Headspace with Gas Chromatography-Mass Spectrometry for the Use of Volatile Organic Compound Profile in Botanical Origin Authentication of Honey
Abstract
1. Introduction
2. Results and Discussion
2.1. HS-GC-MS Method Optimisation
2.2. Monitorisation of VOCs in Honey
2.3. Method Characterisation and Quantification of Identified VOCs
2.4. Non-Targeted Approach Using GC-MS Data
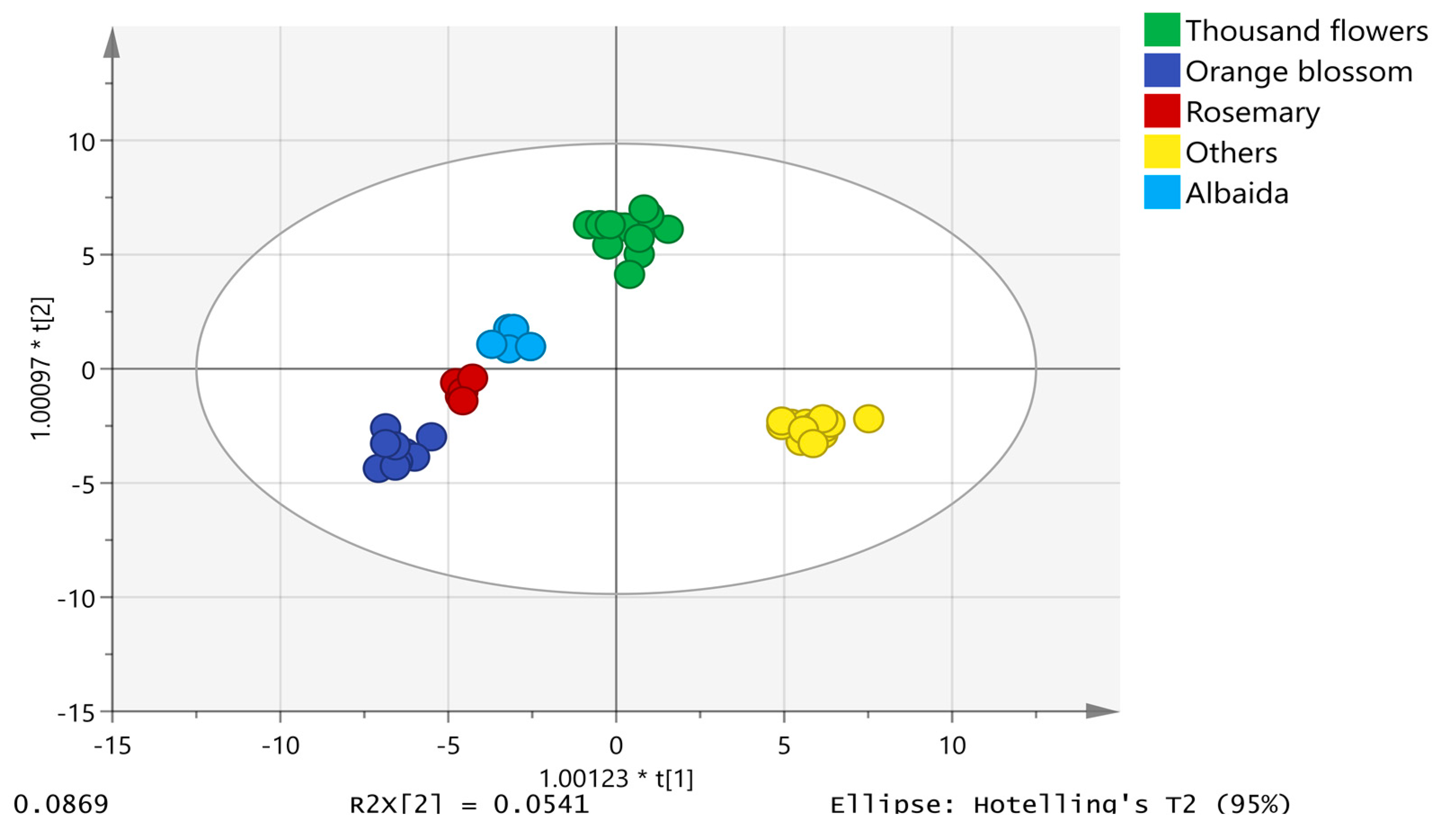
2.5. Chemometric Model for the Classification of Honey According to Botanical Origin
2.6. Application of the Proposed Method
3. Materials and Methods
3.1. Standards and Solvents
3.2. Honey Samples
3.3. Instrumentation and Software
3.4. HS-GC-MS Analysis
3.5. Data Processing
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Cianciosi, D.; Forbes-Hernández, T.Y.; Afrin, S.; Gasparrini, M.; Reboredo-Rodriguez, P.; Manna, P.P.; Zhang, J.; Lamas, L.B.; Flórez, S.M.; Toyos, P.A.; et al. Phenolic compounds in honey and their associated health benefits: A review. Molecules 2018, 23, 2322. [Google Scholar] [CrossRef] [PubMed]
- Hassoun, A.; Måge, I.; Schmidt, W.F.; Temiz, H.T.; Li, L.; Kim, H.-Y.; Nilsen, H.; Biancolillo, A.; Aït-Kaddour, A.; Sikorski, M.; et al. Fraud in animal origin food products: Advances in emerging spectroscopic detection methods over the past five years. Foods 2020, 9, 1069. [Google Scholar] [CrossRef] [PubMed]
- Kafantaris, I.; Amoutzias, G.D.; Mossialos, D. Foodomics in bee product research: A systematic literature review. Eur. Food Res. Technol. 2021, 247, 309–331. [Google Scholar] [CrossRef]
- Zhou, J.; Yao, L.; Li, Y.; Chen, L.; Wu, L.; Zhao, J. Floral classification of honey using liquid chromatography-diode array detection-tandem mass spectrometry and chemometric analysis. Food Chem. 2014, 145, 941–949. [Google Scholar] [CrossRef] [PubMed]
- Ouchemoukh, S.; Amessis-Ouchemoukh, N.; Gómez-Romero, M.; Aboud, F.; Giuseppe, A.; Fernández-Gutiérrez, A.; Segura-Carretero, A. Characterisation of phenolic compounds in Algerian honeys by RP-HPLC coupled to electrospray time-of-flight mass spectrometry. LWT-Food Sci. Technol. 2017, 85, 460–469. [Google Scholar] [CrossRef]
- García-Seval, V.; Martínez-Alfaro, C.; Saurina, J.; Núñez, O.; Sentellas, S. Characterization, classification and authentication of Spanish blossom and honeydew honeys by non-targeted HPLC-UV and off-line SPE HPLC-UV polyphenolic fingerprinting strategies. Foods 2022, 11, 2345. [Google Scholar] [CrossRef]
