Platycodonis Radix Alleviates LPS-Induced Lung Inflammation through Modulation of TRPA1 Channels
Abstract
1. Introduction
2. Results
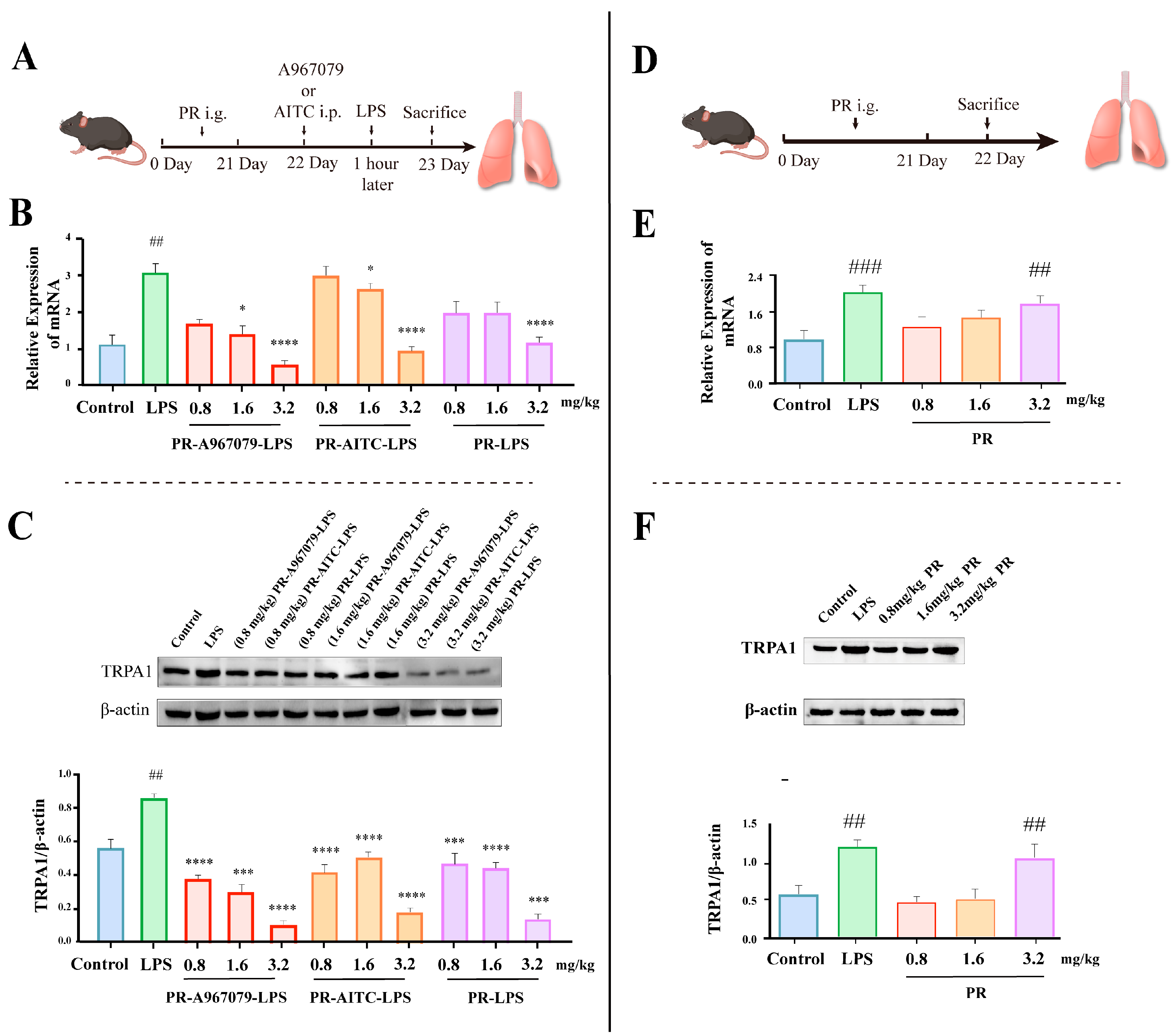
2.1. PR Alleviates LPS-Induced Lung Inflammation through Reducing TRPA1 Expression in Mice
2.2. PR Exhibits a Certain Elevating Effect on TRPA1 Expression in Normal Mice
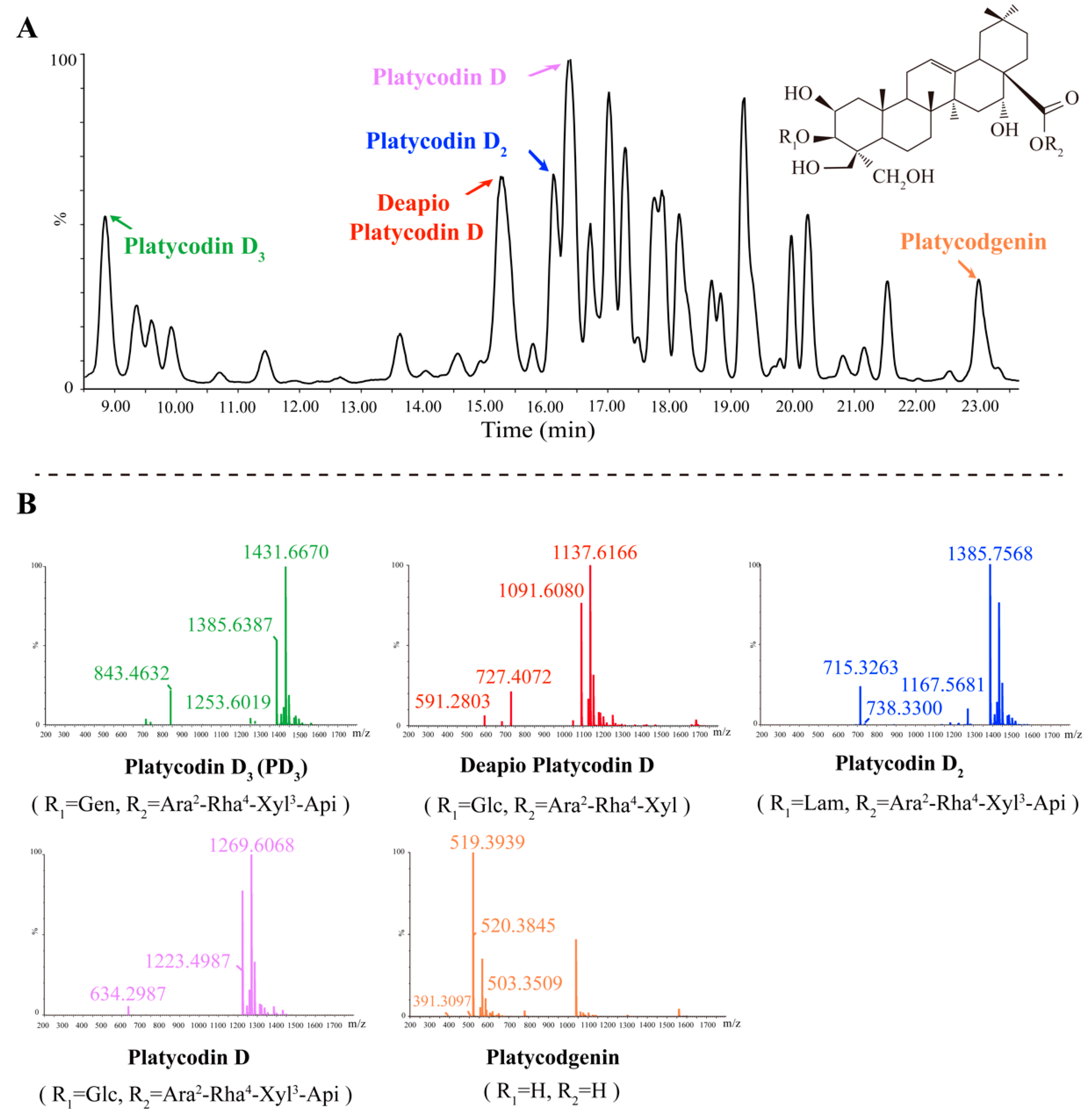
2.3. Five Primary Platycodins Identified in a PR Water Extract
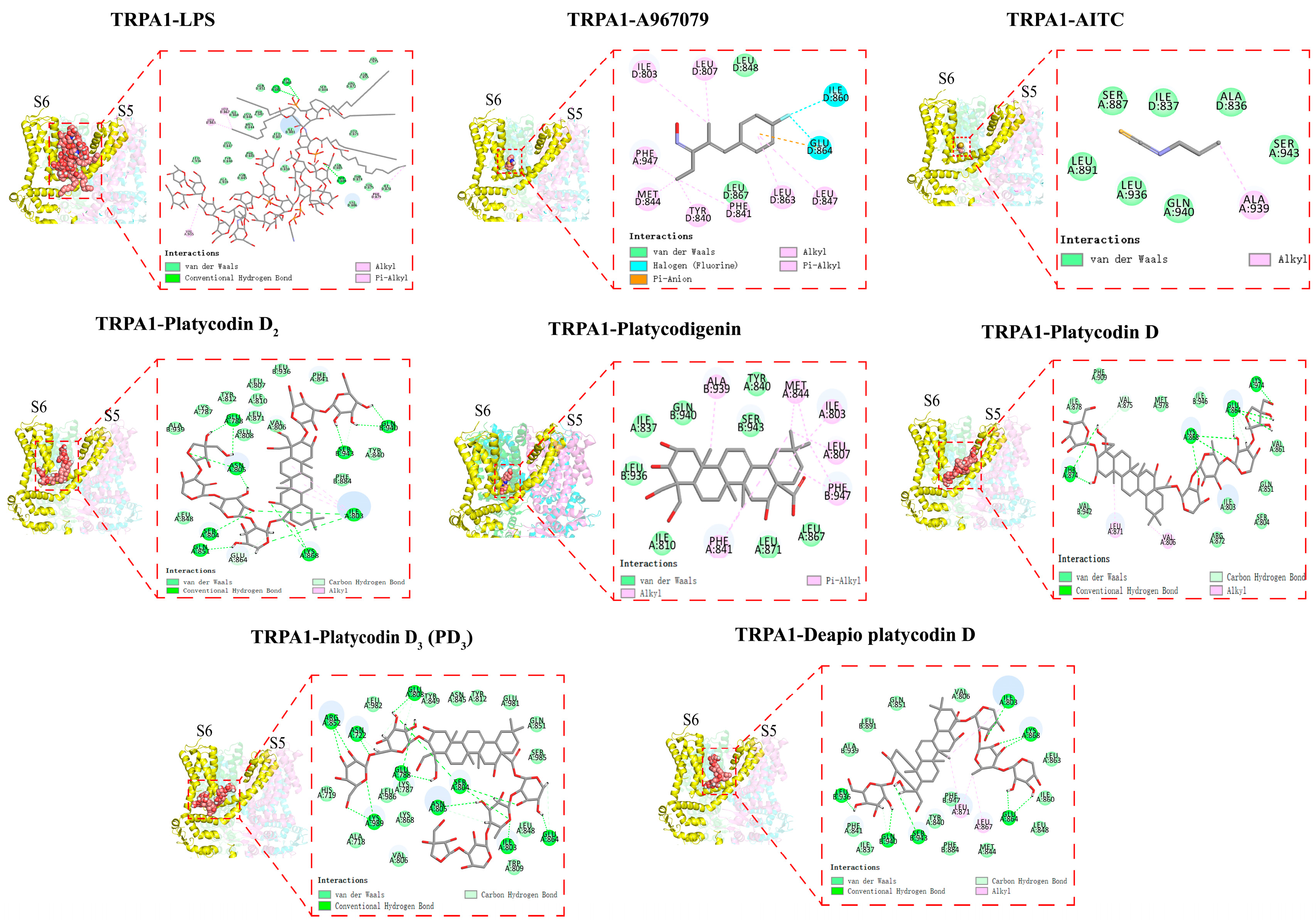
2.4. Platycodins Interaction with TRPA1: Molecular Docking
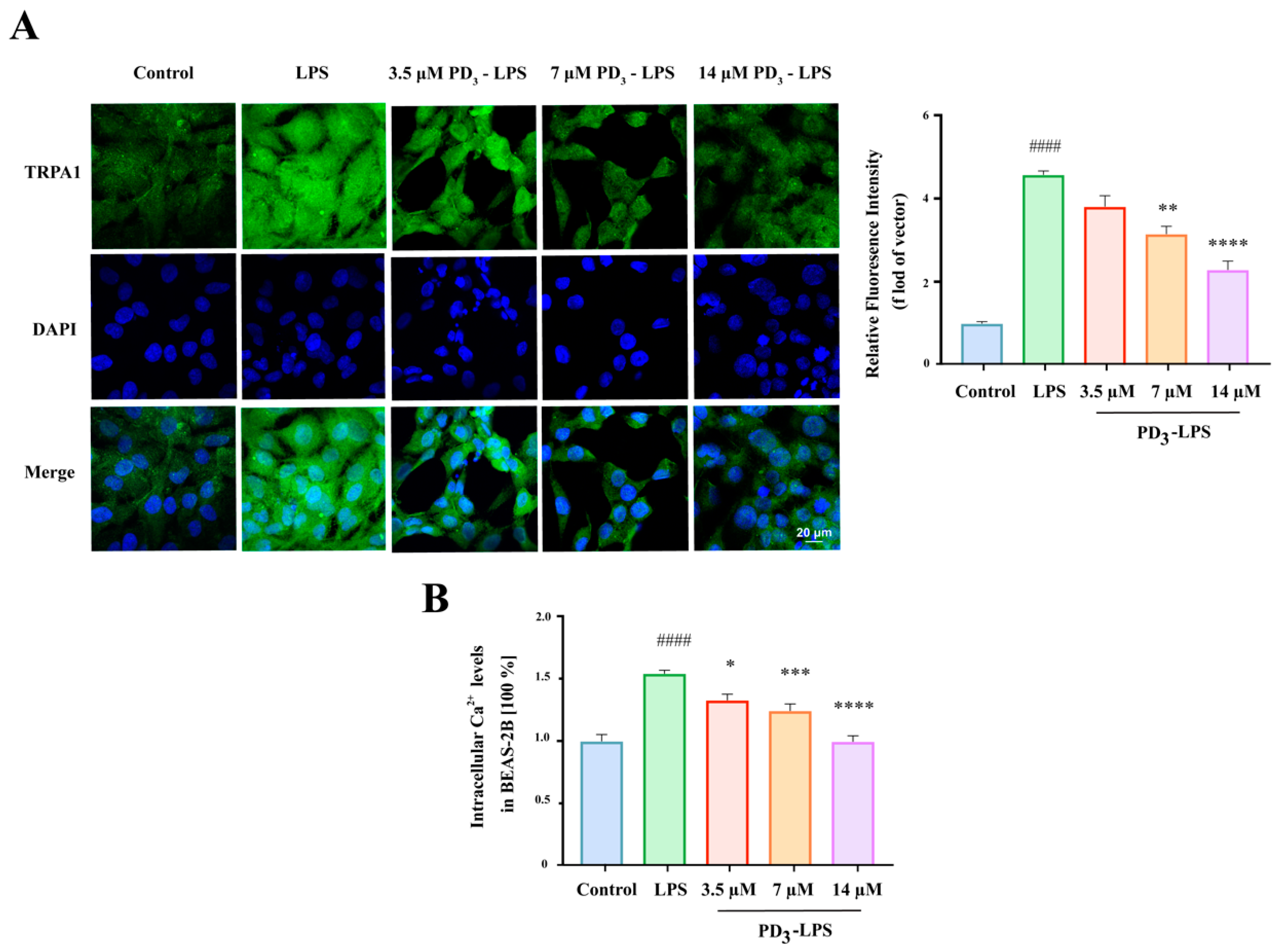
2.5. PD3 Downregulates LPS-Induced TRPA1 Expression and Ca2+ Influx in BEAS-2B Cells
3. Discussion and Conclusions
4. Materials and Methods
4.1. Reagents and Materials
4.2. Animals and Animal Grouping
4.3. Cell Culture
4.4. Histopathological Examination of Lung Tissue
4.5. Analysis of Tumor Necrosis Factor-α (TNF-α) and Interleukin-1β (IL-1β) in the Bronchoalveolar Lavage Fluid
4.6. Western Blotting
4.7. Assay of the mRNA Expression of TRPA1 by qPCR
4.8. Composition Analysis of the PR Water Extract with UPLC-Q-TOF-MS/MS
4.9. Molecular Docking
4.10. Immunofluorescence
4.11. Measurement of Ca2+ Influx
4.12. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Talavera, K.; Startek, J.B.; Alvarez-Collazo, J.; Boonen, B.; Alpizar, Y.A.; Sanchez, A.; Naert, R.; Nilius, B. Mammalian Transient Receptor Potential Trpa1 Channels: From Structure to Disease. Physiol. Rev. 2020, 100, 725–803. [Google Scholar] [CrossRef]
- Jaquemar, D.; Schenker, T.; Trueb, B. An ankyrin-like protein with transmembrane domains is specifically lost after oncogenic transformation of human fibroblasts. J. Biol. Chem. 1999, 274, 7325–7333. [Google Scholar] [CrossRef]
