Surfactant Additives Containing Hydrophobic Fluorocarbon Chains and Hydrophilic Sulfonate Anion for Highly Reversible Zn Anode
Abstract
1. Introduction
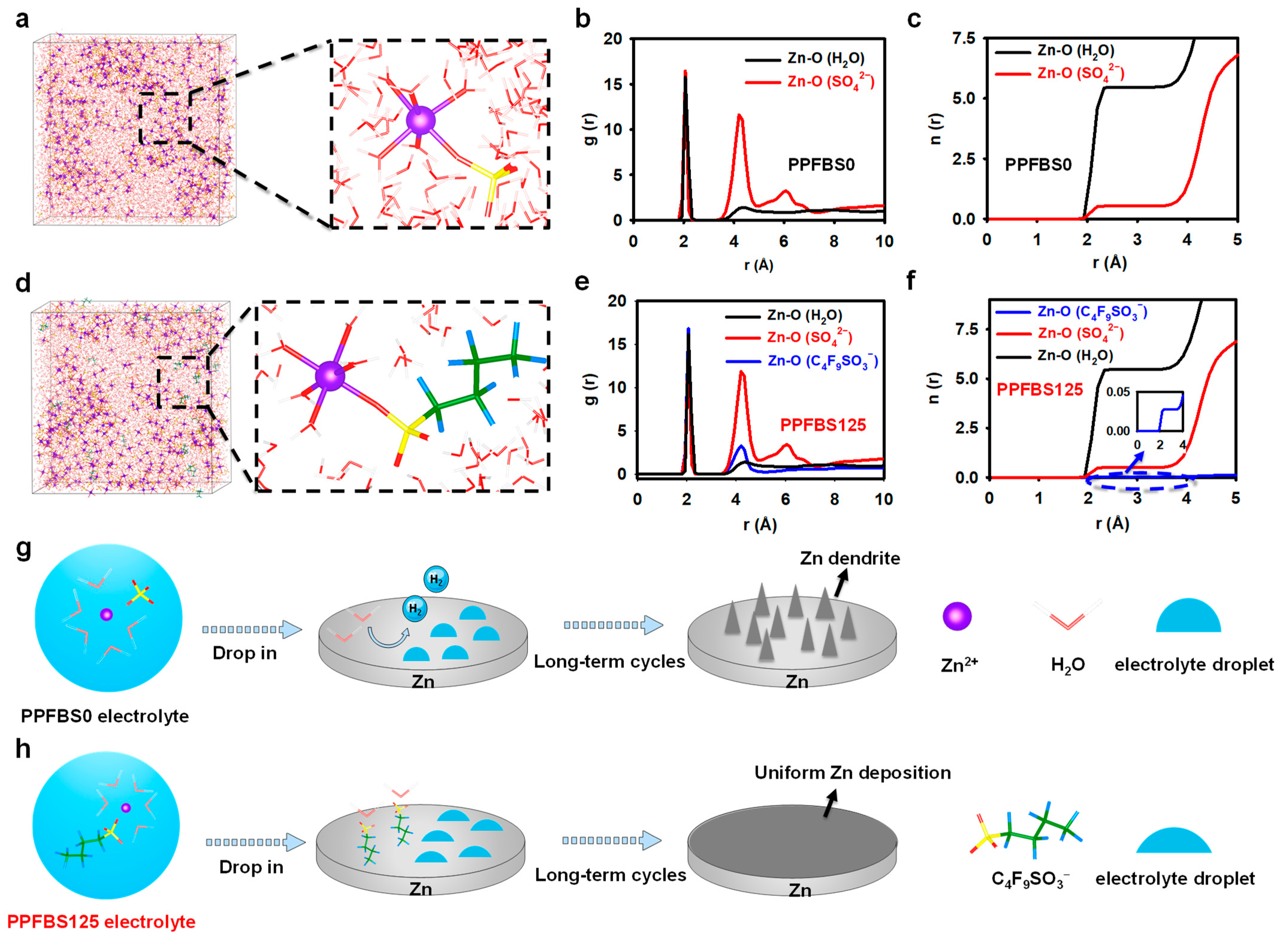
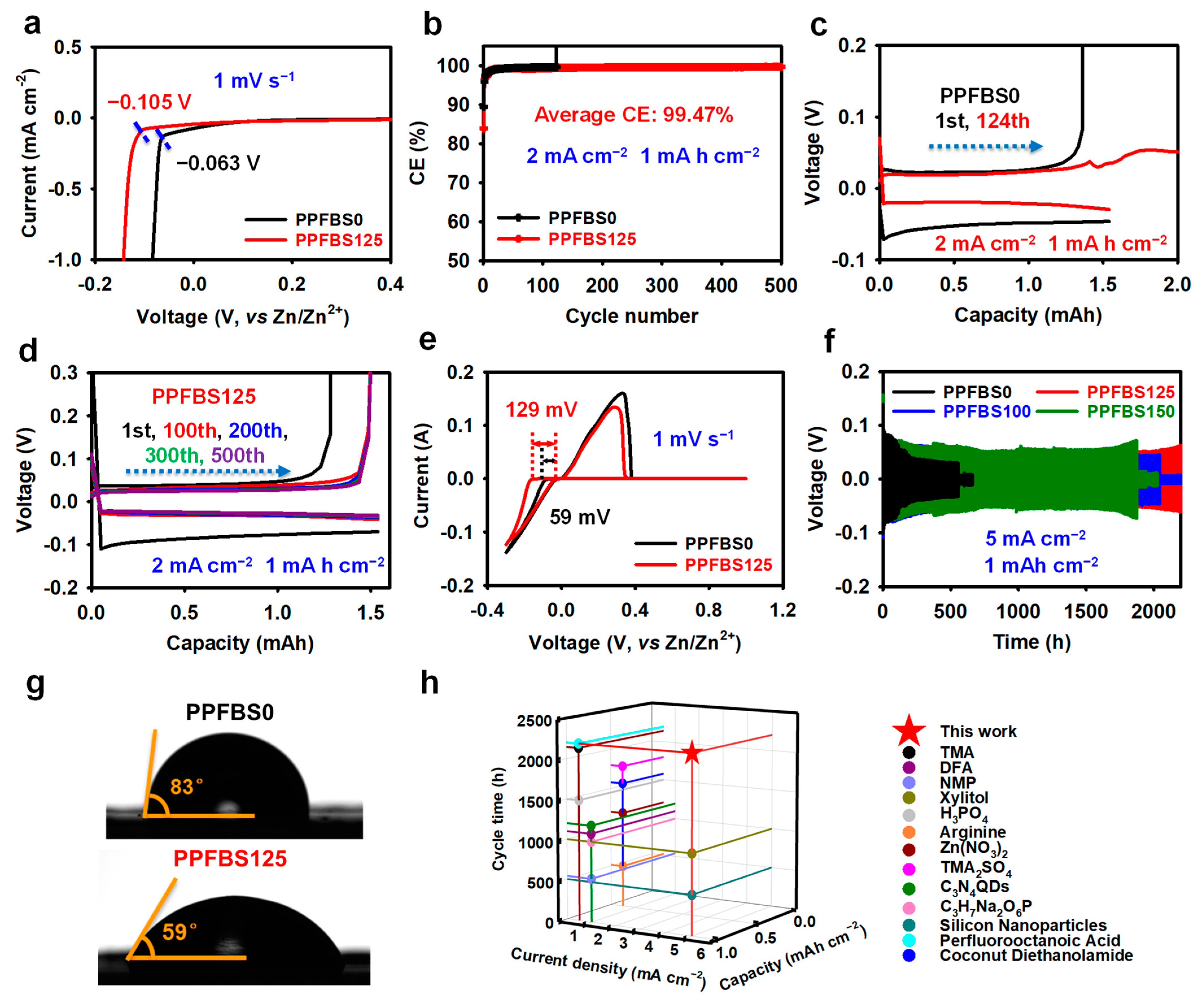
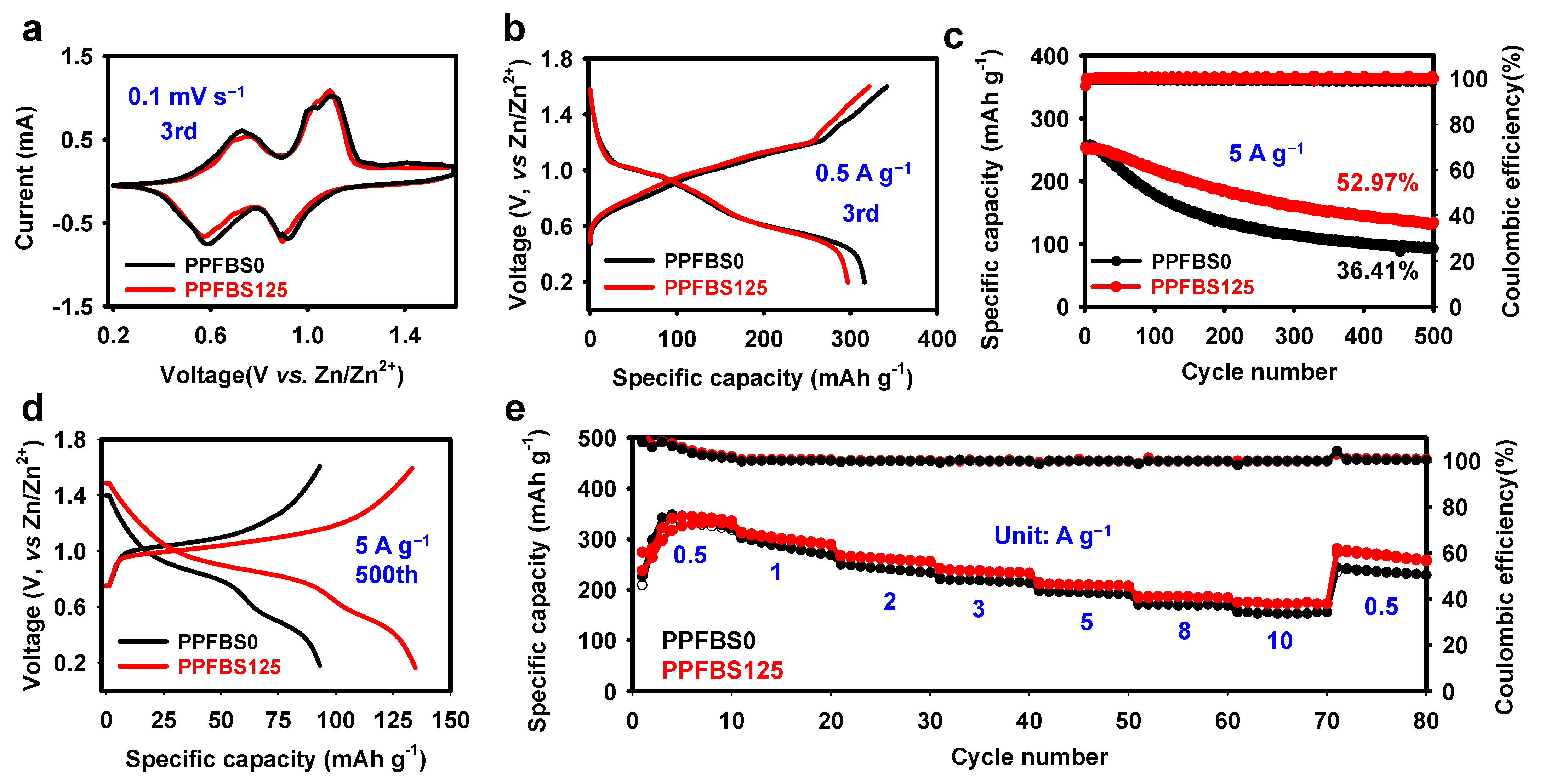
2. Results and Discussion
3. Materials and Methods
3.1. Materials
3.2. Synthesis of Electrolytes
3.3. Synthesis of V2O5 Cathode Material
3.4. Synthesis of the V2O5 Composite Electrode
3.5. Electrochemical Evaluation
3.6. Details of Molecular Dynamics (MD) Simulation
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Armand, M.; Tarascon, J.M. Building better batteries. Nature 2008, 451, 652–657. [Google Scholar] [CrossRef] [PubMed]
- Goodenough, J.B.; Park, K.S. The Li-ion rechargeable battery: A perspective. J. Am. Chem. Soc. 2013, 135, 1167–1176. [Google Scholar] [CrossRef] [PubMed]
- Dunn, B.; Kamath, H.; Tarascon, J.-M. Electrical energy storage for the grid: A battery of choices. Science 2011, 334, 928–935. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Deng, W.Z.; Chen, M.; Wang, Y.B.; Sun, C.F. Mass-producible, quasi-zero-strain, lattice-water-rich inorganic open-frameworks for ultrafast-charging and long-cycling zinc-ion batteries. Adv. Mater. 2020, 32, 2003592. [Google Scholar] [CrossRef]
