A Combined NMR and UV–Vis Approach to Evaluate Radical Scavenging Activity of Rosmarinic Acid and Other Polyphenols
Abstract
:1. Introduction
2. Results
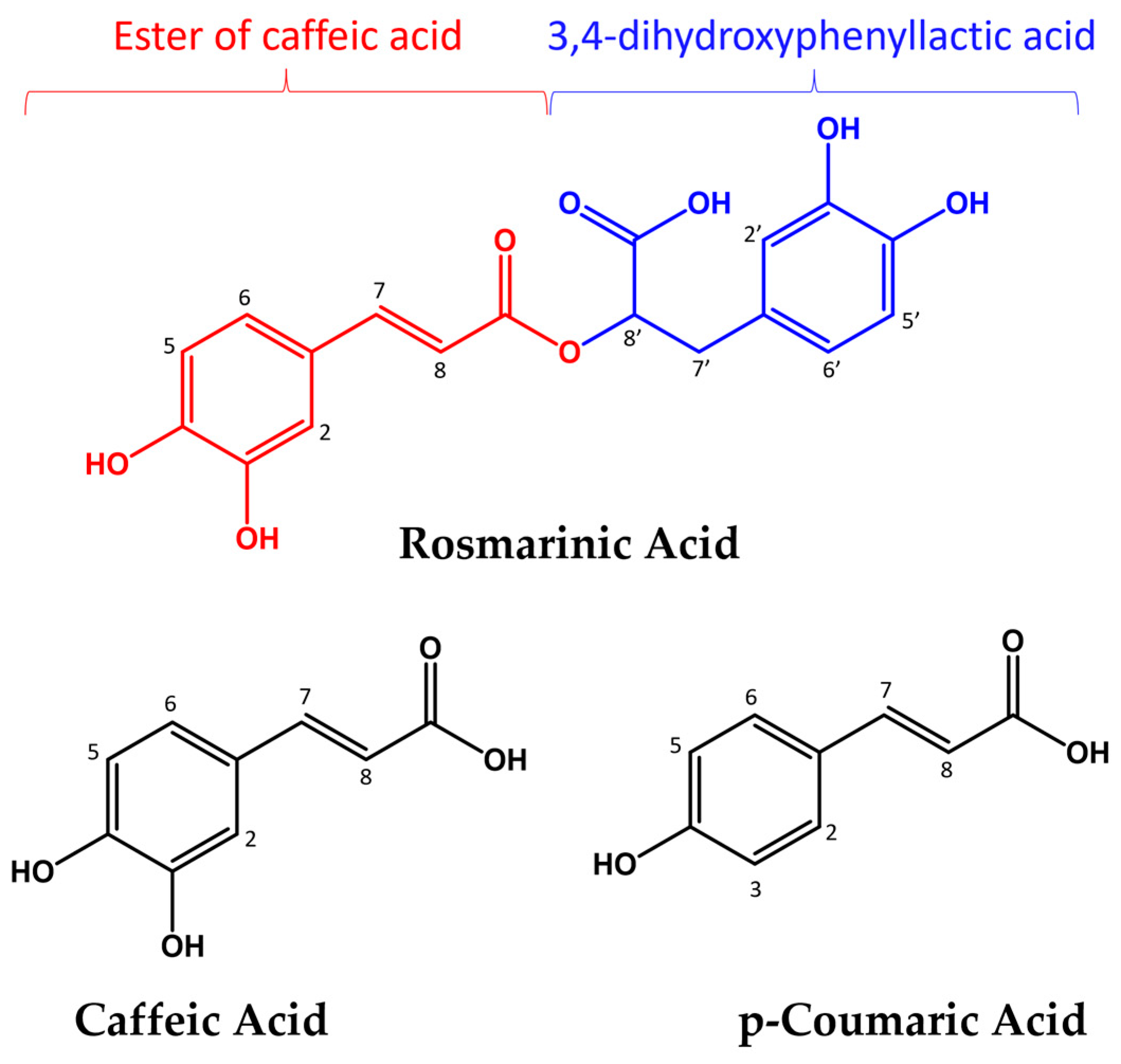
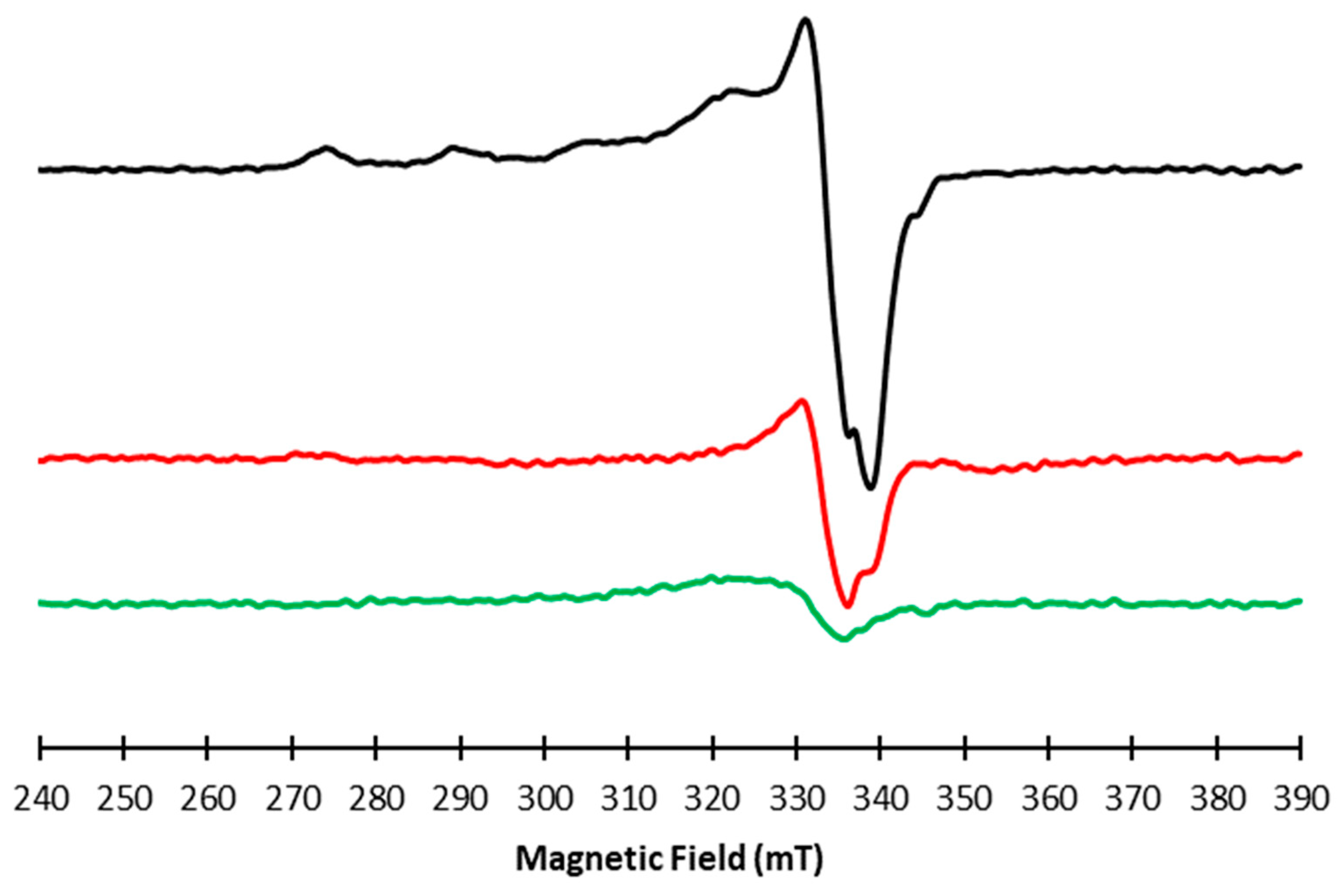
2.1. Copper(II) Interaction with Rosmarinic Acid (RA), Caffeic Acid (CA) and p-Coumaric Acid (pCA)
2.2. Evaluation of the ROS Scavenging Abilities of Rosmarinic Acid (RA), Caffeic Acid (CA), and p-Coumaric Acid (pCA)
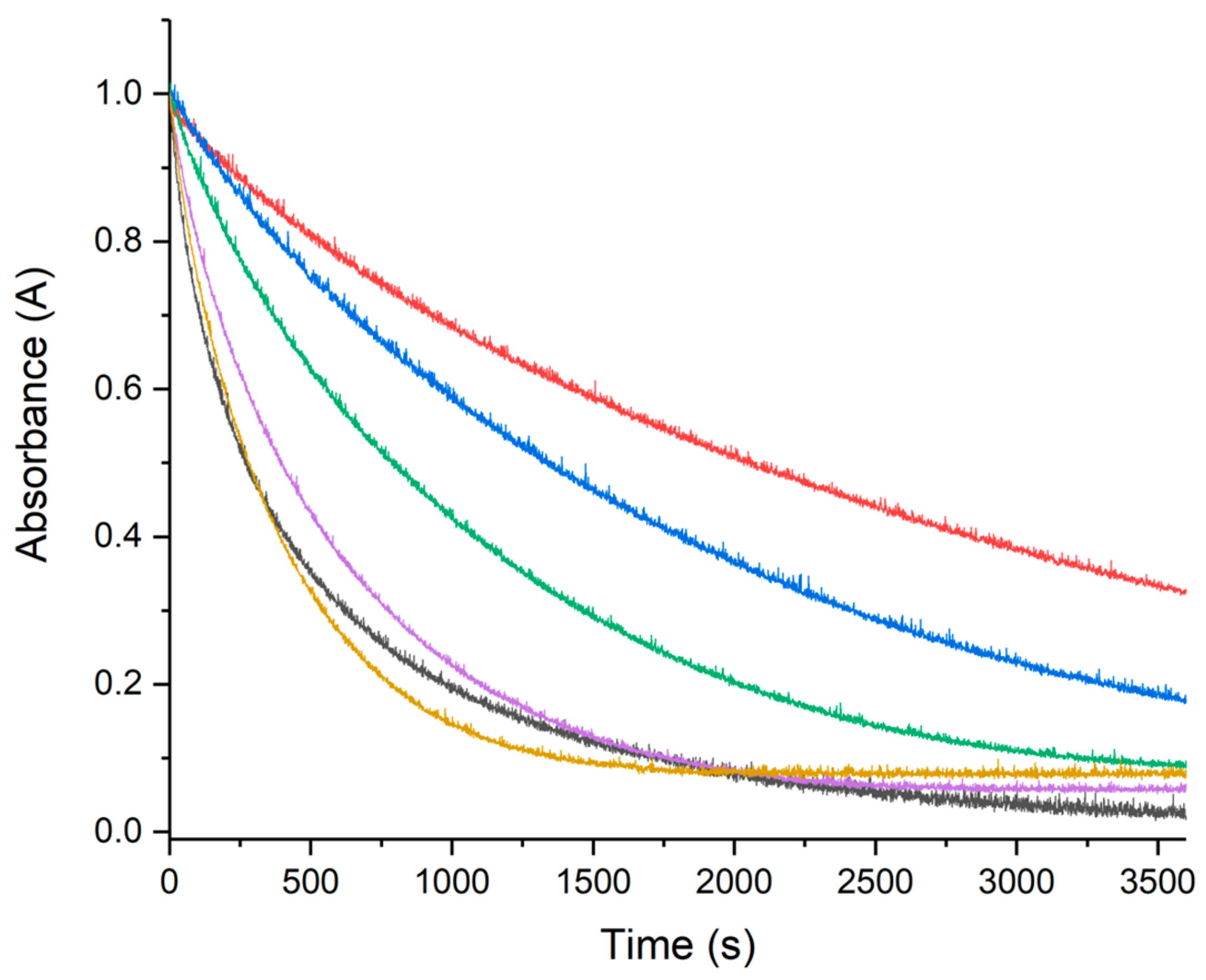
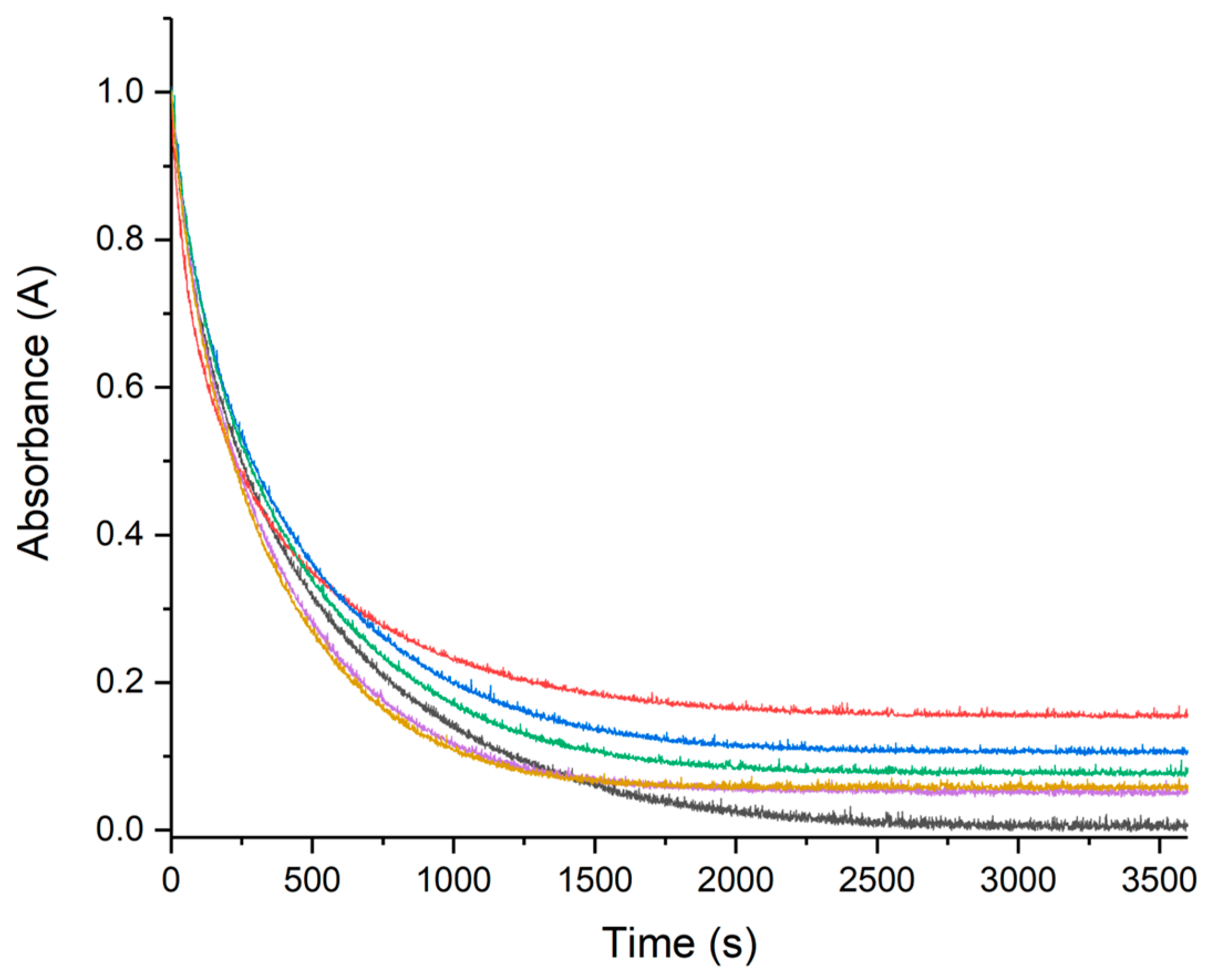
2.2.1. UV–Vis Monitoring of Ascorbate Oxidation Promoted by Copper(II)
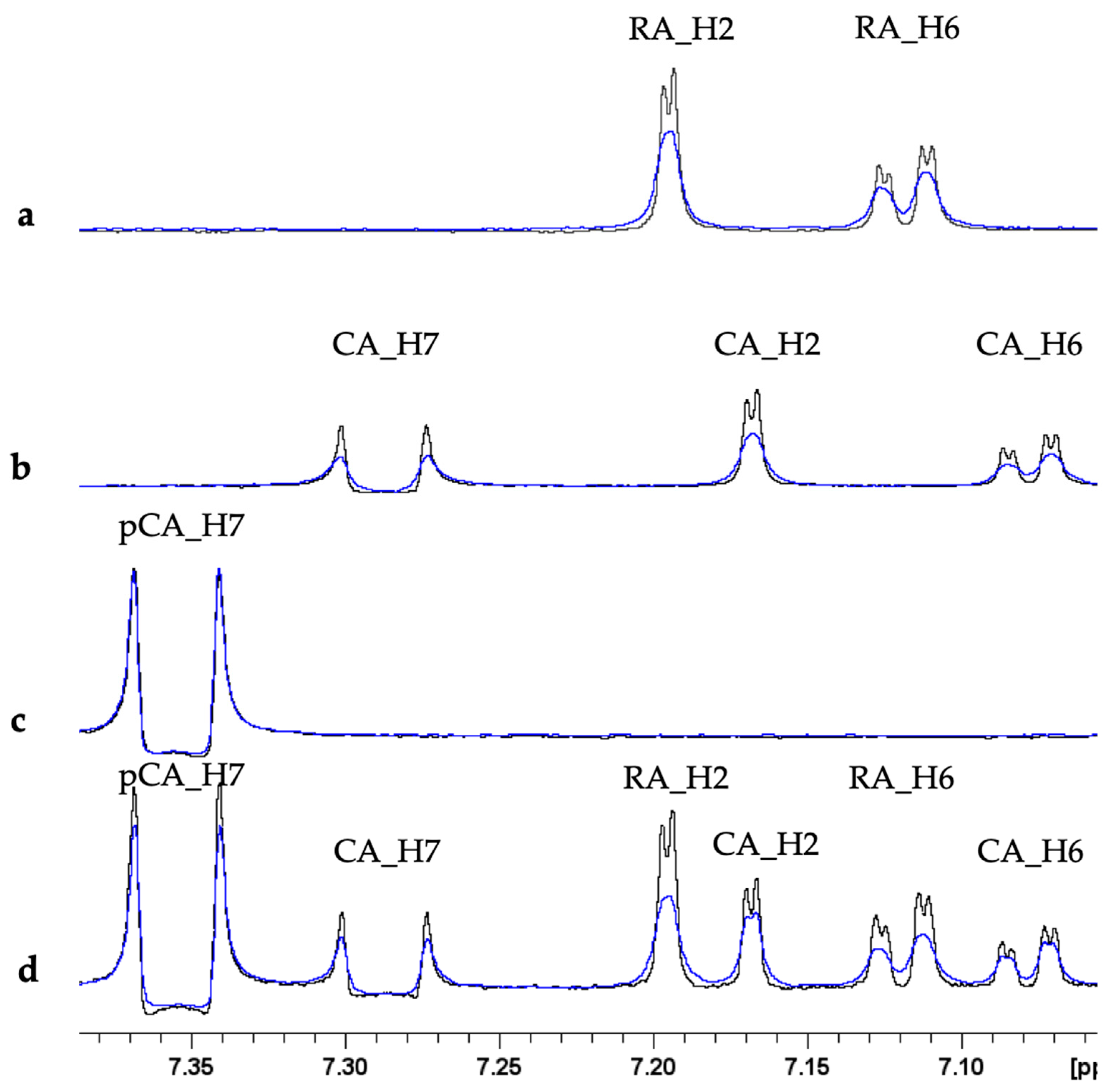
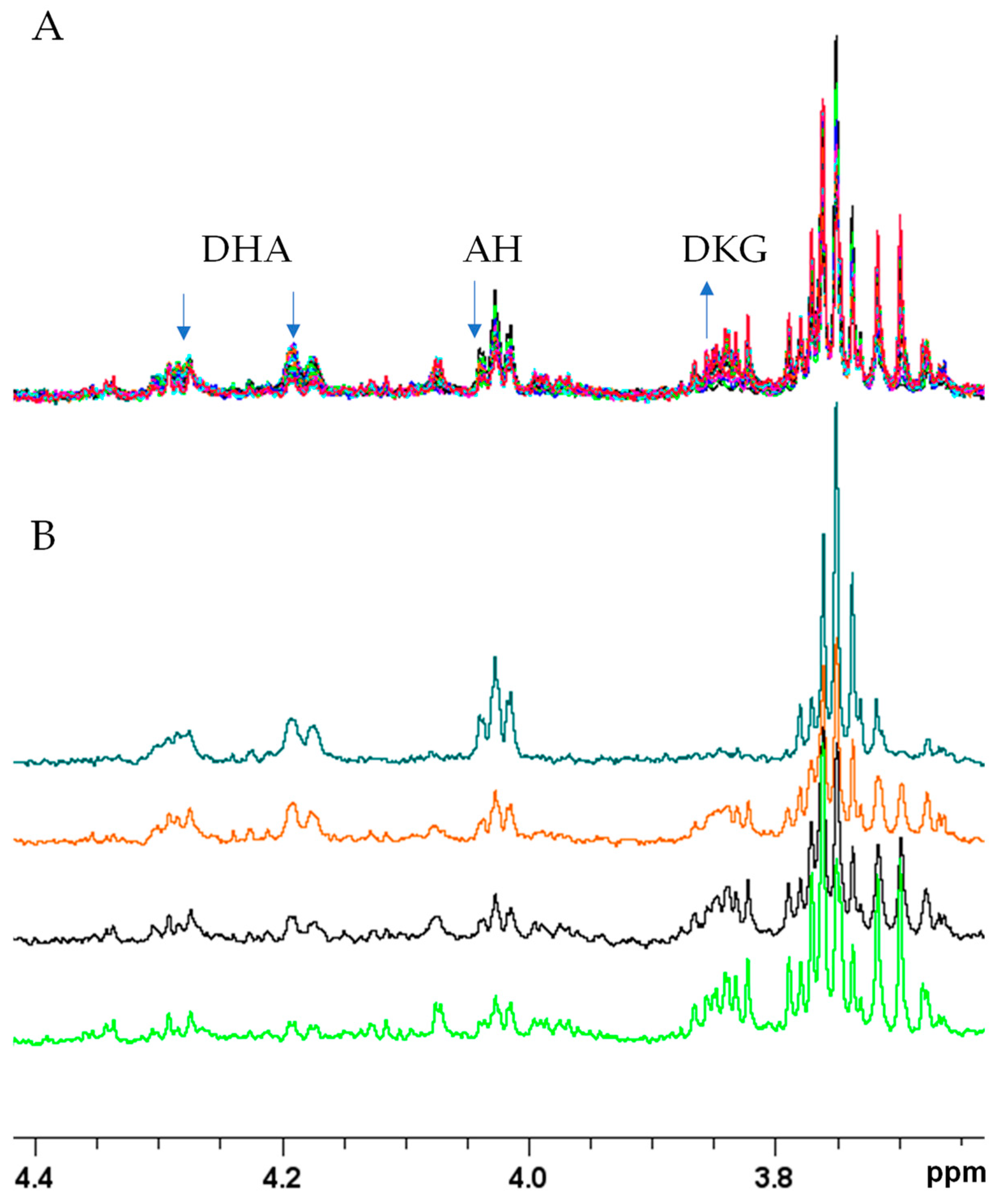
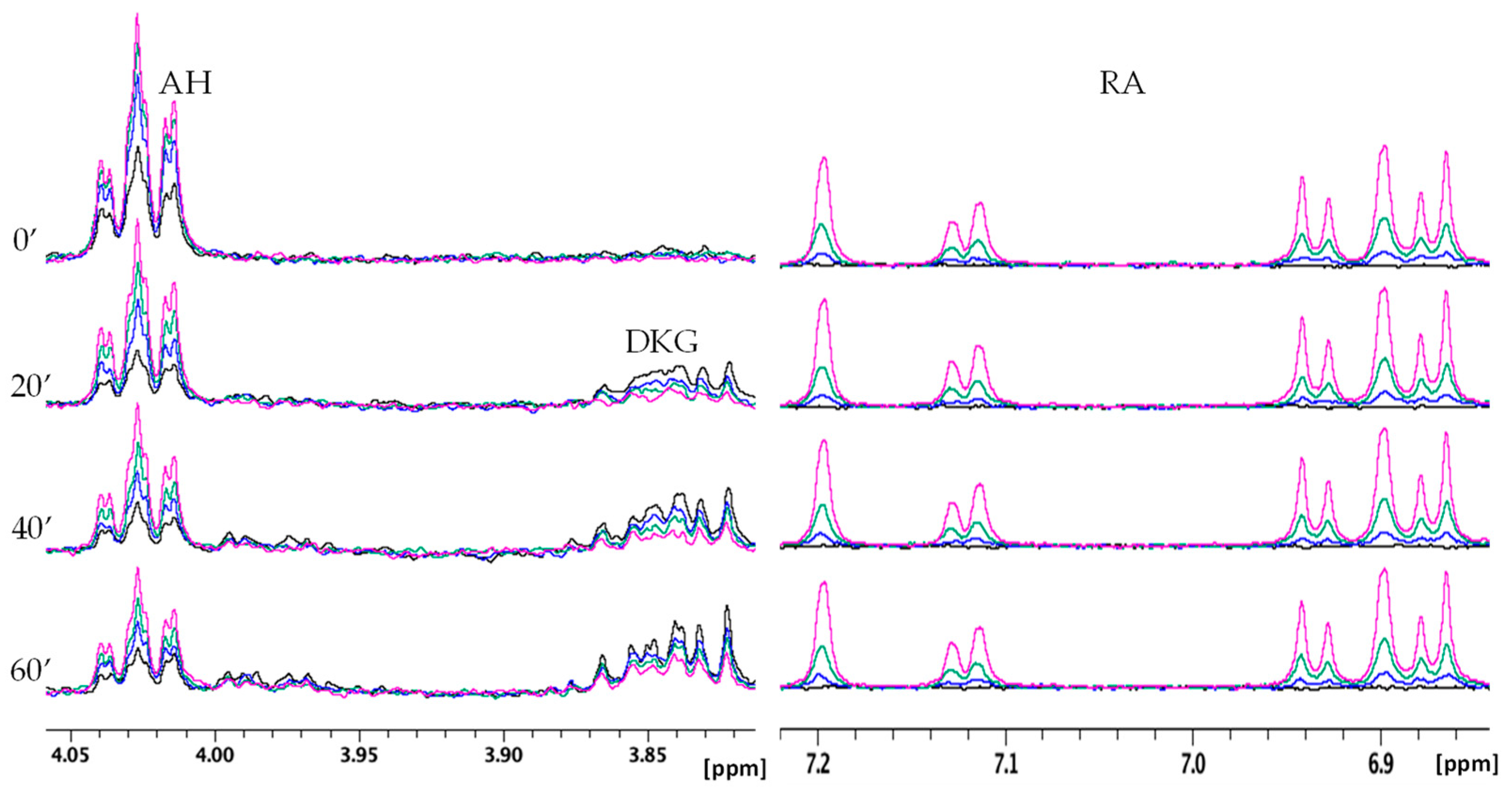
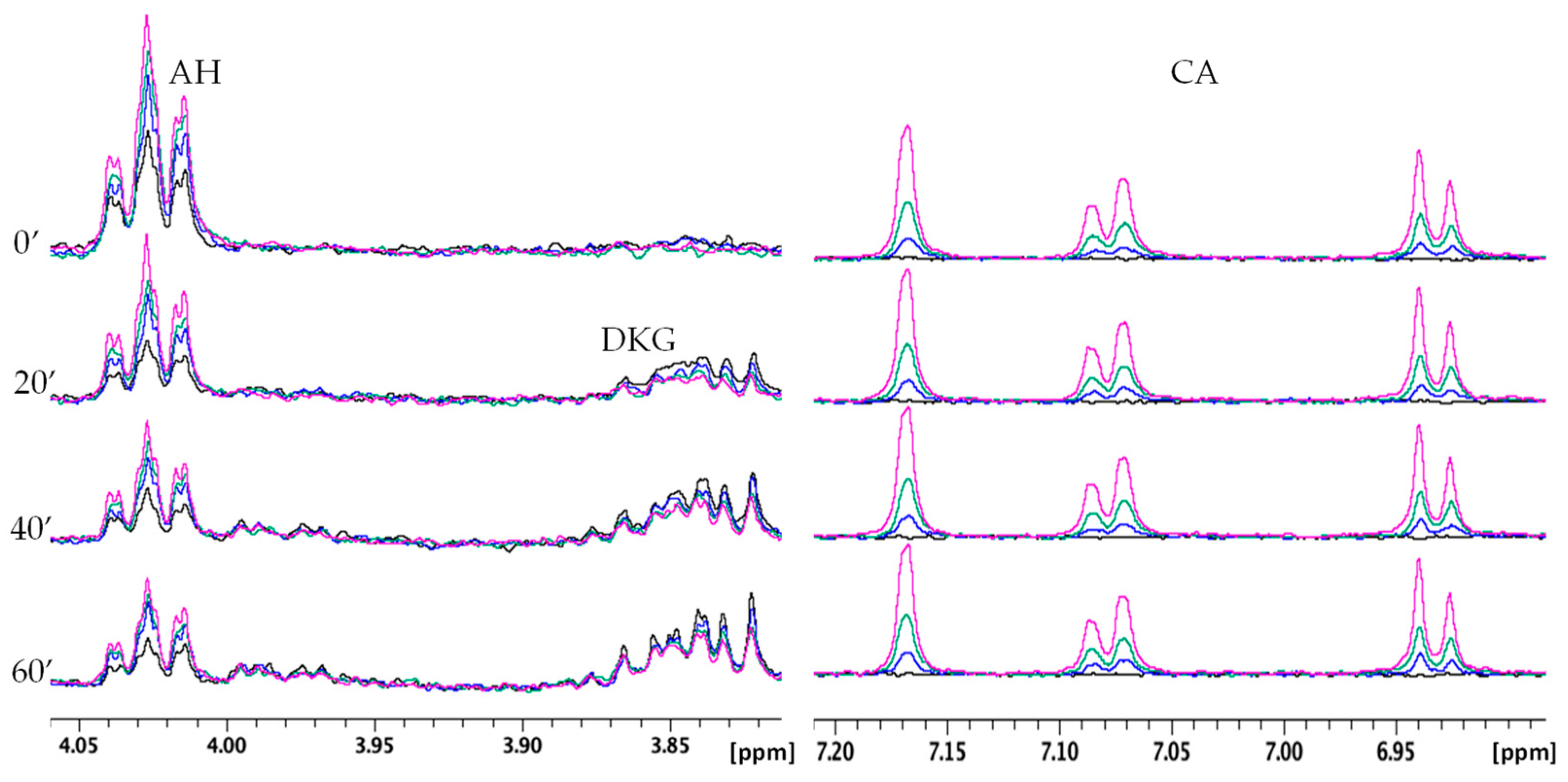
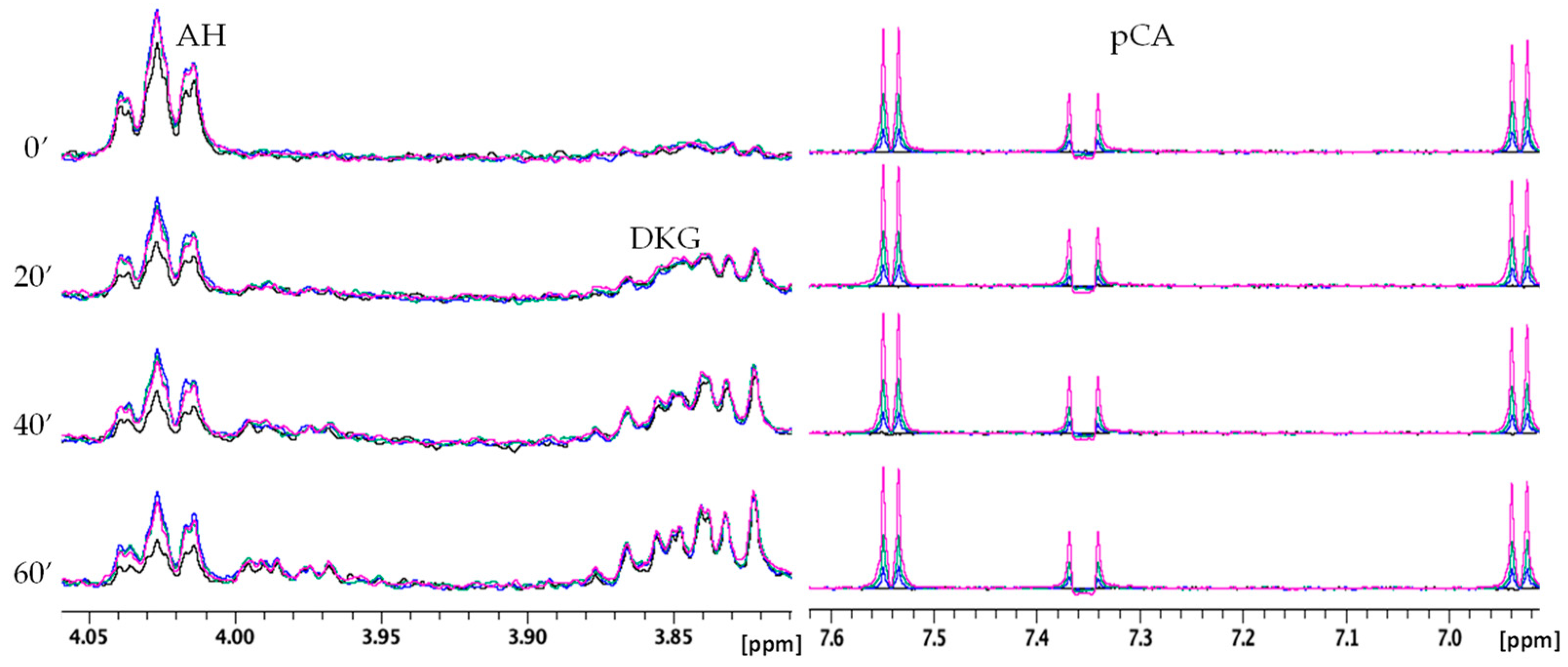
2.2.2. NMR Monitoring of Ascorbate Oxidation Promoted by Copper(II)
3. Discussion
4. Materials and Methods
4.1. Materials
4.2. Sample Preparation
4.3. UV–Vis Measurements
4.4. NMR Experiments
4.5. EPR Spectroscopy
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Sies, H.; Berndt, C.; Jones, D.P. Oxidative Stress. Annu. Rev. Biochem. 2017, 86, 715–748. [Google Scholar] [CrossRef] [PubMed]
- Teleanu, D.M.; Niculescu, A.-G.; Lungu, I.I.; Radu, C.I.; Vladâcenco, O.; Roza, E.; Costăchescu, B.; Grumezescu, A.M.; Teleanu, R.I. An Overview of Oxidative Stress, Neuroinflammation, and Neurodegenerative Diseases. Int. J. Mol. Sci. 2022, 23, 5938. [Google Scholar] [CrossRef] [PubMed]
- Pehlivan, F.E. Vitamin C: An Antioxidant Agent. In Vitamin C; IntechOpen: London, UK, 2017; ISBN 978-953-51-3422-0. [Google Scholar]
- Kola, A.; Nencioni, F.; Valensin, D. Bioinorganic Chemistry of Micronutrients Related to Alzheimer’s and Parkinson’s Diseases. Molecules 2023, 28, 5467. [Google Scholar] [CrossRef] [PubMed]
- Niki, E. Role of Vitamin E as a Lipid-Soluble Peroxyl Radical Scavenger: In Vitro and in Vivo Evidence. Free Radic. Biol. Med. 2014, 66, 3–12. [Google Scholar] [CrossRef] [PubMed]
- Galano, A.; Alvarez-Idaboy, J.R. Glutathione: Mechanism and Kinetics of Its Non-Enzymatic Defense Action against Free Radicals. RSC Adv. 2011, 1, 1763–1771. [Google Scholar] [CrossRef]
- Mirończuk-Chodakowska, I.; Witkowska, A.M.; Zujko, M.E. Endogenous Non-Enzymatic Antioxidants in the Human Body. Adv. Med. Sci. 2018, 63, 68–78. [Google Scholar] [CrossRef]
- Jena, A.B.; Samal, R.R.; Bhol, N.K.; Duttaroy, A.K. Cellular Red-Ox System in Health and Disease: The Latest Update. Biomed. Pharmacother. Biomed. Pharmacother. 2023, 162, 114606. [Google Scholar] [CrossRef]
- Jomova, K.; Raptova, R.; Alomar, S.Y.; Alwasel, S.H.; Nepovimova, E.; Kuca, K.; Valko, M. Reactive Oxygen Species, Toxicity, Oxidative Stress, and Antioxidants: Chronic Diseases and Aging. Arch. Toxicol. 2023, 97, 2499–2574. [Google Scholar] [CrossRef]
