Evaluation the Potential of Onion/Laponite Composites Films for Sustainable Food Packaging with Enhanced UV Protection and Antioxidant Capacity
Abstract
:1. Introduction
2. Results and Discussion
2.1. Physicochemical Properties
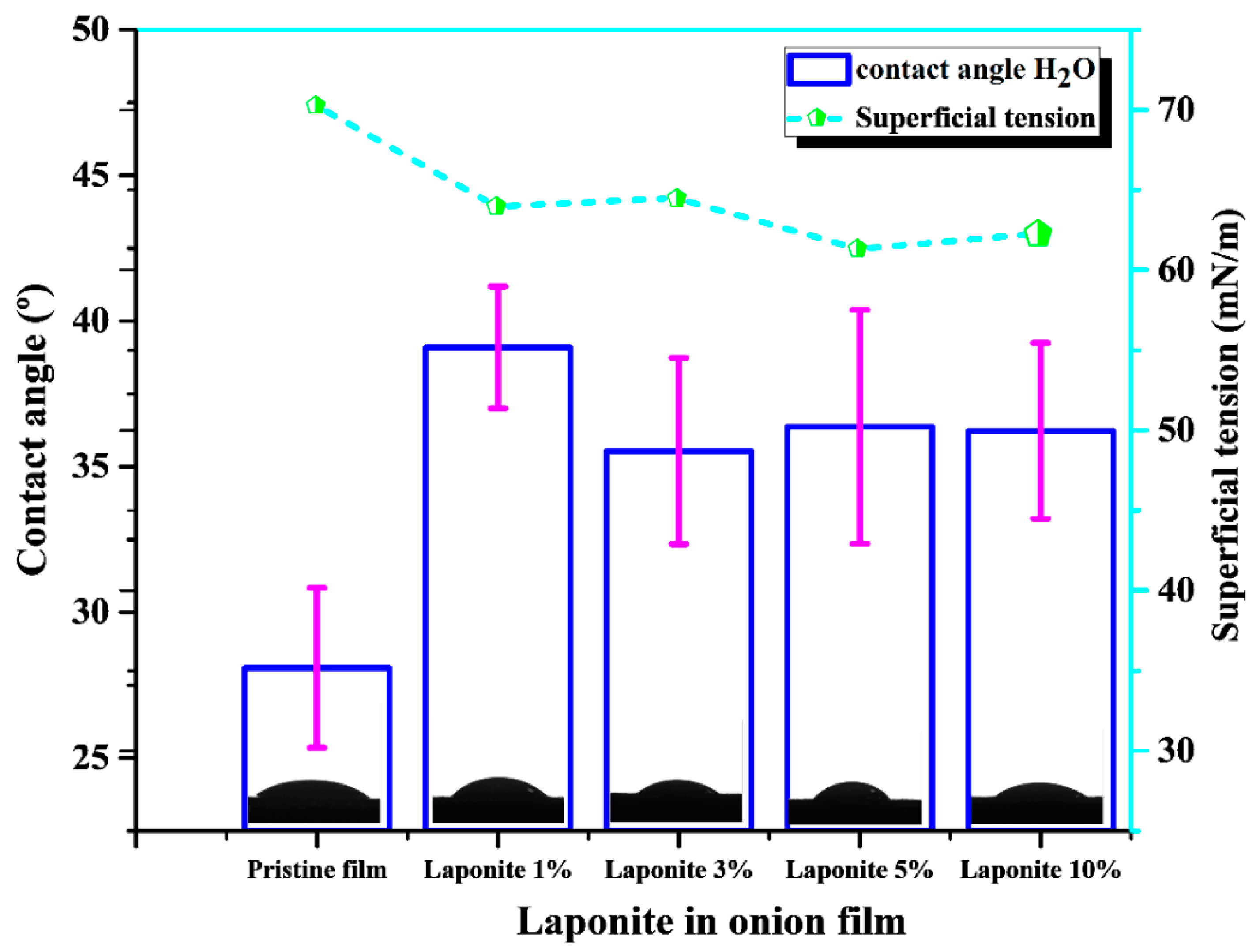
2.2. Surface Wettability Measurements
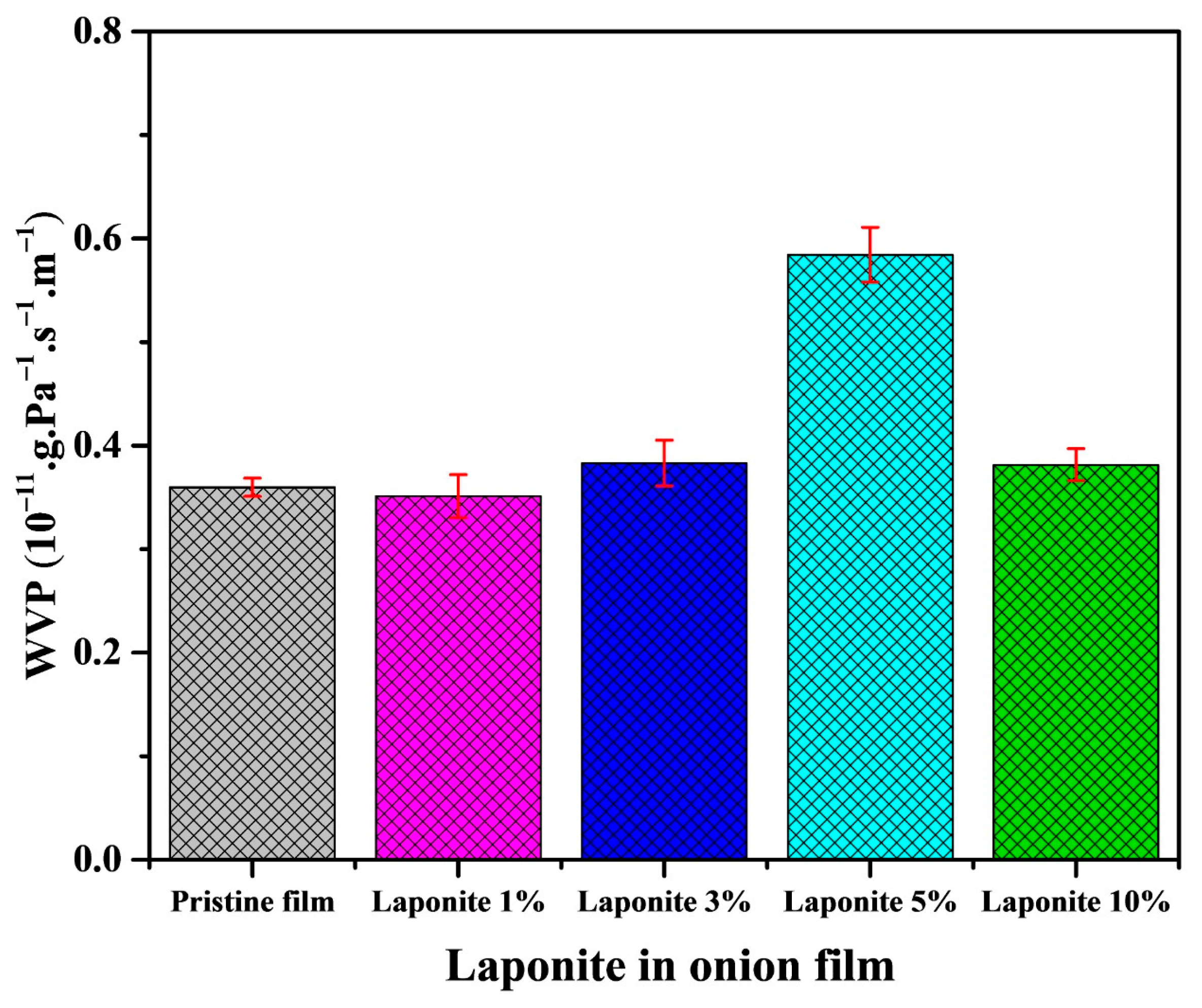
2.3. Water Vapor Permeability
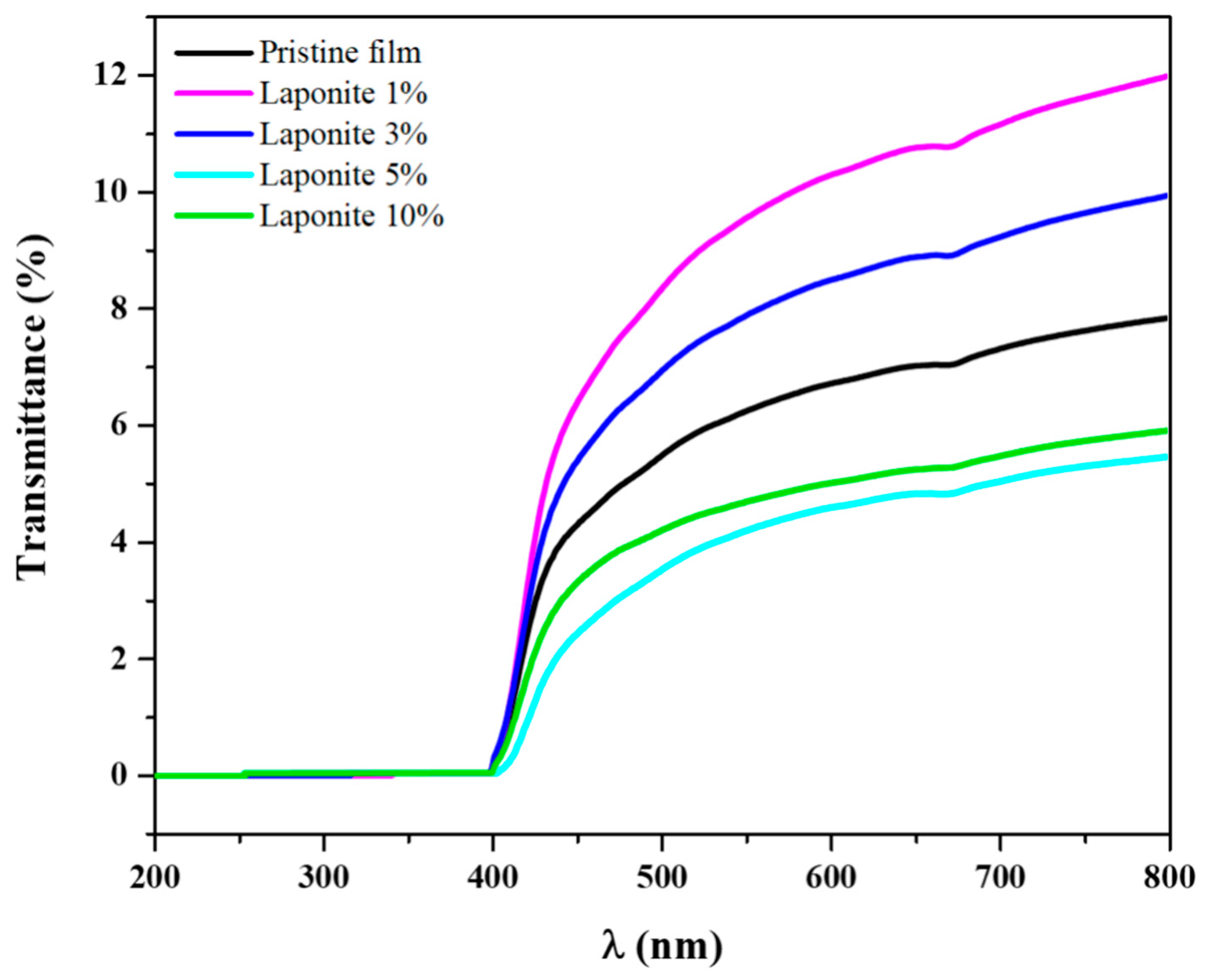
2.4. UV-Vis Spectroscopy
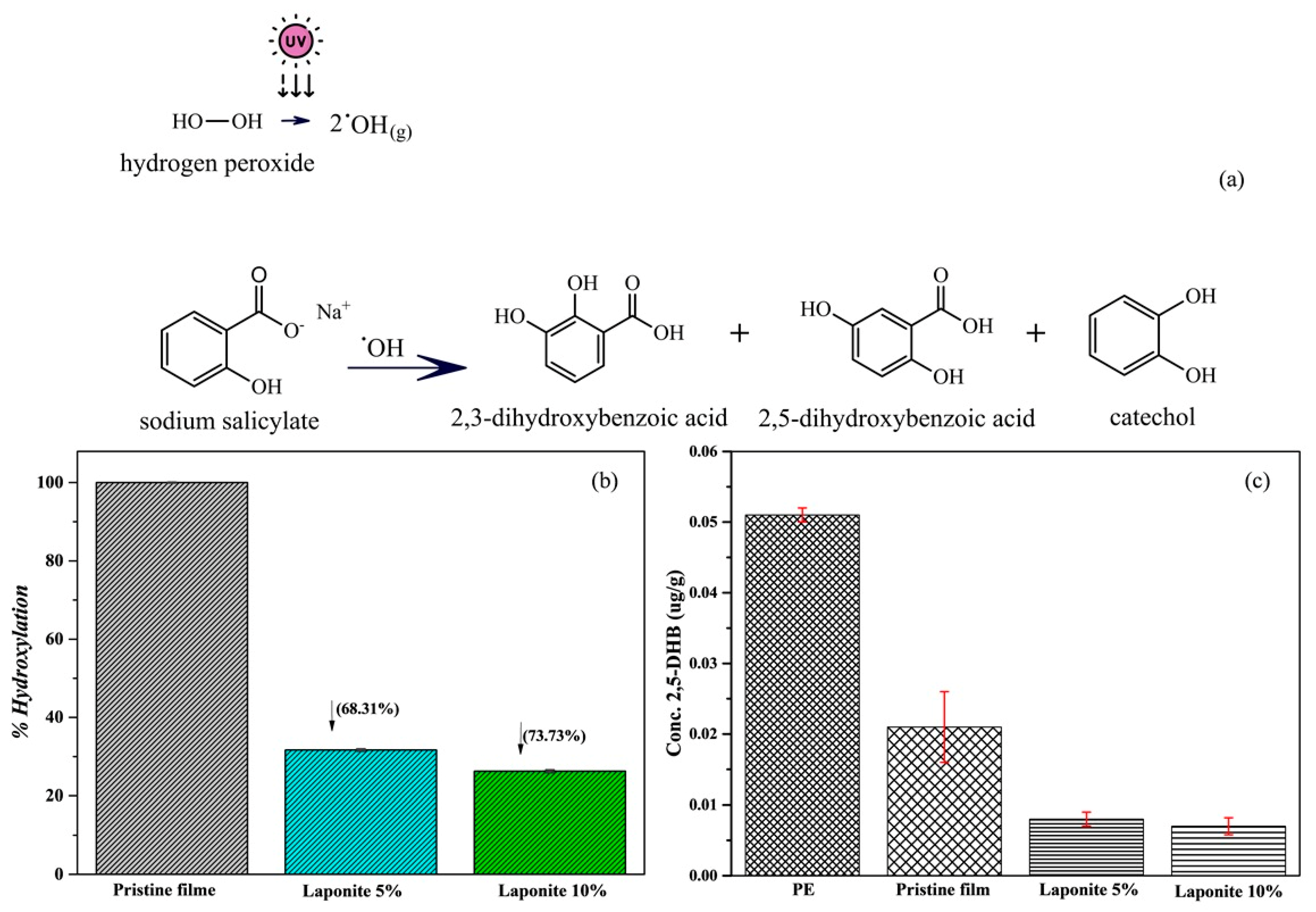
2.5. Antioxidant Properties
3. Materials and Methods
3.1. Materials
3.2. Onion Pulp Preparation and Clay Incorporation
3.3. Characterization of Pristine Onion and Laponite-Modified Films
3.3.1. Physicochemical Properties
3.3.2. Water Vapor Permeability
