Nanoemulsion-Based Multilayer Films for Ground Beef Preservation: Antimicrobial Activity and Physicochemical Properties
Abstract
1. Introduction
2. Results and Discussion
2.1. Particle Size and Homogeneity of Nanoemulsions
2.2. Zeta Potential
2.3. Thickness of Films
2.4. Opacity of Films
2.5. Moisture Absorption
2.6. Mechanical Properties
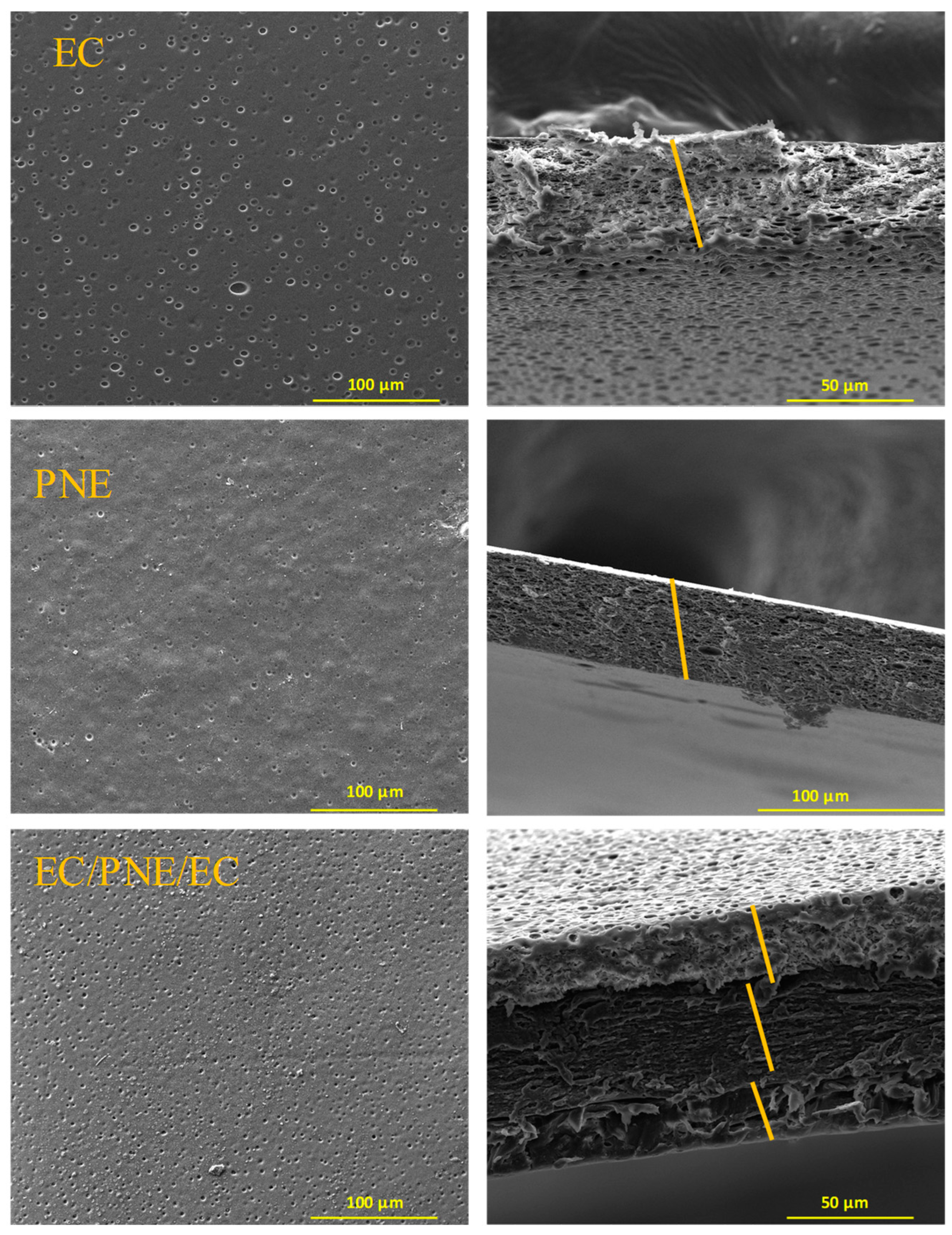
2.7. Morphological Properties of Films
2.8. Antimicrobial Analysis
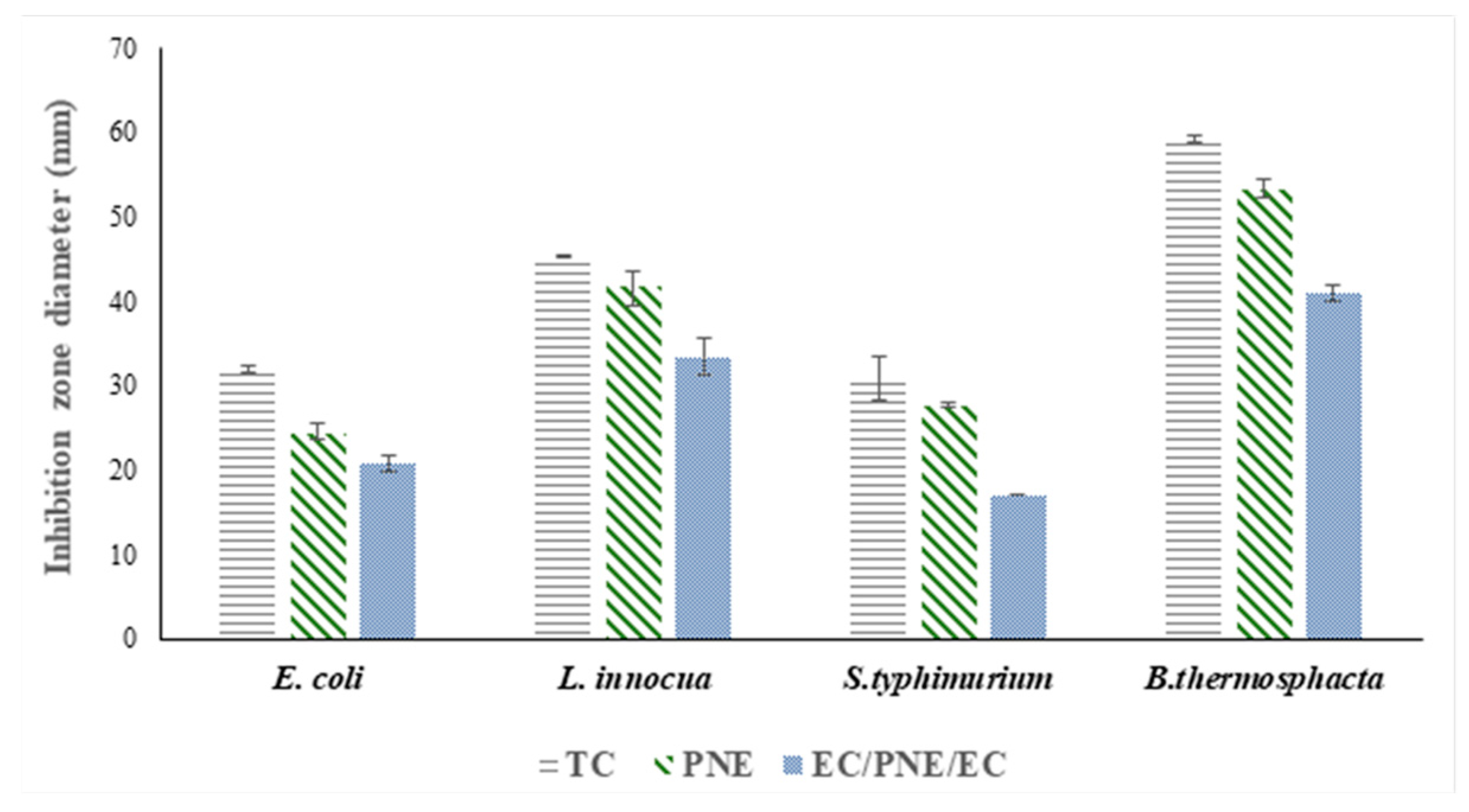
2.8.1. In Vitro Antimicrobial Activity of Films
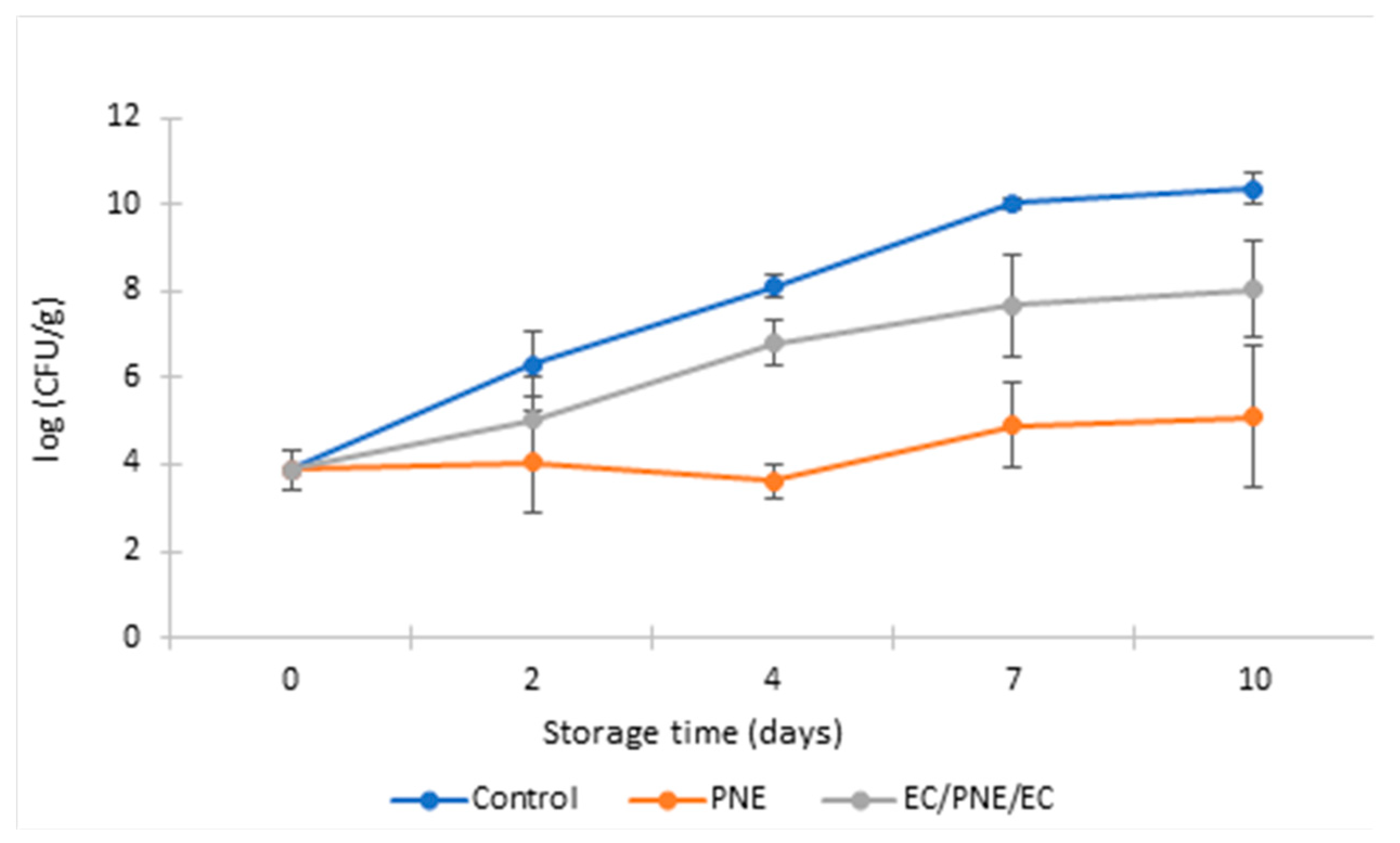
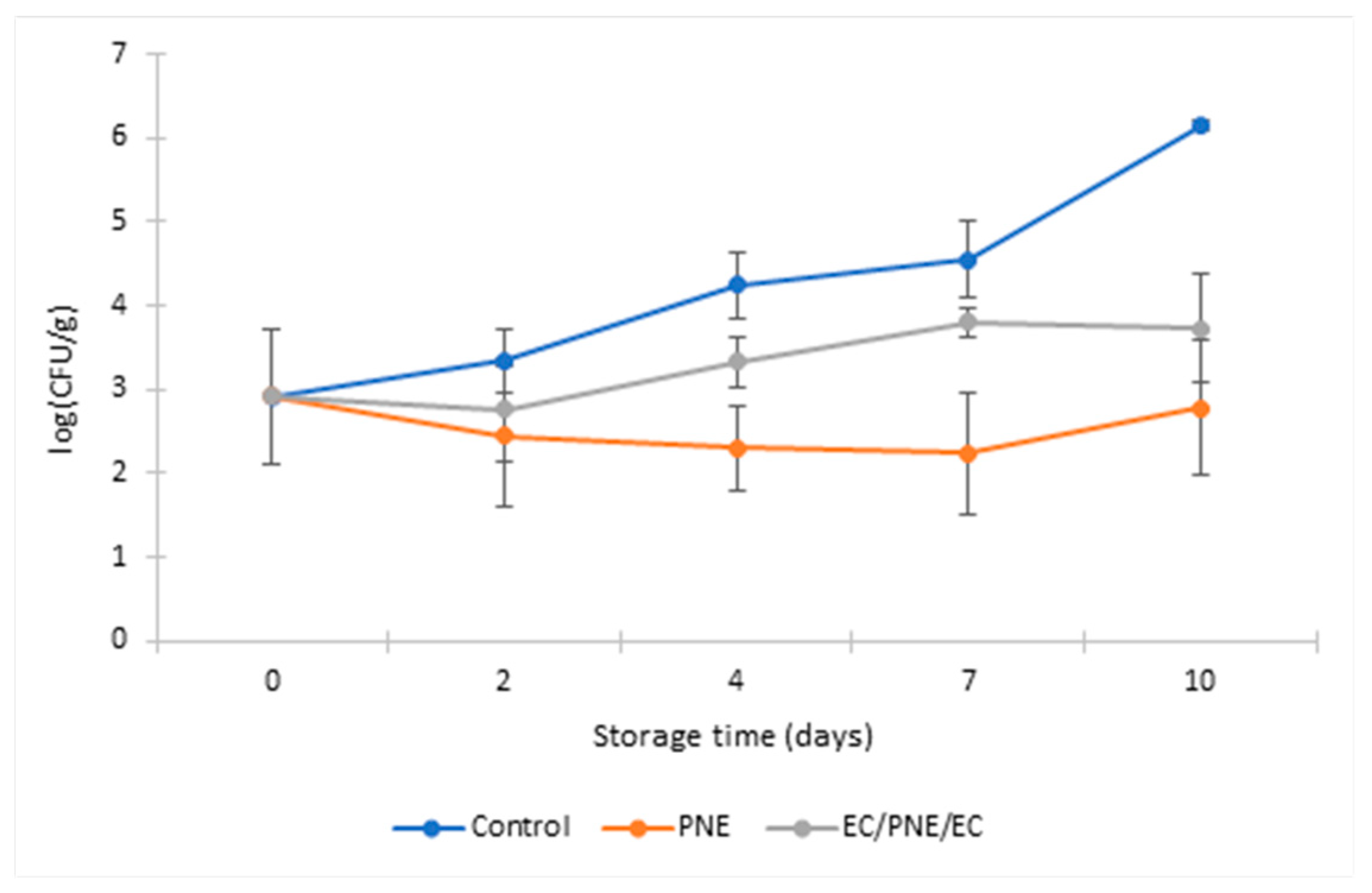
2.8.2. Antimicrobial Activity of Mono and Multilayer Active Films on Refrigerated Stored Ground Beef Patties
3. Materials and Methods
3.1. Materials
3.2. Preparation of the Nanoemulsions (NE)
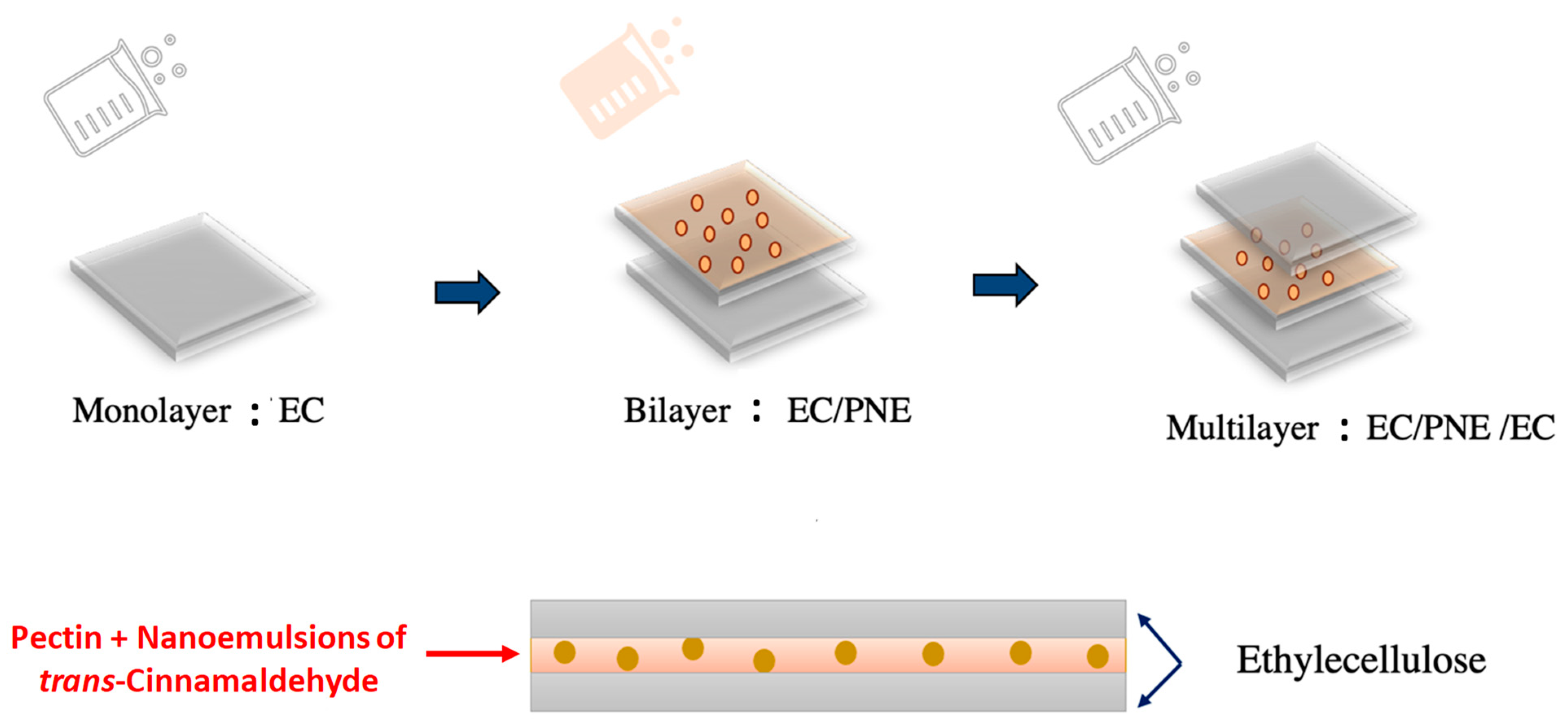
3.3. Preparation of Active Multilayer Films
3.3.1. Preparation of Film-Forming Solutions
3.3.2. Preparation of Films
3.4. Characterization of Nanoemulsions
3.4.1. Size Distribution
3.4.2. Zeta Potential (ζ)
3.5. Physicochemical Characterization of Films
3.5.1. Film Thickness
3.5.2. Light Transmission and Transparency of Films
3.5.3. Moisture Absorption
3.5.4. Mechanical Properties
3.5.5. Scanning Electron Microscopy (SEM)
3.6. Antimicrobial Activity of Films
3.6.1. In vitro Antimicrobial Activity
3.6.2. Efficiency of the Prepared Active Films during the Storage of Packed Ground Beef
3.7. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Walther, B.A.; Kusui, T.; Yen, N.; Hu, C.-S.; Lee, H. Plastic Pollution in East Asia: Macroplastics and Microplastics in the Aquatic Environment and Mitigation Efforts by Various Actors. In Plastics in the Aquatic Environment—Part I: Current Status and Challenges; The Handbook of Environmental Chemistry; Stock, F., Reifferscheid, G., Brennholt, N., Kostianaia, E., Eds.; Springer International Publishing: Cham, Switzerland, 2022; pp. 353–403. ISBN 978-3-030-84118-8. [Google Scholar]