- Hayes, J.D.; Dinkova-Kostova, A.T.; Tew, K.D. Oxidative Stress in Cancer. Cancer Cell 2020, 38, 167–197. [Google Scholar] [CrossRef]
- Abramov, A.Y.; Potapova, E.V.; Dremin, V.V.; Dunaev, A.V. Interaction of Oxidative Stress and Misfolded Proteins in the Mechanism of Neurodegeneration. Life 2020, 10, 101. [Google Scholar] [CrossRef]
- Nakamura, T.; Oh, C.; Zhang, X.; Lipton, S.A. Protein S-Nitrosylation and Oxidation Contribute to Protein Misfolding in Neurodegeneration. Free Radic. Biol. Med. 2021, 172, 562–577. [Google Scholar] [CrossRef] [PubMed]
- Luo, J.; Mills, K.; le Cessie, S.; Noordam, R.; van Heemst, D. Ageing, Age-Related Diseases and Oxidative Stress: What to Do Next? Ageing Res. Rev. 2020, 57, 100982. [Google Scholar] [CrossRef] [PubMed]
- Manoharan, S.; Guillemin, G.J.; Abiramasundari, R.S.; Essa, M.M.; Akbar, M.; Akbar, M.D. The Role of Reactive Oxygen Species in the Pathogenesis of Alzheimer’s Disease, Parkinson’s Disease, and Huntington’s Disease: A Mini Review. Oxid. Med. Cell. Longev. 2016, 2016, 8590578. [Google Scholar] [CrossRef] [PubMed]
- Blesa, J.; Trigo-Damas, I.; Quiroga-Varela, A.; Jackson-Lewis, V.R. Oxidative Stress and Parkinson’s Disease. Front. Neuroanat. 2015, 9, 91. [Google Scholar] [CrossRef]
- Schapira, A.H.V.; Cooper, J.M.; Dexter, D.; Clark, J.B.; Jenner, P.; Marsden, C.D. Mitochondrial Complex I Deficiency in Parkinson’s Disease. J. Neurochem. 1990, 54, 823–827. [Google Scholar] [CrossRef]
- Van der Pol, A.; van Gilst, W.H.; Voors, A.A.; van der Meer, P. Treating Oxidative Stress in Heart Failure: Past, Present and Future. Eur. J. Heart Fail. 2019, 21, 425–435. [Google Scholar] [CrossRef]
- Nishikawa, T.; Araki, E. Impact of Mitochondrial ROS Production in the Pathogenesis of Diabetes Mellitus and Its Complications. Antioxid. Redox Signal. 2007, 9, 343–353. [Google Scholar] [CrossRef]
- Tsao, R. Chemistry and Biochemistry of Dietary Polyphenols. Nutrients 2010, 2, 1231–1246. [Google Scholar] [CrossRef]
- Rudrapal, M.; Khairnar, S.J.; Khan, J.; Dukhyil, A.B.; Ansari, M.A.; Alomary, M.N.; Alshabrmi, F.M.; Palai, S.; Deb, P.K.; Devi, R. Dietary Polyphenols and Their Role in Oxidative Stress-Induced Human Diseases: Insights Into Protective Effects, Antioxidant Potentials and Mechanism(s) of Action. Front. Pharmacol. 2022, 13, 283. [Google Scholar] [CrossRef]
- Rana, A.; Samtiya, M.; Dhewa, T.; Mishra, V.; Aluko, R.E. Health Benefits of Polyphenols: A Concise Review. J. Food Biochem. 2022, 46, e14264. [Google Scholar] [CrossRef]
- Fraga, C.G.; Croft, K.D.; Kennedy, D.O.; Tomás-Barberán, F.A. The Effects of Polyphenols and Other Bioactives on Human Health. Food Funct. 2019, 10, 514–528. [Google Scholar] [CrossRef] [PubMed]
