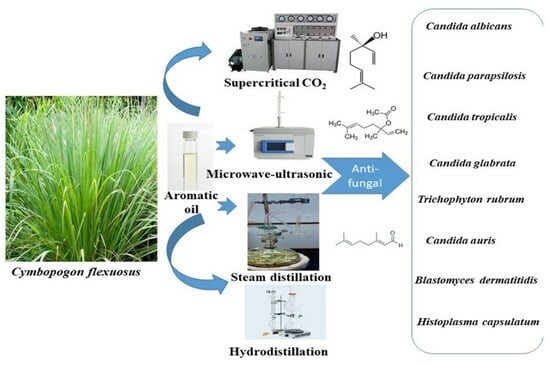
Qualitative and Quantitative Comparison of Aromatic Oil Components and Antifungal Effects of Cymbopogon flexuosus Obtained with Supercritical CO2, Microwave–Ultrasonic, Steam Distillation, and Hydrodistillation Extraction Techniques
Abstract
:1. Introduction
2. Results
2.1. Extraction Yields
2.2. Qualitative and Quantitative Analysis
2.3. Antifungal Activity
3. Discussion
3.1. Yields of Extractions
3.2. Phytochemical Qualitative and Quantitative Analysis
3.3. Antifungal Effect
4. Materials and Methods
4.1. Herbal Material
4.2. Aromatic Oils Extraction Assays
4.2.1. Hydrodistillation Extraction Method
4.2.2. Microwave–Ultrasonic Extraction Method
4.2.3. Supercritical CO2 Extraction Method
4.2.4. Steam Distillation Extraction Method
4.3. GC–MS Analysis
4.4. Antifungal Activity
4.5. Statistical Analysis
5. Conclusions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Revie, N.M.; Iyer, K.R.; Robbins, N.; Cowen, L.E. Antifungal drug resistance: Evolution, mechanisms and impact. Curr. Opin. Microbiol. 2018, 45, 70–76. [Google Scholar] [CrossRef]
- Firacative, C. Invasive fungal disease in humans: Are we aware of the real impact? Memórias Do Inst. Oswaldo Cruz 2020, 115, e200430. [Google Scholar] [CrossRef]
- Ciurea, C.N.; Kosovski, I.-B.; Mare, A.D.; Toma, F.; Pintea-Simon, I.A.; Man, A. Candida and Candidiasis—Opportunism Versus Pathogenicity: A Review of the Virulence Traits. Microorganisms 2020, 8, 857. [Google Scholar] [CrossRef] [PubMed]
- Talapko, J.; Juzbašić, M.; Matijević, T.; Pustijanac, E.; Bekić, S.; Kotris, I.; Škrlec, I. Candida albicans—The virulence factors and clinical manifestations of infection. J. Fungi 2021, 7, 79. [Google Scholar] [CrossRef] [PubMed]
- Jackson, B.R.; Chow, N.; Forsberg, K.; Litvintseva, A.P.; Lockhart, S.R.; Welsh, R.; Vallabhaneni, S.; Chiller, T. On the origins of a species: What might explain the rise of Candida auris? J. Fungi 2019, 5, 58. [Google Scholar] [CrossRef] [PubMed]
- Jenkinson, H.F.; Douglas, L.J. Interactions between Candida species and bacteria in mixed infections. Polymicrob. Dis. 2002, 18, 357–373. [Google Scholar]
- Poulain, D. Candida albicans, plasticity and pathogenesis. Crit. Rev. Microbiol. 2015, 41, 208–217. [Google Scholar] [CrossRef]
- Rodrigues, C.F.; Rodrigues, M.E.; Henriques, M. Candida sp. infections in patients with diabetes mellitus. J. Clin. Med. 2019, 8, 76. [Google Scholar] [CrossRef]
- Trofa, D.; Gácser, A.; Nosanchuk, J.D. Candida parapsilosis, an emerging fungal pathogen. Clin. Microbiol. Rev. 2008, 21, 606–625. [Google Scholar] [CrossRef]
- Zuza-Alves, D.L.; Silva-Rocha, W.P.; Chaves, G.M. An update on Candida tropicalis based on basic and clinical approaches. Front. Microbiol. 2017, 8, 1927. [Google Scholar] [CrossRef]
