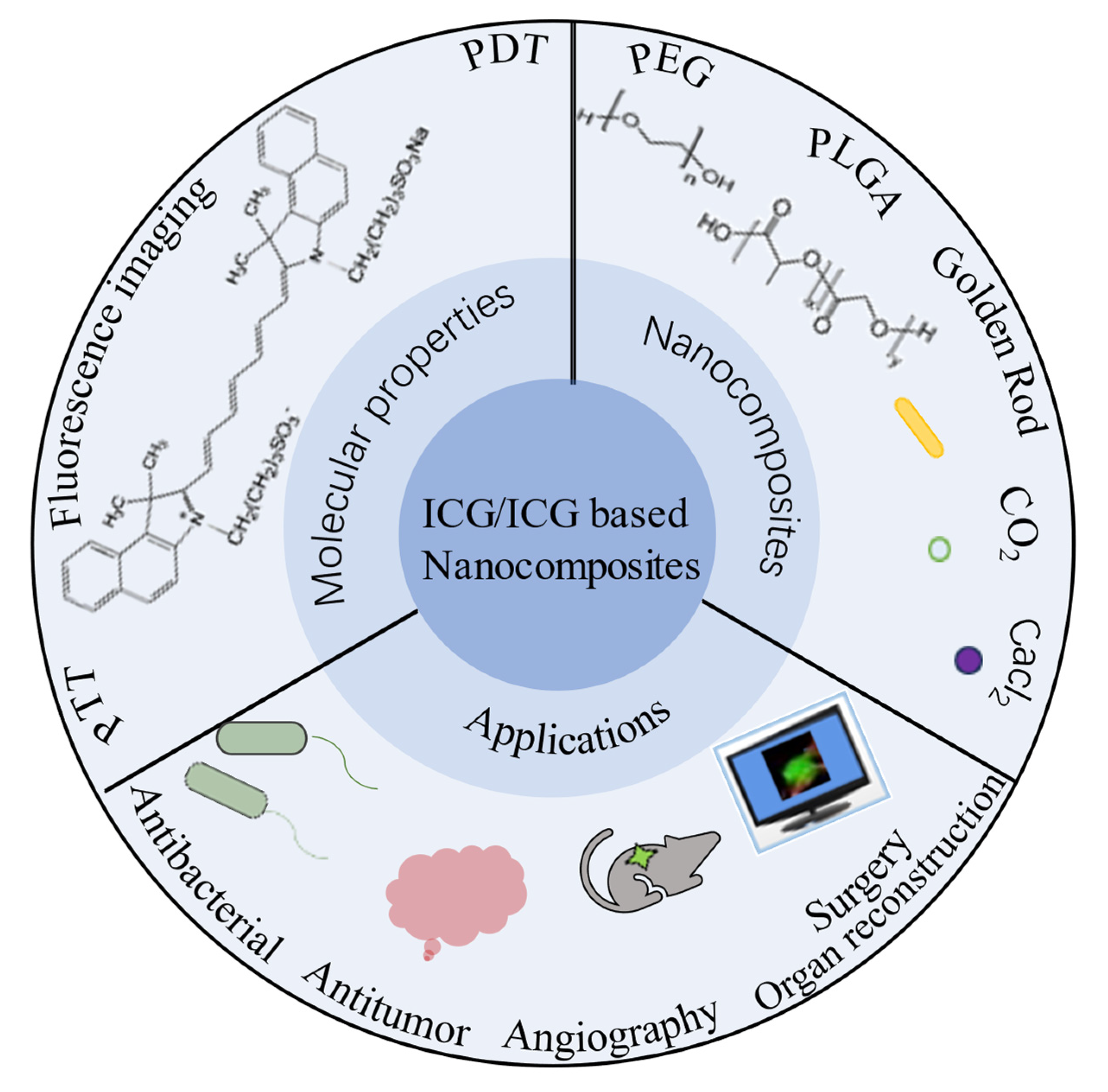
Medical Applications and Advancement of Near Infrared Photosensitive Indocyanine Green Molecules
Abstract
:1. Introduction
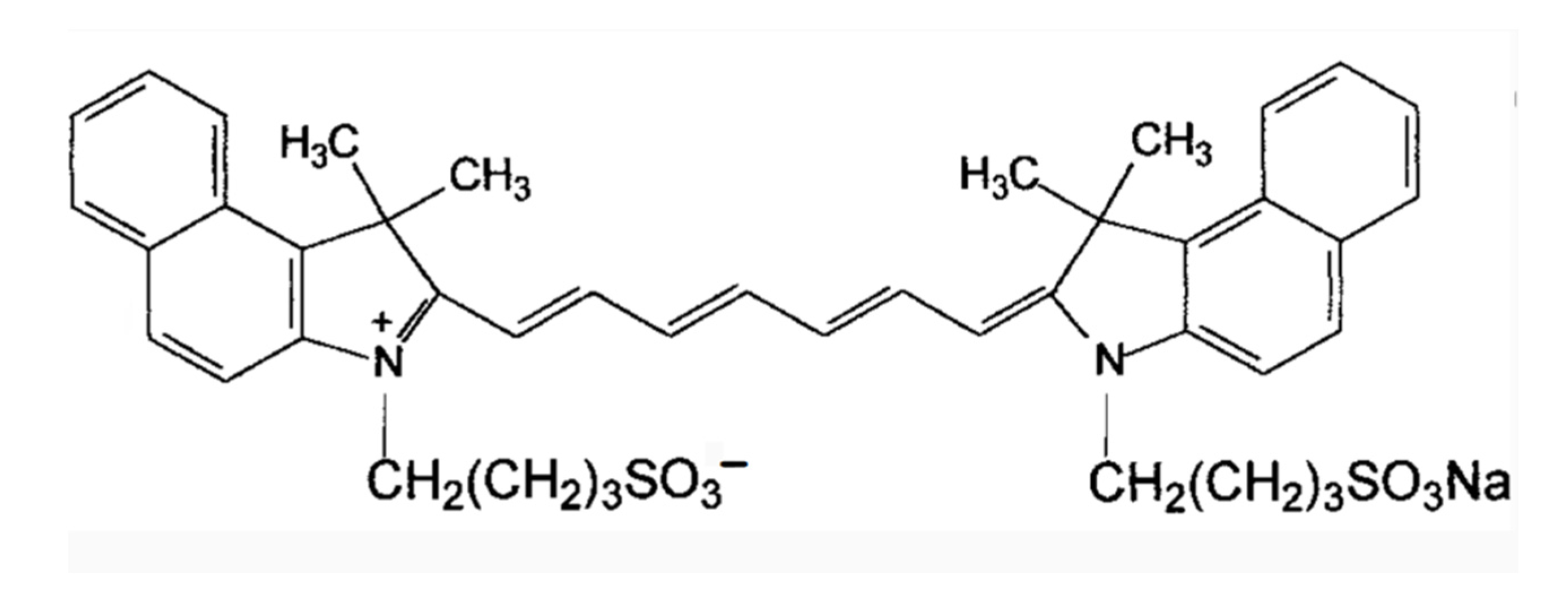
2. Molecular Structure
3. Design of ICG and Composite Nanomaterials
- Enhanced stability in physiological environments;
- Substantially higher photothermal conversion efficiency;
- Prolonged circulation duration in the bloodstream due to the development of nanostructures. Additionally, the enhanced permeability and retention (EPR) effect of solid tumors can potentially facilitate tumor targeting;
- The nanoparticle platform allows for the combination of various diagnostic and therapeutic tools. For instance, simultaneous loading of chemotherapeutic drugs and NIR dyes can be achieved, enabling the concurrent use of photothermal therapy and photothermal-regulated drug therapy to enhance tumor treatment.
