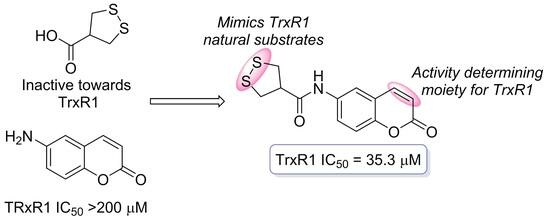
May 1,2-Dithiolane-4-carboxylic Acid and Its Derivatives Serve as a Specific Thioredoxin Reductase 1 Inhibitor?
Abstract
:1. Introduction
2. Results and Discussion
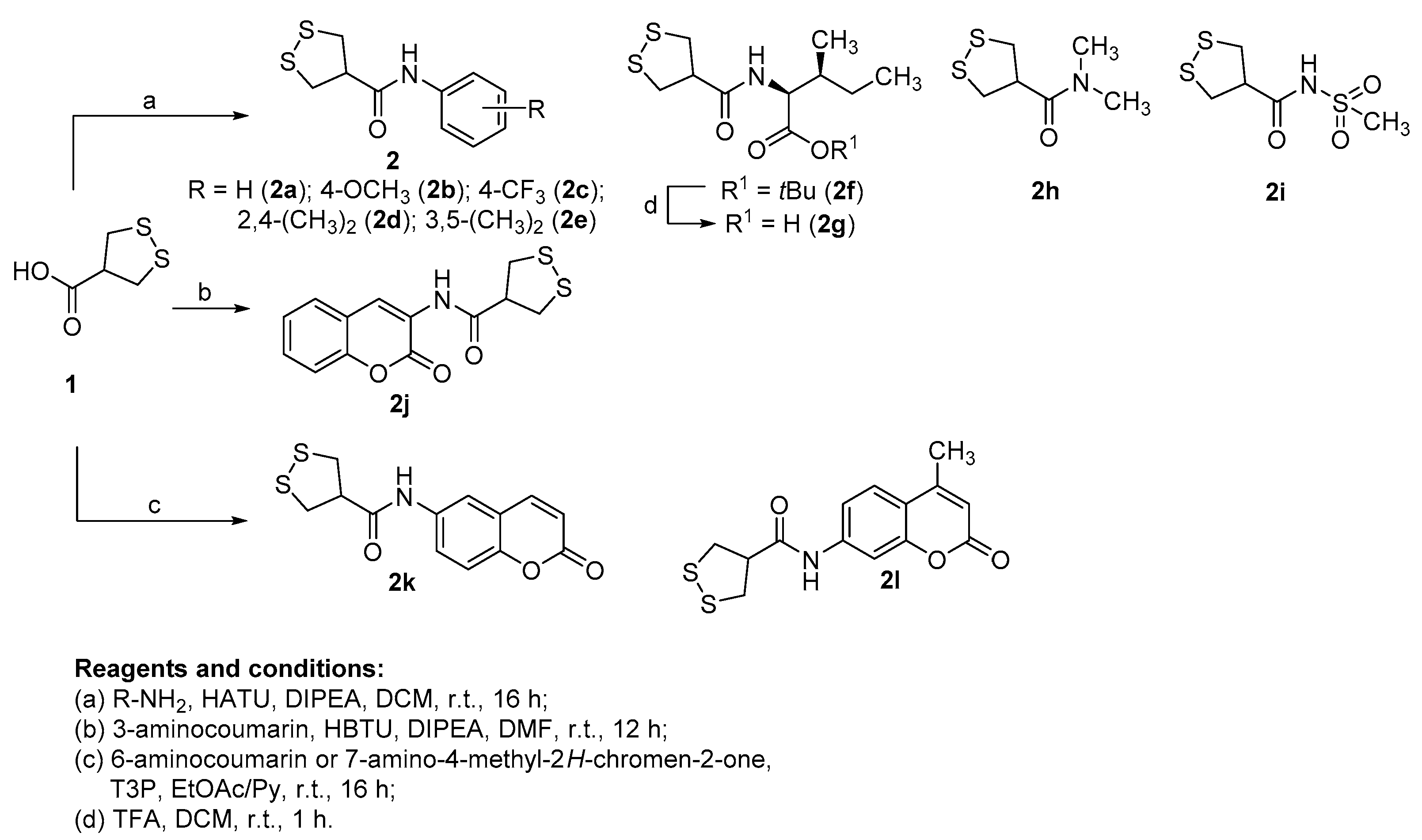
2.1. Synthesis of the Target Compounds
2.2. TrxR1 Inhibitory Activity and SAR Evaluation
2.3. Cytotoxic Effect
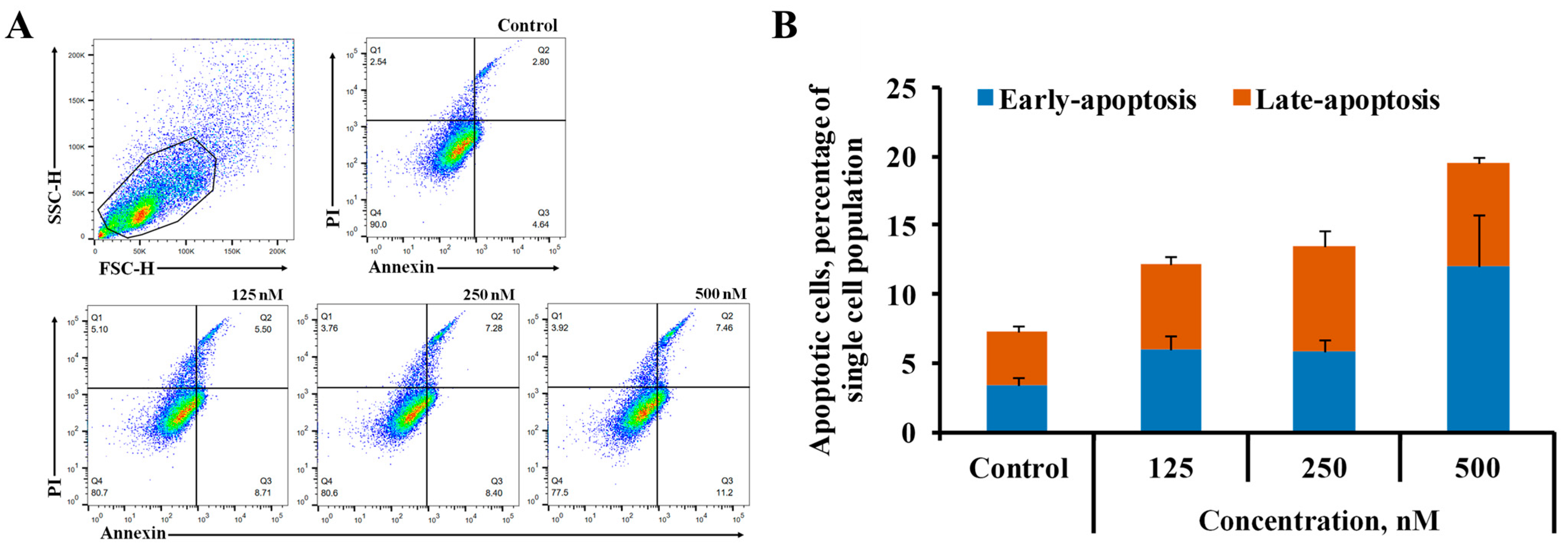
2.4. Cell Death Assay
3. Materials and Methods
3.1. General
3.1.1. General Synthetic Procedure for the Target Compounds 2a–i (Conditions a)
3.1.2. Synthesis of N-(2-Oxo-2H-chromen-3-yl)-1,2-dithiolane-4-carboxamide (2j) (Conditions b)
3.1.3. Synthesis of N-(2-Oxo-2H-chromen-6-yl)-1,2-dithiolane-4-carboxamide (2k) and N-(4-Methyl-2-oxo-2H-chromen-7-yl)-1,2-dithiolane-4-carboxamide (2l) (Conditions c)
3.2. In Vitro Biological Testing
3.2.1. Cell Culture Conditions
3.2.2. Cell Cytotoxicity Assay
3.2.3. Cell Death Assay Using Flow Cytometry Analysis
3.2.4. Colorimetric Detection of Rat TrxR1 Activity
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Park, M.T.; Lee, S.J. Cell cycle and cancer. J. Biochem. Mol. Biol. 2003, 36, 60–65. [Google Scholar] [CrossRef] [PubMed]
- Brandon, M.; Baldi, P.; Wallace, D.C. Mitochondrial mutations in cancer. Oncogene 2006, 25, 4647–4662. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues, M.S.; Reddy, M.M.; Sattler, M. Cell Cycle Regulation by Oncogenic Tyrosine Kinases in Myeloid Neoplasias: From Molecular Redox Mechanisms to Health Implications. Antioxid. Redox Signal. 2008, 10, 1813–1848. [Google Scholar] [CrossRef]
- Hawk, M.A.; McCallister, C.; Schafer, Z.T. Antioxidant Activity during Tumor Progression: A Necessity for the Survival of Cancer Cells? Cancers 2016, 8, 92. [Google Scholar] [CrossRef] [PubMed]
- Galadari, S.; Rahman, A.; Pallichankandy, S.; Thayyullathil, F. Reactive oxygen species and cancer paradox: To promote or to suppress? Free Radic. Biol. Med. 2017, 104, 144–164. [Google Scholar] [CrossRef] [PubMed]
- Moloney, J.N.; Cotter, T.G. ROS signalling in the biology of cancer. Semin. Cell Dev. Biol. 2018, 80, 50–64. [Google Scholar] [CrossRef] [PubMed]
