Unveiling the Aromas and Sensory Evaluation of Hakko Sobacha: A New Functional Non-Dairy Probiotic Fermented Drink
Abstract
:1. Introduction
- Does Sobacha fermentation induce the acidification of the medium towards a pH range allowing the inhibition of pathogenic microorganisms?
- What are the production kinetics of the molecules of interest in the degradation of fermentable sugars? Are the levels of ethanol produced at the end of fermentation in line with the standards for designating non-alcoholic beverages?
- What are the major volatile organic compounds developed during Sobacha fermentation? Among these, which aromas contribute significantly to the product’s final aromatic profile?
- From a sensory point of view, what are the consumer’s opinions and preferences regarding the beverages formulated?
2. Results and Discussion
2.1. Physico-Chemical Characterization of Hakko Sobacha during Fermentation
2.1.1. Evolution of pH during Fermentation
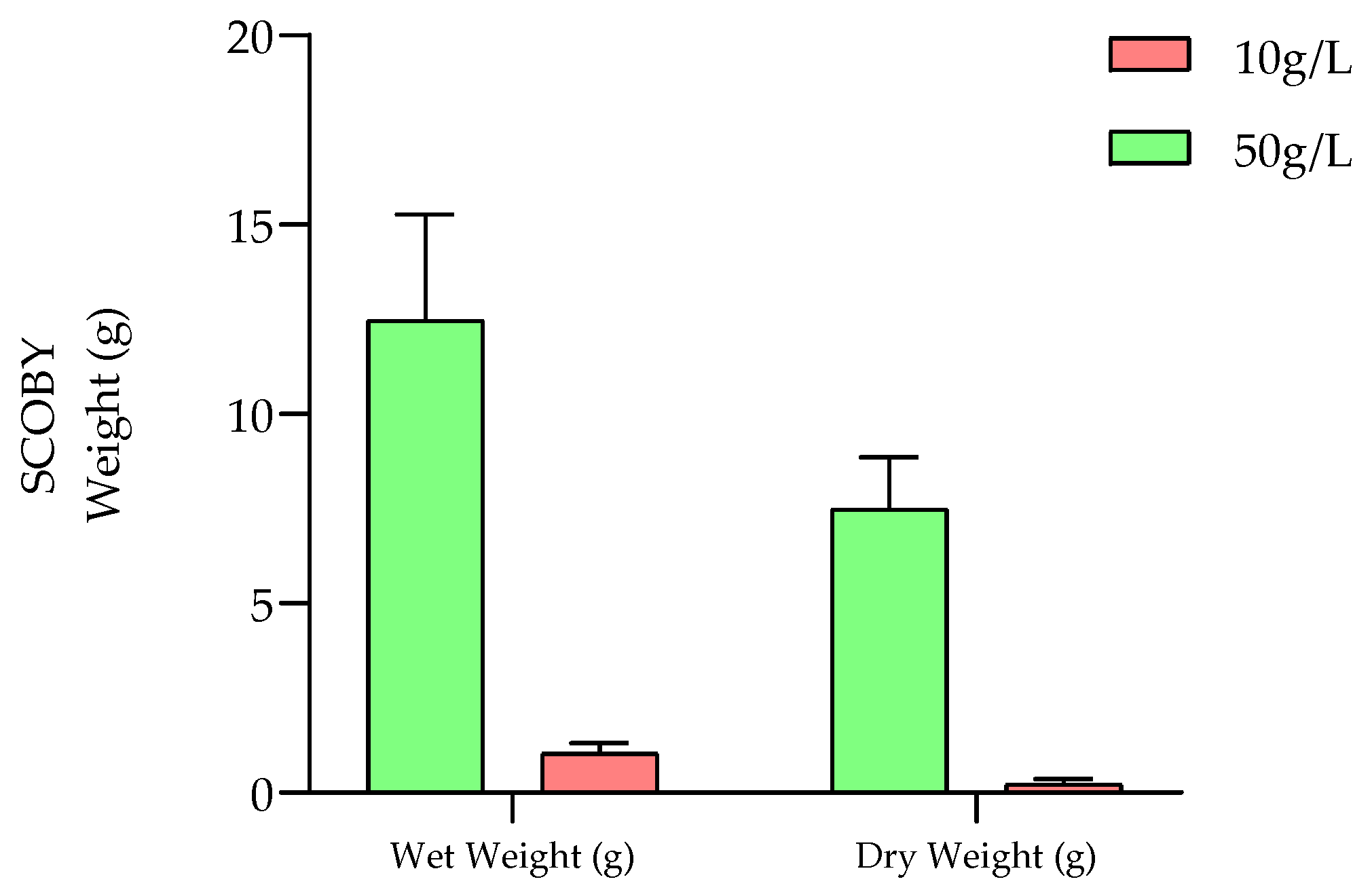
2.1.2. Evolution of SCOBY Production during Fermentation
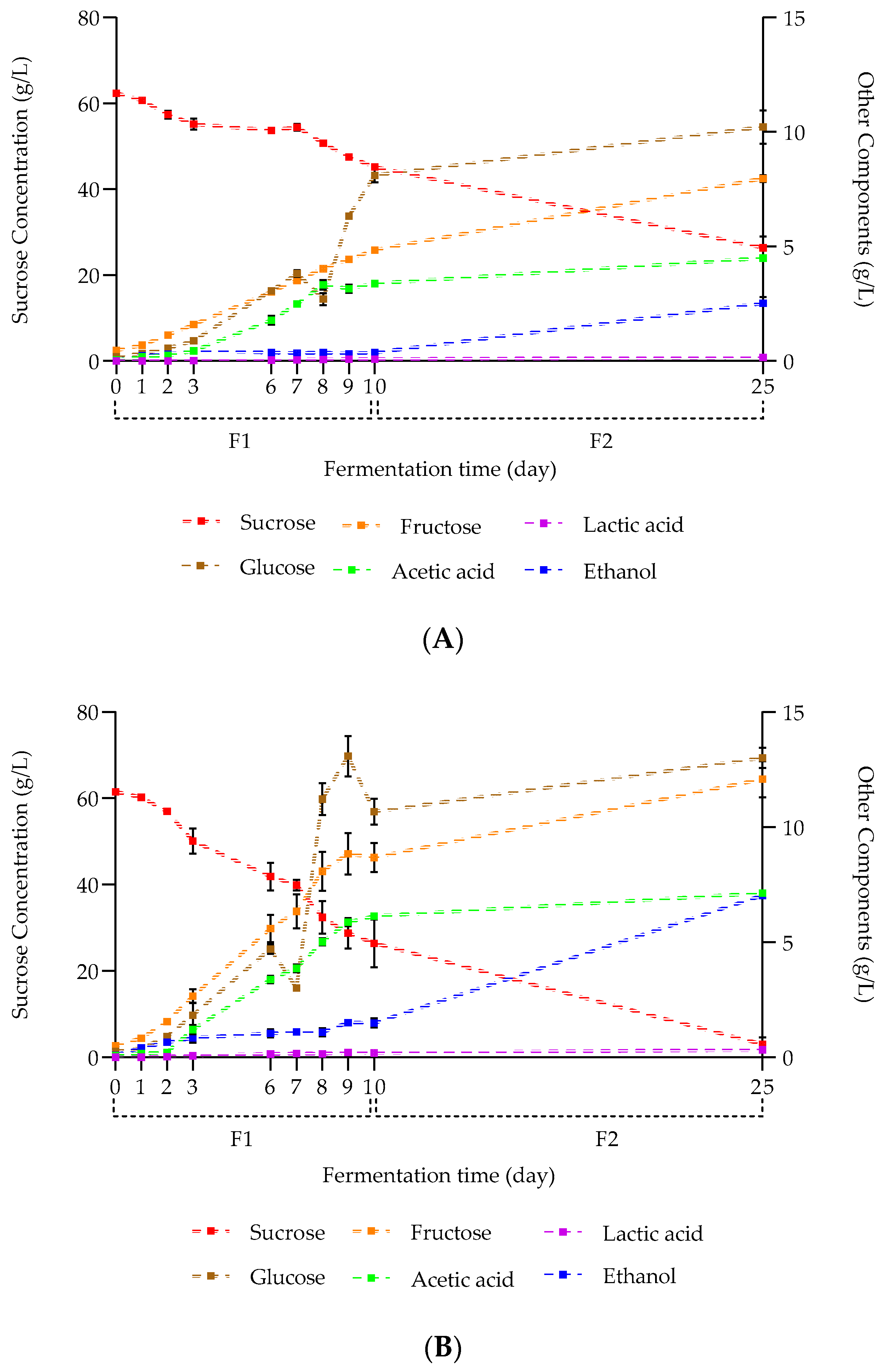
2.1.3. Carbohydrate, Alcohol, and Organic Acid Concentration Kinetics during Hakko Sobacha Fermentation
2.2. Study of the Volatile Organic Compound Development Kinetics during Fermentation
2.2.1. Chemical Analysis of Major VOCs
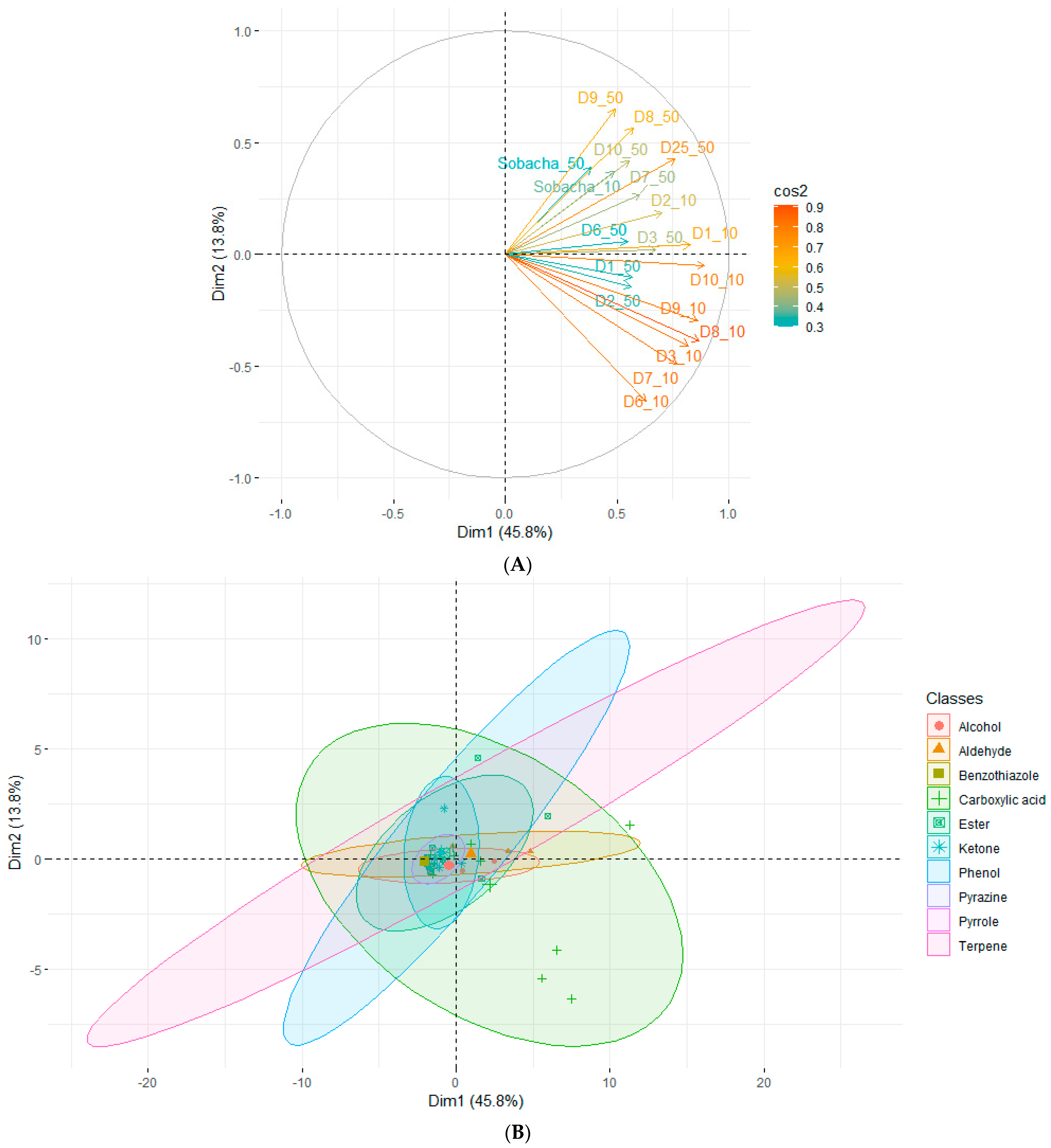
2.2.2. Theoretical Aromatic Characterization of Hakko Sobacha and Estimation of Sensory Profiles
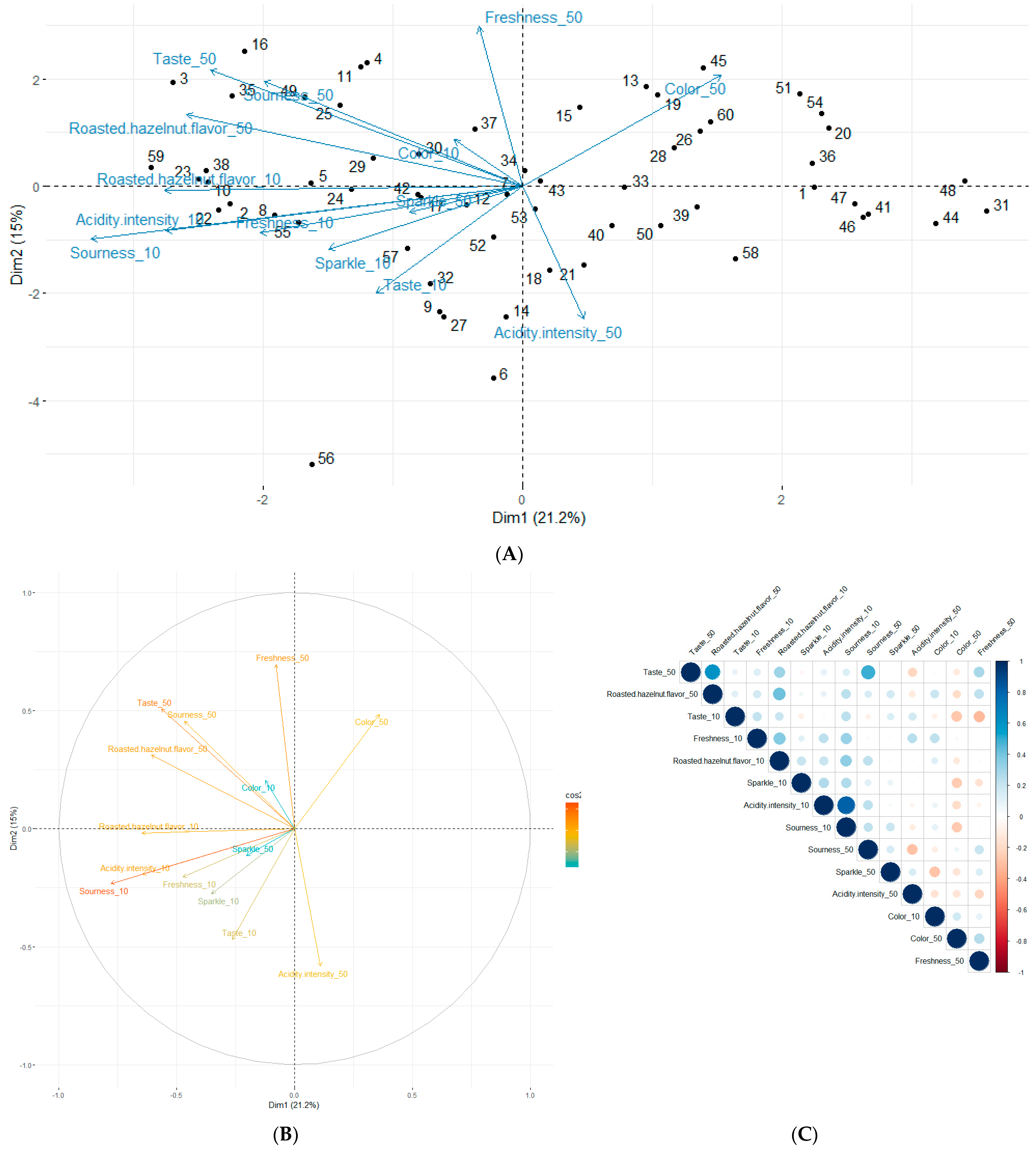
2.3. Study of Sensory Analysis Trends Resulting from the Hakko Sobacha Tasting
3. Materials and Methods
3.1. Materials and Chemical Reagents
3.2. Hakko Sobacha Preparation
3.3. Dynamic Analysis of the Physico-Chemical Parameters of the Drink
3.4. Chromatographic Analysis of the Development of Volatile Organic Compounds
3.5. Sensory Analysis
3.5.1. Triangle Sensory Analysis
3.5.2. Hedonic Evaluation
3.6. Raw Data Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Salmerón, I.; Thomas, K.; Pandiella, S.S. Effect of Substrate Composition and Inoculum on the Fermentation Kinetics and Flavour Compound Profiles of Potentially Non-Dairy Probiotic Formulations. LWT—Food Sci. Technol. 2014, 55, 240–247. [Google Scholar] [CrossRef]
- Lonǎr, E.; Djurić, M.; Malbaša, R.; Kolarov, L.J.; Klašnja, M. Influence of Working Conditions Upon Kombucha Conducted Fermentation of Black Tea. Food Bioprod. Process. 2006, 84, 186–192. [Google Scholar] [CrossRef]
