Effect of Yogurt and Its Components on the Deodorization of Raw and Fried Garlic Volatiles
Abstract
1. Introduction
2. Results and Discussion
2.1. Effect of Frying on Raw Garlic Volatiles
2.2. Effect of Yogurt on Raw and Fried Garlic Volatiles
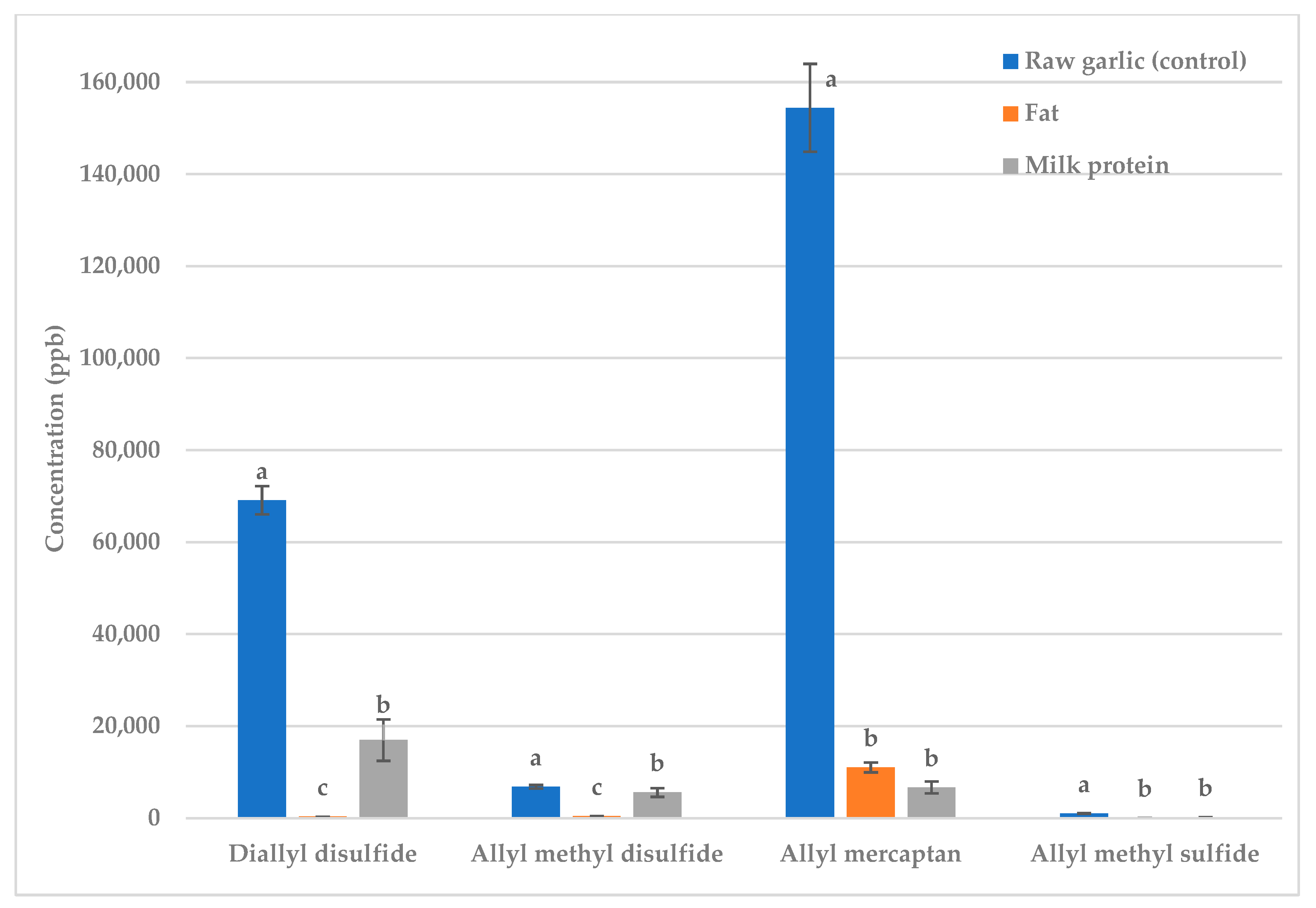
2.3. Effect of Fat Versus Protein on Raw and Fried Garlic Volatiles
2.4. Effect of Water and Protein on Raw and Fried Garlic Volatiles
2.5. Effect of Different Proteins on Raw and Fried Garlic Volatiles
2.6. Effect of Quantity of Butter Fat on Raw and Fried Garlic Volatiles
3. Materials and Methods
3.1. Garlic Sample Preparation
3.2. Treatment Preparation
3.3. Selected-Ion Flow-Tube Mass Spectrometry (SIFT-MS) Headspace Analysis
3.4. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
Appendix A
| Volatiles | Garlic (Control) | Yogurt pH 4.4 | Yogurt pH 7 | Water pH 4.4 | Water pH 7 | Heated Yogurt pH 4.4 |
|---|---|---|---|---|---|---|
| 1,3-dithiane | 4020 ± 1770 | 20.4 ± 6.6 | 154 ± 24 | 589 ± 30 | 524 ± 8 | 71.5 ± 8.5 |
| 2-ethylpyrazine | 29.9 ± 15.7 | 0.67 ± 0.10 | 1.49 ± 0.12 | 3.35 ± 0.13 | 3.81 ± 1.32 | 1.65 ± 0.15 |
| 2-methyl-2-butenal | 93 ± 37 | 2.36 ± 0.39 | 4.44 ± 0.48 | 7.85 ± 0.35 | 6.05 ± 0.91 | 3.04 ± 0.46 |
| 2-methylbenzaldehyde | 51.6 ± 25.1 | 4.2 ± 0.5 | 5.85 ± 1.10 | 2.29 ± 0.49 | 1.89 ± 0.23 | 5.62 ± 0.67 |
| 2-vinyl-4H-1,3-dithiin | 8.59 ± 3.22 | 3.18 ± 1.02 | 4.66 ± 1.02 | 2.38 ± 0.75 | 3.61 ± 0.87 | 5.42 ± 0.74 |
| 2,3-dimethylpyrazine | 38.8 ± 17.1 | 0.44 ± 0.06 | 3.91 ± 0.90 | 2.24 ± 0.09 | 2.55 ± 0.88 | 1.89 ± 0.34 |
| 2,5-dimethylthiophene | 401 ± 212 | 2.59 ± 0.54 | 14.6 ± 1.4 | 85.4 ± 9.84 | 78.4 ± 2.48 | 15.7 ± 3.5 |
| Acetaldehyde | 10,400 ± 617 | 9210 ± 455 | 7390 ± 273 | 1720 ± 237 | 1190 ± 617 | 6722 ± 419 |
| Allicin | 93.9 ± 39.9 | 3.66 ± 2.73 | 2.97 ± 0.61 | 35.8 ± 3.3 | 29.4 ± 1.6 | 9.58 ± 5.20 |
| Allyl mercaptan | 110,000 ± 28,600 | 832 ± 324 | 25,400 ± 1950 | 4240 ± 915 | 5470 ± 1320 | 5621 ± 450 |
| Allyl methyl disulfide | 20,100 ± 6150 | 23.2 ± 8.1 | 615 ± 159 | 3820 ± 236 | 3800 ± 335 | 262 ± 97 |
| Allyl methyl sulfide | 965 ± 275 | 8.39 ± 0.34 | 79.9 ± 11.8 | 63.2 ± 4.8 | 63.8 ± 5.5 | 17.4 ± 3.8 |
| Allyl methyl tetrasulfide | 9.34 ± 4.43 | 2.12 ± 0.30 | 1.7 ± 1.1 | 3.13 ± 0.51 | 2.77 ± 0.87 | 2.08 ± 0.18 |
| Allyl methyl thiosulfinate | 50.8 ± 23.8 | 0.98 ± 0.69 | 1.06 ± 0.26 | 5.81 ± 1.54 | 4.86 ± 0.88 | 1.71 ± 0.55 |
| Allyl methyl trisulfide | 35.3 ± 14.6 | 1.13 ± 0.15 | 2.00 ± 0.11 | 15.6 ± 0.4 | 13.5 ± 2.6 | 1.52 ± 0.20 |
| Aniline | 9.38 ± 3.67 | 0.49 ± 0.09 | 1.04 ± 0.03 | 1.10 ± 0.26 | 1.51 ± 0.22 | 0.57 ± 0.05 |
| Diallyl disulfide | 102,000 ± 56,900 | 28.3 ± 8.7 | 907 ± 251 | 20,300 ± 1350 | 18,700 ± 556 | 945 ± 73 |
| Diallyl sulphide | 1720 ± 841 | 5.96 ± 2.40 | 76.7 ± 14.4 | 243 ± 27 | 189 ± 4 | 46.9 ± 6.4 |
| Diallyl tetrasulfide | 13.3 ± 6.0 | 3.38 ± 0.11 | 3.63 ± 0.62 | 5.74 ± 1.76 | 6.29 ± 2.00 | 3.65 ± 0.61 |
| Diallyl trisulfide | 981 ± 522 | 2.35 ± 0.79 | 9.55 ± 3.60 | 350 ± 18 | 323 ± 21 | 11.0 ± 1.5 |
| Dimethyl disulphide | 487 ± 71 | 2.93 ± 0.92 | 50.4 ± 9.7 | 99.0 ± 10.6 | 98.7 ± 12.6 | 9.20 ± 2.97 |
| Dimethyl sulfide | 2870 ± 1350 | 69.7 ± 4.2 | 109 ± 16 | 815 ± 49 | 560 ± 271 | 72.1 ± 5.4 |
| Dimethyl thioether | 2990 ± 950 | 77.6 ± 7.4 | 109 ± 16 | 846 ± 122 | 567 ± 335 | 72.1 ± 5.4 |
| Dimethyl thiosulfinate | 13.5 ± 6.8 | 1.00 ± 0.51 | 2.10 ± 0.93 | 1.16 ± 0.41 | 1.24 ± 0.23 | 0.87 ± 0.13 |
| Dimethyl trisulfide | 10.6 ± 1.6 | 2.24 ± 0.53 | 2.56 ± 1.23 | 3.11 ± 0.43 | 3.20 ± 1.82 | 2.30 ± 0.21 |
| Dipropyl sulfide | 7900 ± 2530 | 30.5 ± 4.4 | 440 ± 58 | 2220 ± 139 | 2060 ± 50 | 179.0 ± 13.0 |
| Methyl mercaptan | 16,600 ± 1300 | 449 ± 172 | 6280 ± 394 | 1590 ± 262 | 1900 ± 243 | 1061 ± 2 |
| Methyl propyl disulfide | 3410 ± 1130 | 4.92 ± 0.83 | 80.9 ± 22.1 | 588 ± 37 | 519 ± 28 | 37.6 ± 9.4 |
| Pyridine | 20.8 ± 6.6 | 0.78 ± 0.11 | 2.30 ± 0.64 | 2.13 ± 0.20 | 3.13 ± 0.41 | 1.22 ± 0.27 |
| Trimethylpyrazine | 958 ± 364 | 1.80 ± 0.33 | 23.7 ± 5.6 | 160 ± 18 | 147 ± 15 | 10.8 ± 3.08 |
| Volatiles | Fried Garlic (Control) | Yogurt pH 4.4 | Yogurt pH 7 | Water pH 4.4 | Water pH 7 | Heated Yogurt pH 4.4 |
