Ultrasound-Assisted Extraction of Antioxidants from Melastoma malabathricum Linn.: Modeling and Optimization Using Box–Behnken Design
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.1.1. Chemicals and Reagents
2.1.2. Authentication of Species
2.2. Methods
2.2.1. Plant Material and Sample Preparation
2.2.2. Single Factors for Extraction Procedures
2.2.3. Optimization of Extraction Conditions Using Response Surface Methodology (RSM)
Experimental Design
Ultrasound-Assisted Extraction (UAE)
Determination of Antioxidants in M. malabathricum Leaf Extracts
Free Radical Scavenging Activity (DPPH)
Total Phenolic Content (TPC)
Total Flavonoid Content (TFC)
2.2.4. Phytochemical Characterization
Gas Chromatography–Mass Spectrometry (GC-MS) Analysis
Quadrupole Time-of-Flight Mass Spectrometry (QTOF-MS) Analysis
2.2.5. Statistical Analysis
3. Results and Discussion
3.1. Sampling
3.2. One-Factor-at-a-Time (OFAT) Technique
3.3. Optimization of Antioxidant Activities Using a Response Surface Methodology (RSM)
3.3.1. Model Fitting
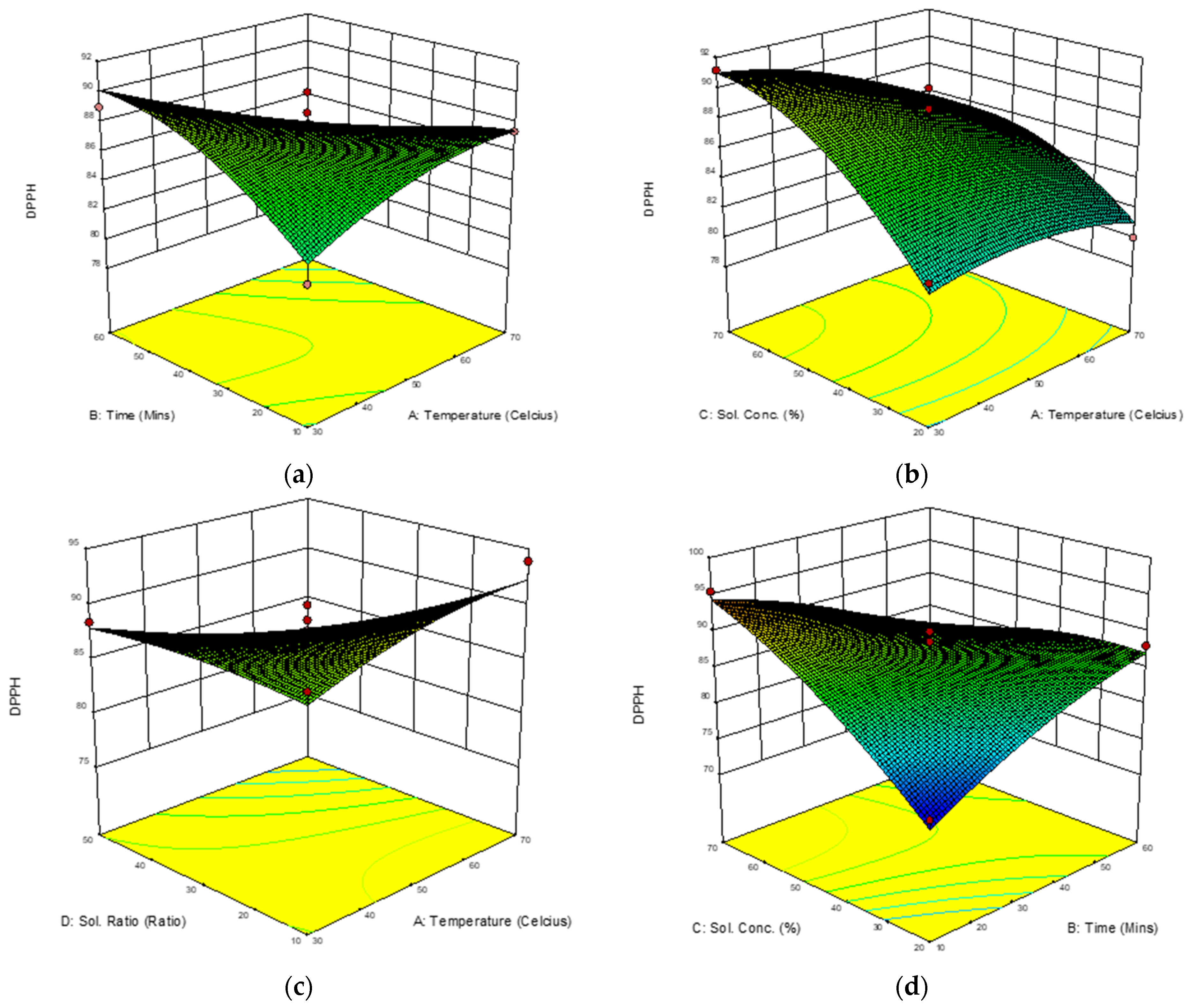
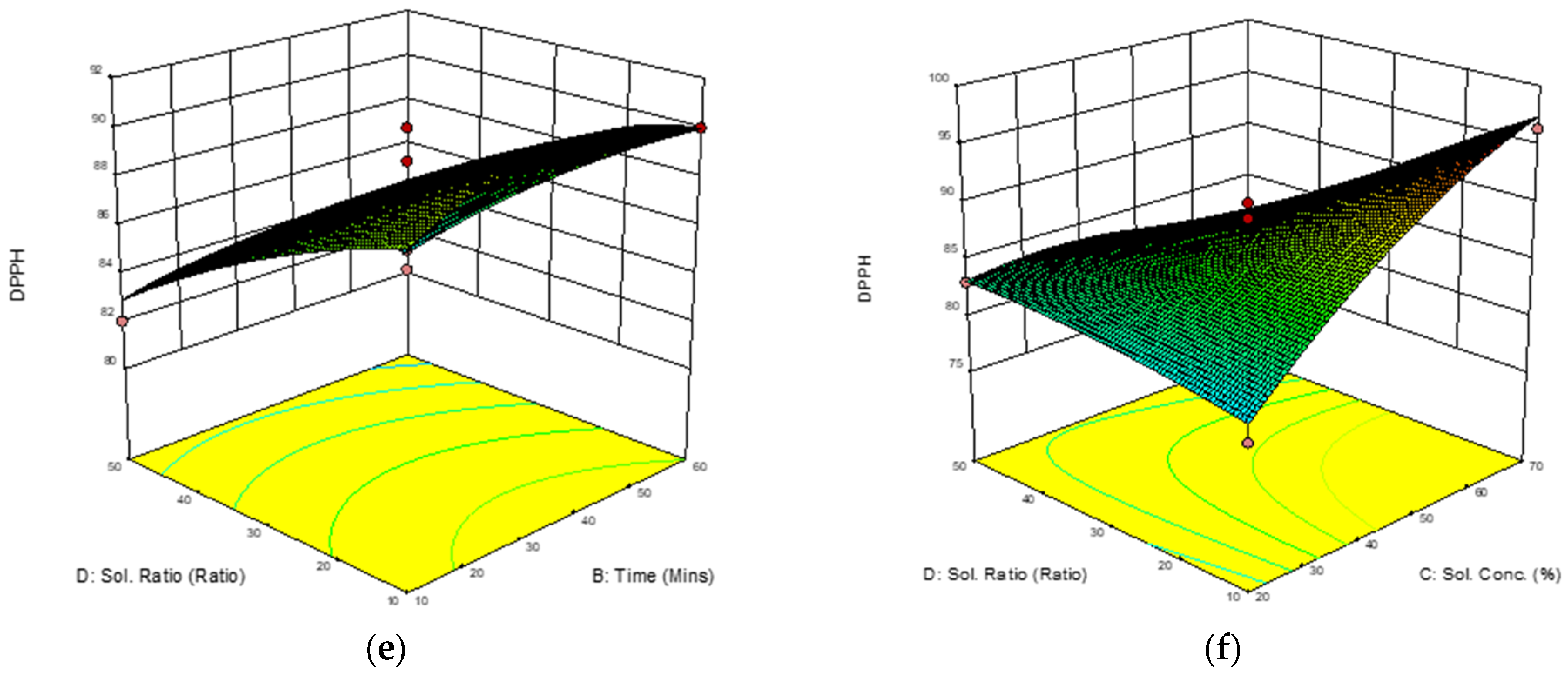
3.3.2. Response Surface Analysis (RSA) of 2,2-Diphenyl-1-Picrylhydrazyl (DPPH) Free Radical Scavenging Ability
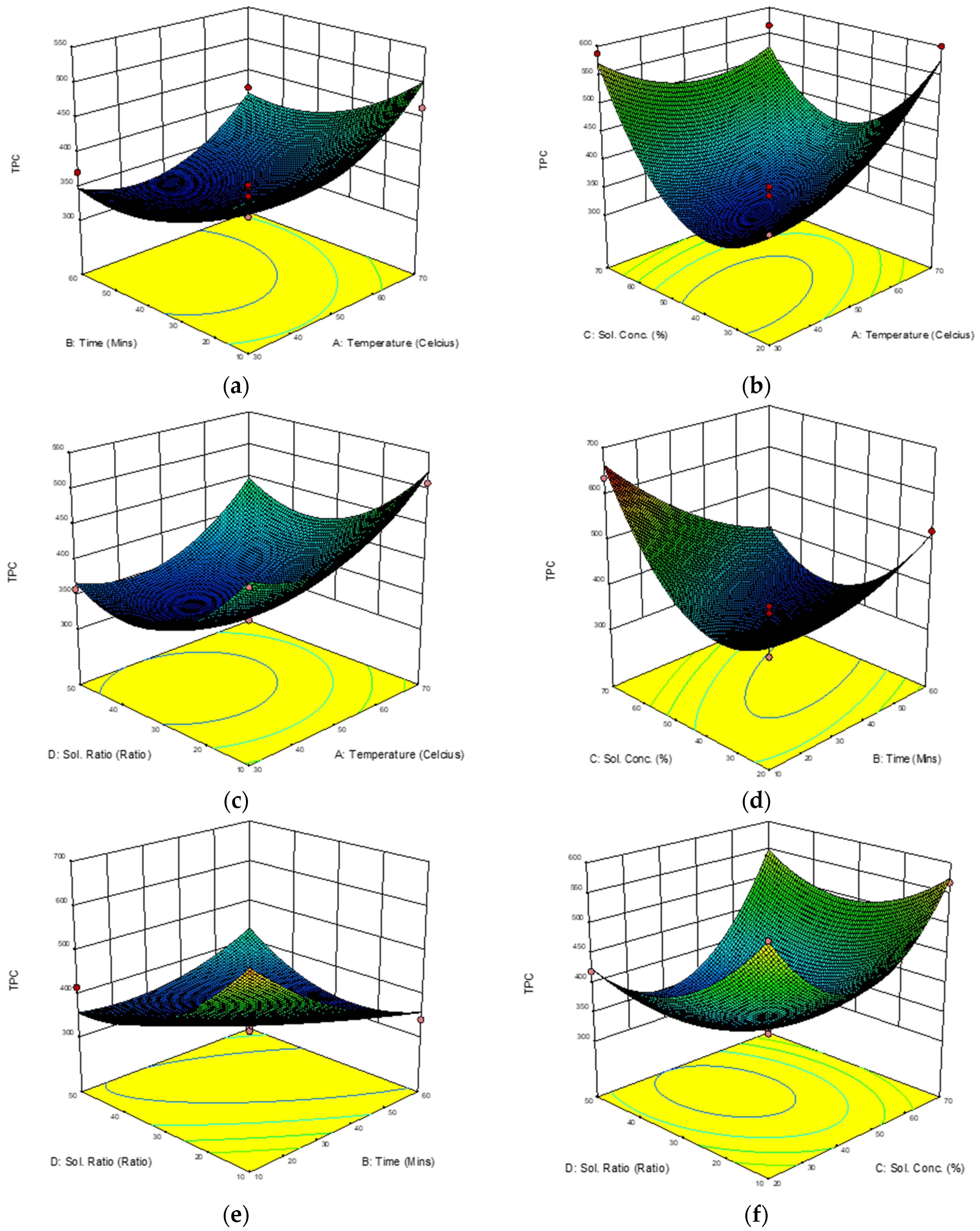
3.3.3. Response Surface Analysis (RSA) of Total Phenolic Content (TPC)
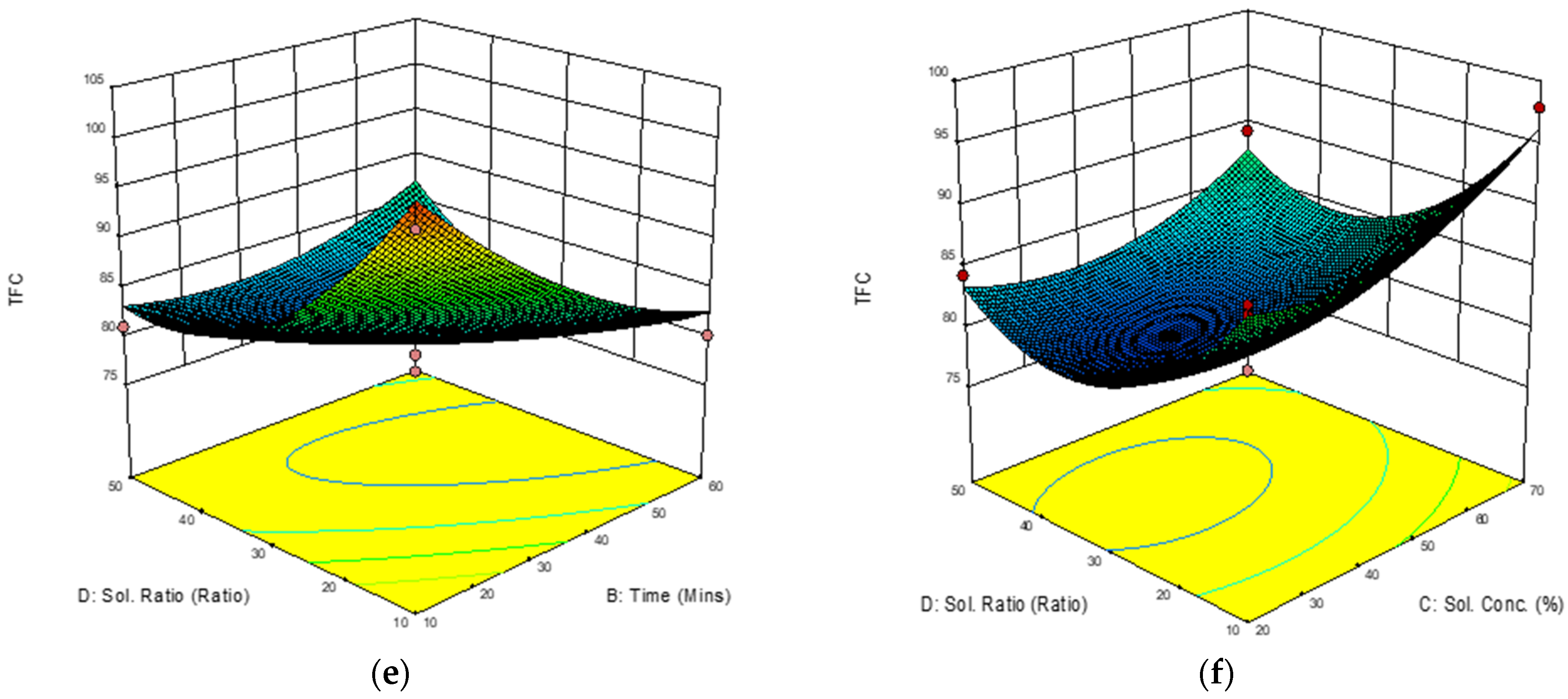
3.3.4. Response Surface Analysis (RSA) of Total Flavonoid Content (TFC)
3.3.5. Verification of Predictive Model