- He, J.; Li, W.; Deng, J.; Lin, Q.; Bai, J.; Zhang, L.; Fang, Y. An Insight into the Health Beneficial of Probiotics Dairy Products: A Critical Review. Crit. Rev. Food Sci. Nutr. 2022, 1–20. [Google Scholar] [CrossRef]
- Qin, H.; Wu, H.; Shen, K.; Liu, Y.; Li, M.; Wang, H.; Qiao, Z.; Mu, Z. Fermented Minor Grain Foods: Classification, Functional Components, and Probiotic Potential. Foods 2022, 11, 3155. [Google Scholar] [CrossRef] [PubMed]
- Nyhan, L.M.; Lynch, K.M.; Sahin, A.W.; Arendt, E.K. Advances in Kombucha Tea Fermentation: A Review. Appl. Microbiol. 2022, 2, 73–103. [Google Scholar] [CrossRef]
- Abaci, N.; Senol Deniz, F.S.; Orhan, I.E. Kombucha—An Ancient Fermented Beverage with Desired Bioactivities: A Narrowed Review. Food Chem. X 2022, 14, 100302. [Google Scholar] [CrossRef]
- Dufresne, C.; Farnworth, E. Tea, Kombucha, and Health: A Review. Food Res. Int. 2000, 33, 409–421. [Google Scholar] [CrossRef]
- Teoh, A.L.; Heard, G.; Cox, J. Yeast Ecology of Kombucha Fermentation. Int. J. Food Microbiol. 2004, 95, 119–126. [Google Scholar] [CrossRef]
- Laavanya, D.; Shirkole, S.; Balasubramanian, P. Current Challenges, Applications and Future Perspectives of SCOBY Cellulose of Kombucha Fermentation. J. Clean. Prod. 2021, 295, 126454. [Google Scholar] [CrossRef]
- Villarreal-Soto, S.A.; Beaufort, S.; Bouajila, J.; Souchard, J.P.; Taillandier, P. Understanding Kombucha Tea Fermentation: A Review. J. Food Sci. 2018, 83, 580–588. [Google Scholar] [CrossRef]
- Staff, D.A.; Register, O.F.; FDA. Code of Federal Regulations: Title 21: Food and Drugs; Office of the Federal Register: Washington, DC, USA, 2005.
- Règlement (UE) no 1169/2011 du Parlement Européen et du Conseil du 25 Octobre 2011 Concernant L’information des Consommateurs sur les Denrées Alimentaires, Modifiant les Règlements (CE) no 1924/2006 et (CE) n o 1925/2006 du Parlement Européen et du Conseil et Abrogeant la Directive 87/250/CEE de la Commission, la Directive 90/496/CEE du Conseil, la Directive 1999/10/CE de la Commission, la Directive 2000/13/CE du Parlement Européen et du Conseil, les Directives 2002/67/CE et 2008/5/CE de la Commission et le Règlement (CE) no 608/2004 de la Commission Texte Présentant de L’intérêt pour l’EEE. 2011, Volume 304. Available online: https://eur-lex.europa.eu/eli/reg/2011/1169/oj/fra (accessed on 10 January 2023).