|---|---|---|---|---|---|---|
| 1,3-dithiane | 119 ± 29 | 11.5 ± 5.6 | 572 ± 148 | 543 ± 60 | 430 ± 32 | 310 ± 103 |
| 2-ethylpyrazine | 31.2 ± 7.3 | 0.91 ± 0.42 | 27.4 ± 9.1 | 12.9 ± 1.8 | 10.0 ± 1.8 | 4.66 ± 1.96 |
| 2-methyl-2-butenal | 39.8 ± 10.4 | 4.66 ± 1.53 | 61.8 ± 15.6 | 34.0 ± 6.4 | 27.7 ± 3.8 | 13.6 ± 7.32 |
| 2-methylbenzaldehyde | 3.21 ± 0.78 | 3.77 ± 0.72 | 5.98 ± 1.14 | 2.89 ± 0.53 | 2.15 ± 0.35 | 5.97 ± 1.15 |
| 2-vinyl-4H-1,3-dithiin | 19.9 ± 4.1 | 2.23 ± 0.22 | 18.4 ± 8.2 | 11.5 ± 0.6 | 10.3 ± 0.9 | 5.55 ± 1.39 |
| 2,3-dimethylpyrazine | 32.2 ± 9.1 | 0.71 ± 0.13 | 20.1 ± 6.9 | 9.07 ± 1.01 | 7.06 ± 1.24 | 3.48 ± 1.93 |
| 2,5-dimethylthiophene | 19.1 ± 5.2 | 2.56 ± 0.82 | 35.8 ± 8.4 | 48.0 ± 4.2 | 39.1 ± 1.4 | 15.1 ± 5.6 |
| Acetaldehyde | 7490 ± 356 | 11,500 ± 1290 | 7300 ± 1780 | 1330 ± 113 | 1140 ± 68 | 10,700 ± 917 |
| Allicin | 6.65 ± 1.75 | 0.93 ± 0.16 | 10.5 ± 4.3 | 9.07 ± 1.13 | 7.43 ± 0.58 | 2.37 ± 0.85 |
| Allyl mercaptan | 654 ± 56 | 93.4 ± 44.5 | 1290 ± 647 | 809 ± 153 | 595 ± 76 | 350 ± 141 |
| Allyl methyl disulfide | 142 ± 34 | 13.1 ± 6.8 | 615 ± 222 | 749 ± 102 | 575 ± 39 | 586 ± 126 |
| Allyl methyl sulfide | 383 ± 60 | 103 ± 60 | 4950 ± 1120 | 3950 ± 663 | 3070 ± 197 | 1990 ± 838 |
| Allyl methyl tetrasulfide | 2.43 ± 0.97 | 1.14 ± 0.44 | 3.86 ± 0.75 | 5.02 ± 1.83 | 4.08 ± 1.16 | 1.89 ± 0.73 |
| Allyl methyl thiosulfinate | 19.1 ± 4.3 | 1.23 ± 0.28 | 20.1 ± 5.5 | 14.9 ± 0.7 | 11.3 ± 1.7 | 8.50 ± 3.67 |
| Allyl methyl trisulfide | 30.1 ± 7.1 | 1.56 ± 0.08 | 36.9 ± 9.4 | 66.5 ± 2.3 | 57.0 ± 2.0 | 50.1 ± 12.3 |
| Aniline | 17.0 ± 4.4 | 0.72 ± 0.31 | 22.0 ± 7.9 | 8.24 ± 1.22 | 5.97 ± 0.60 | 1.97 ± 0.84 |
| Diallyl disulfide | 95.1 ± 26.3 | 4.96 ± 2.36 | 164 ± 42 | 297 ± 28 | 252 ± 14 | 114 ± 36 |
| Diallyl sulphide | 187 ± 40 | 31.0 ± 18.5 | 1500 ± 356 | 1970 ± 127 | 1620 ± 140 | 610 ± 211 |
| Diallyl tetrasulfide | 2.48 ± 0.68 | 1.35 ± 0.43 | 2.48 ± 0.61 | 3.25 ± 0.72 | 2.82 ± 0.49 | 1.84 ± 0.16 |
| Diallyl trisulfide | 47.7 ± 12.6 | 3.21 ± 0.14 | 36.0 ± 8.55 | 102 ± 32 | 83.1 ± 2.7 | 26.9 ± 9.3 |
| Dimethyl disulphide | 91.0 ± 20.3 | 13.0 ± 7.2 | 690 ± 179 | 560 ± 153 | 407 ± 26 | 550 ± 219 |
| Dimethyl sulfide | 524 ± 57 | 190 ± 78 | 1970 ± 243 | 1280 ± 230 | 1040 ± 61 | 259 ± 122 |
| Dimethyl thioether | 655 ± 76 | 223 ± 95 | 2320 ± 296 | 1630 ± 359 | 1360 ± 88 | 300 ± 140 |
| Dimethyl thiosulfinate | 21.4 ± 4.5 | 1.06 ± 0.32 | 16.4 ± 6.6 | 6.95 ± 2.32 | 5.23 ± 0.11 | 1.86 ± 0.82 |
| Dimethyl trisulfide | 25.6 ± 5.7 | 4.06 ± 1.20 | 73.1 ± 17.1 | 94.2 ± 18.9 | 69.7 ± 5.9 | 121 ± 38 |
| Dipropyl sulfide | 209 ± 40 | 34.1 ± 7.5 | 1180 ± 292 | 1470 ± 101 | 1180 ± 101 | 406 ± 150 |
| Methyl mercaptan | 539 ± 54 | 207 ± 87 | 2240 ± 1170 | 1080 ± 420 | 722 ± 119 | 523 ± 161 |
| Methyl propyl disulfide | 41.6 ± 10.3 | 3.41 ± 1.34 | 106 ± 32 | 119 ± 17 | 92.2 ± 6.9 | 83.8 ± 26.5 |
| Pyridine | 12.9 ± 2.91 | 1.38 ± 0.19 | 11.0 ± 3.9 | 4.42 ± 0.77 | 3.46 ± 0.36 | 2.05 ± 0.60 |
| Trimethylpyrazine | 14.2 ± 4.77 | 1.51 ± 0.42 | 35.3 ± 9.66 | 40.3 ± 5.27 | 30.9 ± 2.2 | 24.6 ± 9.54 |
| Volatiles | Raw Garlic (Control) | Whey Protein Isolate | Whey Protein Concentrate | Calcium Caseinate | Micellar Casein | Milk Protein Isolate | Milk Protein Concentrate |
|---|---|---|---|---|---|---|---|
| 1,3-dithiane | 5230 ± 441 | 371 ± 40 | 297 ± 48 | 560 ± 42 | 824 ± 70 | 808 ± 113 | 617 ± 118 |
| 2-ethylpyrazine | 20.4 ± 2.1 | 5.67 ± 1.07 | 4.48 ± 1.04 | 5.18 ± 1.72 | 4.10 ± 0.93 | 4.88 ± 1.44 | 5.23 ± 1.69 |
| 2-methyl-2-butenal | 73 ± 4 | 6.80 ± 0.95 | 7.86 ± 1.62 | 8.78 ± 1.62 | 17.1 ± 5.6 | 16.9 ± 0.3 | 11.9 ± 4.4 |
| 2-methylbenzaldehyde | 70.2 ± 6.7 | 3.29 ± 0.34 | 2.55 ± 0.72 | 3.04 ± 0.99 | 4.07 ± 0.78 | 3.84 ± 1.28 | 3.36 ± 0.48 |
| 2-vinyl-4H-1,3-dithiin | 8.06 ± 2.07 | 2.36 ± 0.85 | 3.74 ± 0.66 | 3.65 ± 0.66 | 2.79 ± 0.94 | 2.82 ± 0.97 | 3.02 ± 0.57 |
| 2,3-dimethylpyrazine | 25.6 ± 1.3 | 8.05 ± 1.26 | 4.24 ± 0.49 | 3.46 ± 1.15 | 2.74 ± 0.62 | 3.26 ± 0.96 | 3.49 ± 1.13 |
| 2,5-dimethylthiophene | 227 ± 18 | 48.7 ± 5.4 | 32.8 ± 5.5 | 70.9 ± 7.8 | 99.9 ± 10.3 | 99.3 ± 17.2 | 74.5 ± 20.7 |