3.3.3. Antioxidant Analysis
Analysis of the Reaction Product
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Tripathi, P.C.; Lawande, K.E. Therapeutic and Medicinal Value of Onion and Garlic; National Research Centre for Onion and Garlic: Nasik, Maharashtra, 2006. [Google Scholar]
- Faraco, T.A.; Silva, O.X.H.; Barud, H.d.S.; Maciel, I.O.; da Silva, R.R.; Quirino, W.G.; Fragneaud, B.; Ribeiro, C.A.; Dias, D.D.S.; Pandoli, O.G.; et al. Ecological Biosubstrates Obtained from Onion Pulp (Allium cepa L.) for Flexible Organic Light-Emitting Diodes. ACS Appl. Mater. Interfaces 2019, 11, 42420–42428. [Google Scholar] [CrossRef] [PubMed]
- Kumari, N.; Kumar, M.; Radha; Lorenzo, J.M.; Sharma, D.; Puri, S.; Pundir, A.; Dhumal, S.; Bhuyan, D.J.; Jayanthy, G.; et al. Onion and garlic polysaccharides: A review on extraction, characterization, bioactivity, and modifications. Int. J. Biol. Macromol. 2022, 219, 1047–1061. [Google Scholar] [CrossRef] [PubMed]
- Kamerling, J.P. Strategies for the Structural Analysis of Carbohydrates. Compr. Glycosci. 2007, 2, 1–68. [Google Scholar] [CrossRef]
- O’Donoghue, E.M.; Somerfield, S.D.; Shaw, M.; Bendall, M.; Hedderly, D.; Eason, J.; Sims, I. Evaluation of Carbohydrates in Pukekohe Longkeeper and Grano Cultivars of Allium cepa. J. Agric. Food Chem. 2005, 53, 6564. [Google Scholar] [CrossRef]
- FAO. Countries by Commodity—Onions. 2017. Available online: http://www.fao.org/faostat/en/#rankings/countries_by_commodity (accessed on 16 September 2023).