3.4. Characterization of Chemical Composition of M. malabathricum Leaf Extracts
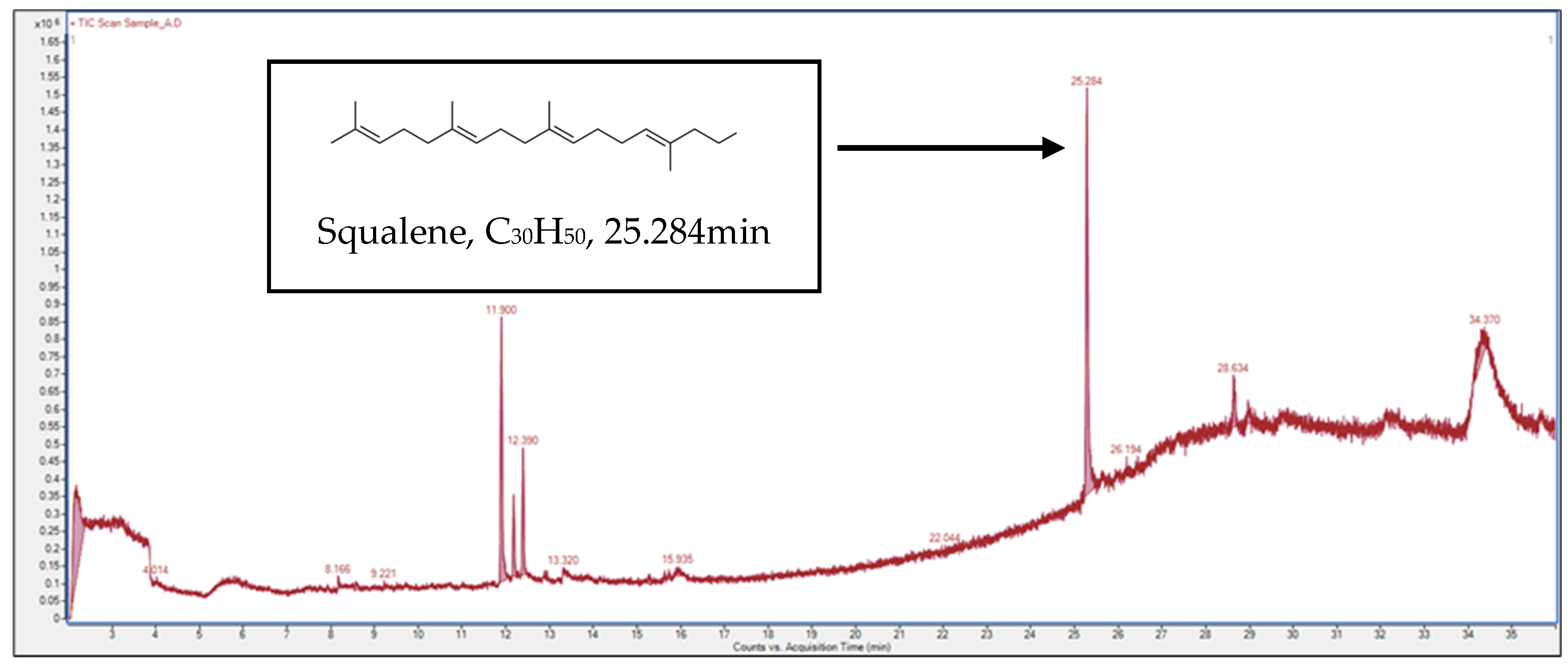
3.4.1. Gas Chromatography–Mass Spectrometry (GC–MS) Analysis
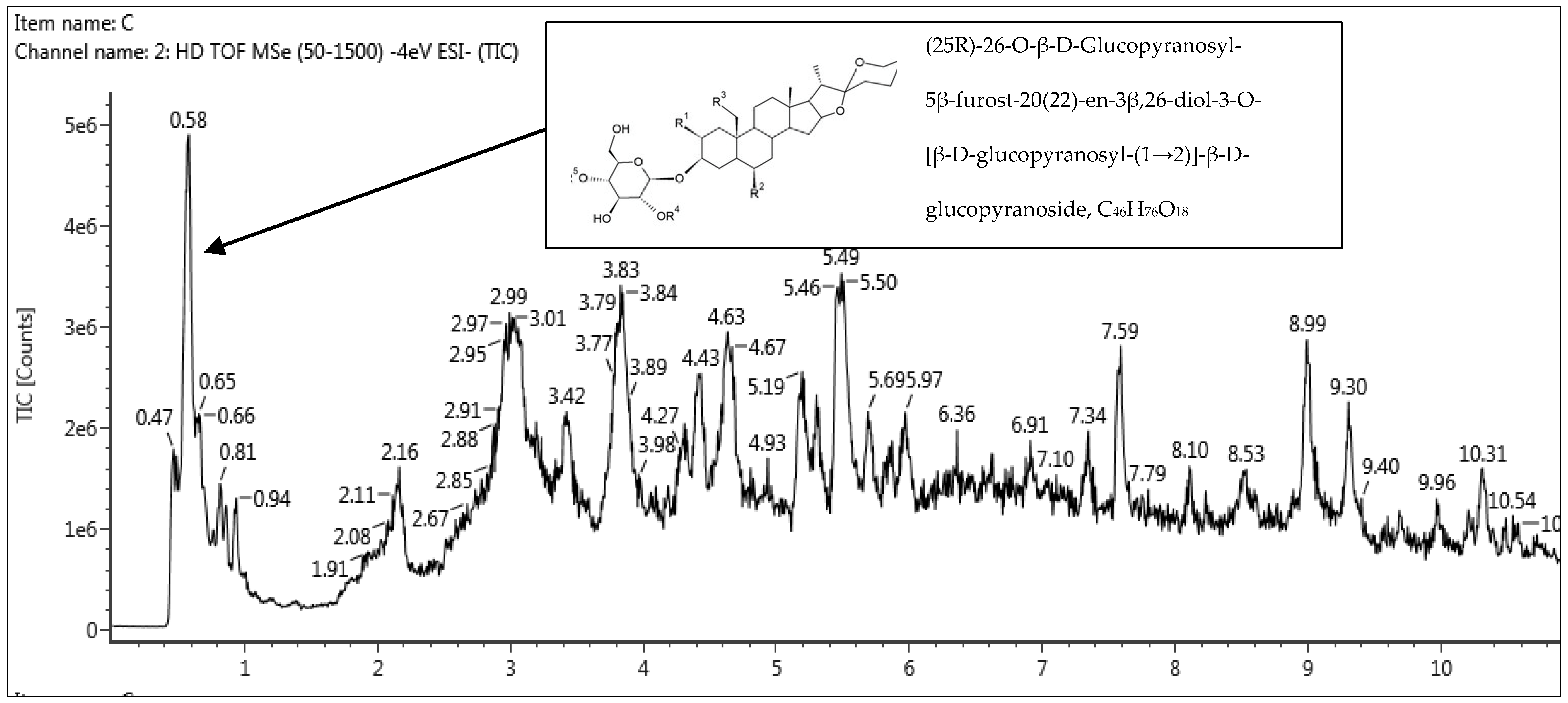
3.4.2. Quadrupole Time-of-Flight Mass Spectrometry (QTOF-MS) Analysis
4. Summary and Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
Sample Availability
References
- Mohd Joffry, S.; Yob, N.J.; Rofiee, M.S.; Meor, M.; Affandi, M.M.; Suhaili, Z.; Othman, F.; Md. Akim, A.; Desa, M.N.M.; Zakaria, Z.A. Melastoma malabathricum (L.) Smith ethnomedicinal uses, chemical constituents, and pharmacological properties: A Review. Evid.-Based Complement. Altern. Med. 2012, 2012, 258434. [Google Scholar] [CrossRef]
- Diris, M.N.; Basri, A.M.; Metali, F.; Ahmad, N.; Taha, H. Phytochemicals and antimicrobial activities of Melastoma malabathricum and Melastoma beccarianum leaf crude extracts. Res. J. Phytochem. 2017, 11, 35–41. [Google Scholar] [CrossRef]
- Rajenderan, M.T. Ethno-medicinal uses and antimicrobial properties of Melastoma malabathricum. SEGi Rev. 2010, 3, 34–44. [Google Scholar]
- Nurdiana, S.; Marziana, N. Wound healing activities of Melastoma malabathricum leaf extract in Sprague Dawley rats. Int. J. Pharm. Sci. Rev. Res. 2013, 20, 20–23. [Google Scholar]
- Carrera, C.; Ruiz-Rodríguez, A.; Palma, M.; Barroso, C.G. Ultrasound assisted extraction of phenolic compounds from grapes. Anal. Chim. Acta 2012, 732, 100–104. [Google Scholar] [CrossRef]