- Fan, Y.; Yu, X.; Feng, Z.; Hu, M.; Zhang, Y. Synthesis of Zn2+-Pre-Intercalated V2O5 nH2O/rGO Composite with Boosted Electrochemical Properties for Aqueous Zn-Ion Batteries. Molecules 2022, 27, 5387. [Google Scholar] [CrossRef]
- Yuan, L.; Hao, J.; Kao, C.-C.; Wu, C.; Liu, H.-K.; Dou, S.-X.; Qiao, S.-Z. Regulation methods for the Zn/electrolyte interphase and the effectiveness evaluation in aqueous Zn-ion batteries. Energy Environ. Sci. 2021, 14, 5669–5689. [Google Scholar] [CrossRef]
- Zhang, S.; Fan, Q.; Liu, Y.; Xi, S.; Liu, X.; Wu, Z.; Hao, J.; Pang, W.K.; Zhou, T.; Guo, Z. Dehydration-triggered ionic channel engineering in potassium niobate for Li/K-ion storage. Adv. Mater. 2020, 32, 2000380. [Google Scholar] [CrossRef]
- Xu, C.; Li, B.; Du, H.; Kang, F. Energetic zinc ion chemistry: The rechargeable zinc ion battery. Angew. Chem. Int. Ed. 2012, 51, 933–935. [Google Scholar] [CrossRef]
- Song, M.; Tan, H.; Chao, D.; Fan, H.J. Recent advances in Zn-Ion batteries. Adv. Funct. Mater. 2018, 28, 1802564. [Google Scholar] [CrossRef]
- Zhou, W.; Zeng, G.; Jin, H.; Jiang, S.; Huang, M.; Zhang, C.; Chen, H. Bio-Template Synthesis of V2O3@Carbonized Dictyophora Composites for Advanced Aqueous Zinc-Ion Batteries. Molecules 2023, 28, 2147. [Google Scholar] [CrossRef]
- Liu, Y.; Tao, F.; Xing, Y.; Pei, Y.; Ren, F. Melamine Foam-Derived Carbon Scaffold for Dendrite-Free and Stable Zinc Metal Anode. Molecules 2023, 28, 1742. [Google Scholar] [CrossRef] [PubMed]
