Influence of Different Rootstocks on Fruit Quality and Primary and Secondary Metabolites Content of Blood Oranges Cultivars
Abstract
1. Introduction
2. Results
2.1. Morphological and Qualitative Parameters
2.2. External Crust and Juice Color
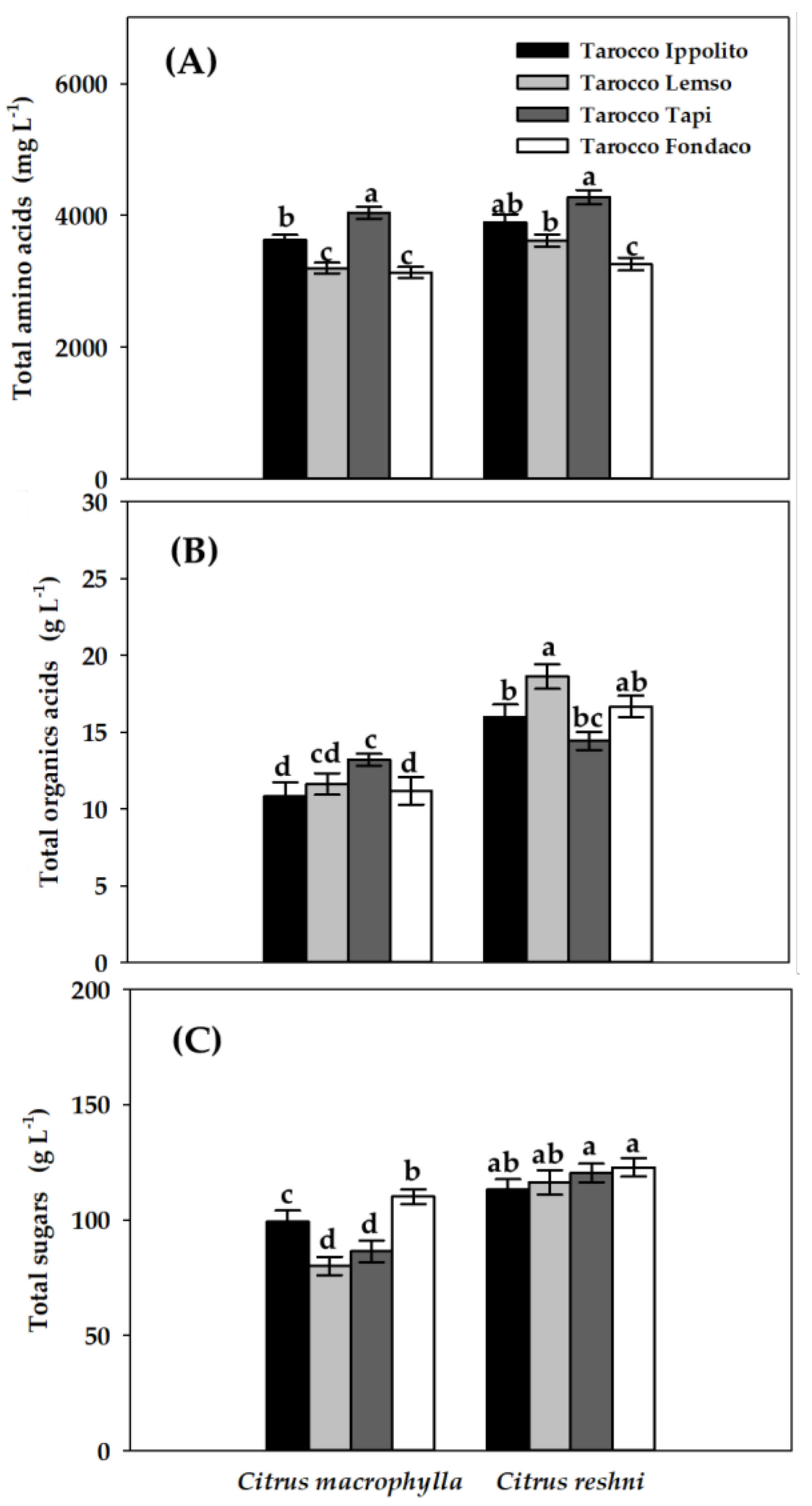
2.3. Primary Metabolites Content in Juice
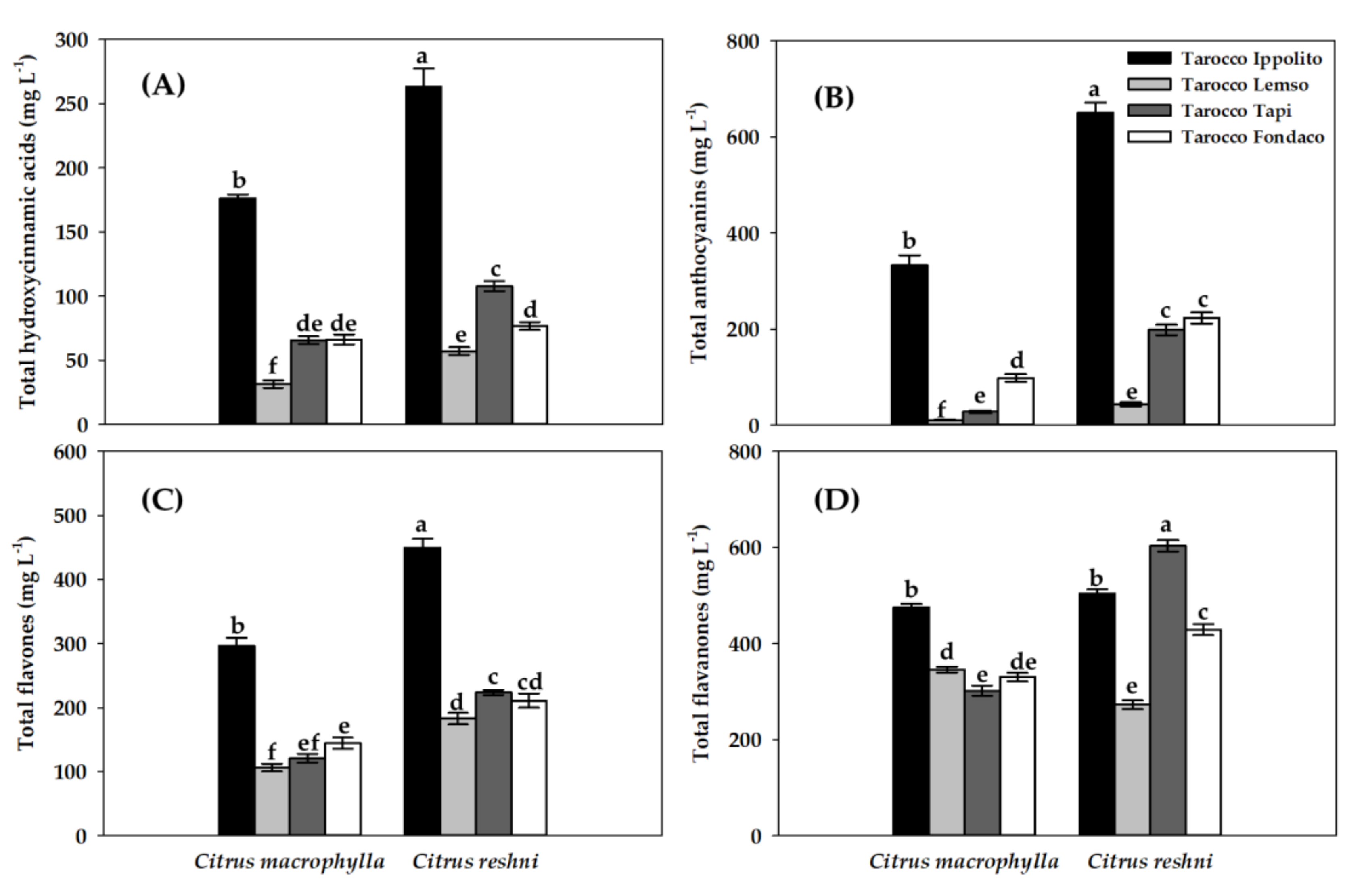
2.4. Secondary Metabolites Content in Juice
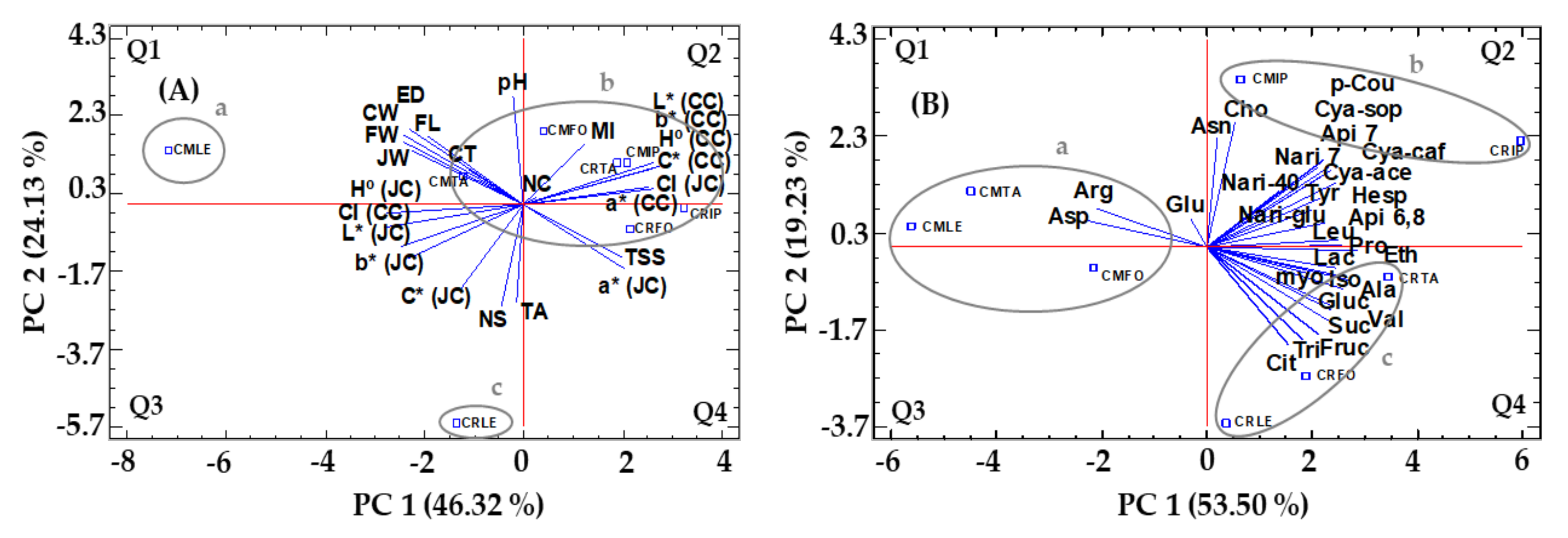
2.5. Principal Component Analysis (PCA)
3. Discussion
4. Materials and Methods
4.1. Plant Material and Sample Preparation
4.2. Fruit Morphological Characterization
4.3. Juice Quality Parameters Dermination
4.4. Analysis of Primary Metabolites by 1H−Nuclear Magnetic Resonance Spectroscopy (1H NMR)
4.5. Analysis of Secondary Metabolites by HPLC−Diode Array Detection−Electrospray Ionization−Mass Spectrometry (HPLC−ESI−DAD−MSn)
4.6. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Food and Agriculture Organization of the United Nations. FAOSTAT Statistical Database. Rome, Italy. 2020. Available online: www.faostat.fao.org (accessed on 30 October 2021).