- Fidel, P.L., Jr.; Vazquez, J.A.; Sobel, J.D. Candida glabrata: Review of epidemiology, pathogenesis, and clinical disease with comparison to C. albicans. Clin. Microbiol. Rev. 1999, 12, 80–96. [Google Scholar] [CrossRef] [PubMed]
- Satoh, K.; Makimura, K.; Hasumi, Y.; Nishiyama, Y.; Uchida, K.; Yamaguchi, H. Candida auris sp. nov., a novel ascomycetous yeast isolated from the external ear canal of an inpatient in a Japanese hospital. Microbiol. Immunol. 2009, 53, 41–44. [Google Scholar] [CrossRef] [PubMed]
- Khalighi, M.; Chang, A. Renal Infectious Diseases. In Pathobiology of Human Disease: A Dynamic Encyclopedia of Disease Mechanisms; Elsevier Inc.: Amsterdam, The Netherlands, 2014; pp. 2857–2868. [Google Scholar]
- Guimarães, A.J.; de Cerqueira, M.D.; Nosanchuk, J.D. Surface architecture of Histoplasma capsulatum. Front Microbiol 2011, 2, 225–232. [Google Scholar] [CrossRef]
- Mrabti, H.N.; Jaradat, N.; Fichtali, I.; Ouedrhiri, W.; Jodeh, S.; Ayesh, S.; Cherrah, Y.; Faouzi, M.E.A. Separation, identification, and antidiabetic activity of catechin isolated from Arbutus unedo L. plant roots. Plants 2018, 7, 31. [Google Scholar] [CrossRef] [PubMed]
- Koparde, A.A.; Doijad, R.C.; Magdum, C.S. Natural products in drug discovery. In Pharmacognosy-Medicinal Plants; IntechOpen: London, UK, 2019. [Google Scholar]
- Qneibi, M.; Hamed, O.; Fares, O.; Jaradat, N.; Natsheh, A.-R.; AbuHasan, Q.; Emwas, N.; Al-Kerm, R.; Al-Kerm, R. The inhibitory role of curcumin derivatives on AMPA receptor subunits and their effect on the gating biophysical properties. Eur. J. Pharm. Sci. 2019, 136, 104951. [Google Scholar] [CrossRef]
- Kalaivani, R.; Devi, V.J.; Umarani, R.; Periyanayagam, K.; Kumaraguru, A.K. Antimicrobial Activity of Some Important Medicinal Plant oils against Human Pathogens. J. Biol. Act. Prod. Nat. 2012, 2, 30–37. [Google Scholar] [CrossRef]
- Ganjewala, D.; Gupta, A.K. Lemongrass (Cymbopogon flexuosus Steud.) Wats essential oil: Overview and biological activities. Recent Prog. Med. Plants 2013, 37, 235–271. [Google Scholar]
- Rajeswara Rao, B. Biological Activities and Medicinal Uses of the Essential Oil and Extracts of Lemongrass (Cymbopogon flexuosus, C. Citratus, C. Pendulus and C. Species); Studium Press LLC: Houston, TX, USA, 2013; Volume 36, pp. 213–257. [Google Scholar]
- Ranade, S.S.; Thiagarajan, P. Lemon grass. Int. J. Pharm. Sci. Rev. Res. 2015, 35, 162–167. [Google Scholar]
- Shah, G.; Shri, R.; Panchal, V.; Sharma, N.; Singh, B.; Mann, A. Scientific basis for the therapeutic use of Cymbopogon citratus, stapf (Lemon grass). J. Adv. Pharm. Technol. Res. 2011, 2, 3. [Google Scholar] [CrossRef]
- Figueiredo, A.C.; Barroso, J.G.; Pedro, L.G.; Scheffer, J.J. Factors affecting secondary metabolite production in plants: Volatile components and essential oils. Flavour Fragr. J. 2008, 23, 213–226. [Google Scholar] [CrossRef]