- Defruyt, S. Towards a New Plastics Economy. In Field Actions Science Reports; Institut Veolia: Aubervilliers, France, 2019; pp. 78–81. [Google Scholar]
- Fabra, M.J.; López-Rubio, A.; Lagaron, J.M. 15—Biopolymers for Food Packaging Applications. In Smart Polymers and their Applications; Aguilar, M.R., San Román, J., Eds.; Woodhead Publishing: Cambridge, UK, 2014; pp. 476–509. ISBN 978-0-85709-695-1. [Google Scholar]
- Luo, S.; Chen, R.; Huang, L.; Liang, R.; Liu, C.; Chen, J. Investigation on the Influence of Pectin Structures on the Pasting Properties of Rice Starch by Multiple Regression. Food Hydrocoll. 2017, 63, 580–584. [Google Scholar] [CrossRef]
- Gao, H.-X.; He, Z.; Sun, Q.; He, Q.; Zeng, W.-C. A Functional Polysaccharide Film Forming by Pectin, Chitosan, and Tea Polyphenols. Carbohydr. Polym. 2019, 215, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Dranca, F.; Oroian, M. Extraction, Purification and Characterization of Pectin from Alternative Sources with Potential Technological Applications. Food Res. Int. 2018, 113, 327–350. [Google Scholar] [CrossRef] [PubMed]
- Espitia, P.J.P.; Du, W.-X.; de Avena-Bustillos, R.J.; de Soares, N.F.F.; McHugh, T.H. Edible Films from Pectin: Physical-Mechanical and Antimicrobial Properties—A Review. Food Hydrocoll. 2014, 35, 287–296. [Google Scholar] [CrossRef]
- Quinto, E.J.; Caro, I.; Villalobos-Delgado, L.H.; Mateo, J.; De-Mateo-Silleras, B.; Redondo-Del-Río, M.P. Food Safety through Natural Antimicrobials. Antibiotics 2019, 8, 208. [Google Scholar] [CrossRef] [PubMed]
- Burt, S. Essential Oils: Their Antibacterial Properties and Potential Applications in Foods—A Review. Int. J. Food Microbiol. 2004, 94, 223–253. [Google Scholar] [CrossRef]