- Harrison, K.; Curtin, C. Microbial Composition of SCOBY Starter Cultures Used by Commercial Kombucha Brewers in North America. Microorganisms 2021, 9, 1060. [Google Scholar] [CrossRef] [PubMed]
- Gu, L.; Huang, X.; Li, Y.; Chen, Q. Production Technology Optimization on Fermented Tea of Golden Buckwheat. Guizhou Agric. Sci. 2014, 42, 169–174. [Google Scholar]
- Dutta, H.; Paul, S.K. Kombucha Drink: Production, Quality, and Safety Aspects. Prod. Manag. Beverages 2019, 1, 259–288. [Google Scholar] [CrossRef]
- Pederson, C.S. Microbiology of Food Fermentations; AVI Publishing Co., Inc.: Westport, CT, USA, 1979; Volume 384. [Google Scholar]
- Arumugam, M.; Raes, J.; Pelletier, E.; Paslier, D.L.; Yamada, T.; Mende, D.R.; Fernandes, G.R.; Tap, J.; Bruls, T.; Batto, J.M.; et al. Enterotypes of the Human Gut Microbiome. Nature 2011, 473, 174–180. [Google Scholar] [CrossRef]
- Behera, S.S.; Ray, R.C.; Das, U.; Panda, S.K.; Saranraj, P. Microorganisms in Fermentation. In Learning Materials in Biosciences; Springer: Cham, Switzerland, 2019; pp. 1–39. [Google Scholar] [CrossRef]
- Jayabalan, R.; Malbaša, R.V.; Lončar, E.S.; Vitas, J.S.; Sathishkumar, M. A Review on Kombucha Tea—Microbiology, Composition, Fermentation, Beneficial Effects, Toxicity, and Tea Fungus. Compr. Rev. Food Sci. Food Saf. 2014, 13, 538–550. [Google Scholar] [CrossRef]
- Liu, Y.; Liu, Z.; Xu, Z.; Li, G. Stir Bar Sorptive Extraction Technology. Prog. Chem. 2020, 32, 1334. [Google Scholar] [CrossRef]
- Reva, O.N.; Zaets, I.E.; Ovcharenko, L.P.; Kukharenko, O.E.; Shpylova, S.P.; Podolich, O.V.; de Vera, J.P.; Kozyrovska, N.O. Metabarcoding of the Kombucha Microbial Community Grown in Different Microenvironments. AMB Express 2015, 5, 35. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Z.J.; Sui, Y.C.; Wu, H.W.; Zhou, C.B.; Hu, X.C.; Zhang, J. Flavour Chemical Dynamics during Fermentation of Kombucha Tea. Emir. J. Food Agric. 2018, 30, 732–741. [Google Scholar] [CrossRef]
- Suffys, S.; Richard, G.; Burgeon, C.; Werrie, P.-Y.; Haubruge, E.; Fauconnier, M.-L.; Goffin, D. Characterization of Aroma Active Compound Production during Kombucha Fermentation: Towards the Control of Sensory Profiles. Foods 2023, 12, 1657. [Google Scholar] [CrossRef]
- Watawana, M.I.; Jayawardena, N.; Gunawardhana, C.B.; Waisundara, V.Y. Health, Wellness, and Safety Aspects of the Consumption of Kombucha. J. Chem. 2015, 2015, 591869. [Google Scholar] [CrossRef]
- Guo, H.; Yang, X.; Zhou, H.; Luo, X.; Qin, P.; Li, J.; Ren, G. Comparison of Nutritional Composition, Aroma Compounds, and Biological Activities of Two Kinds of Tartary Buckwheat Tea. J. Food Sci. 2017, 82, 1735–1741. [Google Scholar] [CrossRef] [PubMed]
- Mondal, S.; Bhar, K.; Sahoo, S.K.; Seru, G.; Ashfaquddin, M.; Pradhan, N.K.; Anjum, M.; Molla, S. Silver Hull Buckwheat (Fagopyrum Esculentum Moench) Is a Part of Nature That Offers Best Health and Honour. J. Complement. Altern. Med. Res. 2021, 15, 22–52. [Google Scholar] [CrossRef]
- Alvarez-Jubete, L.; Arendt, E.K.; Gallagher, E. Nutritive Value of Pseudocereals and Their Increasing Use as Functional Gluten-Free Ingredients. Trends Food Sci. Technol. 2010, 21, 106–113. [Google Scholar] [CrossRef]
- Zou, L.; Wu, D.; Ren, G.; Hu, Y.; Peng, L.; Zhao, J.; Garcia-Perez, P.; Carpena, M.; Prieto, M.A.; Cao, H.; et al. Bioactive Compounds, Health Benefits, and Industrial Applications of Tartary Buckwheat (Fagopyrum tataricum). Crit. Rev. Food Sci. Nutr. 2021, 63, 657–673. [Google Scholar] [CrossRef]
- Krkošková, B.; Mrázová, Z. Prophylactic Components of Buckwheat. Food Res. Int. 2005, 38, 561–568. [Google Scholar] [CrossRef]
- Ignat, M.V.; Salanţă, L.C.; Pop, O.L.; Pop, C.R.; Tofană, M.; Mudura, E.; Coldea, T.E.; Borşa, A.; Pasqualone, A. Current Functionality and Potential Improvements of Non-Alcoholic Fermented Cereal Beverages. Foods 2020, 9, 1031. [Google Scholar] [CrossRef]
- Qin, P.; Ma, T.; Wu, L.; Shan, F.; Ren, G. Identification of Tartary Buckwheat Tea Aroma Compounds with Gas Chromatography-Mass Spectrometry. J. Food Sci. 2011, 76, S401–S407. [Google Scholar] [CrossRef]
- Giménez-Bastida, J.; Piskuła, M.; Zieliński, H. Recent Advances in Processing and Development of Buckwheat Derived Bakery and Non-Bakery Products—A Review. Pol. J. Food Nutr. Sci. 2015, 65, 9–20. [Google Scholar] [CrossRef]
- Zielinska, D.; Szawara-Nowak, D.; Zielinski, H. Antioxidative and Anti-Glycation Activity of Buckwheat Hull Tea Infusion. Int. J. Food Prop. 2012, 16, 228–239. [Google Scholar] [CrossRef]
- Kawai, S. Preparation of Sobacha (Green Tea Blended with Soba (Buckwheat)). JPS5621556A, 6 December 1978. [Google Scholar]
- Nakayama, S. Buckwheat Tea. JPH06284880A, 29 May 1992. [Google Scholar]
- Toshima, T. Production of Buckwheat Green Tea. JPH03201971A, 29 December 1989. [Google Scholar]
- Chen, C.; Liu, B.Y. Changes in Major Components of Tea Fungus Metabolites during Prolonged Fermentation. J. Appl. Microbiol. 2000, 89, 834–839. [Google Scholar] [CrossRef]
- Jarrell, J.A.; Walia, N.; Nemergut, D.; Agadi, A.; Bennett, J.W. Inoculation, Growth and Bactericidal Effects of Three Kombucha Cultures. Microbiol. Res. 2022, 13, 128–136. [Google Scholar] [CrossRef]
- Neffe-Skocińska, K.; Sionek, B.; Ścibisz, I.; Kołożyn-Krajewska, D. Acid Contents and the Effect of Fermentation Condition of Kombucha Tea Beverages on Physicochemical, Microbiological and Sensory Properties. CyTA-J. Food 2017, 15, 601–607. [Google Scholar] [CrossRef]
