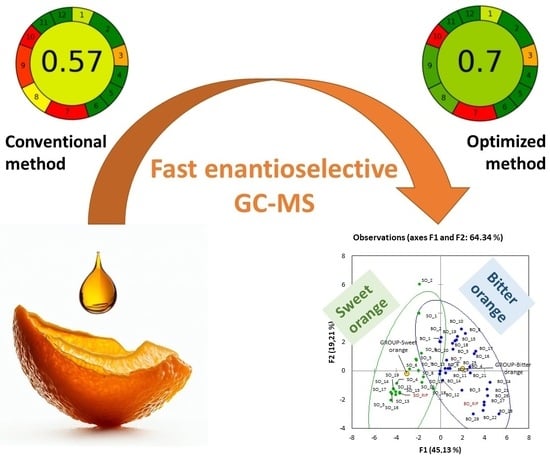
Make the Quality Control of Essential Oils Greener: Fast Enantioselective GC-MS Analysis of Sweet and Bitter Orange as a Case Study
Abstract
:1. Introduction
2. Results and Discussion
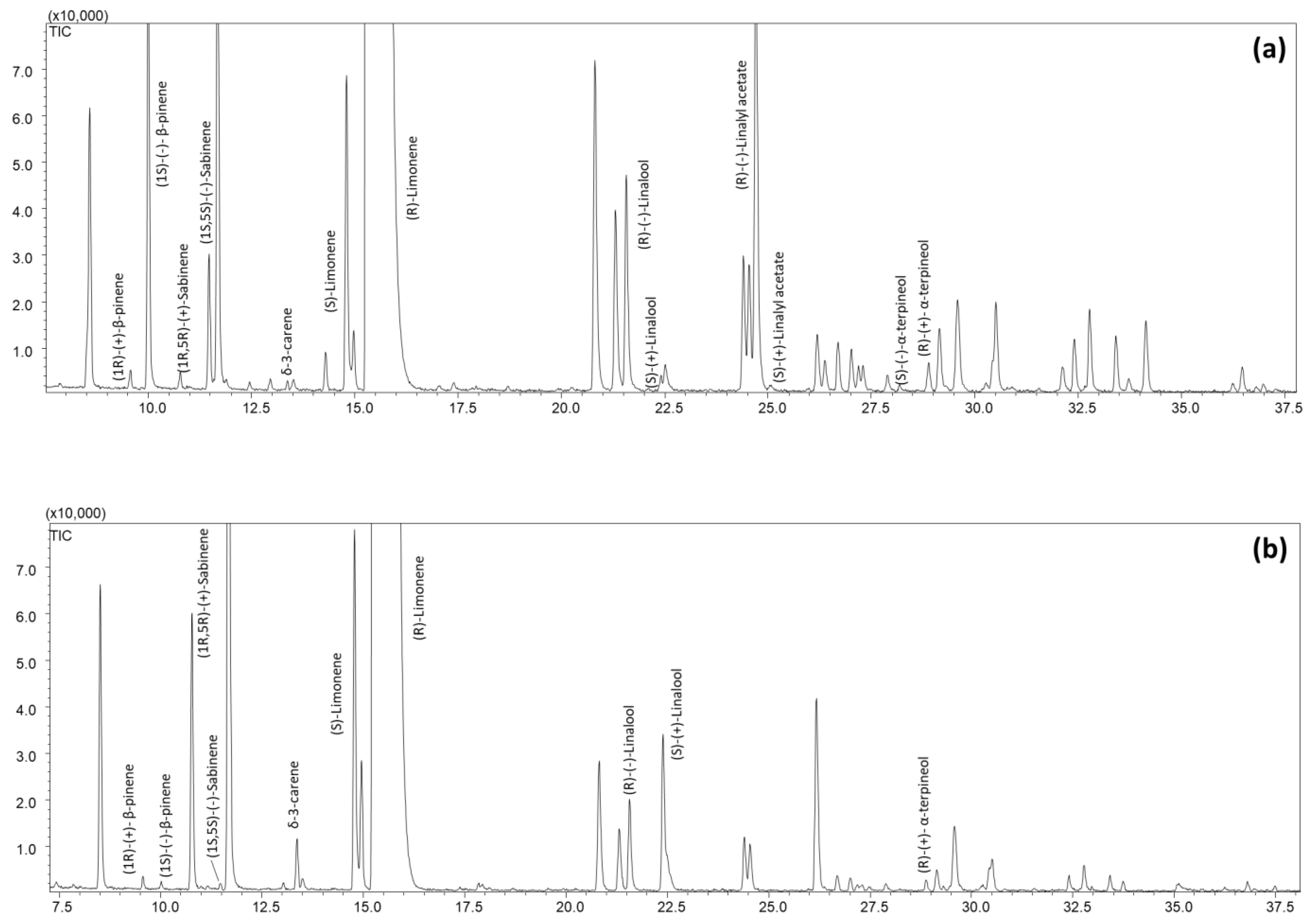
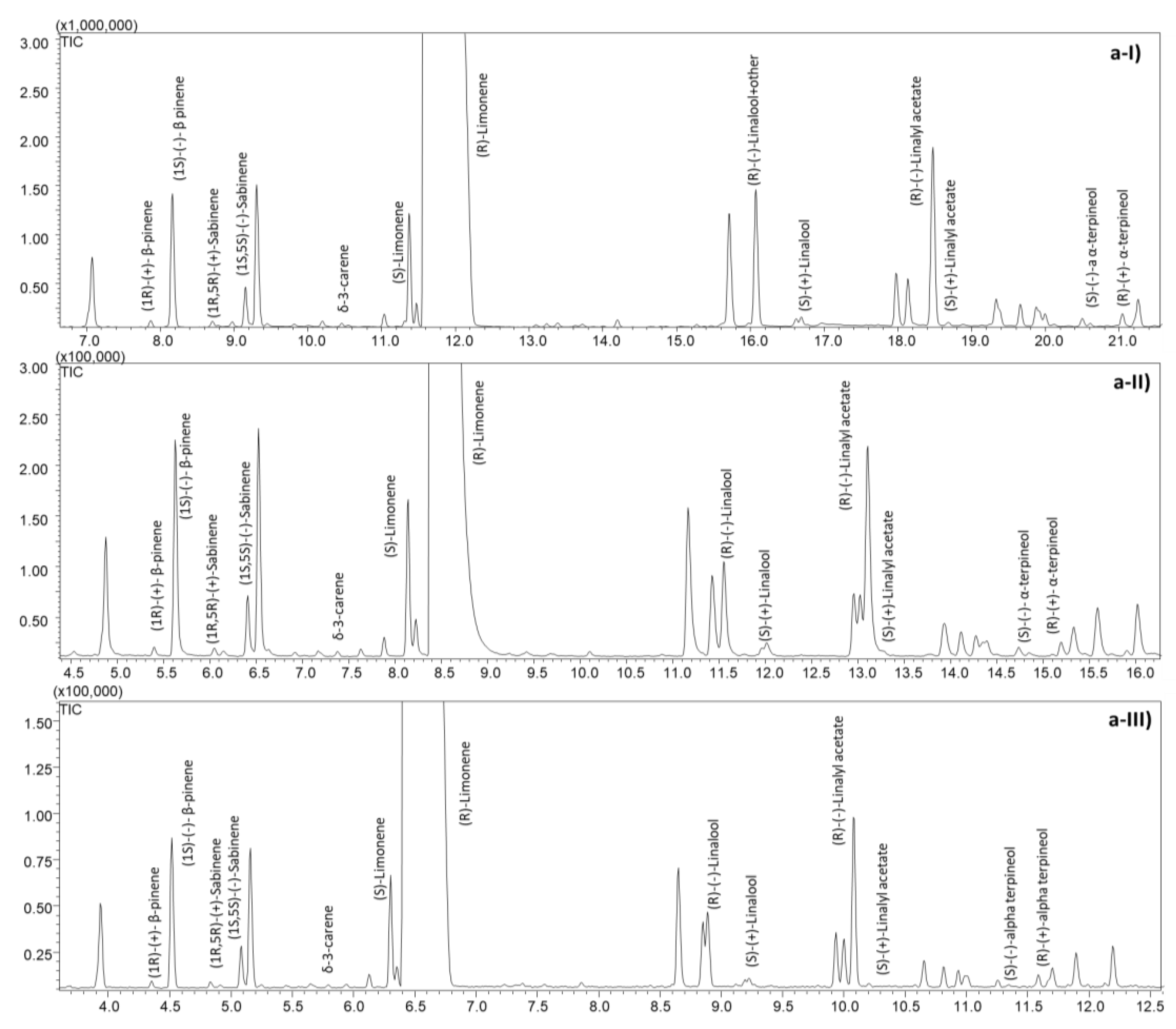
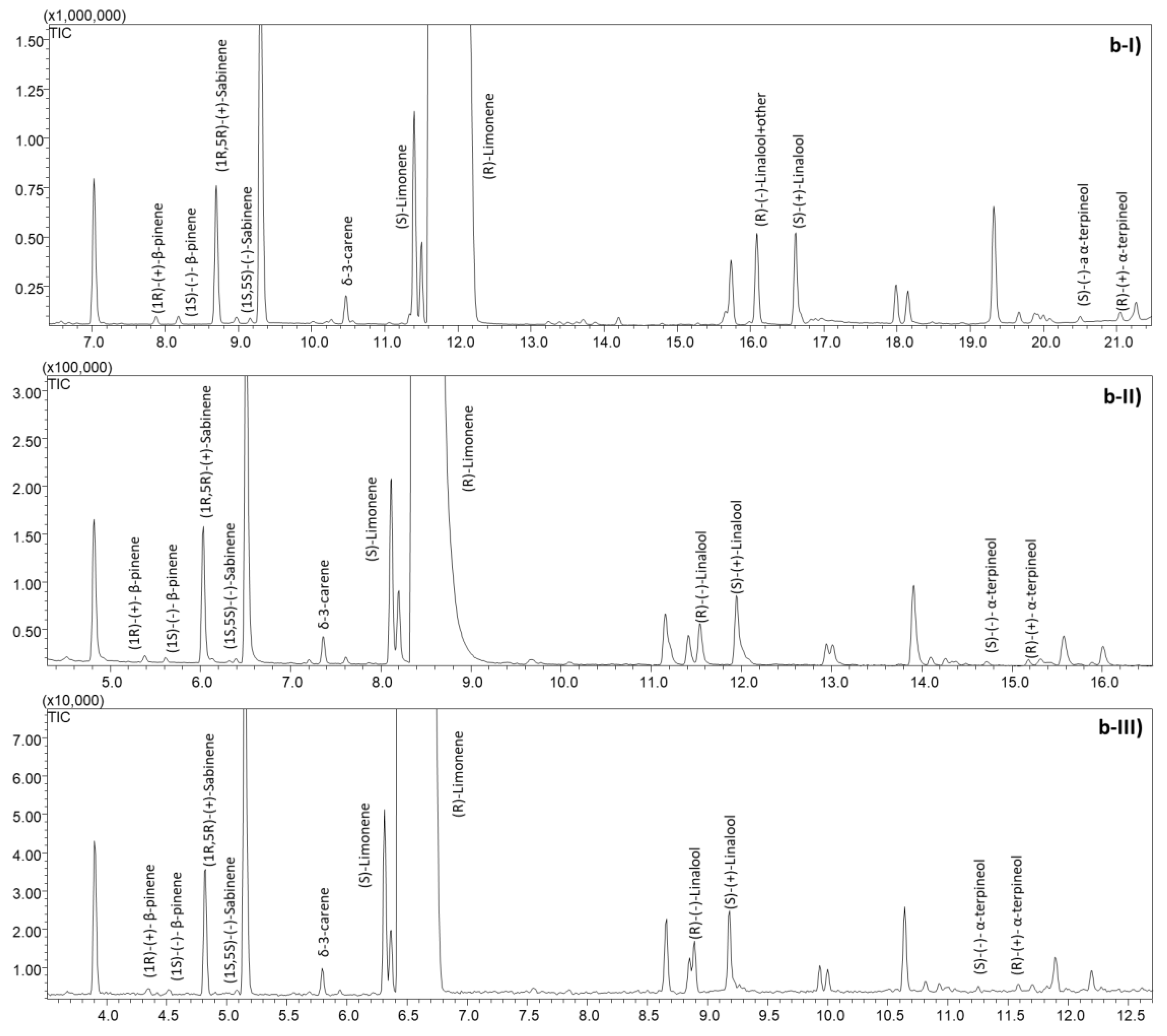
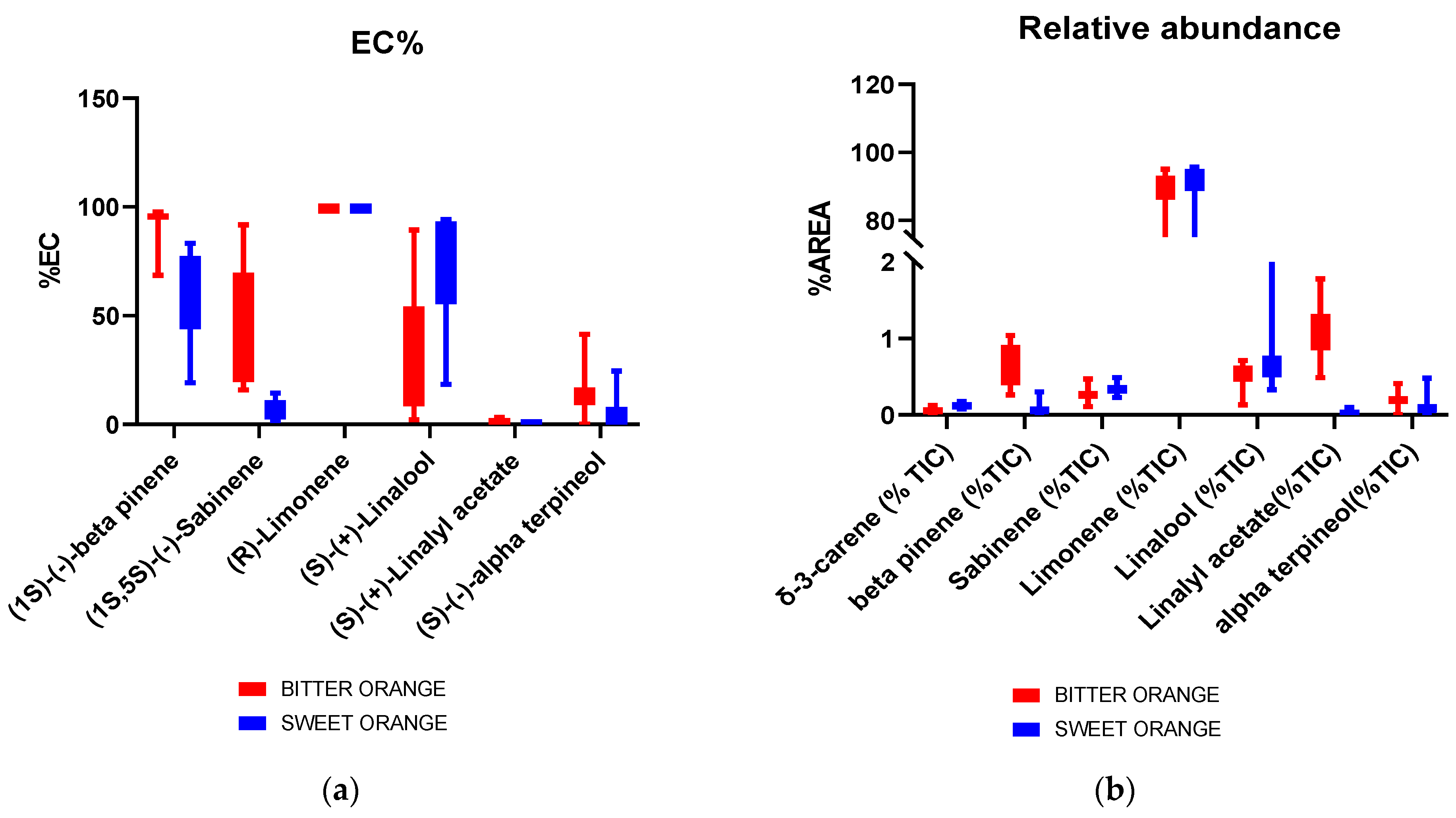
2.1. Sweet and Bitter Orange Essential Oil Characterization