- Memon, T.A.; Nguyen, N.D.; Burrell, K.L.; Scott, A.F.; Almestica-Roberts, M.; Rapp, E.; Deering-Rice, C.E.; Reilly, C.A. Wood Smoke Particles Stimulate MUC5AC Overproduction by Human Bronchial Epithelial Cells through TRPA1 and EGFR Signaling. Toxicol. Sci. 2020, 174, 278–290. [Google Scholar] [CrossRef]
- Nummenmaa, E.; Hamalainen, M.; Pemmari, A.; Moilanen, L.J.; Tuure, L.; Nieminen, R.M.; Moilanen, T.; Vuolteenaho, K.; Moilanen, E. Transient Receptor Potential Ankyrin 1 (TRPA1) Is Involved in Upregulating Interleukin-6 Expression in Osteoarthritic Chondrocyte Models. Int. J. Mol. Sci. 2021, 22, 87. [Google Scholar] [CrossRef]
- Yang, H.; Li, S. Transient Receptor Potential Ankyrin 1 (TRPA1) Channel and Neurogenic Inflammation in Pathogenesis of Asthma. Med. Sci. Monit. 2016, 22, 2917–2923. [Google Scholar] [CrossRef]
- Ko, H.K.; Lin, A.H.; Perng, D.W.; Lee, T.S.; Kou, Y.R. Lung Epithelial TRPA1 Mediates Lipopolysaccharide-Induced Lung Inflammation in Bronchial Epithelial Cells and Mice. Front. Physiol. 2020, 11, 596314. [Google Scholar] [CrossRef]
- Heidarpour, F.; Mohammadabadi, M.R.; Zaidul, I.S.; Maherani, B.; Saari, N.; Hamid, A.A.; Abas, F.; Manap, M.Y.; Mozafari, M.R. Use of prebiotics in oral delivery of bioactive compounds: A nanotechnology perspective. Pharmazie 2011, 66, 319–324. [Google Scholar] [PubMed]
- Mohammadabadi, M.R.; El-Tamimy, M.; Gianello, R.; Mozafari, M.R. Supramolecular assemblies of zwitterionic nanoliposome-polynucleotide complexes as gene transfer vectors: Nanolipoplex formulation and in vitrocharacterisation. J. Liposome Res. 2009, 19, 105–115. [Google Scholar] [CrossRef] [PubMed]