- Linstrom, P.J.; Mallard, W.G. The NIST Chemistry WebBook: A Chemical Data Resource on the Internet. J. Chem. Eng. Data 2001, 46, 1059–1063. [Google Scholar] [CrossRef]
- The Good Scents Company—Flavor, Fragrance, Food and Cosmetics Ingredients Information. Available online: http://www.thegoodscentscompany.com/ (accessed on 10 January 2023).
- Flavornet. Available online: https://www.flavornet.org/flavornet.html (accessed on 31 January 2023).
- Gou, M.; Chen, Q.; Wu, X.; Liu, G.; Fauconnier, M.-L.; Bi, J. Novel Insight into the Evolution of Volatile Compounds during Dynamic Freeze-Drying of Ziziphus Jujuba Cv. Huizao Based on GC–MS Combined with Multivariate Data Analysis. Food Chem. 2023, 410, 135368. [Google Scholar] [CrossRef] [PubMed]
- Rolim, P.M.; Hu, Y.; Gänzle, M.G. Sensory Analysis of Juice Blend Containing Isomalto-Oligosaccharides Produced by Fermentation with Weissella Cibaria. Food Res. Int. 2019, 124, 86–92. [Google Scholar] [CrossRef] [PubMed]
- Lomolino, G.; Marangon, M.; Vincenzi, S.; De Iseppi, A. Sparkling Cider Paired with Italian Cheese: Sensory Analysis and Consumer Assessment. Beverages 2022, 8, 82. [Google Scholar] [CrossRef]
- Rousseau, B.; Meyer, A.; O’mahony, M. Power and Sensitivity of the Same-Different Test: Comparison with Triangle and Duo-Trio Methods. J. Sens. Stud. 1998, 13, 149–173. [Google Scholar] [CrossRef]
- Nguyen, N.K.; Nguyen, P.B.; Nguyen, H.T.; Le, P.H. Screening the Optimal Ratio of Symbiosis between Isolated Yeast and Acetic Acid Bacteria Strain from Traditional Kombucha for High-Level Production of Glucuronic Acid. LWT—Food Sci. Technol. 2015, 64, 1149–1155. [Google Scholar] [CrossRef]
- Meilgaard, M.C.; Carr, B.T.; Civille, G.V. Sensory Evaluation Techniques, 3rd ed.; CRC Press: Boca Raton, FL, USA, 1999; ISBN 978-1-00-304072-9. [Google Scholar]
- Sinkinson, C. Chapter 7—Triangle Test. In Discrimination Testing in Sensory Science; Rogers, L., Ed.; Woodhead Publishing Series in Food Science, Technology and Nutrition; Woodhead Publishing: Sawston, UK, 2017; pp. 153–170. ISBN 978-0-08-101009-9. [Google Scholar]
- Rukman, M.S.; Haerussana, A.N.E.M. The Effect of Different Sweeteners on the Free Radical Scavenging Activities, Alcohol Contents, Sugar Reductions, and Hedonic Properties of Green Tea Kombucha. Pharm. Sci. Asia 2023, 50, 51–58. [Google Scholar] [CrossRef]
- Zubaidah, E.; Yurista, S.; Rahmadani, N.R. Characteristic of Physical, Chemical, and Microbiological Kombucha from Various Varieties of Apples. IOP Conf. Ser. Earth Environ. Sci. 2018, 131, 012040. [Google Scholar] [CrossRef]

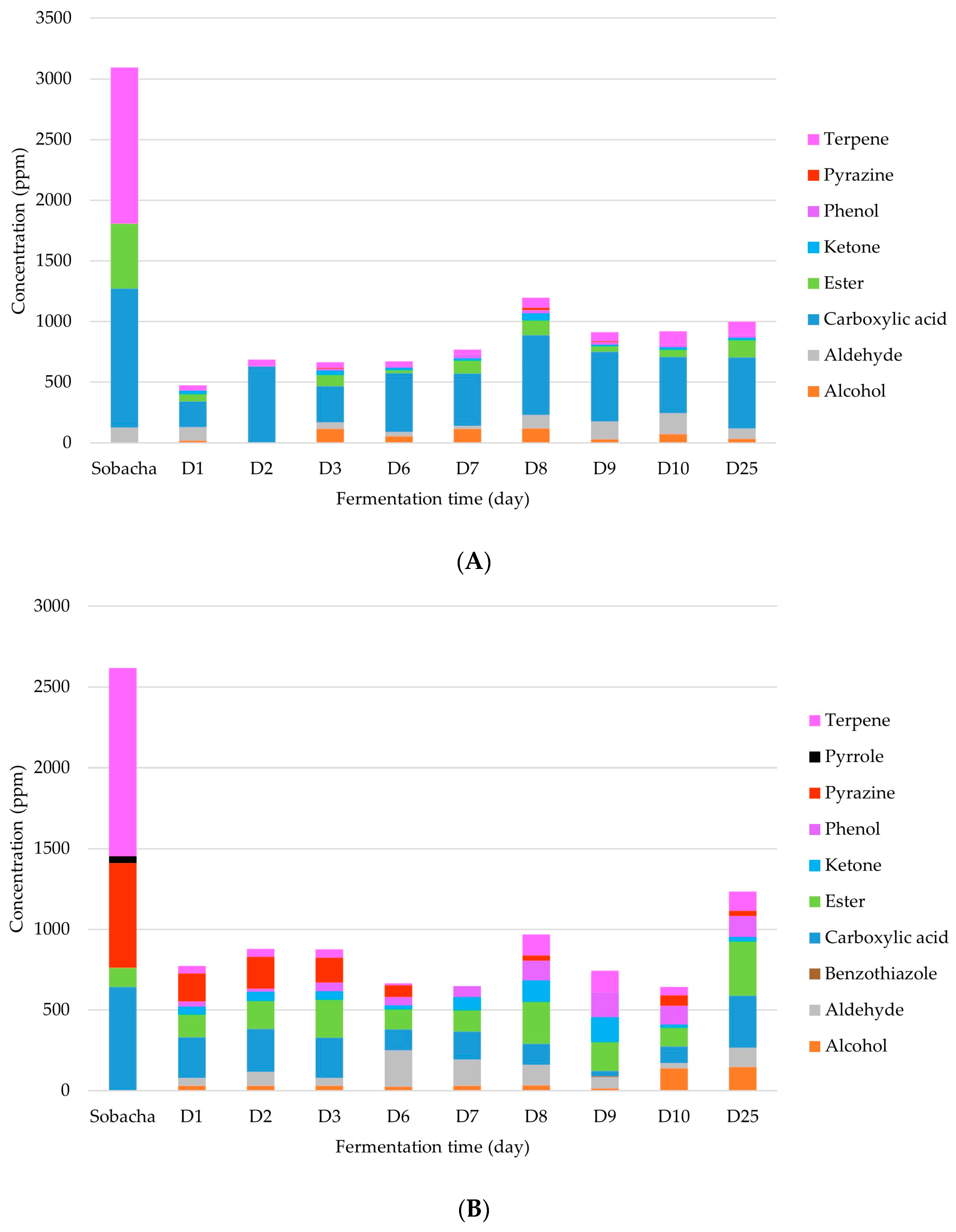
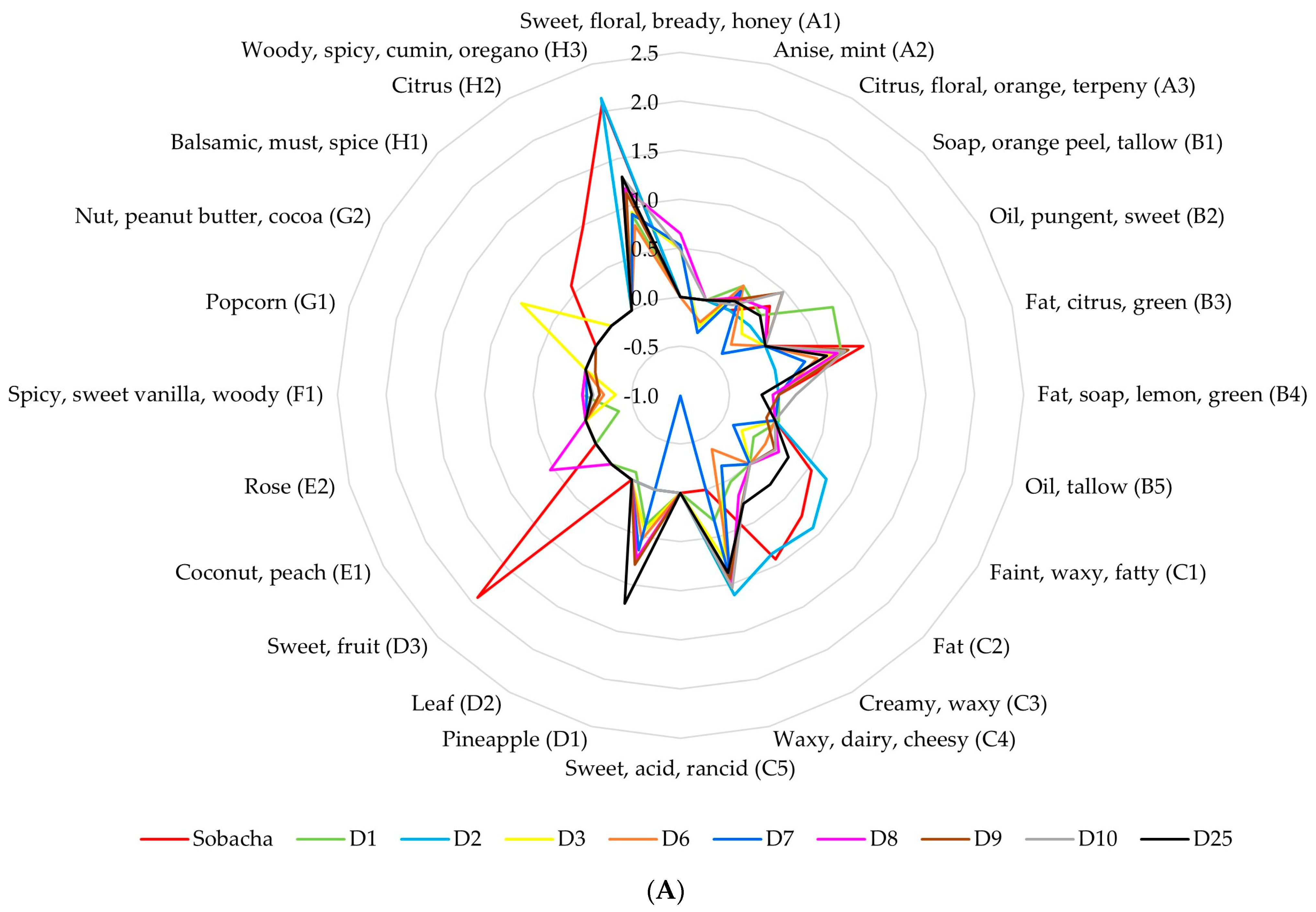
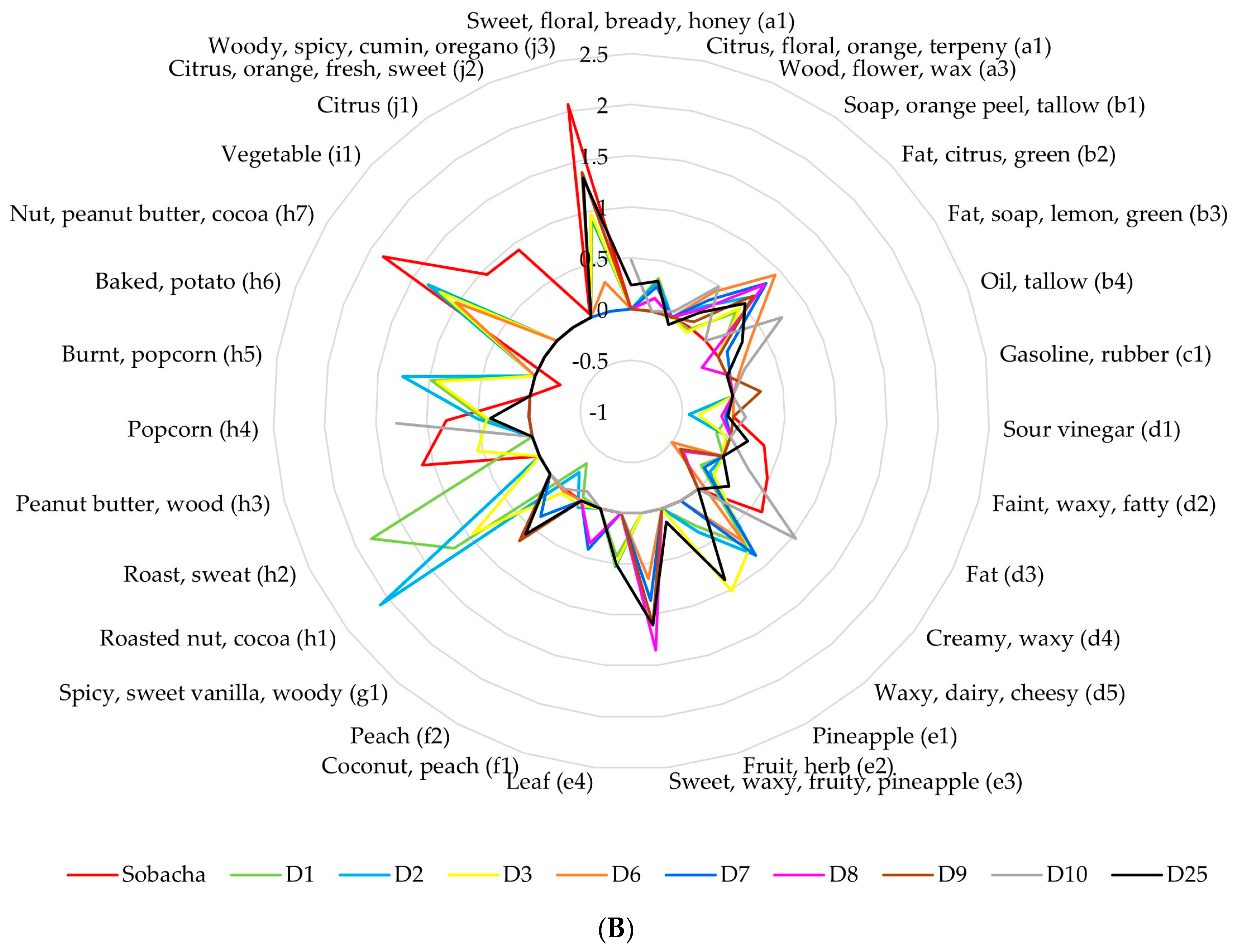
| Hakko Sobacha Fermentation Stages | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Class | Compound | CAS Number | RI | RI lit | Sobacha | D1 | D2 | D3 | D6 | D7 | D8 | D9 | D10 | D25 |
| Alcohol | 2-ethylhexan-1-ol | 104-76-7 | 1016 | 1015 | nd | nd | nd | 14.27 ± 2.68 | 20.28 ± 4.92 | 18.80 ± 3.25 | 16.69 ± 3.19 | 15.82 ± 0.76 | nd | 18.22 ± 0.90 |
| 2-phenylethanol | 60-12-8 | 1116 | 1116 | nd | nd | nd | 60.92 ± 0.11 | nd | 67.78 ± 8.49 | 89.39 ± 8.69 | nd | 60.80 ± 0.20 | nd | |
| alpha-terpineol | 98-55-5 | 1188 | 1190 | nd | nd | nd | 11.73 ± 3.81 | 13.34 ± 6.77 | 10.25 ± 0.22 | nd | nd | nd | nd | |
| linalool | 78-70-6 | 1100 | 1098 | nd | 19.42 ± 0.20 | nd | 17.91 ± 0.46 | 19.27 ± 5.74 | 16.82 ± 0.36 | 14.13 ± 1.44 | 13.28 ± 0.45 | 11.55 ± 0.20 | 12.69 ± 2.32 | |
| nonan-1-ol | 143-08-8 | 1169 | 1169 | nd | nd | nd | 11.01 ± 8.34 | nd | nd | nd | nd | nd | nd | |
| Total | nd | 19.42 | nd | 115.85 | 52.89 | 113.64 | 120.21 | 29.10 | 72.34 | 30.91 | ||||
| Aldehyde | decanal | 112-31-2 | 1203 | 1202 | 58.07 | 42.77 ± 8.30 | nd | 22.97 ± 0.92 | 16.09 ± 7.16 | 11.95 ± 0.75 | 53.22 ± 0.56 | 89.29 ± 1.85 | 90.95 ± 8.30 | 42.23 ± 2.71 |
| dodecanal | 112-54-9 | 1409 | 1412 | nd | 12.41 ± 9.00 | nd | nd | nd | nd | nd | nd | nd | nd | |
| nonanal | 124-19-6 | 1102 | 1102 | 67.90 | 39.94 ± 2.43 | nd | 31.69 ± 2.26 | 22.19 ± 2.60 | 16.44 ± 3.75 | 36.27 ± 7.26 | 47.13 ± 7.30 | 43.47 ± 2.43 | 27.91 ± 4.54 | |
| octanal | 124-13-0 | 1005 | 1001 | nd | nd | nd | nd | nd | nd | 21.88 ± 3.73 | nd | 37.61 ± 0.80 | 16.84 ± 6.09 | |
| undecanal | 112-44-7 | 1305 | 1305 | nd | 13.65 ± 0.80 | nd | nd | nd | nd | nd | 10.10 ± 6.22 | nd | nd | |
| Total | 125.97 | 108.77 | nd | 54.66 | 38.28 | 28.38 | 111.37 | 146.52 | 172.02 | 86.97 | ||||
| Carboxylic acid | 2-ethylhexanoic acid | 149-57-5 | 1115 | 1115 | nd | nd | nd | 11.64 ± 4.94 | 18.49 ± 3.23 | nd | nd | 22.40 ± 5.39 | nd | nd |
| acetic acid | 64-19-7 | 1402 | 1402 | nd | 36.14 ± 2.56 | nd | nd | nd | nd | nd | 36.28 ± 6.35 | nd | 42.54 ± 2.53 | |
| capric acid | 334-48-5 | 1387 | 1387 | nd | 22.78 ± 8.12 | 60.60 ± 7.79 | 60.45 ± 2.77 | 145.32 ± 2.36 | 100.40 ± 8.12 | 147.97 ± 4.29 | 62.11 ± 0.42 | nd | 116.88 ± 0.12 | |
| caprylic acid | 124-07-2 | 1191 | 1191 | nd | 20.47 ± 4.64 | 29.55 ± 5.72 | 101.38 ± 2.27 | 177.36 ± 0.91 | 168.34 ± 7.88 | 183.04 ± 9.80 | 142.89 ± 5.76 | 115.65 ± 4.64 | 81.21 ± 6.59 | |
| myristic acid | 544-63-8 | 1769 | 1769 | 104.02 | 21.86 ± 5.82 | 56.84 ± 2.62 | 15.80 ± 3.65 | nd | 12.47 ± 3.00 | 43.08 ± 8.67 | 38.44 ± 7.19 | 39.45 ± 5.82 | 55.51 ± 1.35 | |
| oleic acid | 112-80-1 | 2102 | 2101 | 84.08 | nd | 22.95 ± 0.41 | nd | nd | nd | nd | nd | nd | 29.27 ± 0.49 | |
| palmitic acid | 57-10-3 | 1973 | 1975 | 650.24 | 79.42 ± 1.60 | 53.56 ± 13.75 | 51.25 ± 7.76 | 32.75 ± 7.57 | 51.11 ± 4.54 | 114.07 ± 2.39 | 137.46 ± 4.18 | 139.28 ± 1.60 | 144.26 ± 5.31 | |