- Legua, P.; Modica, G.; Porras, I.; Conesa, A.; Continella, A. Bioactive compounds, antioxidant activity and fruit quality evaluation of eleven blood orange cultivars. J. Sci. Food Agric. 2021, 102, 2960–2971. [Google Scholar] [CrossRef] [PubMed]
- Habibi, F.; García-Pastor, M.E.; Puente-Moreno, J.; Garrido-Auñón, F.; Serrano, M.; Valero, D. Anthocyanin in blood oranges: A review on postharvest approaches for its enhancement and preservation. Crit. Rev. Food Sci. Nutr. 2022, 12, 1–13. [Google Scholar] [CrossRef]
- Cebadera-Miranda, L.; Domínguez, L.; Dias, M.I.; Barros, L.; Ferreira, I.C.; Igual, M.; Martínez-Navarrete, N.; Fernández-Ruiz, V.; Morales, P.; Cámara, M. Sanguinello and Tarocco (Citrus sinensis [L.] Osbeck): Bioactive compounds and colour appearance of blood oranges. Food Chem. 2018, 270, 395–402. [Google Scholar] [CrossRef]
- Lu, X.; Zhao, C.; Shi, H.; Liao, Y.; Xu, F.; Du, H.; Xiao, H.; Zheng, J. Nutrients and bioactives in citrus fruits: Different citrus varieties, fruit parts, and growth stages. Crit. Rev. Food Sci. Nutr. 2021, 63, 2018–2041. [Google Scholar] [CrossRef] [PubMed]
- Zhao, C.; Wang, F.; Lian, Y.; Xiao, H.; Zheng, J. Biosynthesis of citrus flavonoids and their health effects. Crit. Rev. Food Sci. Nutr. 2020, 60, 566–583. [Google Scholar] [CrossRef] [PubMed]
- Serradilla, M.J.; Akšic, M.F.; Manganaris, G.A.; Ercisli, S.; Gonzalez-Gomez, D.; Valero, D. Fruit chemistry, nutritional benefits and social aspects of cherries. In Cherries: Botany, Production and Uses; CABI: Boston, MA, USA, 2017; pp. 420–441. Available online: https://www.cabi.org/horticulture/ebook/20173212321 (accessed on 23 January 2023).
- Pareek, S.; Valero, D.; Serrano, M. Postharvest biology and technology of pomegranate. J. Sci. Food Agric. 2015, 95, 2360–2379. [Google Scholar] [CrossRef]
- Piero, A.R.L. The State of the Art in Biosynthesis of Anthocyanins and Its Regulation in Pigmented Sweet Oranges [(Citrus sinensis) L. Osbeck]. J. Agric. Food Chem. 2015, 63, 4031–4041. [Google Scholar] [CrossRef] [PubMed]
- Lana, G.; Modica, G.; Casas, G.L.; Siracusa, L.; La Malfa, S.; Gentile, A.; Sicilia, A.; Distefano, G.; Continella, A. Molecular Insights into the Effects of Rootstocks on Maturation of Blood Oranges. Horticulturae 2021, 7, 468. [Google Scholar] [CrossRef]
- Pannitteri, C.; Continella, A.; Cicero, L.L.; Gentile, A.; La Malfa, S.; Sperlinga, E.; Napoli, E.; Strano, T.; Ruberto, G.; Siracusa, L. Influence of postharvest treatments on qualitative and chemical parameters of Tarocco blood orange fruits to be used for fresh chilled juice. Food Chem. 2017, 230, 441–447. [Google Scholar] [CrossRef]
- Habibi, F.; Ramezanian, A.; Guillén, F.; Castillo, S.; Serrano, M.; Valero, D. Changes in Bioactive Compounds, Antioxidant Activity, and Nutritional Quality of Blood Orange Cultivars at Different Storage Temperatures. Antioxidants 2020, 9, 1016. [Google Scholar] [CrossRef] [PubMed]
- Caruso, M.; Ferlito, F.; Licciardello, C.; Allegra, M.; Strano, M.C.; Di Silvestro, S.; Russo, M.P.; Paolo, D.P.; Caruso, P.; Casas, G.L.; et al. Pomological diversity of the Italian blood orange germplasm. Sci. Hortic. 2016, 213, 331–339. [Google Scholar] [CrossRef]
- Modica, G.; Pannitteri, C.; Di Guardo, M.; La Malfa, S.; Gentile, A.; Ruberto, G.; Pulvirenti, L.; Parafati, L.; Continella, A.; Siracusa, L. Influence of rootstock genotype on individual metabolic responses and antioxidant potential of blood orange cv. Tarocco Scirè. J. Food Compos. Anal. 2022, 105, 104246. [Google Scholar] [CrossRef]
- Continella, A.; Pannitteri, C.; La Malfa, S.; Legua, P.; Distefano, G.; Nicolosi, E.; Gentile, A. Influence of different rootstocks on yield precocity and fruit quality of ‘Tarocco Scirè’ pigmented sweet orange. Sci. Hortic. 2018, 230, 62–67. [Google Scholar] [CrossRef]
- Morales, J.; Bermejo, A.; Navarro, P.; Forner-Giner, M.; Salvador, A. Rootstock effect on fruit quality, anthocyanins, sugars, hydroxycinnamic acids and flavanones content during the harvest of blood oranges ‘Moro’ and ‘Tarocco Rosso’ grown in Spain. Food Chem. 2021, 342, 128305. [Google Scholar] [CrossRef]
- Zou, Z.; Xi, W.; Hu, Y.; Nie, C.; Zhou, Z. Antioxidant activity of Citrus fruits. Food Chem. 2016, 196, 885–896. [Google Scholar] [CrossRef] [PubMed]
- Cömert, E.D.; Gökmen, V. Evolution of food antioxidants as a core topic of food science for a century. Food Res. Int. 2018, 105, 76–93. [Google Scholar] [CrossRef]