3.1. ICG/MOFs Nanoparticles
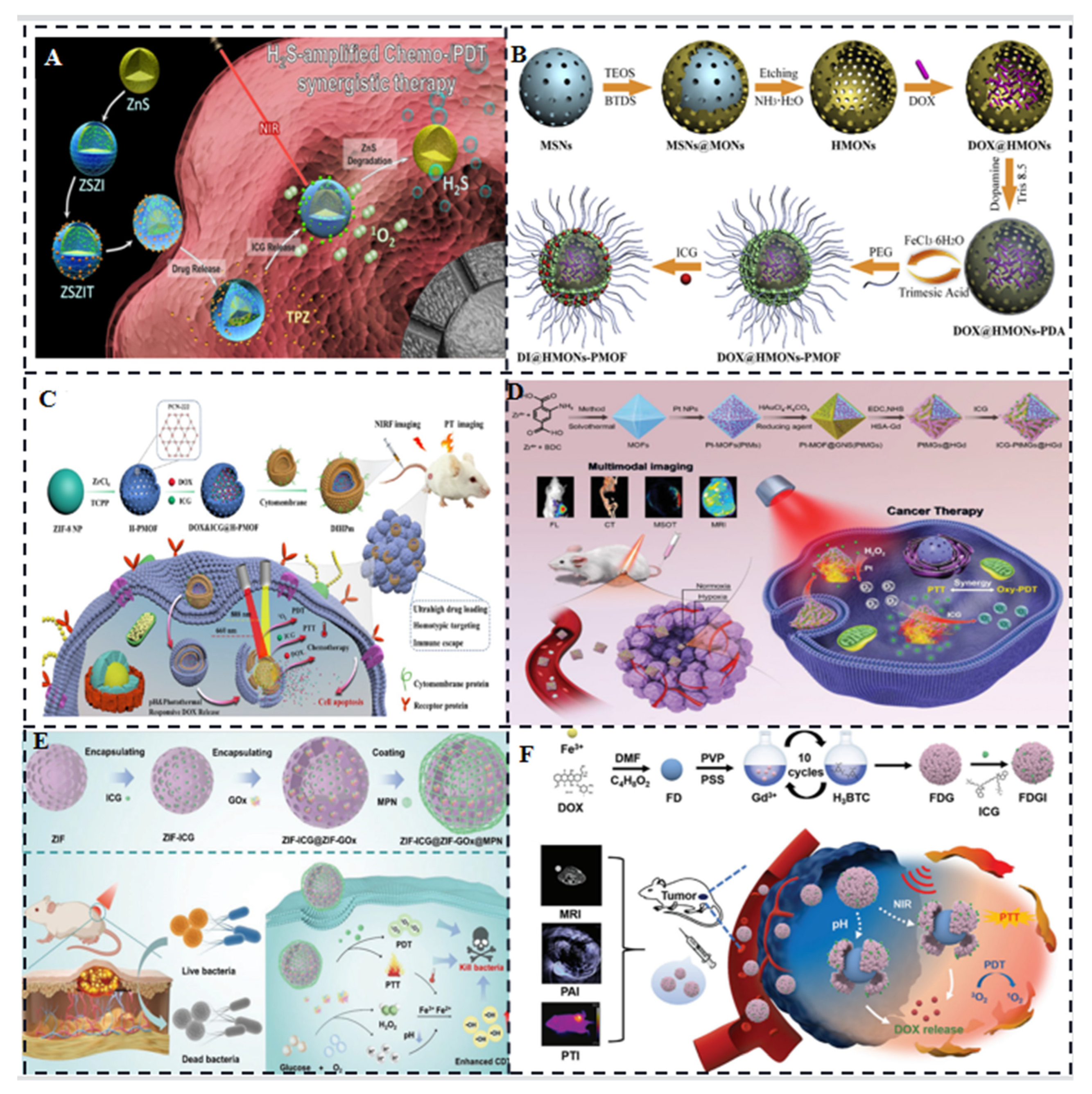
3.2. ICG/Polymers
3.3. Liposome-Coated ICG
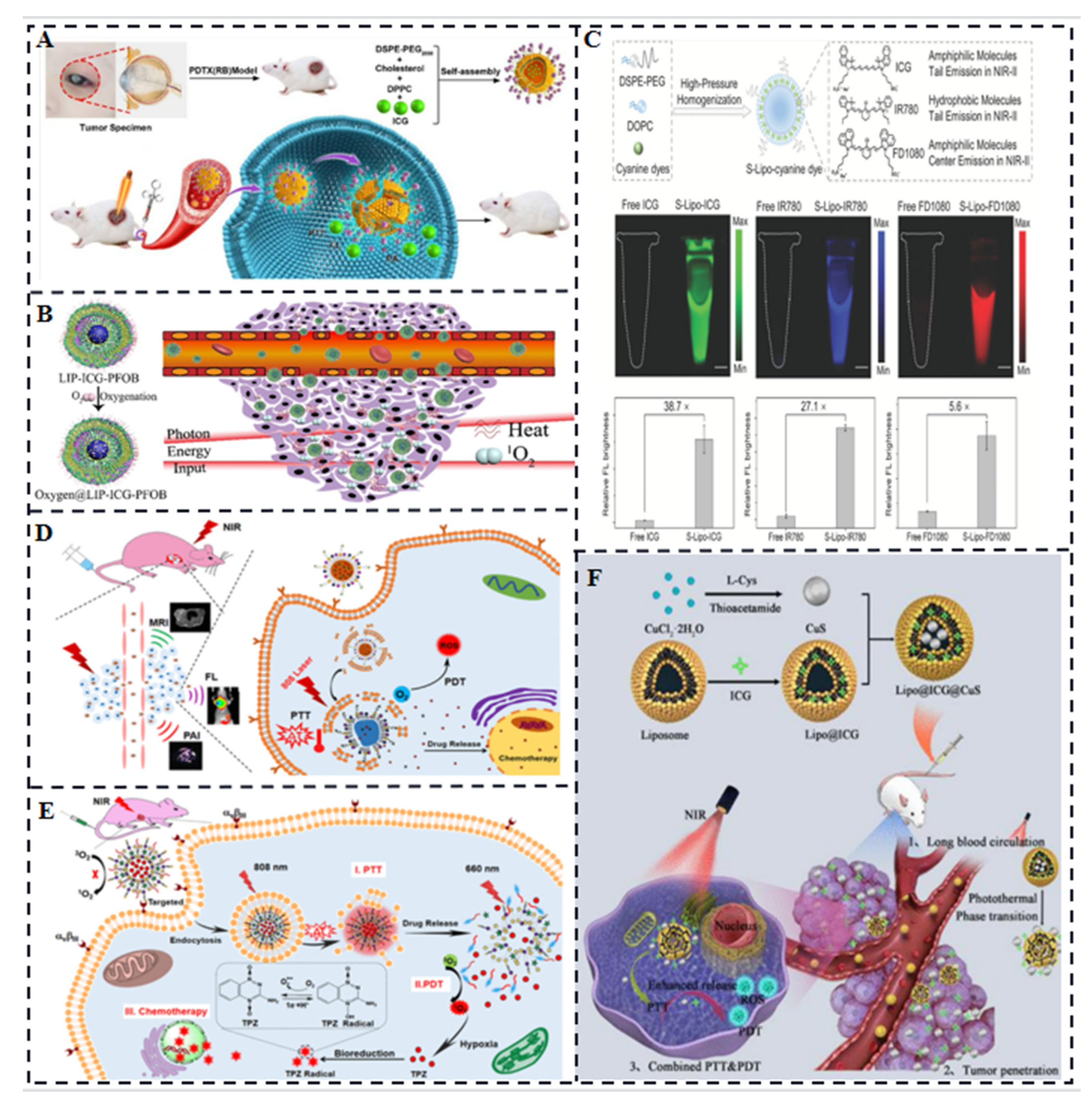
3.4. ICG-Based Micelles Composites
3.5. ICG/Gold Nanocomposites
3.6. ICG/ Silica Nanocomposites
3.7. ICG-Based Multifunctional Composites
4. Imaging and Light Therapy of ICG Molecules
4.1. Imaging and Light Therapy for Tumors

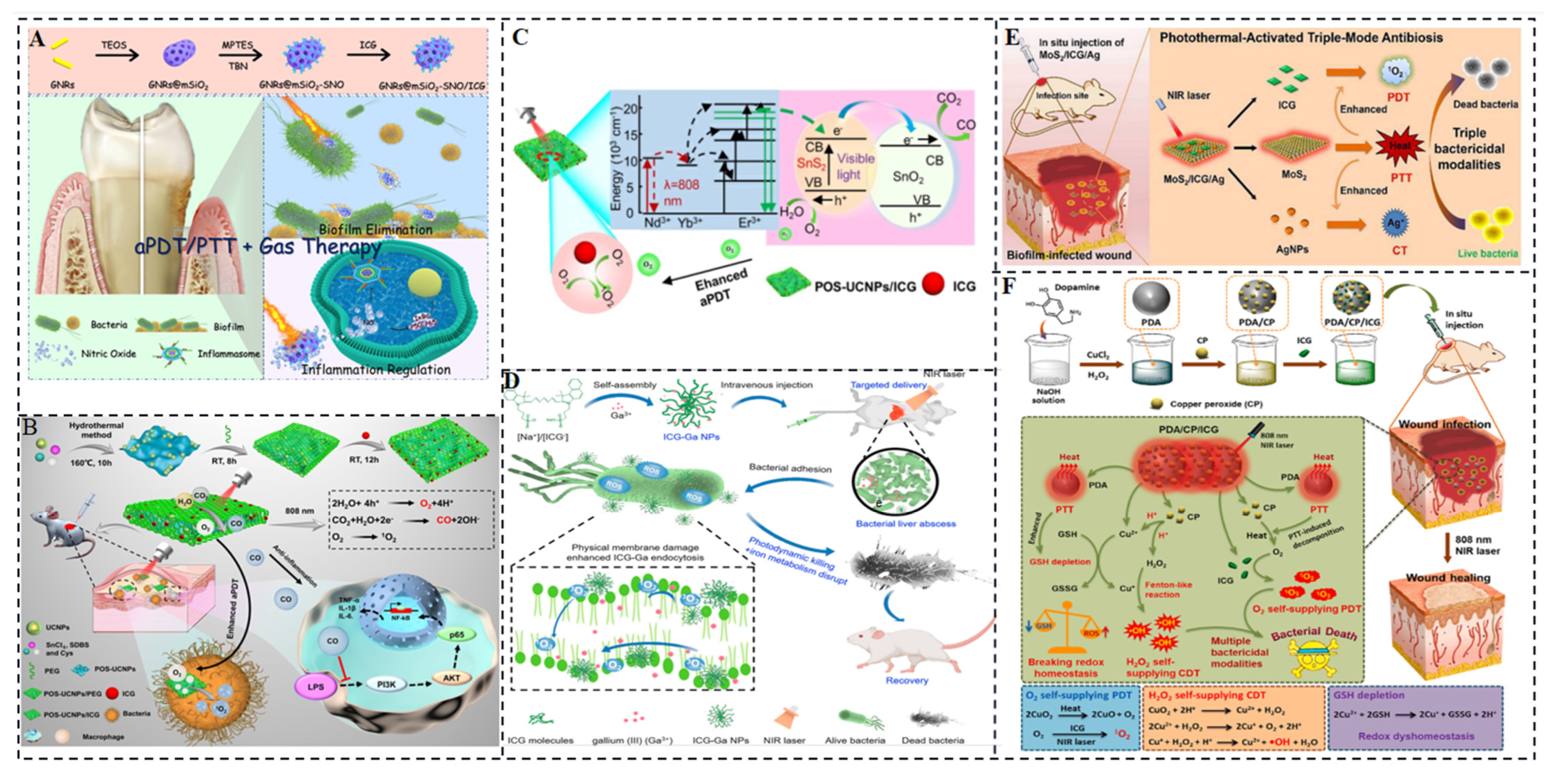
4.2. Antibacterial Phototherapy
5. ICG for Angiography, Surgery, and Organ Reconstruction
5.1. Angiography
5.2. Surgery
5.3. Organ Reconstruction
6. Outlook
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- De Paiva, A.D.C.M.; da Costa Ferreira, M.; da Fonseca, A.D.S. Photodynamic therapy for the treatment of bacterial keratitis. Photodiagn. Photodyn. Ther. 2022, 37, 102717. [Google Scholar] [CrossRef]
- Li, X.; Huang, W.; Zheng, X.; Chang, S.; Liu, C.; Cheng, Q.; Zhu, S. Synergistic in Vitro Effects of Indocyanine Green and Ethylenediamine Tetraacetate-Mediated Antimicrobial Photodynamic Therapy Combined with Antibiotics for Resistant Bacterial Biofilms in Diabetic Foot Infection. Photodiagn. Photodyn. Ther. 2019, 25, 300–308. [Google Scholar] [CrossRef]
- Guo, W.; Ren, Y.; Chen, Z.; Shen, G.; Lu, Y.; Zhou, H.; Li, Z.; Li, Z.; Lu, X.; Li, G.; et al. Targeted Magnetic Resonance Imag-ing/Near-Infrared Dual-Modal Imaging and Ferroptosis/Starvation Therapy of Gastric Cancer with Peritoneal Me-tastasis. Adv. Funct. Mater. 2023, 33, 22139. [Google Scholar] [CrossRef]
- Saxena, V.; Sadoqi, M.; Shao, J. Degradation Kinetics of Indocyanine Green in Aqueous Solution. J. Pharm. Sci. 2003, 92, 2090–2097. [Google Scholar] [CrossRef]
- Higbee-Dempsey, E.; Amirshaghaghi, A.; Case, M.J.; Miller, J.; Busch, T.M.; Tsourkas, A. Indocyanine Green–Coated Gold Nanoclusters for Photoacoustic Imaging and Photothermal Therapy. Adv. Therap. 2019, 2, 1900088. [Google Scholar] [CrossRef]
- Toriumi, N.; Asano, N.; Ikeno, T.; Muranaka, A.; Hanaoka, K.; Urano, Y.; Uchiyama, M. Design of Photostable, Activatable Near-Infrared Photoacoustic Probes Using Tautomeric Benziphthalocyanine as a Platform. Angew. Chem. Int. Ed. 2019, 58, 7788–7791. [Google Scholar] [CrossRef]
- Song, W.; Tang, Z.; Zhang, D.; Burton, N.; Driessen, W.; Chen, X. Comprehensive Studies of Pharmacokinetics and Biodistribution of Indocyanine Green and Liposomal Indocyanine Green by Multispectral Optoacoustic Tomography. RSC Adv. 2015, 5, 3807–3813. [Google Scholar] [CrossRef]
- Wang, H.; Li, X.; Tse, B.W.-C.; Yang, H.; Thorling, C.A.; Liu, Y.; Touraud, M.; Chouane, J.B.; Liu, X.; Roberts, M.S.; et al. Indocyanine Green-Incorporating Nanoparticles for Cancer Theranostics. Theranostics 2018, 8, 1227–1242. [Google Scholar] [CrossRef]
- Landsman, M.L.J.; Kwant, G.; Mordon Mook, J.K.; Zijlstra, G.W. Light-absorbing properties, stability, and spectral stabilization of indocyanine green. J. Appl. Physiol. 1976, 40, 575–583. [Google Scholar] [CrossRef] [PubMed]
- Cheung, C.C.L.; Ma, G.; Karatasos, K.; Seitsonen, J.; Ruokolainen, J.; Koffi, C.-R.; Hassan, H.A.F.M.; Al-Jamal, W.T. Liposome-Templated Indocyanine Green J- Aggregates for In Vivo Near Infrared Imaging and Stable Photothermal Heating. Nanotheranostics 2020, 4, 91–106. [Google Scholar] [CrossRef] [PubMed]
- Sun, C.-Y.; Qin, C.; Wang, X.-L.; Su, Z.-M. Metal-Organic Frameworks as Potential Drug Delivery Systems. Expert. Opin. Drug Deliv. 2013, 10, 89–101. [Google Scholar] [CrossRef] [PubMed]
- Rao, C.; Liao, D.; Pan, Y.; Zhong, Y.; Zhang, W.; Ouyang, Q.; Nezamzadeh-Ejhieh, A.; Liu, J. Novel Formulations of Metal-Organic Frameworks for Controlled Drug Delivery. Expert. Opin. Drug Deliv. 2022, 19, 1183–1202. [Google Scholar] [CrossRef] [PubMed]
- Kang, W.; Tian, Y.; Zhao, Y.; Yin, X.; Teng, Z. Applications of Nanocomposites Based on Zeolitic Imidazolate Framework-8 in Photodynamic and Synergistic Anti-Tumor Therapy. RSC Adv. 2022, 12, 16927–16941. [Google Scholar] [CrossRef] [PubMed]
- Fang, C.; Cen, D.; Wang, Y.; Wu, Y.; Cai, X.; Li, X.; Han, G. ZnS@ZIF-8 Core-Shell Nanoparticles Incorporated with ICG and TPZ to Enable H2S-Amplified Synergistic Therapy. Theranostics 2020, 10, 7671–7682. [Google Scholar] [CrossRef]
- Chen, L.; Zhang, J.; Zhou, X.; Yang, S.; Zhang, Q.; Wang, W.; You, Z.; Peng, C.; He, C. Merging Metal Organic Framework with Hollow Organosilica Nanoparticles as a Versatile Nanoplatform for Cancer Theranostics. Acta Biomater. 2019, 86, 406–415. [Google Scholar] [CrossRef]
