.; Tiwari, B.K. Valorization of Apple Pomace by Extraction of Valuable Compounds. Compr. Rev. Food Sci. Food Saf. 2017, 16, 776–796. [Google Scholar] [CrossRef]
- Bai, X.; Zhang, H.; Ren, S. Antioxidant activity and HPLC analysis of polyphenol-enriched extracts from industrial apple pomace. J. Sci. Food Agric. 2013, 93, 2502–2506. [Google Scholar] [CrossRef]
- Zardo, D.M.; Alberti, A.; Zielinski, A.A.F.; Prestes, A.A.; Esmerino, L.A.; Nogueira, A. Influence of solvents in the extraction of phenolic compounds with antibacterial activity from apple pomace. Sep. Sci. Technol. 2020, 56, 903–911. [Google Scholar] [CrossRef]
- Suárez, B.; Álvarez, Á.L.; García, Y.D.; Barrio, G.d.; Lobo, A.P.; Parra, F. Phenolic profiles, antioxidant activity and in vitro antiviral properties of apple pomace. Food Chem. 2010, 120, 339–342. [Google Scholar] [CrossRef]
- Milan, E.P.; Bertolo, M.R.V.; Martins, V.C.A.; Sobrero, C.E.; Plepis, A.M.G.; Fuhrmann-Lieker, T.; Horn, M.M. Effects of Mangosteen Peel Phenolic Compounds on Tilapia Skin Collagen-Based Mineralized Scaffold Properties. ACS Omega 2022, 7, 34022–34033. [Google Scholar] [CrossRef] [PubMed]
- Nie, L.; Cai, C.; Sun, M.; Zhang, F.; Zheng, L.; Peng, Q.; Shavandi, A.; Yang, S. Iron Oxide Nanoparticles Synthesized Via Green Tea Extract for Doxorubicin Delivery. Curr. Nanosci. 2021, 17, 646–657. [Google Scholar] [CrossRef]
- Hashemi Poor, M.H.; Hosseinzadeh, S.; Aminlari, M. Wound healing potential of pomegranate peel extract in human dermal fibroblasts through regulating the expression of FN1 gene. S. Afr. J. Bot. 2022, 146, 222–229. [Google Scholar] [CrossRef]
- Ahmadian-Kouchaksaraie, Z.; Niazmand, R.; Najafi, M.N. Optimization of the subcritical water extraction of phenolic antioxidants from Crocus sativus petals of saffron industry residues: Box-Behnken design and principal component analysis. Innov. Food Sci. Emerg. Technol. 2016, 36, 234–244. [Google Scholar] [CrossRef]
- Kheirkhah, H.; Baroutian, S.; Quek, S.Y. Evaluation of bioactive compounds extracted from Hayward kiwifruit pomace by subcritical water extraction. Food Bioprod. Process. 2019, 115, 143–153. [Google Scholar] [CrossRef]
- Munir, M.T.; Kheirkhah, H.; Baroutian, S.; Quek, S.Y.; Young, B.R. Subcritical water extraction of bioactive compounds from waste onion skin. J. Clean. Prod. 2018, 183, 487–494. [Google Scholar] [CrossRef]
- Guerrero, M.B.; da Silva Paula, M.M.; Zaragoza, M.M.; Gutiérrez, J.S.; Velderrain, V.G.; Ortiz, A.L.; Collins-Martínez, V. Thermogravimetric study on the pyrolysis kinetics of apple pomace as waste biomass. J. Hydrogen Energy 2014, 39, 16619–16627. [Google Scholar] [CrossRef]
- Gowman, A.C.; Picard, M.C.; Rodriguez-Uribe, A.; Misra, M.; Khalil, H.; Thimmanagari, M.; Mohanty, A.K. Physicochemical analysis of apple and grape pomaces. BioResources 2019, 14, 3210–3230. [Google Scholar] [CrossRef]
- Verma, V.K.; Bram, S.; Gauthier, G.; De Ruyck, J. Evaluation of the performance of a multi-fuel domestic boiler with respect to the existing European standard and quality labels: Part-1. Biomass Bioenergy 2011, 35, 80–89. [Google Scholar] [CrossRef]