- Daneshvand, B.; Ara, K.M.; Raofie, F. Comparison of supercritical fluid extraction and ultrasound-assisted extraction of fatty acids from quince (Cydonia oblonga Miller) seed using response surface methodology and central composite design. J. Chromatogr. A 2012, 1252, 1–7. [Google Scholar] [CrossRef]
- Anbu, J.; Jisha, P.; Varatharajan, R.; Muthappan, M. Antibacterial and wound healing activities of Melastoma malabathricum Linn. Afr. J. Infect. Dis. 2010, 2, 55063. [Google Scholar] [CrossRef]
- Umali-Stuart, G.; Stiuart-Santiago, A. Philippines Medicinal Plants: Family Melastomaceae 2010. Available online: http://www.stuartxchange.org/Malatungaw.html (accessed on 25 March 2022).
- Awang, M.A.; Chua, L.S.; Abdullah, L.C.; Pin, K.Y. Drying Kinetics and Optimization of Quercetrin Extraction from Melastoma malabathricum Leaves. Chem. Eng. Technol. 2021, 44, 1214–1220. [Google Scholar] [CrossRef]
- Belay, K.; Sisay, M. Phytochemical. Constituents and Physicochemical Properties of Medicinal Plant (Moringa oleifera) Around Bule Hora. Chem. Mater. Res. Chem. Mater. 2014, 6, 61–72. [Google Scholar]
- Shannon, E.; Jaiswal, A.K.; Abu-Ghannam, N. Polyphenolic content and antioxidant capacity of white, green, black, and herbal teas: A kinetic study. Food Res. 2018, 2, 11. [Google Scholar] [CrossRef]
- Pakkirisamy, M.; Kalakandan, S.K.; Ravichandran, K. Phytochemical screening, GC-MS, FT-IR Analysis of methanolic extract of Curcuma caesia Roxb (Black turmeric). Pharmacogn. J. 2017, 9, 952–956. [Google Scholar] [CrossRef]