- Sun, X.; He, G.; Xiong, C.; Wang, C.; Lian, X.; Hu, L.; Li, Z.; Dalgarno, S.J.; Yang, Y.-W.; Tian, J. One-Pot Fabrication of Hollow Porphyrinic MOF Nanoparticles with Ultrahigh Drug Loading toward Controlled Delivery and Synergistic Cancer Therapy. ACS Appl. Mater. Interfaces 2021, 13, 3679–3693. [Google Scholar] [CrossRef]
- Jiang, Z.; Pan, Y.; Wang, J.; Li, J.; Yang, H.; Guo, Q.; Liang, S.; Chen, S.; Hu, Y.; Wang, L. Bone-Targeted ICG/Cyt C@ZZF-8 Nanoparticles Based on the Zeolitic Imidazolate Framework-8: A New Synergistic Photodynamic and Protein Therapy for Bone Metastasis. Biomater. Sci. 2022, 10, 2345–2357. [Google Scholar] [CrossRef]
- You, Q.; Zhang, K.; Liu, J.; Liu, C.; Wang, H.; Wang, M.; Ye, S.; Gao, H.; Lv, L.; Wang, C.; et al. Persistent Regulation of Tumor Hypoxia Microenvironment via a Bioinspired Pt-Based Oxygen Nanogenerator for Multimodal Imaging-Guided Synergistic Phototherapy. Adv. Sci. 2020, 7, 1903341. [Google Scholar] [CrossRef]
- Fu, J.; Zhou, Y.; Liu, T.; Wang, W.; Zhao, Y.; Sun, Y.; Zhang, Y.; Qin, W.; Chen, Z.; Lu, C.; et al. A triple-enhanced chemodynamic approach based on glucose-powered hybrid nanoreactors for effective bacteria killing. Nano Res. 2023, 16, 2682–2694. [Google Scholar] [CrossRef]
- Zhu, Y.; Qiao, Z.; Chen, S.; Zeng, L.; Zhang, Y.; Dan, W.; Sun, J.; Fan, H. Bioactive MOFs Based Theranositc Agent for Higly Effective Combination of Multimodal Imaging and Chemo-Phototherapy. Adv. Healthc. Mater. 2020, 9, 2192–2659. [Google Scholar] [CrossRef]
- Han, Y.-H.; Kankala, R.K.; Wang, S.-B.; Chen, A.-Z. Leveraging Engineering of Indocyanine Green-Encapsulated Polymeric Nanocomposites for Biomedical Applications. Nanomaterials 2018, 8, 360. [Google Scholar] [CrossRef] [PubMed]
- Chopra, A. Folic Acid-Indocyanine Green-Poly(d,l-Lactide-Coglycolide)-Lipid Nanoparticles. In Molecular Imaging and Contrast Agent Database (MICAD); National Center for Biotechnology Information (US): Bethesda, MD, USA, 2004. [Google Scholar]
- Hadinoto, K.; Sundaresan, A.; Cheow, W.S. Lipid-Polymer Hybrid Nanoparticles as a New Generation Therapeutic Delivery Platform: A Review. Eur. J. Pharm. Biopharm. 2013, 85 Pt A, 427–443. [Google Scholar] [CrossRef] [PubMed]
- Zheng, C.; Zheng, M.; Gong, P.; Jia, D.; Zhang, P.; Shi, B.; Sheng, Z.; Ma, Y.; Cai, L. Indocyanine Green-Loaded Biodegradable Tumor Targeting Nanoprobes for in Vitro and in Vivo Imaging. Biomaterials 2012, 33, 5603–5609. [Google Scholar] [CrossRef] [PubMed]
- Zhao, P.; Zheng, M.; Yue, C.; Luo, Z.; Gong, P.; Gao, G.; Sheng, Z.; Zheng, C.; Cai, L. Improving Drug Accumulation and Photothermal Efficacy in Tumor Depending on Size of ICG Loaded Lipid-Polymer Nanoparticles. Biomaterials 2014, 35, 6037–6046. [Google Scholar] [CrossRef]
- Zheng, M.; Yue, C.; Ma, Y.; Gong, P.; Zhao, P.; Zheng, C.; Sheng, Z.; Zhang, P.; Wang, Z.; Cai, L. Single-Step Assembly of DOX/ICG Loaded Lipid--Polymer Nanoparticles for Highly Effective Chemo-Photothermal Combination Therapy. ACS Nano 2013, 7, 2056–2067. [Google Scholar] [CrossRef]
- Bahmani, B.; Lytle, C.Y.; Walker, A.M.; Gupta, S.; Vullev, V.I.; Anvari, B. Effects of Nanoencapsulation and PEGylation on Biodistribution of Indocyanine Green in Healthy Mice: Quantitative Fluorescence Imaging and Analysis of Organs. Int. J. Nanomed. 2013, 8, 1609–1620. [Google Scholar]
- Wu, L.; Fang, S.; Shi, S.; Deng, J.; Liu, B.; Cai, L. Hybrid Polypeptide Micelles Loading Indocyanine Green for Tumor Imaging and Photothermal Effect Study. Biomacromolecules 2013, 14, 3027–3033. [Google Scholar] [CrossRef]
- Liu, Y.; Han, Y.; Chen, S.; Liu, J.; Wang, D.; Huang, Y. Liposome-Based Multifunctional Nanoplatform as Effective Therapeutics for the Treatment of Retinoblastoma. Acta Pharm. Sin. B 2022, 12, 2731–2739. [Google Scholar] [CrossRef]
- Sheng, D.; Liu, T.; Deng, L.; Zhang, L.; Li, X.; Xu, J.; Hao, L.; Li, P.; Ran, H.; Chen, H.; et al. Perfluorooctyl Bromide & Indocyanine Green Co-Loaded Nanoliposomes for Enhanced Multimodal Imaging-Guided Phototherapy. Biomaterials 2018, 165, 1–13. [Google Scholar]
- Li, W.; Yang, J.; Luo, L.; Jiang, M.; Qin, B.; Yin, H.; Zhu, C.; Yuan, X.; Zhang, J.; Luo, Z.; et al. Targeting Photodynamic and Photothermal Therapy to the Endoplasmic Reticulum Enhances Immunogenic Cancer Cell Death. Nat. Commun. 2019, 10, 3349. [Google Scholar] [CrossRef]
- Gao, D.; Luo, Z.; He, Y.; Yang, L.; Hu, D.; Liang, Y.; Zheng, H.; Liu, X.; Sheng, Z. Low-Dose NIR-II Preclinical Bioimaging Using Liposome-Encapsulated Cyanine Dyes. Small 2023, 19, e2206544. [Google Scholar] [CrossRef]