- Vidović, S.; Horecki, A.T.; Vladić, J.; Šumić, Z.; Gavarić, A.; Vakula, A. Apple. In Valorization of Fruit Processing By-Products; Elsevier: Amsterdam, The Netherlands, 2020; pp. 17–42. [Google Scholar]
- Wosiacki, G.; Sato, M.F.; Vieira, R.G.; Zardo, D.M.; Falcão, L.D.; Nogueira, A. Apple pomace from eleven cultivars: An approach to identify sources of bioactive compounds. Acta Sci. Agron. 2010, 32, 29–35. [Google Scholar] [CrossRef]
- Lavelli, V.; Kerr, W. Apple pomace is a good matrix for phytochemical retention. J. Agric. Food Chem. 2012, 60, 5660–5666. [Google Scholar] [CrossRef] [PubMed]
- Antonic, B.; Jancikova, S.; Dordevic, D.; Tremlova, B. Apple pomace as food fortification ingredient: A systematic review and meta-analysis. J. Food Sci. 2020, 85, 2977–2985. [Google Scholar] [CrossRef] [PubMed]
- Romelle, F.D.; Rani, A.; Manohar, R.S. Chemical composition of some selected fruit peels. Eur. J. Food Sci. Technol. 2016, 4, 12–21. [Google Scholar]
- Bhushan, S.; Kalia, K.; Sharma, M.; Singh, B.; Ahuja, P.S. Processing of apple pomace for bioactive molecules. Crit. Rev. Biotechnol. 2008, 28, 285–296. [Google Scholar] [CrossRef]
- Giomaro, G.; Karioti, A.; Bilia, A.R.; Bucchini, A.; Giamperi, L.; Ricci, D.; Fraternale, D. Polyphenols profile and antioxidant activity of skin and pulp of a rare apple from Marche region (Italy). Chem. Cent. J. 2014, 8, 45. [Google Scholar] [CrossRef]
- Wolfe, K.; Xianzhong, W.; Rui, H.L. Antioxidant activity of apple peels. J. Agric. Food Chem. 2003, 51, 609–614. [Google Scholar] [CrossRef]
- Butkeviciute, A.; Petrikaite, V.; Jurgaityte, V.; Liaudanskas, M.; Janulis, V. Antioxidant, Anti-Inflammatory, and Cytotoxic Activity of Extracts from Some Commercial Apple Cultivars in Two Colorectal and Glioblastoma Human Cell Lines. Antioxidants 2021, 10, 1098. [Google Scholar] [CrossRef]
- Kalinowska, M.; Gryko, K.; Wroblewska, A.M.; Jablonska-Trypuc, A.; Karpowicz, D. Phenolic content, chemical composition and anti-/pro-oxidant activity of Gold Milenium and Papierowka apple peel extracts. Sci. Rep. 2020, 10, 14951. [Google Scholar] [CrossRef]
- Gunes, R.; Palabiyik, I.; Toker, O.S.; Konar, N.; Kurultay, S. Incorporation of defatted apple seeds in chewing gum system and phloridzin dissolution kinetics. J. Food Eng. 2019, 255, 9–14. [Google Scholar] [CrossRef]
- Zhang, G.Z.; Ji, B.P.; Chen, G. Antioxidant Activities and Phenolic Composition of Apple Peel, Core and Flesh Extracts on Selected Apple Cultivars. Adv. Mater. Res. 2012, 554–556, 1103–1109. [Google Scholar] [CrossRef]
- Manzoor, M.; Singh, J.; Gani, A. Characterization of apple (Malus domestica) seed flour for its structural and nutraceutical potential. LWT 2021, 151, 112138. [Google Scholar] [CrossRef]
- Valavanidis, A.; Vlachogianni, T.; Psomas, A.; Zovoili, A.; Siatis, V. Polyphenolic profile and antioxidant activity of five apple cultivars grown under organic and conventional agricultural practices. Int. J. Food Sci. Technol. 2009, 44, 1167–1175. [Google Scholar] [CrossRef]
