Meldrum’s Acid Furfural Conjugate MAFC: A New Entry as Chromogenic Sensor for Specific Amine Identification
Abstract
:1. Introduction
2. Furan Derivatives as Detection Agents
3. Activated Furans for Colorimetric Sensing
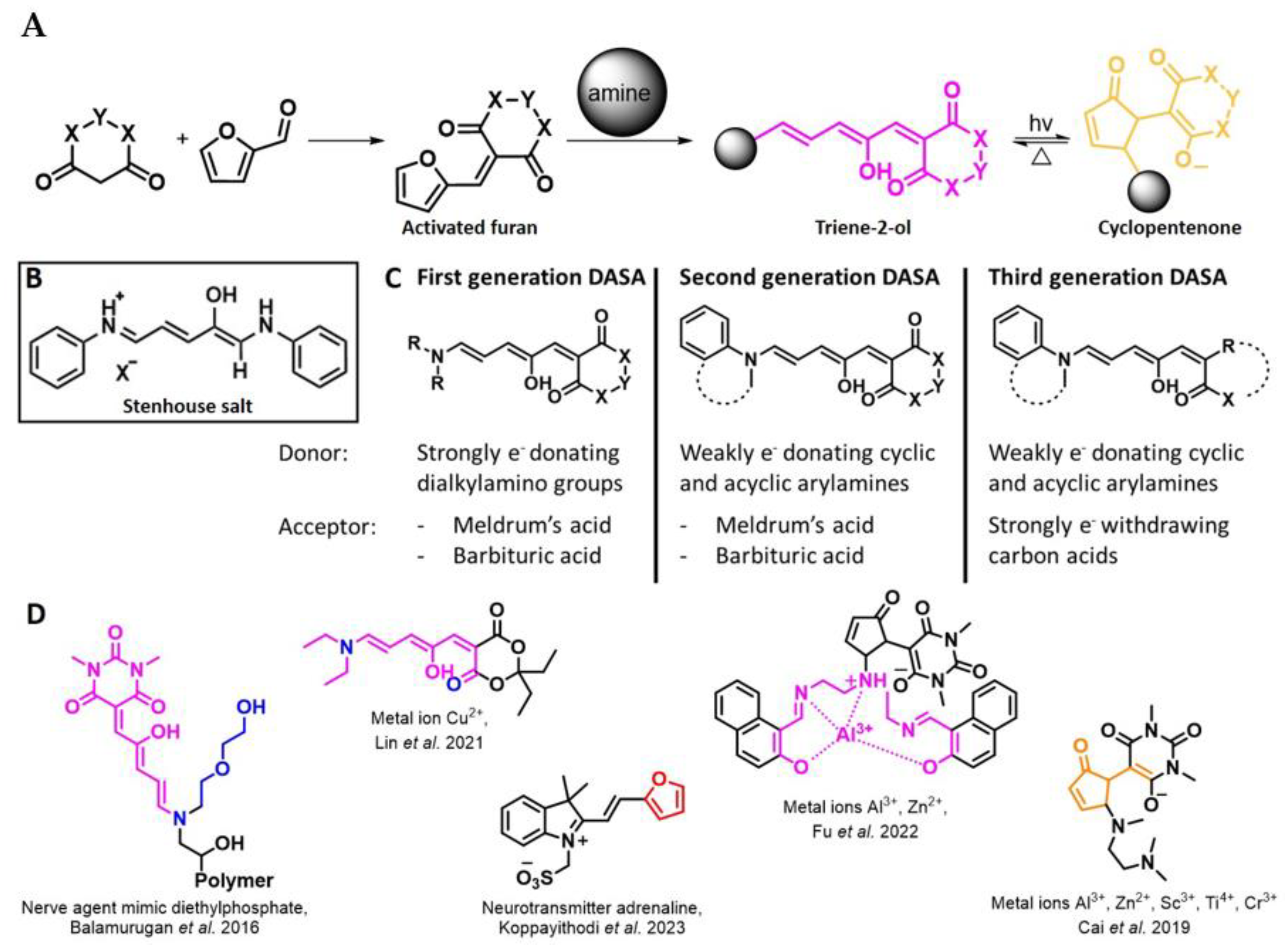
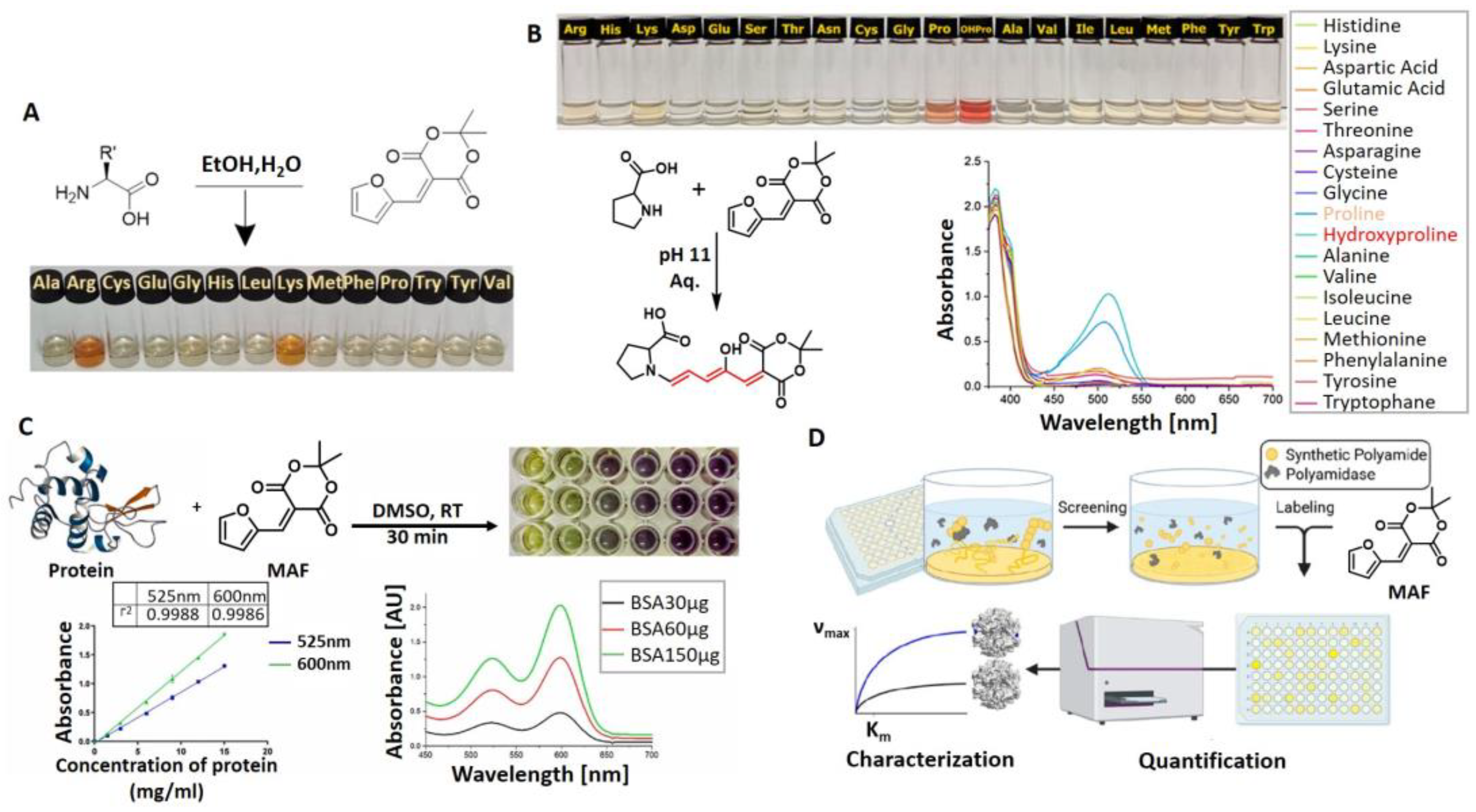
4. Meldrum’s Acid-Activated Furan Conjugate as Amine Detecting Agent
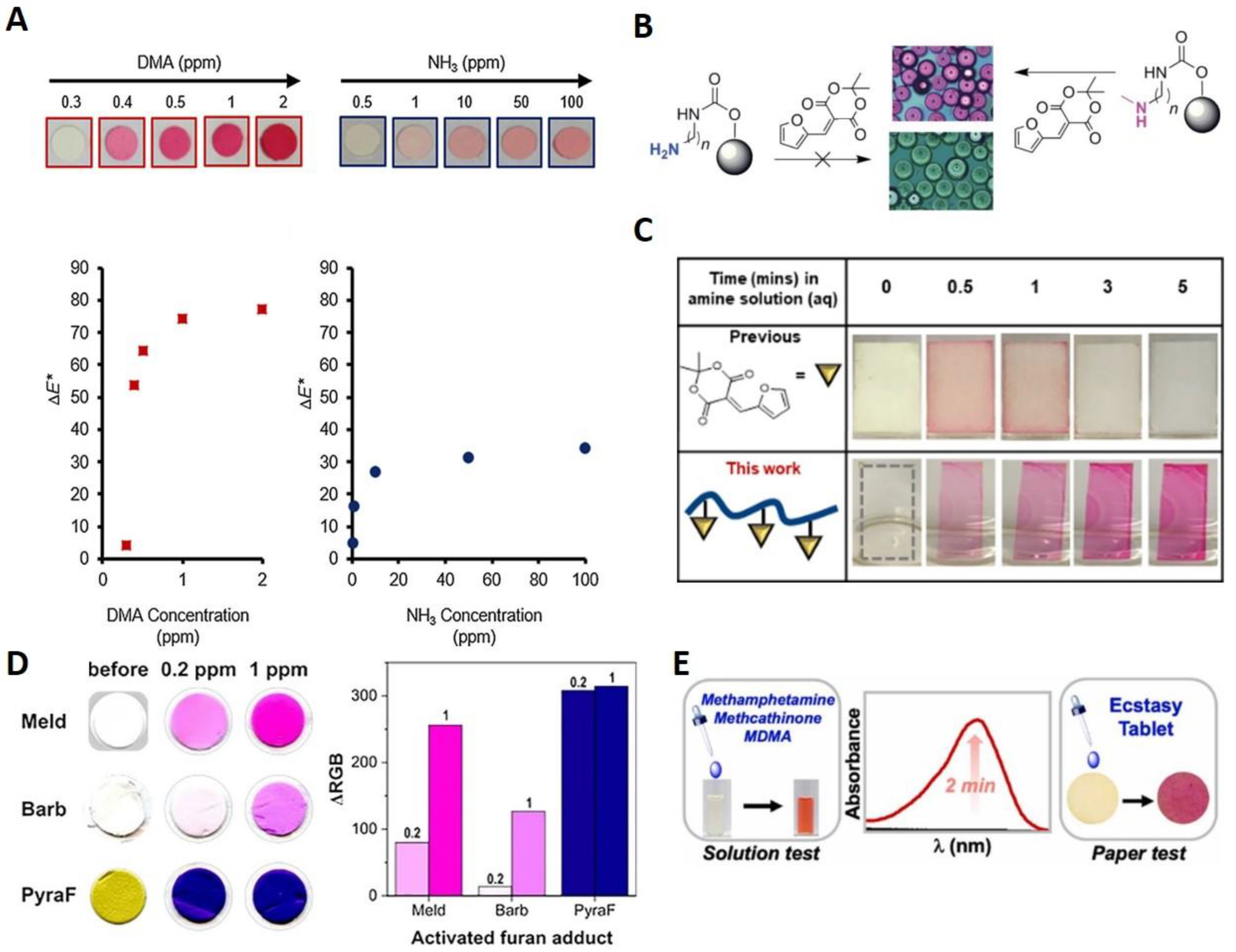
5. Outlook
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Wang, L.; Ran, X.; Tang, H.; Cao, D. Recent advances on reaction-based amine fluorescent probes. Dye. Pigment. 2021, 194, 109634. [Google Scholar] [CrossRef]
- Andre, R.S.; Mercante, L.A.; Facure, M.H.M.; Sanfelice, R.C.; Fugikawa-Santos, L.; Swager, T.M.; Correa, D.S. Recent Progress in Amine Gas Sensors for Food Quality Monitoring: Novel Architectures for Sensing Materials and Systems. ACS Sens. 2022, 7, 2104–2131. [Google Scholar] [CrossRef] [PubMed]
- Danchuk, A.I.; Komova, N.S.; Mobarez, S.N.; Doronin, S.Y.; Burmistrova, N.A.; Markin, A.V.; Duerkop, A. Optical sensors for determination of biogenic amines in food. Anal. Bioanal. Chem. 2020, 412, 4023–4036. [Google Scholar] [CrossRef] [PubMed]
- Grant, M.J.; Wolfe, K.M.; Harding, C.R.; Welch, G.C. Biogenic amine sensors using organic π-conjugated materials as active sensing components and their commercialization potential. J. Mater. Chem. C 2023, 11, 9749–9767. [Google Scholar] [CrossRef]
- Vasconcelos, H.; Coelho, L.C.C.; Matias, A.; Saraiva, C.; Jorge, P.A.S.; de Almeida, J.M.M.M. Biosensors for Biogenic Amines: A Review. Biosensors 2021, 11, 82. [Google Scholar] [CrossRef]
- Gomes Müller, D.; Quadro Oreste, E.; Grazielle Heinemann, M.; Dias, D.; Kessler, F. Biogenic amine sensors and its building materials: A review. Eur. Polym. J. 2022, 175, 111221. [Google Scholar] [CrossRef]
- Joullié, M.M.; Thompson, T.R.; Nemeroff, N.H. Ninhydrin and ninhydrin analogs. Syntheses and applications. Tetrahedron 1991, 47, 8791–8830. [Google Scholar] [CrossRef]
- Brunelle, E.; Huynh, C.; Le, A.M.; Halámková, L.; Agudelo, J.; Halámek, J. New Horizons for Ninhydrin: Colorimetric Determination of Gender from Fingerprints. Anal. Chem. 2016, 88, 2413–2420. [Google Scholar] [CrossRef]
- Oden, S.; Von Hofsten, B. Detection of fingerprints by the ninhydrin reaction. Nature 1954, 173, 449–450. [Google Scholar] [CrossRef]