- Dai, Y.; Wang, B.; Sun, Z.; Cheng, J.; Zhao, H.; Wu, K.; Sun, P.; Shen, Q.; Li, M.; Fan, Q. Multifunctional Theranostic Liposomes Loaded with a Hypoxia-Activated Prodrug for Cascade-Activated Tumor Selective Combination Therapy. ACS Appl. Mater. Interfaces 2019, 11, 39410–39423. [Google Scholar] [CrossRef]
- Dai, Y.; Su, J.; Wu, K.; Ma, W.; Wang, B.; Li, M.; Sun, P.; Shen, Q.; Wang, Q.; Fan, Q. Multifunctional Thermosensitive Liposomes Based on Natural Phase-Change Material: Near-Infrared Light-Triggered Drug Release and Multimodal Imaging-Guided Cancer Combination Therapy. ACS Appl. Mater. Interfaces 2019, 11, 10540–10553. [Google Scholar] [CrossRef]
- Gao, F.; Jiang, L.; Zhang, J.; Chang, Y.; Gao, W.; Ding, L.; Ma, G.; Ma, X.; Guo, Y. Near-Infrared Light-Responsive Nanosystem with Prolonged Circulation and Enhanced Penetration for Increased Photothermal and Photodynamic Therapy. ACS Mater. Lett. 2023, 5, 1–10. [Google Scholar] [CrossRef]
- Zhang, L.; Qin, Y.; Zhang, Z.; Fan, F.; Huang, C.; Lu, L.; Wang, H.; Jin, X.; Zhao, H.; Kong, D.; et al. Dual PH/Reduction-Responsive Hybrid Polymeric Micelles for Targeted Chemo-Photothermal Combination Therapy. Acta Biomater. 2018, 75, 371–385. [Google Scholar] [CrossRef]
- Mundra, V.; Peng, Y.; Rana, S.; Natarajan, A.; Mahato, R.I. Micellar Formulation of Indocyanine Green for Phototherapy of Melanoma. J. Control. Release 2015, 220, 130–140. [Google Scholar] [CrossRef]
- Yang, L.; Hou, X.; Zhang, Y.; Wang, D.; Liu, J.; Huang, F.; Liu, J. NIR-Activated Self-Sensitized Polymeric Micelles for Enhanced Cancer Chemo-Photothermal Therapy. J. Control. Release 2021, 339, 114–129. [Google Scholar] [CrossRef]
- Zhou, T.; Wu, L.; Ma, N.; Tang, F.; Chen, J.; Jiang, Z.; Li, Y.; Ma, T.; Yang, N.; Zong, Z. Photothermally Responsive Theranostic Nanocomposites for Near-infrared Light Triggered Drug Release and Enhanced Synergism of Photothermo-chemotherapy for Gastric Cancer. Bioeng. Transl. Med. 2022, 8, e10368. [Google Scholar] [CrossRef]
- Su, Y.; Liu, Y.; Xu, X.; Zhou, J.; Xu, L.; Xu, X.; Wang, D.; Li, M.; Chen, K.; Wang, W. On-Demand Versatile Prodrug Nanomicelle for Tumor-Specific Bioimaging and Photothermal-Chemo Synergistic Cancer Therapy. ACS Appl. Mater. Interfaces 2018, 10, 38700–38714. [Google Scholar] [CrossRef]
- Wei, R.; Jiang, G.; Lv, M.; Tan, S.; Wang, X.; Zhou, Y.; Cheng, T.; Gao, X.; Chen, X.; Wang, W.; et al. TMTP1-Modified Indocyanine Green-Loaded Polymeric Micelles for Targeted Imaging of Cervical Cancer and Metastasis Sentinel Lymph Node in Vivo. Theranostics 2019, 9, 7325–7344. [Google Scholar] [CrossRef]
- Kuo, W.-S.; Chang, Y.-T.; Cho, K.-C.; Chiu, K.-C.; Lien, C.-H.; Yeh, C.-S.; Chen, S.-J. Gold Nanomaterials Conjugated with Indocyanine Green for Dual-Modality Photodynamic and Photothermal Therapy. Biomaterials 2012, 33, 3270–3278. [Google Scholar] [CrossRef]
- Li, W.; Zhang, H.; Guo, X.; Wang, Z.; Kong, F.; Luo, L.; Li, Q.; Zhu, C.; Yang, J.; Lou, Y.; et al. Gold Nanospheres-Stabilized Indocyanine Green as a Synchronous Photodynamic-Photothermal Therapy Platform That Inhibits Tumor Growth and Metastasis. ACS Appl. Mater. Interfaces 2017, 9, 3354–3367. [Google Scholar] [CrossRef]
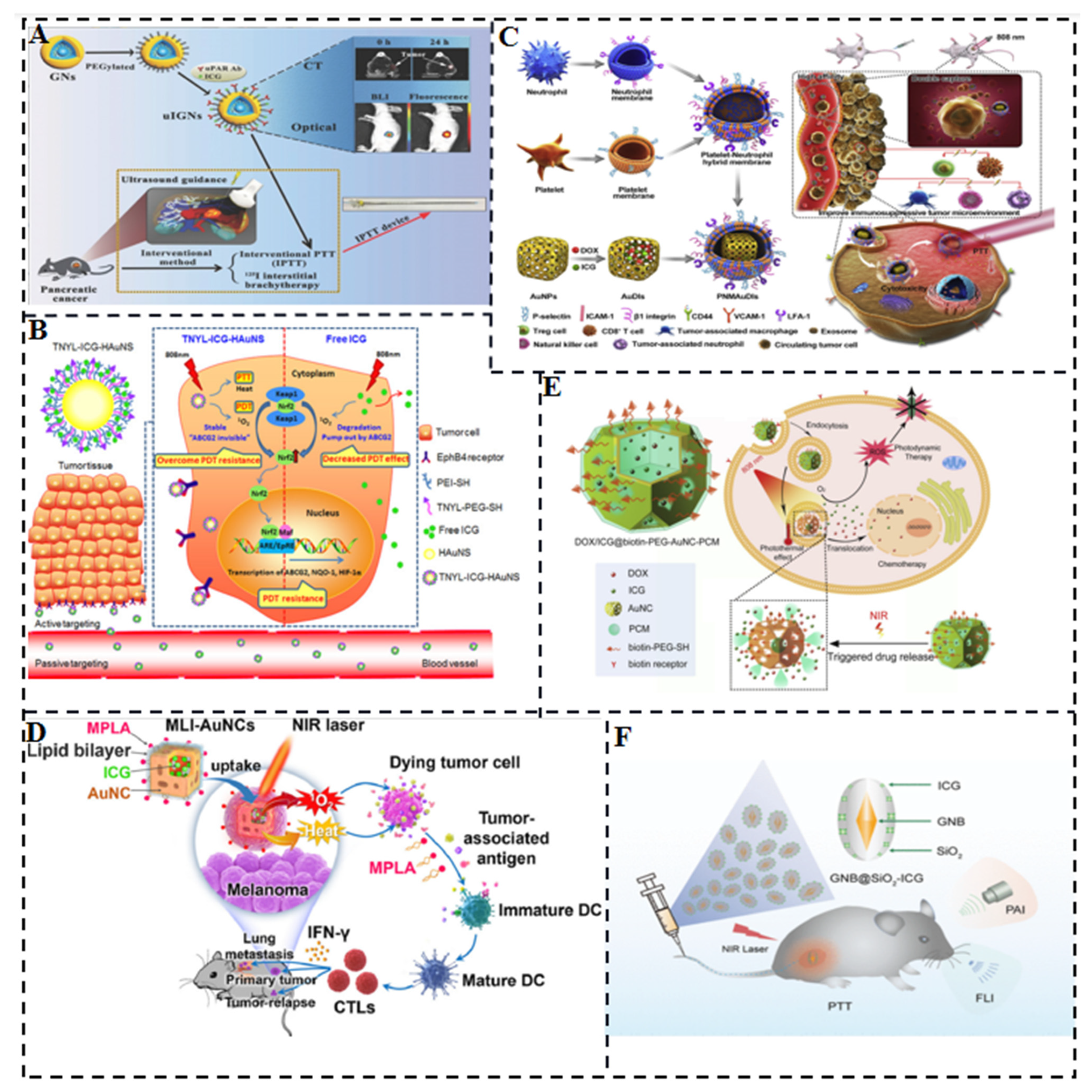
- Hu, Y.; Chi, C.; Wang, L.; Liang, P.; Liu, F.; Shang, W.; Wang, W.; Zhang, F.; Li, S.; Shen, H.; et al. A Comparative Study of Clinical Intervention and Interventional Photothermal Therapy for Pancreatic Cancer. Adv. Mater. 2017, 29, 170048. [Google Scholar] [CrossRef]
- Li, W.; Guo, X.; Kong, F.; Zhang, H.; Luo, L.; Li, Q.; Zhu, C.; Yang, J.; Du, Y.; You, J. Overcoming Photodynamic Resistance and Tumor Targeting Dual-Therapy Mediated by Indocyanine Green Conjugated Gold Nanospheres. J. Control. Release 2017, 258, 171–181. [Google Scholar] [CrossRef]
- Ye, H.; Wang, K.; Lu, Q.; Zhao, J.; Wang, M.; Kan, Q.; Zhang, H.; Wang, Y.; He, Z.; Sun, J. Nanosponges of Circulating Tumor-Derived Exosomes for Breast Cancer Metastasis Inhibition. Biomaterials 2020, 242, 119932. [Google Scholar] [CrossRef]
- Xie, J.; Liang, R.; Li, Q.; Wang, K.; Hussain, M.; Dong, L.; Shen, C.; Li, H.; Shen, G.; Zhu, J.; et al. Photosensitizer-Loaded Gold Nanocages for Immunogenic Phototherapy of Aggressive Melanoma. Acta Biomater. 2022, 142, 264–273. [Google Scholar] [CrossRef]
- Yu, Y.; Zhang, Z.; Wang, Y.; Zhu, H.; Li, F.; Shen, Y.; Guo, S. A New NIR-Triggered Doxorubicin and Photosensitizer Indocyanine Green Co-Delivery System for Enhanced Multidrug Resistant Cancer Treatment through Simultaneous Chemo/Photothermal/Photodynamic Therapy. Acta Biomater. 2017, 59, 170–180. [Google Scholar] [CrossRef]