- Wolfe, K.L.; Liu, R.H. Apple peels as a value-added food ingredient. J. Agric. Food Chem. 2003, 51, 1676–1683. [Google Scholar] [CrossRef]
- Cetkovic, G.; Canadanovic-Brunet, J.; Djilas, S.; Savatovic, S.; Mandic, A.; Tumbas, V. Assessment of polyphenolic content and in vitro antiradical characteristics of apple pomace. Food Chem. 2008, 109, 340–347. [Google Scholar] [CrossRef]
- Zhang, T.; Wei, X.; Miao, Z.; Hassan, H.; Song, Y.; Fan, M. Screening for antioxidant and antibacterial activities of phenolics from Golden Delicious apple pomace. Chem. Cent. J. 2016, 10, 47. [Google Scholar] [CrossRef]
- Okoro, O.V.; Nie, L.; Alimoradi, H.; Shavandi, A.J.F. Waste Apple Pomace Conversion to Acrylic Acid: Economic and Potential Environmental Impact Assessments. Fermentation 2022, 8, 21. [Google Scholar] [CrossRef]
- Halim, N.A.A.; Abidin, Z.Z.; Siajam, S.I.; Hean, C.G.; Harun, M.R. Optimization studies and compositional analysis of subcritical water extraction of essential oil from Citrus hystrix DC. leaves. J. Supercrit. Fluids 2021, 178, 105384. [Google Scholar] [CrossRef]
- Zhang, J.; Wen, C.; Zhang, H.; Duan, Y.; Ma, H. Recent advances in the extraction of bioactive compounds with subcritical water: A review. Trends Food Sci. Technol. 2020, 95, 183–195. [Google Scholar] [CrossRef]
- Teo, C.C.; Tan, S.N.; Yong, J.W.H.; Hew, C.S.; Ong, E.S. Pressurized hot water extraction (PHWE). J. Chromatogr. A 2010, 1217, 2484–2494. [Google Scholar] [CrossRef]
- Xu, H.; Wang, W.; Liu, X.; Yuan, F.; Gao, Y. Antioxidative phenolics obtained from spent coffee grounds (Coffea arabica L.) by subcritical water extraction. Ind. Crops Prod. 2015, 76, 946–954. [Google Scholar] [CrossRef]
- Gong, Y.; Zhang, X.; He, L.; Yan, Q.; Yuan, F.; Gao, Y. Optimization of subcritical water extraction parameters of antioxidant polyphenols from sea buckthorn (Hippophae rhamnoides L.) seed residue. J. Food Sci. Technol. 2015, 52, 1534–1542. [Google Scholar] [CrossRef] [PubMed]
- Tomšik, A.; Pavlić, B.; Vladić, J.; Cindrić, M.; Jovanov, P.; Sakač, M.; Mandić, A.; Vidović, S. Subcritical water extraction of wild garlic (Allium ursinum L.) and process optimization by response surface methodology. J. Supercrit. Fluids 2017, 128, 79–88. [Google Scholar] [CrossRef]
- Tunchaiyaphum, S.; Eshtiaghi, M.N.; Yoswathana, N. Extraction of Bioactive Compounds from Mango Peels Using Green Technology. Int. J. Chem. Eng. Appl. 2013, 4, 194–198. [Google Scholar] [CrossRef]
- Leontowicz, M.; Gorinstein, S.; Leontowicz, H.; Krzeminski, R.; Lojek, A.; Katrich, E.; Číž, M.; Martin-Belloso, O.; Soliva-Fortuny, R.; Haruenkit, R. Apple and pear peel and pulp and their influence on plasma lipids and antioxidant potentials in rats fed cholesterol-containing diets. J. Agric. Food Chem. 2003, 51, 5780–5785. [Google Scholar] [CrossRef]
- Gonçalves Rodrigues, L.G.; Mazzutti, S.; Vitali, L.; Micke, G.A.; Ferreira, S.R.S. Recovery of bioactive phenolic compounds from papaya seeds agroindustrial residue using subcritical water extraction. Biocatal. Agric. Biotechnol. 2019, 22, 101367. [Google Scholar] [CrossRef]
