ACT001 Relieves NMOSD Symptoms by Reducing Astrocyte Damage with an Autoimmune Antibody
Abstract
1. Introduction
2. Results
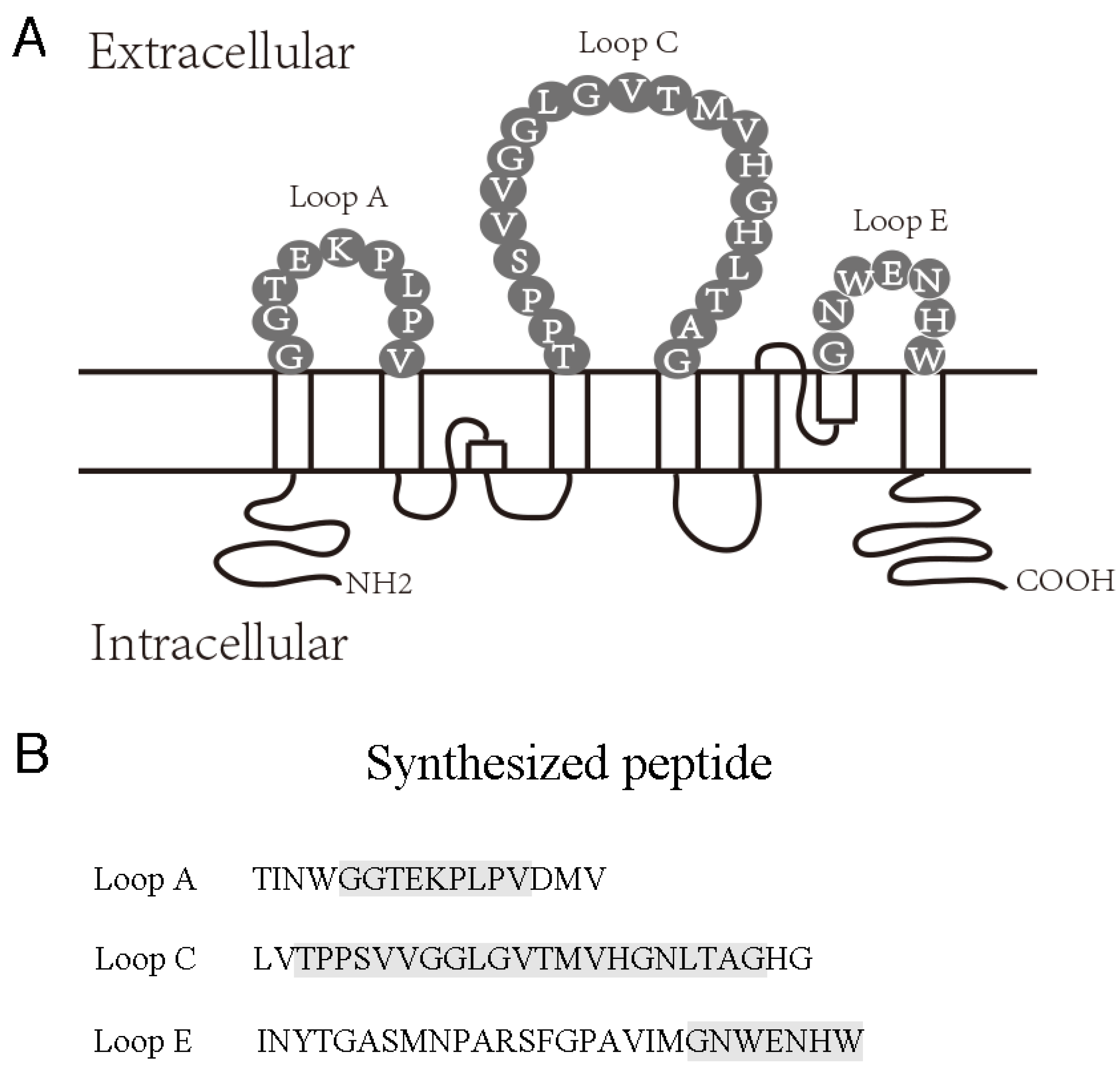
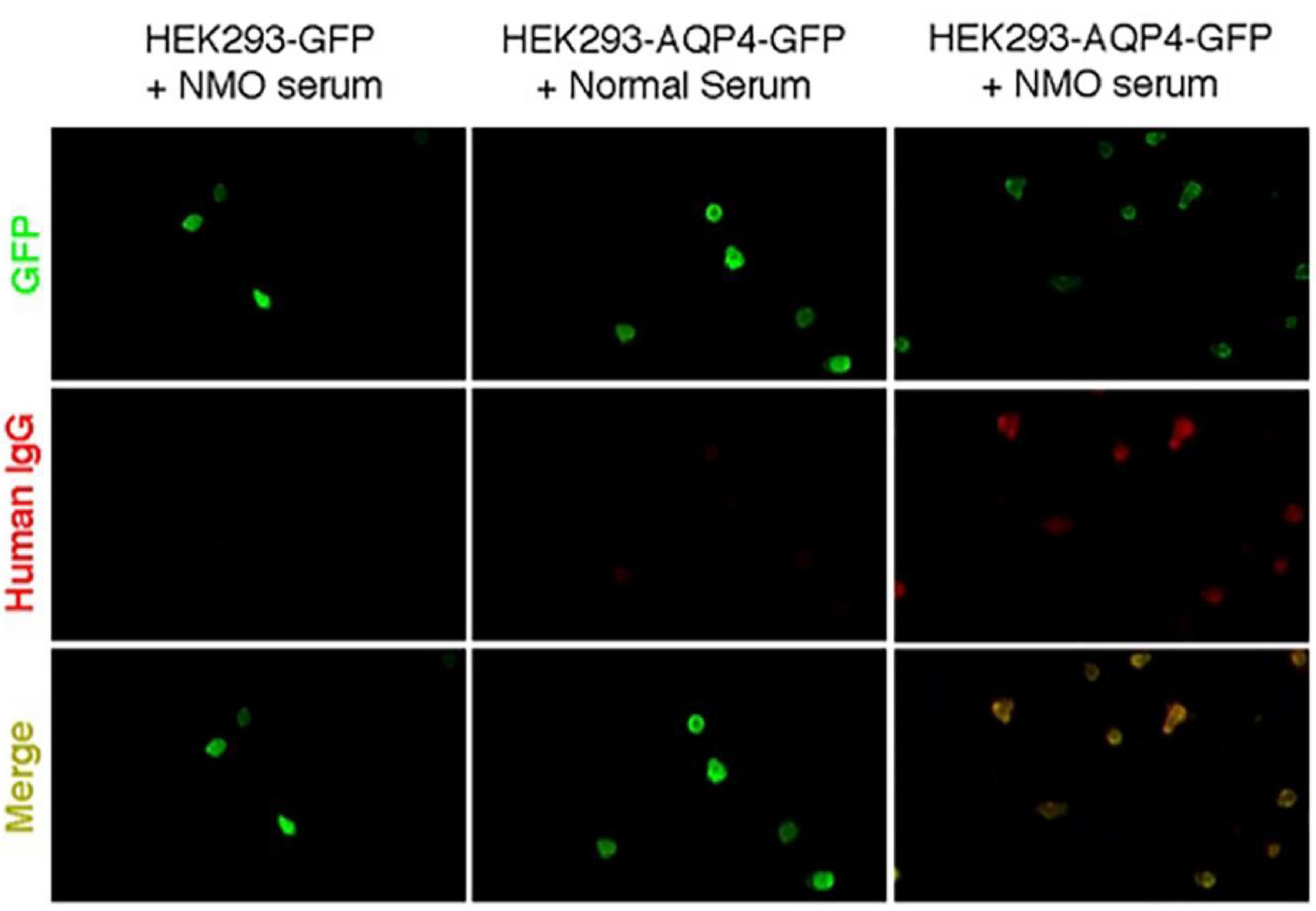
2.1. AQP4 Extracellular Epitope Peptides Reduced the Detectable AQP4-IgG Concentration in NMOSD Patient Serum
2.2. Synthesized Epitope Peptides Reduced the Cytotoxicity of NMOSD Patient Serum on Cultured Astrocytes
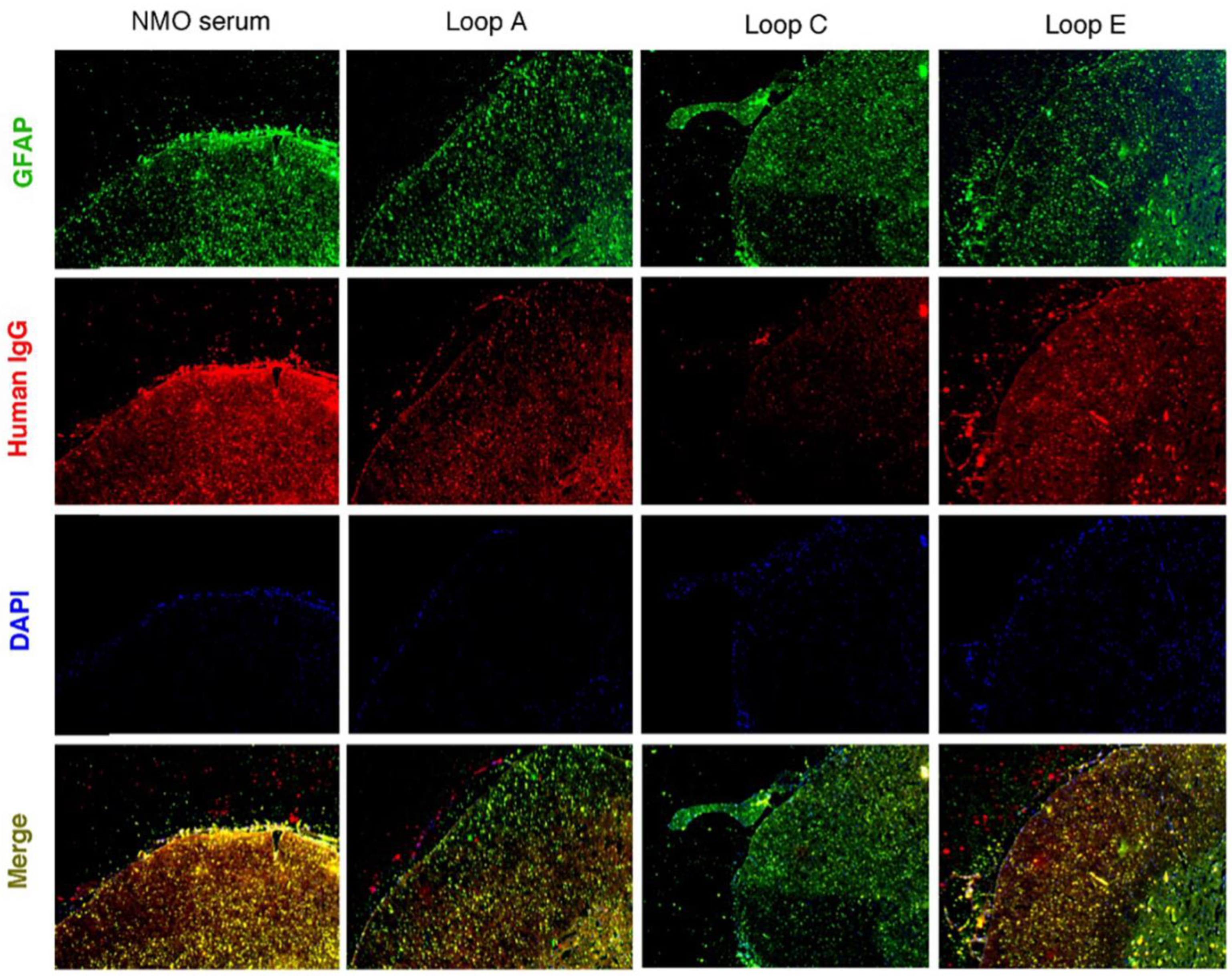
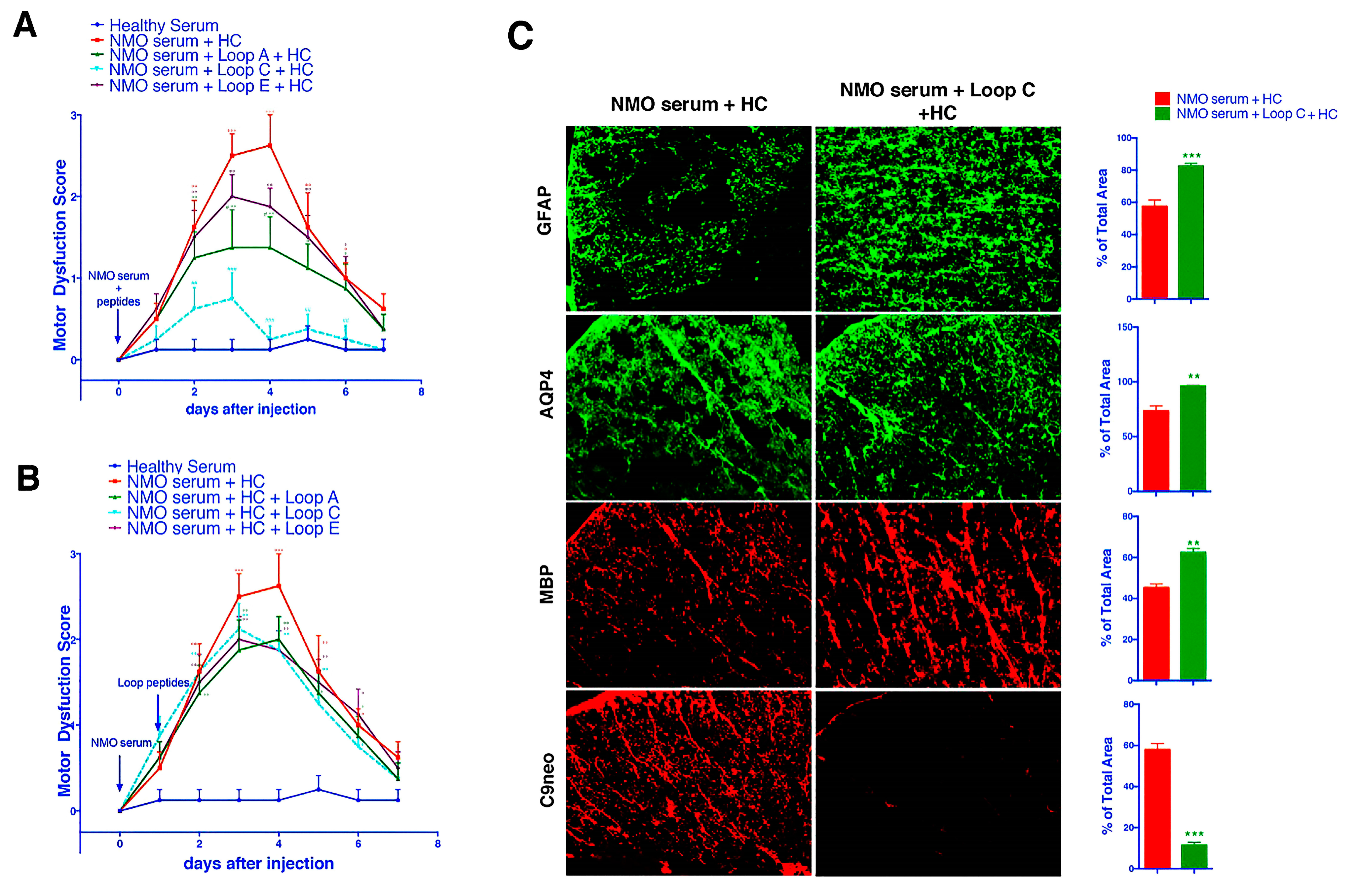
2.3. AQP4 Extracellular Epitope Peptides Blocked the Binding of AQP4-IgG to the Spinal Cord Tissue, Thus Protecting the Animal from the NMOSD Model
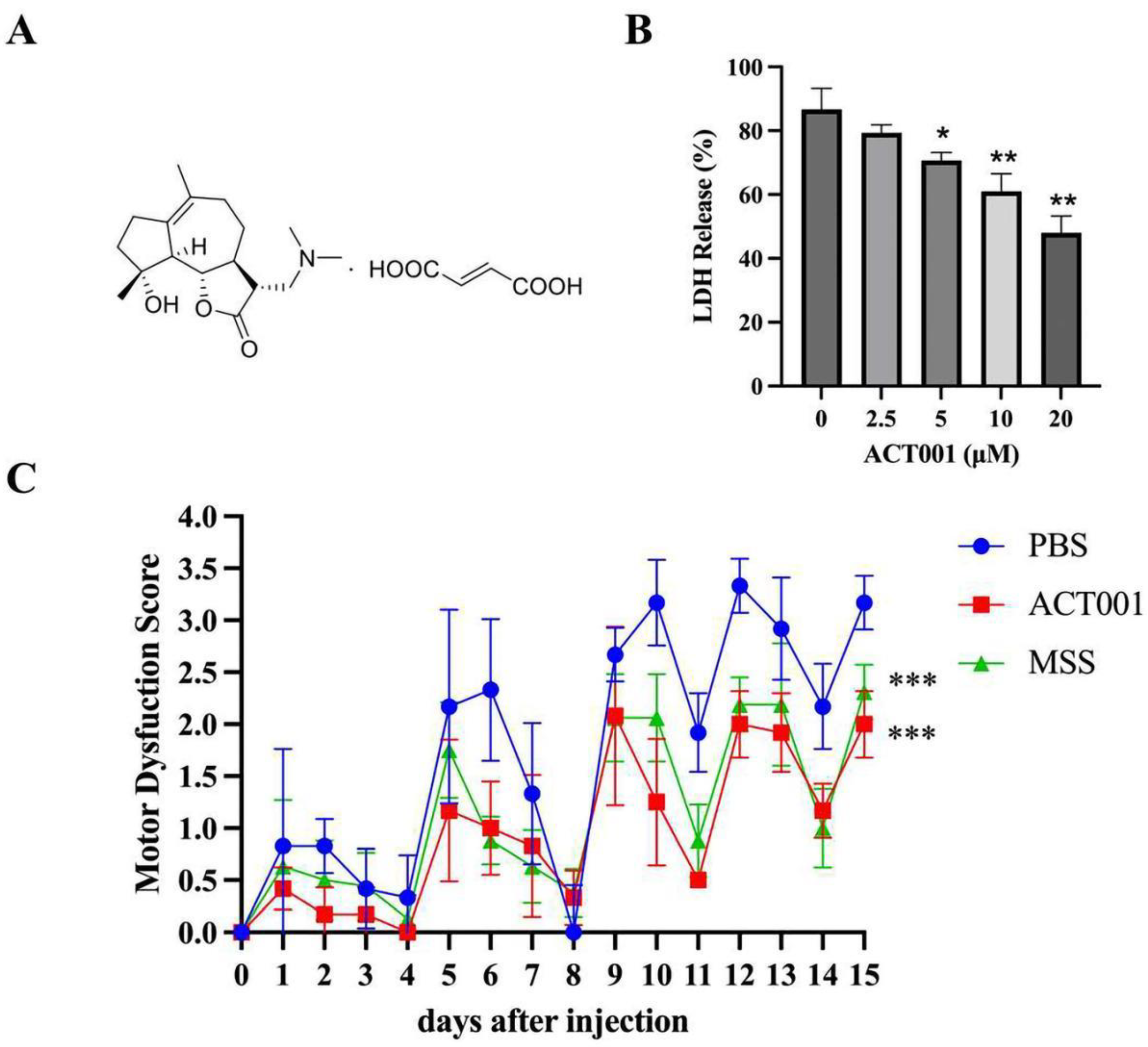
2.4. ACT001 Could Inhibit the Cytotoxicity of AQP4-IgG
2.5. ACT001 Alleviates NMOSD Symptoms in the Animal Model
3. Discussion
4. Materials and Methods
4.1. NMOSD Serum Collection
4.2. Preparation of AQP4 Extracellular Epitope Peptides
4.3. AQP4 Transfection and Indirect Immunofluorescence Cell-Based Assay
4.4. Enzyme-Linked Immunosorbent Assay (ELISA)
4.5. Animals
4.6. Primary Astrocyte Culture
4.7. Cytotoxicity Test
4.8. Live-Astrocyte Immunofluorescence
4.9. Intrathecal Catheter Implantation
4.10. Behavior Test
4.11. Immunofluorescence
4.12. The Therapeutic Effect of ACT001
4.13. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
List of Abbreviations
| NMO | Neuromyelitis optica |
| AQP 4 | Aquaporin 4 |
| ELISA | Enzyme-linked immunosorbent assay |
| CBA | Cell-based assay |
| NMOSD | Neuromyelitis optic spectrum disorder |
| HC | Human complement |
| EAAT2 | Excitatory Amino Acid Transporter 2 |
| MSS | Methylprednisolone sodium succinate |
References