2.2. Improvement in the Enviromental Footprint of the Chiral Analyses of Citrus Essenial Oils
2.2.1. Optimization and Speeding-Up of the Analyses
2.2.2. Assessment of the Environmental Footprint of the Methods
2.3. Analysis of Commercial Essential Oil Samples
2.3.1. Stability of the Enantiomeric Composition over Time
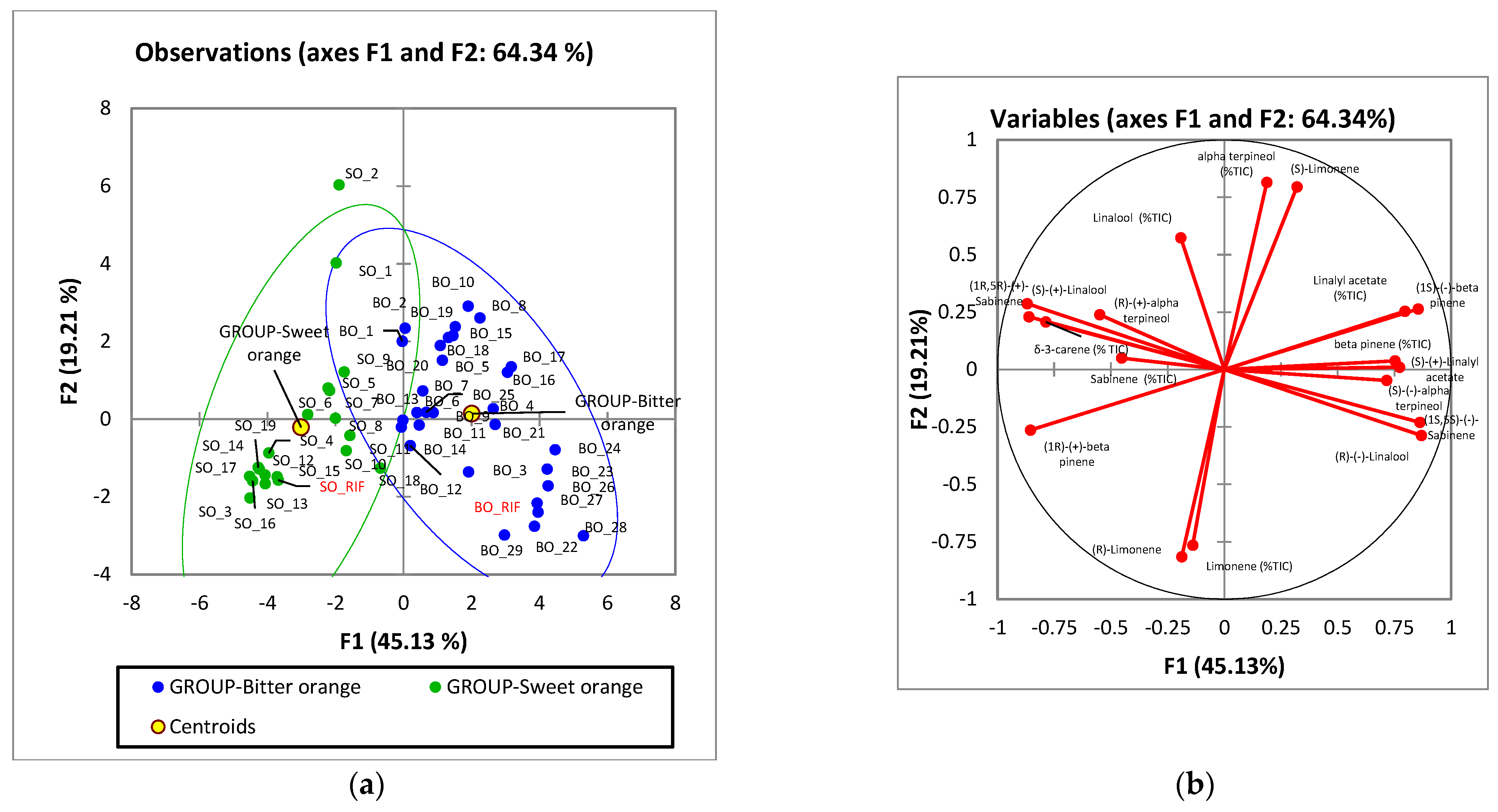
2.3.2. Multivariate Analysis of the Distribution of the Target Compounds in Commercial Samples
3. Materials and Methods
3.1. Samples and Chemicals
3.2. Instruments
3.3. Analysis Conditions
3.4. Data Processing
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- ISO 9235:2021; Aromatic Natural Raw Materials—Vocabulary. ISO: Geneva, Switzerland, 2021.