- Beckwith, A.C.; Paulis, J.W.; Wall, J.S. Direct estimation of lysine in corn meals by the ninhydrin color reaction. J. Agric. Food Chem. 1975, 23, 194–196. [Google Scholar] [CrossRef]
- Leaf, G. Identification and estimation of ninhydrin–positive substances in physio-logical fluids. Proc. Nutr. Soc. 1970, 29, 105–106. [Google Scholar] [CrossRef]
- Li, Z.; Chang, S.; Lin, L.; Li, Y.; An, Q. A colorimetric assay of 1-aminocyclopropane-1-carboxylate (ACC) based on ninhydrin reaction for rapid screening of bacteria containing ACC deaminase. Lett. Appl. Microbiol. 2011, 53, 178–185. [Google Scholar] [CrossRef] [PubMed]
- Abdellatef, H.E.; Khalil, H.M. Colorimetric determination of gabapentin in pharmaceutical formulation. J. Pharm. Biomed. Anal. 2003, 31, 209–214. [Google Scholar] [CrossRef] [PubMed]
- Picton, S.F.; McCluskey, J.T.; Flatt, P.R.; McClenaghan, N.H. Effects of cytotoxic agents on functional integrity and antioxidant enzymes in clonal beta-cells. Diabetes Metab. 2002, 28, 3S70–3S77; discussion 3S108–3S112. [Google Scholar] [PubMed]
- Friedman, M. Applications of the ninhydrin reaction for analysis of amino acids, peptides, and proteins to agricultural and biomedical sciences. J. Agric. Food Chem. 2004, 52, 385–406. [Google Scholar] [CrossRef] [PubMed]
- Dietzen, D.J.; Weindel, A.L.; Carayannopoulos, M.O.; Landt, M.; Normansell, E.T.; Reimschisel, T.E.; Smith, C.H. Rapid comprehensive amino acid analysis by liquid chromatography/tandem mass spectrometry: Comparison to cation exchange with post-column ninhydrin detection. Rapid Commun. Mass Spectrom. 2008, 22, 3481–3488. [Google Scholar] [CrossRef] [PubMed]
- Ajayan, K.; Sainath, S.; Sadik, A.; Nair, M.M.; Nair, A.M.; Karthika, K.S.; Vijayakumar, A.; Nair, S.S.; Nair, B.; Chandran R, P.; et al. Bioconjugation of Meldrum’s acid activated furan: A detergent compatible assay for protein quantitation. Anal. Biochem. 2023, 662, 114998. [Google Scholar] [CrossRef]
- Fu, P.; Yan, Q.; Wang, S.; Wu, H.; Cao, D. A visible-light-gated donor–acceptor Stenhouse adduct chemosensor: Synthesis, photochromism and naked-eye colorimetric/fluorometric sensing of Al3+ and Zn2+. New J. Chem. 2022, 46, 12600–12608. [Google Scholar] [CrossRef]
- Kumpf, J.; Freudenberg, J.; Fletcher, K.; Dreuw, A.; Bunz, U.H.F. Detection of amines with extended distyrylbenzenes by strip assays. J. Org. Chem. 2014, 79, 6634–6645. [Google Scholar] [CrossRef]