| Acetaldehyde | 11,000 ± 1050 | 2630 ± 442 | 4690 ± 260 | 2370 ± 349 | 3670 ± 1060 | 3610 ± 367 | 3320 ± 624 |
| Allicin | 28.7 ± 1.4 | 15.2 ± 1.3 | 24.8 ± 7.5 | 19.3 ± 3.3 | 21.3 ± 4.8 | 31.0 ± 10.4 | 30.1 ± 10.6 |
| Allyl mercaptan | 154,000 ± 9560 | 17,500 ± 1920 | 10,300 ± 794 | 7690 ± 2140 | 6310 ± 456 | 6170 ± 3460 | 6680 ± 1290 |
| Allyl methyl disulfide | 6830 ± 378 | 2600 ± 150 | 2950 ± 516 | 4740 ± 130 | 7380 ± 295 | 7550 ± 369 | 5580 ± 966 |
| Allyl methyl sulfide | 1040 ± 86 | 87.1 ± 8.5 | 80.3 ± 13.1 | 135 ± 7 | 167 ± 3 | 207 ± 9 | 173 ± 14 |
| Allyl methyl tetrasulfide | 6.08 ± 2.10 | 1.96 ± 1.47 | 1.48 ± 0.77 | 2.46 ± 0.20 | 2.40 ± 1.32 | 3.39 ± 0.33 | 2.72 ± 0.69 |
| Allyl methyl thiosulfinate | 55.3 ± 3.9 | 4.20 ± 1.08 | 3.68 ± 0.27 | 4.97 ± 1.03 | 5.13 ± 0.76 | 5.03 ± 0.50 | 4.92 ± 0.18 |
| Allyl methyl trisulfide | 21.5 ± 1.6 | 6.47 ± 1.83 | 8.27 ± 1.10 | 13.5 ± 3.6 | 22.9 ± 1.6 | 20.5 ± 3.4 | 18.2 ± 4.36 |
| Aniline | 21.8 ± 0.4 | 1.40 ± 0.20 | 1.46 ± 0.14 | 1.92 ± 0.64 | 3.01 ± 0.91 | 2.35 ± 0.45 | 2.19 ± 0.22 |
| Diallyl disulfide | 69,100 ± 3100 | 11,200 ± 1090 | 6710 ± 1300 | 17,000 ± 2100 | 24,200 ± 3340 | 22,600 ± 4250 | 17,000 ± 4510 |
| Diallyl sulphide | 3080 ± 253 | 188 ± 13 | 126 ± 17 | 386 ± 31 | 423 ± 35 | 509 ± 62 | 381 ± 76 |
| Diallyl tetrasulfide | 10.5 ± 1.6 | 3.82 ± 1.88 | 4.05 ± 1.09 | 4.67 ± 1.91 | 7.78 ± 0.30 | 5.89 ± 2.68 | 5.98 ± 0.90 |
| Diallyl trisulfide | 566 ± 57 | 187 ± 14.5 | 102 ± 26 | 277 ± 48 | 389 ± 63 | 382 ± 105 | 302 ± 86 |
| Dimethyl disulphide | 174 ± 5 | 73 ± 3.23 | 141 ± 15 | 159 ± 16 | 263 ± 11 | 309 ± 13 | 216 ± 23 |
| Dimethyl sulfide | 1980 ± 68 | 468 ± 46.2 | 309 ± 55 | 707 ± 66 | 984 ± 116 | 938 ± 135 | 718 ± 161 |
| Dimethyl thioether | 2070 ± 123 | 575 ± 58.8 | 377 ± 70 | 913 ± 89 | 1270 ± 153 | 1210 ± 179 | 924 ± 211 |
| Dimethyl thiosulfinate | 12.0 ± 1.1 | 1.16 ± 0.48 | 0.50 ± 0.18 | 1.19 ± 0.29 | 1.02 ± 0.20 | 0.99 ± 0.36 | 0.76 ± 0.18 |
| Dimethyl trisulfide | 5.53 ± 0.65 | 2.71 ± 0.36 | 3.78 ± 0.73 | 3.83 ± 1.33 | 4.75 ± 0.44 | 4.83 ± 0.95 | 5.89 ± 1.10 |
| Dipropyl sulfide | 4480 ± 303 | 1560 ± 165 | 884 ± 178 | 1850 ± 200 | 2540 ± 322 | 2610 ± 414 | 1960 ± 439 |
| Methyl mercaptan | 15,300 ± 1330 | 4370 ± 277 | 3980 ± 170 | 3150 ± 401 | 3080 ± 84 | 2850 ± 1130 | 2970 ± 409 |
| Methyl propyl disulfide | 1130 ± 51 | 331 ± 10 | 373 ± 57 | 637 ± 21 | 1020 ± 43 | 1020 ± 110 | 735 ± 158 |
| Pyridine | 35.8 ± 3.8 | 3.64 ± 0.57 | 2.65 ± 0.38 | 2.88 ± 0.52 | 2.49 ± 0.54 | 3.29 ± 0.14 | 2.70 ± 0.80 |
| Trimethylpyrazine | 343 ± 11 | 96.6 ± 7.52 | 109 ± 23 | 171 ± 3 | 268 ± 10 | 283 ± 15 | 202 ± 25 |
| Volatiles | Fried Garlic (Control) | Whey Protein Isolate | Whey Protein Concentrate | Calcium Caseinate | Micellar Casein | Milk Protein Isolate | Milk Protein Concentrate |
|---|---|---|---|---|---|---|---|
| 1,3-dithiane | 68.2 ± 12.6 | 37.7 ± 5.9 | 36.5 ± 8.8 | 24.8 ± 2.88 | 17 ± 9.85 | 12.5 ± 4.7 | 10.4 ± 4.2 |
| 2-ethylpyrazine | 2.60 ± 0.62 | 0.89 ± 0.11 | 0.93 ± 0.14 | 0.79 ± 0.28 | 0.74 ± 0.17 | 0.50 ± 0.34 | 0.38 ± 0.13 |
| 2-methyl-2-butenal | 38.2 ± 8.35 | 1.92 ± 0.58 | 1.95 ± 0.64 | 1.86 ± 0.52 | 1.66 ± 0.64 | 1.94 ± 0.81 | 1.06 ± 0.45 |
| 2-methylbenzaldehyde | 1.37 ± 0.46 | 0.52 ± 0.11 | 0.80 ± 0.11 | 0.72 ± 0.25 | 0.58 ± 0.11 | 0.43 ± 0.28 | 0.59 ± 0.23 |
| 2-vinyl-4H-1,3-dithiin | 15.9 ± 1.10 | 7.94 ± 1.84 | 5.51 ± 0.27 | 6.6 ± 1.05 | 3.62 ± 0.77 | 4.21 ± 0.31 | 3.65 ± 0.30 |
| 2,3-dimethylpyrazine | 1.72 ± 0.40 | 0.74 ± 0.07 | 0.61 ± 0.09 | 0.51 ± 0.18 | 0.49 ± 0.12 | 0.34 ± 0.24 | 0.38 ± 0.25 |
| 2,5-dimethylthiophene | 17.0 ± 3.45 | 9.32 ± 0.50 | 9.22 ± 1.81 | 7.72 ± 0.5 | 4.56 ± 0.46 | 3.86 ± 0.61 | 3.94 ± 1.10 |
| Acetaldehyde | 3340 ± 748 | 110 ± 5 | 87.2 ± 2.7 | 121 ± 15.1 | 39.3 ± 11.4 | 50.3 ± 7.5 | 37.9 ± 7.4 |
| Allicin | 5.62 ± 2.79 | 1.53 ± 0.16 | 1.61 ± 1.02 | 1.74 ± 0.47 | 0.89 ± 0.3 | 1.48 ± 0.22 | 0.94 ± 0.38 |
| Allyl mercaptan | 46.6 ± 3.30 | 12.4 ± 2.0 | 9.88 ± 2.58 | 14.9 ± 0.7 | 13.4 ± 6.5 | 6.35 ± 0.22 | 5.90 ± 1.17 |
| Allyl methyl disulfide | 83.6 ± 15.4 | 44.6 ± 11.4 | 37.3 ± 9.4 | 27 ± 5 | 19.3 ± 10.1 | 14.8 ± 6.4 | 11.9 ± 5.1 |