- Yi, Z.; Chen, G.; Hou, F.; Wang, L.; Liang, J. Strategies for the stabilization of zn metal anodes for zn-ion batteries. Adv. Energy Mater. 2021, 11, 2003065. [Google Scholar] [CrossRef]
- Yang, H.; Chang, Z.; Qiao, Y.; Deng, H.; Mu, X.; He, P.; Zhou, H. Constructing a super-saturated electrolyte front surface for stable rechargeable aqueous zinc batteries. Angew. Chem. Int. Ed. 2020, 59, 9377–9381. [Google Scholar] [CrossRef] [PubMed]
- Cui, Y.; Zhao, Q.; Wu, X.; Chen, X.; Yang, J.; Wang, Y.; Qin, R.; Ding, S.; Song, Y.; Wu, J.; et al. An interface-bridged organic-inorganic layer that suppresses dendrite formation and side reactions for ultra-long-life aqueous zinc metal anodes. Angew. Chem. Int. Ed. 2020, 59, 16594–16601. [Google Scholar] [CrossRef] [PubMed]
- Jiao, Y.; Li, F.; Jin, X.; Lei, Q.; Li, L.; Wang, L.; Ye, T.; He, E.; Wang, J.; Chen, H.; et al. Engineering polymer glue towards 90% zinc utilization for 1000 hours to make high-performance Zn-ion batteries. Adv. Funct. Mater. 2021, 31, 2107652. [Google Scholar] [CrossRef]
- Zheng, J.; Zhao, Q.; Tang, T.; Yin, J.; Quilty, C.D.; Renderos, G.D.; Liu, X.; Deng, Y.; Wang, L.; Bock, D.C.; et al. Reversible epitaxial electrodeposition of metals in battery anodes. Science 2019, 366, 645–648. [Google Scholar] [CrossRef]