- Karabagias, I.K.; Badeka, A.V.; Kontakos, S.; Karabournioti, S.; Kontominas, M.G. Botanical discrimination of Greek unifloral honeys with physico-chemical and chemometric analyses. Food Chem. 2014, 165, 181–190. [Google Scholar] [CrossRef] [PubMed]
- Yildiz, O.; Gurkan, H.; Sahingil, D.; Degirmenci, A.; Er Kemal, M.; Kolayli, S.; Hayaloglu, A.A. Floral authentication of some monofloral honeys based on volatile composition and physicochemical parameters. Eur. Food Res. Technol. 2022, 248, 2145–2155. [Google Scholar] [CrossRef]
- Karabagias, I.K.; Badeka, A.; Kontominas, M.G. A decisive strategy for monofloral honey authentication using analysis of volatile compounds and pattern recognition techniques. Microchem. J. 2020, 152, 104263. [Google Scholar] [CrossRef]
- Popek, S.; Halagarda, M.; Kursa, K. A new model to identify botanical origin of Polish honeys based on the physicochemical parameters and chemometric analysis. LWT 2017, 77, 482–487. [Google Scholar] [CrossRef]
- Rodopoulou, M.A.; Tananaki, C.; Kanelis, D.; Liolios, V.; Dimou, M.; Thrasyvoulou, A. A chemometric approach for the differentiation of 15 monofloral honeys based on physicochemical parameters. J. Sci. Food Agric. 2022, 102, 139–146. [Google Scholar] [CrossRef] [PubMed]
- Tsagkaris, A.S.; Koulis, G.A.; Danezis, G.P.; Martakos, I.; Dasenaki, M.; Georgiou, C.A.; Thomaidis, N.S. Honey authenticity: Analytical techniques, state of the art and challenges. RSC Adv. 2021, 11, 11273–11294. [Google Scholar] [CrossRef] [PubMed]
- Kivima, E.; Seiman, A.; Pall, R.; Sarapuu, E.; Martverk, K.; Laos, K. Characterization of Estonian honeys by botanical origin. Proc. Est. Acad. Sci. 2014, 63, 183–192. [Google Scholar] [CrossRef]
- Gan, Z.; Yang, Y.; Li, J.; Wen, X.; Zhu, M.; Jiang, Y.; Ni, Y. Using sensor and spectral analysis to classify botanical origin and determine adulteration of raw honey. J. Food Eng. 2016, 178, 151–158. [Google Scholar] [CrossRef]
- Tsagkaris, A.S.; Bechynska, K.; Ntakoulas, D.D.; Pasias, I.N.; Weller, P.; Proestos, C.; Hajslova, J. Investigating the impact of spectral data pre-processing to assess honey botanical origin through Fourier Transform Infrared Spectroscopy (FTIR). J. Food Compos. Anal. 2023, 119, 105276. [Google Scholar] [CrossRef]