| Allyl methyl sulfide | 38.7 ± 6.53 | 7.81 ± 1.75 | 14.5 ± 8.6 | 6.57 ± 0.53 | 17.2 ± 18.1 | 4.99 ± 3.93 | 4.29 ± 2.26 |
| Allyl methyl tetrasulfide | 2.40 ± 1.12 | 1.79 ± 0.27 | 1.97 ± 1.06 | 1.81 ± 0.50 | 1.34 ± 0.40 | 1.27 ± 0.12 | 1.45 ± 0.40 |
| Allyl methyl thiosulfinate | 7.72 ± 3.24 | 2.53 ± 0.71 | 1.88 ± 0.35 | 1.97 ± 0.43 | 1.03 ± 0.44 | 1.17 ± 0.19 | 1.03 ± 0.22 |
| Allyl methyl trisulfide | 21.2 ± 5.05 | 19.3 ± 4.1 | 18.0 ± 2.3 | 20.4 ± 1.8 | 8.13 ± 1.29 | 8.68 ± 1.33 | 8.90 ± 2.70 |
| Aniline | 1.78 ± 0.13 | 0.33 ± 0.08 | 0.28 ± 0.10 | 0.44 ± 0.18 | 0.36 ± 0.26 | 0.25 ± 0.17 | 0.33 ± 0.07 |
| Diallyl disulfide | 184 ± 7 | 151 ± 6.5 | 131 ± 29 | 119 ± 13 | 61.5 ± 8.8 | 63.4 ± 9.6 | 61.1 ± 4.1 |
| Diallyl sulphide | 48.1 ± 7.94 | 25.5 ± 3.4 | 29.1 ± 7.6 | 15.6 ± 1.35 | 13.5 ± 7.9 | 9.87 ± 6.34 | 5.15 ± 1.02 |
| Diallyl tetrasulfide | 2.80 ± 0.55 | 2.23 ± 0.16 | 2.06 ± 0.53 | 3.01 ± 1.00 | 1.88 ± 0.82 | 1.77 ± 1.15 | 2.20 ± 0.60 |
| Diallyl trisulfide | 56.5 ± 24.7 | 46.5 ± 7.2 | 48.5 ± 10.5 | 62 ± 3 | 28.7 ± 3.7 | 38.2 ± 10.3 | 32.1 ± 4.9 |
| Dimethyl disulphide | 14.7 ± 3.8 | 22.5 ± 1.4 | 5.79 ± 2.58 | 3.38 ± 0.90 | 5.30 ± 5.82 | 1.87 ± 0.66 | 2.45 ± 1.89 |
| Dimethyl sulfide | 273 ± 82 | 21.1 ± 1.3 | 25.4 ± 4.3 | 22.1 ± 10.7 | 10.9 ± 4.3 | 9.78 ± 1.44 | 8.75 ± 4.17 |
| Dimethyl thioether | 338 ± 107 | 22.4 ± 3.2 | 25.4 ± 4.3 | 25.1 ± 15.8 | 11.1 ± 4.0 | 10.3 ± 0.6 | 8.75 ± 4.17 |
| Dimethyl thiosulfinate | 2.62 ± 0.81 | 0.60 ± 0.26 | 0.67 ± 0.11 | 0.74 ± 0.17 | 0.97 ± 0.43 | 0.83 ± 0.14 | 0.71 ± 0.28 |
| Dimethyl trisulfide | 12.4 ± 0.5 | 8.09 ± 3.51 | 7.94 ± 1.83 | 6.73 ± 0.31 | 4.94 ± 3.08 | 3.75 ± 1.33 | 3.89 ± 2.03 |
| Dipropyl sulfide | 110 ± 6 | 69.6 ± 3.9 | 74.1 ± 6.36 | 66.3 ± 6.99 | 38.1 ± 6.0 | 44.5 ± 5.3 | 37.8 ± 5.06 |
| Methyl mercaptan | 17.7 ± 4.4 | 6.61 ± 0.81 | 1.70 ± 0.69 | 12.9 ± 1.04 | 15.4 ± 7.7 | 2.73 ± 0.63 | 2.79 ± 0.01 |
| Methyl propyl disulfide | 11.7 ± 2.2 | 6.19 ± 1.52 | 7.09 ± 1.75 | 5.51 ± 0.60 | 3.36 ± 2.11 | 2.75 ± 1.02 | 2.06 ± 0.56 |
| Pyridine | 1.66 ± 0.10 | 0.63 ± 0.22 | 0.29 ± 0.10 | 0.54 ± 0.13 | 0.32 ± 0.17 | 0.46 ± 0.02 | 0.45 ± 0.10 |
| Trimethylpyrazine | 3.60 ± 0.51 | 2.32 ± 0.38 | 1.90 ± 0.14 | 1.60 ± 0.13 | 1.20 ± 0.67 | 0.96 ± 0.33 | 0.71 ± 0.06 |
| Volatiles | Raw Garlic (Control) | 3% Fat | 10% Fat | 20% Fat | 40% Fat | 80% Fat |
|---|---|---|---|---|---|---|
| 1,3-dithiane | 5230 ± 441 | 101 ± 40 | 83.7 ± 17.5 | 102 ± 37 | 107 ± 15 | 11.8 ± 3.4 |
| 2-ethylpyrazine | 20.4 ± 2.1 | 2.10 ± 0.57 | 2.10 ± 0.23 | 2.41 ± 0.45 | 1.87 ± 0.23 | 1.01 ± 0.12 |
| 2-methyl-2-butenal | 73 ± 4 | 2.11 ± 0.20 | 3.26 ± 0.45 | 4.17 ± 0.33 | 3.52 ± 0.58 | 2.91 ± 0.37 |
| 2-methylbenzaldehyde | 70.2 ± 6.7 | 1.38 ± 0.33 | 1.91 ± 0.79 | 2.52 ± 0.99 | 2.31 ± 0.50 | 1.36 ± 0.13 |
| 2-vinyl-4H-1,3-dithiin | 8.06 ± 2.07 | 8.74 ± 0.59 | 8.43 ± 1.13 | 8.27 ± 2.44 | 5.12 ± 0.75 | 3.46 ± 0.56 |
| 2,3-dimethylpyrazine | 25.6 ± 1.3 | 3.36 ± 1.00 | 2.34 ± 0.24 | 2.73 ± 0.60 | 2.07 ± 0.97 | 0.73 ± 0.01 |
| 2,5-dimethylthiophene | 227 ± 18 | 25.2 ± 1.8 | 12.4 ± 0.6 | 9.03 ± 3.50 | 5.29 ± 0.70 | 1.96 ± 0.16 |
| Acetaldehyde | 11,000 ± 1050 | 1640 ± 270 | 2360 ± 307 | 3520 ± 544 | 3680 ± 716 | 3500 ± 544 |
| Allicin | 28.7 ± 1.4 | 26.1 ± 5.1 | 24.1 ± 1.2 | 20.1 ± 2.9 | 12.5 ± 3.1 | 6.40 ± 1.34 |
| Allyl mercaptan | 154,000 ± 9560 | 9730 ± 1100 | 11,000 ± 1080 | 11,000 ± 3690 | 8820 ± 2210 | 2280 ± 247 |
| Allyl methyl disulfide | 6830 ± 378 | 968 ± 579 | 404 ± 49 | 291 ± 63 | 137 ± 32 | 14.9 ± 2.8 |
| Allyl methyl sulfide | 1040 ± 86 | 55.1 ± 10.8 | 53.7 ± 9.7 | 60.8 ± 23.1 | 45.5 ± 15.2 | 10.5 ± 0.2 |
| Allyl methyl tetrasulfide | 6.08 ± 2.10 | 1.24 ± 0.49 | 1.33 ± 0.81 | 1.28 ± 0.32 | 0.87 ± 0.35 | 0.92 ± 0.53 |
| Allyl methyl thiosulfinate | 55.3 ± 3.9 | 20.9 ± 1.4 | 12.9 ± 0.9 | 15.0 ± 2.33 | 14.5 ± 2.5 | 8.52 ± 0.75 |
| Allyl methyl trisulfide | 21.5 ± 1.6 | 1.70 ± 0.22 | 1.25 ± 0.76 | 1.16 ± 0.18 | 1.14 ± 0.57 | 1.00 ± 0.46 |
| Aniline | 21.8 ± 0.4 | 0.95 ± 0.17 | 1.21 ± 0.20 | 2.69 ± 1.49 | 2.17 ± 0.76 | 1.97 ± 0.62 |
| Diallyl disulfide | 69,100 ± 3100 | 964 ± 735 | 322 ± 5 | 213 ± 65 | 119 ± 24 | 19.5 ± 2.3 |