- Mani, P.; Ojha, A.A.; Reddy, V.S.; Mandal, S. “Turn-on” Fluorescence Sensing and Discriminative Detection of Aliphatic Amines Using a 5-Fold-Interpenetrated Coordination Polymer. Inorg. Chem. 2017, 56, 6772–6775. [Google Scholar] [CrossRef]
- Hu, R.; Lin, S.; Hu, D.; Huang, H.; Wang, M.; Li, R.; Tian, M.; Shuai, Z.; Wei, Y. “Turn-on” fluorescence sensor for organic amines fabricated via sustainable processing. Mater. Chem. Front. 2022, 7, 153–159. [Google Scholar] [CrossRef]
- Verbitskiy, E.V.; Kvashnin, Y.A.; Baranova, A.A.; Khokhlov, K.O.; Chuvashov, R.D.; Schapov, I.E.; Yakovleva, Y.A.; Zhilina, E.F.; Shchepochkin, A.V.; Makarova, N.I.; et al. Synthesis and characterization of linear 1,4-diazine-triphenylamine-based selective chemosensors for recognition of nitroaromatic compounds and aliphatic amines. Dye. Pigment. 2020, 178, 108344. [Google Scholar] [CrossRef]
- Givanoudi, S.; Heyndrickx, M.; Depuydt, T.; Khorshid, M.; Robbens, J.; Wagner, P. A Review on Bio- and Chemosensors for the Detection of Biogenic Amines in Food Safety Applications: The Status in 2022. Sensors 2023, 23, 613. [Google Scholar] [CrossRef] [PubMed]
- Gomes, R.F.A.; Coelho, J.A.S.; Afonso, C.A.M. Synthesis and Applications of Stenhouse Salts and Derivatives. Chemistry 2018, 24, 9170–9186. [Google Scholar] [CrossRef] [PubMed]
- Helmy, S.; Leibfarth, F.A.; Oh, S.; Poelma, J.E.; Hawker, C.J.; Read de Alaniz, J. Photoswitching using visible light: A new class of organic photochromic molecules. J. Am. Chem. Soc. 2014, 136, 8169–8172. [Google Scholar] [CrossRef]
- Diaz, Y.J.; Page, Z.A.; Knight, A.S.; Treat, N.J.; Hemmer, J.R.; Hawker, C.J.; Read de Alaniz, J. A Versatile and Highly Selective Colorimetric Sensor for the Detection of Amines. Chemistry 2017, 23, 3562–3566. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Wang, K.; Xu, H.; Yan, W.; Jin, Q.; Cui, D. Detection platforms for point-of-care testing based on colorimetric, luminescent and magnetic assays: A review. Talanta 2019, 202, 96–110. [Google Scholar] [CrossRef]
- Li, L.; Wang, J.; Xu, S.; Li, C.; Dong, B. Recent Progress in Fluorescent Probes for Metal Ion Detection. Front. Chem. 2022, 10, 875241. [Google Scholar] [CrossRef]
- Hu, Y.; Ke, Q.; Yan, C.; Xu, C.-H.; Huang, X.-H.; Hu, S. A new fluorescence chemosensor for selective detection of copper ion in aqueous solution. Tetrahedron Lett. 2016, 57, 2239–2243. [Google Scholar] [CrossRef]