- Mihailović, N.R.; Mihailović, V.B.; Kreft, S.; Ćirić, A.R.; Joksović, L.G.; Đurđević, P.T. Analysis of phenolics in the peel and pulp of wild apples (Malus sylvestris (L.) Mill.). J. Food Compos. Anal. 2018, 67, 1–9. [Google Scholar] [CrossRef]
- Chinnici, F.; Bendini, A.; Gaiani, A.; Riponi, C. Radical scavenging activities of peels and pulps from cv. Golden Delicious apples as related to their phenolic composition. J. Agric. Food Chem. 2004, 52, 4684–4689. [Google Scholar] [CrossRef]
- Lu, Y.; Foo, L.Y. Antioxidant and radical scavenging activities of polyphenols from apple pomace. Food Chem. 2000, 68, 81–85. [Google Scholar] [CrossRef]
- Nile, A.; Nile, S.H.; Shin, J.; Park, G.; Oh, J.W. Quercetin-3-Glucoside Extracted from Apple Pomace Induces Cell Cycle Arrest and Apoptosis by Increasing Intracellular ROS Levels. Int. J. Mol. Sci. 2021, 22, 10749. [Google Scholar] [CrossRef]
- Barbosa, C.H.; Andrade, M.A.; Sendon, R.; Silva, A.S.; Ramos, F.; Vilarinho, F.; Khwaldia, K.; Barbosa-Pereira, L. Industrial Fruits By-Products and Their Antioxidant Profile: Can They Be Exploited for Industrial Food Applications? Foods 2021, 10, 272. [Google Scholar] [CrossRef] [PubMed]
- Duda-Chodak, A.; Tarko, T.; Tuszyński, T. Antioxidant activity of apples–an impact of maturity stage and fruit part. Acta Sci. Pol. Technol. Aliment. 2011, 10, 443–454. [Google Scholar] [PubMed]
- Górnaś, P.; Mišina, I.; Olšteine, A.; Krasnova, I.; Pugajeva, I.; Lācis, G.; Siger, A.; Michalak, M.; Soliven, A.; Segliņa, D. Phenolic compounds in different fruit parts of crab apple: Dihydrochalcones as promising quality markers of industrial apple pomace by-products. Ind. Crops Prod. 2015, 74, 607–612. [Google Scholar] [CrossRef]
- Kschonsek, J.; Wolfram, T.; Stockl, A.; Bohm, V. Polyphenolic Compounds Analysis of Old and New Apple Cultivars and Contribution of Polyphenolic Profile to the In Vitro Antioxidant Capacity. Antioxidants 2018, 7, 20. [Google Scholar] [CrossRef] [PubMed]
- Alonso-Salces, R.M.; Korta, E.; Barranco, A.; Berrueta, L.; Gallo, B.; Vicente, F. Pressurized liquid extraction for the determination of polyphenols in apple. J. Chromatogr. A 2001, 933, 37–43. [Google Scholar] [CrossRef]
- Massini, L.; Rico, D.; Martin-Diana, A.B. Quality attributes of apple juice: Role and effect of phenolic compounds. In Fruit Juices; Elsevier: Amsterdam, The Netherlands, 2018; pp. 45–57. [Google Scholar]
- Diñeiro García, Y.; Valles, B.S.; Picinelli Lobo, A. Phenolic and antioxidant composition of by-products from the cider industry: Apple pomace. Food Chem. 2009, 117, 731–738. [Google Scholar] [CrossRef]
- Lavelli, V.; Corti, S. Phloridzin and other phytochemicals in apple pomace: Stability evaluation upon dehydration and storage of dried product. Food Chem. 2011, 129, 1578–1583. [Google Scholar] [CrossRef]