- Kiokias, S.; Oreopoulou, V. A Review of the Health Protective Effects of Phenolic Acids against a Range of Severe Pathologic Conditions (Including Coronavirus-Based Infections). Molecules 2021, 26, 5405. [Google Scholar] [CrossRef]
- Soobrattee, M.A.; Neergheen, V.S.; Luximon-Ramma, A.; Aruoma, O.I.; Bahorun, T. Phenolics as Potential Antioxidant Therapeutic Agents: Mechanism and Actions. Mutat. Res. Mol. Mech. Mutagen. 2005, 579, 200–213. [Google Scholar] [CrossRef] [PubMed]
- Shahidi, F.; Chandrasekara, A. Hydroxycinnamates and Their in Vitro and in Vivo Antioxidant Activities. Phytochem. Rev. 2010, 9, 147–170. [Google Scholar] [CrossRef]
- Noor, S.; Mohammad, T.; Rub, M.A.; Raza, A.; Azum, N.; Yadav, D.K.; Hassan, M.I.; Asiri, A.M. Biomedical Features and Therapeutic Potential of Rosmarinic Acid. Arch. Pharm. Res. 2022, 45, 205–228. [Google Scholar] [CrossRef]
- Zhou, L.; Huang, Y.; Han, Z.; Wang, J.; Sun, N.; Zhang, R.; Dong, W.; Deng, C.; Zhuang, G. Effects of Rosmarinic Acid on the Inflammatory Response in Allergic Rhinitis Rat Models after PM2.5 Exposure. J. Clin. Lab. Anal. 2022, 36, e24316. [Google Scholar] [CrossRef]
- Kola, A.; Hecel, A.; Lamponi, S.; Valensin, D. Novel Perspective on Alzheimer’s Disease Treatment: Rosmarinic Acid Molecular Interplay with Copper(II) and Amyloid β. Life 2020, 10, 118. [Google Scholar] [CrossRef]
- Nabavi, S.F.; Tenore, G.C.; Daglia, M.; Tundis, R.; Loizzo, M.R.; Nabavi, S.M. The Cellular Protective Effects of Rosmarinic Acid: From Bench to Bedside. Curr. Neurovasc. Res. 2015, 12, 98–105. [Google Scholar] [CrossRef]
- Lamaison, J.L.; Petitjean-Freytet, C.; Carnat, A. Medicinal Lamiaceae with antioxidant properties, a potential source of rosmarinic acid. Pharm. Acta Helv. 1991, 66, 185–188. [Google Scholar]
- Colica, C.; di Renzo, L.; Aiello, V.; de Lorenzo, A.; Abenavoli, L. Rosmarinic Acid as Potential Anti-Inflammatory Agent. Rev. Recent Clin. Trials 2018, 13, 240–242. [Google Scholar] [CrossRef]
- Pham, A.N.; Xing, G.; Miller, C.; Waite, T. Fenton-like Copper Redox Chemistry Revisited: Hydrogen Peroxide and Superoxide Mediation of Copper-Catalyzed Oxidant Production. J. Catal. 2013, 301, 54–64. [Google Scholar] [CrossRef]
- Das, T.K.; Wati, M.R.; Fatima-Shad, K. Oxidative Stress Gated by Fenton and Haber Weiss Reactions and Its Association With Alzheimer’s Disease. Arch. Neurosci. 2015, 2, e20078. [Google Scholar] [CrossRef]
- Eghbaliferiz, S.; Iranshahi, M. Prooxidant Activity of Polyphenols, Flavonoids, Anthocyanins and Carotenoids: Updated Review of Mechanisms and Catalyzing Metals: Prooxidant Activity of Polyphenols and Carotenoids. Phytother. Res. 2016, 30, 1379–1391. [Google Scholar] [CrossRef] [PubMed]