| palmitoleic acid | 373-49-9 | 1953 | 1953 | 180.99 | nd | 66.33 ± 4.72 | nd | nd | nd | 34.84 ± 4.52 | 23.83 ± 10.08 | 24.50 ± 4.62 | nd | |
| pelargonic acid | 112-05-0 | 1271 | 1272 | nd | 21.29 ± 9.61 | 29.86 ± 1.99 | 55.22 ± 7.30 | 110.11 ± 9.81 | 88.29 ± 5.68 | 104.73 ± 3.76 | 87.58 ± 3.79 | 107.95 ± 9.61 | 75.13 ± 0.12 | |
| pentadecanoic acid | 1002-84-2 | 1869 | 1869 | 78.38 | 11.50 ± 4.81 | 14.33 ± 5.32 | nd | nd | nd | 29.52 ± 7.30 | 23.96 ± 2.06 | 35.44 ± 4.81 | 25.35 ± 7.06 | |
| stearic acid | 57-11-4 | 2188 | 2188 | 47.20 | nd | nd | nd | nd | nd | nd | nd | nd | 17.19 ± 4.23 | |
| valeric acid | 109-52-4 | 1744 | 1744 | nd | nd | nd | nd | nd | 10.14 ± 1.32 | nd | nd | nd | nd | |
| Total | 1144.92 | 213.46 | 334.01 | 295.74 | 484.03 | 430.76 | 657.25 | 574.94 | 462.27 | 587.34 | ||||
| Ester | (2,2,4-trimethyl-3-(2-methylpropanoyloxy)pentyl) 2-methylpropanoate | 6846-50-0 | 1587 | 1587 | nd | 36.56 ± 2.46 | nd | 39.66 ± 17.85 | nd | 72.92 ± 0.16 | 82.05 ± 20.16 | nd | 63.12 ± 22.46 | nd |
| 2-phenylethyl acetate | 103-45-7 | 1256 | 1256 | nd | 17.79 ± 8.41 | nd | 20.17 ± 2.75 | 25.37 ± 3.52 | 33.00 ± 13.87 | 40.23 ± 3.90 | 46.38 ± 8.61 | nd | 120.67 ± 8.41 | |
| bis(2-ethylhexyl) hexanedioate | 103-23-1 | 2398 | 2398 | 37.64 | nd | nd | nd | nd | nd | nd | nd | nd | nd | |
| hexyl pivalate | 5434-57-1 | 1162 | 1163 | nd | nd | nd | 27.64 ± 2.42 | nd | nd | nd | nd | nd | nd | |
| isoamyl laurate | 6309-51-9 | 1844 | 1844 | nd | 8.19 ± 1.48 | nd | nd | nd | nd | nd | nd | nd | nd | |
| isopropyl palmitate | 142-91-6 | 1999 | 1999 | 73.33 | nd | nd | nd | nd | nd | nd | nd | nd | nd | |
| linalyl acetate | 115-95-7 | 1258 | 1259 | 425.75 | nd | nd | nd | nd | nd | nd | nd | nd | nd | |
| octyl caprylate | 2306-88-9 | 1777 | 1779 | nd | nd | nd | nd | nd | nd | nd | nd | nd | 23.03 ± 1.02 | |
| triacetin | 102-76-1 | 1344 | 1344 | nd | nd | nd | 8.21 ± 2.00 | nd | nd | nd | nd | nd | nd | |
| Total | 536.72 | 62.54 | nd | 95.69 | 25.37 | 105.92 | 122.28 | 46.38 | 63.12 | 143.70 | ||||
| Ketone | 2,6-ditert-butyl-4-hydroxy-4-methylcyclohexa-2,5-dien-1-one | 10396-80-2 | 1477 | 1478 | nd | 10.20 ± 1.91 | nd | 6.72 ± 1.70 | nd | nd | nd | nd | nd | nd |
| dihydroactinnidiolide | 17092-92-1 | 1525 | 1525 | nd | 12.60 ± 5.39 | nd | 15.65 ± 1.47 | 19.34 ± 7.71 | 20.21 ± 1.79 | 21.50 ± 6.79 | 13.44 ± 5.25 | 16.11 ± 5.39 | 15.64 ± 5.88 | |
| gamma-nonalactone | 104-61-0 | 1362 | 1360 | nd | nd | nd | nd | nd | nd | 34.03 ± 0.19 | nd | nd | nd | |
| geranylacetone | 3796-70-1 | 1455 | 1452 | nd | 5.37 ± 0.19 | nd | 11.94 ± 0.51 | nd | nd | nd | nd | nd | nd | |
| Total | nd | 28.17 | nd | 34.31 | 19.34 | 20.21 | 55.53 | 13.44 | 16.11 | 15.64 | ||||
| Phenol | 2,4-ditert-butylphenol | 96-76-4 | 1512 | 1513 | 72.41 | 16.08 ± 0.35 | nd | 16.79 ± 2.43 | 29.41 ± 7.20 | 9.93 ± 1.35 | nd | 13.20 ± 2.05 | 11.97 ± 0.35 | 14.34 ± 1.30 |
| 4-ethylguaiacol | 2785-87-7 | 1287 | 1285 | nd | nd | nd | 13.83 ± 5.30 | 18.15 ± 1.16 | 27.27 ± 7.51 | 30.19 ± 0.25 | 20.17 ± 8.97 | 23.48 ± 9.80 | 24.38 ± 4.17 | |
| Total | nd | nd | nd | 13.83 | 18.15 | 27.27 | 30.19 | 20.17 | 23.48 | 24.38 | ||||
| Pyrazine | 2,6-diethylpyrazine | 13067-27-1 | 1081 | 1081 | nd | nd | nd | nd | nd | nd | 17.02 ± 2.84 | nd | nd | nd |
| 2-isoamyl-6-methylpyrazine | 13925-00-3 | 915 | 915 | nd | nd | nd | nd | nd | nd | nd | 7.96 ± 2.53 | nd | nd | |
| 3,5-diethyl-2-methylpyrazine | 18138-05-1 | 1159 | 1159 | nd | nd | nd | 7.48 ± 3.27 | nd | nd | nd | nd | nd | nd | |
| Total | nd | nd | nd | 7.48 | nd | nd | 17.02 | 7.96 | nd | nd | ||||
| Terpene | squalene | 111-02-4 | 2834 | 2835 | 62.79 | nd | nd | nd | nd | nd | nd | nd | nd | nd |
| β-myrcene | 535-77-3 | 1023 | 1023 | 510.21 | nd | nd | nd | nd | nd | nd | nd | nd | nd | |
| p-cymene | 99-87-6 | 1025 | 1025 | 676.32 | 42.88 ± 4.14 | 53.51 ± 8.14 | 46.98 ± 7.97 | 34.23 ± 5.19 | 44.80 ± 8.85 | 83.22 ± 8.15 | 75.66 ± 3.63 | 109.48 ± 4.14 | 111.84 ± 3.93 | |
| Total | 1249.32 | 42.88 | 53.51 | 46.98 | 34.23 | 44.80 | 83.22 | 75.66 | 109.48 | 111.84 | ||||
| Hakko Sobacha Fermentation Stages | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Class | Compound | CAS Number | RI | RI lit | Sobacha | D1 | D2 | D3 | D6 | D7 | D8 | D9 | D10 | D25 |
| Alcohol | 2-ethylhexan-1-ol | 104-76-7 | 1016 | 1015 | nd | 8.27 ± 1.18 | 9.19 ± 0.42 | 11.32 ± 2.26 | 11.77 ± 0.99 | 12.97 ± 0.4 | 18.14 ± 3.16 | 14.90 ± 6.49 | 9.69 ± 2.38 | 19.87 ± 1.22 |
| 2-phenylethanol | 60-12-8 | 1116 | 1116 | nd | nd | nd | nd | nd | nd | nd | nd | 117.84 ± 3.84 | 86.29 ± 3.58 | |
| linalool | 78-70-6 | 1100 | 1098 | nd | 21.26 ± 6.64 | 19.67 ± 1.90 | 17.59 ± 1.10 | 13.50 ± 2.3 | 17.61 ± 7.62 | 13.48 ± 0.65 | nd | 10.91 ± 1.03 | 20.00 ± 5.35 | |
| nerolidol | 7212-44-4 | 1567 | 1564 | nd | nd | nd | nd | nd | nd | nd | nd | nd | 20.78 ± 5.93 | |
| Total | nd | 29.52 | 28.87 | 28.91 | 25.27 | 30.58 | 31.62 | 14.90 | 138.44 | 146.94 | ||||
| Aldehyde | decanal | 112-31-2 | 1203 | 1202 | nd | 28.40 ± 9.46 | 53.40 ± 0.58 | 26.29 ± 2.70 | 79.22 ± 1.78 | 63.00 ± 3.41 | 49.67 ± 4.89 | 35.07 ± 5.34 | 17.83 ± 4.50 | 45.02 ± 5.05 |
| nonanal | 124-19-6 | 1102 | 1102 | nd | 20.42 ± 0.23 | 34.11 ± 1.21 | 23.33 ± 8.83 | 68.90 ± 8.0 | 52.91 ± 3.23 | 50.21 ± 7.86 | 35.29 ± 4.38 | 15.34 ± 3.64 | 27.19 ± 4.73 | |
| octanal | 124-13-0 | 1005 | 1001 | nd | nd | nd | nd | 60.69 ± 6.40 | 31.74 ± 9.32 | 16.23 ± 5.74 | nd | nd | 47.52 ± 6.58 | |
| undecanal | 112-44-7 | 1305 | 1305 | nd | nd | nd | nd | 16.90 ± 0.51 | 13.23 ± 4.79 | 13.05 ± 2.43 | nd | nd | nd | |