- Arraibi, A.A.; Liberal, A.; Dias, M.I.; Alves, M.J.; Ferreira, I.; Barros, L.; Barreira, J.C.M. Chemical and Bioactive Characterization of Spanish and Belgian Apple Pomace for Its Potential Use as a Novel Dermocosmetic Formulation. Foods 2021, 10, 1949. [Google Scholar] [CrossRef]
- Karaman, Ş.; Tütem, E.; Başkan, K.S.; Apak, R. Comparison of antioxidant capacity and phenolic composition of peel and flesh of some apple varieties. J. Sci. Food Agric. 2013, 93, 867–875. [Google Scholar] [CrossRef]
- Everette, J.D.; Bryant, Q.M.; Green, A.M.; Abbey, Y.A.; Wangila, G.W.; Walker, R.B. Thorough study of reactivity of various compound classes toward the Folin− Ciocalteu reagent. J. Agric. Food Chem. 2010, 58, 8139–8144. [Google Scholar] [CrossRef]
- Prior, R.L.; Wu, X.; Schaich, K. Standardized methods for the determination of antioxidant capacity and phenolics in foods and dietary supplements. J. Agric. Food Chem. 2005, 53, 4290–4302. [Google Scholar] [CrossRef] [PubMed]
- Ho, H.-Y.; Wei, T.-T.; Cheng, M.-L.; Chiu, D.T.-Y. Green tea polyphenol epigallocatechin-3-gallate protects cells against peroxynitrite-induced cytotoxicity: Modulatory effect of cellular G6PD status. J. Agric. Food Chem. 2006, 54, 1638–1645. [Google Scholar] [CrossRef] [PubMed]
- Spencer, J.P.; Schroeter, H.; Kuhnle, G.; Srai, S.K.S.; Tyrrell, R.M.; Hahn, U.; Rice-Evans, C. Epicatechin and its in vivo metabolite, 3′-O-methyl epicatechin, protect human fibroblasts from oxidative-stress-induced cell death involving caspase-3 activation. Biochem. J. 2001, 354, 493–500. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, T.; Staples, J.; Wataha, J.; Lewis, J.; Lockwood, P.; Schoenlein, P.; Rao, S.; Osaki, T.; Dickinson, D.; Kamatani, T. Protective effects of EGCG on salivary gland cells treated with γ-radiation or cis-platinum (II) diammine dichloride. Anticancer Res. 2004, 24, 3065–3074. [Google Scholar] [PubMed]
- Hedges, J.; Baldock, J.; Gélinas, Y.; Lee, C.; Peterson, M.; Wakeham, S. The biochemical and elemental compositions of marine plankton: A NMR perspective. Mar. Chem. 2002, 78, 47–63. [Google Scholar] [CrossRef]
- ASTM E1756-08(2015); Standard Test Method for Determination of Total Solids in Biomass. ASTM International: West Conshohocken, PA, USA, 2015.
- ASTM D2017-05; Standard Test Method of Accelerated Laboratory Test of Natural Decay Resistance of Woods. ASTM International: West Conshohocken, PA, USA, 2005.
- Kim, J.; Choi, K.; Chung, D. Sample preparation for capillary electrophoretic applications. Compr. Sampl. Sample Prep. 2012, 3, 701–721. [Google Scholar] [CrossRef]
- Carrier, M.; Loppinet-Serani, A.; Denux, D.; Lasnier, J.-M.; Ham-Pichavant, F.; Cansell, F.; Aymonier, C. Thermogravimetric analysis as a new method to determine the lignocellulosic composition of biomass. Biomass Bioenergy 2011, 35, 298–307. [Google Scholar] [CrossRef]
- Okoro, O.; Sun, Z.; Birch, J. Prognostic Assessment of the Viability of Hydrothermal Liquefaction as a Post-Resource Recovery Step after Enhanced Biomethane Generation Using Co-Digestion Technologies. Appl. Sci. 2018, 8, 2290. [Google Scholar] [CrossRef]