- Wingerchuk, D.M.; Lennon, V.A.; Lucchinetti, C.F.; Pittock, S.J.; Weinshenker, B.G. The spectrum of neuromyelitis optica. Lancet Neurol 2007, 6, 805–815. [Google Scholar] [CrossRef]
- Hor, J.; Asgari, N.; Nakashima, I.; Broadley, S.; Leite, M.; Kissani, N.; Jacob, A.; Marignier, R.; Weinshenker, B.; Paul, F.; et al. Epidemiology of Neuromyelitis Optica Spectrum Disorder and Its Prevalence and Incidence Worldwide. Front. Neurol. 2020, 11, 501. [Google Scholar] [CrossRef] [PubMed]
- Brod, S. Review of approved NMO therapies based on mechanism of action, efficacy and long-term effects. Mult. Scler. Relat. Disord. 2020, 46, 102538. [Google Scholar] [CrossRef] [PubMed]
- Waliszewska-Prosół, M.; Chojdak-Łukasiewicz, J.; Budrewicz, S.; Pokryszko-Dragan, A. Neuromyelitis Optica Spectrum Disorder Treatment-Current and Future Prospects. Int. J. Mol. Sci. 2021, 22, 2801. [Google Scholar] [CrossRef] [PubMed]
- Hinson, S.R.; Pittock, S.J.; Lucchinetti, C.F.; Roemer, S.F.; Fryer, J.P.; Kryzer, T.J.; Lennon, V.A. Pathogenic potential of IgG binding to water channel extracellular domain in neuromyelitis optica. Neurology 2007, 69, 2221–2231. [Google Scholar] [CrossRef] [PubMed]
- Weinshenker, B.G.; Wingerchuk, D.M.; Pittock, S.J.; Lucchinetti, C.F.; Lennon, V.A. NMO-IgG: A specific biomarker for neuromyelitis optica. Dis. Markers 2006, 22, 197–206. [Google Scholar] [CrossRef]
- Lennon, V.A.; Kryzer, T.J.; Pittock, S.J.; Verkman, A.S.; Hinson, S.R. IgG marker of optic-spinal multiple sclerosis binds to the aquaporin-4 water channel. J. Exp. Med. 2005, 202, 473–477. [Google Scholar] [CrossRef]