- Wang, Q.-L.; Zhang, H.; Jiang, Y.-B. A highly selective and sensitive turn-on catalytic chemodosimeter for Cu2+ in aqueous solution. Tetrahedron Lett. 2009, 50, 29–31. [Google Scholar] [CrossRef]
- Yang, C.; Gong, D.; Wang, X.; Iqbal, A.; Deng, M.; Guo, Y.; Tang, X.; Liu, W.; Qin, W. A new highly copper-selective fluorescence enhancement chemosensor based on BODIPY excitable with visible light and its imaging in living cells. Sens. Actuators B Chem. 2016, 224, 110–117. [Google Scholar] [CrossRef]
- Jeong, H.Y.; Lee, S.Y.; Kim, C. Furan and Julolidine-Based “Turn-on” Fluorescence Chemosensor for Detection of F- in a Near-Perfect Aqueous Solution. J. Fluoresc. 2017, 27, 1457–1466. [Google Scholar] [CrossRef] [PubMed]
- Saini, N.; Wannasiri, C.; Chanmungkalakul, S.; Prigyai, N.; Ervithayasuporn, V.; Kiatkamjornwong, S. Furan/thiophene-based fluorescent hydrazones as fluoride and cyanide sensors. J. Photochem. Photobiol. A Chem. 2019, 385, 112038. [Google Scholar] [CrossRef]
- Li, C.-R.; Li, S.-L.; Yang, Z.-Y. Development of a coumarin-furan conjugate as Zn2+ ratiometric fluorescent probe in ethanol-water system. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2017, 174, 214–222. [Google Scholar] [CrossRef] [PubMed]
- Boonkitpatarakul, K.; Wang, J.; Niamnont, N.; Liu, B.; Mcdonald, L.; Pang, Y.; Sukwattanasinitt, M. Novel Turn-On Fluorescent Sensors with Mega Stokes Shifts for Dual Detection of Al3+ and Zn2+. ACS Sens. 2016, 1, 144–150. [Google Scholar] [CrossRef]
- Seenan, S.; Manickam, S.; Kulathu Iyer, S. A new furan based fluorescent chemosensor for the recognition of Cr3+ ion and its application in real sample analysis. J. Photochem. Photobiol. A Chem. 2021, 418, 113441. [Google Scholar] [CrossRef]
- Liabsungnoen, A.; Mayurachayakul, P.; Srikittiwanna, K.; Dungchai, W.; Sukwattanasinitt, M.; Srisuwannaket, C.; Mingvanish, W.; Niamnont, N. A novel pyrenyl-furan hydrazone on paper-based device for the selective detection of trinitrotoluene. New J. Chem. 2023, 47, 6880–6888. [Google Scholar] [CrossRef]
- Wakshe, S.B.; Dongare, P.R.; Gore, A.H.; Mote, G.V.; Anbhule, P.V.; Kolekar, G.B. Furan-Dihydroquinazolinone Based Fluorescent Nanoprobe for Selective Recognition of 4-Nitrophenol: A Spectofluorimetric Approach. J. Fluoresc. 2023. [Google Scholar] [CrossRef]