- Hobbi, P.; Okoro, O.V.; Delporte, C.; Alimoradi, H.; Podstawczyk, D.; Nie, L.; Bernaerts, K.V.; Shavandi, A. Kinetic modelling of the solid–liquid extraction process of polyphenolic compounds from apple pomace: Influence of solvent composition and temperature. Bioresour. Bioprocess. 2021, 8, 114. [Google Scholar] [CrossRef]
- He, L.; Zhang, X.; Xu, H.; Xu, C.; Yuan, F.; Knez, Ž.; Novak, Z.; Gao, Y. Subcritical water extraction of phenolic compounds from pomegranate (Punica granatum L.) seed residues and investigation into their antioxidant activities with HPLC–ABTS+ assay. Food Bioprod. Process. 2012, 90, 215–223. [Google Scholar] [CrossRef]
- Pavlić, B.; Vidović, S.; Vladić, J.; Radosavljević, R.; Cindrić, M.; Zeković, Z. Subcritical water extraction of sage (Salvia officinalis L.) by-products—Process optimization by response surface methodology. J. Supercrit. Fluids 2016, 116, 36–45. [Google Scholar] [CrossRef]
- Li, W.; Yang, R.; Ying, D.; Yu, J.; Sanguansri, L.; Augustin, M.A. Analysis of polyphenols in apple pomace: A comparative study of different extraction and hydrolysis procedures. Ind. Crops Prod. 2020, 147, 112250. [Google Scholar] [CrossRef]
- De Torre, M.P.; Cavero, R.Y.; Calvo, M.I.; Vizmanos, J.L. A Simple and a Reliable Method to Quantify Antioxidant Activity In Vivo. Antioxidants 2019, 8, 142. [Google Scholar] [CrossRef] [PubMed]
- Serra, A.T.; Poejo, J.; Matias, A.A.; Bronze, M.R.; Duarte, C.M.M. Evaluation of Opuntia spp. derived products as antiproliferative agents in human colon cancer cell line (HT29). Food Res. Int. 2013, 54, 892–901. [Google Scholar] [CrossRef]

| AP Characterization | Fraction A | Fraction B | Fraction C | Fraction D |
|---|---|---|---|---|
| Moisture (% w/w, wet AP basis) | 67.31 ± 1.06 | 82.77 ± 1.03 | 62.00 ± 0.35 | 85.57 ± 1.37 |
| Lipid (% w/w, dry AP basis) | 1.29 ± 0.52 | 14.80 ± 0.41 | 6.77 ± 0.34 | 5.44 ± 0.04 |
| Carbohydrate (% w/w, dry AP basis) | 71.94 ± 1.30 | 65.9 ± 4.54 | 57.54 ± 5.15 | 71.77 ± 1.12 |
| Protein (% w/w, dry AP basis) | 5.94 ± 0.20 | 4.38 ± 0.00 | 11.88 ± 0.88 | 2.50 ± 0.88 |
| Lignin (% w/w, dry AP basis) | 19.53 ± 1.18 | 14.48 ± 4.54 | 23.82 ± 3.92 | 20.29 ± 1.97 |
| Ash (% w/w, dry AP basis) | 1.30 ± 0.00 | 0.44 ± 0.01 | 1.33 ± 0.05 | 1.08 ± 0.04 |
| Carbon (% w/w, dry AP basis) | 46.15 ± 0.64 | 63.33 ± 0.54 | 44.42 ± 0.44 | 43.90 ± 2.83 |
| Hydrogen (% w/w, dry AP basis) | 6.87 ± 0.11 | 5.27 ± 0.23 | 6.64 ± 0.06 | 6.31 ± 0.11 |
| Nitrogen (% w/w, dry AP basis) | 0.95 ± 0.03 | 0.70 ± 0.00 | 1.9 ± 0.14 | 0.4 ± 0.14 |
| Oxygen (% w/w, dry AP basis) | 44.67 ± 0.65 | 30.18 ± 0.31 | 45.70 ± 0.46 | 48.29 ± 2.88 |
| Sulfur (% w/w, dry AP basis) | 0.07 ± 0.01 | 0.09 ± 0.01 | 0.03 ± 0.01 | 0.03 ± 0.01 |
| Run | T: Temperature (◦C) | t: Time (min) | S: Mean Sample Particle Size (µm) | W: Solid-to-Solvent Ratio (g/100 mL) | Y: TPC (mg GAE/g db) |
|---|---|---|---|---|---|
| 1 | 100 | 75 | 750 | 10 | 5.30 |
| 2 | 220 | 75 | 500 | 5.5 | 18.90 |
| 3 | 100 | 120 | 750 | 5.5 | 7.19 |
| 4 | 100 | 75 | 1000 | 5.5 | 6.36 |
| 5 | 160 | 120 | 1000 | 5.5 | 18.33 |
| 6 | 160 | 120 | 750 | 1 | 36.26 |