- Jafari, A.S.A.A.; Askari-Hemmat, H.; Mohammadabadi, M.; Asadi, F.M.; Mansouri, B.M. The effect of Cannabis seed on DLK1 gene expression in heart tissue of Kermani lambs. Agric. Biotechnol. J. 2023, 15, 217–234. [Google Scholar]
- Zhang, L.; Wang, Y.; Yang, D.; Zhang, C.; Zhang, N.; Li, M.; Liu, Y. Platycodon grandiflorus—An ethnopharmacological, phytochemical and pharmacological review. J. Ethnopharmacol. 2015, 164, 147–161. [Google Scholar] [CrossRef]
- Li, Q.Q.; Yang, T.; Zhao, S.; Zheng, Q.F.; Li, Y.X.; Zhang, Z.Y.; Sun, X.Y.; Liu, Y.; Zhang, Y.Q.; Xie, J.B. Distribution, Biotransformation, Pharmacological Effects, Metabolic Mechanism and Safety Evaluation of Platycodin D: A Comprehensive Review. Curr. Drug Metab. 2022, 23, 21–29. [Google Scholar] [CrossRef] [PubMed]
- Choi, S.I.; Choi, Y.E.; Han, X.G.; Men, X.; Lee, S.J.; Park, B.W.; Kim, J.J.; Kim, S.H.; Lee, O.H. Fermented Platycodon grandiflorum extract alleviates TNF-alpha/IFN-gamma-induced inflammatory response in HaCaT cells and modulates immune balance on 1-chloro-2,4-dinitrobenzene-induced atopic dermatitis in NC/Nga mice. J. Funct. Foods 2021, 85, 104617. [Google Scholar] [CrossRef]
- Gao, W.; Guo, Y.; Yang, H. Platycodin D protects against cigarette smoke-induced lung inflammation in mice. Int. Immunopharmacol. 2017, 47, 53–58. [Google Scholar] [CrossRef] [PubMed]
- King, J.V.L.; Emrick, J.J.; Kelly, M.J.S.; Herzig, V.; King, G.F.; Medzihradszky, K.F.; Julius, D. A Cell-Penetrating Scorpion Toxin Enables Mode-Specific Modulation of TRPA1 and Pain. Cell 2019, 178, 1362–1374. [Google Scholar] [CrossRef] [PubMed]