- Oroian, M.; Prisacaru, A.; Hretcanu, E.C.; Stroe, S.G.; Leahu, A.; Buculei, A. Heavy metals profile in honey as a potential indicator of botanical and geographical origin. Int. J. Food Prop. 2015, 19, 1825–1836. [Google Scholar] [CrossRef]
- Chen, H.; Fan, C.; Chang, Q.; Pang, G.; Hu, X.; Lu, M.; Wang, W. Chemometric determination of the botanical origin for Chinese honeys on the basis of mineral elements determined by ICP-MS. J. Agric. Food Chem. 2014, 62, 2443–2448. [Google Scholar] [CrossRef]
- Karabagias, I.K.; Louppis, A.P.; Badeka, A.; Papastephanou, C.; Kontominas, M.G. Nutritional aspects and botanical origin recognition of Mediterranean honeys based on the “mineral imprint’’ with the application of supervised and non-supervised statistical techniques. Eur. Food Res. Technol. 2019, 245, 1939–1949. [Google Scholar] [CrossRef]
- Czipa, N.; Alexa, L.; Phillips, C.J.C.; Kovács, B. Macro-element ratios provide improved identification of the botanical origin of mono-floral honeys. Eur. Food Res. Technol. 2018, 244, 1439–1445. [Google Scholar] [CrossRef]
- Montenegro, G.; Gómez, M.; Casaubon, G.; Belancic, A.; Mujica, A.M.; Peña, R.C. Analysis of volatile compounds in three unifloral native Chilean honeys. Phyton-Int. J. Exp. Bot. 2009, 78, 61–65. [Google Scholar] [CrossRef]
- Rahman, M.M.; Alam, M.N.; Fatima, N.; Shahjalal, H.M.; Gan, S.H.; Khalil, M.I. Chemical composition and biological properties of aromatic compounds in honey: An overview. J. Food Biochem. 2017, 41, e12405. [Google Scholar] [CrossRef]
- Machado, A.M.; Miguel, M.G.; Vilas-Boas, M.; Figueiredo, A.C. Honey volatiles as a fingerprint for botanical origin—A review on their occurrence on monofloral honeys. Molecules 2020, 25, 374. [Google Scholar] [CrossRef] [PubMed]
- Wardencki, W.; Chmiel, T.; Dymerski, T.; Biernacka, P.; Plutowska, B. Application of gas chromatography, mass spectrometry and olfactometry for quality assessment of selected food products. Ecol. Chem. Eng. S 2009, 16, 287–300. [Google Scholar]
- Gerhardt, N.; Birkenmeier, M.; Schwolow, S.; Rohn, S.; Weller, P. Volatile-compound fingerprinting by headspace-gas-chromatography ion-mobility spectrometry (HS-GC-IMS) as a benchtop alternative to 1H NMR profiling for assessment of the authenticity of honey. Anal. Chem. 2018, 90, 1777–1785. [Google Scholar] [CrossRef]