| 7 | 160 | 75 | 750 | 5.5 | 24.13 |
| 8 | 100 | 30 | 750 | 5.5 | 6.38 |
| 9 | 220 | 120 | 750 | 5.5 | 14.02 |
| 10 | 160 | 75 | 500 | 1 | 36.45 |
| 11 | 160 | 120 | 750 | 10 | 15.04 |
| 12 | 160 | 75 | 1000 | 1 | 27.97 |
| 13 | 160 | 30 | 750 | 10 | 12.19 |
| 14 | 100 | 75 | 750 | 1 | 15.58 |
| 15 | 220 | 75 | 750 | 10 | 13.54 |
| 16 | 160 | 75 | 1000 | 10 | 17.98 |
| 17 | 160 | 75 | 750 | 5.5 | 23.33 |
| 18 | 160 | 75 | 750 | 5.5 | 20.10 |
| 19 | 160 | 30 | 1000 | 5.5 | 18.01 |
| 20 | 220 | 75 | 750 | 1 | 39.01 |
| 21 | 160 | 30 | 500 | 5.5 | 18.45 |
| 22 | 160 | 75 | 500 | 10 | 16.44 |
| 23 | 100 | 75 | 500 | 5.5 | 6.63 |
| 24 | 160 | 30 | 750 | 1 | 27.77 |
| 25 | 220 | 30 | 750 | 5.5 | 20.42 |
| 26 | 220 | 75 | 1000 | 5.5 | 15.62 |
| 27 | 160 | 120 | 500 | 5.5 | 16.56 |
| Polyphenolic Compounds | Concentration of Polyphenol in Apple Pomace Fractions (mg/100 g db Extract) | |||
|---|---|---|---|---|
| Fraction A | Fraction B | Fraction C | Fraction D | |
| Phloridzin * | 33.02 ± 1.04 | 167.48 ± 12.93 | 856.87 ± 17.94 | 18.17 ± 0.46 |
| Chlorogenic acid * | 6.89 ± 0.65 | 26.37 ± 1.13 | 97.05 ± 2.45 | 17.26 ± 0.66 |
| Quercetin | 6.42 ± 0.44 | 22.10 ± 1.88 | 0.81 ± 0.36 | 1.84 ± 0.45 |
| Gallic acid | 0.91 ± 0.39 | nd | 1.36 ± 0.37 | 0.84 ± 0.20 |
| p-coumaric acid | 0.22 ± 0.02 | nd | 0.22 ± 0.06 | 0.23 ± 0.11 |
| Ferulic acid | 0.2 ± 0.04 | 0.61 ± 0.03 | nd | nd |
| (+)-catechin * | nd | nd | 6.82 ± 4.17 | nd |
| (−)-epicatechin | 2.98 ± 0.67 | nd | nd | nd |
| Phloretin | nd | nd | nd | nd |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hobbi, P.; Okoro, O.V.; Hajiabbas, M.; Hamidi, M.; Nie, L.; Megalizzi, V.; Musonge, P.; Dodi, G.; Shavandi, A. Chemical Composition, Antioxidant Activity and Cytocompatibility of Polyphenolic Compounds Extracted from Food Industry Apple Waste: Potential in Biomedical Application. Molecules 2023, 28, 675. https://doi.org/10.3390/molecules28020675
Hobbi P, Okoro OV, Hajiabbas M, Hamidi M, Nie L, Megalizzi V, Musonge P, Dodi G, Shavandi A. Chemical Composition, Antioxidant Activity and Cytocompatibility of Polyphenolic Compounds Extracted from Food Industry Apple Waste: Potential in Biomedical Application. Molecules. 2023; 28(2):675. https://doi.org/10.3390/molecules28020675
Chicago/Turabian StyleHobbi, Parinaz, Oseweuba Valentine Okoro, Maryam Hajiabbas, Masoud Hamidi, Lei Nie, Véronique Megalizzi, Paul Musonge, Gianina Dodi, and Amin Shavandi. 2023. "Chemical Composition, Antioxidant Activity and Cytocompatibility of Polyphenolic Compounds Extracted from Food Industry Apple Waste: Potential in Biomedical Application" Molecules 28, no. 2: 675. https://doi.org/10.3390/molecules28020675
APA StyleHobbi, P., Okoro, O. V., Hajiabbas, M., Hamidi, M., Nie, L., Megalizzi, V., Musonge, P., Dodi, G., & Shavandi, A. (2023). Chemical Composition, Antioxidant Activity and Cytocompatibility of Polyphenolic Compounds Extracted from Food Industry Apple Waste: Potential in Biomedical Application. Molecules, 28(2), 675. https://doi.org/10.3390/molecules28020675