- Sagar, N.A.; Kumar, Y.; Singh, R.; Nickhil, C.; Kumar, D.; Sharma, P.; Om Pandey, H.; Bhoj, S.; Tarafdar, A. Onion waste based-biorefinery for sustainable generation of value-added products. Bioresour. Technol. 2022, 362, 127870. [Google Scholar] [CrossRef]
- Vojvodić, C.A.; Šeremet, D.; Mandura, A.; Martinić, A.; Komes, D. Onion solid waste as a potential source of functional food ingredients. Eng. Power Bull. Croat. Acad. Eng. 2020, 15, 7–13. [Google Scholar]
- Osojnik Črnivec, I.G.; Skrt, M.; Šeremet, D.; Sterniša, M.; Farčnik, D.; Štrumbelj, E.; Poljanšek, A.; Cebin, N.; Pogačnik, L.; Smole Možina, S.; et al. Waste streams in onion production: Bioactive compounds, quercetin and use of antimicrobial and antioxidative properties. Waste Manag. 2021, 126, 476–486. [Google Scholar] [CrossRef]
- Teshika, J.D.; Zakariyyah, A.M.; Zaynab, T.; Zengin, G.; Rengasamy, K.R.; Pandian, S.K.; Fawzi, M.M. Traditional and modern uses of onion bulb (Allium cepa L.): A systematic review. Crit. Rev. Food Sci. Nutr. 2018, 59 (Suppl. 1), S39–S70. [Google Scholar] [CrossRef]
- Dias, D.D.S.; Otoni, C.G.; da Silva, R.R.; Meneguin, A.B.; Mattoso, L.H.C.; da Silva Barud, H.; Ribeiro, C.A. Large scale manufacturing of puree-only edible films from onion bulb (Allium cepa L.): Probing production and structure–processing–property correlations. Ind. Crop. Prod. 2020, 145, 111847. [Google Scholar] [CrossRef]
- Barreto, M.R.; Aleixo, N.A.; Silvestre, R.B.; Fregonezi, N.F.; Barud, H.d.S.; Dias, D.d.S.; Ribeiro, C.A.; Resende, F.A. Genotoxicological safety assessment of puree-only edible films from onion bulb (Allium cepa L.) for use in food packaging-related applications. J. Food Sci. 2019, 85, 201–208. [Google Scholar] [CrossRef]
- Soares, K.S.; Souza, M.P.; Silva-Filho, E.C.; Barud, H.S.; Ribeiro, C.A.; Santos, D.D.; Rocha, K.N.S.; de Moura, J.F.P.; Oliveira, R.L.; Bezerra, L.R. Effect of Edible Onion (Allium cepa L.) Film on Quality, Sensory Properties and Shelf Life of Beef Burger Patties. Molecules 2021, 26, 7202. [Google Scholar] [CrossRef] [PubMed]
- Castro, F.V.R.; Andrade, M.A.; Sanches Silva, A.; Vaz, M.F.; Vilarinho, F. The Contribution of a Whey Protein Film Incorporated with Green Tea Extract to Minimize the Lipid Oxidation of Salmon (Salmo salar L.). Foods 2019, 8, 327. [Google Scholar] [CrossRef] [PubMed]
- Ait Ouahioune, L.; Wrona, M.; Becerril, R.; Salafranca, J.; Nerín, C.; Djenane, D. Ceratonia siliqua L. kibbles, seeds and leaves as a source of volatile bioactive compounds for antioxidant food biopackaging applications. Food Packag. Shelf Life 2022, 31, 100764. [Google Scholar] [CrossRef]
