Polyphenol Profiling by LC QTOF/ESI-MS and Biological Activity of Purple Passion Fruit Epicarp Extract
Abstract
:1. Introduction
2. Results and Discussion
2.1. Content of Polyphenolic Compounds in Purple Passion Fruits Epicarp Extracts—Identification and Quantification
2.2. In Vitro Biological Activity of Passion Fruit Epicarp Extract
2.2.1. Antidiabetic Activity
2.2.2. Inhibition of Acetylcholinesterase (AChE) and Butyrylcholinesterase (BuChE)
2.2.3. Anti-Inflammatory Activity
2.2.4. Antioxidant Capacity
3. Materials and Methods
3.1. Materials
3.2. Identification and Quantification of Phenolic Compounds of Passion Fruit Epicarp Extract
3.3. Analysis of Polymeric Procyanidins by Phloroglucinolysis
3.4. In Vitro Biological Activity of Passion Fruit Epicarp Extract
3.4.1. Inhibition of α-Amylase, α-Glucosidase, and Pancreatic Lipase
3.4.2. Inhibition of Acetylcholinesterase (AChE) and Butyrylcholinesterase (BuChE)
3.4.3. Anti-Inflammatory Activity
3.4.4. Antioxidant Capacity
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- García-Villegas, A.; Rojas-García, A.; Villegas-Aguilar, M.D.C.; Fernández-Moreno, P.; Fernández-Ochoa, Á.; Cádiz-Gurrea, M.D.L.L.; Arráez-Román, D.; Segura-Carretero, A. Cosmeceutical Potential of Major Tropical and Subtropical Fruit By-Products for a Sustainable Revalorization. Antioxidants 2022, 11, 203. [Google Scholar] [CrossRef]
- Niu, H.; Yuan, L.; Zhou, H.; Yun, Y.; Li, J.; Tian, J.; Zhong, K.; Zhou, L. Comparison of the Effects of High Pressure Processing, Pasteurization and High Temperature Short Time on the Physicochemical Attributes, Nutritional Quality, Aroma Profile and Sensory Characteristics of Passion Fruit Purée. Foods 2022, 11, 632. [Google Scholar] [CrossRef]
- Leite, I.B.; Magalhães, C.D.; Monteiro, M.; Fialho, E. Addition of Honey to an Apple and Passion Fruit Mixed Beverage Improves Its Phenolic Compound Profile. Foods 2021, 10, 1525. [Google Scholar] [CrossRef]
- dos Reis, L.C.R.; Facco, E.M.P.; Salvador, M.; Flôres, S.H.; de Oliveira Rios, A. Antioxidant Potential and Physicochemical Characterization of Yellow, Purple and Orange Passion Fruit. J. Food Sci. Technol. 2018, 55, 2679–2691. [Google Scholar] [CrossRef] [PubMed]
- Dilucia, F.; Lacivita, V.; Conte, A.; Del Nobile, M.A. Sustainable Use of Fruit and Vegetable By-Products to Enhance Food Packaging Performance. Foods 2020, 9, 857. [Google Scholar] [CrossRef] [PubMed]
- Kruczek, M.; Drygaś, B.; Habryka, C. Pomace in Fruit Industry and Their Contemporary Potential Application. World Sci. News 2016, 48, 259–265. [Google Scholar]
- Yu, J.; Ahmedna, M. Functional Components of Grape Pomace: Their Composition, Biological Properties and Potential Applications. Int. J. Food Sci. Technol. 2013, 48, 221–237. [Google Scholar] [CrossRef]
- Skinner, R.C.; Gigliotti, J.C.; Ku, K.M.; Tou, J.C. A Comprehensive Analysis of the Composition, Health Benefits, and Safety of Apple Pomace. Nutr. Rev. 2018, 76, 893–909. [Google Scholar] [CrossRef]
- Vasyliev, G.S.; Vorobyova, V.I.; Linyucheva, O.V. Evaluation of Reducing Ability and Antioxidant Activity of Fruit Pomace Extracts by Spectrophotometric and Electrochemical Methods. J. Anal. Methods Chem. 2020, 2020, 8869436. [Google Scholar] [CrossRef] [PubMed]
