Application of Semiconductor Metal Oxide in Chemiresistive Methane Gas Sensor: Recent Developments and Future Perspectives
Abstract
1. Introduction
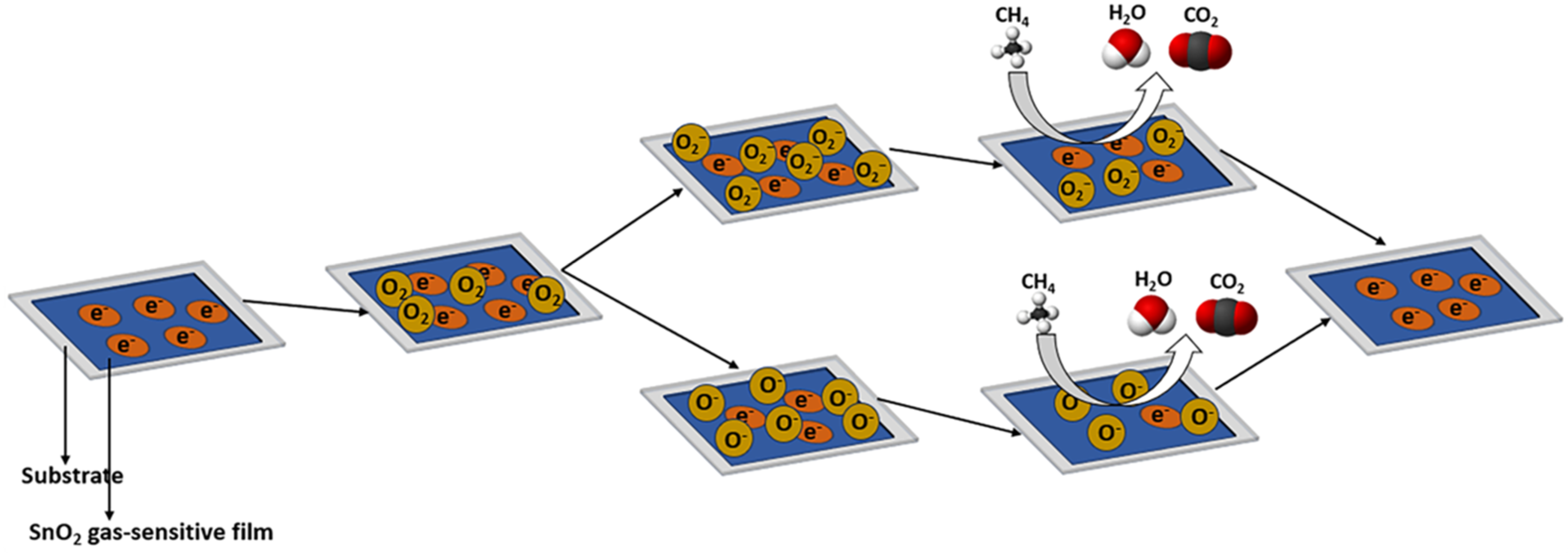
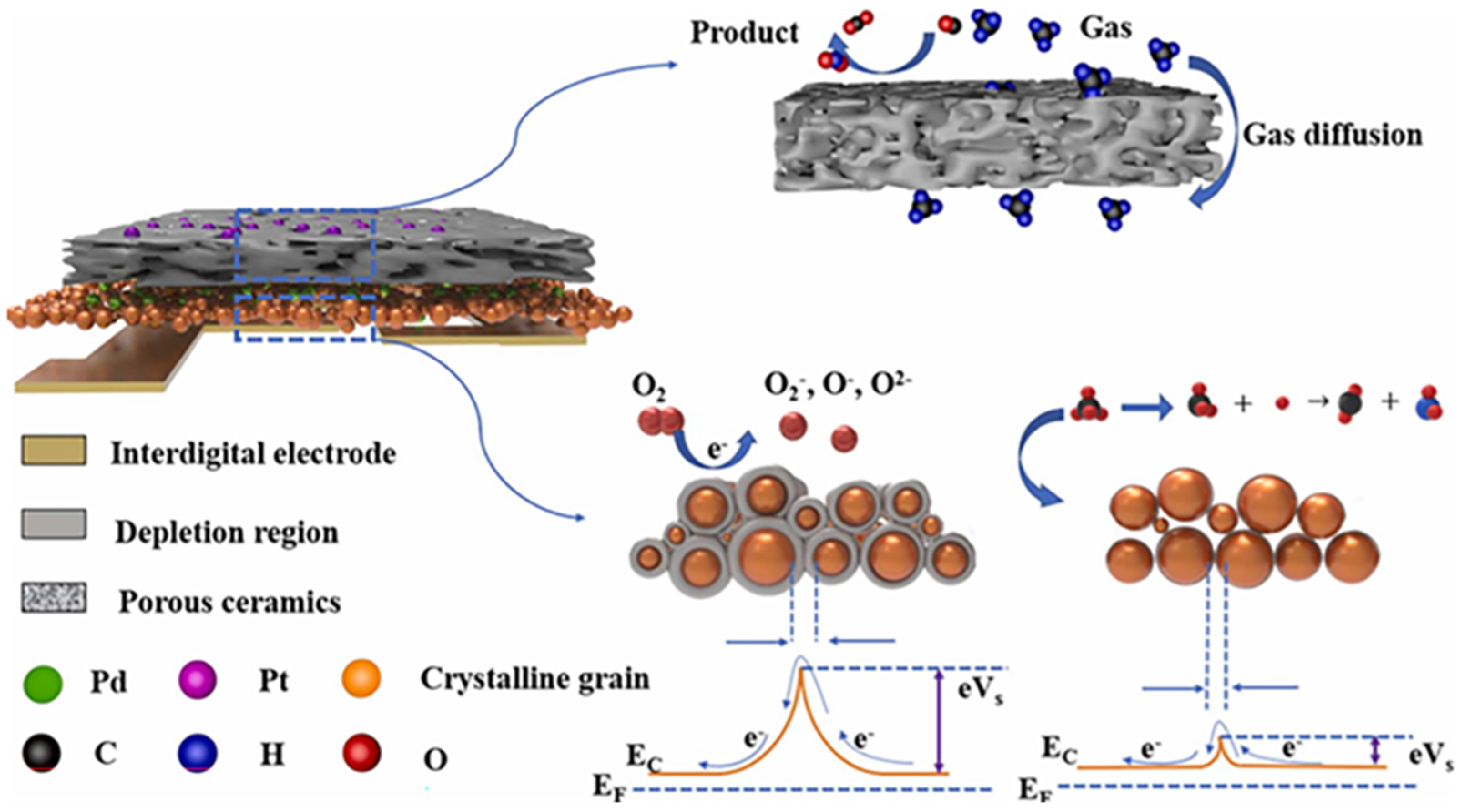
2. Chemiresistive Gas Sensors: Fundamentals and Mechanisms
3. Types of Metal Oxide Semiconductors for Methane Sensing
4. Recent Developments in Metal Oxide Semiconductors for Methane Sensing
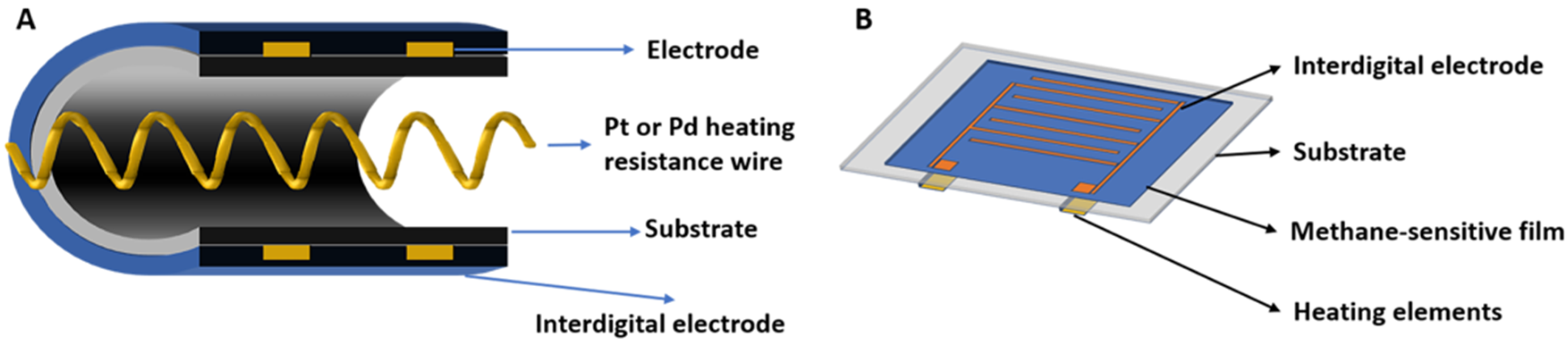
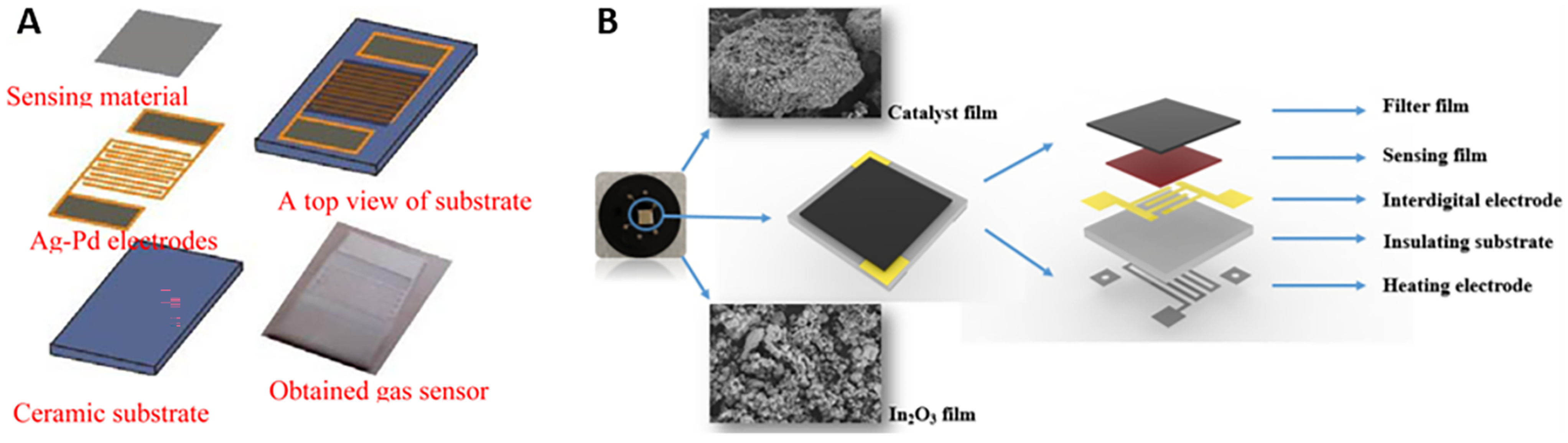
5. Sensor Fabrication Techniques and Integration
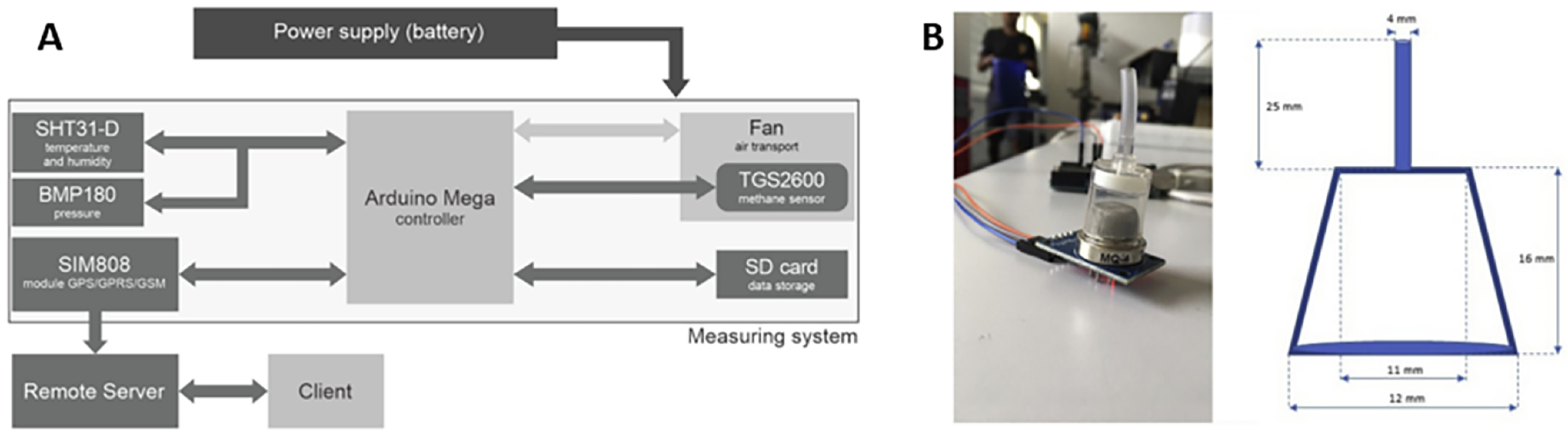
6. Challenges and Limitations
7. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Majhi, S.M.; Mirzaei, A.; Kim, H.W.; Kim, S.S.; Kim, T.W. Recent Advances in Energy-Saving Chemiresistive Gas Sensors: A Review. Nano Energy 2021, 79, 105369. [Google Scholar] [CrossRef] [PubMed]
- Bag, A.; Lee, N.-E. Gas Sensing with Heterostructures Based on Two-Dimensional Nanostructured Materials: A Review. J. Mater. Chem. C 2019, 7, 13367–13383. [Google Scholar] [CrossRef]
- Asadzadeh, S.; de Oliveira, W.J.; de Souza Filho, C.R. UAV-Based Remote Sensing for the Petroleum Industry and Environmental Monitoring: State-of-the-Art and Perspectives. J. Pet. Sci. Eng. 2022, 208, 109633. [Google Scholar] [CrossRef]
- Nagahage, I.S.P.; Nagahage, E.A.A.D.; Fujino, T. Assessment of the Applicability of a Low-Cost Sensor–Based Methane Monitoring System for Continuous Multi-Channel Sampling. Environ. Monit. Assess. 2021, 193, 509. [Google Scholar] [CrossRef]
- Shaw, J.T.; Shah, A.; Yong, H.; Allen, G. Methods for Quantifying Methane Emissions Using Unmanned Aerial Vehicles: A Review. Philos. Trans. R. Soc. A 2021, 379, 20200450. [Google Scholar] [CrossRef]
- Engram, M.; Walter Anthony, K.M.; Sachs, T.; Kohnert, K.; Serafimovich, A.; Grosse, G.; Meyer, F.J. Remote Sensing Northern Lake Methane Ebullition. Nat. Clim. Change 2020, 10, 511–517. [Google Scholar] [CrossRef]
- Gong, W.; Hu, J.; Wang, Z.; Wei, Y.; Li, Y.; Zhang, T.; Zhang, Q.; Liu, T.; Ning, Y.; Zhang, W. Recent Advances in Laser Gas Sensors for Applications to Safety Monitoring in Intelligent Coal Mines. Front. Phys. 2022, 10, 1058475. [Google Scholar] [CrossRef]
- Bezdek, M.J.; Luo, S.-X.L.; Ku, K.H.; Swager, T.M. A Chemiresistive Methane Sensor. Proc. Natl. Acad. Sci. USA 2021, 118, e2022515118. [Google Scholar] [CrossRef]
- Zabelin, D.; Zabelina, A.; Guselnikova, O.; Miliutina, E.; Kolska, Z.; Stulik, J.; Polansky, R.; Elashnikov, R.; Kalachyova, Y.; Svorcik, V.; et al. Selective Methane Chemiresistive Detection Using MWCNTs Array Decorated by Metal Organic Framework Layer. Surf. Interfaces 2023, 40, 103105. [Google Scholar] [CrossRef]
- Naganaboina, V.R.; Anandkumar, M.; Deshpande, A.S.; Singh, S.G. Single-Phase High-Entropy Oxide-Based Chemiresistor: Toward Selective and Sensitive Detection of Methane Gas for Real-Time Applications. Sens. Actuators B Chem. 2022, 357, 131426. [Google Scholar] [CrossRef]
- Chesler, P.; Hornoiu, C.; Anastasescu, M.; Calderon-Moreno, J.M.; Gheorghe, M.; Gartner, M. Cobalt- and Copper-Based Chemiresistors for Low Concentration Methane Detection, a Comparison Study. Gels 2022, 8, 721. [Google Scholar] [CrossRef] [PubMed]
- Mounasamy, V.; Mani, G.K.; Ponnusamy, D.; Tsuchiya, K.; Reshma, P.R.; Prasad, A.K.; Madanagurusamy, S. Cadmium Metavanadate Mixed Oxide Nanorods for the Chemiresistive Detection of Methane Molecules. New J. Chem. 2020, 44, 12473–12485. [Google Scholar] [CrossRef]
- Shah, V.; Bhaliya, J.; Patel, G.M.; Joshi, P. Room-Temperature Chemiresistive Gas Sensing of SnO2 Nanowires: A Review. J. Inorg. Organomet. Polym. Mater. 2022, 32, 741–772. [Google Scholar] [CrossRef]
- Thangamani, G.J.; Pasha, S.K.K. Titanium Dioxide (TiO2) Nanoparticles Reinforced Polyvinyl Formal (PVF) Nanocomposites as Chemiresistive Gas Sensor for Sulfur Dioxide (SO2) Monitoring. Chemosphere 2021, 275, 129960. [Google Scholar] [CrossRef] [PubMed]