- Wingerchuk, D.; Banwell, B.; Bennett, J.; Cabre, P.; Carroll, W.; Chitnis, T.; de Seze, J.; Fujihara, K.; Greenberg, B.; Jacob, A.; et al. International consensus diagnostic criteria for neuromyelitis optica spectrum disorders. Neurology 2015, 85, 177–189. [Google Scholar] [CrossRef]
- Flanagan, E.P.; Weinshenker, B.G. Neuromyelitis optica spectrum disorders. Curr. Neurol. Neurosci. Rep. 2014, 14, 483. [Google Scholar] [CrossRef]
- Crane, J.M.; Tajima, M.; Verkman, A.S. Live-cell imaging of aquaporin-4 diffusion and interactions in orthogonal arrays of particles. Neuroscience 2010, 168, 892–902. [Google Scholar] [CrossRef]
- Iorio, R.; Fryer, J.P.; Hinson, S.R.; Fallier-Becker, P.; Wolburg, H.; Pittock, S.J.; Lennon, V.A. Astrocytic autoantibody of neuromyelitis optica (NMO-IgG) binds to aquaporin-4 extracellular loops, monomers, tetramers and high order arrays. J. Autoimmun. 2013, 40, 21–27. [Google Scholar] [CrossRef] [PubMed]
- Verkman, A.S.; Ratelade, J.; Rossi, A.; Zhang, H.; Tradtrantip, L. Aquaporin-4: Orthogonal array assembly, CNS functions, and role in neuromyelitis optica. Acta Pharmacol. Sin. 2011, 32, 702–710. [Google Scholar] [CrossRef] [PubMed]
- Jarius, S.; Wildemann, B. AQP4 antibodies in neuromyelitis optica: Diagnostic and pathogenetic relevance. Nat. Rev. Neurol. 2010, 6, 383–392. [Google Scholar] [CrossRef] [PubMed]
- Phuan, P.W.; Ratelade, J.; Rossi, A.; Tradtrantip, L.; Verkman, A.S. Complement-dependent cytotoxicity in neuromyelitis optica requires aquaporin-4 protein assembly in orthogonal arrays. J. Biol. Chem. 2012, 287, 13829–13839. [Google Scholar] [CrossRef]
- Tradtrantip, L.; Zhang, H.; Anderson, M.O.; Saadoun, S.; Phuan, P.W.; Papadopoulos, M.C.; Bennett, J.L.; Verkman, A.S. Small-molecule inhibitors of NMO-IgG binding to aquaporin-4 reduce astrocyte cytotoxicity in neuromyelitis optica. FASEB J. 2012, 26, 2197–2208. [Google Scholar] [CrossRef]