- American Public Health Association. American Public Health Association (APHA). Standard Methods for the Examination of Water and Wastewater. In Apha, WEF and AWWA; Greenberg, A.E., Clesceri, L.S., Eaton, A.D., Eds.; American Public Health Association: Washington, DC, USA, 1992; p. 1134. [Google Scholar]
- Ma, J.; Wu, S.; Shekhar, N.V.R.; Biswas, S.; Sahu, A.K. Determination of physicochemical parameters and levels of heavy metals in food waste water with environmental effects. Bioinorg. Chem. Appl. 2020, 2020, 1–9. [Google Scholar] [CrossRef]
- Shyama, P.S.; Deepika, M. Optimization of process parameter for alpha-amylase produced by Bacillus cereus amy3 using one factor at a time (OFAT) and central composite rotatable (CCRD) design based response surface methodology (RSM). Biocatal. Agric. Biotechnol. 2019, 19, 101168. [Google Scholar] [CrossRef]
- Aydar, A.Y. Utilization of Response Surface Methodology in Optimization of Extraction of Plant Materials. 2018. Available online: https://books.google.com.hk/books?hl=zh-TW&lr=&id=QemPDwAAQBAJ&oi=fnd&pg=PA157&dq=Utilization+of+Response+Surface+Methodology+in+Optimization+of+Extraction+of+Plant+Materials&ots=MQXJj10uSM&sig=7_NNGjsezgK-8R6PP2-fCSq9h7I&redir_esc=y#v=onepage&q=Utilization%20of%20Response%20Surface%20Methodology%20in%20Optimization%20of%20Extraction%20of%20Plant%20Materials&f=false (accessed on 28 October 2022).