- Domeneguetti, R.R.; Sakai, V.Y.; Perotti, G.F.; Silva, I.C.; Tercjak, A.; Barud, H.S.; Pavan, F.; Constantino, V.R.L.; Ribeiro, S.J. Structural and morphological properties of in-situ biosynthesis of biocompatible bacterial cellulose/Laponite nanocomposites. Appl. Clay Sci. 2023, 234, 106851. [Google Scholar] [CrossRef]
- Silva, J.M.; Barud, H.S.; Meneguin, A.B.; Constantino, V.R.L.; Ribeiro, S.J.L. Inorganic-organic bio-nanocomposite films based on Laponite and Cellulose Nanofibers (CNF). Appl. Clay Sci. 2019, 168, 428–435. [Google Scholar] [CrossRef]
- Valencia, G.A.; Lourenço, R.V.; Bittante, A.M.Q.B.; do Amaral Sobral, P.J. Physical and morphological properties of nanocomposite films based on gelatin and Laponite. Appl. Clay Sci. 2016, 124–125, 260–266. [Google Scholar] [CrossRef]
- Li, X.; Liu, A.; Ye, R.; Wang, Y.; Wang, W. Fabrication of gelatin–laponite composite films: Effect of the concentration of laponite on physical properties and the freshness of meat during storage. Food Hydrocoll. 2015, 44, 390–398. [Google Scholar] [CrossRef]
- Yang, F.; Chen, G.; Li, J.; Zhang, C.; Ma, Z.; Zhao, M.; Yang, Y.; Han, Y.; Huang, Z.; Weng, Y. Effects of Quercetin and Organically Modified Montmorillonite on the Properties of Poly(butylene adipate-co-terephthalate)/Thermoplastic Starch Active Packaging Films. ACS Omega 2022, 8, 663–672. [Google Scholar] [CrossRef]
- Lee, M.H.; Kim, S.Y.; Park, H.J. Effect of halloysite nanoclay on the physical, mechanical, and antioxidant properties of chitosan films incorporated with clove essential oil. Food Hydrocoll. 2018, 84, 58–67. [Google Scholar] [CrossRef]
- Sivasangar, S.; Taufiq-Yap, Y.H.; Zainal, Z.; Kitagawa, K. Thermal behavior of lignocellulosic materials under aerobic/anaerobic environments. Int. J. Hydrog. Energy 2013, 38, 16011–16019. [Google Scholar] [CrossRef]
- Liu, Z.; Zhao, Z.; Jin, X.; Wang, L.-M.; Liu, Y.D. Preparation of Cellulose/Laponite Composite Particles and Their Enhanced Electrorheological Responses. Molecules 2021, 26, 1482. [Google Scholar] [CrossRef] [PubMed]
- Daniel, L.M.; Frost, R.L.; Zhu, H.Y. Edge-modification of laponite with dimethyl-octylmethoxysilane. J. Colloid Interface Sci. 2008, 321, 302–309. [Google Scholar] [CrossRef] [PubMed]