- Iqbal, A.; Schulz, P.; Rizvi, S.S.H. Valorization of Bioactive Compounds in Fruit Pomace from Agro-Fruit Industries: Present Insights and Future Challenges. Food Biosci. 2021, 44, 101384. [Google Scholar] [CrossRef]
- Bober, I.; Oszmiański, J. Wytłoków Aronii Do Naparów Herbat Owocowych. Acta Sci. Pol. Technol. Aliment. 2004, 3, 63–72. [Google Scholar]
- Ghada, B.; Pereira, E.; Pinela, J.; Prieto, M.A.; Pereira, C.; Calhelha, R.C.; Stojkovic, D.; Sokóvic, M.; Zaghdoudi, K.; Barros, L.; et al. Recovery of Anthocyanins from Passion Fruit Epicarp for Food Colorants: Extraction Process Optimization and Evaluation of Bioactive Properties. Molecules 2020, 25, 3203. [Google Scholar] [CrossRef] [PubMed]
- Plainfossé, H.; Trinel, M.; Verger-Dubois, G.; Azoulay, S.; Burger, P.; Fernandez, X. Valorisation of Ribes nigrum L. Pomace, an Agri-Food by-Product to Design a New Cosmetic Active. Cosmetics 2020, 7, 56. [Google Scholar] [CrossRef]
- Gołębiewska, E.; Kalinowska, M.; Yildiz, G. Sustainable Use of Apple Pomace (AP) in Different Industrial Sectors. Materials 2022, 15, 1788. [Google Scholar] [CrossRef] [PubMed]
- Sharma, M.; Usmani, Z.; Gupta, V.K.; Bhat, R. Valorization of Fruits and Vegetable Wastes and By-Products to Produce Natural Pigments. Crit. Rev. Biotechnol. 2021, 41, 535–563. [Google Scholar] [CrossRef] [PubMed]
- Roopchand, D.E.; Krueger, C.G.; Moskal, K.; Fridlender, B.; Lila, M.A.; Raskin, I. Food-Compatible Method for the Efficient Extraction and Stabilization of Cranberry Pomace Polyphenols. Food Chem. 2013, 141, 3664–3669. [Google Scholar] [CrossRef]
- Fernandes, P.A.R.; Ferreira, S.S.; Bastos, R.; Ferreira, I.; Cruz, M.T.; Pinto, A.; Coelho, E.; Passos, C.P.; Coimbra, M.A.; Cardoso, S.M.; et al. Apple Pomace Extract as a Sustainable Food Ingredient. Antioxidants 2019, 8, 189. [Google Scholar] [CrossRef]
- Song, Y.; Wei, X.Q.; Li, M.Y.; Duan, X.W.; Sun, Y.M.; Yang, R.L.; Su, X.D.; Huang, R.M.; Wang, H. Nutritional Composition and Antioxidant Properties of the Fruits of a Chinese Wild Passiflora foetida. Molecules 2018, 23, 459. [Google Scholar] [CrossRef]
- Liew, S.Q.; Chin, N.L.; Yusof, Y.A. Extraction and Characterization of Pectin from Passion Fruit Peels. Agric. Agric. Sci. Procedia 2014, 2, 231–236. [Google Scholar] [CrossRef]
- Freitas de Oliveira, C.; Giordani, D.; Lutckemier, R.; Gurak, P.D.; Cladera-Olivera, F.; Ferreira Marczak, L.D. Extraction of Pectin from Passion Fruit Peel Assisted by Ultrasound. LWT 2016, 71, 110–115. [Google Scholar] [CrossRef]
- Leoro, M.G.V.; Clerici, M.T.P.S.; Chang, Y.K.; Steel, C.J. Evaluation of the In Vitro Glycemic Index of a Fiber-Rich Extruded Breakfast Cereal Produced with Organic Passion Fruit Fiber and Corn Flour. Ciência Tecnol. Aliment. 2010, 30, 964–968. [Google Scholar] [CrossRef]
- Kandandapani, S.; Balaraman, A.K.; Ahamed, H.N. Extracts of Passion Fruit Peel and Seed of Passiflora edulis (Passifloraceae) Attenuate Oxidative Stress in Diabetic Rats. Chin. J. Nat. Med. 2015, 13, 680–686. [Google Scholar] [CrossRef] [PubMed]