- Legua, P.; Forner, J.; Hernández, F.; Forner-Giner, M. Total phenolics, organic acids, sugars and antioxidant activity of mandarin (Citrus clementina Hort. ex Tan.): Variation from rootstock. Sci. Hortic. 2014, 174, 60–64. [Google Scholar] [CrossRef]
- Legua, P.; Bellver, R.; Forner, J.; Forner-Giner, M.A. Plant growth, yield and fruit quality of ‘Lane Late’ navel orange on four Citrus rootstocks. Span. J. Agric. Res. 2011, 9, 271–279. [Google Scholar] [CrossRef]
- Forner-Giner, M.; Santos, M.B.-D.L.; Melgarejo, P.; Martínez-Nicolás, J.J.; Melián-Navarro, A.; Ruíz-Canales, A.; Continella, A.; Legua, P. Fruit Quality and Primary and Secondary Metabolites Content in Eight Cultivars of Blood Oranges. Agronomy 2023, 13, 1037. [Google Scholar] [CrossRef]
- Bowman, K.D.; Joubert, J.; Talon, M.; Caruso, M.; Gmitter, F. Citrus Rootstocks. In The Genus Citrus; Woodhead Publishing: Duxford, UK; Elsevier: Duxford, UK, 2020; pp. 105–127. [Google Scholar]
- Alfaro, J.M.; Bermejo, A.; Navarro, P.; Quiñones, A.; Salvador, A. Effect of Rootstock on Citrus Fruit Quality: A Review. Food Rev. Int. 2021, 1, 19. [Google Scholar] [CrossRef]
- Musacchi, S.; Serra, S. Apple fruit quality: Overview on pre-harvest factors. Sci. Hortic. 2018, 234, 409–430. [Google Scholar] [CrossRef]
- Ordóñez-Díaz, J.L.; Hervalejo, A.; Pereira-Caro, G.; Muñoz-Redondo, J.M.; Romero-Rodríguez, E.; Arenas-Arenas, F.J.; Moreno-Rojas, J.M. Effect of Rootstock and Harvesting Period on the Bioactive Compounds and Antioxidant Activity of Two Orange Cultivars (‘Salustiana’ and ‘Sanguinelli’) Widely Used in Juice Industry. Processes 2020, 8, 1212. [Google Scholar] [CrossRef]
- Al-Jaleel, A.; Zekri, M.; Hammam, Y. Yield, fruit quality, and tree health of ‘Allen Eureka’ lemon on seven rootstocks in Saudi Arabia. Sci. Hortic. 2005, 105, 457–465. [Google Scholar] [CrossRef]
- Legua, P.; Martinez-Cuenca, M.R.; Bellver, R.; Forner-Giner, M. Rootstock’s and scion’s impact on lemon quality in southeast Spain. Int. Agrophysics 2018, 32, 325–333. [Google Scholar] [CrossRef]
- Emmanouilidou, M.G.; Kyriacou, M.C. Rootstock-modulated yield performance, fruit maturation and phytochemical quality of ‘Lane Late’ and ‘Delta’ sweet orange. Sci. Hortic. 2017, 225, 112–121. [Google Scholar] [CrossRef]
- Sau, S.; Ghosh, S.N.; Sarkar, S.; Gantait, S. Effect of rootstocks on growth, yield, quality, and leaf mineral composition of Nagpur mandarin (Citrus reticulata Blanco.), grown in red lateritic soil of West Bengal, India. Sci. Hortic. 2018, 237, 142–147. [Google Scholar] [CrossRef]
- de Carvalho, L.M.; de Carvalho, H.W.L.; de Barros, I.; Martins, C.R.; Soares Filho, W.d.S.; Girardi, E.A.; Passos, O.S. New scion-rootstock combinations for diversification of sweet orange orchards in tropical hardsetting soils. Sci. Hortic. 2019, 243, 169–176. [Google Scholar] [CrossRef]
- Baldwin, E.A.; Bai, J.; Plotto, A.; Ritenour, M.A. Citrus fruit quality assessment; producer and consumer perspectives. Stewart Postharvest Rev. 2014, 10, 1–7. [Google Scholar]
- Barry, G.H.; Castle, W.S.; Davies, F.S. Rootstocks and Plant Water Relations Affect Sugar Accumulation of Citrus Fruit Via Osmotic Adjustment. J. Am. Soc. Hortic. Sci. 2004, 129, 881–889. [Google Scholar] [CrossRef]
- Cheng, J.; Wei, L.; Mei, J.; Wu, J. Effect of rootstock on phenolic compounds and antioxidant properties in berries of grape (Vitis vinifera L.) cv. ‘Red Alexandria’. Sci. Hortic. 2017, 217, 137–144. [Google Scholar] [CrossRef]
- Speer, H.; D’cunha, N.M.; Alexopoulos, N.I.; McKune, A.J.; Naumovski, N. Anthocyanins and Human Health—A Focus on Oxidative Stress, Inflammation and Disease. Antioxidants 2020, 9, 366. [Google Scholar] [CrossRef] [PubMed]
- Incesu, M.; Çimen, B.; Yesiloglu, T.; Yilmaz, B. Rootstock effects on yield, fruit quality, rind and juice color of “Moro” blood orange. J. Food Agric. Environ. 2013, 11, 867–871. [Google Scholar]
- Nishiyama, Y.; Yun, C.-S.; Matsuda, F.; Sasaki, T.; Saito, K.; Tozawa, Y. Expression of bacterial tyrosine ammonia-lyase creates a novel p-coumaric acid pathway in the biosynthesis of phenylpropanoids in Arabidopsis. Planta 2010, 232, 209–218. [Google Scholar] [CrossRef]
- EN 32011R0543; Commission Implementing Regulation (EC) N_543/2011 of 7 June 2011 Laying Down Detailed Rules for the Application of the Council Regulation (EC) No 1243/2007 in Respect of the Fruit and Vegetables Processed Fruit and Vegetables Sectors. European Union: Luxembourg, 2011.