- Nazzaro, F.; Fratianni, F.; Coppola, R.; De Feo, V. Essential oils and antifungal activity. Pharmaceuticals 2017, 10, 86. [Google Scholar] [CrossRef] [PubMed]
- Soylu, E.M.; Kurt, Ş.; Soylu, S. In vitro and in vivo antifungal activities of the essential oils of various plants against tomato grey mould disease agent Botrytis cinerea. Int. J. Food Microbiol. 2010, 143, 183–189. [Google Scholar] [CrossRef]
- Mugnaini, L.; Nardoni, S.; Pinto, L.; Pistelli, L.; Leonardi, M.; Pisseri, F.; Mancianti, F. In vitro and in vivo antifungal activity of some essential oils against feline isolates of Microsporum canis. J. De Mycol. Medicale 2012, 22, 179–184. [Google Scholar] [CrossRef]
- Saleh, E.S.; Al-Thbity, S.M.; Gobouri, A.A. Impact of Extraction Methods on The Chemical Composition and Biological Activity of Small Cardamom Essential Oils. Egypt. J. Chem. 2023, 66, 101–109. [Google Scholar] [CrossRef]
- Sharifi-Rad, J.; Sureda, A.; Tenore, G.C.; Daglia, M.; Sharifi-Rad, M.; Valussi, M.; Tundis, R.; Sharifi-Rad, M.; Loizzo, M.R.; Ademiluyi, A.O. Biological activities of essential oils: From plant chemoecology to traditional healing systems. Molecules 2017, 22, 70. [Google Scholar] [CrossRef] [PubMed]
- Bolouri, P.; Salami, R.; Kouhi, S.; Kordi, M.; Asgari Lajayer, B.; Hadian, J.; Astatkie, T. Applications of essential oils and plant extracts in different industries. Molecules 2022, 27, 8999. [Google Scholar] [CrossRef]
- Abd Rashed, A.; Rathi, D.-N.G.; Ahmad Nasir, N.A.H.; Abd Rahman, A.Z. Antifungal properties of essential oils and their compounds for application in skin fungal infections: Conventional and nonconventional approaches. Molecules 2021, 26, 1093. [Google Scholar] [CrossRef]
- Swamy, M.K.; Akhtar, M.S.; Sinniah, U.R. Antimicrobial properties of plant essential oils against human pathogens and their mode of action: An updated review. Evid Based Complement. Alternat. Med. 2016, 2016, 3012462. [Google Scholar] [CrossRef]
- Kant, R.; Kumar, A. Review on essential oil extraction from aromatic and medicinal plants: Techniques, performance and economic analysis. Sustain. Chem. Pharm. 2022, 30, 100829. [Google Scholar] [CrossRef]
- Schaneberg, B.T.; Khan, I.A. Comparison of extraction methods for marker compounds in the essential oil of lemon grass by GC. J. Agric. Food Chem. 2002, 50, 1345–1349. [Google Scholar] [CrossRef]
- Singh, A.; Ahmad, A.; Bushra, R. Supercritical carbon dioxide extraction of essential oils from leaves of Eucalyptus globulus L., their analysis and application. Anal. Methods 2016, 8, 1339–1350. [Google Scholar] [CrossRef]