- Karimi-Maleh, H.; Liu, Y.; Li, Z.; Darabi, R.; Orooji, Y.; Karaman, C.; Karimi, F.; Baghayeri, M.; Rouhi, J.; Fu, L.; et al. Calf Thymus Ds-DNA Intercalation with Pendimethalin Herbicide at the Surface of ZIF-8/Co/rGO/C3N4/Ds-DNA/SPCE; A Bio-Sensing Approach for Pendimethalin Quantification Confirmed by Molecular Docking Study. Chemosphere 2023, 332, 138815. [Google Scholar] [CrossRef]
- Li, X.; Fu, L.; Chen, F.; Zhao, S.; Zhu, J.; Yin, C. Application of Heterogeneous Catalytic Ozonation in Wastewater Treatment: An Overview. Catalysts 2023, 13, 342. [Google Scholar] [CrossRef]
- Karimi-Maleh, H.; Darabi, R.; Karimi, F.; Karaman, C.; Shahidi, S.A.; Zare, N.; Baghayeri, M.; Fu, L.; Rostamnia, S.; Rouhi, J.; et al. State-of-Art Advances on Removal, Degradation and Electrochemical Monitoring of 4-Aminophenol Pollutants in Real Samples: A Review. Environ. Res. 2023, 222, 115338. [Google Scholar] [CrossRef]
- Franco, M.A.; Conti, P.P.; Andre, R.S.; Correa, D.S. A Review on Chemiresistive ZnO Gas Sensors. Sens. Actuators Rep. 2022, 4, 100100. [Google Scholar] [CrossRef]
- Rath, R.J.; Farajikhah, S.; Oveissi, F.; Dehghani, F.; Naficy, S. Chemiresistive Sensor Arrays for Gas/Volatile Organic Compounds Monitoring: A Review. Adv. Eng. Mater. 2023, 25, 2200830. [Google Scholar] [CrossRef]
- Baharuddin, A.A.; Ang, B.C.; Haseeb, A.S.M.A.; Wong, Y.C.; Wong, Y.H. Advances in Chemiresistive Sensors for Acetone Gas Detection. Mater. Sci. Semicond. Process. 2019, 103, 104616. [Google Scholar] [CrossRef]
- Yang, B.; Myung, N.V.; Tran, T.-T. 1D Metal Oxide Semiconductor Materials for Chemiresistive Gas Sensors: A Review. Adv. Electron. Mater. 2021, 7, 2100271. [Google Scholar] [CrossRef]
- Chen, L.; Yu, Q.; Pan, C.; Song, Y.; Dong, H.; Xie, X.; Li, Y.; Liu, J.; Wang, D.; Chen, X. Chemiresistive Gas Sensors Based on Electrospun Semiconductor Metal Oxides: A Review. Talanta 2022, 246, 123527. [Google Scholar] [CrossRef] [PubMed]
- Sharma, A.; Ahmed, A.; Singh, A.; Oruganti, S.K.; Khosla, A.; Arya, S. Review—Recent Advances in Tin Oxide Nanomaterials as Electrochemical/Chemiresistive Sensors. J. Electrochem. Soc. 2021, 168, 027505. [Google Scholar] [CrossRef]
- Nasiri, N.; Clarke, C. Nanostructured Chemiresistive Gas Sensors for Medical Applications. Sensors 2019, 19, 462. [Google Scholar] [CrossRef]
- Tang, Y.; Zhao, Y.; Liu, H. Room-Temperature Semiconductor Gas Sensors: Challenges and Opportunities. ACS Sens. 2022, 7, 3582–3597. [Google Scholar] [CrossRef] [PubMed]
- Jeong, S.-Y.; Kim, J.-S.; Lee, J.-H. Rational Design of Semiconductor-Based Chemiresistors and Their Libraries for Next-Generation Artificial Olfaction. Adv. Mater. 2020, 32, 2002075. [Google Scholar] [CrossRef]
- Mondal, B.; Gogoi, P.K. Nanoscale Heterostructured Materials Based on Metal Oxides for a Chemiresistive Gas Sensor. ACS Appl. Electron. Mater. 2022, 4, 59–86. [Google Scholar] [CrossRef]
- Kim, H.-J.; Lee, J.-H. Highly Sensitive and Selective Gas Sensors Using P-Type Oxide Semiconductors: Overview. Sens. Actuators B Chem. 2014, 192, 607–627. [Google Scholar] [CrossRef]
- Kang, K.-S.; Lee, S.P. CO Gas Sensors Operating at Room Temperature. J. Mater. Sci. 2003, 38, 4319–4323. [Google Scholar] [CrossRef]
- Wang, X.; Yee, S.S.; Carey, W.P. Transition between Neck-Controlled and Grain-Boundary-Controlled Sensitivity of Metal-Oxide Gas Sensors. Sens. Actuators B Chem. 1995, 25, 454–457. [Google Scholar] [CrossRef]
- Gomri, S.; Seguin, J.-L.; Guerin, J.; Aguir, K. A Mobility and Free Carriers Density Fluctuations Based Model of Adsorption–Desorption Noise in Gas Sensor. J. Phys. Appl. Phys. 2008, 41, 065501. [Google Scholar] [CrossRef]
- Morrison, S.R. Mechanism of Semiconductor Gas Sensor Operation. Sens. Actuators 1987, 11, 283–287. [Google Scholar] [CrossRef]
- Xu, C.; Tamaki, J.; Miura, N.; Yamazoe, N. Grain Size Effects on Gas Sensitivity of Porous SnO2-Based Elements. Sens. Actuators B Chem. 1991, 3, 147–155. [Google Scholar] [CrossRef]
- Rothschild, A.; Komem, Y. The Effect of Grain Size on the Sensitivity of Nanocrystalline Metal-Oxide Gas Sensors. J. Appl. Phys. 2004, 95, 6374–6380. [Google Scholar] [CrossRef]
- Xu, C.; Tamaki, J.; Miura, N.; Yamazoe, N. Relationship between Gas Sensitivity and Microstructure of Porous SnO2. Denki Kagaku Oyobi Kogyo Butsuri Kagaku 1990, 58, 1143–1148. [Google Scholar] [CrossRef]