- Macagnan, F.T.; Dos Santos, L.R.; Roberto, B.S.; De Moura, F.A.; Bizzani, M.; Da Silva, L.P. Biological Properties of Apple Pomace, Orange Bagasse and Passion Fruit Peel as Alternative Sources of Dietary Fibre. Bioact. Carbohydr. Diet. Fibre 2015, 6, 1–6. [Google Scholar] [CrossRef]
- Farid, R.; Rezaieyazdi, Z.; Mirfeizi, Z.; Hatef, M.R.; Mirheidari, M.; Mansouri, H.; Esmaelli, H.; Bentley, G.; Lu, Y.; Foo, Y.; et al. Oral Intake of Purple Passion Fruit Peel Extract Reduces Pain and Stiffness and Improves Physical Function in Adult Patients with Knee Osteoarthritis. Nutr. Res. 2010, 30, 601–606. [Google Scholar] [CrossRef]
- de Souza, C.G.; Rodrigues, T.H.S.; e Silva, L.M.A.; Ribeiro, P.R.V.; de Brito, E.S. Sequential Extraction of Flavonoids and Pectin from Yellow Passion Fruit Rind Using Pressurized Solvent or Ultrasound. J. Sci. Food Agric. 2018, 98, 1362–1368. [Google Scholar] [CrossRef]
- Es-Safi, N.E.; Gómez-Cordovés, C. Characterization of Flavonoid Glycosides from Fenugreek (Trigonella foenum-graecum) Crude Seeds by HPLC-DAD-ESI/MS Analysis. Int. J. Mol. Sci. 2014, 15, 20668. [Google Scholar] [CrossRef]
- Simirgiotis, M.J.; Schmeda-Hirschmann, G.; Bórquez, J.; Kennelly, E.J. The Passiflora tripartita (Banana Passion) Fruit: A Source of Bioactive Flavonoid C-Glycosides Isolated by HSCCC and Characterized by HPLC-DAD-ESI/MS/MS. Molecules 2013, 18, 1672. [Google Scholar] [CrossRef]
- Zucolotto, S.M.; Fagundes, C.; Reginatto, F.H.; Ramos, F.A.; Castellanos, L.; Duque, C.; Schenkel, E.P. Analysis of C-Glycosyl Flavonoids from South American Passiflora Species by HPLC-DAD and HPLC-MS. Phytochem. Anal. 2012, 23, 232–239. [Google Scholar] [CrossRef]
- Cao, J.; Yin, C.; Qin, Y.; Cheng, Z.; Chen, D. Approach to the Study of Flavone Di-C-Glycosides by High Performance Liquid Chromatography-Tandem Ion Trap Mass Spectrometry and Its Application to Characterization of Flavonoid Composition in Viola Yedoensis. J. Mass Spectrom. 2014, 49, 1010–1024. [Google Scholar] [CrossRef]
- Cavaliere, C.; Foglia, P.; Pastorini, E.; Samperi, R.; Laganà, A. Identification and Mass Spectrometric Characterization of Glycosylated Flavonoids in Triticum durum Plants by High-Performance Liquid Chromatography with Tandem Mass Spectrometry. Rapid Commun. Mass Spectrom. 2005, 19, 3143–3158. [Google Scholar] [CrossRef]
- Lin, L.Z.; Sun, J.; Chen, P.; Harnly, J. UHPLC-PDA-ESI/HRMS/MSn Analysis of Anthocyanins, Flavonol Glycosides, and Hydroxycinnamic Acid Derivatives in Red Mustard Greens (Brassica Juncea Coss Variety). J. Agric. Food Chem. 2011, 59, 12059–12072. [Google Scholar] [CrossRef] [PubMed]
- Gu, D.; Yang, Y.; Abdulla, R.; Aisa, H.A. Characterization and Identification of Chemical Compositions in the Extract of Artemisia rupestris L. by Liquid Chromatography Coupled to Quadrupole Time-of-Flight Tandem Mass Spectrometry. Rapid Commun. Mass Spectrom. 2012, 26, 83–100. [Google Scholar] [CrossRef] [PubMed]
- Hassan, W.H.B.; Abdelaziz, S.; Al Yousef, H.M. Chemical Composition and Biological Activities of the Aqueous Fraction of Parkinsonea aculeata L. Growing in Saudi Arabia. Arab. J. Chem. 2019, 12, 377–387. [Google Scholar] [CrossRef]