- Aljaafari, M.N.; AlAli, A.O.; Baqais, L.; Alqubaisy, M.; AlAli, M.; Molouki, A.; Ong-Abdullah, J.; Abushelaibi, A.; Lai, K.-S.; Lim, S.-H.E. An Overview of the Potential Therapeutic Applications of Essential Oils. Molecules 2021, 26, 628. [Google Scholar] [CrossRef]
- Chen, H.; Hu, X.; Chen, E.; Wu, S.; McClements, D.J.; Liu, S.; Li, B.; Li, Y. Preparation, Characterization, and Properties of Chitosan Films with Cinnamaldehyde Nanoemulsions. Food Hydrocoll. 2016, 61, 662–671. [Google Scholar] [CrossRef]
- Mukurumbira, A.R.; Shellie, R.A.; Keast, R.; Palombo, E.A.; Jadhav, S.R. Encapsulation of Essential Oils and Their Application in Antimicrobial Active Packaging. Food Control 2022, 136, 108883. [Google Scholar] [CrossRef]
- Jafari, S.M. 1—An Overview of Nanoencapsulation Techniques and Their Classification. In Nanoencapsulation Technologies for the Food and Nutraceutical Industries; Jafari, S.M., Ed.; Academic Press: Cambridge, MA, USA, 2017; pp. 1–34. ISBN 978-0-12-809436-5. [Google Scholar]
- Sharma, R.; Jafari, S.M.; Sharma, S. Antimicrobial Bio-Nanocomposites and Their Potential Applications in Food Packaging. Food Control 2020, 112, 107086. [Google Scholar] [CrossRef]
- Nisar, T.; Wang, Z.-C.; Yang, X.; Tian, Y.; Iqbal, M.; Guo, Y. Characterization of Citrus Pectin Films Integrated with Clove Bud Essential Oil: Physical, Thermal, Barrier, Antioxidant and Antibacterial Properties. Int. J. Biol. Macromol. 2018, 106, 670–680. [Google Scholar] [CrossRef] [PubMed]
- Almasi, H.; Azizi, S.; Amjadi, S. Development and Characterization of Pectin Films Activated by Nanoemulsion and Pickering Emulsion Stabilized Marjoram (Origanum majorana L.) Essential Oil. Food Hydrocoll. 2020, 99, 105338. [Google Scholar] [CrossRef]
- Alvarez, M.V.; Ortega-Ramirez, L.A.; Gutierrez-Pacheco, M.M.; Bernal-Mercado, A.T.; Rodriguez-Garcia, I.; Gonzalez-Aguilar, G.A.; Ponce, A.; del Moreira, M.R.; Roura, S.I.; Ayala-Zavala, J.F. Oregano Essential Oil-Pectin Edible Films as Anti-Quorum Sensing and Food Antimicrobial Agents. Front. Microbiol. 2014, 5, 699. [Google Scholar] [CrossRef] [PubMed]