- Zhang, H.; Penn, R.L.; Hamers, R.J.; Banfield, J.F. Enhanced Adsorption of Molecules on Surfaces of Nanocrystalline Particles. J. Phys. Chem. B 1999, 103, 4656–4662. [Google Scholar] [CrossRef]
- Basu, P.K.; Bhattacharyya, P.; Saha, N.; Saha, H.; Basu, S. The Superior Performance of the Electrochemically Grown ZnO Thin Films as Methane Sensor. Sens. Actuators B Chem. 2008, 133, 357–363. [Google Scholar] [CrossRef]
- Sakai, G.; Baik, N.S.; Miura, N.; Yamazoe, N. Gas Sensing Properties of Tin Oxide Thin Films Fabricated from Hydrothermally Treated Nanoparticles: Dependence of CO and H2 Response on Film Thickness. Sens. Actuators B Chem. 2001, 77, 116–121. [Google Scholar] [CrossRef]
- Tiemann, M. Porous Metal Oxides as Gas Sensors. Chem.—Eur. J. 2007, 13, 8376–8388. [Google Scholar] [CrossRef]
- Korotcenkov, G.; Brinzari, V.; Schwank, J.; DiBattista, M.; Vasiliev, A. Peculiarities of SnO2 Thin Film Deposition by Spray Pyrolysis for Gas Sensor Application. Sens. Actuators B Chem. 2001, 77, 244–252. [Google Scholar] [CrossRef]
- Sakai, G.; Matsunaga, N.; Shimanoe, K.; Yamazoe, N. Theory of Gas-Diffusion Controlled Sensitivity for Thin Film Semiconductor Gas Sensor. Sens. Actuators B Chem. 2001, 80, 125–131. [Google Scholar] [CrossRef]
- Hossein-Babaei, F.; Orvatinia, M. Analysis of Thickness Dependence of the Sensitivity in Thin Film Resistive Gas Sensors. Sens. Actuators B Chem. 2003, 89, 256–261. [Google Scholar] [CrossRef]
- Akasaka, S. Thin Film YSZ-Based Limiting Current-Type Oxygen and Humidity Sensor on Thermally Oxidized Silicon Substrates. Sens. Actuators B Chem. 2016, 236, 499–505. [Google Scholar] [CrossRef]
- Waitz, T.; Becker, B.; Wagner, T.; Sauerwald, T.; Kohl, C.-D.; Tiemann, M. Ordered Nanoporous SnO2 Gas Sensors with High Thermal Stability. Sens. Actuators B Chem. 2010, 150, 788–793. [Google Scholar] [CrossRef]
- Zhou, Q.; Chen, W.; Xu, L.; Peng, S. Hydrothermal Synthesis of Various Hierarchical ZnO Nanostructures and Their Methane Sensing Properties. Sensors 2013, 13, 6171–6182. [Google Scholar] [CrossRef]
- Xu, K.; Liao, N.; Zhang, M.; Xue, W. Selective Methane Sensing Properties of VO2 at Different Temperatures: A First Principles Study. Appl. Surf. Sci. 2021, 536, 147969. [Google Scholar] [CrossRef]
- Shaalan, N.M.; Rashad, M.; Moharram, A.H.; Abdel-Rahim, M.A. Promising Methane Gas Sensor Synthesized by Microwave-Assisted Co3O4 Nanoparticles. Mater. Sci. Semicond. Process. 2016, 46, 1–5. [Google Scholar] [CrossRef]
- Wang, Y.; Sun, X.; Cao, J. Enhanced Methane Sensing Performance of Ag Modified In2O3 Microspheres. J. Alloys Compd. 2022, 895, 162557. [Google Scholar] [CrossRef]
- Stankova, M.; Vilanova, X.; Llobet, E.; Calderer, J.; Bittencourt, C.; Pireaux, J.J.; Correig, X. Influence of the Annealing and Operating Temperatures on the Gas-Sensing Properties of Rf Sputtered WO3 Thin-Film Sensors. Sens. Actuators B Chem. 2005, 105, 271–277. [Google Scholar] [CrossRef]
- Debeda, H.; Dulau, L.; Dondon, P.; Menil, F.; Lucat, C.; Massok, P. Development of a Reliable Methane Detector. Sens. Actuators B Chem. 1997, 44, 248–256. [Google Scholar] [CrossRef]
- Xue, D.; Zhang, S.; Zhang, Z. Hydrothermally Prepared Porous 3D SnO2 Microstructures for Methane Sensing at Lower Operating Temperature. Mater. Lett. 2019, 237, 336–339. [Google Scholar] [CrossRef]
- Kumar, P.; Chiu, Y.-H.; Deng, Z.-I.; Kumar, U.; Chen, K.-L.; Huang, W.-M.; Wu, C.-H. Surface Modification of ZnO Nanopillars to Enhance the Sensitivity towards Methane: The Studies of Experimental and First-Principle Simulation. Appl. Surf. Sci. 2021, 568, 150817. [Google Scholar] [CrossRef]
- Comert, B.; Akin, N.; Donmez, M.; Saglam, S.; Ozcelik, S. Titanium Dioxide Thin Films as Methane Gas Sensors. IEEE Sens. J. 2016, 16, 8890–8896. [Google Scholar] [CrossRef]
- Wu, R.; Tian, F.; Liu, Z.; Xue, X.; Zhang, J.; Zu, J. CH4 Activation and Sensing on Hexagonal WO3 (001) and (110) Surfaces. Appl. Surf. Sci. 2019, 481, 1154–1159. [Google Scholar] [CrossRef]
- Zhang, H.; Chang, J.; Wang, Y.; Cao, J. Synthesis of Porous Hierarchical In2O3 Nanostructures with High Methane Sensing Property at Low Working Temperature. Nanomaterials 2022, 12, 3081. [Google Scholar] [CrossRef] [PubMed]