| Diallyl sulphide | 3080 ± 253 | 45.2 ± 23.6 | 24.0 ± 3.2 | 27.3 ± 7.7 | 38.4 ± 7.6 | 7.33 ± 1.58 |
| Diallyl tetrasulfide | 10.5 ± 1.6 | 2.92 ± 1.11 | 3.74 ± 0.14 | 3.05 ± 0.38 | 4.06 ± 2.29 | 1.58 ± 0.41 |
| Diallyl trisulfide | 566 ± 57 | 12.8 ± 8.1 | 6.13 ± 0.97 | 3.41 ± 1.69 | 3.49 ± 0.11 | 1.78 ± 0.09 |
| Dimethyl disulphide | 174 ± 5 | 96.6 ± 29.9 | 69.2 ± 11.7 | 65.9 ± 14.7 | 31.2 ± 8.6 | 2.93 ± 1.00 |
| Dimethyl sulfide | 1980 ± 68 | 79.6 ± 28.0 | 59.6 ± 4.7 | 68.7 ± 7.4 | 49.2 ± 6.3 | 24.5 ± 1.4 |
| Dimethyl thioether | 2070 ± 123 | 91.2 ± 38.5 | 72.1 ± 6.4 | 85.9 ± 10.9 | 54.4 ± 15.0 | 24.5 ± 1.4 |
| Dimethyl thiosulfinate | 12 ± 1 | 0.79 ± 0.45 | 1.22 ± 0.38 | 1.14 ± 0.21 | 0.93 ± 0.16 | 0.65 ± 0.25 |
| Dimethyl trisulfide | 5.53 ± 0.65 | 1.60 ± 0.34 | 2.15 ± 0.46 | 1.65 ± 1.11 | 1.58 ± 0.19 | 1.41 ± 0.30 |
| Dipropyl sulfide | 4480 ± 303 | 278 ± 152 | 148 ± 11 | 132 ± 33 | 112 ± 20 | 74 ± 13 |
| Methyl mercaptan | 15,300 ± 1330 | 4540 ± 1100 | 6360 ± 1120 | 7530 ± 1890 | 7920 ± 1350 | 2410 ± 239 |
| Methyl propyl disulfide | 1130 ± 51 | 114 ± 67 | 49.9 ± 5.2 | 41.3 ± 9.2 | 19.5 ± 5.1 | 2.69 ± 0.39 |
| Pyridine | 35.8 ± 3.8 | 1.86 ± 0.36 | 2.56 ± 0.19 | 3.27 ± 0.89 | 2.13 ± 0.45 | 0.97 ± 0.44 |
| Trimethylpyrazine | 343 ± 11 | 35.3 ± 19.6 | 16.5 ± 2.8 | 12.1 ± 3.3 | 6.12 ± 1.04 | 1.19 ± 0.15 |
| Volatiles | Fried Garlic (Control) | 3% Fat | 10% Fat | 20% Fat | 40% Fat | 80% Fat |
|---|---|---|---|---|---|---|
| 1,3-dithiane | 115 ± 25 | 14.0 ± 1.8 | 8.16 ± 4.57 | 3.50 ± 0.27 | 2.53 ± 0.50 | 0.87 ± 0.39 |
| 2-ethylpyrazine | 2.08 ± 1.32 | 0.68 ± 0.07 | 0.76 ± 0.64 | 0.45 ± 0.41 | 0.45 ± 0.29 | 0.48 ± 0.05 |
| 2-methyl-2-butenal | 50.2 ± 13.0 | 6.88 ± 1.62 | 5.21 ± 1.27 | 3.76 ± 0.30 | 3.74 ± 0.77 | 2.03 ± 0.24 |
| 2-methylbenzaldehyde | 1.82 ± 0.53 | 0.40 ± 0.22 | 0.43 ± 0.35 | 0.87 ± 0.37 | 0.63 ± 0.16 | 0.42 ± 0.24 |
| 2-vinyl-4H-1,3-dithiin | 29.2 ± 6.7 | 3.25 ± 1.07 | 1.52 ± 0.11 | 1.44 ± 0.55 | 1.12 ± 0.45 | 1.71 ± 0.44 |
| 2,3-dimethylpyrazine | 1.34 ± 0.79 | 0.76 ± 0.36 | 0.59 ± 0.56 | 0.41 ± 0.43 | 0.28 ± 0.17 | 0.34 ± 0.03 |
| 2,5-dimethylthiophene | 26.8 ± 4.7 | 3.57 ± 0.97 | 2.04 ± 0.67 | 1.45 ± 0.08 | 1.98 ± 0.40 | 1.50 ± 0.61 |
| Acetaldehyde | 2540 ± 600 | 218 ± 42 | 272 ± 7 | 311 ± 21 | 330 ± 29 | 261 ± 7 |
| Allicin | 15.7 ± 9.1 | 8.48 ± 5.03 | 2.08 ± 1.18 | 0.97 ± 0.39 | 0.80 ± 0.12 | 0.62 ± 0.35 |
| Allyl mercaptan | 80.7 ± 9.5 | 43.7 ± 6.8 | 20.3 ± 2.7 | 16.7 ± 3.5 | 17.0 ± 3.2 | 11.3 ± 1.1 |
| Allyl methyl disulfide | 266 ± 39 | 26.6 ± 9.0 | 10.2 ± 4.4 | 3.59 ± 0.28 | 2.99 ± 0.23 | 1.00 ± 0.24 |
| Allyl methyl sulfide | 104 ± 8 | 30.6 ± 3.3 | 14.0 ± 6.7 | 8.63 ± 0.93 | 7.50 ± 1.60 | 7.42 ± 0.70 |
| Allyl methyl tetrasulfide | 2.42 ± 1.25 | 1.05 ± 0.30 | 0.89 ± 0.30 | 0.76 ± 0.25 | 0.49 ± 0.11 | 0.64 ± 0.21 |
| Allyl methyl thiosulfinate | 27.0 ± 5.1 | 8.94 ± 3.77 | 6.56 ± 0.23 | 6.39 ± 0.76 | 3.49 ± 0.63 | 3.36 ± 1.30 |
| Allyl methyl trisulfide | 43.5 ± 11.4 | 3.77 ± 1.43 | 2.00 ± 0.47 | 1.44 ± 0.56 | 1.02 ± 0.33 | 0.40 ± 0.09 |
| Aniline | 2.38 ± 0.63 | 1.03 ± 0.35 | 0.67 ± 0.09 | 0.52 ± 0.10 | 0.48 ± 0.10 | 0.63 ± 0.10 |
| Diallyl disulfide | 355 ± 61 | 20.3 ± 5.53 | 6.23 ± 1.40 | 3.42 ± 0.40 | 1.58 ± 0.48 | 0.82 ± 0.30 |
| Diallyl sulphide | 68.1 ± 10.5 | 12.3 ± 2.1 | 5.14 ± 2.59 | 3.21 ± 0.55 | 1.99 ± 0.22 | 1.54 ± 0.45 |
| Diallyl tetrasulfide | 2.60 ± 0.34 | 1.84 ± 0.84 | 2.35 ± 1.75 | 1.12 ± 0.68 | 1.18 ± 0.11 | 1.52 ± 0.40 |
| Diallyl trisulfide | 81.5 ± 19.9 | 8.18 ± 4.25 | 2.34 ± 1.62 | 1.02 ± 0.41 | 0.90 ± 0.20 | 1.08 ± 0.12 |
| Dimethyl disulphide | 56.5 ± 6.9 | 14.9 ± 0.2 | 5.87 ± 2.34 | 2.96 ± 0.89 | 2.12 ± 0.40 | 1.00 ± 0.17 |
| Dimethyl sulfide | 281 ± 46 | 122 ± 26 | 73.0 ± 26.3 | 50.5 ± 17.5 | 48.1 ± 5.8 | 28.8 ± 2.1 |
| Dimethyl thioether | 355 ± 50 | 151 ± 31 | 88.4 ± 33.6 | 56.2 ± 12.7 | 56.5 ± 4.1 | 31.5 ± 1.0 |
| Dimethyl thiosulfinate | 4.71 ± 0.77 | 0.94 ± 0.24 | 0.56 ± 0.22 | 0.78 ± 0.28 | 0.56 ± 0.20 | 0.70 ± 0.18 |
| Dimethyl trisulfide | 42.9 ± 16.3 | 6.75 ± 1.00 | 3.46 ± 2.21 | 2.67 ± 0.85 | 1.55 ± 0.40 | 1.30 ± 0.63 |