- Guerra-Rosas, M.I.; Morales-Castro, J.; Cubero-Márquez, M.A.; Salvia-Trujillo, L.; Martín-Belloso, O. Antimicrobial Activity of Nanoemulsions Containing Essential Oils and High Methoxyl Pectin during Long-Term Storage. Food Control 2017, 77, 131–138. [Google Scholar] [CrossRef]
- Yang, D.; Peng, X.; Zhong, L.; Cao, X.; Chen, W.; Zhang, X.; Liu, S.; Sun, R. “Green” Films from Renewable Resources: Properties of Epoxidized Soybean Oil Plasticized Ethyl Cellulose Films. Carbohydr. Polym. 2014, 103, 198–206. [Google Scholar] [CrossRef] [PubMed]
- Ju, J.; Chen, X.; Xie, Y.; Yu, H.; Guo, Y.; Cheng, Y.; Qian, H.; Yao, W. Application of Essential Oil as a Sustained Release Preparation in Food Packaging. Trends Food Sci. Technol. 2019, 92, 22–32. [Google Scholar] [CrossRef]
- Wang, Q.; Chen, W.; Zhu, W.; McClements, D.J.; Liu, X.; Liu, F. A Review of Multilayer and Composite Films and Coatings for Active Biodegradable Packaging. Npj Sci. Food 2022, 6, 18. [Google Scholar] [CrossRef]
- Almasi, H.; Jahanbakhsh Oskouie, M.; Saleh, A. A Review on Techniques Utilized for Design of Controlled Release Food Active Packaging. Crit. Rev. Food Sci. Nutr. 2021, 61, 2601–2621. [Google Scholar] [CrossRef]
- Mastromatteo, M.; Barbuzzi, G.; Conte, A.; Del Nobile, M.A. Controlled Release of Thymol from Zein Based Film. Innov. Food Sci. Emerg. Technol. 2009, 10, 222–227. [Google Scholar] [CrossRef]
- Stroescu, M.; Stoica-Guzun, A.; Jipa, I.M. Vanillin Release from Poly(Vinyl Alcohol)-Bacterial Cellulose Mono and Multilayer Films. J. Food Eng. 2013, 114, 153–157. [Google Scholar] [CrossRef]