- Alberga, D.; Trisciuzzi, D.; Lattanzi, G.; Bennett, J.L.; Verkman, A.S.; Mangiatordi, G.F.; Nicolotti, O. Comparative molecular dynamics study of neuromyelitis optica-immunoglobulin G binding to aquaporin-4 extracellular domains. Biochim. Biophys. Acta 2017, 1859, 1326–1334. [Google Scholar] [CrossRef]
- Mangiatordi, G.F.; Alberga, D.; Siragusa, L.; Goracci, L.; Lattanzi, G.; Nicolotti, O. Challenging AQP4 druggability for NMO-IgG antibody binding using molecular dynamics and molecular interaction fields. Biochim. Biophys. Acta 2015, 1848, 1462–1471. [Google Scholar] [CrossRef]
- Tradtrantip, L.; Asavapanumas, N.; Phuan, P.W.; Verkman, A.S. Potential therapeutic benefit of C1-esterase inhibitor in neuromyelitis optica evaluated in vitro and in an experimental rat model. PLoS ONE 2014, 9, e106824. [Google Scholar] [CrossRef]
- Ratelade, J.; Smith, A.J.; Verkman, A.S. Human immunoglobulin G reduces the pathogenicity of aquaporin-4 autoantibodies in neuromyelitis optica. Exp. Neurol. 2014, 255, 145–153. [Google Scholar] [CrossRef]
- Phuan, P.W.; Zhang, H.; Asavapanumas, N.; Leviten, M.; Rosenthal, A.; Tradtrantip, L.; Verkman, A.S. C1q-targeted monoclonal antibody prevents complement-dependent cytotoxicity and neuropathology in in vitro and mouse models of neuromyelitis optica. Acta Neuropathol. 2013, 125, 829–840. [Google Scholar] [CrossRef]
- Tradtrantip, L.; Ratelade, J.; Zhang, H.; Verkman, A.S. Enzymatic deglycosylation converts pathogenic neuromyelitis optica anti-aquaporin-4 immunoglobulin G into therapeutic antibody. Ann. Neurol. 2013, 73, 77–85. [Google Scholar] [CrossRef]