- Blokhina, O.; Virolainen, E.; Fagerstedt, K.V. Antioxidants, Oxidative Damage and Oxygen Deprivation Stress: A Review. Ann. Bot. 2003, 91, 179–194. [Google Scholar] [CrossRef] [PubMed]
- Gupte, A.; Mumper, R.J. Elevated Copper and Oxidative Stress in Cancer Cells as a Target for Cancer Treatment. Cancer Treat. Rev. 2009, 35, 32–46. [Google Scholar] [CrossRef]
- Hadi, S.M.; Ullah, M.F.; Shamim, U.; Bhatt, S.H.; Azmi, A.S. Catalytic Therapy of Cancer by Ascorbic Acid Involves Redox Cycling of Exogenous/Endogenous Copper Ions and Generation of Reactive Oxygen Species. Chemotherapy 2010, 56, 280–284. [Google Scholar] [CrossRef]
- Ullah, M.F.; Khan, H.Y.; Zubair, H.; Shamim, U.; Hadi, S.M. The Antioxidant Ascorbic Acid Mobilizes Nuclear Copper Leading to a Prooxidant Breakage of Cellular DNA: Implications for Chemotherapeutic Action against Cancer. Cancer Chemother. Pharmacol. 2011, 67, 103–110. [Google Scholar] [CrossRef]
- Bhat, S.H.; Azmi, A.S.; Hanif, S.; Hadi, S.M. Ascorbic Acid Mobilizes Endogenous Copper in Human Peripheral Lymphocytes Leading to Oxidative DNA Breakage: A Putative Mechanism for Anticancer Properties. Int. J. Biochem. Cell Biol. 2006, 38, 2074–2081. [Google Scholar] [CrossRef]
- Chen, L.; Min, J.; Wang, F. Copper Homeostasis and Cuproptosis in Health and Disease. Signal Transduct. Target. Ther. 2022, 7, 378. [Google Scholar] [CrossRef]
- Murekhina, A.E.; Yarullin, D.N.; Sovina, M.A.; Kitaev, P.A.; Gamov, G.A. Copper (II)-Catalyzed Oxidation of Ascorbic Acid: Ionic Strength Effect and Analytical Use in Aqueous Solution. Inorganics 2022, 10, 102. [Google Scholar] [CrossRef]
- Noël, S.; Perez, F.; Pedersen, J.T.; Alies, B.; Ladeira, S.; Sayen, S.; Guillon, E.; Gras, E.; Hureau, C. A New Water-Soluble Cu(II) Chelator That Retrieves Cu from Cu(Amyloid-β) Species, Stops Associated ROS Production and Prevents Cu(II)-Induced Aβ Aggregation. J. Inorg. Biochem. 2012, 117, 322–325. [Google Scholar] [CrossRef] [PubMed]
- Guilloreau, L.; Combalbert, S.; Sournia-Saquet, A.; Mazarguil, H.; Faller, P. Redox Chemistry of Copper–Amyloid-β: The Generation of Hydroxyl Radical in the Presence of Ascorbate Is Linked to Redox-Potentials and Aggregation State. ChemBioChem 2007, 8, 1317–1325. [Google Scholar] [CrossRef] [PubMed]
- Khaw, K.T.; Woodhouse, P. Interrelation of Vitamin C, Infection, Haemostatic Factors, and Cardiovascular Disease. BMJ 1995, 310, 1559–1563. [Google Scholar] [CrossRef]
- Kola, A.; Dudek, D.; Valensin, D. Metal Complexation Mechanisms of Polyphenols Associated to Alzheimer’s Disease. Curr. Med. Chem. 2021, 28, 7278–7294. [Google Scholar] [CrossRef]
- Lakey-Beitia, J.; Burillo, A.M.; la Penna, G.; Hegde, M.L.; Rao, K.S. Polyphenols as Potential Metal Chelation Compounds Against Alzheimer’s Disease. J. Alzheimers Dis. JAD 2021, 82, S335–S357. [Google Scholar] [CrossRef] [PubMed]