- Buonocore, G.G.; Conte, A.; Corbo, M.R.; Sinigaglia, M.; Del Nobile, M.A. Mono- and Multilayer Active Films Containing Lysozyme as Antimicrobial Agent. Innov. Food Sci. Emerg. Technol. 2005, 6, 459–464. [Google Scholar] [CrossRef]
- Jipa, I.M.; Stoica-Guzun, A.; Stroescu, M. Controlled Release of Sorbic Acid from Bacterial Cellulose Based Mono and Multilayer Antimicrobial Films. LWT 2012, 47, 400–406. [Google Scholar] [CrossRef]
- Xiong, Y.; Li, S.; Warner, R.D.; Fang, Z. Effect of Oregano Essential Oil and Resveratrol Nanoemulsion Loaded Pectin Edible Coating on the Preservation of Pork Loin in Modified Atmosphere Packaging. Food Control 2020, 114, 107226. [Google Scholar] [CrossRef]
- Bogdanović, U.; Lazić, V.; Vodnik, V.; Budimir, M.; Marković, Z.; Dimitrijević, S. Copper Nanoparticles with High Antimicrobial Activity. Mater. Lett. 2014, 128, 75–78. [Google Scholar] [CrossRef]
- Mudalige, T.; Qu, H.; Van Haute, D.; Ansar, S.M.; Paredes, A.; Ingle, T. Chapter 11—Characterization of Nanomaterials: Tools and Challenges. In Nanomaterials for Food Applications; Micro and Nano Technologies; López Rubio, A., Fabra Rovira, M.J., Martínez Sanz, M., Gómez-Mascaraque, L.G., Eds.; Elsevier: Amsterdam, The Netherlands, 2019; pp. 313–353. ISBN 978-0-12-814130-4. [Google Scholar]
- Duffy, J.; Larsson, M.; Hill, A. Suspension Stability; Why Particle Size, Zeta Potential and Rheology Are Important. Annu. Trans. Nord. Rheol. Soc. 2012, 20, 6. [Google Scholar]
- Galdeano, M.C.; Wilhelm, A.E.; Mali, S.; Grossmann, M.V.E. Influence of Thickness on Properties of Plasticized Oat Starch Films. Braz. Arch. Biol. Technol. 2013, 56, 637–644. [Google Scholar] [CrossRef]
- Escamilla-García, M.; Reyes-Basurto, A.; García-Almendárez, B.E.; Hernández-Hernández, E.; Calderón-Domínguez, G.; Rossi-Márquez, G.; Regalado-González, C. Modified Starch-Chitosan Edible Films: Physicochemical and Mechanical Characterization. Coatings 2017, 7, 224. [Google Scholar] [CrossRef]