- Meseguer, V.; Alpizar, Y.A.; Luis, E.; Tajada, S.; Denlinger, B.; Fajardo, O.; Manenschijn, J.A.; Fernandez-Pena, C.; Talavera, A.; Kichko, T.; et al. TRPA1 channels mediate acute neurogenic inflammation and pain produced by bacterial endotoxins. Nat. Commun. 2014, 5, 3125. [Google Scholar] [CrossRef]
- Ma, X.Q.; Li, S.M.; Chan, C.L.; Su, T.; Li, W.D.; Cao, H.; Fong, W.F.; Yu, Z.L. Influence of sulfur fumigation on glycoside profile in Platycodonis Radix (Jiegeng). Chin. Med. 2016, 11, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.L.; Huang, M.Y.; Yang, Y.; Huang, M.Q.; Shi, J.J.; Zou, L.; Lu, J.J. Bioactive platycodins from Platycodonis Radix: Phytochemistry, pharmacological activities, toxicology and pharmacokinetics. Food Chem. 2020, 327, 127029. [Google Scholar] [CrossRef]
- Deng, Y.; Ye, X.; Chen, Y.; Ren, H.; Xia, L.; Liu, Y.; Liu, M.; Liu, H.; Zhang, H.; Wang, K.; et al. Chemical Characteristics of Platycodon grandiflorum and its Mechanism in Lung Cancer Treatment. Front. Pharmacol. 2020, 11, 609825. [Google Scholar] [CrossRef]
- Paulsen, C.E.; Armache, J.P.; Gao, Y.; Cheng, Y.F.; Julius, D. Structure of the TRPA1 Ion Channel Suggests Regulatory Mechanisms. Biophys. J. 2016, 110, 26a. [Google Scholar] [CrossRef]
- Chen, J.; Zhang, X.F.; Kort, M.E.; Huth, J.R.; Sun, C.; Miesbauer, L.J.; Cassar, S.C.; Neelands, T.; Scott, V.E.; Moreland, R.B.; et al. Molecular determinants of species-specific activation or blockade of TRPA1 channels. J. Neurosci. 2008, 28, 5063–5071. [Google Scholar] [CrossRef]