- Stenhouse, J. Ueber Furfuranilin und Furfurtoluidin. Ann. Chem. Pharm. 1870, 156, 197–205. [Google Scholar] [CrossRef]
- Henschke, J.P.; Liu, Y.; Huang, X.; Chen, Y.; Meng, D.; Xia, L.; Wei, X.; Xie, A.; Li, D.; Huang, Q.; et al. The Manufacture of a Homochiral 4-Silyloxycyclopentenone Intermediate for the Synthesis of Prostaglandin Analogues. Org. Process Res. Dev. 2012, 16, 1905–1916. [Google Scholar] [CrossRef]
- Ulbrich, K.; Kreitmeier, P.; Reiser, O. Microwave- or Microreactor-Assisted Conversion of Furfuryl Alcohols into 4-Hydroxy-2-cyclopentenones. Synlett 2010, 2010, 2037–2040. [Google Scholar] [CrossRef]
- Michalak, K.; Wicha, J. An Enantioselective Total Synthetic Approach to (+)-Heptemerone G and (+)-Guanacastepene A from 2-Furyl Methyl Carbinol. Synlett 2013, 24, 1387–1390. [Google Scholar] [CrossRef]
- Li, S.-W.; Batey, R.A. Mild lanthanide(III) catalyzed formation of 4,5-diaminocyclopent-2-enones from 2-furaldehyde and secondary amines: A domino condensation/ring-opening/electrocyclization process. Chem. Commun. 2007, 3759–3761. [Google Scholar] [CrossRef]
- Sroda, M.M.; Stricker, F.; Peterson, J.A.; Bernal, A.; Read de Alaniz, J. Donor-Acceptor Stenhouse Adducts: Exploring the Effects of Ionic Character. Chemistry 2021, 27, 4183–4190. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Du, Y.; Yuan, L.; Chu, Z.; He, L. Donor-acceptor Stenhouse adducts as new emerging photoswitches: Synthesis, light-responsive properties, and applications in polymers science. J. Macromol. Sci. Part A 2021, 58, 717–724. [Google Scholar] [CrossRef]
- Balamurugan, A.; Lee, H. A Visible Light Responsive On-Off Polymeric Photoswitch for the Colorimetric Detection of Nerve Agent Mimics in Solution and in the Vapor Phase. Macromolecules 2016, 49, 2568–2574. [Google Scholar] [CrossRef]
- Koppayithodi, S.; Jana, P.; Bandyopadhyay, S. Highly Selective and Quantitative Point-of-Care Diagnostic Method for Adrenaline. Chemistry 2023, 29, e202300327. [Google Scholar] [CrossRef] [PubMed]
- Cai, Y.-D.; Chen, T.-Y.; Chen, X.Q.; Bao, X. Multiresponsive Donor-Acceptor Stenhouse Adduct: Opportunities Arise from a Diamine Donor. Org. Lett. 2019, 21, 7445–7449. [Google Scholar] [CrossRef]
- Lin, Z.; Wang, S.; Yan, Q.; Yan, Q.; Cao, D. Visible light/Cu2+ dual triggered photochromic donor-acceptor stenhouse adducts: Synthesis, properties and applications. Dye. Pigment. 2021, 191, 109384. [Google Scholar] [CrossRef]