- Prasad, A.K.; Amirthapandian, S.; Dhara, S.; Dash, S.; Murali, N.; Tyagi, A.K. Novel Single Phase Vanadium Dioxide Nanostructured Films for Methane Sensing near Room Temperature. Sens. Actuators B Chem. 2014, 191, 252–256. [Google Scholar] [CrossRef]
- Wu, R.; Tian, L.; Li, H.; Liu, H.; Luo, J.; Tian, X.; Hua, Z.; Wu, Y.; Fan, S. A Selective Methane Gas Sensor Based on Metal Oxide Semiconductor Equipped with an On-Chip Microfilter. Sens. Actuators B Chem. 2022, 359, 131557. [Google Scholar] [CrossRef]
- Oliaee, S.N.; Khodadadi, A.; Mortazavi, Y.; Alipour, S. Highly Selective Pt/SnO2 Sensor to Propane or Methane in Presence of CO and Ethanol, Using Gold Nanoparticles on Fe2O3 Catalytic Filter. Sens. Actuators B Chem. 2010, 147, 400–405. [Google Scholar] [CrossRef]
- Fateminia, F.S.; Mortazavi, Y.; Khodadadi, A.A. Au-Promoted Ce-Zr Catalytic Filter for Pt/SnO2 Sensor to Selectively Detect Methane and Ethanol in the Presence of Interfering Indoor Gases. Mater. Sci. Semicond. Process. 2019, 90, 182–189. [Google Scholar] [CrossRef]
- Li, H.; Wu, R.; Tian, X.; Han, L.; Chen, T.; Yang, B.; Zhi, Z.; Hua, Z.; Fan, S. A Catalytic Filter Based on Pt-CeO2 for Selective Methane Detection with SnO2 Sensors. Sens. Actuators B Chem. 2023, 389, 133872. [Google Scholar] [CrossRef]
- JoŢca, J.; Harmel, J.; Joanny, L.; Ryzhikov, A.; Kahn, M.L.; Fau, P.; Chaudret, B.; Fajerwerg, K. Au/MOx (M = Zn, Ti) Nanocomposites as Highly Efficient Catalytic Filters for Chemical Gas Sensing at Room Temperature and in Humid Atmosphere. Sens. Actuators B Chem. 2017, 249, 357–363. [Google Scholar] [CrossRef]
- Lu, N.; Fan, S.; Zhao, Y.; Yang, B.; Hua, Z.; Wu, Y. A Selective Methane Gas Sensor with Printed Catalytic Films as Active Filters. Sens. Actuators B Chem. 2021, 347, 130603. [Google Scholar] [CrossRef]
- Shaposhnik, A.; Moskalev, P.; Sizask, E.; Ryabtsev, S.; Vasiliev, A. Selective Detection of Hydrogen Sulfide and Methane by a Single MOX-Sensor. Sensors 2019, 19, 1135. [Google Scholar] [CrossRef] [PubMed]
- Govardhan, K.; Grace, A.N. Metal/Metal Oxide Doped Semiconductor Based Metal Oxide Gas Sensors—A Review. Sens. Lett. 2016, 14, 741–750. [Google Scholar] [CrossRef]
- Nikolic, M.V.; Milovanovic, V.; Vasiljevic, Z.Z.; Stamenkovic, Z. Semiconductor Gas Sensors: Materials, Technology, Design, and Application. Sensors 2020, 20, 6694. [Google Scholar] [CrossRef]
- Ben Saber, N.; Mezni, A.; Alrooqi, A.; Altalhi, T. A Review of Ternary Nanostructures Based Noble Metal/Semiconductor for Environmental and Renewable Energy Applications. J. Mater. Res. Technol. 2020, 9, 15233–15262. [Google Scholar] [CrossRef]
- Karimi-Maleh, H.; Ghalkhani, M.; Saberi Dehkordi, Z.; Mohsenpour Tehran, M.; Singh, J.; Wen, Y.; Baghayeri, M.; Rouhi, J.; Fu, L.; Rajendran, S. MOF-Enabled Pesticides as Developing Approach for Sustainable Agriculture and Reducing Environmental Hazards. J. Ind. Eng. Chem. 2023, in press. [Google Scholar] [CrossRef]
- Karimi-Maleh, H.; Darabi, R.; Baghayeri, M.; Karimi, F.; Fu, L.; Rouhi, J.; Niculina, D.E.; Gündüz, E.S.; Dragoi, E.N. Recent Developments in Carbon Nanomaterials-Based Electrochemical Sensors for Methyl Parathion Detection. J. Food Meas. Charact. 2023, 17, 5371–5389. [Google Scholar] [CrossRef]
- Xie, K.; Xu, S.; Xu, K.; Hao, W.; Wang, J.; Wei, Z. BiOCl Heterojunction Photocatalyst: Construction, Photocatalytic Performance, and Applications. Chemosphere 2023, 317, 137823. [Google Scholar] [CrossRef]
- Xie, K.; Wang, W.; Li, Y.; Xu, M.; Han, Z.; Zhang, Y.; Gao, W. Study on Structure-Performance Relationship of RGO Enhanced Polypropylene Composites with Improved Atomic Oxygen Resistance. Compos. Part B Eng. 2022, 239, 109970. [Google Scholar] [CrossRef]
- Zheng, Y.; Zhang, P.; Fu, L. Advances on Polysaccharides from Cactus: Analysis and Review Based on Bibliometrics. J. Prof. Assoc. Cactus Dev. 2023, 25, 1–22. [Google Scholar] [CrossRef]
- Shi, H.; Fu, L.; Chen, F.; Zhao, S.; Lai, G. Preparation of Highly Sensitive Electrochemical Sensor for Detection of Nitrite in Drinking Water Samples. Environ. Res. 2022, 209, 112747. [Google Scholar] [CrossRef] [PubMed]