- Danh, L.T.; Han, L.N.; Triet, N.D.A.; Zhao, J.; Mammucari, R.; Foster, N. Comparison of chemical composition, antioxidant and antimicrobial activity of lavender (Lavandula angustifolia L.) essential oils extracted by supercritical CO2, hexane and hydrodistillation. Food Bioprocess Technol. 2013, 6, 3481–3489. [Google Scholar] [CrossRef]
- Janghel, A.; Deo, S.; Raut, P.; Bhosle, D.; Verma, C.; Kumar, S.S.; Agrawal, M.; Amit, N.; Sharma, M.; Giri, T. Supercritical fluid extraction (SFE) techniques as an innovative green technologies for the effective extraction of the active phytopharmaceuticals. Res. J. Pharm. Technol. 2015, 8, 775–786. [Google Scholar] [CrossRef]
- Uwineza, P.A.; Waśkiewicz, A. Recent advances in supercritical fluid extraction of natural bioactive compounds from natural plant materials. Molecules 2020, 25, 3847. [Google Scholar] [CrossRef]
- Gao, S.; Liu, G.; Li, J.; Chen, J.; Li, L.; Li, Z.; Zhang, X.; Zhang, S.; Thorne, R.F.; Zhang, S. Antimicrobial activity of lemongrass essential oil (Cymbopogon flexuosus) and its active component citral against dual-species biofilms of Staphylococcus aureus and Candida species. Front. Cell. Infect. Microbiol. 2020, 10, 603858. [Google Scholar] [CrossRef] [PubMed]
- Vinutha, M.; Thara Saraswathi, K. Study on the essential oil of aerial and sub-aerial parts of Cymbopogon flexuosus (Nees ex Steud) Wats. Int. J. Curr. Sci. 2013, 7, 42–47. [Google Scholar]
- Balti, M.A.; Hadrich, B.; Kriaa, K.; Kechaou, N. Lab-scale extraction of essential oils from Tunisian lemongrass (Cymbopogon flexuosus). Chem. Eng. Process.-Process Intensif. 2018, 124, 164–173. [Google Scholar] [CrossRef]
- Rajeswara Rao, B.R.; Adinarayana, G.; Kumar, A.N.; Rajput, D.K.; Syamasundar, K.V. Chemical-profile variations in essential oils isolated from lemongrass (Cymbopogon flexuosus) biomass and condensate wastewater by re-distillation and solvent extraction techniques. J. EssEntial Oil Res. 2016, 28, 557–564. [Google Scholar] [CrossRef]
- Bhattacharya, R.; Rolta, R.; Dev, K.; Sourirajan, A. Synergistic potential of essential oils with antibiotics to combat fungal pathogens: Present status and future perspectives. Phytother. Res. 2021, 35, 6089–6100. [Google Scholar] [CrossRef] [PubMed]
- Goel, N.; Hashmi, Z.; Khan, N.; Ahmad, R.; Khan, W.H. Recent Strategies to Combat Multidrug Resistance. In Non-Traditional Approaches to Combat Antimicrobial Drug Resistance; Springer: Berlin/Heidelberg, Germany, 2023; pp. 1–27. [Google Scholar]
- Al-Ghanayem, A.A. Phytochemical analysis of Cymbopogon flexuosus (lemongrass) oil, its antifungal activity, and role in inhibiting biofilm formation in Candida albicans MTCC854. J. King Saud Univ.-Sci. 2022, 34, 102072. [Google Scholar] [CrossRef]
- Hasim, S.; Coleman, J. Targeting the fungal cell wall: Current therapies and implications for development of alternative antifungal agents. Future Med. Chem. 2019, 11, 869–883. [Google Scholar] [CrossRef]