- Ghanbarzadeh, B.; Almasi, H. Physical Properties of Edible Emulsified Films Based on Carboxymethyl Cellulose and Oleic Acid. Int. J. Biol. Macromol. 2011, 48, 44–49. [Google Scholar] [CrossRef]
- Fasihi, H.; Fazilati, M.; Hashemi, M.; Noshirvani, N. Novel Carboxymethyl Cellulose-Polyvinyl Alcohol Blend Films Stabilized by Pickering Emulsion Incorporation Method. Carbohydr. Polym. 2017, 167, 79–89. [Google Scholar] [CrossRef]
- Sánchez-González, L.; Chiralt, A.; González-Martínez, C.; Cháfer, M. Effect of Essential Oils on Properties of Film Forming Emulsions and Films Based on Hydroxypropylmethylcellulose and Chitosan. J. Food Eng. 2011, 105, 246–253. [Google Scholar] [CrossRef]
- Martucci, J.F.; Ruseckaite, R.A. Biodegradation of Three-Layer Laminate Films Based on Gelatin under Indoor Soil Conditions. Polym. Degrad. Stab. 2009, 94, 1307–1313. [Google Scholar] [CrossRef]
- Xia, C.; Wang, W.; Wang, L.; Liu, H.; Xiao, J. Multilayer Zein/Gelatin Films with Tunable Water Barrier Property and Prolonged Antioxidant Activity. Food Packag. Shelf Life 2019, 19, 76–85. [Google Scholar] [CrossRef]
- Walsh, S.E.; Maillard, J.-Y.; Russell, A.D.; Catrenich, C.E.; Charbonneau, D.L.; Bartolo, R.G. Activity and Mechanisms of Action of Selected Biocidal Agents on Gram-Positive and -Negative Bacteria. J. Appl. Microbiol. 2003, 94, 240–247. [Google Scholar] [CrossRef] [PubMed]
- Fisher, K.; Phillips, C.A. The Effect of Lemon, Orange and Bergamot Essential Oils and Their Components on the Survival of Campylobacter Jejuni, Escherichia Coli O157, Listeria Monocytogenes, Bacillus Cereus and Staphylococcus Aureus in Vitro and in Food Systems. J. Appl. Microbiol. 2006, 101, 1232–1240. [Google Scholar] [CrossRef] [PubMed]
- Srisa, A.; Harnkarnsujarit, N. Antifungal Films from Trans-Cinnamaldehyde Incorporated Poly(Lactic Acid) and Poly(Butylene Adipate-Co-Terephthalate) for Bread Packaging. Food Chem. 2020, 333, 127537. [Google Scholar] [CrossRef] [PubMed]