- Choi, J.S.; Islam, M.N.; Ali, M.Y.; Kim, Y.M.; Park, H.J.; Sohn, H.S.; Jung, H.A. The Effects of C-Glycosylation of Luteolin on Its Antioxidant, Anti-Alzheimer’s Disease, Anti-Diabetic, and Anti-Inflammatory Activities. Arch. Pharm. Res. 2014, 37, 1354–1363. [Google Scholar] [CrossRef]
- Zhou, X.; Wang, F.; Zhou, R.; Song, X.; Xie, M. Apigenin: A Current Review on Its Beneficial Biological Activities. J. Food Biochem. 2017, 41, e12376. [Google Scholar] [CrossRef]
- Gates, M.A.; Vitonis, A.F.; Tworoger, S.S.; Rosner, B.; Titus-Ernstoff, L.; Hankinson, S.E.; Cramer, D.W. Flavonoid Intake and Ovarian Cancer Risk in a Population-Based Case-Control Study. Int. J. Cancer 2009, 124, 1918–1925. [Google Scholar] [CrossRef]
- Ayvaz, H.; Cabaroglu, T.; Akyildiz, A.; Pala, C.U.; Temizkan, R.; Ağçam, E.; Ayvaz, Z.; Durazzo, A.; Lucarini, M.; Direito, R.; et al. Anthocyanins: Metabolic Digestion, Bioavailability, Therapeutic Effects, Current Pharmaceutical/Industrial Use, and Innovation Potential. Antioxidants 2023, 12, 48. [Google Scholar] [CrossRef]
- Fallah, A.A.; Sarmast, E.; Jafari, T. Effect of Dietary Anthocyanins on Biomarkers of Oxidative Stress and Antioxidative Capacity: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. J. Funct. Foods 2020, 68, 103912. [Google Scholar] [CrossRef]
- Aron, P.M.; Kennedy, J.A. Flavan-3-Ols: Nature, Occurrence and Biological Activity. Mol. Nutr. Food Res. 2008, 52, 79–104. [Google Scholar] [CrossRef]
- Kumar, N.; Goel, N. Phenolic Acids: Natural Versatile Molecules with Promising Therapeutic Applications. Biotechnol. Rep. 2019, 24, e00370. [Google Scholar] [CrossRef]
- Fonseca, A.M.A.; Geraldi, M.V.; Junior, M.R.M.; Silvestre, A.J.D.; Rocha, S.M. Purple Passion Fruit (Passiflora edulis f. edulis): A Comprehensive Review on the Nutritional Value, Phytochemical Profile and Associated Health Effects. Food Res. Int. 2022, 160, 111665. [Google Scholar] [CrossRef] [PubMed]
- KidØy, L.; Nygård, A.M.; Andersen, Ø.M.; Pedersen, A.T.; Aksnes, D.W.; Kiremire, B.T. Anthocyanins in Fruits of Passiflora edulis and P. suberosa. J. Food Compos. Anal. 1997, 10, 49–54. [Google Scholar] [CrossRef]
- Zhang, L.; Tu, Z.C.; Yuan, T.; Wang, H.; Xie, X.; Fu, Z.F. Antioxidants and α-Glucosidase Inhibitors from Ipomoea batatas Leaves Identified by Bioassay-Guided Approach and Structure-Activity Relationships. Food Chem 2016, 208, 61–67. [Google Scholar] [CrossRef] [PubMed]
- Sebastian, J.; Jose, S. Antidiabetic and Antioxidant Profile of Three Varieties of Passion Fruit. FoodSci Indian J. Res. Food Sci. Nutr. 2018, 5, 16–21. [Google Scholar] [CrossRef]
- Nair, S.S.; Kavrekar, V.; Mishra, A. Evaluation of In Vitro Anti Diabetic Activity of Selected Plant Extracts. Int. J. Pharm. Sci. Invent. 2013, 2, 12–19. [Google Scholar]
- Mukherjee, M. Human Digestive and Metabolic Lipases—A Brief Review. J. Mol. Catal. B Enzym. 2003, 22, 369–376. [Google Scholar] [CrossRef]