- Maki-Opas, I.; Hamalainen, M.; Moilanen, L.J.; Haavikko, R.; Ahonen, T.J.; Alakurtti, S.; Moreira, V.M.; Muraki, K.; Yli-Kauhaluoma, J.; Moilanen, E. Pyrazine-Fused Triterpenoids Block the TRPA1 Ion Channel In Vitro and Inhibit TRPA1-Mediated Acute Inflammation In Vivo. ACS Chem. Neurosci. 2019, 10, 2848–2857. [Google Scholar] [CrossRef]
- Lee, G.; Choi, J.; Nam, Y.J.; Song, M.J.; Kim, J.K.; Kim, W.J.; Kim, P.; Lee, J.S.; Kim, S.; No, K.T.; et al. Identification and characterization of saikosaponins as antagonists of transient receptor potential A1 channel. Phytother. Res. 2020, 34, 788–795. [Google Scholar] [CrossRef]
- Chung, J.W.; Noh, E.J.; Zhao, H.L.; Sim, J.S.; Ha, Y.W.; Shin, E.M.; Lee, E.B.; Cheong, C.S.; Kim, Y.S. Anti-inflammatory activity of prosapogenin methyl ester of platycodin D via nuclear factor-kappaB pathway inhibition. Biol. Pharm. Bull. 2008, 31, 2114–2120. [Google Scholar] [CrossRef] [PubMed]
- Yap, J.M.G.; Ueda, T.; Takeda, N.; Fukumitsu, K.; Fukuda, S.; Uemura, T.; Tajiri, T.; Ohkubo, H.; Maeno, K.; Ito, Y.; et al. An inflammatory stimulus sensitizes TRPA1 channel to increase cytokine release in human lung fibroblasts. Cytokine 2020, 129, 155027. [Google Scholar] [CrossRef] [PubMed]
- Bautista, D.M.; Pellegrino, M.; Tsunozaki, M. TRPA1: A gatekeeper for inflammation. Annu. Rev. Physiol. 2013, 75, 181–200. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.; Han, E.H.; Lim, M.K.; Lee, S.H.; Yu, H.J.; Lim, Y.H.; Kang, S. Fermented Platycodon grandiflorum Extracts Relieve Airway Inflammation and Cough Reflex Sensitivity In Vivo. J. Med. Food 2020, 23, 1060–1069. [Google Scholar] [CrossRef]