- Ünalan, İ.U.; Korel, F.; Yemenicioğlu, A. Active Packaging of Ground Beef Patties by Edible Zein Films Incorporated with Partially Purified Lysozyme and Na2EDTA. Int. J. Food Sci. Technol. 2011, 46, 1289–1295. [Google Scholar] [CrossRef]
- Xavier, L.O.; Sganzerla, W.G.; Rosa, G.B.; da Rosa, C.G.; Agostinetto, L.; de Veeck, A.P.L.; Bretanha, L.C.; Micke, G.A.; Dalla Costa, M.; Bertoldi, F.C.; et al. Chitosan Packaging Functionalized with Cinnamodendron Dinisii Essential Oil Loaded Zein: A Proposal for Meat Conservation. Int. J. Biol. Macromol. 2021, 169, 183–193. [Google Scholar] [CrossRef] [PubMed]
- dos Caetano, K.S.; Hessel, C.T.; Tondo, E.C.; Flôres, S.H.; Cladera-Olivera, F. Application of Active Cassava Starch Films Incorporated with Oregano Essential Oil and Pumpkin Residue Extract on Ground Beef. J. Food Saf. 2017, 37, e12355. [Google Scholar] [CrossRef]
- Quesada, J.; Sendra, E.; Navarro, C.; Sayas-Barberá, E. Antimicrobial Active Packaging Including Chitosan Films with Thymus vulgaris L. Essential Oil for Ready-to-Eat Meat. Foods 2016, 5, 57. [Google Scholar] [CrossRef]
- Peng, Y.; Li, Y. Combined Effects of Two Kinds of Essential Oils on Physical, Mechanical and Structural Properties of Chitosan Films. Food Hydrocoll. 2014, 36, 287–293. [Google Scholar] [CrossRef]
- Ghadetaj, A.; Almasi, H.; Mehryar, L. Development and Characterization of Whey Protein Isolate Active Films Containing Nanoemulsions of Grammosciadium Ptrocarpum Bioss. Essential Oil. Food Packag. Shelf Life 2018, 16, 31–40. [Google Scholar] [CrossRef]

| Film Samples | Thickness (μm) | Opacity (A × mm−1) | Moisture Absorption (%) |
|---|---|---|---|
| P | 44.33 ± 2.08 c | 1.20 ± 0.02 b | 22.54 ± 1.81 a |
| PNE | 62.00 ± 2.65 c | 15.19 ± 1.55 a | 9.40 ± 2.28 c |
| EC | 105.33 ± 7.02 b | 0.97 ± 0.06 b | 7.21 ± 0.20 c |