- Arroyo-Manzanares, N.; García-Nicolás, M.; Zafra-Navarro, F.; Campillo, N.; Viñas, P. A non-targeted metabolomic strategy for characterization of the botanical origin of honey samples using headspace gas chromatography—Ion mobility spectrometry. Anal. Methods 2022, 14, 5047–5055. [Google Scholar] [CrossRef] [PubMed]
- Guyot, C.; Scheirman, V.; Collin, S. Floral origin markers of heather honeys: Calluna vulgaris and Erica arborea. Food Chem. 1999, 64, 3–11. [Google Scholar] [CrossRef]
- Castro-Vázquez, L.; Díaz-Maroto, M.C.; González-Viñas, M.A.; Pérez-Coello, M.S. Differentiation of monofloral citrus, rosemary, eucalyptus, lavender, thyme and heather honeys based on volatile composition and sensory descriptive analysis. Food Chem. 2009, 112, 1022–1030. [Google Scholar] [CrossRef]
- Jerković, I.; Mastelić, J.; Marijanović, Z. A variety of volatile compounds as markers in unifloral honey from Dalmatian Sage (Salvia officinalis L.). Chem. Biodivers. 2006, 3, 1307–1316. [Google Scholar] [CrossRef]
- Cuevas-Glory, L.F.; Pino, J.A.; Santiago, L.S.; Sauri-Duch, E. A review of volatile analytical methods for determining the botanical origin of honey. Food Chem. 2007, 103, 1032–1043. [Google Scholar] [CrossRef]
- Vieira da Costa, A.C.; Barbosa Sousa, J.M.; Alencar Bezerra, T.K.; Honorato da Silva, F.L.; Pastore, G.M.; Azevedo Pereira da Silva, M.A.; Suely Madruga, M. Volatile profile of monofloral honeys produced in Brazilian semiarid region by stingless bees and key volatile compounds. LWT 2018, 94, 198–207. [Google Scholar] [CrossRef]
- Manyi-Loh, C.E.; Ndip, R.N.; Clarke, A.M. Volatile compounds in honey: A review on their involvement in aroma, botanical origin determination and potential biomedical activities. Int. J. Mol. Sci. 2011, 12, 9514–9532. [Google Scholar] [CrossRef] [PubMed]
- Karabagias, I.K.; Nikolaou, C.; Karabagias, V.K. Volatile fingerprints of common and rare honeys produced in Greece: In search of PHVMs with implementation of the honey code. Eur. Food Res. Technol. 2019, 245, 23–39. [Google Scholar] [CrossRef]