- Lobo, V.; Patil, A.; Phatak, A.; Chandra, N. Free radicals, antioxidants and functional foods: Impact on human health. Pharm. Rev. 2010, 4, 118–126. [Google Scholar] [CrossRef]
- Behera, S.K.; Meena, H.; Chakraborty, S.; Meikap, B.C. Application of response surface methodology (RSM) for optimization of leaching parameters for ash reduction from low-grade coal. Int. J. Mining Sci. Technol. 2018, 28, 621–629. [Google Scholar] [CrossRef]
- Bewick, V.; Cheek, L.; Ball, J. Statistics review 7: Correlation and regression. Crit. Care 2003, 7, 451–459. [Google Scholar] [CrossRef]
- David, I.J.; Adubisi, O.D.; Ogbaji, O.E.; Eghwerido, J.T.; Umar, Z.A. Resistant measures in assessing the adequacy of regression models. Sci. Afr. 2020, 8, e00437. [Google Scholar] [CrossRef]
- Rajewski, J.; Dobrzyńska-Inger, A. Application of Response Surface Methodology (RSM) for the Optimization of Chromium (III) Synergistic Extraction by Supported Liquid Membrane. Membranes 2021, 11, 854. [Google Scholar] [CrossRef]
- Noordin, M.Y.; Venkatesh, V.C.; Sharif, S.; Elting, S.A. Abdullah, Application of response surface methodology in describing the performance of coated carbide tools when turning AISI 1045 steel. J. Mater. Process. Technol. 2004, 145, 46–58. [Google Scholar] [CrossRef]
- Gupta, V.; Gupta, A.K.; Dhingra, A.K. Development of Surface Roughness Model Using Response Surface Methodology. Int. J. Eng. Sci. 2012, 1. [Google Scholar]
- Kedare, S.B.; Singh, R.P. Genesis and development of DPPH method of antioxidant assay. J. Food Sci. Technol. 2011, 48, 412–422. [Google Scholar] [CrossRef] [PubMed]
- Vardanega, R.; Santos, D.T.; Meireles, M.A. Intensification of bioactive compounds extraction from medicinal plants using ultrasonic irradiation. Pharmacogn. Rev. 2014, 8, 88–95. [Google Scholar] [CrossRef] [PubMed]
- Buitink, J.; Claessens, M.M.; Hemminga, M.A.; Hoekstra, F.A. Influence of water content and temperature on molecular mobility and intracellular glasses in seeds and pollen. Plant. Physiol. 1998, 118, 531–541. [Google Scholar] [CrossRef] [PubMed]
- Che Sulaiman, I.S.; Basri, M.; Fard Masoumi, H.R.; Chee, W.J.; Ashari, S.E.; Ismail, M. Effects of temperature, time, and solvent ratio on the extraction of phenolic compounds and the anti-radical activity of Clinacanthus nutans Lindau leaves by response surface methodology. Chem. Cent. J. 2017, 11, 54. [Google Scholar] [CrossRef] [PubMed]
- Le, A.V.; Parks, S.E.; Nguyen, M.H.; Roach, P.D. Effect of Solvents and Extraction Methods on Recovery of Bioactive Compounds from Defatted Gac (Momordica cochinchinensis Spreng.) Seeds. Separations 2018, 5, 39. [Google Scholar] [CrossRef]
- Khadhraoui, B.; Ummat, V.; Tiwari, B.K.; Fabiano-Tixier, A.S.; Chemat, F. Review of ultrasound combinations with hybrid and innovative techniques for extraction and processing of food and natural products. Ultrason. Sonochemistry 2021, 76, 105625. [Google Scholar] [CrossRef]
- Azahar, N.F.; Abd Gani, S.S.; Zaidan, U.H.; Bawon, P.; Halmi, M.I.E. Optimization of the Antioxidant Activities of Mixtures of Melastomataceae Leaves Species (M. malabathricum Linn. Smith, M. decemfidum, and M. hirta) Using a Simplex Centroid Design and Their Anti-Collagenase and Elastase Properties. Appl. Sci. 2020, 10, 7002. [Google Scholar] [CrossRef]
- Yim, H.S.; Chye, F.Y.; Rao, V.; Low, J.Y.; Matanjun, P.; How, S.E.; Ho, C.W. Optimization of extraction time and temperature on antioxidant activity of Schizophyllum commune aqueous extract using response surface methodology. J. Food Sci. Technol. 2013, 50, 275–283. [Google Scholar] [CrossRef]
- Lee, C.; Hun, L.; Yaakob, H.; Wong, S.L.; Hichem, B.J. Optimization of Ultrasound-Assisted Extraction of Total Flavonoids Content from the White Flowering Variety of Melastoma Malabathricum. J. Kejuruter. 2019, 2, 91–102. [Google Scholar]
- Lee, T.H.; Lee, C.H.; Ong, P.Y.; Wong, S.L.; Hamdan, N.; Ya’akob, H.; Azmi, N.A.; Khoo, S.C.; Zakaria, Z.A.; Cheng, K.K. Comparison of extraction methods of phytochemical compounds from white flower variety of Melastoma malabathricum. S. Afr. J. Botany 2022, 148, 170–179. [Google Scholar] [CrossRef]
- Chaves, J.O.; De Souza, M.C.; Da Silva, L.C.; Lachos-Perez, D.; Torres-Mayanga, P.C.; Machado, A.P.; Forster-Carneiro, T.; Vázquez-Espinosa, M.; González-de-Peredo, A.V.; Barbero, G.F.; et al. Extraction of flavonoids from natural sources using modern techniques. Front. Chem. 2020, 25, 8. [Google Scholar] [CrossRef]
- Zhang, Z.; Li, X.; Sang, S.; McClements, D.J.; Chen, L.; Long, J.; Jiao, A.; Jin, Z.; Qiu, C. Polyphenols as Plant-Based Nutraceuticals: Health Effects, Encapsulation, Nano-Delivery, and Application. Foods 2022, 11, 2189. [Google Scholar] [CrossRef]
- Sánchez-Quesada, C.; López-Biedma, A.; Toledo, E.; Gaforio, J.J. Squalene Stimulates a Key Innate Immune Cell to Foster Wound Healing and Tissue Repair. Evid.-Based Complement. Altern. Med. 2018, 2018, 9473094. [Google Scholar] [CrossRef]
- Gonzalez, A.C.; Costa, T.F.; Andrade, Z.A.; Medrado, A.R. Wound healing—A literature review. Anais Bras. Dermatol. 2016, 91, 614–620. [Google Scholar] [CrossRef]
- Huang, Z.R.; Lin, Y.K.; Fang, J.Y. Biological and pharmacological activities of squalene and related compounds: Potential uses in cosmetic dermatology. Molecules 2009, 14, 540–554. [Google Scholar] [CrossRef]