- Do, T.K.T.; Hadji-Minaglou, F.; Antoniotti, S.; Fernandez, X. Authenticity of essential oils. TrAC Trends Anal. Chem. 2015, 66, 146–157. [Google Scholar] [CrossRef]
- Industry Report. Available online: https://www.fortunebusinessinsights.com/industry-reports/essential-oils-market-101063 (accessed on 30 June 2023).
- Market Report. Available online: https://www.marketsandmarkets.com/Market-Reports/essential-oil-market-119674487.html (accessed on 30 June 2023).
- Dugo, G.; Mondello, L. Citrus Oils: Composition, Advanced Analytical Techniques, Contaminants, and Biological Activity; CRC Press: Boca Raton, FL, USA, 2010. [Google Scholar]
- Tranchida, P.Q.; Bonaccorsi, I.; Dugo, P.; Mondello, L.; Dugo, G. Analysis of Citrus essential oils: State of the art and future perspectives. A review. Flavour Fragr. J. 2012, 27, 98–123. [Google Scholar] [CrossRef]
- Yang, K.-M.; Chen, C.-W.; Chen, M.-H.; Chen, H.-C.; Lin, L.-Y. Authenticity Analysis of Cold-Pressed Orange Essential Oils by GC/MS on Polymethoxyflavone Components. Agriculture 2023, 13, 179. [Google Scholar] [CrossRef]
- Cohen, S.M.; Eisenbrand, G.; Fukushima, S.; Gooderham, N.J.; Guengerich, F.P.; Hecht, S.S.; Rietjens, I.M.C.M.; Bastaki, M.; Davidsen, J.M.; Harman, C.L.; et al. FEMA GRAS assessment of natural flavor complexes: Citrus-derived flavoring ingredients. Food Chem. Toxicol. 2019, 124, 192–218. [Google Scholar] [CrossRef] [PubMed]
- WFO—The World Fora Online. Available online: http://www.worldfloraonline.org (accessed on 30 June 2023).
- Farag, M.A.; Abib, B.; Ayad, L.; Khattab, A.R. Sweet and bitter oranges: An updated comparative review of their bioactives, nutrition, food quality, therapeutic merits and biowaste valorization practices. Food Chem. 2020, 331, 127306. [Google Scholar] [CrossRef] [PubMed]
- Raccary, B.; Loubet, P.; Peres, C.; Sonnemann, G. Evaluating the environmental impacts of analytical chemistry methods: From a critical review towards a proposal using a life cycle approach. TrAC Trends Anal. Chem. 2022, 147, 116525. [Google Scholar] [CrossRef]
- Gałuszka, A.; Migaszewski, Z.; Namieśnik, J. The 12 principles of green analytical chemistry and the SIGNIFICANCE mnemonic of green analytical practices. TrAC Trends Anal. Chem. 2013, 50, 78–84. [Google Scholar] [CrossRef]
- Cagliero, C.; Bicchi, C.; Cordero, C.; Rubiolo, P.; Sgorbini, B.; Liberto, E. Fast headspace-enantioselective GC-mass spectrometric-multivariate statistical method for routine authentication of flavoured fruit foods. Food Chem. 2012, 132, 1071–1079. [Google Scholar] [CrossRef]
- Cagliero, C.; Sgorbini, B.; Cordero, C.; Liberto, E.; Rubiolo, P.; Bicchi, C. Enantioselective Gas Chromatography with Derivatized Cyclodextrins in the Flavour and Fragrance Field. Isr. J. Chem. 2016, 56, 925–939. [Google Scholar] [CrossRef]
- Armenta, S.; de la Guardia, M. Green chromatography for the analysis of foods of animal origin. TrAC Trends Anal. Chem. 2016, 80, 517–530. [Google Scholar] [CrossRef]
- Napolitano-Tabares, P.I.; Negrín-Santamaría, I.; Gutiérrez-Serpa, A.; Pino, V. Recent efforts to increase greenness in chromatography. Curr. Opin. Green Sustain. Chem. 2021, 32, 100536. [Google Scholar] [CrossRef]