- Gomez-Gamez, A.B.; Yebra-Rodriguez, A.; Peñas-Sanjuan, A.; Soriano-Cuadrado, B.; Jimenez-Millan, J. Influence of clay percentage on the technical properties of montmorillonite/polylactic acid nanocomposites. Appl. Clay Sci. 2020, 198, 105818. [Google Scholar] [CrossRef]
- Brunier, B.; Sheibat-Othman, N.; Chniguir, M.; Chevalier, Y.; Bourgeat-Lami, E. Investigation of Four Different Laponite Clays as Stabilizers in Pickering Emulsion Polymerization. Langmuir 2016, 32, 6046–6057. [Google Scholar] [CrossRef] [PubMed]
- Ninciuleanu, C.M.; Ianchiş, R.; Alexandrescu, E.; Mihăescu, C.I.; Scomoroşcenco, C.; Nistor, C.L.; Preda, S.; Petcu, C.; Teodorescu, M. The Effects of Monomer, Crosslinking Agent, and Filler Concentrations on the Viscoelastic and Swelling Properties of Poly(methacrylic acid) Hydrogels: A Comparison. Materials 2021, 14, 2305. [Google Scholar] [CrossRef]
- Schulz, H.; Baranska, M. Identification and quantification of valuable plant substances by IR and Raman spectroscopy. Vib. Spectrosc. 2007, 43, 13–25. [Google Scholar] [CrossRef]
- Abboud, Y.; Eddahbi, A.; El Bouari, A.; Aitenneite, H.; Brouzi, K.; Mouslim, J. Microwave-assisted approach for rapid and green phytosynthesis of silver nanoparticles using aqueous onion (Allium cepa) extract and their antibacterial activity. J. Nanostructure Chem. 2013, 3, 84. [Google Scholar] [CrossRef]
- Wilson, R.H.; Smith, A.C.; Kačuráková, M.; Saunders, P.K.; Wellner, N.; Waldron, K.W. The Mechanical Properties and Molecular Dynamics of Plant Cell Wall Polysaccharides Studied by Fourier-Transform Infrared Spectroscopy. Plant Physiol. 2000, 124, 397–406. [Google Scholar] [CrossRef]
- Lu, X.; Wang, J.; Al-Qadiri, H.M.; Ross, C.F.; Powers, J.R.; Tang, J.; Rasco, B.A. Determination of total phenolic content and antioxidant capacity of onion (Allium cepa) and shallot (Allium oschaninii) using infrared spectroscopy. Food Chem. 2011, 129, 637–644. [Google Scholar] [CrossRef]
- Van der Sman, R.G.M.; van den Hoek, I.A.F.; Renzetti, S. Sugar replacement with zwitterionic plasticizers like amino acids. Food Hydrocoll. 2020, 109, 106113. [Google Scholar] [CrossRef]
- Kirtil, E.; Aydogdu, A.; Svitova, T.; Radke, C.J. Assessment of the performance of several novel approaches to improve physical properties of guar gum based biopolymer films. Food Packag. Shelf Life 2021, 29, 100687. [Google Scholar] [CrossRef]
- Rouf, T.B.; Schmidt, G.; Kokini, J.L. Zein–Laponite nanocomposites with improved mechanical, thermal and barrier properties. J. Mater. Sci. 2018, 53, 7387–7402. [Google Scholar] [CrossRef]