| Total | nd | 48.81 | 87.51 | 49.63 | 225.70 | 160.87 | 129.17 | 70.36 | 33.17 | 119.73 | ||||
| Benzothiazole | 1,3-benzothiazole | 95-16-9 | 1227 | 1224 | nd | nd | nd | nd | nd | nd | nd | 5.68 ± 2.56 | nd | nd |
| Total | nd | nd | nd | nd | nd | nd | nd | 5.68 | nd | nd | ||||
| Carboxylic acid | acetic acid | 64-19-7 | 1402 | 1402 | nd | 44.55 ± 3.85 | 18.55 ± 2.14 | 23.12 ± 6.27 | nd | 41.86 ± 3.87 | 38.49 ± 6.69 | nd | 10.51 ± 3.34 | 44.09 ± 1.91 |
| capric acid | 334-48-5 | 1387 | 1387 | nd | 67.21 ± 2.07 | 78.79 ± 7.33 | 48.58 ± 7.89 | 57.52 ± 4.19 | nd | nd | nd | nd | nd | |
| caprylic acid | 124-07-2 | 1191 | 1191 | nd | nd | nd | nd | nd | nd | 58.35 ± 8.37 | nd | nd | 67.41 ± 9.18 | |
| myristic acid | 544-63-8 | 1769 | 1769 | 65.62 | 21.67 ± 3.00 | 27.27 ± 2.53 | 26.61 ± 4.60 | nd | nd | nd | nd | 19.57 ± 4.75 | 44.89 ± 2.73 | |
| oleic acid | 112-80-1 | 2102 | 2101 | 44.98 | nd | nd | nd | nd | nd | nd | nd | nd | nd | |
| palmitic acid | 57-10-3 | 1973 | 1975 | 304.18 | 54.14 ± 8.37 | 69.62 ± 4.05 | 78.80 ± 0.45 | 23.65 ± 4.01 | 59.47 ± 3.70 | 32.54 ± 0.67 | 30.09 ± 3.39 | 61.46 ± 8.72 | 119.47 ± 4.46 | |
| palmitoleic acid | 373-49-9 | 1953 | 1953 | 144.70 | nd | nd | nd | nd | nd | nd | nd | nd | 22.97 ± 4.02 | |
| pelargonic acid | 112-05-0 | 1271 | 1272 | nd | 54.86 ± 6.66 | 71.38 ± 6.19 | 58.74 ± 5.98 | 47.52 ± 6.73 | 72.43 ± 1.72 | nd | nd | nd | nd | |
| pentadecanoic acid | 1002-84-2 | 1869 | 1869 | 50.19 | 9.28 ± 2.64 | nd | 13.18 ± 8.18 | nd | nd | nd | nd | 11.09 ± 2.32 | 23.63 ± 5.48 | |
| stearic acid | 57-11-4 | 2188 | 2188 | 32.16 | nd | nd | nd | nd | nd | nd | nd | nd | nd | |
| Total | 641.83 | 251.72 | 265.60 | 249.04 | 128.70 | 173.76 | 129.37 | 30.09 | 102.64 | 322.45 | ||||
| Ester | (2-decanoyloxy-3-octanoyloxypropyl) decanoate | 82426-88-8 | 3348 | 3348 | nd | 6.79 ± 0.78 | nd | 7.78 ± 0.19 | nd | nd | nd | nd | nd | nd |
| [2,2,4-trimethyl-3-(2-methylpropanoyloxy)pentyl] 2-methylpropanoate | 6846-50-0 | 1587 | 1587 | nd | 30.84 ± 1.52 | 36.09 ± 2.72 | 50.89 ± 6.86 | 93.09 ± 2.01 | 80.65 ± 0.74 | 93.85 ± 2.60 | 84.85 ± 6.78 | 50.19 ± 2.21 | 78.92 ± 0.65 | |
| 1,3-di(octanoyloxy)propan-2-yl decanoate | 33368-87-5 | 3137 | 3137 | nd | 24.43 ± 4.83 | 34.95 ± 2.27 | 25.74 ± 4.79 | nd | nd | nd | nd | nd | 40.61 ± 8.29 | |
| 2-phenylethyl acetate | 103-45-7 | 1256 | 1256 | nd | 14.24 ± 4.31 | 16.72 ± 3.25 | 77.44 ± 8.65 | nd | nd | nd | nd | 22.58 ± 5.83 | 57.55 ± 2.20 | |
| ethyl 2-phenylacetate | 101-97-3 | 1244 | 1244 | nd | nd | nd | nd | nd | nd | nd | nd | nd | 10.25 ± 3.45 | |
| ethyl caprylate | 106-32-1 | 1196 | 1196 | nd | nd | nd | nd | 33.19 ± 5.69 | 54.40 ± 6.93 | 167.58 ± 8.85 | 94.33 ± 3.06 | 43.93 ± 8.48 | 95.52 ± 1.29 | |
| isoamyl laurate | 6309-51-9 | 1844 | 1844 | nd | 26.73 ± 5.38 | 33.72 ± 1.53 | 32.48 ± 1.83 | nd | nd | nd | nd | nd | 31.54 ± 8.18 | |
| methyl caproate | 106-70-7 | 924 | 924 | 119.45 | nd | nd | nd | nd | nd | nd | nd | nd | nd | |
| octan-2-yl hexadecanoate | 55194-81-5 | 2501 | 2501 | nd | 13.14 ± 2.19 | 17.60 ± 0.84 | 14.91 ± 3.36 | nd | nd | nd | nd | nd | 20.94 ± 3.97 | |
| triacetin | 102-76-1 | 1344 | 1344 | nd | 9.10 ± 1.61 | 9.27 ± 0.63 | 9.49 ± 1.11 | nd | nd | nd | nd | nd | nd | |
| tricaprylin | 538-23-8 | 2858 | 2859 | nd | 17.36 ± 4.71 | 25.28 ± 7.75 | 18.15 ± 2.89 | nd | nd | nd | nd | nd | nd | |
| Total | 119.45 | 142.63 | 173.63 | 236.89 | 126.28 | 135.06 | 261.43 | 179.18 | 116.70 | 335.34 | ||||
| Ketone | 2,6-ditert-butyl-4-hydroxy-4-methylcyclohexa-2,5-dien-1-one | 10396-80-2 | 1477 | 1478 | nd | 9.06 ± 2.72 | 12.09 ± 1.60 | 7.30 ± 1.43 | nd | 12.82 ± 2.75 | 16.61 ± 1.08 | 11.60 ± 7.57 | 7.18 ± 1.67 | 15.69 ± 5.78 |
| dihydroactinnidiolide | 17092-92-1 | 1525 | 1525 | nd | 10.71 ± 3.04 | 10.93 ± 0.15 | 11.12 ± 1.21 | 22.28 ± 9.41 | 20.00 ± 2.39 | 24.24 ± 4.97 | 17.79 ± 8.18 | 12.23 ± 3.21 | 12.47 ± 2.54 | |
| gamma-nonalactone | 104-61-0 | 1362 | 1360 | nd | nd | nd | nd | nd | 25.93 ± 5.62 | 22.55 ± 3.87 | nd | nd | nd | |
| gamma-undecalactone | 104-67-6 | 1573 | 1573 | nd | 27.81 ± 0.31 | 35.72 ± 8.00 | 33.90 ± 3.55 | nd | nd | nd | nd | nd | nd | |
| 3,5-dihydroxy-6-methyl-2,3-dihydropyran-4-one | 28564-83-2 | 1154 | 1154 | nd | nd | nd | nd | nd | 20.38 ± 9.86 | 68.11 ± 7.99 | 127.31 ± 7.95 | nd | nd | |
| Total | nd | 47.58 | 58.74 | 52.32 | 22.28 | 79.12 | 131.52 | 156.70 | 19.40 | 28.16 | ||||
| Phenol | 2,4-ditert-butylphenol | 96-76-4 | 1512 | 1513 | nd | 8.74 ± 2.76 | nd | 7.04 ± 0.98 | 19.62 ± 6.12 | nd | nd | 10.84 ± 5.99 | 7.47 ± 1.58 | nd |
| 4-ethylguaiacol | 2785-87-7 | 1287 | 1285 | nd | 14.13 ± 5.91 | 18.31 ± 8.59 | 33.73 ± 5.84 | 31.81 ± 5.07 | 68.07 ± 0.13 | 122.98 ± 0.62 | 141.96 ± 8.29 | 63.55 ± 9.73 | 116.18 ± 3.33 | |
| dihydroeugenol | 2785-89-9 | 1261 | 1260 | nd | nd | nd | nd | nd | nd | nd | nd | 35.09 ± 3.67 | 12.39 ± 0.25 | |
| isochavibetol | 19784-98-6 | 1312 | 1312 | nd | 10.19 ± 0.93 | nd | 13.79 ± 2.19 | nd | nd | nd | nd | 10.32 ± 2.00 | nd | |
| Total | nd | 33.06 | 18.31 | 54.55 | 51.43 | 68.07 | 122.98 | 152.80 | 116.44 | 128.56 | ||||
| Pyrazine | 2,5-dimethyl-3-propylpyrazine | 18433-97-1 | 1162 | 1162 | nd | 15.43 ± 4.90 | 125.65 ± 7.86 | 9.00 ± 1.57 | nd | nd | nd | nd | nd | nd |
| 2,6-diethylpyrazine | 13067-27-1 | 1081 | 1081 | nd | nd | nd | 63.59 ± 1.39 | 68.41 ± 7.27 | nd | 32.98 ± 1.22 | nd | 23.98 ± 5.70 | 16.76 ± 7.98 | |
| 2-ethyl-5-methylpyrazine | 13360-64-0 | 1001 | 1000 | nd | 66.98 ± 7.88 | nd | nd | nd | nd | nd | nd | 23.41 ± 9.21 | nd | |