- Bajoub, A.; Pacchiarotta, T.; Hurtado-Fernández, E.; Olmo-García, L.; García-Villalba, R.; Fernández-Gutiérrez, A.; Mayboroda, O.A.; Carrasco-Pancorbo, A. Comparing two metabolic profiling approaches (liquid chromatography and gas chromatography coupled to mass spectrometry) for extra-virgin olive oil phenolic compounds analysis: A botanical classification perspective. J. Chromatogr. A 2016, 1428, 267–279. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Gao, P.; Wang, X.; Duan, Y. The early diagnosis and monitoring of squamous cell carcinoma via saliva metabolomics. Sci. Rep. 2014, 4, 6802. [Google Scholar] [CrossRef] [PubMed]
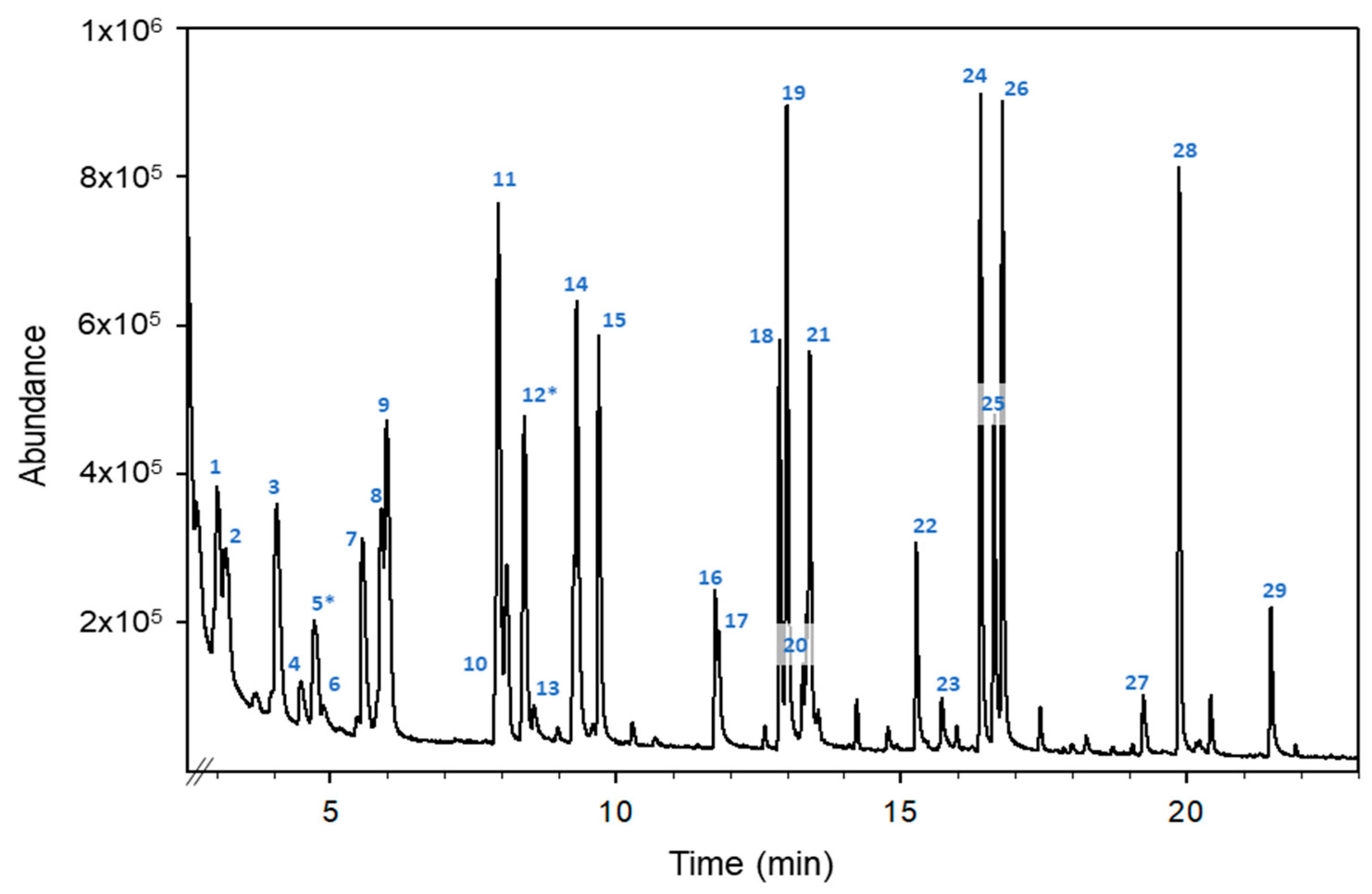
| Compound | RT 1 (min) | Target Ion (m/z) | Qualifier Ions (m/z) |
|---|---|---|---|
| 2-Pentanone | 2.99 | 43 | 71, 86 |
| Valeraldehyde | 3.16 | 44 | 29, 86 |
| 4-Methylpentan-2-one | 4.03 | 43 | 58, 85 |
| Trans-2-pentenal | 4.46 | 55 | 84, 41 |
| Toluene * | 4.70 | 91 | 92, 65 |
| 1-Pentanol | 4.80 | 42 | 31, 70 |
| 2-Hexanone | 5.54 | 43 | 58, 85 |
| Hexanal | 5.88 | 44 | 57, 82 |
| Ethyl butyrate | 5.96 | 71 | 43, 88 |
| Trans-2-hexen-1-al | 7.86 | 41 | 69, 83 |
| Ethyl isovalerate | 7.93 | 88 | 57, 115 |
| p-Xylene * | 8.39 | 91 | 105, 106 |
| 1-Hexanol | 8.54 | 56 | 43, 84 |
| 2-Heptanone | 9.32 | 43 | 58, 71 |
| Heptanal | 9.69 | 70 | 44, 96 |
| Trans-2-heptenal | 11.73 | 41 | 70, 83 |
| Benzaldehyde | 11.79 | 106 | 77, 107 |
| 6-Methyl-5-hepten-2-one | 12.86 | 43 | 69, 108 |
| 2-Octanone | 12.99 | 43 | 71, 85 |
| 2-Octanol | 13.33 | 45 | 70, 97 |
| Octanal | 13.39 | 41 | 44, 128 |
| Trans-2-octenal | 15.26 | 41 | 83, 97 |
| 1-Octanol | 15.70 | 56 | 41, 84 |
| 2-Nonanone | 16.39 | 43 | 71, 99 |
| Linalool | 16.63 | 71 | 93, 136 |
| Nonanal | 16.77 | 57 | 43, 98 |
| 4-Methylacetophenone | 19.25 | 119 | 91, 65 |
| Decanal | 19.87 | 43 | 82, 112 |
| Trans-2-decenal | 21.48 | 43 | 82, 110 |
| Compound | Linear Range (μg g−1) | R2 | LOD 1 (LOQ 2) (μg g−1) |
|---|---|---|---|
| 2-Pentanone | 0.016–1.00 | 0.993 | 0.005 (0.016) |
| Valeraldehyde | 0.016–1.00 | 0.992 | 0.005 (0.016) |
| 4-Methylpentan-2-one | 0.016–1.00 | 0.993 | 0.005 (0.016) |
| Trans-2-pentenal | 0.216–1.00 | 0.993 | 0.065 (0.216) |
| 1-Pentanol | 0.249–1.00 | 0.990 | 0.075 (0.249) |
| 2-Hexanone | 0.218–1.00 | 0.991 | 0.065 (0.218) |
| Hexanal | 0.015–1.00 | 0.991 | 0.005 (0.015) |
| Ethyl butyrate | 0.040–1.00 | 0.997 | 0.012 (0.040) |