- Plotka, J.; Tobiszewski, M.; Sulej, A.M.; Kupska, M.; Gorecki, T.; Namiesnik, J. Green chromatography. J. Chromatogr. A 2013, 1307, 1–20. [Google Scholar] [CrossRef] [PubMed]
- Nowak, P.M.; Bis, A.; Rusin, M.; Woźniakiewicz, M. Carbon footprint of the analytical laboratory and the three-dimensional approach to its reduction. Green Anal. Chem. 2023, 4, 100051. [Google Scholar] [CrossRef]
- Pena-Pereira, F.; Wojnowski, W.; Tobiszewski, M. AGREE—Analytical GREEnness Metric Approach and Software. Anal. Chem. 2020, 92, 10076–10082. [Google Scholar] [CrossRef] [PubMed]
- Brenna, E.; Fuganti, C.; Serra, S. Enantioselective perception of chiral odorants. Tetrahedron-Asymmetry 2003, 14, 1–42. [Google Scholar] [CrossRef]
- Liberto, E.; Cagliero, C.; Sgorbini, B.; Bicchi, C.; Sciarrone, D.; Zellner, B.D.; Mondello, L.; Rubiolo, P. Enantiomer identification in the flavour and fragrance fields by “interactive” combination of linear retention indices from enantio selective gas chromatography and mass spectrometry. J. Chromatogr. A 2008, 1195, 117–126. [Google Scholar] [CrossRef] [PubMed]
- Agilent. GC Calculators and Method Translation Software. Available online: https://www.agilent.com/en/support/gas-chromatography/gccalculators (accessed on 30 June 2023).
- Klee, M.S.; Blumberg, L.M. Theoretical and Practical Aspects of Fast Gas Chromatography and Method Translation. J. Chromatogr. Sci. 2002, 40, 234–247. [Google Scholar] [CrossRef] [PubMed]
- Turek, C.; Stintzing, F.C. Stability of Essential Oils: A Review. Compr. Rev. Food Sci. Food Saf. 2013, 12, 40–53. [Google Scholar] [CrossRef]

| Sample | Year of Production | Original EC% | EC% in 2023 |
|---|---|---|---|
| Bitter orange essential oil | 2006 | (R)-Linalool 72.3% (S)-Linalool 27.7% | (R)-Linalool 72.7% (S)-Linalool 27.3% |
| Sweet orange essential oil | 2006 | (R)-Linalool 7.7% (S)-Linalool 92.3% | (R)-Linalool 7.8% (S)-Linalool 92.2% |
| Linalool standard (isolated) | 2016 | (R)-Linalool 96.1% (S)-Linalool 3.9% | (R)-Linalool 95.7% (S)-Linalool 4.3% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bechis, G.; Minteguiaga, M.A.; Sgorbini, B.; Marengo, A.; Rubiolo, P.; Cagliero, C. Make the Quality Control of Essential Oils Greener: Fast Enantioselective GC-MS Analysis of Sweet and Bitter Orange as a Case Study. Molecules 2023, 28, 6231. https://doi.org/10.3390/molecules28176231
Bechis G, Minteguiaga MA, Sgorbini B, Marengo A, Rubiolo P, Cagliero C. Make the Quality Control of Essential Oils Greener: Fast Enantioselective GC-MS Analysis of Sweet and Bitter Orange as a Case Study. Molecules. 2023; 28(17):6231. https://doi.org/10.3390/molecules28176231
Chicago/Turabian StyleBechis, Gaia, Manuel A. Minteguiaga, Barbara Sgorbini, Arianna Marengo, Patrizia Rubiolo, and Cecilia Cagliero. 2023. "Make the Quality Control of Essential Oils Greener: Fast Enantioselective GC-MS Analysis of Sweet and Bitter Orange as a Case Study" Molecules 28, no. 17: 6231. https://doi.org/10.3390/molecules28176231
APA StyleBechis, G., Minteguiaga, M. A., Sgorbini, B., Marengo, A., Rubiolo, P., & Cagliero, C. (2023). Make the Quality Control of Essential Oils Greener: Fast Enantioselective GC-MS Analysis of Sweet and Bitter Orange as a Case Study. Molecules, 28(17), 6231. https://doi.org/10.3390/molecules28176231