| 2-ethylpyrazine | 13360-65-1 | 1077 | 1078 | 129.11 | nd | nd | 35.45 ± 5.61 | nd | nd | nd | nd | 14.85 ± 3.17 | nd | |
| 2-isoamyl-6-methylpyrazine | 13925-00-3 | 915 | 915 | 64.64 | 27.91 ± 8.79 | 31.81 ± 6.60 | 25.66 ± 7.56 | nd | nd | nd | nd | 16.90 ± 0.11 | 23.85 ± 6.77 | |
| 2-isobutyl-3-methylpyrazine | 13925-06-9 | 1114 | 1114 | nd | 9.50 ± 1.10 | 18.06 ± 2.11 | 8.33 ± 1.28 | nd | nd | nd | nd | nd | nd | |
| 5-ethyl-2,3-dimethylpyrazine | 15707-34-3 | 1104 | 1105 | nd | nd | 9.89 ± 0.59 | 10.03 ± 0.02 | nd | nd | nd | nd | nd | nd | |
| 2-methylpyrazine | 109-08-0 | 1151 | 1150 | 41.55 | nd | nd | nd | nd | nd | nd | nd | nd | nd | |
| 3,5-diethyl-2-methylpyrazine | 18138-05-1 | 1159 | 1159 | 72.24 | 22.13 ± 8.12 | 21.66 ± 2.82 | 16.06 ± 6.01 | 10.43 ± 0.62 | nd | nd | nd | 6.40 ± 0.97 | nd | |
| 2-methoxy-5-methylpyrazine | 2882-22-6 | 971 | 971 | 342.21 | 62.80 ± 2.30 | nd | nd | nd | nd | nd | nd | nd | nd | |
| Total | 649.76 | 174.11 | 197.76 | 152.95 | 75.37 | nd | 32.98 | nd | 65.02 | 35.02 | ||||
| Pyrrole | 1-(furan-2-ylmethyl)pyrrole | 1438-94-4 | 1185 | 1185 | 43.78 | nd | nd | nd | nd | nd | nd | nd | nd | nd |
| Total | 43.78 | nd | nd | nd | nd | nd | nd | nd | nd | nd | ||||
| Terpene | squelene | 111-02-4 | 2834 | 2835 | 53.88 | nd | nd | nd | nd | nd | nd | nd | nd | nd |
| β-myrcene | 535-77-3 | 1023 | 1023 | 439.92 | nd | nd | nd | nd | nd | nd | nd | nd | nd | |
| D-limonene | 5989-27-5 | 1036 | 1035 | 32.43 | nd | nd | nd | nd | nd | nd | nd | nd | nd | |
| p-cymene | 99-87-6 | 1025 | 1025 | 635.14 | 44.66 ± 8.94 | 49.16 ± 9.68 | 51.50 ± 7.49 | 10.72 ± 2.29 | nd | 129.58 ± 5.24 | 133.63 ± 9.15 | 50.63 ± 9.19 | 117.51 ± 2.37 | |
| Total | 1161.38 | 44.66 | 49.16 | 51.50 | 10.72 | nd | 129.58 | 133.63 | 50.63 | 117.51 | ||||
| Hakko Sobacha Fermentation Stages | |||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Class | Compound | CAS Number | RI | RI lit | Perception Threshold (ppm) | Aroma Description | Sobacha | D1 | D2 | D3 | D6 | D7 | D8 | D9 | D10 | D25 | |
| Alcohol | 2-phenylethanol | A1 | 60-12-8 | 1116 | 1116 | 20 | Sweet, floral, bready, honey | nd | nd | nd | 3.05 | nd | 3.39 | 4.47 | nd | 3.04 | nd |
| alpha-terpineol | A2 | 98-55-5 | 1188 | 1190 | 22.5 | Anise, mint | nd | nd | nd | 0.52 | 0.59 | 0.46 | nd | nd | nd | nd | |
| linalool | A3 | 78-70-6 | 1100 | 1098 | 10 | Citrus, floral, orange, terpene | nd | 1.94 | nd | 1.79 | 1.93 | 1.68 | 1.41 | 1.33 | 1.15 | 1.27 | |
| Aldehyde | decanal | B1 | 112-31-2 | 1203 | 1202 | 30 | Soap, orange peel, tallow | 1.94 | 1.43 | nd | 0.77 | 0.54 | 0.40 | 1.77 | 2.98 | 3.03 | 1.41 |
| dodecanal | B2 | 112-54-9 | 1409 | 1412 | 2 | Oil, pungent, sweet | nd | 6.21 | nd | nd | nd | nd | nd | nd | nd | nd | |
| nonanal | B3 | 124-19-6 | 1102 | 1102 | 8 | Fat, citrus, green | 8.49 | 4.99 | nd | 3.96 | 2.77 | 2.05 | 4.53 | 5.89 | 5.43 | 3.49 | |
| octanal | B4 | 124-13-0 | 1005 | 1001 | 25 | Fat, soap, lemon, green | nd | nd | nd | nd | nd | nd | 0.88 | nd | 1.50 | 0.67 | |
| undecanal | B5 | 112-44-7 | 1305 | 1305 | 12.5 | Oil, tallow | nd | 1.09 | nd | nd | nd | nd | nd | 0.81 | nd | nd | |
| Carboxylic acid | myristic acid | C1 | 544-63-8 | 1769 | 1769 | 30 | Faint, waxy, fatty | 3.47 | 0.73 | 5.23 | 0.53 | nd | 0.42 | 1.44 | 1.28 | 1.31 | 1.85 |
| oleic acid | C2 | 112-80-1 | 2102 | 2101 | 15 | Fat | 5.61 | nd | 8.20 | nd | nd | nd | nd | nd | nd | 1.95 | |
| palmitic acid | C3 | 57-10-3 | 1973 | 1975 | 75 | Creamy, waxy | 8.67 | 1.06 | 7.38 | 0.68 | 0.44 | 0.68 | 1.52 | 1.83 | 1.86 | 1.92 | |
| pelargonic acid | C4 | 112-05-0 | 1271 | 1272 | 10 | Waxy, dairy, cheesy | nd | 2.13 | 12.99 | 5.52 | 11.01 | 8.83 | 10.47 | 8.76 | 10.79 | 7.51 | |
| valeric acid | C5 | 109-52-4 | 1744 | 1744 | 100 | Sweet, acid, rancid | nd | nd | nd | nd | nd | 0.10 | nd | nd | nd | nd | |
| Ester | 2-phenylethyl acetate | D1 | 103-45-7 | 1256 | 1256 | 7.5 | Pineapple | nd | 2.37 | nd | 2.69 | 3.38 | 4.40 | 5.36 | 6.18 | nd | 16.09 |
| isoamyl laurate | D2 | 6309-51-9 | 1844 | 1844 | 10 | Leaf | nd | 0.82 | nd | nd | nd | nd | nd | nd | nd | nd | |
| linalyl acetate | D3 | 115-95-7 | 1258 | 1259 | 5 | Sweet, fruit | 85.15 | nd | nd | nd | nd | nd | nd | nd | nd | nd | |
| Ketone | gamma-nonalactone | E1 | 104-61-0 | 1362 | 1360 | 10 | Coconut, peach | nd | nd | nd | nd | nd | nd | 3.40 | nd | nd | nd |
| geranylacetone | E2 | 3796-70-1 | 1455 | 1452 | 12 | Rose | nd | 0.45 | nd | 0.99 | nd | nd | nd | nd | nd | nd | |
| Phenol | 4-ethylguaiacol | F1 | 2785-87-7 | 1287 | 1285 | 30 | Spicy, sweet vanilla, woody | nd | nd | nd | 0.46 | 0.60 | 0.91 | 1.01 | 0.67 | 0.78 | 0.81 |
| Pyrazine | 2-isoamyl-6-methylpyrazine | G1 | 13925-00-3 | 915 | 915 | 10 | Popcorn | nd | nd | nd | nd | nd | nd | nd | 0.80 | nd | nd |
| 3,5-diethyl-2-methylpyrazine | G2 | 18138-05-1 | 1159 | 1159 | 1 | Nut, peanut butter, cocoa | nd | nd | nd | 7.48 | nd | nd | nd | nd | nd | nd | |
| Terpene | beta-myrcene | H1 | 123-35-3 | 990 | 990 | 10 | Balsamic, must, spice | 3.75 | nd | nd | nd | nd | nd | nd | nd | nd | nd |
| β-myrcene | H2 | 535-77-3 | 1023 | 1023 | 52.5 | Citrus | 9.72 | nd | nd | nd | nd | nd | nd | nd | nd | nd | |
| p-cymene | H3 | 99-87-6 | 1025 | 1025 | 5.5 | Woody, spicy, cumin, oregano | 122.97 | 7.80 | 137.00 | 8.54 | 6.22 | 8.15 | 15.13 | 13.76 | 19.90 | 20.33 | |
| Hakko Sobacha Fermentation Stages | |||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Class | Compound | CAS Number | RI | RI lit | Perception Threshold (ppm) | Aroma Description | Sobacha | D1 | D2 | D3 | D6 | D7 | D8 | D9 | D10 | D25 | |