- De Pascual-Teresa, S.; Sanchez-Ballesta, M.T. Anthocyanins: From plant to health. Phytochemistry 2008, 7, 281–299. [Google Scholar] [CrossRef]
- Machado, F.L.D.C.; Da Costa, J.M.C.; Teixeira, A.; Costa, J.D.P.D. The influence of rootstock and time of harvest on the fruit quality during storage of in two grapefruit cultivars. Acta Sci. Agron. 2015, 37, 339–346. [Google Scholar] [CrossRef]
- Lo Cicero, L.; Puglisi, I.; Nicolosi, E.; Gentile, A.; Ferlito, F.; Continella, A.; Lo Piero, A.R. Anthocyanin levels and expresión analysis of biosynthesis-related genes during ripening of sicilian and international grape berries subjected to leaf removal and water deficit. J. Agric. Sci. Technol. 2016, 18, 1333–1344. Available online: http://hdl.handle.net/123456789/3829 (accessed on 24 January 2023).
- AIJN. The AIJN Code of Practice, AIJN—European Fruit Juice Association; AIJN: Antwerp, Belgium, 2018. [Google Scholar]
- Schiffman, S.S.; Sennewald, K.; Gagnon, J. Comparison of taste qualities and thresholds of D- and L-amino acids. Physiol. Behav. 1981, 27, 51–59. [Google Scholar] [CrossRef] [PubMed]
- Rambla, J.L.; Tikunov, Y.M.; Monforte, A.J.; Bovy, A.G.; Granell, A. The expanded tomato fruit volatile landscape. J. Exp. Bot. 2014, 65, 4613–4623. [Google Scholar] [CrossRef]
- Wistaff, E.A.; Beller, S.; Schmid, A.; Neville, J.J.; Nietner, T. Chemometric analysis of amino acid profiles for detection of fruit juice adulterations—Application to verify authenticity of blood orange juice. Food Chem. 2021, 343, 128452. [Google Scholar] [CrossRef]
- Kelebek, H.; Canbas, A.; Selli, S. Determination of phenolic composition and antioxidant capacity of blood orange juices obtained from cvs. Moro and Sanguinello (Citrus sinensis (L.) Osbeck) grown in Turkey. Food Chem. 2008, 107, 1710–1716. [Google Scholar] [CrossRef]
- Bassal, M.A. Growth, yield and fruit quality of ‘Marisol’ clementine grown on four rootstocks in Egypt. Sci. Hortic. 2009, 119, 132–137. [Google Scholar] [CrossRef]
- Lee, H.S.; Coates, G.A. Quantitative study of free sugars and myoinositol in Citrus juices by HPLC and literature compilation. J. Liq. Chromatogr. Relat. Technol. 2014, 14, 2123–2141. [Google Scholar] [CrossRef]
- Hussain, S.; Curk, F.; Anjum, M.A.; Pailly, O.; Tison, G. Performance evaluation of common clementine on various Citrus rootstocks. Sci. Hortic. 2013, 150, 278–282. [Google Scholar] [CrossRef]
- Castellari, M.; Versari, A.; Spinabelli, U.; Galassi, S.; Amati, A. An improved hplc method for the analysis of organic acids, carbohydrates, and alcohols in grape musts and wines. J. Liq. Chromatogr. Relat. Technol. 2000, 23, 2047–2056. [Google Scholar] [CrossRef]
- Sicari, V.; Pellicanò, T.M.; Giuffrè, A.M.; Zappia, C.; Capocasale, M. Bioactive compounds and antioxidant activity of Citrus juices produced from varieties cultivated in Calabria. J. Food Meas. Charact. 2016, 10, 773–780. [Google Scholar] [CrossRef]
- López-Sobaler, A.M.; Mora, A.M.L.; González, M.D.S.; Suárez, P.; Aparicio, A.; Ortega, R.M. Importance of choline in cognitive function. Nutr. Hosp. 2020, 37, 2. [Google Scholar] [CrossRef]
- Shah, K.R.; Vyas, R.; Patel, G. Bioethanol Production from Pulp of Fruits. Biosci. Biotechnol. Res. Commun. 2019, 12, 464–471. [Google Scholar] [CrossRef]
- Nazir-Lone, A.; Tanveer-Malik, A.; Shahid-Naikoo, H.; Sharma-Raghu, R.; Sheikh, A. Trigonelline, a naturally occurring alkaloidal agent protects ultraviolet-B (UV-B) irradiation induced apoptotic cell death in human skin fibroblasts via attenuation of oxidative stress, restoration of cellular calcium homeostasis and prevention of endoplasmic reticulum (ER) stress. J. Photochem. Photobiol. B Biol. 2020, 202, 111720. [Google Scholar] [CrossRef]
- Konstantinidis, N.; Franke, H.; Schwarz, S.; Lachenmeier, D.W. Risk Assessment of Trigonelline in Coffee and Coffee By-Products. Molecules 2023, 28, 3460. [Google Scholar] [CrossRef]
- Sdiri, S.; Salvador, A.; Farhat, I.; Navarro, P.; Besada, C. Influence of postharvest handling on antioxidant compounds of citrusfruits. In Citrus: Molecular Phylogeny, Antioxidant Properties and Medicinal Uses; Nova Science Publishers: New York, NY, USA, 2014; pp. 83–93. Available online: http://hdl.handle.net/20.500.11939/4508 (accessed on 24 January 2023).