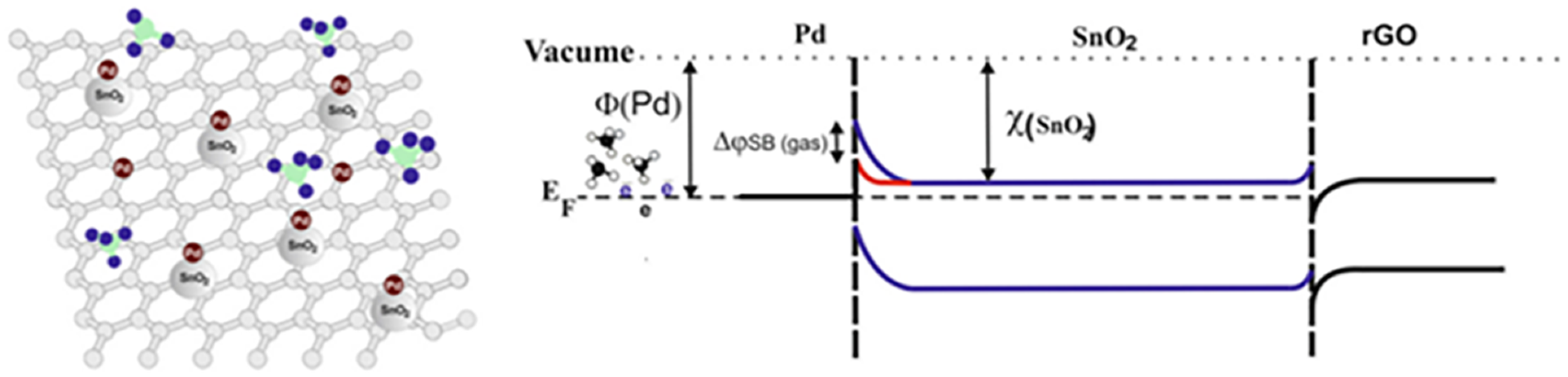
- Yao, L.; Li, Y.; Ran, Y.; Yang, Y.; Zhao, R.; Su, L.; Kong, Y.; Ma, D.; Chen, Y.; Wang, Y. Construction of Novel Pd–SnO2 Composite Nanoporous Structure as a High-Response Sensor for Methane Gas. J. Alloys Compd. 2020, 826, 154063. [Google Scholar] [CrossRef]
- Oleksenko, L.P.; Fedorenko, G.V.; Maksymovych, N.P. Platinum-Containing Adsorption-Semiconductor Sensors Based on Nanosized Tin Dioxide for Methane Detection. Theor. Exp. Chem. 2017, 53, 259–264. [Google Scholar] [CrossRef]
- Oleksenko, L.; Fedorenko, G.; Maksymovych, N. Effect of Heterogeneous Catalytic Methane Oxidation on Kinetics of Conductivity Response of Adsorption Semiconductor Sensors Based on Pd/SnO2 Nanomaterial. Res. Chem. Intermed. 2019, 45, 4101–4111. [Google Scholar] [CrossRef]
- Oleksenko, L.P.; Fedorenko, G.V.; Maksymovych, N.P. Highly Sensitive to Methane Sensor Materials Based on Nano-Pd/SnO2. Theor. Exp. Chem. 2019, 55, 132–136. [Google Scholar] [CrossRef]
- Fedorenko, G.V.; Oleksenko, L.P.; Maksymovych, N.P.; Matushko, I.P. Semiconductor Adsorption Sensors Based on Nanosized Pt/SnO2 Materials and Their Sensitivity to Methane. Russ. J. Phys. Chem. A 2015, 89, 2259–2262. [Google Scholar] [CrossRef]
- Kim, J.C.; Jun, H.K.; Huh, J.-S.; Lee, D.D. Tin Oxide-Based Methane Gas Sensor Promoted by Alumina-Supported Pd Catalyst. Sens. Actuators B Chem. 1997, 45, 271–277. [Google Scholar] [CrossRef]
- Yang, L.; Zhou, X.; Song, L.; Wang, Y.; Wu, X.; Han, N.; Chen, Y. Noble Metal/Tin Dioxide Hierarchical Hollow Spheres for Low-Concentration Breath Methane Sensing. ACS Appl. Nano Mater. 2018, 1, 6327–6336. [Google Scholar] [CrossRef]
- Navazani, S.; Hassanisadi, M.; Eskandari, M.M.; Talaei, Z. Design and Evaluation of SnO2-Pt/MWCNTs Hybrid System as Room Temperature-Methane Sensor. Synth. Met. 2020, 260, 116267. [Google Scholar] [CrossRef]
- Fedorenko, G.; Oleksenko, L.; Maksymovych, N.; Skolyar, G.; Ripko, O. Semiconductor Gas Sensors Based on Pd/SnO2 Nanomaterials for Methane Detection in Air. Nanoscale Res. Lett. 2017, 12, 329. [Google Scholar] [CrossRef] [PubMed]
- Haridas, D.; Gupta, V. Enhanced Response Characteristics of SnO2 Thin Film Based Sensors Loaded with Pd Clusters for Methane Detection. Sens. Actuators B Chem. 2012, 166–167, 156–164. [Google Scholar] [CrossRef]
- Liang, J.; Liu, J.; Li, W.; Hu, M. Preparation and Room Temperature Methane Sensing Properties of Platinum-Decorated Vanadium Oxide Films. Mater. Res. Bull. 2016, 84, 332–339. [Google Scholar] [CrossRef]
- Liang, J.; Liu, J.; Li, N.; Li, W. Magnetron Sputtered Au-Decorated Vanadium Oxides Composite Thin Films for Methane-Sensing Properties at Room Temperature. J. Alloys Compd. 2016, 671, 283–290. [Google Scholar] [CrossRef]
- Xue, D.; Zhang, Z.; Wang, Y. Enhanced Methane Sensing Performance of SnO2 Nanoflowers Based Sensors Decorated with Au Nanoparticles. Mater. Chem. Phys. 2019, 237, 121864. [Google Scholar] [CrossRef]
- Sekizawa, K.; Widjaja, H.; Maeda, S.; Ozawa, Y.; Eguchi, K. Low Temperature Oxidation of Methane over Pd/SnO2 Catalyst. Appl. Catal. Gen. 2000, 200, 211–217. [Google Scholar] [CrossRef]
- Kolmakov, A.; Klenov, D.O.; Lilach, Y.; Stemmer, S.; Moskovits, M. Enhanced Gas Sensing by Individual SnO2 Nanowires and Nanobelts Functionalized with Pd Catalyst Particles. Nano Lett. 2005, 5, 667–673. [Google Scholar] [CrossRef]
- Xue, D.; Wang, P.; Zhang, Z.; Wang, Y. Enhanced Methane Sensing Property of Flower-like SnO2 Doped by Pt Nanoparticles: A Combined Experimental and First-Principle Study. Sens. Actuators B Chem. 2019, 296, 126710. [Google Scholar] [CrossRef]
- Wang, M.; Feng, Y. Palladium–Silver Thin Film for Hydrogen Sensing. Sens. Actuators B Chem. 2007, 123, 101–106. [Google Scholar] [CrossRef]
- Hughes, R.; Schubert, W.; Zipperian, T.; Rodriguez, J.; Plut, T. Thin-film Palladium and Silver Alloys and Layers for Metal-insulator-semiconductor Sensors. J. Appl. Phys. 1987, 62, 1074–1083. [Google Scholar] [CrossRef]