| Alcohol | 2-phenylethanol | a1 | 60-12-8 | 1116 | 1116 | 50 | Sweet, floral, bready, honey | nd | nd | nd | nd | nd | nd | nd | nd | 2.36 | 1.73 |
| linalool | a2 | 78-70-6 | 1100 | 1098 | 10 | Citrus, floral, orange, terpene | nd | 2.13 | 1.97 | 1.76 | 1.35 | 1.76 | 1.35 | nd | 1.09 | 2.00 | |
| nerolidol | a3 | 7212-44-4 | 1567 | 1564 | 25 | Wood, flower, wax | nd | nd | nd | nd | nd | nd | nd | nd | nd | 0.83 | |
| Aldehyde | decanal | b1 | 112-31-2 | 1203 | 1202 | 30 | Soap, orange peel, tallow | nd | 0.95 | 1.78 | 0.88 | 2.64 | 2.10 | 1.66 | 1.17 | 0.59 | 1.50 |
| nonanal | b2 | 124-19-6 | 1102 | 1102 | 8 | Fat, citrus, green | nd | 2.55 | 4.26 | 2.92 | 8.61 | 6.61 | 6.28 | 4.41 | 1.92 | 3.40 | |
| octanal | b3 | 124-13-0 | 1005 | 1001 | 25 | Fat, soap, lemon, green | nd | nd | nd | nd | 2.43 | 1.27 | 0.65 | nd | nd | 1.90 | |
| undecanal | b4 | 112-44-7 | 1305 | 1305 | 12.5 | Oil, tallow | nd | nd | nd | nd | 1.35 | 1.06 | 1.04 | nd | nd | nd | |
| Benzothiazole | 1,3-benzothiazole | c1 | 95-16-9 | 1227 | 1224 | 3 | Gasoline, rubber | nd | nd | nd | nd | nd | nd | nd | 1.89 | nd | nd |
| Carboxylic acid | acetic acid | d1 | 64-19-7 | 1402 | 1402 | 50.5 | Sour vinegar | nd | 0.88 | 0.37 | 0.46 | nd | 0.83 | 0.76 | nd | 0.21 | 0.87 |
| myristic acid | d2 | 544-63-8 | 1769 | 1769 | 30 | Faint, waxy, fatty | 2.19 | 0.72 | 0.91 | 0.89 | nd | nd | nd | nd | 0.65 | 1.50 | |
| oleic acid | d3 | 112-80-1 | 2102 | 2101 | 15 | Fat | 3.00 | nd | nd | nd | nd | nd | nd | nd | nd | nd | |
| palmitic acid | d4 | 57-10-3 | 1973 | 1975 | 75 | Creamy, waxy | 4.06 | 0.72 | 0.93 | 1.05 | 0.32 | 0.79 | 0.43 | 0.40 | 0.82 | 1.59 | |
| pelargonic acid | d5 | 112-05-0 | 1271 | 1272 | 10 | Waxy, dairy, cheesy | nd | 5.49 | 7.14 | 5.87 | 4.75 | 7.24 | nd | nd | nd | nd | |
| Ester | 2-phenylethyl acetate | e1 | 103-45-7 | 1256 | 1256 | 7.5 | Pineapple | nd | 1.90 | 2.23 | 10.33 | nd | nd | nd | nd | 3.01 | 7.67 |
| ethyl 2-phenylacetate | e2 | 101-97-3 | 1244 | 1244 | 7.5 | Fruit, herb | nd | nd | nd | nd | nd | nd | nd | nd | nd | 1.37 | |
| ethyl caprylate | e3 | 106-32-1 | 1196 | 1196 | 7.5 | Sweet, waxy, fruity, pineapple | nd | nd | nd | nd | 4.43 | 7.25 | 22.34 | 12.58 | 5.86 | 12.74 | |
| isoamyl laurate | e4 | 6309-51-9 | 1844 | 1844 | 10 | Leaf | nd | 2.67 | 3.37 | 3.25 | nd | nd | nd | nd | nd | 3.15 | |
| Ketone | gamma-nonalactone | f1 | 104-61-0 | 1362 | 1360 | 10 | Coconut, peach | nd | nd | nd | nd | nd | 2.59 | 2.26 | nd | nd | nd |
| gamma-undecalactone | f2 | 104-67-6 | 1573 | 1573 | 30 | Peach | nd | 0.93 | 1.19 | 1.13 | nd | nd | nd | nd | nd | nd | |
| Phenol | 4-ethylguaiacol | g1 | 2785-87-7 | 1287 | 1285 | 30 | Spicy, sweet vanilla, woody | nd | 0.47 | 0.61 | 1.12 | 1.06 | 2.27 | 4.10 | 4.73 | 2.12 | 3.87 |
| Pyrazine | 2,5-dimethyl-3-propylpyrazine | h1 | 18433-97-1 | 1162 | 1162 | 1 | Roasted nut, cocoa | nd | 15.43 | 125.65 | 9.00 | nd | nd | nd | nd | nd | nd |
| 2-ethyl-5-methylpyrazine | h2 | 13360-64-0 | 1001 | 1000 | 1 | Roast, sweat | nd | 66.98 | nd | nd | nd | nd | nd | nd | 23.41 | nd | |
| 2-ethylpyrazine | h3 | 13360-65-1 | 1077 | 1078 | 10 | Peanut butter, wood | 12.91 | nd | nd | 3.54 | nd | nd | nd | nd | 1.49 | nd | |
| 2-isoamyl-6-methylpyrazine | h4 | 13925-00-3 | 915 | 915 | 10 | Popcorn | 6.46 | 2.79 | 3.18 | 2.57 | nd | nd | nd | nd | 1.69 | 2.38 | |
| 2-isobutyl-3-methylpyrazine | h5 | 13925-06-9 | 1114 | 1114 | 1 | Burnt, popcorn | nd | 9.50 | 18.06 | 8.33 | nd | nd | nd | nd | nd | nd | |
| 2-methylpyrazine | h6 | 109-08-0 | 1151 | 1150 | 75 | Baked, potato | 0.55 | nd | nd | nd | nd | nd | nd | nd | nd | nd | |
| 3,5-diethyl-2-methylpyrazine | h7 | 18138-05-1 | 1159 | 1159 | 1 | Nut, peanut butter, cocoa | 72.24 | 22.13 | 21.66 | 16.06 | 10.43 | nd | nd | nd | 6.40 | nd | |
| Pyrrole | 1-(furan-2-ylmethyl)pyrrole | i1 | 1438-94-4 | 1185 | 1185 | 5 | Vegetable | 8.76 | nd | nd | nd | nd | nd | nd | nd | nd | nd |
| Terpene | β-myrcene | j1 | 535-77-3 | 1023 | 1023 | 52.5 | Citrus | 8.38 | nd | nd | nd | nd | nd | nd | nd | nd | nd |
| D-limonene | j2 | 5989-27-5 | 1036 | 1035 | 30 | Citrus, orange, fresh, sweet | 1.08 | nd | nd | nd | nd | nd | nd | nd | nd | nd | |
| p-cymene | j3 | 99-87-6 | 1025 | 1025 | 5.5 | Woody, spicy, cumin, oregano | 115.48 | 8.12 | 8.94 | 9.36 | 1.95 | nd | 23.56 | 24.30 | 9.20 | 21.36 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Suffys, S.; Goffin, D.; Richard, G.; Francis, A.; Haubruge, E.; Fauconnier, M.-L. Unveiling the Aromas and Sensory Evaluation of Hakko Sobacha: A New Functional Non-Dairy Probiotic Fermented Drink. Molecules 2023, 28, 6084. https://doi.org/10.3390/molecules28166084
Suffys S, Goffin D, Richard G, Francis A, Haubruge E, Fauconnier M-L. Unveiling the Aromas and Sensory Evaluation of Hakko Sobacha: A New Functional Non-Dairy Probiotic Fermented Drink. Molecules. 2023; 28(16):6084. https://doi.org/10.3390/molecules28166084
Chicago/Turabian StyleSuffys, Sarah, Dorothée Goffin, Gaëtan Richard, Adrien Francis, Eric Haubruge, and Marie-Laure Fauconnier. 2023. "Unveiling the Aromas and Sensory Evaluation of Hakko Sobacha: A New Functional Non-Dairy Probiotic Fermented Drink" Molecules 28, no. 16: 6084. https://doi.org/10.3390/molecules28166084
APA StyleSuffys, S., Goffin, D., Richard, G., Francis, A., Haubruge, E., & Fauconnier, M.-L. (2023). Unveiling the Aromas and Sensory Evaluation of Hakko Sobacha: A New Functional Non-Dairy Probiotic Fermented Drink. Molecules, 28(16), 6084. https://doi.org/10.3390/molecules28166084