- Hou, Y.; Sun, B.; Liu, W.; Yu, B.; Shi, Q.; Luo, F.; Bai, Y.; Feng, H. Targeting of glioma stem-like cells with a parthenolide derivative ACT001 through inhibition of AEBP1/PI3K/AKT signaling. Theranostics 2021, 11, 555–566. [Google Scholar] [CrossRef] [PubMed]
- Cai, L.; Gong, Q.; Qi, L.; Xu, T.; Suo, Q.; Li, X.; Wang, W.; Jing, Y.; Yang, D.; Xu, Z.; et al. ACT001 attenuates microglia-mediated neuroinflammation after traumatic brain injury via inhibiting AKT/NFκB/NLRP3 pathway. Cell Commun. Signal. CCS 2022, 20, 56. [Google Scholar] [CrossRef] [PubMed]
- Jaffar, J.; Glaspole, I.; Symons, K.; Westall, G. Inhibition of NF-κB by ACT001 reduces fibroblast activity in idiopathic pulmonary fibrosis. Biomed. Pharmacother. 2021, 138, 111471. [Google Scholar] [CrossRef]
- Lai, C.; Tian, G.; Takahashi, T.; Liu, W.; Yang, L.; Zhang, X. Neuromyelitis optica antibodies in patients with severe optic neuritis in China. J. Neuroophthalmol. 2011, 31, 16–19. [Google Scholar] [CrossRef]
- Hinson, S.R.; Romero, M.F.; Popescu, B.F.; Lucchinetti, C.F.; Fryer, J.P.; Wolburg, H.; Fallier-Becker, P.; Noell, S.; Lennon, V.A. Molecular outcomes of neuromyelitis optica (NMO)-IgG binding to aquaporin-4 in astrocytes. Proc. Natl. Acad. Sci. USA 2012, 109, 1245–1250. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Verkman, A.S. Longitudinally extensive NMO spinal cord pathology produced by passive transfer of NMO-IgG in mice lacking complement inhibitor CD59. J. Autoimmun. 2014, 53, 67–77. [Google Scholar] [CrossRef] [PubMed]
- Asavapanumas, N.; Verkman, A.S. Neuromyelitis optica pathology in rats following intraperitoneal injection of NMO-IgG and intracerebral needle injury. Acta Neuropathol. Commun. 2014, 2, 48. [Google Scholar] [CrossRef] [PubMed]