- Tian, F.; Woo, S.Y.; Lee, S.Y.; Park, S.B.; Zheng, Y.; Chun, H.S. Antifungal Activity of Essential Oil and Plant-Derived Natural Compounds against Aspergillus flavus. Antibiotics 2022, 11, 1727. [Google Scholar] [CrossRef] [PubMed]
- Pisoschi, A.M.; Pop, A.; Georgescu, C.; Turcuş, V.; Olah, N.K.; Mathe, E. An overview of natural antimicrobials role in food. Eur. J. Med. Chem. 2018, 143, 922–935. [Google Scholar] [CrossRef] [PubMed]
- Marino, A.; Nostro, A.; Mandras, N.; Roana, J.; Ginestra, G.; Miceli, N. Evaluation of antimicrobial activity of the hydrolate of Coridothymus capitatus (L.) Reichenb. fil.(Lamiaceae) alone and in combination with antimicrobial agents. BMC Complement. Med. Ther. 2020, 20, 89. [Google Scholar] [CrossRef] [PubMed]
- Medeiros, C.; de Sousa, M.; Uchoa, D.; Nobre, M.; Bezerra, A.; Rolim, L.; Morais, A.; Nogueira, T.; Nogueira, R.; Lima, E. Antifungal activity of linalool against fluconazole-resistant clinical strains of vulvovaginal Candida albicans and its predictive mechanism of action. Braz. J. Med. Biol. 2022, 55, e11831. [Google Scholar] [CrossRef]
- Kumar Singh, V.; Das, S.; Kumar Dwivedy, A.; Kumar Chaudhari, A.; Upadhyay, N.; Dubey, N.K. Assessment of chemically characterized Salvia sclarea L. essential oil and its combination with linalyl acetate as novel plant based antifungal, antiaflatoxigenic and antioxidant agent against herbal drugs contamination and probable mode of action. Nat. Prod. Res. 2021, 35, 782–787. [Google Scholar] [CrossRef] [PubMed]
- Leite, M.C.A.; Bezerra, A.P.d.B.; Sousa, J.P.d.; Guerra, F.Q.S.; Lima, E.d.O. Evaluation of antifungal activity and mechanism of action of citral against Candida albicans. Evid.-Based Complement. Altern. Med. 2014, 2014, 378280. [Google Scholar] [CrossRef]
- Wuryatmo, E.; Klieber, A.; Scott, E.S. Inhibition of citrus postharvest pathogens by vapor of citral and related compounds in culture. J. Agric. Food Chem. 2003, 51, 2637–2640. [Google Scholar] [CrossRef] [PubMed]
- Belletti, N.; Kamdem, S.S.; Tabanelli, G.; Lanciotti, R.; Gardini, F. Modeling of combined effects of citral, linalool and β-pinene used against Saccharomyces cerevisiae in citrus-based beverages subjected to a mild heat treatment. Int. J. Food Microbiol. 2010, 136, 283–289. [Google Scholar] [CrossRef] [PubMed]
- Jaradat, N.; Adwan, L.; K’aibni, S.; Shraim, N.; Zaid, A.n. Chemical composition, anthelmintic, antibacterial and antioxidant effects of Thymus bovei essential oil. BMC Complement. Altern. Med. 2016, 16, 418. [Google Scholar] [CrossRef]
- Wang, Y.; Li, R.; Jiang, Z.-T.; Tan, J.; Tang, S.-H.; Li, T.-T.; Liang, L.-L.; He, H.-J.; Liu, Y.-M.; Li, J.-T. Green and solvent-free simultaneous ultrasonic-microwave assisted extraction of essential oil from white and black peppers. Ind. Crop. Prod. 2018, 114, 164–172. [Google Scholar] [CrossRef]