| Dipropyl sulfide | 205 ± 51 | 41.6 ± 11.6 | 21.3 ± 4.9 | 20.6 ± 1.2 | 13.0 ± 1.97 | 16.0 ± 2.74 |
| Methyl mercaptan | 35.0 ± 5.5 | 11.3 ± 2.6 | 6.54 ± 0.96 | 6.88 ± 2.35 | 4.70 ± 3.40 | 2.82 ± 3.08 |
| Methyl propyl disulfide | 36.7 ± 9.5 | 5.24 ± 1.09 | 2.78 ± 0.45 | 2.19 ± 0.28 | 0.86 ± 0.28 | 1.17 ± 0.17 |
| Pyridine | 2.22 ± 0.14 | 0.58 ± 0.18 | 0.47 ± 0.26 | 0.29 ± 0.05 | 0.36 ± 0.12 | 0.57 ± 0.24 |
| Trimethylpyrazine | 9.60 ± 1.19 | 1.50 ± 0.24 | 1.08 ± 0.32 | 0.78 ± 0.12 | 0.45 ± 0.09 | 0.52 ± 0.26 |
References
- Ahmad, J.I. Garlic—A panacea for health and good taste? Nutr. Food Sci. 1996, 96, 32–35. [Google Scholar] [CrossRef]
- Block, E. The Chemistry of Garlic and Onions. Sci. Am. 1985, 252, 114–118. [Google Scholar] [CrossRef] [PubMed]
- Sasi, M.; Kumar, S.; Kumar, M.; Thapa, S.; Prajapati, U.; Tak, Y.; Changan, S.; Saurabh, V.; Kumari, S.; Kumar, A.; et al. Garlic (Allium sativum L.) Bioactives and Its Role in Alleviating Oral Pathologies. Antioxidants 2021, 10, 1847. [Google Scholar] [CrossRef]
- Borlinghaus, J.; Albrecht, F.; Gruhlke, M.C.H.; Nwachukwu, I.D.; Slusarenko, A.J. Allicin: Chemistry and Biological Properties. Molecules 2014, 19, 12591–12618. [Google Scholar] [CrossRef] [PubMed]
- Palazzolo, C.T.; Ramirez, D.; Locatelli, D.A.; Manucha, W.; Castro, C.; Camargo, A. Bioaccessibility and permeability of bioactive compounds in raw and cooked garlic. J. Food Compos. Anal. 2018, 70, 49–53. [Google Scholar] [CrossRef]
- Castada, H.Z.; Mirondo, R.; Sigurdson, G.T.; Giusti, M.M.; Barringer, S. Deodorization of garlic odor by spearmint, peppermint, and chocolate mint leaves and rosmarinic acid. LWT 2017, 84, 160–167. [Google Scholar] [CrossRef]
- Negishi, O.; Negishi, Y. Enzymatic Deodorization with Raw Fruits, Vegetables and Mushrooms. Food Sci. Technol. Res. 1999, 5, 176–180. [Google Scholar] [CrossRef]
- Sinir, G.Ö.; Barrınger, S. Deodorization of garlic odor by fresh and dried herbs using SIFT-MS. GIDA J. Food 2021, 46, 358–366. [Google Scholar] [CrossRef]
- Munch, R.; Barringer, S.A. Deodorization of Garlic Breath Volatiles by Food and Food Components. J. Food Sci. 2014, 79, C526–C533. [Google Scholar] [CrossRef]
- Negishi, O.; Negishi, Y.; Ozawa, T. Effects of Food Materials on Removal of Allium-Specific Volatile Sulfur Compounds. J. Agric. Food Chem. 2002, 50, 3856–3861. [Google Scholar] [CrossRef]
- Mirondo, R.; Barringer, S. Deodorization of Garlic Breath by Foods, and the Role of Polyphenol Oxidase and Phenolic Compounds. J. Food Sci. 2016, 81, C2425–C2430. [Google Scholar] [CrossRef] [PubMed]
- Hansanugrum, A.; Barringer, S.A. Effect of Milk on the Deodorization of Malodorous Breath after Garlic Ingestion. J. Food Sci. 2010, 75, C549–C558. [Google Scholar] [CrossRef] [PubMed]
- Kühn, J.; Considine, T.; Singh, H. Interactions of Milk Proteins and Volatile Flavor Compounds: Implications in the Development of Protein Foods. J. Food Sci. 2006, 71, R72–R82. [Google Scholar] [CrossRef]
- Druaux, C.; Le Thanh, M.; Seuvre, A.M.; Voilley, A. Application of headspace analysis to the study of aroma compounds-lipids interactions. J. Am. Oil Chem. Soc. 1998, 75, 127–130. [Google Scholar] [CrossRef]
- Ramesh, C.C. Chapter 2—An Overview of Yogurt Production and Composition. In Yogurt in Health and Disease Prevention; Nagendra, P.S., Ed.; Academic Press: Cambridge, MA, USA, 2017; pp. 31–47. ISBN 9780128051344. [Google Scholar] [CrossRef]
- Tamaki, K.; Sonoki, S.; Tamaki, T.; Ehara, K. Measurement of odour after in vitro or in vivo ingestion of raw or heated garlic, using electronic nose, gas chromatography and sensory analysis. Int. J. Food Sci. Technol. 2008, 43, 130–139. [Google Scholar] [CrossRef]
- Sato, S.; Sekine, Y.; Kakumu, Y. Measurement of diallyl disulfide and allyl methyl sulfide emanating from human skin surface and influence of ingestion of grilled garlic. Sci. Rep. 2020, 10, 465. [Google Scholar] [CrossRef]
- National Center for Biotechnology Information. PubChem Compound Summary for CID 11617, Diallyl Sulfide. 2023. Available online: https://pubchem.ncbi.nlm.nih.gov/compound/Diallyl-sulfide (accessed on 5 June 2023).