- Bertini, I.; Luchinat, C.; Parigi, G. Paramagnetic Constraints: An Aid for Quick Solution Structure Determination of Paramagnetic Metalloproteins. Concepts Magn. Reson. 2002, 14, 259–286. [Google Scholar] [CrossRef]
- Gaggelli, E.; Bernardi, F.; Molteni, E.; Pogni, R.; Valensin, D.; Valensin, G.; Remelli, M.; Luczkowski, M.; Kozlowski, H. Interaction Of The Human Prion PrP(106−126) Sequence With Copper(II), Manganese(II), And Zinc(II): NMR and EPR Studies. J. Am. Chem. Soc. 2005, 127, 996–1006. [Google Scholar] [CrossRef]
- Gaggelli, E.; Kozlowski, H.; Valensin, D.; Valensin, G. NMR Studies on Cu(II)-Peptide Complexes: Exchange Kinetics and Determination of Structures in Solution. Mol. Biosyst. 2005, 1, 79–84. [Google Scholar] [CrossRef]
- Migliorini, C.; Porciatti, E.; Luczkowski, M.; Valensin, D. Structural Characterization of Cu2+, Ni2+ and Zn2+ Binding Sites of Model Peptides Associated with Neurodegenerative Diseases. Coord. Chem. Rev. 2012, 256, 352–368. [Google Scholar] [CrossRef]
- Solomon, I. Relaxation Processes in a System of Two Spins. Phys. Rev. 1955, 99, 559–565. [Google Scholar] [CrossRef]
- De Ricco, R.; Potocki, S.; Kozlowski, H.; Valensin, D. NMR Investigations of Metal Interactions with Unstructured Soluble Protein Domains. Coord. Chem. Rev. 2014, 269, 1–12. [Google Scholar] [CrossRef]
- Peisach, J.; Blumberg, W.E. Structural Implications Derived from the Analysis of Electron Paramagnetic Resonance Spectra of Natural and Artificial Copper Proteins. Arch. Biochem. Biophys. 1974, 165, 691–708. [Google Scholar] [CrossRef] [PubMed]
- Atrián-Blasco, E.; del Barrio, M.; Faller, P.; Hureau, C. Ascorbate Oxidation by Cu(Amyloid-β) Complexes: Determination of the Intrinsic Rate as a Function of Alterations in the Peptide Sequence Revealing Key Residues for Reactive Oxygen Species Production. Anal. Chem. 2018, 90, 5909–5915. [Google Scholar] [CrossRef]
- Shen, J.; Griffiths, P.T.; Campbell, S.J.; Utinger, B.; Kalberer, M.; Paulson, S.E. Ascorbate Oxidation by Iron, Copper and Reactive Oxygen Species: Review, Model Development, and Derivation of Key Rate Constants. Sci. Rep. 2021, 11, 7417. [Google Scholar] [CrossRef] [PubMed]
- Aruoma, O.I.; Halliwell, B. Superoxide-Dependent and Ascorbate-Dependent Formation of Hydroxyl Radicals from Hydrogen Peroxide in the Presence of Iron. Are Lactoferrin and Transferrin Promoters of Hydroxyl-Radical Generation? Biochem. J. 1987, 241, 273–278. [Google Scholar] [CrossRef]
- Scarpa, M.; Stevanato, R.; Viglino, P.; Rigo, A. Superoxide Ion as Active Intermediate in the Autoxidation of Ascorbate by Molecular Oxygen. Effect of Superoxide Dismutase. J. Biol. Chem. 1983, 258, 6695–6697. [Google Scholar] [CrossRef]
- Human Metabolome Database. Available online: https://hmdb.ca/ (accessed on 2 August 2023).