- Li, C.; Mei, E.; Chen, C.; Li, Y.; Nugasur, B.; Hou, L.; Ding, X.; Hu, M.; Zhang, Y.; Su, Z.; et al. Gold-Nanobipyramid-Based Nanotheranostics for Dual-Modality Imaging-Guided Phototherapy. ACS Appl. Mater. Interfaces 2020, 12, 12541–12548. [Google Scholar] [CrossRef]
- Kang, J.; Wang, J.; Hariri, A.; Kim, D.; Han, Y.; Park, J.-H.; Zuidema, J.M.; Jokerst, J.V.; Sailor, M.J. Enhanced Performance of a Molecular Photoacoustic Imaging Agent by Encapsulation in Mesoporous Silicon Nanoparticles. Adv. Mater. 2018, 30, e1800512. [Google Scholar] [CrossRef]
- Peng, D.; Du, Y.; Shi, Y.; Mao, D.; Jia, X.; Li, H.; Zhu, Y.; Wang, K.; Tian, J. Precise Diagnosis in Different Scenarios Using Photoacoustic and Fluorescence Imaging with Dual-Modality Nanoparticles. Nanoscale 2016, 8, 14480–14488. [Google Scholar] [CrossRef]
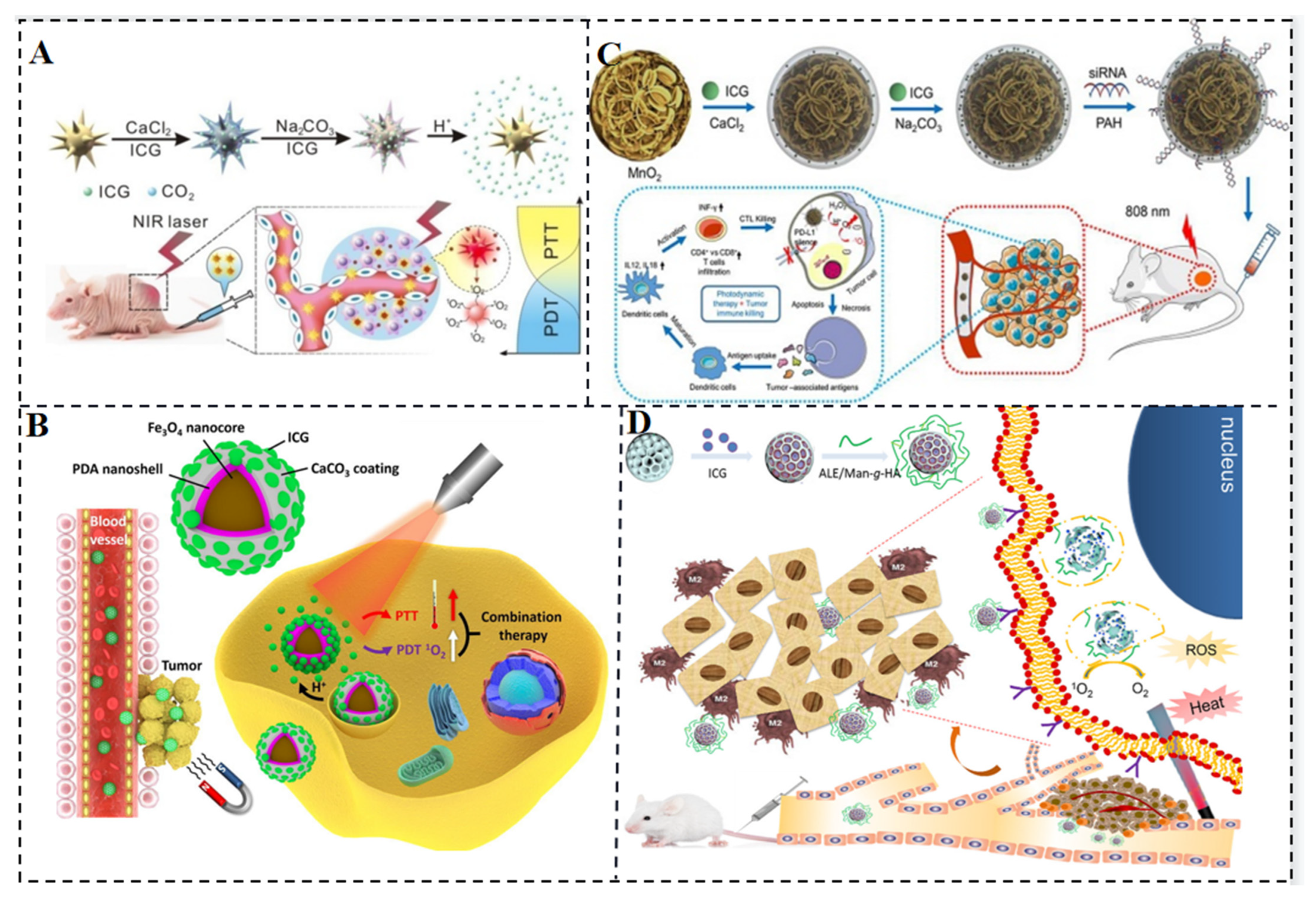
- Xue, P.; Hou, M.; Sun, L.; Li, Q.; Zhang, L.; Xu, Z.; Kang, Y. Calcium-Carbonate Packaging Magnetic Polydopamine Nanoparticles Loaded with Indocyanine Green for near-Infrared Induced Photothermal/Photodynamic Therapy. Acta Biomater. 2018, 81, 242–255. [Google Scholar] [CrossRef]
- Huang, X.; Wu, J.; He, M.; Hou, X.; Wang, Y.; Cai, X.; Xin, H.; Gao, F.; Chen, Y. Combined Cancer Chemo-Photodynamic and Photothermal Therapy Based on ICG/PDA/TPZ-Loaded Nanoparticles. Mol. Pharm. 2019, 16, 2172–2183. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Pan, Y.; Cao, W.; Xia, F.; Liu, B.; Niu, J.; Alfranca, G.; Sun, X.; Ma, L.; de la Fuente, J.M.; et al. A Tumor Microenvironment Responsive Biodegradable CaCO3/MnO2- Based Nanoplatform for the Enhanced Photodynamic Therapy and Improved PD-L1 Immunotherapy. Theranostics 2019, 9, 6867–6884. [Google Scholar] [CrossRef] [PubMed]
- Gao, S.; Liu, Y.; Liu, M.; Yang, D.; Zhang, M.; Shi, K. Biodegradable Mesoporous Nanocomposites with Dual-Targeting Function for Enhanced Anti-Tumor Therapy. J. Control. Release 2022, 341, 383–398. [Google Scholar] [CrossRef]
- Deng, H.; Zhou, Z.; Yang, W.; Lin, L.-S.; Wang, S.; Niu, G.; Song, J.; Chen, X. Endoplasmic Reticulum Targeting to Amplify Immunogenic Cell Death for Cancer Immunotherapy. Nano Lett. 2020, 20, 1928–1933. [Google Scholar] [CrossRef] [PubMed]
- Ma, H.; Lu, Y.; Huang, Z.; Long, S.; Cao, J.; Zhang, Z.; Zhou, X.; Shi, C.; Sun, W.; Du, J.; et al. ER-Targeting Cyanine Dye as an NIR Photoinducer to Efficiently Trigger Photoimmunogenic Cancer Cell Death. J. Am. Chem. Soc. 2022, 144, 3477–3486. [Google Scholar] [CrossRef]
- Dai, L.; Yu, Y.; Luo, Z.; Li, M.; Chen, W.; Shen, X.; Chen, F.; Sun, Q.; Zhang, Q.; Gu, H.; et al. Photosensitizer Enhanced Disassembly of Amphiphilic Micelle for ROS-Response Targeted Tumor Therapy in Vivo. Biomaterials 2016, 104, 1–17. [Google Scholar] [CrossRef]
- Chen, Y.; Li, Y.; Liu, J.; Zhu, Q.; Ma, J.; Zhu, X. Erythrocyte Membrane Bioengineered Nanoprobes via Indocyanine Green-Directed Assembly for Single NIR Laser-Induced Efficient Photodynamic/Photothermal Theranostics. J. Control. Release 2021, 335, 345–358. [Google Scholar] [CrossRef]
- Du, B.; Chong, Y.; Jiang, X.; Yu, M.; Lo, U.-G.; Dang, A.; Chen, Y.-A.; Li, S.; Hernandez, E.; Lin, J.C.; et al. Hyperfluorescence Imaging of Kidney Cancer Enabled by Renal Secretion Pathway Dependent Efflux Transport. Angew. Chem. Int. Ed. Engl. 2021, 60, 351–359. [Google Scholar] [CrossRef]
- Naito, S.; Sakamoto, N.; Kotoh, S.; Goto, K.; Matsumoto, T.; Kumazawa, J. Expression of P-Glycoprotein and Multidrug Resistance in Renal Cell Carcinoma. Eur. Urol. 1993, 24, 156–160. [Google Scholar]
- Bianchi, M.; Sun, M.; Jeldres, C.; Shariat, S.F.; Trinh, Q.-D.; Briganti, A.; Tian, Z.; Schmitges, J.; Graefen, M.; Perrotte, P.; et al. Distribution of Metastatic Sites in Renal Cell Carcinoma: A Population-Based Analysis. Ann. Oncol. 2012, 23, 973–980. [Google Scholar] [CrossRef]
- Liu, R.; Tang, J.; Xu, Y.; Zhou, Y.; Dai, Z. Nano-Sized Indocyanine Green J-Aggregate as a One-Component Theranostic Agent. Nanotheranostics 2017, 1, 430–439. [Google Scholar] [CrossRef] [PubMed]
- Farrakhova, D.; Maklygina, Y.; Romanishkin, I.; Yakovlev, D.; Plyutinskaya, A.; Bezdetnaya, L.; Loschenov, V. Fluorescence Imaging Analysis of Distribution of Indocyanine Green in Molecular and Nanoform in Tumor Model. Photodiagn. Photodyn. Ther. 2022, 37, 102636. [Google Scholar] [CrossRef] [PubMed]
- Kraft, J.C.; Ho, R.J.Y. Interactions of Indocyanine Green and Lipid in Enhancing Near-Infrared Fluorescence Properties: The Basis for near-Infrared Imaging in Vivo. Biochemistry 2014, 53, 1275–1283. [Google Scholar] [CrossRef]