- Ghosh, S.; RoyChaudhuri, C.; Bhattacharya, R.; Saha, H.; Mukherjee, N. Palladium–Silver-Activated ZnO Surface: Highly Selective Methane Sensor at Reasonably Low Operating Temperature. ACS Appl. Mater. Interfaces 2014, 6, 3879–3887. [Google Scholar] [CrossRef] [PubMed]
- Chakraborty, S.; Sen, A.; Maiti, H.S. Selective Detection of Methane and Butane by Temperature Modulation in Iron Doped Tin Oxide Sensors. Sens. Actuators B Chem. 2006, 115, 610–613. [Google Scholar] [CrossRef]
- Quaranta, F.; Rella, R.; Siciliano, P.; Capone, S.; Epifani, M.; Vasanelli, L.; Licciulli, A.; Zocco, A. A Novel Gas Sensor Based on SnO2/Os Thin Film for the Detection of Methane at Low Temperature. Sens. Actuators B Chem. 1999, 58, 350–355. [Google Scholar] [CrossRef]
- Shi, J.; Liu, S.; Zhang, P.; Sui, N.; Cao, S.; Zhou, T.; Zhang, T. Sb/Pd Co-Doped SnO2 Nanoparticles for Methane Detection: Resistance Reduction and Sensing Performance Studies. Nanotechnology 2021, 32, 475506. [Google Scholar] [CrossRef]
- Li, G.; Wang, X.; Yan, L.; Wang, Y.; Zhang, Z.; Xu, J. PdPt Bimetal-Functionalized SnO2 Nanosheets: Controllable Synthesis and Its Dual Selectivity for Detection of Carbon Monoxide and Methane. ACS Appl. Mater. Interfaces 2019, 11, 26116–26126. [Google Scholar] [CrossRef]
- Gagaoudakis, E.; Michail, G.; Katerinopoulou, D.; Moschovis, K.; Iliopoulos, E.; Kiriakidis, G.; Binas, V.; Aperathitis, E. Transparent P-Type NiO:Al Thin Films as Room Temperature Hydrogen and Methane Gas Sensors. Mater. Sci. Semicond. Process. 2020, 109, 104922. [Google Scholar] [CrossRef]
- Chen, R.; Luo, S.; Xie, D.; Yu, Y.; Xiang, L. Highly Dispersive Palladium Loading on ZnO by Galvanic Replacements with Improved Methane Sensing Performance. Chemosensors 2022, 10, 329. [Google Scholar] [CrossRef]
- Zhang, B.; Wang, Y.; Meng, X.; Zhang, Z.; Mu, S. High Response Methane Sensor Based on Au-Modified Hierarchical Porous Nanosheets-Assembled ZnO Microspheres. Mater. Chem. Phys. 2020, 250, 123027. [Google Scholar] [CrossRef]
- Zhang, Y.; Cao, J.; Wang, Y. Ultrahigh Methane Sensing Properties Based on Ni-Doped Hierarchical Porous In2O3 Microspheres at Low Temperature. Vacuum 2022, 202, 111149. [Google Scholar] [CrossRef]
- Wang, F.; Liu, H.; Hu, K.; Li, Y.; Zeng, W.; Zeng, L. Hierarchical Composites of MoS2 Nanoflower Anchored on SnO2 Nanofiber for Methane Sensing. Ceram. Int. 2019, 45, 22981–22986. [Google Scholar] [CrossRef]
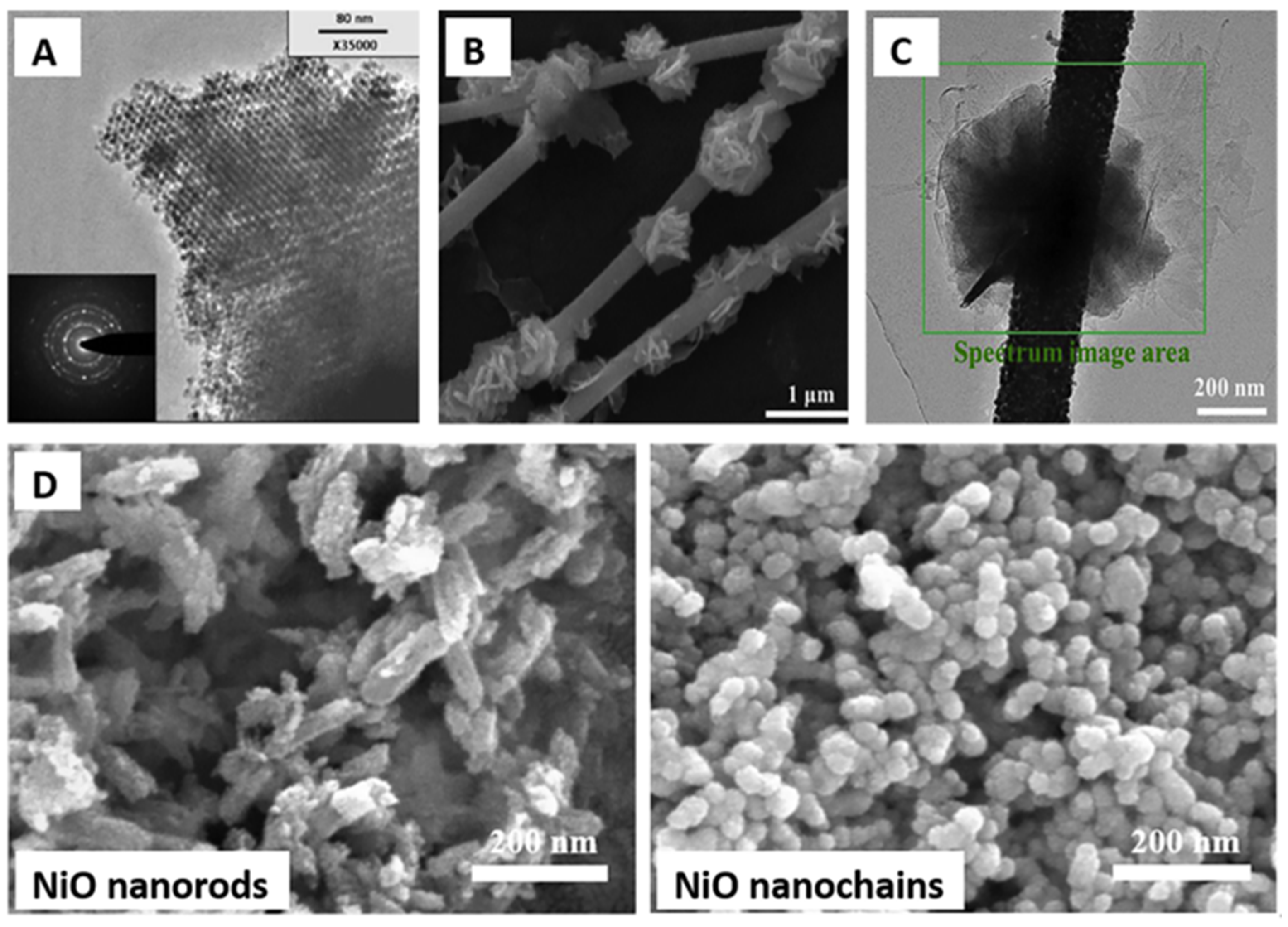
- Zhang, S.; Zhang, B.; Zhang, B.; Wang, Y.; Bala, H.; Zhang, Z. Structural Evolution of NiO from Porous Nanorods to Coral-like Nanochains with Enhanced Methane Sensing Performance. Sens. Actuators B Chem. 2021, 334, 129645. [Google Scholar] [CrossRef]
- Chimowa, G.; Tshabalala, Z.P.; Akande, A.A.; Bepete, G.; Mwakikunga, B.; Ray, S.S.; Benecha, E.M. Improving Methane Gas Sensing Properties of Multi-Walled Carbon Nanotubes by Vanadium Oxide Filling. Sens. Actuators B Chem. 2017, 247, 11–18. [Google Scholar] [CrossRef]
- Nasresfahani, S.; Sheikhi, M.H.; Tohidi, M.; Zarifkar, A. Methane Gas Sensing Properties of Pd-Doped SnO2/Reduced Graphene Oxide Synthesized by a Facile Hydrothermal Route. Mater. Res. Bull. 2017, 89, 161–169. [Google Scholar] [CrossRef]
- Navazani, S.; Shokuhfar, A.; Hassanisadi, M.; Askarieh, M.; Di Carlo, A.; Agresti, A. Facile Synthesis of a SnO2@rGO Nanohybrid and Optimization of Its Methane-Sensing Parameters. Talanta 2018, 181, 422–430. [Google Scholar] [CrossRef] [PubMed]
- Navazani, S.; Shokuhfar, A.; Hassanisadi, M.; Di Carlo, A.; Shahcheraghi, N. Fabrication and Characterization of a Sensitive, Room Temperature Methane Sensor Based on SnO2@reduced Graphene Oxide-Polyaniline Ternary Nanohybrid. Mater. Sci. Semicond. Process. 2018, 88, 139–147. [Google Scholar] [CrossRef]
- Kooti, M.; Keshtkar, S.; Askarieh, M.; Rashidi, A. Progress toward a Novel Methane Gas Sensor Based on SnO2 Nanorods-Nanoporous Graphene Hybrid. Sens. Actuators B Chem. 2019, 281, 96–106. [Google Scholar] [CrossRef]
- Chen, A.; Bai, S.; Shi, B.; Liu, Z.; Li, D.; Liu, C.C. Methane Gas-Sensing and Catalytic Oxidation Activity of SnO2–In2O3 Nanocomposites Incorporating TiO2. Sens. Actuators B Chem. 2008, 135, 7–12. [Google Scholar] [CrossRef]