- de Mello e Silva, G.N.; Batista Rodrigues, E.S.; Lopes de Macêdo, I.Y.; Vicente Gil, H.P.; Campos, H.M.; Ghedini, P.C.; da Silva Cardozo, L.; Batista, E.A.; Lopes de Araújo, G.; Vaz, B.G.; et al. Blackberry Jam Fruit (Randia formosa (Jacq.) K. Schum): An Amazon Superfruit with in Vitro Neuroprotective Properties. Food Biosci. 2022, 50, 102084. [Google Scholar] [CrossRef]
- Lim, Y.J.; Oh, C.S.; Park, Y.D.; Eom, S.H.; Kim, D.O.; Kim, U.J.; Cho, Y.S. Physiological Components of Kiwifruits with In Vitro Antioxidant and Acetylcholinesterase Inhibitory Activities. Food Sci. Biotechnol. 2014, 23, 943–949. [Google Scholar] [CrossRef]
- Jabir, N.R.; Khan, F.R.; Tabrez, S. Cholinesterase Targeting by Polyphenols: A Therapeutic Approach for the Treatment of Alzheimer’s Disease. CNS Neurosci. Ther. 2018, 24, 753–762. [Google Scholar] [CrossRef]
- Khan, M.T.H.; Orhan, I.; Şenol, F.S.; Kartal, M.; Şener, B.; Dvorská, M.; Šmejkal, K.; Šlapetová, T. Cholinesterase Inhibitory Activities of Some Flavonoid Derivatives and Chosen Xanthone and Their Molecular Docking Studies. Chem. Biol. Interact. 2009, 181, 383–389. [Google Scholar] [CrossRef]
- Esmaeili, S.; Ara, L.; Hajimehdipoor, H.; Kolivand, H.; Motamed, S.M. Acetylcholinesterase Inhibitory Effects of Some Plants from Rosaceae. Res. J. Pharmacogn. 2015, 2, 33–37. [Google Scholar]
- Lopez-Lazaro, M. Distribution and Biological Activities of the Flavonoid Luteolin. Mini-Rev. Med. Chem. 2008, 9, 31–59. [Google Scholar] [CrossRef]
- Sezen Karaoğlan, E.; Hanci, H.; Koca, M.; Kazaz, C. Some Bioactivities of Isolated Apigenin-7-O-Glucoside and Luteolin-7-O-Glucoside. Appl. Sci. 2023, 13, 1503. [Google Scholar] [CrossRef]
- Mira, A.; Yamashita, S.; Katakura, Y.; Shimizu, K. In Vitro Neuroprotective Activities of Compounds from Angelica shikokiana Makino. Molecules 2015, 20, 4813. [Google Scholar] [CrossRef]
- Wu, M.; Liu, M.; Wang, F.; Cai, J.; Luo, Q.; Li, S.; Zhu, J.; Tang, Z.; Fang, Z.; Wang, C.; et al. The Inhibition Mechanism of Polyphenols from Phyllanthus emblica Linn. Fruit on Acetylcholinesterase: A Interaction, Kinetic, Spectroscopic, and Molecular Simulation Study. Food Res. Int. 2022, 158, 111497. [Google Scholar] [CrossRef] [PubMed]
- Ulbrich, H.; Fiebich, B.; Dannhardt, G. Cyclooxygenase-1/2 (COX-1/COX-2) and 5-Lipoxygenase (5-LOX) Inhibitors of the 6,7-Diaryl-2,3-1H-Dihydropyrrolizine Type. Eur. J. Med. Chem. 2002, 37, 953–959. [Google Scholar] [CrossRef] [PubMed]
- Baysal, T.; Demirdöven, A. Lipoxygenase in Fruits and Vegetables: A Review. Enzym. Microb. Technol. 2007, 40, 491–496. [Google Scholar] [CrossRef]
- Martel-Pelletier, J.; Lajeunesse, D.; Reboul, P.; Pelletier, J.P. Therapeutic Role of Dual Inhibitors of 5-LOX and COX, Selective and Non-Selective Non-Steroidal Anti-Inflammatory Drugs. Ann. Rheum. Dis. 2003, 62, 501–509. [Google Scholar] [CrossRef] [PubMed]
- de Albuquerque, M.A.C.; Levit, R.; Beres, C.; Bedani, R.; de Moreno de LeBlanc, A.; Saad, S.M.I.; LeBlanc, J.G. Tropical Fruit By-Products Water Extracts of Tropical Fruit by-Products as Sources of Soluble Fibres and Phenolic Compounds with Potential Antioxidant, Anti-Inflammatory, and Functional Properties. J. Funct. Foods 2019, 52. [Google Scholar] [CrossRef]