- Elsayed, H.E.; El-Deeb, E.M.; Taha, H.; Taha, H.S.; Elgindi, M.R.; Moharram, F.A. Essential oils of Psidium cattleianum Sabine leaves and flowers: Anti-inflammatory and cytotoxic activities. Front. Chem. 2023, 11, 1120432. [Google Scholar] [CrossRef] [PubMed]
- Boutekedjiret, C.; Bentahar, F.; Belabbes, R.; Bessiere, J. Extraction of rosemary essential oil by steam distillation and hydrodistillation. Flavour Fragr. J. 2003, 18, 481–484. [Google Scholar] [CrossRef]
- Adams, R.P. Quadrupole Mass Spectra of Compounds Listed in Order of Their Retention Time on DB-5, Identification of Essential Oils Components by Gas Chromatography/Quadrupole Mass Spectroscopy; Allured Publishing Corporation: Carol Stream, IL, USA, 2001; 456p. [Google Scholar]
- Clinical and Laboratory Standards Institute. Performance Standard for Antimicrobial Susceptibility Testing. Available online: https://www.nih.org.pk/wp-content/uploads/2021/02/CLSI-2020.pdf (accessed on 11 July 2023).
| Names | Retention Time | Retention Index | Reference Retention Index | Microwave–Ultrasonic Method % (v/v) of AO | Steam Distillation Method % (v/v) of AO | Hydrodistillation Method % (v/v) of AO | Supercritical CO2 Method % (v/v) of AO |
|---|---|---|---|---|---|---|---|
| Camphene | 10.9 | 955 | 951 | 0.29 | 0.3 | 0.34 | 0.37 |
| Methyl heptenone | 12.39 | 988 | 986 | 1.31 | 1.38 | 1.44 | 1.4 |
| β-Myrcene | 12.56 | 992 | 993 | 1.34 | 0.88 | 2.12 | 2.32 |
| Hexyl acetate | 13.51 | 1013 | 1015 | 0.48 | 0.51 | 0.37 | 0.35 |
| Limonene | 14.28 | 1030 | 1030 | 1.71 | 1.76 | 1.71 | 1.69 |
| Eucalyptol | 14.42 | 1033 | 1033 | 1.39 | 1.35 | 1.11 | 1.21 |
| Cis-β-ocimene | 14.64 | 1038 | 1040 | 0.18 | 0.18 | 0.14 | 0.1 |
| Linalool | 17.26 | 1102 | 1105 | 21.91 | 22.21 | 16.87 | 17.7 |
| Camphor | 19.25 | 1149 | 1148 | 1.16 | 1.16 | 0.88 | 0.9 |
| Citronellal | 19.55 | 1156 | 1156 | 0.43 | 0.47 | 0.51 | 0.43 |
| Borneol | 20.23 | 1172 | 1172 | 0.41 | 0.43 | 0.37 | 0.38 |
| Terpinen-4-ol | 20.7 | 1183 | 1180 | 0.59 | 0.6 | 0.45 | 0.47 |
| α-Terpineol | 21.3 | 1197 | 1198 | 2.55 | 2.53 | 1.98 | 2.13 |
| Neral (β-citral) | 22.87 | 1246 | 1248 | 17.41 | 16.93 | 22.09 | 21.49 |
| Linalyl acetate | 23.34 | 1258 | 1257 | 24.61 | 24.34 | 18.77 | 20.86 |
| Geranial (α-citral) | 24.00 | 1275 | 1278 | 20.64 | 20.96 | 27.01 | 25.6 |
| Geranyl acetate | 27.29 | 1373 | 1383 | 0.84 | 0.94 | 0.97 | 0.77 |
| Isocaryophyllene | 28.14 | 1404 | 1405 | 1.35 | 1.28 | 1.18 | 1.19 |
| Total | 98.6% | 98.21% | 98.31% | 99.36% | |||
| Yields | 1.55± 0.05 (w/w) | 1.24 ± 0.04 (w/w) | 1.17 ± 0.01 (w/w) | 1.62 ± 0.03 (w/w) | |||
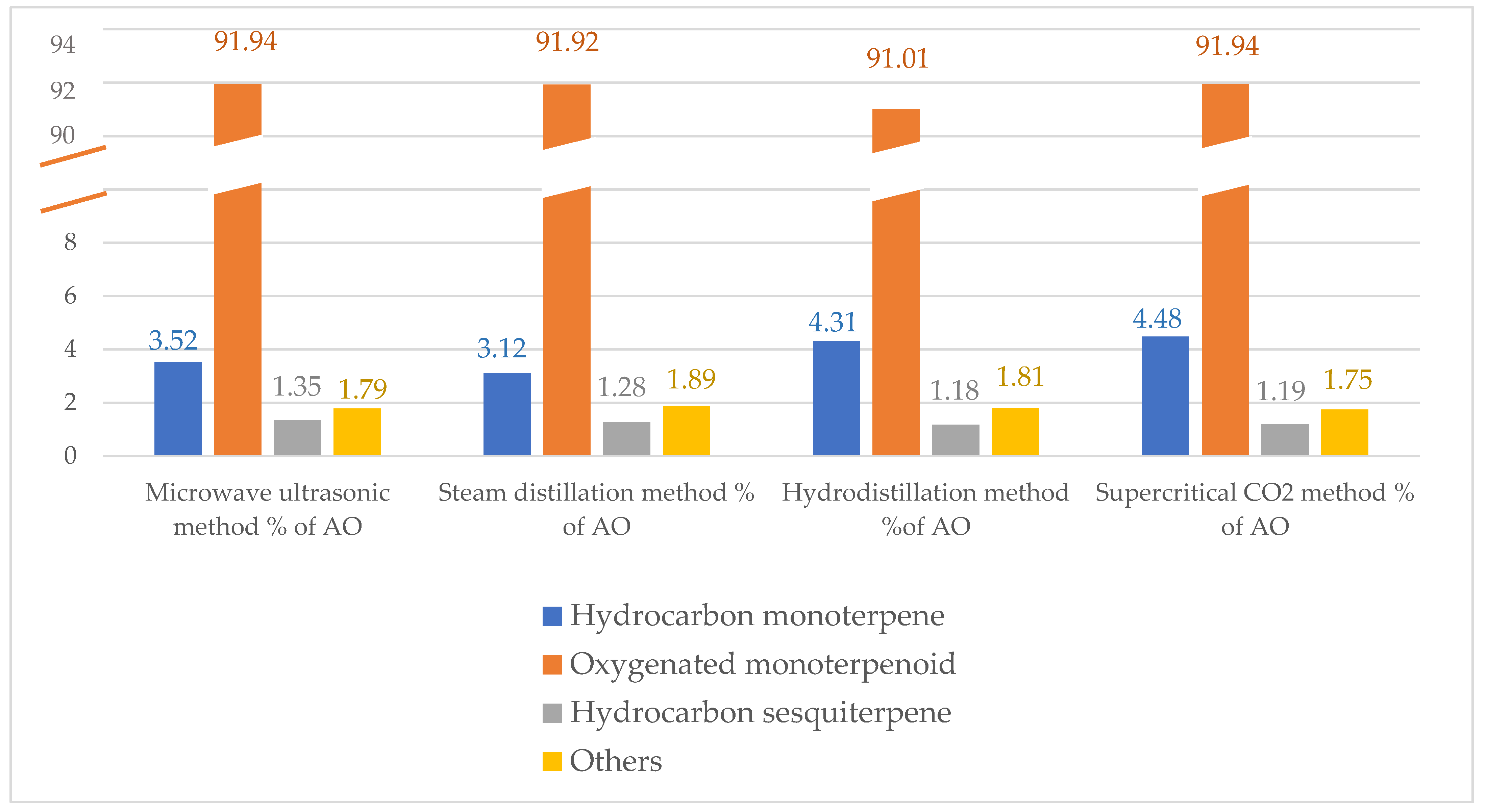
| Phytochemical Groups | |||||||
| Hydrocarbon monoterpene | 3.52 | 3.12 | 4.31 | 4.48 | |||