- Bishnoi, S.; Rehman, S.; Dutta, S.B.; De, S.K.; Chakraborty, A.; Nayak, D.; Gupta, S. Optical-Property-Enhancing Novel Near-Infrared Active Niosome Nanoformulation for Deep-Tissue Bioimaging. ACS Omega 2021, 6, 22616–22624. [Google Scholar] [CrossRef]
- Wood, C.A.; Han, S.; Kim, C.S.; Wen, Y.; Sampaio, D.R.T.; Harris, J.T.; Homan, K.A.; Swain, J.L.; Emelianov, S.Y.; Sood, A.K.; et al. Clinically Translatable Quantitative Molecular Photoacoustic Imaging with Liposome-Encapsulated ICG J-Aggregates. Nat. Commun. 2021, 12, 5410. [Google Scholar] [CrossRef]
- Zhang, R.; Li, Y.; Zhou, M.; Wang, C.; Feng, P.; Miao, W.; Huang, H. Photodynamic Chitosan Nano-Assembly as a Potent Alternative Candidate for Combating Antibiotic-Resistant Bacteria. ACS Appl. Mater. Interfaces 2019, 11, 26711–26721. [Google Scholar] [CrossRef]
- Gnanasekar, S.; Kasi, G.; He, X.; Zhang, K.; Xu, L.; Kang, E.-T. Recent Advances in Engineered Polymeric Materials for Efficient Photodynamic Inactivation of Bacterial Pathogens. Bioact. Mater. 2023, 21, 157–174. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Lu, S.; Liu, W.; Dai, T.; Ke, J.; Li, X.; Li, R.; Zhang, Y.; Chen, Z.; Chen, X. Synergistic Lysozyme-Photodynamic Therapy Against Resistant Bacteria Based on an Intelligent Upconversion Nanoplatform. Angew. Chem. Int. Ed. Engl. 2021, 60, 19201–19206. [Google Scholar] [CrossRef]
- Casu, C.; Orrù, G.; Scano, A. Curcumin/H2O2 Photodynamically Activated: An Antimicrobial Time-Response Assessment against an MDR Strain of Candida Albicans. Eur. Rev. Med. Pharmacol. Sci. 2022, 26, 8841–8851. [Google Scholar] [PubMed]
- Detty, M.R.; Gibson, S.L.; Wagner, S.J. Current Clinical and Preclinical Photosensitizers for Use in Photodynamic Therapy. J. Med. Chem. 2004, 47, 3897–3915. [Google Scholar] [CrossRef]
- Schmitt, J.; Heitz, V.; Sour, A.; Bolze, F.; Ftouni, H.; Nicoud, J.-F.; Flamigni, L.; Ventura, B. Diketopyrrolopyrrole-Porphyrin Conjugates with High Two-Photon Absorption and Singlet Oxygen Generation for Two-Photon Photodynamic Therapy. Angew. Chem. Int. Ed. Engl. 2015, 54, 169–173. [Google Scholar] [CrossRef]
- Sahu, A.; Choi, W.I.; Lee, J.H.; Tae, G. Graphene Oxide Mediated Delivery of Methylene Blue for Combined Photodynamic and Photothermal Therapy. Biomaterials 2013, 34, 6239–6248. [Google Scholar] [CrossRef] [PubMed]
- Yue, L.; Zheng, M.; Wang, M.; Khan, I.M.; Ding, X.; Zhang, Y.; Wang, Z. Water-Soluble Chlorin E6-Hydroxypropyl Chitosan as a High-Efficiency Photoantimicrobial Agent against Staphylococcus Aureus. Int. J. Biol. Macromol. 2022, 208, 669–677. [Google Scholar] [CrossRef] [PubMed]
- Sun, S.; Chen, J.; Jiang, K.; Tang, Z.; Wang, Y.; Li, Z.; Liu, C.; Wu, A.; Lin, H. Ce6-Modified Carbon Dots for Multimodal-Imaging-Guided and Single-NIR-Laser-Triggered Photothermal/Photodynamic Synergistic Cancer Therapy by Reduced Irradiation Power. ACS Appl. Mater. Interfaces 2019, 11, 5791–5803. [Google Scholar] [CrossRef] [PubMed]
- Qi, M.; Ren, X.; Li, W.; Sun, Y.; Sun, X.; Li, C.; Yu, S.; Xu, L.; Zhou, Y.; Song, S.; et al. NIR Responsive Nitric Oxide Nanogenerator for Enhanced Biofilm Eradication and Inflammation Immunotherapy against Periodontal Diseases. Nano Today 2022, 43, 101447. [Google Scholar] [CrossRef]
- Zhou, B.; Sun, X.; Dong, B.; Yu, S.; Cheng, L.; Hu, S.; Liu, W.; Xu, L.; Bai, X.; Wang, L.; et al. Antibacterial PDT Nanoplatform Capable of Releasing Therapeutic Gas for Synergistic and Enhanced Treatment against Deep Infections. Theranostics 2022, 12, 2580–2597. [Google Scholar] [CrossRef]
- Feng, L.; Chen, M.; Li, R.; Zhou, L.; Wang, C.; Ye, P.; Hu, X.; Yang, J.; Sun, Y.; Zhu, Z.; et al. Biodegradable Oxygen-Producing Manganese-Chelated Metal Organic Frameworks for Tumor-Targeted Synergistic Chemo/Photothermal/Photodynamic Therapy. Acta Biomater. 2022, 138, 463–477. [Google Scholar] [CrossRef]
- Dutta, D.; Wang, J.; Li, X.; Zhou, Q.; Ge, Z. Covalent Organic Framework Nanocarriers of Singlet Oxygen for Oxygen-Independent Concurrent Photothermal/Photodynamic Therapy to Ablate Hypoxic Tumors. Small 2022, 18, e2202369. [Google Scholar] [CrossRef]
- Zheng, X.; Zhang, L.; Ju, M.; Liu, L.; Ma, C.; Huang, Y.; Wang, B.; Ding, W.; Luan, X.; Shen, B. Rational Modulation of BODIPY Photosensitizers to Design Metal-Organic Framework-Based NIR Nanocomposites for High-Efficiency Photodynamic Therapy in a Hypoxic Environment. ACS Appl. Mater. Interfaces 2022, 14, 46262–46272. [Google Scholar] [CrossRef]