- Tradtrantip, L.; Yao, X.; Su, T.; Smith, A.J.; Verkman, A.S. Bystander mechanism for complement-initiated early oligodendrocyte injury in neuromyelitis optica. Acta Neuropathol. 2017, 134, 35–44. [Google Scholar] [CrossRef]
- Hinson, S.R.; Clift, I.C.; Luo, N.; Kryzer, T.J.; Lennon, V.A. Autoantibody-induced internalization of CNS AQP4 water channel and EAAT2 glutamate transporter requires astrocytic Fc receptor. Proc. Natl. Acad. Sci. USA 2017, 114, 5491–5496. [Google Scholar] [CrossRef]
- Pittock, S.J.; Lennon, V.A.; McKeon, A.; Mandrekar, J.; Weinshenker, B.G.; Lucchinetti, C.F.; O’Toole, O.; Wingerchuk, D.M. Eculizumab in AQP4-IgG-positive relapsing neuromyelitis optica spectrum disorders: An open-label pilot study. Lancet Neurol 2013, 12, 554–562. [Google Scholar] [CrossRef] [PubMed]
- Tradtrantip, L.; Asavapanumas, N.; Verkman, A.S. Therapeutic cleavage of anti-aquaporin-4 autoantibody in neuromyelitis optica by an IgG-selective proteinase. Mol. Pharmacol. 2013, 83, 1268–1275. [Google Scholar] [CrossRef]
- Son, M.; Kim, D.; Park, K.S.; Hong, S.; Park, T.H. Detection of aquaporin-4 antibody using aquaporin-4 extracellular loop-based carbon nanotube biosensor for the diagnosis of neuromyelitis optica. Biosens. Bioelectron. 2016, 78, 87–91. [Google Scholar] [CrossRef] [PubMed]
- Raveendra, B.L.; Wu, H.; Baccala, R.; Reddy, M.M.; Schilke, J.; Bennett, J.L.; Theofilopoulos, A.N.; Kodadek, T. Discovery of peptoid ligands for anti-aquaporin 4 antibodies. Chem. Biol. 2013, 20, 351–359. [Google Scholar] [CrossRef] [PubMed]