- Kim, J.Y.; Hwang, Y.P.; Kim, D.H.; Han, E.H.; Chung, Y.C.; Roh, S.H.; Jeong, H.G. Inhibitory effect of the saponins derived from roots of Platycodon grandiflorum on carrageenan-induced inflammation. Biosci. Biotech. Bioch. 2006, 70, 858–864. [Google Scholar] [CrossRef]
- Chen, S.T.; Wang, Q.; Ming, S.; Zheng, H.G.; Hua, B.J.; Yang, H.S. Platycodin D induces apoptosis through JNK1/AP-1/PUMA pathway in non-small cell lung cancer cells: A new mechanism for an old compound. Front. Pharmacol. 2022, 13, 1045375. [Google Scholar] [CrossRef]
- Phillips, D.R.; Rasbery, J.M.; Bartel, B.; Matsuda, S.P.T. Biosynthetic diversity in plant triterpene cyclization. Curr. Opin. Plant Biol. 2006, 9, 305–314. [Google Scholar] [CrossRef]
- Saito, S.; Tominaga, M. Functional diversity and evolutionary dynamics of thermoTRP channels. Cell Calcium 2015, 57, 214–221. [Google Scholar] [CrossRef]
- Qiao, J.Y.; Chen, L.X.; Huang, X.J.; Guo, F.F. Effects of nebulized N-acetylcystein on the expression of HMGB1 and RAGE in rats with hyperoxia-induced lung injury. J. Cell Physiol. 2019, 234, 10547–10553. [Google Scholar] [CrossRef] [PubMed]
- Liou, C.J.; Huang, W.C.; Kuo, M.L.; Yang, R.C.; Shen, J.J. Long-term oral administration of Gynostemma pentaphyllum extract attenuates airway inflammation and Th2 cell activities in ovalbumin-sensitized mice. Food Chem. Toxicol. 2010, 48, 2592–2598. [Google Scholar] [CrossRef]
- Zhao, X.; Hou, P.; Xin, H.; Zhang, Y.; Zhou, A.; Lai, C.; Xie, J. A glucogalactomanan polysaccharide isolated from Agaricus bisporus causes an inflammatory response via the ERK/MAPK and IκB/NFκB pathways in macrophages. Int. J. Biol. Macromol. 2020, 151, 1067–1073. [Google Scholar] [CrossRef] [PubMed]