- Zhang, P.; Wu, Q.; Yang, J.; Hou, M.; Zheng, B.; Xu, J.; Chai, Y.; Xiong, L.; Zhang, C. Tumor Microenvironment-Responsive Nanohybrid for Hypoxia Amelioration with Photodynamic and near-Infrared II Photothermal Combination Therapy. Acta Biomater. 2022, 146, 450–464. [Google Scholar] [CrossRef]
- Xie, T.; Qi, Y.; Li, Y.; Zhang, F.; Li, W.; Zhong, D.; Tang, Z.; Zhou, M. Ultrasmall Ga-ICG Nanoparticles Based Gallium Ion/Photodynamic Synergistic Therapy to Eradicate Biofilms and against Drug-Resistant Bacterial Liver Abscess. Bioact. Mater. 2021, 6, 3812–3823. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Gong, M.; Xiao, J.; Hai, L.; Luo, Y.; He, L.; Wang, Z.; Deng, L.; He, D. Photothermally Activated Multifunctional MoS2 Bactericidal Nanoplatform for Combined Chemo/Photothermal/Photodynamic Triple-Mode Therapy of Bacterial and Biofilm Infections. Chem. Eng. J. 2022, 429, 132600. [Google Scholar] [CrossRef]
- Xiao, J.; Hai, L.; Yang, K.; Luo, Y.; Wang, Z.; Li, J.; Ou, C.; Wang, L.; Deng, L.; He, D. Self-Enhanced ROS Generation by Responsive Co-Delivery of H2O2 and O2 Based on a Versatile Composite Biomaterial for Hypoxia-Irrelevant Multimodal Antibiofilm Therapy. Chem. Eng. J. 2023, 465, 142958. [Google Scholar] [CrossRef]
- Shaitelman, S.F.; Cromwell, K.D.; Rasmussen, J.C.; Stout, N.L.; Armer, J.M.; Lasinski, B.B.; Cormier, J.N. Recent Progress in the Treatment and Prevention of Cancer-Related Lymphedema: Lymphedema Treatment and Prevention. CA Cancer J. Clin. 2015, 65, 55–81. [Google Scholar] [CrossRef]
- Chan, A.; Kow, A.; Hibi, T.; Di Benedetto, F.; Serrablo, A. Liver Resection in Cirrhotic Liver: Are There Any Limits? Int. J. Surg. 2020, 82S, 109–114. [Google Scholar] [CrossRef] [PubMed]
- Noura, S.; Ohue, M.; Seki, Y.; Yamamoto, T.; Idota, A.; Fujii, J.; Yamasaki, T.; Nakajima, H.; Murata, K.; Kameyama, M.; et al. Evaluation of the Lateral Sentinel Node by Indocyanine Green for Rectal Cancer Based on Micrometastasis Determined by Reverse Transcriptase-Polymerase Chain Reaction. Oncol. Rep. 2008, 20, 745–750. [Google Scholar] [CrossRef]
- Murawa, D.; Hirche, C.; Dresel, S.; Hünerbein, M. Sentinel Lymph Node Biopsy in Breast Cancer Guided by Indocyanine Green Fluorescence. Br. J. Surg. 2009, 96, 1289–1294. [Google Scholar] [CrossRef]
- Fujiwara, M.; Mizukami, T.; Suzuki, A.; Fukamizu, H. Sentinel Lymph Node Detection in Skin Cancer Patients Using Real-Time Fluorescence Navigation with Indocyanine Green: Preliminary Experience. J. Plast. Reconstr. Aesthet. Surg. 2009, 62, e373–e378. [Google Scholar] [CrossRef]
- Mitsumori, N.; Nimura, H.; Takahashi, N.; Kawamura, M.; Aoki, H.; Shida, A.; Omura, N.; Yanaga, K. Sentinel Lymph Node Navigation Surgery for Early Stage Gastric Cancer. World J. Gastroenterol. 2014, 20, 5685–5693. [Google Scholar] [CrossRef]
- Hirche, C.; Dresel, S.; Krempien, R.; Hünerbein, M. Sentinel Node Biopsy by Indocyanine Green Retention Fluorescence Detection for Inguinal Lymph Node Staging of Anal Cancer: Preliminary Experience. Ann. Surg. Oncol. 2010, 17, 2357–2362. [Google Scholar] [CrossRef] [PubMed]
- Park, D.J.; Lee, H.-J.; Lee, H.S.; Kim, W.H.; Kim, H.-H.; Lee, K.U.; Choe, K.J.; Yang, H.-K. Sentinel Node Biopsy for CT1 and CT2a Gastric Cancer. Eur. J. Surg. Oncol. 2006, 32, 48–54. [Google Scholar] [CrossRef] [PubMed]
- Olsen, T.W.; Lim, J.I.; Capone, A.; Myles, R.A.; Gilman, J.P. Anaphylactic Shock Following Indocyanine Green Angiography. Arch. Ophthalmol. 1996, 114, 97. [Google Scholar] [CrossRef] [PubMed]
- Guyer, D.R.; Yannuzzi, L.A.; Slakter, J.S.; Sorenson, J.A.; Hope-Ross, M.; Orlock, D.R. Digital Indocyanine-Green Videoangiography of Occult Choroidal Neovascularization. Ophthalmology 1994, 101, 1727–1735; discussion 1735–1737. [Google Scholar] [CrossRef]
- Avvad, F.K.; Duker, J.S.; Reichel, E.; Margolis, T.I.; Puliafito, C.A. The Digital Indocyanine Green Videoangiography Characteristics of Well-Defined Choroidal Neovascularization. Ophthalmology 1995, 102, 401–405. [Google Scholar] [CrossRef]
- Guyer, D.R.; Yannuzzi, L.A.; Slakter, J.S.; Sorenson, J.A.; Hanutsaha, P.; Spaide, R.F.; Schwartz, S.G.; Hirschfeld, J.M.; Orlock, D.A. Classification of Choroidal Neovascularization by Digital Indocyanine Green Videoangiography. Ophthalmology 1996, 103, 2054–2060. [Google Scholar] [CrossRef]
- Chen, Q.-Y.; Xie, J.-W.; Zhong, Q.; Wang, J.-B.; Lin, J.-X.; Lu, J.; Cao, L.-L.; Lin, M.; Tu, R.-H.; Huang, Z.-N.; et al. Safety and Efficacy of Indocyanine Green Tracer-Guided Lymph Node Dissection During Laparoscopic Radical Gastrectomy in Patients With Gastric Cancer: A Randomized Clinical Trial. JAMA Surg. 2020, 155, 300–311. [Google Scholar] [CrossRef]