- Aleksanyan, M.S. Methane Sensor Based on SnO2/In2O3/TiO2 Nanostructure. J. Contemp. Phys. Armen. Acad. Sci. 2010, 45, 77–80. [Google Scholar] [CrossRef]
- Chakraborty, S.; Sen, A.; Maiti, H.S. Complex Plane Impedance Plot as a Figure of Merit for Tin Dioxide-Based Methane Sensors. Sens. Actuators B Chem. 2006, 119, 431–434. [Google Scholar] [CrossRef]
- Wang, Y.; Yao, M.; Guan, R.; Zhang, Z.; Cao, J. Enhanced Methane Sensing Performance of NiO Decorated In2O3 Nanospheres Composites at Low Temperature. J. Alloys Compd. 2021, 854, 157169. [Google Scholar] [CrossRef]
- Zhang, S.; Li, Y.; Sun, G.; Zhang, B.; Wang, Y.; Cao, J.; Zhang, Z. Enhanced Methane Sensing Properties of Porous NiO Nanaosheets by Decorating with SnO2. Sens. Actuators B Chem. 2019, 288, 373–382. [Google Scholar] [CrossRef]
- Vuong, N.M.; Hieu, N.M.; Hieu, H.N.; Yi, H.; Kim, D.; Han, Y.-S.; Kim, M. Ni2O3-Decorated SnO2 Particulate Films for Methane Gas Sensors. Sens. Actuators B Chem. 2014, 192, 327–333. [Google Scholar] [CrossRef]
- Xue, D.; Wang, Y.; Cao, J.; Sun, G.; Zhang, Z. Improving Methane Gas Sensing Performance of Flower-like SnO2 Decorated by WO3 Nanoplates. Talanta 2019, 199, 603–611. [Google Scholar] [CrossRef] [PubMed]
- Xue, D.; Wang, J.; Wang, Y.; Sun, G.; Cao, J.; Bala, H.; Zhang, Z. Enhanced Methane Sensing Properties of WO3 Nanosheets with Dominant Exposed (200) Facet via Loading of SnO2 Nanoparticles. Nanomaterials 2019, 9, 351. [Google Scholar] [CrossRef] [PubMed]
- Bagheri, M.; Khodadadi, A.A.; Mahjoub, A.R.; Mortazavi, Y. Gallia–ZnO Nanohybrid Sensors with Dramatically Higher Sensitivity to Ethanol in Presence of CO, Methane and VOCs. Sens. Actuators B Chem. 2016, 223, 576–585. [Google Scholar] [CrossRef]
- Bagheri, M.; Khodadadi, A.A.; Mahjoub, A.R.; Mortazavi, Y. Highly Sensitive Gallia-SnO2 Nanocomposite Sensors to CO and Ethanol in Presence of Methane. Sens. Actuators B Chem. 2013, 188, 45–52. [Google Scholar] [CrossRef]
- Ghosh, S.; Bhattacharyya, R.; Saha, H.; Chaudhuri, C.R.; Mukherjee, N. Functionalized ZnO/ZnO2 n–N Straddling Heterostructure Achieved by Oxygen Plasma Bombardment for Highly Selective Methane Sensing. Phys. Chem. Chem. Phys. 2015, 17, 27777–27788. [Google Scholar] [CrossRef]
- Yang, B.; Zhang, Z.; Tian, C.; Yuan, W.; Hua, Z.; Fan, S.; Wu, Y.; Tian, X. Selective Detection of Methane by HZSM-5 Zeolite/Pd-SnO2 Gas Sensors. Sens. Actuators B Chem. 2020, 321, 128567. [Google Scholar] [CrossRef]
- Zhang, D.; Chang, H.; Sun, Y.; Jiang, C.; Yao, Y.; Zhang, Y. Fabrication of Platinum-Loaded Cobalt Oxide/Molybdenum Disulfide Nanocomposite toward Methane Gas Sensing at Low Temperature. Sens. Actuators B Chem. 2017, 252, 624–632. [Google Scholar] [CrossRef]
- Bhattacharyya, P.; Basu, P.K.; Saha, H.; Basu, S. Fast Response Methane Sensor Using Nanocrystalline Zinc Oxide Thin Films Derived by Sol–Gel Method. Sens. Actuators B Chem. 2007, 124, 62–67. [Google Scholar] [CrossRef]
- Zhao, Y.; Wang, S.; Yuan, W.; Fan, S.; Hua, Z.; Wu, Y.; Tian, X. Selective Detection of Methane by Pd-In2O3 Sensors with a Catalyst Filter Film. Sens. Actuators B Chem. 2021, 328, 129030. [Google Scholar] [CrossRef]
- Somov, A.; Baranov, A.; Savkin, A.; Spirjakin, D.; Spirjakin, A.; Passerone, R. Development of Wireless Sensor Network for Combustible Gas Monitoring. Sens. Actuators Phys. 2011, 171, 398–405. [Google Scholar] [CrossRef]
- Furst, L.; Feliciano, M.; Frare, L.; Igrejas, G. A Portable Device for Methane Measurement Using a Low-Cost Semiconductor Sensor: Development, Calibration and Environmental Applications. Sensors 2021, 21, 7456. [Google Scholar] [CrossRef] [PubMed]
- Butturini, A.; Fonollosa, J. Use of Metal Oxide Semiconductor Sensors to Measure Methane in Aquatic Ecosystems in the Presence of Cross-Interfering Compounds. Limnol. Oceanogr. Methods 2022, 20, 710–720. [Google Scholar] [CrossRef]
- Fakra, D.A.H.; Andriatoavina, D.A.S.; Razafindralambo, N.A.M.N.; Amarillis, K.A.; Andriamampianina, J.M.M. A Simple and Low-Cost Integrative Sensor System for Methane and Hydrogen Measurement. Sens. Int. 2020, 1, 100032. [Google Scholar] [CrossRef]