- Chen, T.; Lennon, V.A.; Liu, Y.U.; Bosco, D.B.; Li, Y.; Yi, M.H.; Zhu, J.; Wei, S.; Wu, L.J. Astrocyte-microglia interaction drives evolving neuromyelitis optica lesion. J. Clin. Investig. 2020, 130, 4025–4038. [Google Scholar] [CrossRef]
- Waters, P.J.; McKeon, A.; Leite, M.I.; Rajasekharan, S.; Lennon, V.A.; Villalobos, A.; Palace, J.; Mandrekar, J.N.; Vincent, A.; Bar-Or, A.; et al. Serologic diagnosis of NMO: A multicenter comparison of aquaporin-4-IgG assays. Neurology 2012, 78, 665–671; discussion 669. [Google Scholar] [CrossRef]
- Wingerchuk, D.M.; Lennon, V.A.; Pittock, S.J.; Lucchinetti, C.F.; Weinshenker, B.G. Revised diagnostic criteria for neuromyelitis optica. Neurology 2006, 66, 1485. [Google Scholar] [CrossRef]
- Tingjun, C.; Zhaohui, L.; Zhaocai, J.; Zihao, L.; Quangang, X.; Dehui, H.; Qing, L.; Shihui, W. Changes of CXCL12, CXCL14 and PDGF levels in the brain of patients with idiopathic demyelinating optic neuritis and neuromyelitis optica. J. Neuroimmunol. 2015, 279, 1–6. [Google Scholar] [CrossRef]
- Kang, H.; Cao, S.; Chen, T.; Jiang, Z.; Liu, Z.; Li, Z.; Wei, Y.; Ai, N.; Xu, Q.; Lin, Q.; et al. The poor recovery of neuromyelitis optica spectrum disorder is associated with a lower level of CXCL12 in the human brain. J. Neuroimmunol. 2015, 289, 56–61. [Google Scholar] [CrossRef] [PubMed]

| LC50% | R2 | p | |
|---|---|---|---|
| Healthy control | 3.602 ng/mL ± 0.1653 | 0.9555 | |
| AQP4-IgG + Complement | 0.396 ng/mL ± 0.056 | 0.9638 | *** |
| AQP4-IgG + Complement + Loop A + C + E | 3.052 ng/mL ± 0.1128 | 0.9512 | ### |
| AQP4-IgG + Complement + Loop A | 1.253 ng/mL ± 0.1781 | 0.9019 | *,### |
| AQP4-IgG + Complement + Loop C | 1.450 ng/mL ± 0.1879 | 0.8902 | *,### |
| AQP4-IgG + Complement + Loop E | 0.5903 ng/mL ± 0.8902 | 0.8801 | *** |