- Arnér, E.S.J. Perspectives of TrxR1-based cancer therapies. In Oxidative Stress Eustress and Distress; Sies, H., Ed.; Academic Press: Cambridge, MA, USA, 2020; pp. 639–667. ISBN 978-0-12-818606-0. [Google Scholar]
- Karlenius, T.C.; Tonissen, K.F. Thioredoxin and Cancer: A Role for Thioredoxin in all States of Tumor Oxygenation. Cancers 2010, 2, 209–232. [Google Scholar] [CrossRef]
- Zhang, J.; Zhang, B.; Li, X.; Han, X.; Liu, R.; Fang, J. Small molecule inhibitors of mammalian thioredoxin reductase as potential anticancer agents: An update. Med. Res. Rev. 2019, 39, 5–39. [Google Scholar] [CrossRef]
- Holmgren, A.; Lu, J. Thioredoxin and thioredoxin reductase: Current research with special reference to human disease. Biochem. Biophys. Res. Commun. 2010, 396, 120–124. [Google Scholar] [CrossRef]
- Lu, J.; Chew, E.-H.; Holmgren, A. Targeting thioredoxin reductase is a basis for cancer therapy by arsenic trioxide. Proc. Natl. Acad. Sci. USA 2007, 104, 12288–12293. [Google Scholar] [CrossRef]
- Krasavin, M.; Žalubovskis, R.; Grandāne, A.; Domračeva, I.; Zhmurov, P.; Supuran, C.T. Sulfocoumarins as dual inhibitors of human carbonic anhydrase isoforms IX/XII and of human thioredoxin reductase. J. Enzym. Inhib. Med. Chem. 2020, 35, 506–510. [Google Scholar] [CrossRef]
- Jovanović, M.; Zhukovsky, D.; Podolski-Renić, A.; Domračeva, I.; Žalubovskis, R.; Senćanski, M.; Glišić, S.; Sharoyko, V.; Tennikova, T.; Dar’in, D.; et al. Novel electrophilic amides amenable by the Ugi reaction perturb thioredoxin system via thioredoxin reductase 1 (TrxR1) inhibition: Identification of DVD-445 as a new lead compound for anticancer therapy. Eur. J. Med. Chem. 2019, 181, 111580. [Google Scholar] [CrossRef] [PubMed]
- Jovanović, M.; Zhukovsky, D.; Podolski-Renić, A.; Žalubovskis, R.; Dar’in, D.; Sharoyko, V.; Tennikova, T.; Pešić, M.; Krasavin, M. Further exploration of DVD-445 as a lead thioredoxin reductase (TrxR) inhibitor for cancer therapy: Optimization of potency and evaluation of anticancer potential. Eur. J. Med. Chem. 2020, 191, 112119. [Google Scholar] [CrossRef]
- Zhang, J.; Li, X.; Han, X.; Liu, R.; Fang, J. Targeting the Thioredoxin System for Cancer Therapy. Trends Pharmacol. Sci. 2017, 38, 794–808. [Google Scholar] [CrossRef] [PubMed]