- Iberl, B.; Winkler, G.; Müller, B.; Knobloch, K. Quantitative Determination of Allicin and Alliin from Garlic by HPLC*. Planta Medica 1990, 56, 320–326. [Google Scholar] [CrossRef]
- Locatelli, D.A.; Altamirano, J.C.; González, R.E.; Camargo, A.B. Home-cooked garlic remains a healthy food. J. Funct. Foods 2015, 16, 1–8. [Google Scholar] [CrossRef]
- Wang, J.; Qiao, L.; Wang, R.; Zhang, N.; Liu, Y.; Chen, H.; Sun, J.; Wang, S.; Zhang, Y. Effect of Frying Process on the Flavor Variations of Allium Plants. Foods 2023, 12, 1371. [Google Scholar] [CrossRef] [PubMed]
- Reineccius, G. Flavor Chemistry and Technology, 2nd ed.; CRC Press: Boca Raton, FL, USA, 2005. [Google Scholar] [CrossRef]
- Yu, T.H.; Wu, C.M.; Ho, C.T. Volatile compounds of deep-oil fried, microwave-heated and oven-baked garlic slices. J. Agric. Food Chem. 1993, 41, 800–805. [Google Scholar] [CrossRef]
- Abe, K.; Hori, Y.; Myoda, T. Volatile compounds of fresh and processed garlic. Exp. Ther. Med. 2020, 19, 1585–1593. [Google Scholar] [CrossRef] [PubMed]
- Buttery, R.G.; Dante, G.G.; Louisa, C.L. Flavor Compounds. Volatilities in Vegetable Oil and Oil-Water Mixtures. Estimation of Odor Thresholds. J. Agric. Food Chem. 1973, 21, 198–201. [Google Scholar] [CrossRef]
- Alberts, B.; Johnson, A.; Lewis, J. Protein Function. In Molecular Biology of the Cell, 4th ed.; Garland Science: New York, NY, USA, 2002. Available online: https://www.ncbi.nlm.nih.gov/books/NBK26911/ (accessed on 26 May 2023).
- Anema, S.G.; Li, Y. Effect of pH on the Association of Denatured Whey Proteins with Casein Micelles in Heated Reconstituted Skim Milk. J. Agric. Food Chem. 2003, 51, 1640–1646. [Google Scholar] [CrossRef]
- Mahomud, M.S.; Katsuno, N.; Nishizu, T. Role of whey protein-casein complexes on yogurt texture. Rev. Agric. Sci. 2017, 5, 1–12. [Google Scholar] [CrossRef]
- Vasbinder, A.J.; Alting, A.C.; Visschers, R.W.; de Kruif, C.G. Texture of acid milk gels: Formation of disulfide cross-links during acidification. Int. Dairy J. 2003, 13, 29–38. [Google Scholar] [CrossRef]
- Kühn, J.; Considine, T.; Singh, H. Binding of Flavor Compounds and Whey Protein Isolate as Affected by Heat and High Pressure Treatments. J. Agric. Food Chem. 2008, 56, 10218–10224. [Google Scholar] [CrossRef]
- Hansen, A.P. A Review of the Interactions between Milk Proteins and Dairy Flavor Compounds. In Food Proteins and Lipids. Advances in Experimental Medicine and Biology; Damodaran, S., Ed.; Springer: Boston, MA, USA, 1997; Volume 415. [Google Scholar] [CrossRef]
- Eshpari, H.; Tong, P.S.; Corredig, M. Changes in the physical properties, solubility, and heat stability of milk protein concentrates prepared from partially acidified milk. J. Dairy Sci. 2014, 97, 7394–7401. [Google Scholar] [CrossRef]
- Jocelyn, P.C. Chapter 16: Thioldisulfide exchange methods. In Biochemistry of SH Group: The Occurrence, Chemical Properties, Metabolism and Biological Function of Thiols and Disulphides; Academic Press: New York, NY, USA, 1972; pp. 148–154. [Google Scholar]
- Adams, R.L.; Mottram, D.S.; Parker, J.K.; Brown, H.M. Flavor-protein binding: Disulfide interchange reactions between ovalbumin and volatile disulfides. J. Agric. Food Chem. 2001, 49, 4333–4336. [Google Scholar] [CrossRef]
- Parker, J.K.; Mottram, D.S.; Adams, R.L. Interaction of sulphur-containing aroma compounds with proteins in both model systems and real food systems. In Flavour Research at the Dawn of the Twenty-First Century: Proceedings of the 10th Weurman Flavour Research Symposium; Le Quéré, J.L., Étiévant, P.X., Eds.; Intercept: London, UK, 2002; pp. 45–50. ISBN 2743006390. [Google Scholar]
- Wiedemann, C.; Kumar, A.; Lang, A.; Ohlenschläger, O. Cysteines and Disulfide Bonds as Structure-Forming Units: Insights from Different Domains of Life and the Potential for Characterization by NMR. Front. Chem. 2020, 8, 280. [Google Scholar] [CrossRef]
- Whitt, D.M.; Pranata, J.; Carter, B.G.; Barbano, D.M.; Drake, M.A. Effects of micellar casein concentrate purity and milk fat on sulfur/eggy flavor in ultrapasteurized milk-based beverages. J. Dairy Sci. 2022, 105, 5700–5713. [Google Scholar] [CrossRef]
- Zhang, L.; Zhou, R.; Zhang, J.; Zhou, P. Heat-induced denaturation and bioactivity changes of whey proteins. Int. Dairy J. 2021, 123, 105175. [Google Scholar] [CrossRef]
- Hoffman, J.R.; Falvo, M.J. Protein—Which is Best? J. Sports Sci. Med. 2004, 3, 118–130. [Google Scholar] [PubMed]
- Esteghlal, S.; Gahruie, H.H.; Niakousari, M.; Barba, F.J.; Bekhit, A.E.-D.; Mallikarjunan, K.; Roohinejad, S. Bridging the Knowledge Gap for the Impact of Non-Thermal Processing on Proteins and Amino Acids. Foods 2019, 8, 262. [Google Scholar] [CrossRef]
- Aguilera, J.M. The food matrix: Implications in processing, nutrition and health. Crit. Rev. Food Sci. Nutr. 2019, 59, 3612–3629. [Google Scholar] [CrossRef]







| Volatiles | Raw Garlic | Fried Garlic |
|---|---|---|
| 1,3-dithiane | 1240 ± 879 | 65.9 ± 31.8 |
| 2-ethylpyrazine | 7.20 ± 5.83 | 11.0 ± 4.5 |
| 2-methyl-2-butenal | 60.9 ± 49.1 | 23.6 ± 8.67 |
| 2-methylbenzaldehyde | 5.97 ± 3.37 | 1.36 ± 0.77 |
| 2-vinyl-4H-1,3-dithiin | 4.21 ± 2.94 | 8.26 ± 3.26 |
| 2,3-dimethylpyrazine | 4.81 ± 3.89 | 15.5 ± 10.7 |
| 2,5-dimethylthiophene | 134 ± 100 | 9.85 ± 1.62 |
| Acetaldehyde | 19,100 ± 11,300 | 7990 ± 3390 |
| Allicin | 1330 ± 1190 | 81.4 ± 42.1 |
| Allyl mercaptan | 116,000 ± 20,200 | 121 ± 8 |
| Allyl methyl disulfide | 13,400 ± 8780 | 79.8 ± 29.2 |
| Allyl methyl sulfide | 1380 ± 385 | 73.3 ± 9.3 |
| Allyl methyl tetrasulfide | 2.56 ± 1.25 | 1.66 ± 0.57 |
| Allyl methyl thiosulfinate | 89.4 ± 73.8 | 8.06 ± 2.02 |
| Allyl methyl trisulfide | 35.7 ± 18.5 | 7.46 ± 0.76 |
| Aniline | 2.56 ± 1.56 | 5.93 ± 3.34 |
| Diallyl disulfide | 26,200 ± 19,000 | 69.1 ± 43.5 |