- Yanase, Y.; Miura, M.; Fujii, Y.; Okumura, S.; Yoshimura, T. Evaluation of the Concentrations of Hydrogen and Methane Emitted by Termite Using a Semiconductor Gas Sensor. J. Wood Sci. 2013, 59, 243–248. [Google Scholar] [CrossRef][Green Version]
- Dobrzyniewski, D.; Szulczyński, B.; Dymerski, T.; Gębicki, J. Development of Gas Sensor Array for Methane Reforming Process Monitoring. Sensors 2021, 21, 4983. [Google Scholar] [CrossRef] [PubMed]
- Barriault, M.; Alexander, I.; Tasnim, N.; O’Brien, A.; Najjaran, H.; Hoorfar, M. Classification and Regression of Binary Hydrocarbon Mixtures Using Single Metal Oxide Semiconductor Sensor With Application to Natural Gas Detection. Sens. Actuators B Chem. 2021, 326, 129012. [Google Scholar] [CrossRef]


| Sensor Model | Manufacturer | Typical Detection Range |
|---|---|---|
| TGS2611 | Figaro | 500~10,000 ppm |
| MQ-4 | Zhengzhou Winsen | 300~10,000 ppm |
| MPS005 | Nevada Nano Methane Gas Sensor | 500~1500 ppm |
| Cubic SJH-100 | CO2Meter | 0~100% LEL |
| RS-CH4-*-2 | Renke | 0~100% LEL |
| Sensor Types | Mechanisms | Advantages | Disadvantages |
|---|---|---|---|
| Optical sensors | Detect changes in light waves due to interaction of analyte with receptor. | Non-destructive; Immune to electromagnetic interference; Can operate without oxygen. | High cost; High power consumption; Lack of significance and distinctiveness of methane optical absorption region. |
| Calorimetric sensors | Measure heat produced from chemical reaction and correlate to reactant concentration. | Simplistic design; Portable; Easy to manufacture; Easy to manufacture; Good selectivity; Can operate in harsh environments. | Low detection accuracy; Susceptible to cracking, catalyst poisoning, and oversaturation; High power consumption; short lifespan. |
| Pyroelectric sensors | Convert thermal energy into electrical energy based on pyroelectricity. | Non-destructive; Can operate without oxygen; Good sensitivity and responsivity; Wide measuring range; Operate at room temperature. | High cost; High power consumption; Immobile; Difficult to manufacture. |
| Chemiresistive sensors | Absorption of gas on the surface of metal oxide surface changes its conductivity, which is measured to determine gas concentration. | Low cost; Lightweight and robust; Long lifespan; Resistant to poisoning. | Poor selectivity; Small and high operational temperature range; Slow recovery rate; Significant additive dependency; Affected by temperature, humidity, degradation; Sensitive to humidity. |
| Electrochemical sensors | Measure target gas concentration by oxidizing/reducing the gas at an electrode and measuring current. | Low cost; Non-hazardous materials; High boiling points and low volatility; Good selectivity; Can detect small leaks. Solid-state-based: No leakage; Safe; Robust; Good selectivity for methane. | Amperometric-based: susceptible to leakage and evaporation; Hazardous materials; Slow response time. Ionic liquid-based: Susceptible to leakage; Slow response time. Solid-state-based: Requires high temperature; Unable to detect low gas concentrations; Susceptible to degradation or loss of electrolyte. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fu, L.; You, S.; Li, G.; Li, X.; Fan, Z. Application of Semiconductor Metal Oxide in Chemiresistive Methane Gas Sensor: Recent Developments and Future Perspectives. Molecules 2023, 28, 6710. https://doi.org/10.3390/molecules28186710
Fu L, You S, Li G, Li X, Fan Z. Application of Semiconductor Metal Oxide in Chemiresistive Methane Gas Sensor: Recent Developments and Future Perspectives. Molecules. 2023; 28(18):6710. https://doi.org/10.3390/molecules28186710
Chicago/Turabian StyleFu, Li, Shixi You, Guangjun Li, Xingxing Li, and Zengchang Fan. 2023. "Application of Semiconductor Metal Oxide in Chemiresistive Methane Gas Sensor: Recent Developments and Future Perspectives" Molecules 28, no. 18: 6710. https://doi.org/10.3390/molecules28186710
APA StyleFu, L., You, S., Li, G., Li, X., & Fan, Z. (2023). Application of Semiconductor Metal Oxide in Chemiresistive Methane Gas Sensor: Recent Developments and Future Perspectives. Molecules, 28(18), 6710. https://doi.org/10.3390/molecules28186710