- Pham-Huy, L.A.; He, H.; Pham-Huy, C. Free Radicals, Antioxidants in Disease and Health. Int. J. Biomed. Sci. 2008, 4, 89. [Google Scholar]
- Suleria, H.A.R.; Barrow, C.J.; Dunshea, F.R. Screening and Characterization of Phenolic Compounds and Their Antioxidant Capacity in Different Fruit Peels. Foods 2020, 9, 1206. [Google Scholar] [CrossRef] [PubMed]
- Septembre-Malaterre, A.; Stanislas, G.; Douraguia, E.; Gonthier, M.P. Evaluation of Nutritional and Antioxidant Properties of the Tropical Fruits Banana, Litchi, Mango, Papaya, Passion Fruit and Pineapple Cultivated in Réunion French Island. Food Chem. 2016, 212, 225–233. [Google Scholar] [CrossRef] [PubMed]
- Moo-Huchin, V.M.; Moo-Huchin, M.I.; Estrada-León, R.J.; Cuevas-Glory, L.; Estrada-Mota, I.A.; Ortiz-Vázquez, E.; Betancur-Ancona, D.; Sauri-Duch, E. Antioxidant Compounds, Antioxidant Activity and Phenolic Content in Peel from Three Tropical Fruits from Yucatan, Mexico. Food Chem. 2015, 166, 17–22. [Google Scholar] [CrossRef] [PubMed]
- Tian, C.; Liu, X.; Chang, Y.; Wang, R.; Lv, T.; Cui, C.; Liu, M. Investigation of the Anti-Inflammatory and Antioxidant Activities of Luteolin, Kaempferol, Apigenin and Quercetin. S. Afr. J. Bot. 2021, 137, 257–264. [Google Scholar] [CrossRef]
- Wojdyło, A.; Nowicka, P. Profile of Phenolic Compounds of Prunus armeniaca L. Leaf Extract Determined by LC-ESI-QTOF-MS/MS and Their Antioxidant, Anti-Diabetic, Anti-Cholinesterase, and Anti-Inflammatory Potency. Antioxidants 2021, 10, 1869. [Google Scholar] [CrossRef] [PubMed]
- Wojdyło, A.; Nowicka, P.; Tkacz, K.; Turkiewicz, I.P. Sprouts vs. Microgreens as Novel Functional Foods: Variation of Nutritional and Phytochemical Profiles and Their in Vitro Bioactive Properties. Molecules 2020, 25, 4648. [Google Scholar] [CrossRef]
- Nowicka, P.; Wojdyło, A. Anti-Hyperglycemic and Anticholinergic Effects of Natural Antioxidant Contents in Edible Flowers. Antioxidants 2019, 8, 308. [Google Scholar] [CrossRef]
- Wojdyło, A.; Nowicka, P.; Bąbelewski, P. Phenolic and Carotenoid Profile of New Goji Cultivars and Their Anti-Hyperglycemic, Anti-Aging and Antioxidant Properties. J. Funct. Foods 2018, 48, 632–642. [Google Scholar] [CrossRef]
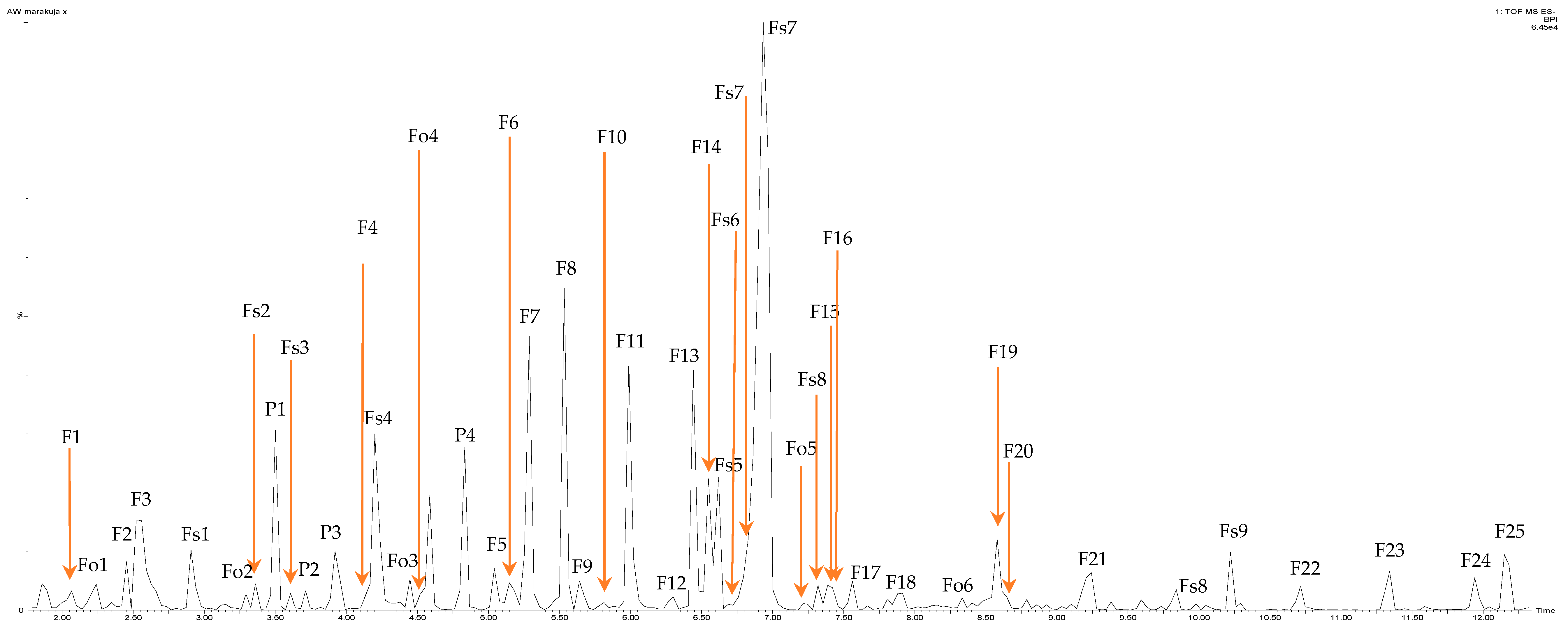
| Peak No | tR (min) | Assigned Identity | Molecular Ion [M-H]− (m/z) | Main Ions MS/MS (m/z) | Phenolic Content [mg/100 g dw] |
|---|---|---|---|---|---|
| Flavan-3-ol | |||||
| Fo1 | 2.30 | (+)-catechin † | 289.0143 | 245.0367 | 35.02 ± 1.43 |
| Fo2 | 4.40 | (−)-epicatechin † | 289.0143 | 245.0363 | 38.43 ± 2.65 |
| Fo3 | 3.30 | Procyanidin dimer (B2) † | 577.0538 | 289.0143 | 943.60 ± 35.27 ¥ |
| Fo4 | 4.50 | Procyanidin dimer | 577.0311 | 289.0152 | |
| Fo5 | 7.28 | Procyanidin dimer | 577.0237 | 289.0153 | |
| Fo6 | 8.20 | Procyanidin dimer | 577.0538 | 289.0143 | |
| Σ flavan-3-ols | 1017.05 | ||||
| Degree of polymerization | 7.03 ± 0.23 | ||||
| Phenolic acid | |||||
| P1 | 3.50 | Ferulic acid 4-O-glucoside | 355.0124 | 313.0664/269.0807/193.0318/161.0304 | 51.95 ± 2.43 |
| P2 | 3.71 | Sinapic acid hexoside | 385.0844 | 190.0087 | 121.53 ± 1.45 |
| P3 | 3.92 | Salicylic acid glucoside | 299.0522 | 137.0487 | 152.12 ± 2.76 |