- Huang, L.; Luo, J.; Song, N.; Gao, W.; Zhu, L.; Yao, W. CRISPR/Cas9-Mediated Knockout of miR-130b Affects Mono- and Polyunsaturated Fatty Acid Content via PPARG-PGC1alpha Axis in Goat Mammary Epithelial Cells. Int. J. Mol. Sci. 2022, 23, 3640. [Google Scholar] [CrossRef] [PubMed]
- Huang, W.C.; Fang, L.W.; Liou, C.J. Phloretin Attenuates Allergic Airway Inflammation and Oxidative Stress in Asthmatic Mice. Front. Immunol. 2017, 8, 134. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
- Bar Oz, M.; Kumar, A.; Elayyan, J.; Reich, E.; Binyamin, M.; Kandel, L.; Liebergall, M.; Steinmeyer, J.; Lefebvre, V.; Dvir-Ginzberg, M. Acetylation reduces SOX9 nuclear entry and ACAN gene transactivation in human chondrocytes. Aging Cell 2016, 15, 499–508. [Google Scholar] [CrossRef]
- Fazi, R.; Tintori, C.; Brai, A.; Botta, L.; Selvaraj, M.; Garbelli, A.; Maga, G.; Botta, M. Homology Model-Based Virtual Screening for the Identification of Human Helicase DDX3 Inhibitors. J. Chem. Inf. Model 2015, 55, 2443–2454. [Google Scholar] [CrossRef]
- Liu, J.; Huang, W.; Ren, C.; Wen, Q.; Liu, W.; Yang, X.; Wang, L.; Zhu, B.; Zeng, L.; Feng, X.; et al. Flotillin-2 promotes metastasis of nasopharyngeal carcinoma by activating NF-κB and PI3K/Akt3 signaling pathways. Sci. Rep. 2015, 5, 11614. [Google Scholar] [CrossRef]

| Components | Rt (Min) | Formula | R1 | R2 | Ion Fragmentation |
|---|---|---|---|---|---|
| Platycodin D3 | 8.768 | C63H102O33 | Gen | Ara2-Rha4-Xyl3-Api | 1431.6670, 1385.6387, 1253.6019, 843.4632 |
| Deapio-Platycodin D | 15.36 | C52H84O24 | Glc | Ara2-Rha4-Xyl | 1137.6166, 1091.6080, 727.4072, 591.2803 |
| Platycodin D2 | 16.17 | C63H102O33 | Lam | Ara2-Rha4-Xyl3-Api | 1385.7568, 1167.5681, 738.3300, 715.3263 |