- Good, R.J. Contact Angles and the Surface Free Energy of Solids. In Surface and Colloid Science; Springer: Boston, MA, USA, 1979; pp. 1–29. [Google Scholar] [CrossRef]
- Carrier, O.; Bonn, D. Contact Angles and the Surface Free Energy of Solids. In Droplet Wetting and Evaporation; Academic Press: Cambridge, MA, USA, 2015; pp. 15–23. [Google Scholar]
- Calambas, H.L.; Fonseca, A.; Adames, D.; Aguirre-Loredo, Y.; Caicedo, C. Physical-Mechanical Behavior and Water-Barrier Properties of Biopolymers-Clay Nanocomposites. Molecules 2021, 26, 6734. [Google Scholar] [CrossRef] [PubMed]
- Condés, M.C.; Añón, M.C.; Dufresne, A.; Mauri, A.N. Composite and nanocomposite films based on amaranth biopolymers. Food Hydrocoll. 2018, 74, 159–167. [Google Scholar] [CrossRef]
- Ezati, P.; Khan, A.; Priyadarshi, R.; Bhattacharya, T.; Tammina, S.K.; Rhim, J.-W. Biopolymer-based UV protection functional films for food packaging. Food Hydrocoll. 2023, 142, 108771. [Google Scholar] [CrossRef]
- Lockhart, H.; Paine, F.A. Child-resistant packaging. In Packaging of Pharmaceuticals and Healthcare Products; Springer: Boston, MA, USA, 1996; pp. 151–171. [Google Scholar] [CrossRef]
- Zanta, C.L.P.S.; Martínez-Huitle, C.A. Degradation of 2-hydroxybenzoic acid by advanced oxidation processes. Braz. J. Chem. Eng. 2009, 26, 503–513. [Google Scholar] [CrossRef]
- Colletti, C.G.; Massaro, M.; Lazzara, G.; Cavallaro, G.; Milioto, S.; Pibiri, I.; Noto, R.; Riela, S. Synthesis, characterization and study of covalently modified triazole LAPONITE® edges. Appl. Clay Sci. 2020, 187, 105489. [Google Scholar] [CrossRef]
- Pálková, H.; Madejová, J.; Zimowska, M.; Serwicka, E.M. Laponite-derived porous clay heterostructures: II. FTIR study of the structure evolution. Microporous Mesoporous Mater. 2010, 127, 237–244. [Google Scholar] [CrossRef]
- Sidhu, J.S.; Ali, M.; Al-Rashdan, A.; Ahmed, N. Onion (Allium cepa L.) is potentially a good source of important antioxidants. J. Food Sci. Technol. 2019, 56, 1811–1819. [Google Scholar] [CrossRef]
- Mukoyama, T. Fitting of Gaussian peaks by simulated annealing. X-ray Spectrom. 2016, 46, 63–68. [Google Scholar] [CrossRef]
- Wu, S. Calculation of interfacial tension in polymer systems. J. Polym. Sci. 2007, 34, 19–30. [Google Scholar] [CrossRef]
- Pezo, D.; Salafranca, J.; Nerín, C. Determination of the antioxidant capacity of active food packagings by in situ gas-phase hydroxyl radical generation and high-performance liquid chromatography–fluorescence detection. J. Chromatogr. A 2008, 1178, 126–133. [Google Scholar] [CrossRef] [PubMed]