- Zhou, P.; Zhang, J.; Zhang, Y.; Liu, Y.; Liang, J.; Liu, B.; Zhang, W. Generation of Hydrogen Peroxide and Hydroxyl Radical Resulting from Oxygen-Dependent Oxidation of L-Ascorbic Acid via Copper Redox-Catalyzed Reactions. RSC Adv. 2016, 6, 38541–38547. [Google Scholar] [CrossRef]
- Simpson, G.L.; Ortwerth, B.J. The Non-Oxidative Degradation of Ascorbic Acid at Physiological Conditions. Biochim. Biophys. Acta 2000, 1501, 12–24. [Google Scholar] [CrossRef]
- Tikekar, R.V.; Anantheswaran, R.C.; Elias, R.J.; LaBorde, L.F. Ultraviolet-Induced Oxidation of Ascorbic Acid in a Model Juice System: Identification of Degradation Products. J. Agric. Food Chem. 2011, 59, 8244–8248. [Google Scholar] [CrossRef]
- Halliwell, B. Vitamin C: Antioxidant or pro-Oxidant in Vivo? Free Radic. Res. 1996, 25, 439–454. [Google Scholar] [CrossRef]
- Podmore, I.D.; Griffiths, H.R.; Herbert, K.E.; Mistry, N.; Mistry, P.; Lunec, J. Vitamin C Exhibits Pro-Oxidant Properties. Nature 1998, 392, 559. [Google Scholar] [CrossRef]
- Asplund, K.U.M.; Jansson, P.J.; Lindqvist, C.; Nordström, T. Measurement of Ascorbic Acid (Vitamin C) Induced Hydroxyl Radical Generation in Household Drinking Water. Free Radic. Res. 2002, 36, 1271–1276. [Google Scholar] [CrossRef] [PubMed]
- Jansson, P.J.; Asplund, K.U.M.; Mäkelä, J.C.; Lindqvist, C.; Nordström, T. Vitamin C (Ascorbic Acid) Induced Hydroxyl Radical Formation in Copper Contaminated Household Drinking Water: Role of Bicarbonate Concentration. Free Radic. Res. 2003, 37, 901–905. [Google Scholar] [CrossRef] [PubMed]
- Bielski, B.H.J.; Allen, A.O.; Schwarz, H.A. Mechanism of the Disproportionation of Ascorbate Radicals. J. Am. Chem. Soc. 1981, 103, pp. 3516–33518. [CrossRef]
- Jung, C.-H.; Wells, W.W. Spontaneous Conversion Ofl-Dehydroascorbic Acid Tol-Ascorbic Acid Andl-Erythroascorbic Acid. Arch. Biochem. Biophys. 1998, 355, 9–14. [Google Scholar] [CrossRef] [PubMed]
- Yuan, J.-P.; Chen, F. Degradation of Ascorbic Acid in Aqueous Solution. J. Agric. Food Chem. 1998, 46, 5078–5082. [Google Scholar] [CrossRef]
- Buettner, G.R. The Pecking Order of Free Radicals and Antioxidants: Lipid Peroxidation, α-Tocopherol, and Ascorbate. Arch. Biochem. Biophys. 1993, 300, 535–543. [Google Scholar] [CrossRef]
- Buettner, G.R.; Czapski, P.G. Ascorbate Autoxidation in the Presence of Iron and Copper Chelates. Free Radic. Res. Commun. 1986, 1, 349–353. [Google Scholar] [CrossRef]
- Fry, S.C. Oxidative Scission of Plant Cell Wall Polysaccharides by Ascorbate-Induced Hydroxyl Radicals. Biochem. J. 1998, 332, 507–515. [Google Scholar] [CrossRef]
- Davies, M.B.; Austin, J.; Partridge, D.A. Vitamin C: Its Chemistry and Biochemistry; Royal Society of Chemistry: London, UK, 1991; ISBN 978-0-85186-333-7. [Google Scholar]