- Ņikitjuka, A.; Žalubovskis, R. Asparagusic Acid—A Unique Approach toward Effective Cellular Uptake of Therapeutics: Application, Biological Targets, and Chemical Properties. ChemMedChem 2023, 18, e202300143. [Google Scholar] [CrossRef] [PubMed]
- Parry, R.J.; Mizusawa, A.E.; Chiu, A.E.; Naidu, M.V.; Ricciardone, M. Biosynthesis of sulfur Compounds. Investigations of the Biosynthesis of Asparagusic Acid. J. Am. Chem. Soc. 1985, 107, 2512–2521. [Google Scholar]
- Abegg, D.; Gasparini, G.; Hoch, D.G.; Shuster, A.; Bartolami, E.; Matile, S.; Adibekian, A. Strained cyclic Disulfides Enable Cellular Uptake by Reacting with the Transferrin Receptor. J. Am. Chem. Soc. 2016, 139, 231–238. [Google Scholar] [CrossRef]
- Li, X.; Zhang, B.; Yan, C.; Li, J.; Wang, S.; Wei, X.; Jiang, X.; Zhou, P.; Fang, J. A fast and specific fluorescent probe for thioredoxin reductase that works via disulphide bond cleavage. Nat. Commun. 2019, 10, 2745. [Google Scholar] [CrossRef]
- Zhang, L.; Duan, D.; Liu, Y.; Ge, C.; Cui, X.; Sun, J.; Fang, J. Highly Selective Off–On Fluorescent Probe for Imaging Thioredoxin Reductase in Living Cells. J. Am. Chem. Soc. 2014, 136, 226–233. [Google Scholar] [CrossRef]
- Felber, J.G.; Poczka, L.; Scholzen, K.C.; Zeisel, L.; Maier, M.S.; Busker, S.; Theisen, U.; Brandstädter, C.; Becker, K.; Arnér, E.S.J.; et al. Cyclic 5-membered disulfides are not selective substrates of thioredoxin reductase, but are opened nonspecifically. Nat. Commun. 2022, 13, 1754. [Google Scholar] [CrossRef]
- Tirla, A.; Hansen, M.; Rivera-Fuentes, P. Synthesis of Asparagusic Acid Modified Lysine and its Application in Solid-Phase Synthesis of Peptides with Enhanced Cellular Uptake. Synlett 2018, 29, 1289–1292. [Google Scholar]
- Zhang, B.; Liu, Y.; Li, X.; Xu, J.; Fang, J. Small Molecules to Target the Selenoprotein Thioredoxin Reductase. Chemistry 2018, 13, 3593–3600. [Google Scholar] [CrossRef] [PubMed]
- Prasad, C.P.; Tripathi, S.C.; Kumar, M.; Mohapatra, P. Passage number of cancer cell lines: Importance, intricacies, and way-forward. Biotechnol. Bioeng. 2023, 120, 2049–2055. [Google Scholar] [CrossRef] [PubMed]
- Mosmann, T. Rapid colorimetric assay for cellular growth and survival: Application to proliferation and cytotoxicity assays. J. Immunol. Methods 1983, 65, 55–63. [Google Scholar] [CrossRef] [PubMed]