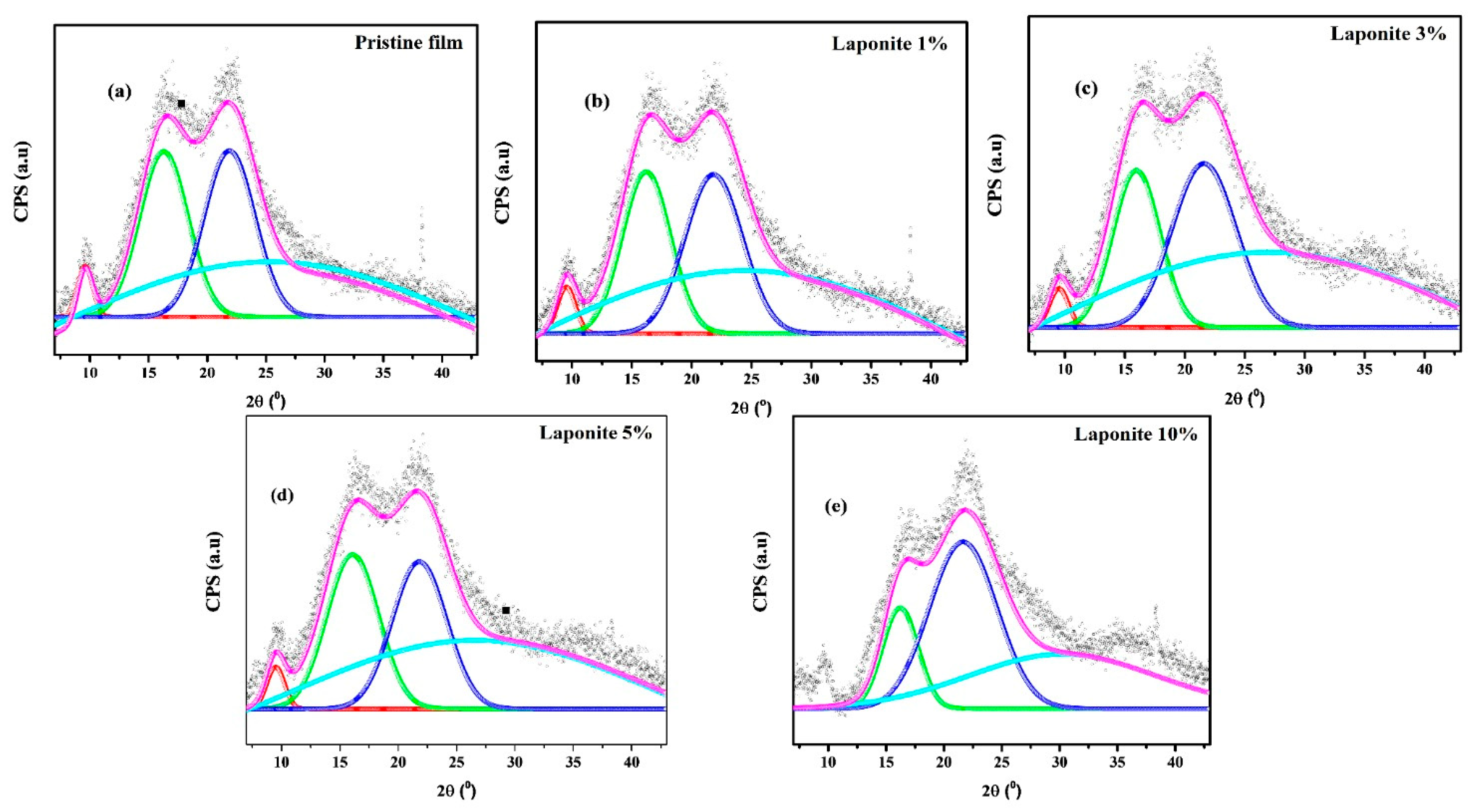
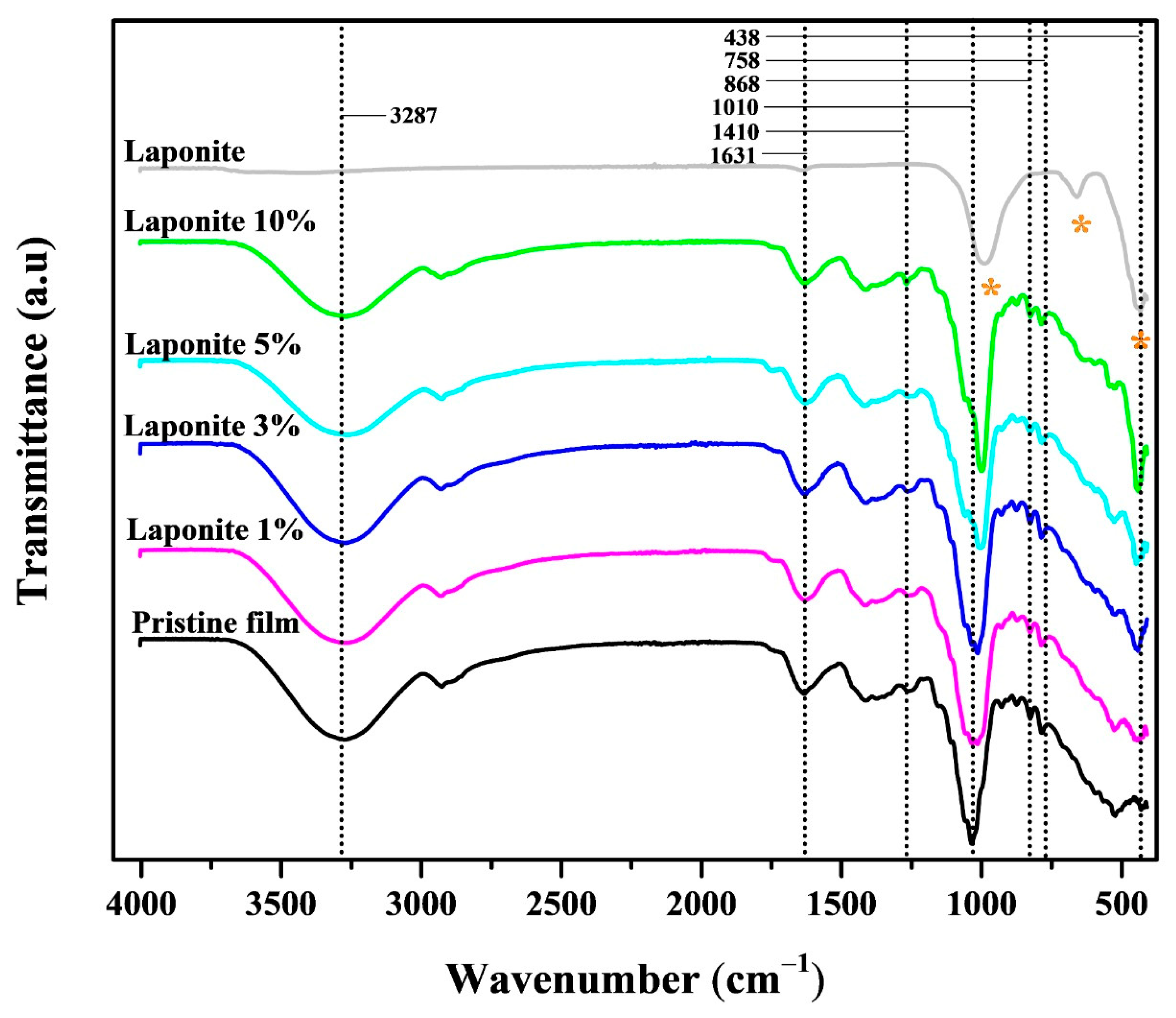
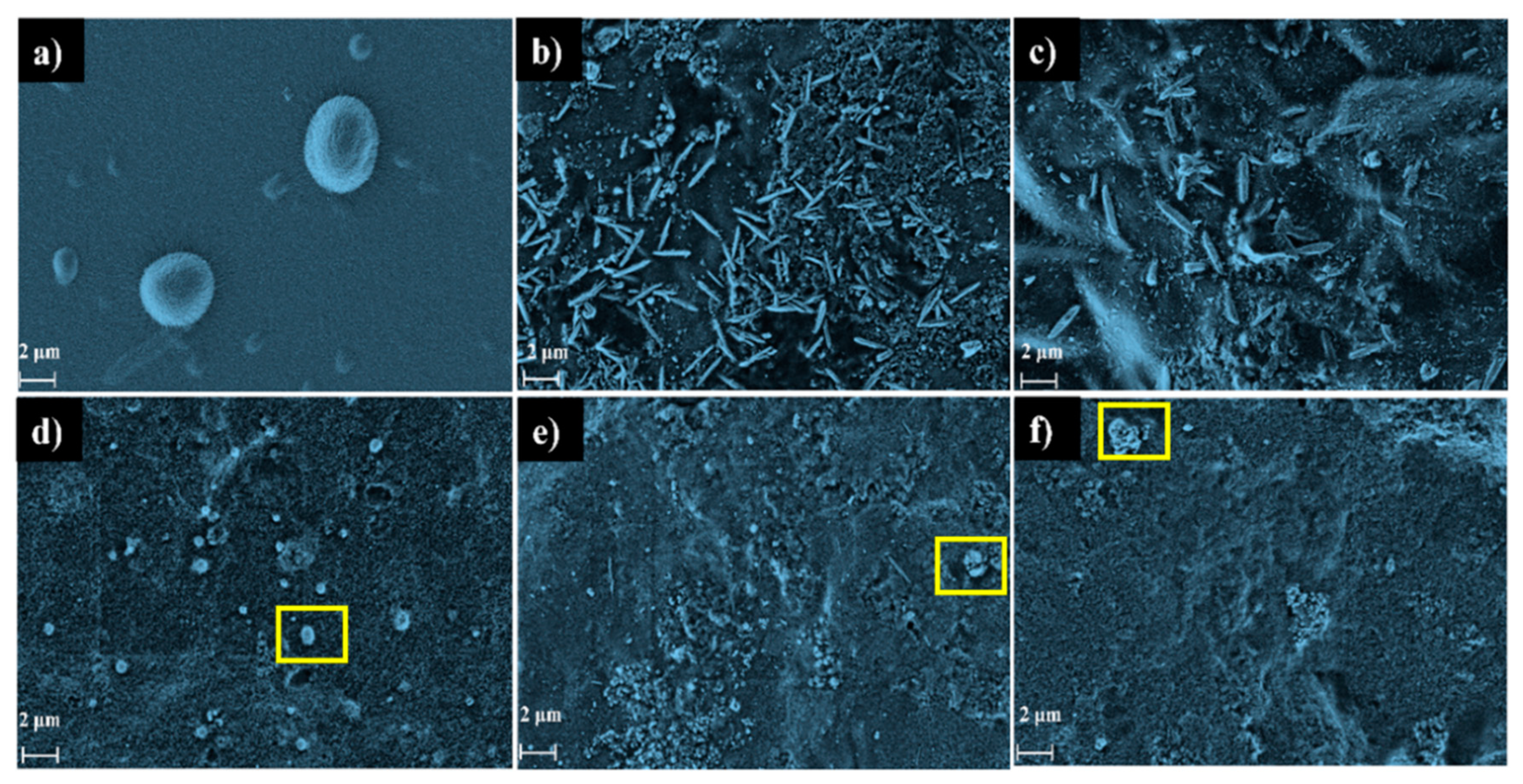
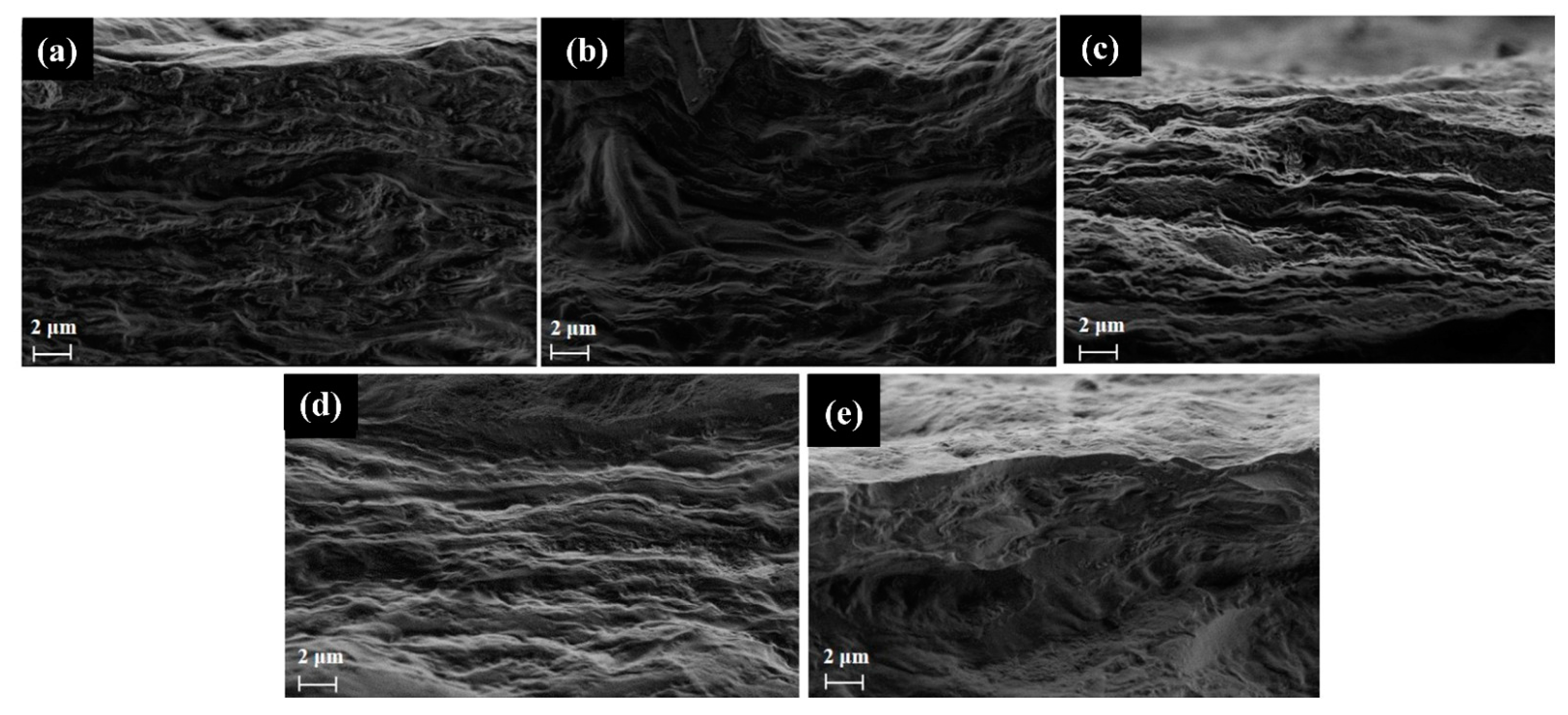
| Film | Crl (%) | Thickness (μm) | Opacity (a.u/mm) |
|---|---|---|---|
| Pristine film | 20.3 | 0.153 ± 0.02 | 7.7 |
| Laponite 1% | 17.5 | 0.152 ± 0.01 | 6.5 |
| Laponite 3% | 19.7 | 0.142 ± 0.01 | 7.5 |
| Laponite 5% | 25.5 | 0.149 ± 0.01 | 9.0 |
| Laponite 10% | 59.2 | 0.142 ± 0.02 | 9.1 |
| Liquids | Water | Diiodomethane | |
|---|---|---|---|
| Coef. Liq/vap | γL (mN/m) | 72.8 | 50.8 |
| Polar Component Liq/Vap | (mN/m) | 51.0 | 0.0 |
| Dispersive Component | (mN/m) | 21.8 | 50.8 |
| Parameters | Value |
|---|---|
| Linearity range (µg/g) | 0.0014–0.14 |
| Correlation coefficient (r) | 1.0000 |
| Limit of Detection—LOD (ng/g) | 0.41 |
| Limit of Quantification—LOQ (ng/g) | 13.75 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Barbosa, M.L.; Oliveira, L.M.d.; Paiva, R.; Dametto, A.C.; Dias, D.d.S.; Ribeiro, C.A.; Wrona, M.; Nerín, C.; Barud, H.d.S.; Cruz, S.A. Evaluation the Potential of Onion/Laponite Composites Films for Sustainable Food Packaging with Enhanced UV Protection and Antioxidant Capacity. Molecules 2023, 28, 6829. https://doi.org/10.3390/molecules28196829
Barbosa ML, Oliveira LMd, Paiva R, Dametto AC, Dias DdS, Ribeiro CA, Wrona M, Nerín C, Barud HdS, Cruz SA. Evaluation the Potential of Onion/Laponite Composites Films for Sustainable Food Packaging with Enhanced UV Protection and Antioxidant Capacity. Molecules. 2023; 28(19):6829. https://doi.org/10.3390/molecules28196829
Chicago/Turabian StyleBarbosa, Maciel L., Leticia M. de Oliveira, Robert Paiva, Alessandra C. Dametto, Diogenes dos S. Dias, Clovis A. Ribeiro, Magdalena Wrona, Cristina Nerín, Hernane da S. Barud, and Sandra A. Cruz. 2023. "Evaluation the Potential of Onion/Laponite Composites Films for Sustainable Food Packaging with Enhanced UV Protection and Antioxidant Capacity" Molecules 28, no. 19: 6829. https://doi.org/10.3390/molecules28196829
APA StyleBarbosa, M. L., Oliveira, L. M. d., Paiva, R., Dametto, A. C., Dias, D. d. S., Ribeiro, C. A., Wrona, M., Nerín, C., Barud, H. d. S., & Cruz, S. A. (2023). Evaluation the Potential of Onion/Laponite Composites Films for Sustainable Food Packaging with Enhanced UV Protection and Antioxidant Capacity. Molecules, 28(19), 6829. https://doi.org/10.3390/molecules28196829