| Oxygenated monoterpenoid | 91.94 | 91.92 | 91.01 | 91.94 | |||
| Hydrocarbon sesquiterpene | 1.35 | 1.28 | 1.18 | 1.19 | |||
| Others | 1.79 | 1.89 | 1.81 | 1.75 | |||
| Total | 98.6 | 98.21 | 98.31 | 99.36 | |||
| Fungus | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Source of Fungi | ATCC | Clinical Species | |||||||
| Assigned name/number | 90028 | 145 | 154 | 201 | 204 | 210 | 240 | 253 | 261 |
| Microbe | Candida albicans | Candida parapsilosis | Candida tropicalis | Candida albicans | Candida glabrata | Trichophyton rubrum | Candida auris | Blastomyces dermatitidis | Histoplasma capsulatum |
| AO extracted with hydrodistillation method | 12.5 ± 0.33 | 50 ± 1.24 | 12.5 ± 0.33 | 12.5 ± 0.33 | 6.25 ± 0.91 | 25 ± 1.15 | 50 ± 1.24 | 50 ± 1.24 | 25 ± 1.15 |
| AO extracted with steam distillation method | 3.13 ± 0.01 | 3.13 ± 0.01 | 6.25 ± 0.91 | 6.25 ± 0.91 | 3.13 ± 0.01 | 6.25 ± 0.91 | 25 ± 1.15 | 25 ± 1.15 | 12.5 ± 0.33 |
| AO extracted with microwave–ultrasonic method | 6.25 ± 0.91 | 3.13 ± 0.01 | 6.25± 0.91 | 6.25 ± 0.91 | 3.13 ± 0.01 | 6.25 ± 0.91 | 25 ± 1.15 | 25 ± 1.15 | 12.5 ± 0.33 |
| AO extracted with supercritical CO2 method | 25 ± 1.15 | 25 ± 1.15 | 12.5 ± 0.33 | 25 ± 1.15 | 12.5 ± 0.33 | 25 ± 1.15 | 50 ± 1.24 | 50 ± 1.24 | 25 ± 1.15 |
| Fluconazole | 1.56 ± 0.03 | 6.25 ± 0.91 | 6.25 ± 0.91 | 3.13 ± 0.01 | 3.13 ± 0.01 | 12.5 ± 0.33 | 25 ± 1.15 | 25 ± 1.15 | 12.5 ± 0.33 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jaradat, N. Qualitative and Quantitative Comparison of Aromatic Oil Components and Antifungal Effects of Cymbopogon flexuosus Obtained with Supercritical CO2, Microwave–Ultrasonic, Steam Distillation, and Hydrodistillation Extraction Techniques. Molecules 2023, 28, 6870. https://doi.org/10.3390/molecules28196870
Jaradat N. Qualitative and Quantitative Comparison of Aromatic Oil Components and Antifungal Effects of Cymbopogon flexuosus Obtained with Supercritical CO2, Microwave–Ultrasonic, Steam Distillation, and Hydrodistillation Extraction Techniques. Molecules. 2023; 28(19):6870. https://doi.org/10.3390/molecules28196870
Chicago/Turabian StyleJaradat, Nidal. 2023. "Qualitative and Quantitative Comparison of Aromatic Oil Components and Antifungal Effects of Cymbopogon flexuosus Obtained with Supercritical CO2, Microwave–Ultrasonic, Steam Distillation, and Hydrodistillation Extraction Techniques" Molecules 28, no. 19: 6870. https://doi.org/10.3390/molecules28196870
APA StyleJaradat, N. (2023). Qualitative and Quantitative Comparison of Aromatic Oil Components and Antifungal Effects of Cymbopogon flexuosus Obtained with Supercritical CO2, Microwave–Ultrasonic, Steam Distillation, and Hydrodistillation Extraction Techniques. Molecules, 28(19), 6870. https://doi.org/10.3390/molecules28196870