| P4 | 4.83 | Ferulic acid 4-O-galactoside † | 355.0754 | 269.0807 | 83.54 ± 2.99 |
| Σ phenolic acid | 409.14 | ||||
| Flavone | |||||
| F1 | 2.21 | Acacetin-rhamnoside | 429.0596 | 283.0466 | 499.34 ± 10.32 |
| F2 | 2.45 | Apigenin derivatives | 455.1432 | 305.0297/287.0388/269.0135/168.9985 | 75.73 ± 2.43 |
| F3 | 2.52 | Acacetin-rhamnoside | 429.05 | 283.0432/207.0354/151.0095/165.0272 | 305.34 ± 6.54 |
| F4 | 4.15 | Luteolin-8-C-glucoside (orientin) † | 447.0599 | 417.0670/285.0168/151.9977 | 223.54 ± 11.32 |
| F5 | 5.04 | Apigenin-7-O-(6″-galloyl)-hexoside | 583.1554 | 357.1140/327.0977/269.0874/165.0430 | 273.23 ± 15.21 |
| F6 | 5.18 | Luteolin-7-O-(6″-galloyl)-rhamnoside | 583.1653 | 537.1574/357.1180/281.1142/285.0874 | 288.34 ± 16.32 |
| F7 | 5.29 | Apigenin-6-C-(6″-O-crotonyl)-pentoside | 469.1075 | 361.1308/340.0760/294.0700/269.0807/161.0304 | 360.23 ± 10.32 |
| F8 | 5.53 | Di-hydroxy-di-methoxy-flavone-di-hexose (chrysoerior derivatives) | 683.2097 | 486.1270/440.1263/307.0784/300.9991 | 968.45 ± 21.27 |
| F9 | 5.64 | Apigenin-6-C-xyloside-6-C-glucoside (vicenin) | 563.1574 | 537.1557/341.0632/269.0269/203.0748/165.0351 | 55.43 ± 1.32 |
| F10 | 5.81 | Apigenin-6-C-xyloside-6-C-glucoside (vicenin) | 563.1557 | 424.0364/327.0903/269.0269 | 192.32 ± 2.74 |
| F11 | 5.99 | Luteolin-7-O-rutinoside † | 593.1002 | 285.0168 | 199.48 ± 5.29 |
| F12 | 6.30 | Luteolin-8-C-digitoxopyranosyl-4′-O-hexoside | 577.1099 | 285.0168 | 104.23 ± 3.22 |
| F13 | 6.44 | Luteolin-O-di-pentoside-O-hexoside | 711.1668 | 355.0754/311.0916/285.0168 | 460.56 ± 5.25 |
| F14 | 6.55 | Apigenin-2″-O-deoxyhexosyl-C-hexoside | 577.1148 | 413.0587/336.0821/269.0807 | 505.29 ± 2.76 |
| F15 | 7.34 | Luteolin-7-O-rutinoside † | 593.1102 | 285.0098/284.0084 | 178.44 ± 5.11 |
| F16 | 7.38 | Luteolin-O-deoxyhexosyl-C-pentoside | 561.1247 | 523.1760/320.1076/359.1804/285.0168/277.0204/195.0452 | 66.85 ± 2.88 |
| F17 | 7.56 | Luteolin-8-C-glucoside (orientin) † | 447.0555 | 285.0168 | 114.69 ± 10.32 |
| F18 | 7.91 | Luteolin-6-hydroxy-O-glucuronide | 477.0694 | 359.1804/303.1126/285.0168 | 94.38 ± 3.54 |
| F19 | 8.16 | Luteolin-8-C-digitoxopyranosyl-4′-O-hexoside | 577.1198 | 285.0098 | 93.26 ± 2.55 |
| F20 | 8.58 | Acacetin-di-rhamnoside | 575.1001 | 411.0390/301.0133/283.0432 | 193.48 ± 6.44 |
| F21 | 9.21 | Luteolin-8-C-digitoxo-pyranosyl-4′-O-hexoside | 577.1148 | 415.0667/353.0414/311.0339/285.0202 | 337.48 ± 8.45 |
| F22 | 10.71 | Luteolin-8-C-digitoxo-pyranosyl | 415.0709 | 311.0339/285.0202 | 135.32 ± 1.34 |
| F23 | 11.34 | Luteolin derivatives | 697.3668 | 859.4095/285.9161 | 430.96 ± 4.21 |
| F24 | 11.94 | Apigenin-C-hexosyl-O-rhamnoside-O-hexoside | 741.3559 | 533.3114/399.2210/271.0691/269.0202 | 401.00 ± 11.54 |
| F25 | 12.15 | Apigenin-6-C-(6″-O-crotonyl)-glucoside | 499.2872 | 533.2972/269.0202/171.0862/127.0954 | 555.99 ± 24.12 |
| Σ Acacetin dervatives | 998.16 | ||||
| Σ Apigenin derivatives | 2419.22 | ||||
| Σ Luteolin derivatives | 2727.53 | ||||
| Σ Chrysoerior derivatives | 968.45 | ||||
| Σ flavone | 7341.47 | ||||
| Flavonols | |||||