- Newmark, H.L. Squalene, olive oil, and cancer risk. Rev. Hypothesis. Ann. N. Y. Acad. Sci. 1999, 889, 193–203. [Google Scholar] [CrossRef]
- Ishikawa, T.; Sasaki, D.; Aizawa, R.; Yamamoto, M.; Yaegashi, T.; Irié, T.; Sasaki, M. The Role of Lactic Acid on Wound Healing, Cell Growth, Cell Cycle Kinetics, and Gene Expression of Cultured Junctional Epithelium Cells in the Pathophysiology of Periodontal Disease. Pathogens 2021, 10, 1507. [Google Scholar] [CrossRef]
- Purnamawati, S.; Indrastuti, N.; Danarti, R.; Saefudin, T. The Role of Moisturizers in Addressing Various Kinds of Dermatitis: A Review. Clin. Med. Res. 2017, 15, 75–87. [Google Scholar] [CrossRef]
- Raman, B.V.; Samuel, L.A.; Saradhi, M.P.; Rao, B.N.; Krishna, N.V.; Sudhakar, M.; Radhakrishnan, T.M. Antibacterial, antioxidant activity and GC-MS analysis of Eupatorium odoratum. Asian J. Pharm. Clin. Res. 2012, 5, 99–106. [Google Scholar]
- Sadiq, A.; Zeb, A.; Ullah, F.; Ahmad, S.; Ayaz, M.; Rashid, U.; Muhammad, N. Chemical Characterization, Analgesic, Antioxidant, and Anticholinesterase Potentials of Essential Oils from Isodon rugosus Wall. ex. Benth. Front. Pharmacol. 2018, 9, 623. [Google Scholar] [CrossRef]
- Guo, S.; Dipietro, L.A. Factors affecting wound healing. J. Dent. Res. 2010, 89, 219–229. [Google Scholar] [CrossRef] [PubMed]
- Anjukrishna, S.R.; Chandrika, P.; Lekhya, G.; Rao, B.; Shyla, H. Pharmacological properties, phytochemical and GC-MS analysis of Bauhinia acuminata Linn. J. Chem. Pharm. Res. 2015, 2015, 372–380. [Google Scholar]
- Sawada, Y.; Akiyama, K.; Sakata, A.; Kuwahara, A.; Otsuki, H.; Sakurai, T.; Saito, K.; Hirai, M.Y. Widely Targeted Metabolomics Based on Large-Scale MS/MS Data for Elucidating Metabolite Accumulation Patterns in Plants. Plant Cell Physiol. 2008, 50, 37–47. [Google Scholar] [CrossRef] [PubMed]
- Samad, N.; Dutta, S.; Sodunke, T.E.; Fairuz, A.; Sapkota, A.; Miftah, Z.F.; Jahan, I.; Sharma, P.; Abubakar, A.R.; Rowaiye, A.B.; et al. Fat-Soluble Vitamins and the Current Global Pandemic of COVID-19: Evidence-Based Efficacy from Literature Review. J. Inflamm. Res. 2021, 14, 2091–2110. [Google Scholar] [CrossRef] [PubMed]
- Elufioye, T.; Obuotor, E.M.; Agbedahunsi, J.; Adesanya, S.A. Anticholinesterase constituents from the leaves of Spondias mombin L. (Anacardiaceae). Biol. Targets Ther. 2017, 11, 107–114. [Google Scholar] [CrossRef]
- Jivishov, E.; Keusgen, M. Can Allium chemical chest be a source of anticancer compounds? Phytochem. Rev. 2020, 19, 1503–1523. [Google Scholar] [CrossRef]
- Panche, A.N.; Diwan, A.D.; Chandra, S.R. Flavonoids: An overview. J. Nutr. Sci. 2016, 5, e47. [Google Scholar] [CrossRef]
- Anand David, A.V.; Arulmoli, R.; Parasuraman, S. Overviews of Biological Importance of Quercetin: A Bioactive Flavonoid. Pharm. Rev. 2016, 10, 84–89. [Google Scholar] [CrossRef]
- Calderón-Montaño, J.M.; Burgos-Morón, E.; Pérez-Guerrero, C.; López-Lázaro, M. A review on the dietary flavonoid kaempferol. Mini Rev. Med. Chem. 2011, 11, 298–344. [Google Scholar] [CrossRef]
- Podolak, I.; Galanty, A.; Sobolewska, D. Saponins as cytotoxic agents: A review. Phytochem. Rev. 2010, 9, 425–474. [Google Scholar] [CrossRef]
- Chhetri, D.R. Myo-Inositol and Its Derivatives: Their Emerging Role in the Treatment of Human Diseases. Front Pharmacol. 2019, 10, 1172. [Google Scholar] [CrossRef]
- Shi, J.; Arunasalam, K.; Yeung, D.; Kakuda, Y.; Mittal, G.; Jiang, Y. Saponins from edible legumes: Chemistry, processing, and health benefits. J. Med. Food. 2004, 7, 67–78. [Google Scholar] [CrossRef]
- Küpeli Akkol, E.; Genç, Y.; Karpuz, B.; Sobarzo-Sánchez, E.; Capasso, R. Coumarins and Coumarin-Related Compounds in Pharmacotherapy of Cancer. Cancers 2020, 12, 1959. [Google Scholar] [CrossRef]
- Sharifi-Rad, J.; Quispe, C.; Castillo, C.M.S.; Caroca, R.; Lazo-Vélez, M.A.; Antonyak, H.; Polishchuk, A.; Lysiuk, R.; Oliinyk, P.; De Masi, L.; et al. Ellagic Acid: A Review on Its Natural Sources, Chemical Stability, and Therapeutic Potential. Oxid. Med. Cell. Longev. 2022, 2022, 3848084. [Google Scholar] [CrossRef]
- Jamal, A.; Kausar Wizarat, K.M.; Shamsuddin, A.Z.; Joseph, D. Connolly, Jangomolide, a novel limonoid from flacourtia jangomas. Phytochemistry 1984, 23, 1269–1270. [Google Scholar]
- Omoruyi, F.O.; Budiaman, A.; Eng, Y.; Olumese, F.E.; Hoesel, J.L.; Ejilemele, A.; Okorodudu, A.O. The potential benefits and adverse effects of phytic Acid supplement in streptozotocin-induced diabetic rats. Adv. Pharm. Sci. 2013, 2013, 172494. [Google Scholar] [CrossRef]
- Bai, J.; Zhang, Y.; Tang, C.; Hou, Y.; Ai, X.; Chen, X.; Zhang, Y.; Wang, X.; Meng, X. Gallic acid: Pharmacological activities and molecular mechanisms involved in inflammation-related diseases. Biomed. Pharmacother. 2021, 133, 110985. [Google Scholar] [CrossRef]
- Soares, S.; Brandão, E.; Guerreiro, C.; Soares, S.; Mateus, N.; de Freitas, V. Tannins in Food: Insights into the Molecular Perception of Astringency and Bitter Taste. Molecules 2020, 25, 2590. [Google Scholar] [CrossRef]
- Pandey, K.B.; Rizvi, S.I. Plant polyphenols as dietary antioxidants in human health and disease. Oxid. Med. Cell. Longev. 2009, 2, 270–278. [Google Scholar] [CrossRef]