| Trans-2-hexen-1-al | 0.129–1.00 | 0.980 | 0.039 (0.129) |
| Ethyl isovalerate | 0.040–1.00 | 0.995 | 0.012 (0.040) |
| 1-Hexanol | 0.083–1.00 | 0.994 | 0.025 (0.083) |
| 2-Heptanone | 0.083–1.00 | 0.994 | 0.025 (0.083) |
| Heptanal | 0.016–1.00 | 0.991 | 0.005 (0.016) |
| Trans-2-heptenal | 0.130–1.00 | 0.999 | 0.039 (0.130) |
| Benzaldehyde | 0.016–1.00 | 0.992 | 0.005 (0.016) |
| 6-Methyl-5-hepten-2-one | 0.016–1.00 | 0.995 | 0.005 (0.016) |
| 2-Octanone | 0.016–1.00 | 0.996 | 0.005 (0.016) |
| 2-Octanol | 0.083–1.00 | 0.990 | 0.025 (0.083) |
| Octanal | 0.016–1.00 | 0.995 | 0.005 (0.016) |
| Trans-2-octenal | 0.016–1.00 | 0.991 | 0.005 (0.016) |
| 1-Octanol | 0.016–1.00 | 0.991 | 0.005 (0.016) |
| 2-Nonanone | 0.016–1.00 | 0.997 | 0.005 (0.016) |
| Linalool | 0.015–1.00 | 0.994 | 0.005 (0.015) |
| Nonanal | 0.016–1.00 | 0.998 | 0.005 (0.016) |
| 4-Methylacetophenone | 0.016–1.00 | 0.994 | 0.005 (0.016) |
| Decanal | 0.016–1.00 | 0.998 | 0.005 (0.016) |
| Trans-2-decenal | 0.218–1.00 | 0.995 | 0.065 (0.218) |
| Compound | Albaida | Orange Blossom | Thousand Flowers | Rosemary | Others | |
|---|---|---|---|---|---|---|
| 2-Pentanone | Mean (ng g−1) | 26.0 ± 1.3 a | 33 ± 8 a,b | 32 ± 9 b | 34 ± 7 b | 36 ± 10 b |
| Range (ng g−1) | NQ 1–27.4 | NQ–45.8 | 19.4–49.4 | 26.7–46.0 | 0.00–61.4 | |
| Incidence (%) | 66.7 | 83.3 | 100.0 | 100.0 | 86.4 | |
| Valeraldehyde | Mean (ng g−1) | 208 ± 38 b | 35 ± 15 a | 41 ± 24 a,b | 46 ± 9 a,b | 42 ± 18 a |
| Range (ng g−1) | NQ–235.2 | NQ–69.4 | 0.00–86.3 | 34.0–56.9 | 0.00–69.0 | |
| Incidence (%) | 33.3 | 75.0 | 94.1 | 100.0 | 63.6 | |
| 4-Methylpentan-2-one | Mean (ng g−1) | ND 2,a | ND a | 46 ± 10 b | ND a | 43 ± 8 b |
| Range (ng g−1) | – | – | 0.00–59.1 | – | 0.00–48.3 | |
| Incidence (%) | – | – | 35.3 | – | 54.5 | |
| Trans-2-pentenal | Mean (ng g−1) | ND a,b | ND a | ND a | ND a,b | 287 ± 38 b |
| Range (ng g−1) | – | – | – | – | 0.00–334.8 | |
| Incidence (%) | – | – | – | – | 18.2 | |
| 2-Hexanone | Mean (ng g−1) | NQ | NQ | NQ | NQ | NQ |
| Range (ng g−1) | NQ | 0.00–NQ | 0.00–NQ | 0.00–NQ | 0.00–NQ | |
| Incidence (%) | – | – | – | – | – | |
| Hexanal | Mean (ng g−1) | 15.4 a | 69 ± 17 a | 70 ± 24 a | 83 ± 11 a | 173 ± 140 b |
| Range (ng g−1) | 0.00–15.4 | 0.00–94.0 | 0.00–99.1 | 74.9–90.5 | 0.00–463.7 | |