- Cornelissen, M.; Philippeé, J.; De Sitter, S.; De Ridder, L. Annexin V expression in apoptotic peripheral blood lymphocytes: An lectron microscopic evaluation. Apoptosis 2002, 7, 41–47. [Google Scholar] [PubMed]
- Smith, A.D.; Morris, V.C.; Levander, O.A. Rapid determination of glutathione peroxidase and thioredoxin reductase activities using a 96-well microplate format: Comparison to standard cuvette-based assays. Int. J. Vitam. Nutr. Res. 2001, 71, 87–92. [Google Scholar] [CrossRef] [PubMed]

| Compound | % TrxR1 Activity Inhibition at 200 µM | IC50 ± SD, µM | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| TrxR1 | HEK293 | HepG2 | HeLa | 4T1 | MDA MB 231 | MDA MB 435 | A549 | HT1080 | ||
| 2g | 50 | 186.4± 14.9 | 1.74 ± 0.57 | >2 | >2 | >2 | >2 | >2 | >2 | >2 |
| 2j | 100 | 5.3 ± 0.4 | 0.53 ± 0.23 | 1.56 ± 0.39 | >2 | >2 | >2 | >2 | >2 | 1.80 ± 0.56 |
| 2k | 89 | 36.3 ± 2.9 | 0.41 ± 0.12 | 0.29 ± 0.08 | 0.17 ± 0.03 | 0.24 ± 0.06 | 0.62 ± 0.14 | 0.25 ± 0.05 | 0.28 ± 0.04 | 0.31 ± 0.07 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nikitjuka, A.; Krims-Davis, K.; Kaņepe-Lapsa, I.; Ozola, M.; Žalubovskis, R. May 1,2-Dithiolane-4-carboxylic Acid and Its Derivatives Serve as a Specific Thioredoxin Reductase 1 Inhibitor? Molecules 2023, 28, 6647. https://doi.org/10.3390/molecules28186647
Nikitjuka A, Krims-Davis K, Kaņepe-Lapsa I, Ozola M, Žalubovskis R. May 1,2-Dithiolane-4-carboxylic Acid and Its Derivatives Serve as a Specific Thioredoxin Reductase 1 Inhibitor? Molecules. 2023; 28(18):6647. https://doi.org/10.3390/molecules28186647
Chicago/Turabian StyleNikitjuka, Anna, Kristaps Krims-Davis, Iveta Kaņepe-Lapsa, Melita Ozola, and Raivis Žalubovskis. 2023. "May 1,2-Dithiolane-4-carboxylic Acid and Its Derivatives Serve as a Specific Thioredoxin Reductase 1 Inhibitor?" Molecules 28, no. 18: 6647. https://doi.org/10.3390/molecules28186647
APA StyleNikitjuka, A., Krims-Davis, K., Kaņepe-Lapsa, I., Ozola, M., & Žalubovskis, R. (2023). May 1,2-Dithiolane-4-carboxylic Acid and Its Derivatives Serve as a Specific Thioredoxin Reductase 1 Inhibitor? Molecules, 28(18), 6647. https://doi.org/10.3390/molecules28186647