| EC/PNE/EC | 285.67 ± 18.65 a | 2.02 ± 0.14 b | 15.35 ± 0.64 b |
| Symbol of Samples | Tensile Strength (MPa) | Elongation at Break (%) | Young Module (MPa) |
|---|---|---|---|
| P | 3.03 ± 0.27 b | 8.16 ± 1.08 ab | 42.18 ± 3.10 c |
| PNE | 1.61 ± 0.28 b | 4.14 ± 1.43 c | 48.15 ± 11.69 c |
| EC | 13.69 ± 1.38 a | 7.52 ± 0.76 b | 220.37 ± 24.14 a |
| EC/PNE/EC | 14.98 ± 0.90 a | 10.20 ± 0.98 a | 179.22 ± 23.02 b |
| Sample Names | Composition of the Film |
|---|---|
| P | Monolayer of Pectin |
| PNE | Monolayer of pectin incorporated by nanoemulsion of trans-Cinnamaldehyde |
| EC | Monolayer of ethyl cellulose |
| EC/PNE/EC | Multilayer including two outer layers of EC and one intermediate layer of PNE |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Baghi, F.; Ghnimi, S.; Dumas, E.; Chihib, N.-E.; Gharsallaoui, A. Nanoemulsion-Based Multilayer Films for Ground Beef Preservation: Antimicrobial Activity and Physicochemical Properties. Molecules 2023, 28, 4274. https://doi.org/10.3390/molecules28114274
Baghi F, Ghnimi S, Dumas E, Chihib N-E, Gharsallaoui A. Nanoemulsion-Based Multilayer Films for Ground Beef Preservation: Antimicrobial Activity and Physicochemical Properties. Molecules. 2023; 28(11):4274. https://doi.org/10.3390/molecules28114274
Chicago/Turabian StyleBaghi, Fatemeh, Sami Ghnimi, Emilie Dumas, Nour-Eddine Chihib, and Adem Gharsallaoui. 2023. "Nanoemulsion-Based Multilayer Films for Ground Beef Preservation: Antimicrobial Activity and Physicochemical Properties" Molecules 28, no. 11: 4274. https://doi.org/10.3390/molecules28114274
APA StyleBaghi, F., Ghnimi, S., Dumas, E., Chihib, N.-E., & Gharsallaoui, A. (2023). Nanoemulsion-Based Multilayer Films for Ground Beef Preservation: Antimicrobial Activity and Physicochemical Properties. Molecules, 28(11), 4274. https://doi.org/10.3390/molecules28114274