- Roberts, C.A.; Allen, S.; Helmy, S. Using Donor-Acceptor Stenhouse Adducts to Teach Photochromism in the Undergraduate Laboratory. J. Chem. Educ. 2021, 98, 1736–1740. [Google Scholar] [CrossRef]
- Singh, S.; Friedel, K.; Himmerlich, M.; Lei, Y.; Schlingloff, G.; Schober, A. Spatiotemporal Photopatterning on Polycarbonate Surface through Visible Light Responsive Polymer Bound DASA Compounds. ACS Macro Lett. 2015, 4, 1273–1277. [Google Scholar] [CrossRef] [PubMed]
- Singh, S.; Mai, P.; Borowiec, J.; Zhang, Y.; Lei, Y.; Schober, A. Donor-acceptor Stenhouse adduct-grafted polycarbonate surfaces: Selectivity of the reaction for secondary amine on surface. R. Soc. Open Sci. 2018, 5, 180207. [Google Scholar] [CrossRef] [PubMed]
- Chen, Q.; Diaz, Y.J.; Hawker, M.C.; Martinez, M.R.; Page, Z.A.; Zhang, S.X.-A.; Hawker, C.J.; Read de Alaniz, J. Stable Activated Furan and Donor-Acceptor Stenhouse Adduct Polymer Conjugates as Chemical and Thermal Sensors. Macromolecules 2019, 52, 4370–4375. [Google Scholar] [CrossRef]
- Ulrich, S.; Moura, S.O.; Diaz, Y.; Clerc, M.; Guex, A.G.; de Alaniz, J.R.; Martins, A.; Neves, N.M.; Rottmar, M.; Rossi, R.M.; et al. Electrospun colourimetric sensors for detecting volatile amines. Sens. Actuators B Chem. 2020, 322, 128570. [Google Scholar] [CrossRef]
- Sabahi-Agabager, L.; Eskandari, H.; Nasiri, F.; Shamkhali, A.N.; Baghi Sefidan, S. Properties of a furan ring-opening reaction in aqueous micellar solutions for selective sensing of mesalazine. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2021, 258, 119846. [Google Scholar] [CrossRef] [PubMed]
- Cho, S.; Kim, Y. Donor-acceptor Stenhouse adduct formation for the simple and rapid colorimetric detection of amphetamine-type stimulants. Sens. Actuators B Chem. 2022, 355, 131274. [Google Scholar] [CrossRef]
- Zeußel, L.; Mai, P.; Sharma, S.; Schober, A.; Ren, S.; Singh, S. Colorimetric Method for Instant Detection of Lysine and Arginine Using Novel Meldrum’s Acid-Furfural Conjugate. ChemistrySelect 2021, 6, 6834–6840. [Google Scholar] [CrossRef]
- Zeußel, L.; Aziz, C.; Schober, A.; Singh, S. pH-Dependent Selective Colorimetric Detection of Proline and Hydroxyproline with Meldrum’s Acid-Furfural Conjugate. Chemosensors 2021, 9, 343. [Google Scholar] [CrossRef]