| Fs1 | 2.91 | Quercetin-3-O-pentoside | 433.0339 | 380.1268/313.0664/241.0921/146.0696 | 89.93 ± 3.56 |
| Fs2 | 3.36 | Quercetin-3-O-hexoside (glucoside I) † | 463.0523 | 301.0062 | 59.45 ± 5.32 |
| Fs3 | 3.60 | Quercetin-3-O-hexoside | 463.0523 | 301.0062/385.0443/137.0100 | 64.32 ± 2.14 |
| Fs4 | 4.20 | Quercetin-3-O-hexoside | 465.0722 | 301.0666 | 228.11 ± 6.89 |
| Fs5 | 6.62 | Quercetin-3-O-rutinoside † | 609.1018 | 301.0133 | 219.45 ± 6.99 |
| Fs6 | 6.78 | Quercetin-3-O-galactoside † | 463.0523 | 301.0054/276.0603/245.07 | 140.41 ± 5.27 |
| Fs7 | 6.93 | Quercetin-3-di-sinapoyl-tri-glucoside-7-di-glucoside | 761.1526 | 336.0821/301.0054/276.0671/230.0474 | 1504.15 ± 2.56 |
| Fs8 | 7.32 | Quercetin-3-O-acetylglucoside | 505.0633 | 301.0054 | 89.79 ± 3.76 |
| Fs9 | 10.22 | Quercetin-O-tri-pentoside-3-O-hexoside | 859.4095 | 694.0944/301.1803/271.0724 | 156.76 ± 5.21 |
| Σ flavonols | 2324.11 | ||||
| Anthocyanins | |||||
| A1 | 4.59 | Cyanidin-3-O-glucoside † | 449.0603 | 287.0180 | 737.69 ± 3.11 |
| A2 | 6.29 | Cyanidin-3-O-(6″-p-coumaroyl)-glucoside | 595.1019 | 449.0646/299.0216/287.0180/249.0563 | 789.9 ± 5.74 |
| A3 | 6.55 | Delphinidin-3-O-(6″-p-coumaroyl)-glucoside | 611.1003 | 465.0489/303.0134 | 774.82 ± 6.32 |
| A4 | 6.72 | Delphinidin-3-O-glucoside † | 465.0577 | 303.0098 | 85.72 ± 2.45 |
| A5 | 6.86 | Cyanidin-3-O-(6″-p-coumaroyl)-glucoside | 595.0870 | 449.0646/287.0146 | 98.44 ± 4.11 |
| A6 | 8.48 | Cyanidin-3-O-hexoside-rhamnoside | 595.1069 | 431.0513/287.0180 | 500.43 ± 5.72 |
| A7 | 8.84 | Delphinidin-di-C,C-hexosyl-O-rhamnoside | 759.0952 | 456.0952/303.0062 | 47.96 ± 3.87 |
| Σ anthocyanins | 3034.96 | ||||
| Total polyphenols | 14 126.73 | ||||
| Name of Analysis | Biological Activity |
|---|---|
| Antioxidant activity [mmol TE/100 g] | |
| -ABTSo+ | 1004.40 ± 20.80 |
| -ORAC | 160.70 ± 16.35 |
| Antidiabetic activity [IC50; mg/mL] | |
| -α-amylase | 7.99 ± 0.23 |
| -α-glucosidase | 12.80 ± 1.01 |
| -pancreatic lipase | 0.42 ± 0.02 |
| Cholinesterase activity [IC50; mg/mL] | |
| -acetylocholinesterase | 18.29 ± 1.76 |
| -butyrylcholinesterse | 14.22 ± 1.11 |
| Anti-inflamatory activity [IC50; mg/mL] | |
| -cyclooxygenase 1 | 6.00 ± 0.74 |
| -cyclooxygenase 2 | 0.90 ± 0.63 |
| -15-lipoxygenase | 4.90 ± 0.39 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Siniawska, M.; Wojdyło, A. Polyphenol Profiling by LC QTOF/ESI-MS and Biological Activity of Purple Passion Fruit Epicarp Extract. Molecules 2023, 28, 6711. https://doi.org/10.3390/molecules28186711
Siniawska M, Wojdyło A. Polyphenol Profiling by LC QTOF/ESI-MS and Biological Activity of Purple Passion Fruit Epicarp Extract. Molecules. 2023; 28(18):6711. https://doi.org/10.3390/molecules28186711
Chicago/Turabian StyleSiniawska, Monika, and Aneta Wojdyło. 2023. "Polyphenol Profiling by LC QTOF/ESI-MS and Biological Activity of Purple Passion Fruit Epicarp Extract" Molecules 28, no. 18: 6711. https://doi.org/10.3390/molecules28186711
APA StyleSiniawska, M., & Wojdyło, A. (2023). Polyphenol Profiling by LC QTOF/ESI-MS and Biological Activity of Purple Passion Fruit Epicarp Extract. Molecules, 28(18), 6711. https://doi.org/10.3390/molecules28186711