- Wakabayashi, T.; Cacciaguerra, A.B.; Abe, Y.; Bona, E.D.; Nicolini, D.; Mocchegiani, F.; Kabeshima, Y.; Vivarelli, M.; Wakabayashi, G.; Kitagawa, Y. Indocyanine Green Fluorescence Navigation in Liver Surgery: A Systematic Review on Dose and Timing of Administration. Ann. Surg. 2022, 275, 1025–1034. [Google Scholar] [CrossRef]
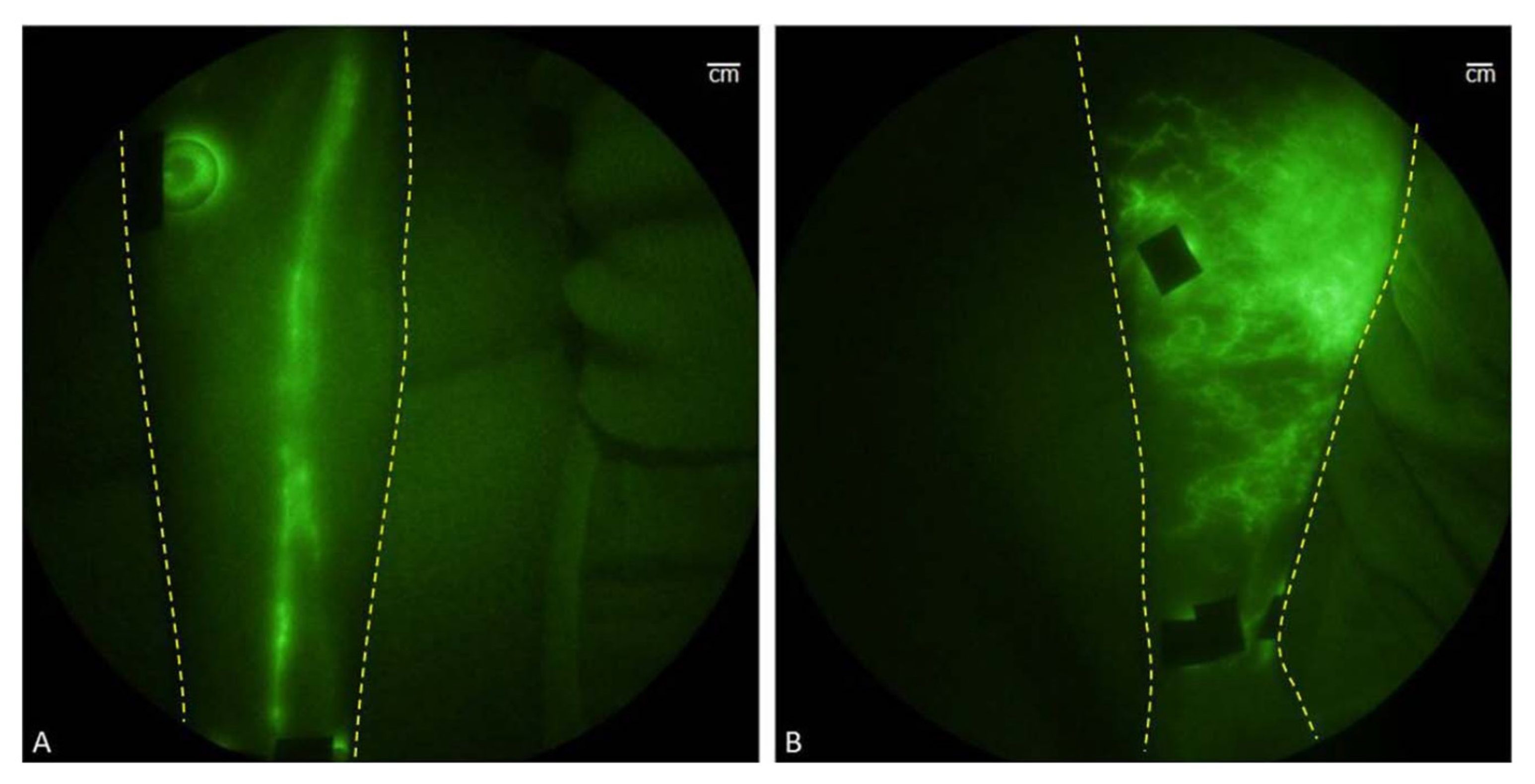
- Siddighi, S.; Yune, J.J.; Hardesty, J. Indocyanine Green for Intraoperative Localization of Ureter. Am. J. Obstet. Gynecol. 2014, 211, 436.e1–436.e2. [Google Scholar] [CrossRef]
- Lee, Z.; Moore, B.; Giusto, L.; Eun, D.D. Use of Indocyanine Green during Robot-Assisted Ureteral Reconstructions. Eur. Urol. 2015, 67, 291–298. [Google Scholar] [CrossRef]

| ICG Nanocomposites | |||||||
|---|---|---|---|---|---|---|---|
| Nanoplatforms | Functions | Types | Trigger | Target | Advantages | References | |
| ICG/MOFs | ZSZIT | H2S gas therapy | vivo | PH | Large surface area/Adjustable pore size and shape/Adjustable composition/Functionalised surface | [14] | |
| DI@HMONs-PMOF | PDT/PTT | vivo | PH/660/808 nm | [15] | |||
| DOX&ICG@H-PMOFm | CT/PTT/PDT&NIRF/PTI | vivo | PH/660&808 nm | [16] | |||
| ICG-PtMOFs@GNSs@HSA-Gd | PT/FL/MSOT/X-ray CT/MRI | vivo | PH | [18] | |||
| ZIF-ICG@ZIF-GOx@MPN | PTT/PDT/CDT | vivo | PH/NIR | [19] | |||
| Fe-DOX@Gd-MOF-ICG | MRI/PTI/PAI&PTT/PDT | vivo | PH/NIR | [20] | |||
| ICG/Polymer | FA-ICG-PLGA-lipid | FLI | vitro | NIR | Biodegradable | [24] | |
| DOX&ICG-PLGA-lecithin-PEG | CT/ PTT | vitro | NIR | [26] | |||
| ICG-PEG-PLL-PLLeu | FLI | vivo | NIR | Protection of enclosed drugs, high bioavailability, and good biocompatibility | [28] | ||
| Liposome-coated ICG | ICG -Lipid | PTT&Imaging | vivo | NIR | [29] | ||
| FAL-ICG-HAuNS | PDT/PTT | vivo | NIR | ER | [31] | ||
| Lipo-cyanine dyes | NIR-II Imaging | vitro | NIR-II | [32] | |||
| FA-ICG&DOX-Gd-Lipo | PT/CT&MRI/FL/PAI | vivo | NIR | FRa | [33] | ||
| ICG&Ce6&TPZ-Lipo | PTT/PDT | vivo | 660/808 nm | [34] | |||
| Lipo@ICG@CuS | PDT/PTT | vivo | NIR | [35] | |||
| ICG based Micelles Nanocomposites | Micelles -DOX & ICG | PTT/CT&NIR Imaging | vivo | PH/NIR/GSH | Nucleus/DNA Cleavage | Small size, Ease of assembly and versatility | [36] |
| ICG-NH2-PEG-PCC | PTT&NIR Imaging | vivo | NIR | [37] | |||
| GA-PEG-TK-ICG PMs | PTT/CT | vivo | NIR/ROS | HSP90 | [38] | ||
| ICG&PCL&5-FU&MEO2MA-b-HMAM | PTT/CT | vivo | NIR | [39] | |||
| ICG-HA-PTX | PTT/CT | vivo | NIR | CD44 | [40] | ||
| TMTP1-PEG-PLGA-ICG | CT&NIR-FL | vivo | NIR | T-SLN | [41] | ||
| ICG/Gold Composites | TNYL-ICG-HAuNS | PDT/PTT | vivo | NIR | Nrf2&NQO-1&HIF-1α | photothermal effect, porous mesoporous structure, uniform size | [45] |
| PNM@AuNC@ICG&DOX | PTT/Immunotherapy | vivo | NIR | Tumor-associated macrophage/neutrophil/Natural killer cell&CD44/VCAM-1/LFA-1 | [46] | ||
| MPLA & ICG-AUNCs | PTT/PDT | vivo | NIR | Tumor-associated antigen | [47] | ||
| DOX/ICG@biotin-PEG-AuNC-PCM | PDT/CT | vivo | NIR | Endocytosis | [48] | ||
| GNB@SiO2-ICG | PTT&FLI/PAI | vivo | NIR | [49] | |||
| ICG based multifunctional Composites | GNS@CaCO3/ICG | PT&FL Imaging | vivo | PH/NIR | Acid degradation, Immunomodulation | [52] | |
| Fe3O4@PDA@CaCO3/ICG | PDT/PTT | vivo | PH/NIR | [53] | |||
| Mn@CaCO3/ICG-siRNA | PDT/Immunotherapy | vivo | PH/NIR | PD-L1 | [54] | ||
| ALE/Man-g-HA | PDT/PTT | vivo | PH/NIR | CD44 | [55] | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mahmut, Z.; Zhang, C.; Ruan, F.; Shi, N.; Zhang, X.; Wang, Y.; Zheng, X.; Tang, Z.; Dong, B.; Gao, D.; et al. Medical Applications and Advancement of Near Infrared Photosensitive Indocyanine Green Molecules. Molecules 2023, 28, 6085. https://doi.org/10.3390/molecules28166085
Mahmut Z, Zhang C, Ruan F, Shi N, Zhang X, Wang Y, Zheng X, Tang Z, Dong B, Gao D, et al. Medical Applications and Advancement of Near Infrared Photosensitive Indocyanine Green Molecules. Molecules. 2023; 28(16):6085. https://doi.org/10.3390/molecules28166085
Chicago/Turabian StyleMahmut, Zulpya, Chunmei Zhang, Fei Ruan, Nan Shi, Xinyao Zhang, Yuda Wang, Xianhong Zheng, Zixin Tang, Biao Dong, Donghui Gao, and et al. 2023. "Medical Applications and Advancement of Near Infrared Photosensitive Indocyanine Green Molecules" Molecules 28, no. 16: 6085. https://doi.org/10.3390/molecules28166085
APA StyleMahmut, Z., Zhang, C., Ruan, F., Shi, N., Zhang, X., Wang, Y., Zheng, X., Tang, Z., Dong, B., Gao, D., & Sun, J. (2023). Medical Applications and Advancement of Near Infrared Photosensitive Indocyanine Green Molecules. Molecules, 28(16), 6085. https://doi.org/10.3390/molecules28166085