- Puetz, H.; Janknecht, C.; Contreras, F.; Vorobii, M.; Kurkina, T.; Schwaneberg, U. Validated High-Throughput Screening System for Directed Evolution of Nylon-Depolymerizing Enzymes. ACS Sustain. Chem. Eng. 2023. [Google Scholar] [CrossRef]
- Mason, B.P.; Whittaker, M.; Hemmer, J.; Arora, S.; Harper, A.; Alnemrat, S.; McEachen, A.; Helmy, S.; Read de Alaniz, J.; Hooper, J.P. A temperature-mapping molecular sensor for polyurethane-based elastomers. Appl. Phys. Lett. 2016, 108, 041906. [Google Scholar] [CrossRef]
- Zhong, D.; Cao, Z.; Wu, B.; Zhang, Q.; Wang, G. Polymer dots of DASA-functionalized polyethyleneimine: Synthesis, visible light/pH responsiveness, and their applications as chemosensors. Sens. Actuators B Chem. 2018, 254, 385–392. [Google Scholar] [CrossRef]
- Chen, T.-Y.; Cai, Y.-D.; Jiang, S.-Q.; Cai, W.; Tong, M.-L.; Bao, X. Light- and Chemical-Stimuli-Induced Isomerization of Donor-Acceptor Stenhouse Adducts. ChemPhotoChem 2021, 5, 559–564. [Google Scholar] [CrossRef]

| Activated Furan Species Involved in Sensing | Analyte | Application | LOD | Source |
|---|---|---|---|---|
 | Cu2+ | Metal ion chemosensing | 0.168 µM | [29] |
 | Cu2+ | Metal ion chemosensing | 15 nM | [30] |
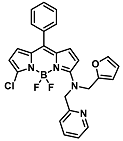 | Cu2+ | Metal ion chemosensing in living cells | 5 µM | [31] |
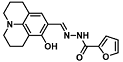 | F− | Dental health | 10.02 µM | [32] |
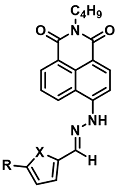 | F−, CN- | Sensing of biologically and environmentally pertinent species | <0.3 ppm | [33] |
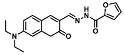 | Zn2+ | Metal ion chemosensing | 0.113 µM | [34] |
 | Zn2+, Âl3+ | Metal ion chemosensing | 3.1 nM | [35] |
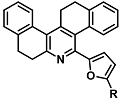 | Cr3+ | Metal ion chemosensing | 142 nM | [36] |
 | Trinitrotoluene | Identification of explosives | 30 µM | [37] |
 | 4-Nitrophenol | Environmental protection | 16.11 nM | [38] |
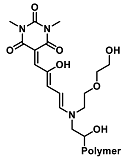 | Diethylcyanophosphate | Nerve agent detection | 1.0 mM | [46] |
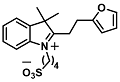 | Adrenaline | Neurotransmitter detection | 1.37 nM | [47] |
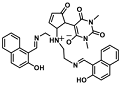 | Al3+, Zn2+ | Metal ion chemosensing | 0.365 µM, 0.1 µM | [18] |
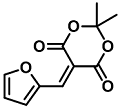
 | Primary Amines Sc3+, Ti4+, Cr3+, Al3+ | Colorimetric sensing of primary amines and high-charged Lewis acids | 1.75 µM | [48] |
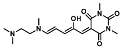