- Sousa, W.R.; da Rocha, C.; Cardoso, C.L.; Silva, D.H.S.; Zanoni, M.V.B. Determination of the relative contribution of phenolic antioxidants in orange juice by voltammetric methods. J. Food Compos. Anal. 2004, 17, 619–633. [Google Scholar] [CrossRef]
- Agut, B.; Gamir, J.; Jaques, J.A.; Flors, V. Systemic resistance in citrus to Tetranychus urticae induced by conspecifics is transmitted by grafting and mediated by mobile amino acids. J. Exp. Bot. 2016, 67, 5711–5723. [Google Scholar] [CrossRef]
- Tavarini, S.; Gil, M.; Tomas-Barberan, F.; Buendia, B.; Remorini, D.; Massai, R.; Degl’Innocenti, E.; Guidi, L. Effects of water stress and rootstocks on fruit phenolic composition and physical/chemical quality in Suncrest peach. Ann. Appl. Biol. 2011, 158, 226–233. [Google Scholar] [CrossRef]
- Yıldırım, F.; Yıldırım, A.N.; San, B.; Ercişli, S. The Relationship Between Growth Vigour of Rootstock and Phenolic Contents in Apple (Malus × domestica). Erwerbs-Obstbau 2016, 58, 25–29. [Google Scholar] [CrossRef]
- Hemmati, N.; Ghasemnezhad, A.; Moghaddam, J.F.; Ebrahimi, P. Variation in the content of bioflavonoids of orange as affected by scion, rootstock, and fruit part. Acta Physiol. Plant. 2018, 40, 83. [Google Scholar] [CrossRef]
- Saini, M.K.; Capalash, N.; Kaur, C.; Singh, S.P. Comprehensive metabolic profiling to decipher the influence of rootstocks on fruit juice metabolome of Kinnow (C. nobilis × C. deliciosa). Sci. Hortic. 2019, 257, 108673. [Google Scholar] [CrossRef]
- Feng, S.; Niu, L.; Suh, J.H.; Hung, W.-L.; Wang, Y. Comprehensive Metabolomics Analysis of Mandarins (Citrus reticulata) as a Tool for Variety, Rootstock, and Grove Discrimination. J. Agric. Food Chem. 2018, 66, 10317–10326. [Google Scholar] [CrossRef]
- McGuire, R.G. Reporting of Objective Color Measurements. Hortscience 1992, 27, 1254–1255. [Google Scholar] [CrossRef]
- Jimenez-Cuesta, M.; Cuquerella, J.; Martínez-Javega, J.M. Determination of a colour index for Citrus fruit degreening. In Proceedings of the International Society of Citriculture Citriculture, IV Congress, Tokio, Japan, 9–12 November 1981; Volume 2, pp. 750–753. Available online: https://agris.fao.org/agris-search/search.do?recordID=US201302640817 (accessed on 30 January 2023).
- Choi, Y.H.; Kim, H.K.; Linthorst, H.J.M.; Hollander, J.G.; Lefeber, A.W.M.; Erkelens, C.; Nuzillard, J.-M.; Verpoorte, R. NMR Metabolomics to Revisit the Tobacco Mosaic Virus Infection in Nicotiana tabacum Leaves. J. Nat. Prod. 2006, 69, 742–748. [Google Scholar] [CrossRef] [PubMed]
- Legua, P.; Forner-Giner, M.Á.; Nuncio-Jáuregui, N.; Hernández, F. Polyphenolic compounds, anthocyanins and antioxidant activity of nineteen pomegranate fruits: A rich source of bioactive compounds. J. Funct. Foods 2016, 23, 628–636. [Google Scholar] [CrossRef]

| Parameters | Citrus macrophylla | Citrus reshni | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Tarocco Ippolito | Tarocco Lempso | Tarocco Tapi | Tarocco Fondaconuovo | Tarocco Ippolito | Tarocco Lempso | Tarocco Tapi | Tarocco Fondaconuovo | ANOVA−Factors A: Root. B: Vari. A*B | |||
| (A) Morphological parameters * | |||||||||||
| FW (g) | 137.56 cd | 275.78 a | 177.33 b | 203.44 b | 136.67 cd | 127.89 d | 169.89 bc | 167.78 bc | * | *** | NS |
| ED (mm) | 67.08 cd | 81.37 a | 73.21 b | 72.81 b | 64.98 cd | 63.17 d | 70.37 bc | 68.96 bc | ** | *** | NS |
| FL (mm) | 57.76 c | 76.79 a | 66.58 b | 73.77 a | 60.90 bc | 57.64 c | 64.27 b | 65.97 b | * | *** | *** |
| CT (mm) | 4.68 b | 4.65 bc | 6.25 a | 4.59 bc | 4.14 bc | 4.02 bc | 3.66 bc | 3.39 c | *** | ** | ** |
| NC | 10.11 a | 9.78 ab | 10.22 a | 9.67 ab | 9.78 ab | 10.0 a | 10.67 a | 9.44 b | NS | NS | NS |
| NS | 0.11 c | 0.22 bc | 0.0 c | 0.33 b | 0.22 b | 0.78 a | 0.0 c | 0.33 b | NS | NS | NS |
| CW (g) | 58.15 c | 113.67 a | 82.33 b | 81.56 b | 57.56 c | 45.98 c | 60.43 c | 59.44 c | *** | *** | ** |
| JW (w:w) | 79.40 d | 162.11 a | 95.00 cd | 121.89 b | 79.11 d | 81.91 d | 109.46 c | 108.33 c | NS | *** | * |
| (B) Qualitative parameters ** | |||||||||||