| Incidence (%) | 16.7 | 50.0 | 47.1 | 33.3 | 72.7 | |
| Ethyl butyrate | Mean (ng g−1) | 289 ± 16 b | ND a | ND a | ND a | ND a |
| Range (ng g−1) | 0.00–300.8 | – | – | – | – | |
| Incidence (%) | 33.3 | – | – | – | – | |
| 2-Heptanone | Mean (ng g−1) | ND a,b | 94 ± 12 b | ND a | ND a,b | ND a |
| Range (ng g−1) | – | 0.00–85.4 | – | – | – | |
| Incidence (%) | – | 16.7 | – | – | – | |
| Heptanal | Mean (ng g−1) | 28 ± 3 a,b | 27 ± 2 a,b | 46 ± 40 b | 34 ± 5 a,b | 31 ± 5 a |
| Range (ng g−1) | 23.3–32.9 | 22.2–30.8 | 0.00–142.1 | 26.6–39.9 | 0.00–38.6 | |
| Incidence (%) | 100.0 | 100.0 | 82.4 | 100.0 | 63.6 | |
| Trans-2-heptenal | Mean (ng g−1) | ND a | ND a | ND a | ND a | 84 ± 3 a |
| Range (ng g−1) | – | – | – | – | 0.00–86.8 | |
| Incidence (%) | – | – | – | – | 9.1 | |
| Benzaldehyde | Mean (ng g−1) | NQ a | 43 ± 2 a | 42 ± 19 a,b | 42 ± 11 a,b | 102 ± 72 b |
| Range (ng g−1) | 0.00–NQ | 0.00–44.4 | 0.0–80.2 | 0.00–50.5 | 0.00–213.9 | |
| Incidence (%) | – | 16.7 | 70.6 | 66.7 | 50.0 | |
| 6-Methyl-5-hepten-2-one | Mean (ng g−1) | 46.7 ± 0.4 a,b | 43 ± 6 b | 41.6 ± 0.1 a | 41.5 ± 0.3 a,b | 48 ± 2 a |
| Range (ng g−1) | 0.00–47.0 | 0.00–49.1 | 0.00–41.6 | 0.00–41.7 | 0.00–50.1 | |
| Incidence (%) | 33.3 | 66.7 | 11.8 | 33.3 | 18.2 | |
| 2-Octanone | Mean (ng g−1) | 25.5 ± 1.4 a,b | 20 ± 2 a,b | 20.5 ± 0.4 a | 56.2 ± 0.3 b | 20.8 ± 1.8 a,b |
| Range (ng g−1) | 0.00–26.5 | 0.00–23.5 | 0.00–20.8 | 0.00–56.4 | 0.00–24.9 | |
| Incidence (%) | 33.3 | 50.0 | 11.8 | 33.3 | 36.4 | |
| Octanal | Mean (ng g−1) | 79 ± 69 a | 84 ± 61 a | 72 ± 81 a | 41 ± 6 a | 142 ± 394 a |
| Range (ng g−1) | 31.1–175.7 | 0.00–161.7 | 0.00–37.2 | 0.00–50.6 | 0.00–1717.8 | |
| Incidence (%) | 100.0 | 75.0 | 64.7 | 66.7 | 81.8 | |
| Trans-2-octenal | Mean (ng g−1) | 34 ± 2 a,b | 31 ± 2 a,b | 36 ± 3 b | 39 ± 9 b | 32 ± 5 a |
| Range (ng g−1) | 28.7–36.3 | 27.0–32.9 | 0.00–44.3 | 31.3–55.4 | 0.00–40.7 | |
| Incidence (%) | 100.0 | 100.0 | 88.2 | 100.0 | 72.7 | |
| 1-Octanol | Mean (ng g−1) | 61 ± 2 a,b | 89.2 ± 1.8 a,b | 84 ± 19 a,b | 77 ± 14 b | 155 ± 2 a |
| Range (ng g−1) | 0.00–63.2 | 0.00–90.5 | 0.00–105.3 | 0.00–92.0 | 0.00–156.7 | |
| Incidence (%) | 33.3 | 16.7 | 23.5 | 66.7 | 9.1 | |
| 2-Nonanone | Mean (ng g−1) | 38.1 ± 0.2 b | ND a | ND a | ND a | 48.3 ± 0.9 a,b |