 | Diethylamine, dimethylamine, piperidine, butylamine, ammonia, cadaverine | Biogenic amine sensing in solution and vapor phase | 0.4 ppm, 10 ppm, 10 ppm, 4.4 ppm, 13.2 ppm, 100 ppm | [26] |
| 2° amines | Amine sensing on polymer surface | na | [52] | |
| diethylamine, n-butylamine, indoline, p-methoxyaniline | Chemical and thermal amine sensing in aqueous solution | 20 ppm, 10 ppm, 20 ppm, 100 ppm | [53] | |
| Mesalazine | Amine sensing in pharmaceutical products | 0.04 µg/mL | [55] | |
| Methamphetamine, 3,4-methylenedioxymethamphetamine | Sensing of amphetamine-type stimulants | 0.36 µg/mL, 0.57 µg/mL | [56] | |
| lysine | Sensing of amino acids | 100 µM | [57] | |
| Proline, 4-hydroxyproline | Sensing of amino acids | 11 µM, 6 µM | [58] | |
| Bovine Serum Albumin | Quantification of proteins | 125 µg/mL | [17] | |
| Polyamide and Polyurethane degradation products: ’6-aminohexanoic acid, hexamethylenediamine, 2,4-toluenediamine, 2,6-toluenediamine, 4,4′-methylenedianiline | Process control | 49 µM, 23.8 µM, 0.6 µM, 10.6 µM, 5.9 µM | [59] | |
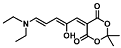
 | Temperature mapping | [60] | ||
 | Cu2+, Fe3+ | Metal ion chemosensing | 1.3 nM, 10.1 nM | [61] |
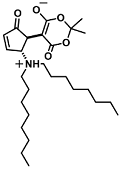
 | Cu2+ | Metal ion chemosensing | 100 µM | [49] |
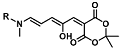 | Cu2+ | Metal ion chemosensing | 3.1 µM | [62] |
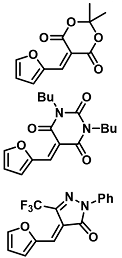 | Diethylamine | Volatile amine sensing with electrospun meshes | <1 ppm, 1–10 ppm, 0.1 ppm | [54] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zeußel, L.; Singh, S. Meldrum’s Acid Furfural Conjugate MAFC: A New Entry as Chromogenic Sensor for Specific Amine Identification. Molecules 2023, 28, 6627. https://doi.org/10.3390/molecules28186627
Zeußel L, Singh S. Meldrum’s Acid Furfural Conjugate MAFC: A New Entry as Chromogenic Sensor for Specific Amine Identification. Molecules. 2023; 28(18):6627. https://doi.org/10.3390/molecules28186627
Chicago/Turabian StyleZeußel, Lisa, and Sukhdeep Singh. 2023. "Meldrum’s Acid Furfural Conjugate MAFC: A New Entry as Chromogenic Sensor for Specific Amine Identification" Molecules 28, no. 18: 6627. https://doi.org/10.3390/molecules28186627
APA StyleZeußel, L., & Singh, S. (2023). Meldrum’s Acid Furfural Conjugate MAFC: A New Entry as Chromogenic Sensor for Specific Amine Identification. Molecules, 28(18), 6627. https://doi.org/10.3390/molecules28186627