- Jiang, D.; Li, X.; Liu, L.; Yagnik, G.B.; Zhou, F. Reaction Rates and Mechanism of the Ascorbic Acid Oxidation by Molecular Oxygen Facilitated by Cu(II)-Containing Amyloid-Beta Complexes and Aggregates. J. Phys. Chem. B 2010, 114, 4896–4903. [Google Scholar] [CrossRef]
- Timoshnikov, V.A.; Kobzeva, T.V.; Polyakov, N.E.; Kontoghiorghes, G.J. Redox Interactions of Vitamin C and Iron: Inhibition of the Pro-Oxidant Activity by Deferiprone. Int. J. Mol. Sci. 2020, 21, 3967. [Google Scholar] [CrossRef]
- Zhang, L.; Liu, Y.; Wang, Y.; Xu, M.; Hu, X. UV–Vis Spectroscopy Combined with Chemometric Study on the Interactions of Three Dietary Flavonoids with Copper Ions. Food Chem. 2018, 263, 208–215. [Google Scholar] [CrossRef] [PubMed]
- Kasprzak, M.M.; Erxleben, A.; Ochocki, J. Properties and Applications of Flavonoid Metal Complexes. RSC Adv. 2015, 5, 45853–45877. [Google Scholar] [CrossRef]
- Nardini, M.; D’Aquino, M.; Tomassi, G.; Gentili, V.; di Felice, M.; Scaccini, C. Inhibition of Human Low-Density Lipoprotein Oxidation by Caffeic Acid and Other Hydroxycinnamic Acid Derivatives. Free Radic. Biol. Med. 1995, 19, 541–552. [Google Scholar] [CrossRef]
- Damasceno, S.S.; Dantas, B.B.; Ribeiro-Filho, J.; Antônio, M.; Araújo, D.; Galberto, M.; Da Costa, J. Chemical Properties of Caffeic and Ferulic Acids in Biological System: Implications in Cancer Therapy. A Review. Curr. Pharm. Des. 2017, 23, 3015–3023. [Google Scholar] [CrossRef]
- Espíndola, K.M.M.; Ferreira, R.G.; Narvaez, L.E.M.; Silva Rosario, A.C.R.; da Silva, A.H.M.; Silva, A.G.B.; Vieira, A.P.O.; Monteiro, M.C. Chemical and Pharmacological Aspects of Caffeic Acid and Its Activity in Hepatocarcinoma. Front. Oncol. 2019, 9, 541. [Google Scholar] [CrossRef]
- Grazul, M.; Budzisz, E. Biological Activity of Metal Ions Complexes of Chromones, Coumarins and Flavones. Coord. Chem. Rev. 2009, 253, 2588–2598. [Google Scholar] [CrossRef]
- Hahn, H.J.; Kim, K.B.; An, I.-S.; Ahn, K.J.; Han, H.J. Protective Effects of Rosmarinic Acid against Hydrogen Peroxide-induced Cellular Senescence and the Inflammatory Response in Normal Human Dermal Fibroblasts. Mol. Med. Rep. 2017, 16, 9763–9769. [Google Scholar] [CrossRef]
- Lee, H.J.; Cho, H.-S.; Park, E.; Kim, S.; Lee, S.-Y.; Kim, C.-S.; Kim, D.K.; Kim, S.-J.; Chun, H.S. Rosmarinic Acid Protects Human Dopaminergic Neuronal Cells against Hydrogen Peroxide-Induced Apoptosis. Toxicology 2008, 250, 109–115. [Google Scholar] [CrossRef]
- Zhou, H.; Fu, B.; Xu, B.; Mi, X.; Li, G.; Ma, C.; Xie, J.; Li, J.; Wang, Z. Rosmarinic Acid Alleviates the Endothelial Dysfunction Induced by Hydrogen Peroxide in Rat Aortic Rings via Activation of AMPK. Oxidative Med. Cell. Longev. 2017, 2017, 7091904. [Google Scholar] [CrossRef]