| pH | 3.68 a | 3.63 ab | 3.43 c | 3.67 a | 3.48 bc | 3.15 d | 3.49 bc | 3.36 c | *** | *** | *** |
| TSS (Brix) | 13.67 bc | 12.00 de | 11.63 e | 12.87 cd | 14.33 ab | 14.70 ab | 15.40 a | 15.07 a | *** | *** | *** |
| TA (g citric acid L−1) | 7.86 d | 11.50 c | 11.47 c | 9.37 d | 13.07 bc | 16.22 a | 12.84 bc | 13.54 b | *** | *** | *** |
| MI (TSS/TA) | 17.4 a | 10.5 cd | 10.2 de | 13.7 b | 11.0 cd | 9.1 e | 12.0 c | 11.1 cd | *** | *** | *** |
| Color | Citrus macrophylla | Citrus reshni | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Tarocco Ippolito | Tarocco Lempso | Tarocco Tapi | Tarocco Fondaconuovo | Tarocco Ippolito | Tarocco Lempso | Tarocco Tapi | Tarocco Fondaconuovo | ANOVA−Factors A: Root. B: Vari. A*B | |||
| (A) Crust | |||||||||||
| L* | 54.40 b | 37.17 d | 59.43 a | 60.78 a | 61.55 a | 45.11 c | 60.31 a | 59.41 a | * | *** | ** |
| a* | 32.25 ab | 21.90 c | 35.25 a | 35.00 a | 32.65 as | 29.07 b | 33.82 a | 33.88 a | NS | *** | NS |
| b* | 37.89 b | 10.68 c | 45.82 ab | 48.19 a | 50.21 a | 21.28 c | 48.87 a | 46.18 ab | ** | *** | *** |
| C* | 50.05 b | 24.37 d | 57.85 a | 59.73 a | 60.24 a | 36.22 c | 59.76 a | 57.43 a | ** | *** | *** |
| H° | 49.00 b | 25.77 d | 52.35 ab | 53.80 ab | 56.18 a | 35.22 c | 54.90 ab | 53.48 ab | NS | *** | ** |
| CI | 16.58 c | 56.17 a | 13.06 c | 12.28 c | 11.52 c | 33.32 b | 12.06 c | 12.69 c | NS | *** | NS |
| (B) Juice | |||||||||||
| L* | 36.53 bc | 41.08 a | 41.57 a | 38.49 b | 34.58 c | 41.93 a | 35.85 c | 36.00 c | *** | *** | *** |
| a* | 8.37 a | 4.69 b | 6.73 ab | 8.91 a | 8.21 a | 9.61 a | 6.76 ab | 9.05 a | NS | *** | NS |
| b* | 4.76 bc | 9.56 a | 10.56 a | 6.47 b | 3.83 c | 11.36 a | 4.14 c | 4.84 bc | *** | *** | *** |
| C* | 9.93 bc | 10.68 bc | 12.53 ab | 11.02 bc | 9.06 bc | 14.90 a | 8.00 c | 10.28 bc | *** | *** | * |
| H° | 29.76 de | 64.23 a | 57.49 ab | 35.95 cde | 24.96 e | 49.75 abc | 42.73 bcd | 27.98 de | *** | *** | NS |
| CI | 55.57 a | 11.80 d | 15.36 d | 35.88 bc | 62.22 a | 20.36 cd | 35.36 bc | 52.85 ab | *** | *** | NS |
| Primary Metabolites | Citrus macrophylla | Citrus reshni | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Tarocco Ippolito | Tarocco Lempso | Tarocco Tapi | Tarocco Fondaconuovo | Tarocco Ippolito | Tarocco Lempso | Tarocco Tapi | Tarocco Fondaconuovo | ANOVA−Factors A: Root. B: Vari. A*B | |||
| (A) Amino acids (mg L−1) | |||||||||||
| Alanine | 74.15 cd | 41.43 e | 49.08 e | 49.81 de | 104.03 ab | 88.40 bc | 117.44 a | 95.35 abc | *** | *** | * |
| Arginine | 522.13 de | 752.62 ab | 846.84 a | 602.0 bcd | 428.66 e | 408.86 e | 669.0 abc | 464.12 e | *** | *** | * |
| Asparagine | 384.23 c | 686.22 a | 663.12 ab | 390.46 c | 431.0 abc | 415.14 bc | 311.07 c | 417.06 bc | ** | * | ** |
| Aspartate | 1001.34 a | 743.36 bc | 921.67 ab | 787.0 abc | 838.0 abc | 741.01 bc | 905.21 ab | 650.68 c | * | *** | NS |
| Glutamine | 205.90 ab | 214.51 ab | 216.39 ab | 231.10 ab | 199.70 ab | 193.06 ab | 256.75 a | 178.96 b | NS | NS | * |
| Isoleucine | 6.01 cd | 3.14 e | 4.02 de | 4.11 de | 7.65 b | 7.02 bc | 9.86 a | 8.32 ab | *** | * | ** |
| Leucine | 2.96 c | 2.09 c | 1.86 c | 3.37 bc | 5.85 a | 2.50 c | 4.05 b | 4.85 ab | *** | *** | * |
| Proline | 1329.03 c | 712.37 e | 1266.0 cd | 987.70 d | 1749.91 a | 1667.0 ab | 1917.35 a | 1354.0 bc | *** | *** | ** |
| Tyrosine | 91.25 ab | 37.68 c | 64.48 bc | 69.04 bc | 113.05 a | 77.52 ab | 60.73 bc | 70.06 bc | * | *** | * |
| Valine | 11.37 bc | 7.36 c | 8.08 c | 8.05 c | 16.35 b | 17.71 b | 23.37 a | 18.32 b | *** | * | *** |
| (B) Organic acids (g L−1) | |||||||||||
| Citrate | 10.83 c | 11.61 c | 13.19 bc | 11.18 c | 16.00 ab | 18.62 a | 14.42 b | 16.66 ab | *** | NS | ** |
| Lactate | 0.01 b | 0.01 b | 0.01 b | 0.01 b | 0.02 a | 0.01 b | 0.02 a | 0.02 a | *** | *** | NS |
| (C) Sugars (g L−1) | |||||||||||
| Fructose | 26.86 abc | 21.89 c | 25.15 bc | 30.99 ab | 30.58 a | 33.54 a | 33.51 a | 33.49 a | *** | ** | ** |
| Glucose | 25.96 b | 19.57 c | 21.76 c | 28.80 ab | 30.13 a | 28.92 ab | 28.75 ab | 32.02 a | *** | *** | ** |