| Diallyl sulfide | 1900 ± 711 | 51.8 ± 5.7 |
| Diallyl tetrasulfide | 2.80 ± 2.12 | 1.18 ± 0.51 |
| Diallyl trisulfide | 236 ± 175 | 14.7 ± 4.2 |
| Dimethyl disulfide | 554 ± 367 | 47.9 ± 24.8 |
| Dimethyl sulfide | 981 ± 692 | 384 ± 159 |
| Dimethyl thioether | 1130 ± 794 | 447 ± 174 |
| Dimethyl thiosulfinate | 10.7 ± 4.7 | 3.63 ± 0.08 |
| Dimethyl trisulfide | 10.9 ± 4.94 | 9.58 ± 4.06 |
| Dipropyl sulfide | 5130 ± 4240 | 16.3 ± 4.1 |
| Methyl mercaptan | 24,400 ± 3580 | 55.9 ± 7.15 |
| Methyl propyl disulfide | 1970 ± 1350 | 19.5 ± 9.9 |
| Pyridine | 2.42 ± 1.65 | 6.91 ± 3.19 |
| Trimethylpyrazine | 493 ± 344 | 6.14 ± 2.86 |
| Volatiles | Raw Garlic | Yogurt + Raw Garlic | Fried Garlic | Yogurt + Fried Garlic |
|---|---|---|---|---|
| 1,3-dithiane | 4020 ± 1770 | 20.4 ± 6.6 | 203 ± 2 | 16.6 ± 15.6 |
| 2-ethylpyrazine | 29.9 ± 15.7 | 0.67 ± 0.10 | 25.4 ± 4.6 | 1.08 ± 0.76 |
| 2-methyl-2-butenal | 93.0 ± 37.9 | 2.36 ± 0.39 | 36.3 ± 1.5 | 3.59 ± 2.81 |
| 2-methylbenzaldehyde | 51.6 ± 25.1 | 4.20 ± 0.50 | 2.32 ± 0.09 | 2.91 ± 2.11 |
| 2-vinyl-4H-1,3-dithiin | 8.59 ± 3.22 | 3.18 ± 1.02 | 13.0 ± 0.2 | 1.76 ± 0.97 |
| 2,3-dimethylpyrazine | 38.8 ± 17.1 | 0.44 ± 0.06 | 51.9 ± 3.1 | 0.95 ± 0.84 |
| 2,5-dimethylthiophene | 401 ± 212 | 2.59 ± 0.54 | 19.9 ± 0.7 | 1.77 ± 1.10 |
| Acetaldehyde | 10,400 ± 617 | 9210 ± 455 | 3380 ± 322 | 6770 ± 5310 |
| Allicin | 93.9 ± 39.9 | 3.66 ± 2.73 | 3.86 ± 1.03 | 0.41 ± 0.24 |
| Allyl mercaptan | 110,000 ± 28,600 | 832 ± 324 | 169 ± 5 | 29.0 ± 27.8 |
| Allyl methyl disulfide | 20,100 ± 6150 | 23.2 ± 8.1 | 350 ± 18 | 18.1 ± 16.2 |
| Allyl methyl sulfide | 965 ± 275 | 8.39 ± 0.34 | 742 ± 20 | 75.3 ± 90.1 |
| Allyl methyl tetrasulfide | 9.34 ± 4.43 | 2.12 ± 0.30 | 2.90 ± 0.16 | 1.27 ± 0.17 |
| Allyl methyl thiosulfinate | 50.8 ± 23.8 | 0.98 ± 0.69 | 28.9 ± 1.9 | 1.29 ± 0.83 |
| Allyl methyl trisulfide | 35.3 ± 14.6 | 1.13 ± 0.15 | 54.0 ± 2.4 | 3.54 ± 2.78 |
| Aniline | 9.38 ± 3.67 | 0.49 ± 0.09 | 15.7 ± 2.6 | 0.29 ± 0.27 |
| Diallyl disulfide | 102,000 ± 56,900 | 28.3 ± 8.7 | 84.9 ± 2.3 | 4.64 ± 3.92 |
| Diallyl sulfide | 1720 ± 841 | 5.96 ± 2.40 | 250 ± 14 | 18.1 ± 21.8 |
| Diallyl tetrasulfide | 13.3 ± 6.03 | 3.38 ± 0.11 | 1.44 ± 0.23 | 1.20 ± 0.66 |
| Diallyl trisulfide | 981 ± 522 | 2.35 ± 0.79 | 28.4 ± 1.8 | 2.11 ± 1.49 |
| Dimethyl disulfide | 487 ± 71.3 | 2.93 ± 0.92 | 293 ± 3 | 23.1 ± 27.6 |
| Dimethyl sulfide | 2870 ± 1350 | 69.7 ± 4.21 | 151 ± 16 | 37.0 ± 33.8 |
| Dimethyl thioether | 2990 ± 950 | 77.6 ± 7.43 | 182 ± 43 | 43.4 ± 40.3 |
| Dimethyl thiosulfinate | 13.5 ± 6.8 | 1.00 ± 0.51 | 15.8 ± 0.2 | 0.81 ± 0.26 |
| Dimethyl trisulfide | 10.6 ± 1.6 | 2.24 ± 0.53 | 110 ± 15 | 7.00 ± 5.40 |
| Dipropyl sulfide | 7900 ± 2530 | 30.5 ± 4.40 | 194 ± 15 | 23.6 ± 19.2 |
| Methyl mercaptan | 16,600 ± 1300 | 449 ± 172 | 218 ± 29 | 102 ± 103 |
| Methyl propyl disulfide | 3410 ± 1130 | 4.92 ± 0.83 | 71.1 ± 1.7 | 4.07 ± 3.59 |
| Pyridine | 20.8 ± 6.6 | 0.78 ± 0.11 | 16.6 ± 0.8 | 0.76 ± 0.65 |
| Trimethylpyrazine | 958 ± 364 | 1.80 ± 0.33 | 24.0 ± 1.5 | 1.38 ± 0.96 |
| Volatile Compound | Ion Product | Reagent Ion | m/z | Reaction Rate (k) 10−9 cm3 s−1 |
|---|---|---|---|---|
| 1-3-dithiane | C4H7S+ | O2+ | 87 | 2.3 |
| 2-ethylpyrazine | C6H8N2.H+ | H3O+ | 109 | 3 |
| 2-Methyl-2-butenal | C5H7O+ | NO+ | 83 | 4 |
| 2-methylbenzyaldehyde | C8H7O+ | NO+ | 119 | 3.3 |
| 2-vinyl-4H-1,3-dithiin | C6H8S2+ | NO+ | 144 | 2.4 |
| 2,3-dimethylpyrazine | C6N2H8.H+ | H3O+ | 109 | 3.4 |
| 2,5-dimethylthiophene | C6H8S.H+ | H3O+ | 113 | 3 |
| Acetaldehyde | CH3CO+ | NO+ | 43 | 6 |
| C2H5O+ | H3O+ | 45 | 3.7 | |
| Allicin | C6H10OS2 | NO+ | 162 | 2.4 |
| Allyl mercaptan | C3H6S | NO+ | 74 | 2.4 |
| C3H6S.H+ | H3O+ | 75 | 2.6 | |
| Allyl methyl disulfide | C4H8S2 | NO+ | 120 | 2.4 |
| C4H8S2.H+ | H3O+ | 121 | 2.6 | |
| Allyl methyl sulfide | C4H8S+ | NO+ | 88 | 2.5 |
| C4H8S.H+ | H3O+ | 89 | 3 | |
| Allyl methyl tetrasulfide | C4H8S4+ | NO+ | 184 | 2.4 |
| Allyl methyl thiosulfinate | C4H8S2O | NO+ | 136 | 2.4 |
| Allyl methyl trisulfide | C4H8S3+ | NO+ | 152 | 2.4 |
| Aniline | C6H5NH2.H+ | H3O+ | 94 | 2.8 |
| Diallyl disulfide | (C3H5)2S2.H+ | H3O+ | 147 | 3 |
| Diallyl sulfide | (C3H5)2S.H+ | H3O+ | 115 | 2.9 |
| Diallyl tetrasulfide | C6H10S4+ | NO+ | 210 | 2.4 |
| Diallyl trisulfide | C6H10S3 | NO+ | 178 | 2.4 |
| Dimethyl disulfide | (CH3)2S2.H+ | H3O+ | 95 | 2.6 |
| Dimethyl sulfide | (CH3)2S+ | O2+ | 62 | 2.2 |
| Dimethyl thioether | (CH3)2S+ | O2+ | 62 | 2.2 |
| (CH3)2S+ | NO+ | 62 | 2.2 | |
| Dimethyl thiosulfinate | C2H6OS2+ | NO+ | 110 | 2.4 |
| Dimethyl trisulfide | C2H6S3+ | NO+ | 126 | 1.9 |
| Dipropyl sulfide | C3H4S+ | O2+ | 72 | 2.4 |
| Methyl mercaptan | CH4S.H+ | H3O+ | 49 | 1.8 |
| Methyl propyl disulfide | C4H10S2+ | NO+ | 122 | 1.5 |
| C4H10S2.H+ | H3O+ | 123 | 2.5 | |
| Pyridine | C5H5N.H+ | H3O+ | 80 | 3.3 |
| Trimethylpyrazine | C7H10N2+ | O2+ | 122 | 2.5 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kaur, M.; Barringer, S. Effect of Yogurt and Its Components on the Deodorization of Raw and Fried Garlic Volatiles. Molecules 2023, 28, 5714. https://doi.org/10.3390/molecules28155714
Kaur M, Barringer S. Effect of Yogurt and Its Components on the Deodorization of Raw and Fried Garlic Volatiles. Molecules. 2023; 28(15):5714. https://doi.org/10.3390/molecules28155714
Chicago/Turabian StyleKaur, Manpreet, and Sheryl Barringer. 2023. "Effect of Yogurt and Its Components on the Deodorization of Raw and Fried Garlic Volatiles" Molecules 28, no. 15: 5714. https://doi.org/10.3390/molecules28155714
APA StyleKaur, M., & Barringer, S. (2023). Effect of Yogurt and Its Components on the Deodorization of Raw and Fried Garlic Volatiles. Molecules, 28(15), 5714. https://doi.org/10.3390/molecules28155714