- Luo, C.; Zou, L.; Sun, H.; Peng, J.; Gao, C.; Bao, L.; Ji, R.; Jin, Y.; Sun, S. A Review of the Anti-Inflammatory Effects of Rosmarinic Acid on Inflammatory Diseases. Front. Pharmacol. 2020, 11, 153. [Google Scholar] [CrossRef]
- Guan, H.; Luo, W.; Bao, B.; Cao, Y.; Cheng, F.; Yu, S.; Fan, Q.; Zhang, L.; Wu, Q.; Shan, M. A Comprehensive Review of Rosmarinic Acid: From Phytochemistry to Pharmacology and Its New Insight. Molecules 2022, 27, 3292. [Google Scholar] [CrossRef]
- Dahchour, A. Anxiolytic and Antidepressive Potentials of Rosmarinic Acid: A Review with a Focus on Antioxidant and Anti-Inflammatory Effects. Pharmacol. Res. 2022, 184, 106421. [Google Scholar] [CrossRef] [PubMed]
- Hwang, T.L.; Shaka, A.J. Multiple-Pulse Mixing Sequences That Selectively Enhance Chemical Exchange or Cross-Relaxation Peaks in High-Resolution NMR Spectra. J. Magn. Reson. 1998, 135, 280–287. [Google Scholar] [CrossRef] [PubMed]
- Rakhit, G.; Antholine, W.E.; Froncisz, W.; Hyde, J.S.; Pilbrow, J.R.; Sinclair, G.R.; Sarkar, B. Direct Evidence of Nitrogen Coupling in the Copper(II) Complex of Bovine Serum Albumin by S-Band Electron Spin Resonance Technique. J. Inorg. Biochem. 1985, 25, 217–224. [Google Scholar] [CrossRef] [PubMed]

| Polyphenol | Proton | ppm | I/I0 Alone | I/I0 Mix |
|---|---|---|---|---|
| RA | H2 | 7.19 | 0.70 | 0.63 |
| H6 | 7.12 | 0.83 | 0.77 | |
| H7 | 7.59 | 0.68 | 0.60 | |
| H8 | 6.39 | 0.79 | 0.70 | |
| H2′ | 6.90 | 0.86 | 0.77 | |
| H5′ | 6.87 | 0.67 | 0.55 | |
| H6′ | 6.80 | 0.63 | 0.49 | |
| CA | H2 | 7.17 | 0.56 | 0.86 |
| H6 | 7.08 | 0.61 | 0.89 | |
| H7 | 7.29 | 0.52 | 0.80 | |
| H8 | 6.35 | 0.55 | 0.75 | |
| pCA | H2 | 7.54 | 1.01 | 0.95 |
| H6 | 7.54 | 1.01 | 0.95 | |
| H7 | 7.35 | 1.01 | 0.98 | |
| H8 | 6.38 | 1.00 | 0.98 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kola, A.; Vigni, G.; Baratto, M.C.; Valensin, D. A Combined NMR and UV–Vis Approach to Evaluate Radical Scavenging Activity of Rosmarinic Acid and Other Polyphenols. Molecules 2023, 28, 6629. https://doi.org/10.3390/molecules28186629
Kola A, Vigni G, Baratto MC, Valensin D. A Combined NMR and UV–Vis Approach to Evaluate Radical Scavenging Activity of Rosmarinic Acid and Other Polyphenols. Molecules. 2023; 28(18):6629. https://doi.org/10.3390/molecules28186629
Chicago/Turabian StyleKola, Arian, Ginevra Vigni, Maria Camilla Baratto, and Daniela Valensin. 2023. "A Combined NMR and UV–Vis Approach to Evaluate Radical Scavenging Activity of Rosmarinic Acid and Other Polyphenols" Molecules 28, no. 18: 6629. https://doi.org/10.3390/molecules28186629
APA StyleKola, A., Vigni, G., Baratto, M. C., & Valensin, D. (2023). A Combined NMR and UV–Vis Approach to Evaluate Radical Scavenging Activity of Rosmarinic Acid and Other Polyphenols. Molecules, 28(18), 6629. https://doi.org/10.3390/molecules28186629