| Sucrose | 44.81 bc | 36.97 c | 37.73 c | 48.10 bc | 49.79 bc | 51.61 ab | 56.18 a | 55.39 a | *** | * | ** |
| Myo−inostol | 1.63 d | 1.56 d | 1.72 cd | 2.32 ab | 2.75 a | 2.25 abc | 1.98 bcd | 1.99 bcd | *** | * | *** |
| Glu/Fru | 0.97 a | 0.89 b | 0.87 b | 0.93 a | 0.99 a | 0.86 b | 0.86 b | 0.96 a | ** | * | * |
| (D) Others (mg L−1) | |||||||||||
| Choline | 23.25 a | 11.07 c | 17.67 b | 15.43 b | 18.25 b | 10.71 c | 14.58 bc | 10.67 c | *** | *** | * |
| Ethanol | 575.0 bcd | 150.53 f | 124.50 f | 371.27 ef | 1047.64 a | 492.81 de | 738.29 bc | 838.81 ab | *** | *** | NS |
| Trigonelline | 3.91 f | 5.66 f | 4.90 f | 6.56 ef | 10.90 bc | 14.42 a | 12.77 ab | 8.92 cd | *** | ** | *** |
| Secondary Metabolites | Citrus macrophylla | Citrus reshni | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Tarocco Ippolito | Tarocco Lempso | Tarocco Tapi | Tarocco Fondaconuovo | Tarocco Ippolito | Tarocco Lempso | Tarocco Tapi | Tarocco Fondaconuovo | ANOVA−Factors A: Root. B: Var. A*B | |||
| (A) Hydroxycinnamic acids (mg L−1) | |||||||||||
| p−coumaric acid 4−O−glucoside | 176.25 b | 31.31 d | 65.55 cd | 66.01 bc | 263.34 a | 57.09 cd | 107.78 c | 76.66 bc | *** | *** | ** |
| (B) Anthocyanins (mg L−1) | |||||||||||
| Cyanidin 3−O−(6″−caffeoyl−glucoside) | 24.55 b | 0.16 d | 0.06 d | 6.57 cd | 64.91 a | 2.42 d | 7.15 cd | 14.69 bc | *** | *** | *** |
| Cyanidin 3−O−sophoroside | 134.60 b | 5.19 e | 11.00 e | 48.15 de | 237.76 a | 13.17 e | 80.92 c | 70.26 cd | *** | *** | *** |
| Cyanidin 3−O−(6″−acetyl−glucoside) | 174.10 b | 5.61 d | 16.59 cd | 43.51 c | 347.76 a | 27.91 c | 110.23 b | 137.79 b | *** | *** | *** |
| (C) Flavones (mg L−1) | |||||||||||
| Apigenin 6,8−di−C−glycoside | 110.54 c | 75.56 d | 68.73 d | 70.74 d | 173.02 a | 134.99 b | 110.24 c | 100.56 c | *** | *** | ** |
| Apigenin 7−O−(6″−malonyl−apiosyl−glucoside) | 186.35 b | 30.43 e | 52.29 d | 73.68 d | 276.73 a | 47.71 de | 113.30 c | 110.19 c | *** | *** | ** |
| (D) Flavanones (mg L−1) | |||||||||||
| Naringenin−glucosyl−rutinoside | 31.43 ab | 8.04 d | 15.80 cd | 16.30 cd | 29.19 abc | 27.04 abc | 32.97 a | 20.75 bcd | *** | *** | ** |
| Narirutin, naringenin−7−rutinoside | 101.85 a | 62.65 b | 75.35 b | 53.49 b | 117.66 a | 61.97 b | 114.34 a | 67.79 b | *** | *** | ** |
| Hesperidin, 7−rutinoside | 324.16 b | 247.89 bc | 198.25 c | 247.68 bc | 337.13 ab | 176.29 c | 428.41 a | 324.85 b | *** | *** | *** |
| Didymin, naringenin−40−methyl−ether 7−rutinoside | 17.81 b | 17.25 b | 12.30 bc | 12.55 bc | 9.96 d | 7.35 d | 27.35 a | 15.67 b | ** | *** | *** |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Forner-Giner, M.Á.; Ballesta-de los Santos, M.; Melgarejo, P.; Martínez-Nicolás, J.J.; Núñez-Gómez, D.; Continella, A.; Legua, P. Influence of Different Rootstocks on Fruit Quality and Primary and Secondary Metabolites Content of Blood Oranges Cultivars. Molecules 2023, 28, 4176. https://doi.org/10.3390/molecules28104176
Forner-Giner MÁ, Ballesta-de los Santos M, Melgarejo P, Martínez-Nicolás JJ, Núñez-Gómez D, Continella A, Legua P. Influence of Different Rootstocks on Fruit Quality and Primary and Secondary Metabolites Content of Blood Oranges Cultivars. Molecules. 2023; 28(10):4176. https://doi.org/10.3390/molecules28104176
Chicago/Turabian StyleForner-Giner, María Ángeles, Manuel Ballesta-de los Santos, Pablo Melgarejo, Juan José Martínez-Nicolás, Dámaris Núñez-Gómez, Alberto Continella, and Pilar Legua. 2023. "Influence of Different Rootstocks on Fruit Quality and Primary and Secondary Metabolites Content of Blood Oranges Cultivars" Molecules 28, no. 10: 4176. https://doi.org/10.3390/molecules28104176
APA StyleForner-Giner, M. Á., Ballesta-de los Santos, M., Melgarejo, P., Martínez-Nicolás, J. J., Núñez-Gómez, D., Continella, A., & Legua, P. (2023). Influence of Different Rootstocks on Fruit Quality and Primary and Secondary Metabolites Content of Blood Oranges Cultivars. Molecules, 28(10), 4176. https://doi.org/10.3390/molecules28104176