| Score Value | Disease Signs |
|---|---|
| 0 | Can walk along a bridge without losing balance and can lower itself back onto the ground gracefully using its paws. |
| 1 | Loss of footing while walking along the ledge but otherwise good coordination. |
| 2 | Ineffective use of hind legs and landing on its head rather than paws when descending onto the ground. |
| 3 | Falling while walking or attempting to lower itself or shaking and refusing to move despite encouragement. |
| 4 | Both hind legs are paralyzed. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, H.; Yang, M.; Song, H.; Sun, M.; Zhou, H.; Fu, J.; Zhou, D.; Bai, W.; Chen, B.; Lai, M.; et al. ACT001 Relieves NMOSD Symptoms by Reducing Astrocyte Damage with an Autoimmune Antibody. Molecules 2023, 28, 1412. https://doi.org/10.3390/molecules28031412
Li H, Yang M, Song H, Sun M, Zhou H, Fu J, Zhou D, Bai W, Chen B, Lai M, et al. ACT001 Relieves NMOSD Symptoms by Reducing Astrocyte Damage with an Autoimmune Antibody. Molecules. 2023; 28(3):1412. https://doi.org/10.3390/molecules28031412
Chicago/Turabian StyleLi, Hongen, Mo Yang, Honglu Song, Mingming Sun, Huanfen Zhou, Junxia Fu, Di Zhou, Wenhao Bai, Biyue Chen, Mengying Lai, and et al. 2023. "ACT001 Relieves NMOSD Symptoms by Reducing Astrocyte Damage with an Autoimmune Antibody" Molecules 28, no. 3: 1412. https://doi.org/10.3390/molecules28031412
APA StyleLi, H., Yang, M., Song, H., Sun, M., Zhou, H., Fu, J., Zhou, D., Bai, W., Chen, B., Lai, M., Kang, H., & Wei, S. (2023). ACT001 Relieves NMOSD Symptoms by Reducing Astrocyte Damage with an Autoimmune Antibody. Molecules, 28(3), 1412. https://doi.org/10.3390/molecules28031412