- Niaz, K.; Khan, F. Analysis of polyphenolics. Recent Adv. Nat. Prod. Anal. 2020, 39–197. [Google Scholar] [CrossRef]
- Pott, D.M.; Osorio, S.; Vallarino, J.G. From Central to Specialized Metabolism: An Overview of Some Secondary Compounds Derived from the Primary Metabolism for Their Role in Conferring Nutritional and Organoleptic Characteristics to Fruit. Front. Plant Sci. 2019, 10, 835. [Google Scholar] [CrossRef] [PubMed]
- Okuda, T.; Yoshida, T.; Hatano, T.; Ito, H. Ellagitannins Renewed the Concept of Tannins. Chem. Biol. Ellagitannins 2009, 122, 1–54. [Google Scholar] [CrossRef]



| Minimum Level | Maximum Level | |
|---|---|---|
| −1 | 1 | |
| Extraction temperature, XET | 30 | 70 |
| Ultrasonic time, XUT | 10 | 60 |
| Solvent concentration, XSC | 20 | 70 |
| Sample-to-liquid ratio, XSLR | 10 | 50 |
| Run Order a | Extraction Temperature, °C | Ultrasonic Time, min | Solvent Concentration, % | Sample-to-Liquid Ratio |
|---|---|---|---|---|
| 1 | 50 | 35 | 45 | 1:30 |
| 2 | 50 | 60 | 20 | 1:30 |
| 3 | 30 | 35 | 70 | 1:30 |
| 4 | 50 | 10 | 45 | 1:10 |
| 5 | 50 | 60 | 45 | 1:50 |
| 6 | 30 | 35 | 45 | 1:10 |
| 7 | 50 | 10 | 70 | 1:30 |
| 8 | 30 | 60 | 45 | 1:30 |
| 9 | 50 | 10 | 20 | 1:30 |
| 10 | 50 | 35 | 20 | 1:10 |
| 11 | 70 | 35 | 20 | 1:30 |
| 12 | 50 | 35 | 70 | 1:50 |
| 13 | 50 | 35 | 45 | 1:30 |
| 14 | 50 | 35 | 20 | 1:50 |
| 15 | 50 | 60 | 70 | 1:30 |
| 16 | 30 | 35 | 45 | 1:50 |
| 17 | 50 | 35 | 70 | 1:10 |
| 18 | 50 | 35 | 45 | 1:30 |
| 19 | 70 | 60 | 45 | 1:30 |
| 20 | 50 | 35 | 45 | 1:30 |
| 21 | 70 | 35 | 45 | 1:50 |
| 22 | 70 | 35 | 70 | 1:30 |
| 23 | 50 | 10 | 45 | 1:50 |
| 24 | 50 | 60 | 45 | 1:10 |
| 25 | 50 | 35 | 45 | 1:30 |
| 26 | 30 | 35 | 20 | 1:30 |
| 27 | 30 | 10 | 45 | 1:30 |
| 28 | 70 | 10 | 45 | 1:30 |
| 29 | 70 | 35 | 45 | 1:10 |
| Power: | |
|---|---|
| Capillary Voltage | 1.50 kV |
| Reference Capillary Voltage | 3.00 kV |
| Cone Flow Rate (L/H): | |
| Source Temperature | 120 °C |
| Desolvation Gas Temperature | 550 °C |
| Desolvation Gas Flow | 800 L/H |
| Cone Gas Flow | 50 L/H |
| Run Order a | DPPH b | TPC c | TFC d | |||
|---|---|---|---|---|---|---|
| Exp. | Pred. | Exp. | Pred. | Exp. | Pred. | |
| 1 | 90.00 | 87.89 | 352.31 | 334.09 | 81.00 | 79.43 |
| 2 | 88.10 | 87.11 | 519.99 | 515.92 | 85.10 | 83.92 |
| 3 | 91.17 | 90.99 | 587.43 | 570.74 | 94.66 | 97.32 |
| 4 | 88.99 | 89.06 | 646.21 | 595.68 | 100.43 | 102.98 |
| 5 | 80.09 | 80.93 | 421.00 | 432.24 | 85.01 | 87.18 |
| 6 | 88.88 | 87.80 | 449.80 | 457.74 | 104.68 | 103.24 |
| 7 | 95.44 | 94.32 | 634.40 | 663.91 | 98.36 | 97.43 |
| 8 | 89.00 | 90.15 | 371.16 | 347.57 | 92.11 | 90.63 |
| 9 | 75.89 | 74.71 | 399.90 | 419.68 | 82.99 | 81.54 |
| 10 | 78.99 | 80.56 | 564.14 | 564.47 | 90.88 | 89.91 |
| 11 | 79.99 | 81.08 | 599.63 | 577.03 | 88.99 | 91.04 |
| 12 | 80.00 | 79.64 | 535.50 | 549.01 | 89.26 | 87.62 |
| 13 | 86.88 | 87.89 | 348.80 | 334.09 | 76.33 | 79.43 |
| 14 | 83.00 | 83.15 | 420.00 | 425.95 | 84.33 | 83.33 |
| 15 | 81.78 | 80.84 | 400.00 | 405.67 | 79.36 | 78.70 |
| 16 | 88.43 | 87.92 | 357.77 | 367.63 | 83.97 | 82.92 |
| 17 | 96.36 | 97.41 | 567.50 | 575.39 | 97.88 | 96.28 |
| 18 | 86.25 | 87.89 | 321.30 | 334.09 | 79.87 | 79.43 |
| 19 | 79.94 | 79.90 | 423.29 | 415.26 | 80.00 | 78.63 |
| 20 | 87.68 | 87.89 | 335.67 | 334.09 | 78.06 | 79.43 |
| 21 | 78.00 | 76.96 | 431.05 | 448.56 | 95.34 | 94.67 |
| 22 | 83.66 | 85.20 | 565.25 | 525.35 | 83.87 | 86.04 |
| 23 | 82.00 | 82.92 | 415.53 | 357.45 | 80.99 | 83.19 |
| 24 | 89.99 | 89.98 | 340.11 | 358.90 | 80.11 | 82.63 |
| 25 | 88.64 | 87.89 | 312.38 | 334.09 | 81.88 | 79.43 |
| 26 | 82.41 | 81.78 | 384.47 | 385.08 | 79.11 | 81.65 |
| 27 | 82.44 | 83.69 | 401.10 | 422.98 | 89.00 | 87.76 |
| 28 | 87.38 | 87.44 | 464.40 | 501.84 | 98.99 | 97.86 |
| 29 | 93.88 | 92.27 | 507.77 | 523.35 | 90.66 | 89.60 |
| Variance Source | df | DPPH | TPC | TFC | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Sum of Squares | Mean Square | F-Value | Sum of Squares | Mean Square | F-Value | Sum of Squares | Mean Square | F-Value | |||||
| Model | 14 | 765.99 | 54.71 | 25.09 | ** | 2.66 × 105 | 18,978.35 | 17.98 | ** | 1597.5 | 114.11 | 18.04 | ** |
| Extraction temp., XET | 1 | 31.62 | 31.62 | 14.50 | ** | 16,108.06 | 16,108.06 | 15.26 | ** | 2.69 | 2.69 | 0.43 | ns |
| Ultrasonic time, XUT | 1 | 0.87 | 0.87 | 0.40 | ns | 19,681.54 | 19,681.54 | 18.65 | ** | 200.63 | 200.63 | 31.72 | ** |
| Solvent conc., XSC | 1 | 133.51 | 133.51 | 61.22 | ** | 13,463.4 | 13,463.4 | 12.76 | ** | 85.24 | 85.24 | 13.48 | ** |
| Sample-to-liquid ratio, XSLR | 1 | 173.02 | 173.02 | 79.34 | ** | 20,392.5 | 20,392.5 | 19.32 | ** | 174.3 | 174.3 | 27.56 | ** |
| XET XUT | 1 | 49.00 | 49.00 | 22.47 | ** | 31.22 | 31.22 | 0.03 | ns | 122.14 | 122.14 | 19.31 | ** |
| XET XSC | 1 | 6.48 | 6.48 | 2.97 | ns | 14,082.36 | 14,082.36 | 13.34 | ** | 106.82 | 106.82 | 16.89 | ** |
| XET XSLR | 1 | 59.52 | 59.52 | 27.29 | ** | 58.63 | 58.63 | 0.056 | ns | 161.18 | 161.18 | 25.48 | ** |
| XUT XSC | 1 | 167.31 | 167.31 | 76.73 | ** | 31,415.77 | 31,415.77 | 29.77 | ** | 111.42 | 111.42 | 17.62 | ** |
| XUT XSLR | 1 | 2.12 | 2.12 | 0.97 | ns | 24,269.44 | 24,269.44 | 23 | ** | 148.12 | 148.12 | 23.42 | ** |
| XSC XSLR | 1 | 103.69 | 103.69 | 47.55 | ** | 3143.87 | 3143.87 | 2.98 | ns | 1.07 | 1.07 | 0.17 | ns |