| Range (ng g−1) | 0.00–38.2 | – | – | – | 0.00–48.9 | |
| Incidence (%) | 33.3 | – | – | – | 9.1 | |
| Linalool | Mean (ng g−1) | NQ a | 35 ± 19 b | 20 ± 2 a | 23.8 ± 1.9 a | 19.1 ± 1.6 a |
| Range (ng g−1) | NQ | NQ–72.0 | 0.00–21.8 | NQ–25.1 | NQ–21.7 | |
| Incidence (%) | – | 66.7 | 29.4 | 33.3 | 27.3 | |
| Nonanal | Mean (ng g−1) | 25.7 ± 1.7 a | 29 ± 12 a | 72 ± 2 a | 30 ± 14 a | 97 ± 75 a |
| Range (ng g−1) | 0.00–26.8 | 0.00–45.9 | 0.00–96.1 | NQ–45.0 | 0.00–232.4 | |
| Incidence (%) | 33.3 | 50.0 | 35.3 | 66.7 | 36.4 | |
| 4-Methylacetophenone | Mean (ng g−1) | NQ a | 57 ± 23 a | 70 ± 2 a | 82 ± 6 a | 161 ± 75 a |
| Range (ng g−1) | 0.00–NQ | 0.00–88.1 | 0.00–72.9 | 77.2–86.3 | 0.00–232.4 | |
| Incidence (%) | – | 50.0 | 11.8 | 33.3 | 18.2 | |
| Decanal | Mean (ng g−1) | 35.6 ± 0.6 a | 36.0 ± 1.0 a | 36 ± 2 a | 260 ± 446 b | 36 ± 2 a |
| Range (ng g−1) | 0.00–36.4 | 0.00–37.5 | 0.00–39.1 | 35.1–929.4 | 0.00–41.0 | |
| Incidence (%) | 66.7 | 83.3 | 35.3 | 66.7 | 81.8 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Castell, A.; Arroyo-Manzanares, N.; Guerrero-Núñez, Y.; Campillo, N.; Viñas, P. Headspace with Gas Chromatography-Mass Spectrometry for the Use of Volatile Organic Compound Profile in Botanical Origin Authentication of Honey. Molecules 2023, 28, 4297. https://doi.org/10.3390/molecules28114297
Castell A, Arroyo-Manzanares N, Guerrero-Núñez Y, Campillo N, Viñas P. Headspace with Gas Chromatography-Mass Spectrometry for the Use of Volatile Organic Compound Profile in Botanical Origin Authentication of Honey. Molecules. 2023; 28(11):4297. https://doi.org/10.3390/molecules28114297
Chicago/Turabian StyleCastell, Ana, Natalia Arroyo-Manzanares, Yolanda Guerrero-Núñez, Natalia Campillo, and Pilar Viñas. 2023. "Headspace with Gas Chromatography-Mass Spectrometry for the Use of Volatile Organic Compound Profile in Botanical Origin Authentication of Honey" Molecules 28, no. 11: 4297. https://doi.org/10.3390/molecules28114297
APA StyleCastell, A., Arroyo-Manzanares, N., Guerrero-Núñez, Y., Campillo, N., & Viñas, P. (2023). Headspace with Gas Chromatography-Mass Spectrometry for the Use of Volatile Organic Compound Profile in Botanical Origin Authentication of Honey. Molecules, 28(11), 4297. https://doi.org/10.3390/molecules28114297