| Platycodin D | 16.27 | C57H92O28 | Glc | Ara2-Rha4-Xyl3-Api | 1269.6068, 1223.4987, 634.2987 |
| Platycodigenin | 22.96 | C32H48O7 | H | H | 520.3845, 519.3939, 503.3509, 391.3097 |
| Gene | Sequence (5′-3′) | Product Length/bp | Annealing Temperature | Cycle Number |
|---|---|---|---|---|
| TRPA1 | F: CGAGAGTCCTTCCTAGAACCAT R: CCTCAGCAATGTCGCCAAC | 145 | 62 °C | 40 |
| GAPDH | F: TGCCCCCATGTTTGTGATG R: TGTGGTCATGAGCCCTTCC | 126 | 62 °C | 40 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, T.; Zhao, S.; Yuan, Y.; Zhao, X.; Bu, F.; Zhang, Z.; Li, Q.; Li, Y.; Wei, Z.; Sun, X.; et al. Platycodonis Radix Alleviates LPS-Induced Lung Inflammation through Modulation of TRPA1 Channels. Molecules 2023, 28, 5213. https://doi.org/10.3390/molecules28135213
Yang T, Zhao S, Yuan Y, Zhao X, Bu F, Zhang Z, Li Q, Li Y, Wei Z, Sun X, et al. Platycodonis Radix Alleviates LPS-Induced Lung Inflammation through Modulation of TRPA1 Channels. Molecules. 2023; 28(13):5213. https://doi.org/10.3390/molecules28135213
Chicago/Turabian StyleYang, Tan, Shuang Zhao, Yu Yuan, Xiaotong Zhao, Fanjie Bu, Zhiyuan Zhang, Qianqian Li, Yaxin Li, Zilu Wei, Xiuyan Sun, and et al. 2023. "Platycodonis Radix Alleviates LPS-Induced Lung Inflammation through Modulation of TRPA1 Channels" Molecules 28, no. 13: 5213. https://doi.org/10.3390/molecules28135213
APA StyleYang, T., Zhao, S., Yuan, Y., Zhao, X., Bu, F., Zhang, Z., Li, Q., Li, Y., Wei, Z., Sun, X., Zhang, Y., & Xie, J. (2023). Platycodonis Radix Alleviates LPS-Induced Lung Inflammation through Modulation of TRPA1 Channels. Molecules, 28(13), 5213. https://doi.org/10.3390/molecules28135213