| XET 2 | 1 | 7.01 | 7.01 | 3.21 | ns | 16,565.59 | 16,565.59 | 15.7 | ** | 270.17 | 270.17 | 42.72 | ** |
| XUT 2 | 1 | 15.72 | 15.72 | 7.21 | * | 9015.33 | 9015.33 | 8.54 | ** | 52.24 | 52.24 | 8.26 | ** |
| XSC 2 | 1 | 28.30 | 28.30 | 12.98 | ** | 1.10 × 105 | 1.10 × 105 | 103.74 | ** | 63.58 | 63.58 | 10.05 | ** |
| XSLR 2 | 1 | 2.42 | 2.42 | 1.11 | ns | 27,146.35 | 27,146.35 | 25.72 | ** | 293.38 | 293.38 | 46.39 | ** |
| Residual | 14 | 30.53 | 2.18 | 14,775.56 | 1055.4 | 88.54 | 6.32 | ||||||
| Lack of Fit | 10 | 21.76 | 2.18 | 0.99 | ns | 13,589.96 | 1359 | 4.59 | ns | 68.4 | 6.84 | 1.36 | ns |
| Pure Error | 4 | 8.77 | 2.19 | 1185.6 | 296.4 | 20.14 | 5.04 | ||||||
| Total | 28 | 796.52 | 2.81 × 105 | 1686.04 | |||||||||
| R-Squared | 0.9617 | 0.9473 | 0.9475 | ||||||||||
| Adj. R-Squared | 0.9233 | 0.8946 | 0.8950 | ||||||||||
| Pred. R-Squared | 0.8254 | 0.7143 | 0.7477 | ||||||||||
| Adeq. Precision | 21.377 | 14.116 | 13.606 | ||||||||||
| C.V. % | 1.72 | 7.2 | 2.88 | ||||||||||
| PRESS | 139.05 | 80,130.68 | 425.45 | ||||||||||
| Variable | Equation | No. | |
|---|---|---|---|
| Y1 (DPPH) | = | 87.89 – 1.62A – 0.27B + 3.34C – 3.80D – 3.50AB – 1.27AC – 3.86AD – 6.47BC – 0.73BD – 5.09CD – 1.04A2 – 1.56B2 – 2.09C2 – 0.61D2 | (4) |
| Y2 (TPC) | = | 334.09A + 36.64A – 40.50B + 33.50C – 41.22D – 2.79AB – 59.33AC + 3.83AD – 88.62BC + 77.89BD + 28.04CD + 50.54A2 + 37.28B2 + 129.92C2 + 64.69D2 | (5) |
| Y3 (TFC) | = | 79.43 – 0.47A – 4.09B + 2.67C – 3.81D – 5.53AB – 5.17AC + 6.35AD – 5.28BC + 6.09BD – 0.52CD + 6.45A2 + 2.84B2 + 3.13C2 + 6.73D2 | (6) |
| No. | Name | Formula | RT, min | m/z, % |
|---|---|---|---|---|
| 1 | Squalene | C30H50 | 25.284 | 31.7% |
| 2 | Lactic acid | C3H6O3 | 2.183 | 22.4% |
| 3 | Neophytadiene | C20H38 | 11.900 | 22.2% |
| 4 | Cyclotrisiloxane, hexamethyl- | C6H18O3Si3 | 22.044 | 12.1% |
| 5 | 3,7,11,15-Tetramethyl-2-hexadecen-1-ol | C20H40O | 12.390 | 7.4% |
| 6 | Cyclohexane,1,1’-(2-propyl-1,3-propanediyl) bis- | C18H34 | 15.935 | 0.8% |
| 7 | 3-Chloropropionic acid, octadecyl ester | C21H41ClO2 | 8.566 | 0.7% |
| 8 | 1-Octadecyne | C18H34 | 15.291 | 0.7% |
| 9 | Pentanoic acid, 5-hydroxy-, 2,4-di-t-butylphenyl esters | C19H30O3 | 8.166 | 0.5% |
| 10 | 11,13-Dimethyl-12-tetradecen-1-ol acetate | C18H34O2 | 15.727 | 0.4% |
| 11 | 1H-Indene, 5-butyl-6-hexyloctahydro- | C19H36 | 12.945 | 0.3% |
| 12 | Oleic Acid | C18H34O2 | 13.320 | 0.3% |
| 13 | 6-Acetyl-beta-d-mannose | C8H14O7 | 4.014 | 0.2% |
| 14 | E-7-Octadecene | C18H36 | 9.221 | 0.2% |
| No. | Observed | Component Name | Formula | Neutral Mass (Da) | Observed (m/z) | Mass Error (ppm) |
|---|---|---|---|---|---|---|
| RT (min) | ||||||
| 1 | 0.53 | (25R)-26-O-β-D-Glucopyranosyl-5β-furost-20(22)-en-3β,26-diol-3-O-[β-D glucopyranosyl -(1→2)]-β-D-glucopyranoside | C46H76O18 | 916.50317 | 915.4956 | −0.4 |
| 2 | 0.53 | Prosapogenin 5 (Julibroside A1) | C53H84O22 | 1072.54542 | 1071.5399 | 1.7 |
| 3 | 0.53 | Meso-inositol | C6H12O6 | 180.06339 | 179.0557 | −2.2 |
| 4 | 0.55 | Macrostemonoside D | C53H86O24 | 1106.5509 | 1105.5392 | −4 |
| 5 | 0.55 | Calycanthoside | C17H20O10 | 384.10565 | 383.0994 | 2.8 |
| 6 | 3.84 | Castalagin | C41H26O26 | 934.07123 | 933.066 | 2.2 |
| 7 | 5.68 | Gallic acid | C7H6O5 | 170.02152 | 169.0139 | −2.2 |
| 8 | 5.68 | Bistortaside | C22H24O14 | 512.11661 | 511.1083 | −2 |
| 9 | 5.68 | Gemin D | C27H22O18 | 634.08061 | 633.074 | 1 |
| 10 | 5.94 | Geraniin | C41H28O27 | 952.0818 | 951.071 | −3.7 |
| 11 | 5.94 | Potentillin | C41H28O26 | 936.08688 | 935.0821 | 2.7 |
| 12 | 5.94 | Curculigo saponin K | C48H82O19 | 962.54503 | 961.5371 | −0.7 |
| 13 | 7.55 | Jangomolide | C26H28O8 | 468.17842 | 467.1691 | −4.4 |
| 14 | 10.27 | Isopimpinellin | C13H10O5 | 246.05282 | 245.045 | −2.2 |
| 15 | 10.27 | 3,8,9-Trihydeoxy-6H-benzo[c]chromen-6-one | C13H8O5 | 244.03717 | 243.0291 | −3.3 |
| 16 | 10.27 | Quercetin_1 | C15H10O7 | 302.04265 | 301.0343 | v3.5 |
| 17 | 10.27 | Kaempferol-3-O-β-D-glucopyranoside | C21H20O11 | 448.10056 | 447.0936 | 0.7 |
| 18 | 10.27 | Munjistin | C15H8O6 | 284.03209 | 283.0253 | 1.8 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hosni, S.; Gani, S.S.A.; Orsat, V.; Hassan, M.; Abdullah, S. Ultrasound-Assisted Extraction of Antioxidants from Melastoma malabathricum Linn.: Modeling and Optimization Using Box–Behnken Design. Molecules 2023, 28, 487. https://doi.org/10.3390/molecules28020487
Hosni S, Gani SSA, Orsat V, Hassan M, Abdullah S. Ultrasound-Assisted Extraction of Antioxidants from Melastoma malabathricum Linn.: Modeling and Optimization Using Box–Behnken Design. Molecules. 2023; 28(2):487. https://doi.org/10.3390/molecules28020487
Chicago/Turabian StyleHosni, Suzziyana, Siti Salwa Abd Gani, Valérie Orsat, Masriana Hassan, and Sumaiyah Abdullah. 2023. "Ultrasound-Assisted Extraction of Antioxidants from Melastoma malabathricum Linn.: Modeling and Optimization Using Box–Behnken Design" Molecules 28, no. 2: 487. https://doi.org/10.3390/molecules28020487
APA StyleHosni, S., Gani, S. S. A., Orsat, V., Hassan, M., & Abdullah, S. (2023). Ultrasound-Assisted Extraction of Antioxidants from Melastoma malabathricum Linn.: Modeling and Optimization Using Box–Behnken Design. Molecules, 28(2), 487. https://doi.org/10.3390/molecules28020487