- Ma, L.; Li, Q.; Ying, Y.; Ma, F.; Chen, S.; Li, Y.; Huang, H.; Zhi, C. Toward practical high-areal-capacity aqueous zinc-metal batteries: Quantifying hydrogen evolution and a solid-ion conductor for stable zinc anodes. Adv. Mater. 2021, 33, 2007406. [Google Scholar] [CrossRef]
- Zhao, Z.; Wang, R.; Peng, C.; Chen, W.; Wu, T.; Hu, B.; Weng, W.; Yao, Y.; Zeng, J.; Chen, Z.; et al. Horizontally arranged zinc platelet electrodeposits modulated by fluorinated covalent organic framework film for high-rate and durable aqueous zinc ion batteries. Nat. Commun. 2021, 12, 6606. [Google Scholar] [CrossRef]
- Cao, J.; Zhang, D.; Gu, C.; Wang, X.; Wang, S.; Zhang, X.; Qin, J.; Wu, Z.-S. Manipulating crystallographic orientation of zinc deposition for dendrite-free zinc ion batteries. Adv. Energy Mater. 2021, 11, 2101299. [Google Scholar] [CrossRef]
- Fang, Y.; Xie, X.; Zhang, B.; Chai, Y.; Lu, B.; Liu, M.; Zhou, J.; Liang, S. Regulating zinc deposition behaviors by the conditioner of pan separator for zinc-ion batteries. Adv. Funct. Mater. 2022, 32, 2109671. [Google Scholar] [CrossRef]
- Zhang, X.; Li, J.; Qi, K.; Yang, Y.; Liu, D.; Wang, T.; Liang, S.; Lu, B.; Zhu, Y.; Zhou, J. An ion-sieving janus separator toward planar electrodeposition for deeply rechargeable Zn-metal anodes. Adv. Mater. 2022, 34, 2205175. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Li, X.; Fan, L.; Shuai, Y.; Zhang, N. Ultrathin and super-tough membrane for anti-dendrite separator in aqueous zinc-ion batteries. Cell Rep. Phys. Sci. 2022, 3, 100824. [Google Scholar] [CrossRef]
- Zhu, J.; Bie, Z.; Cai, X.; Jiao, Z.; Wang, Z.; Tao, J.; Song, W.; Fan, H.J. A molecular-sieve electrolyte membrane enables separator-free zinc batteries with ultralong cycle life. Adv. Mater. 2022, 34, 2207209. [Google Scholar] [CrossRef]
- Luo, Y.; Yang, Y.; Tao, Y.; Huang, D.; Huang, B.; Chen, H. Directing the preferred crystal orientation by a cellulose acetate/graphene oxide composite separator for dendrite-free Zn-metal anodes. ACS Appl. Mater. Interfaces 2021, 4, 14599–14607. [Google Scholar] [CrossRef]
- Parker, J.F.; Chervin, C.N.; Pala, I.R.; Machler, M.; Burz, M.F.; Long, J.W.; Rolison, D.R. Rechargeable nickel-3D zinc batteries: An energy-dense, safer alternative to lithium-ion. Science 2017, 356, 414–417. [Google Scholar] [CrossRef]
- Ko, J.S.; Geltmacher, A.B.; Hopkins, B.J.; Rolison, D.R.; Long, J.W.; Parker, J.F. Robust 3D Zn sponges enable high-power, energy-dense alkaline batteries. ACS Appl. Mater. Interfaces 2019, 2, 212–216. [Google Scholar]
- Guo, W.; Cong, Z.; Guo, Z.; Chang, C.; Liang, X.; Liu, Y.; Hu, W.; Pu, X. Dendrite-free Zn anode with dual channel 3D porous frameworks for rechargeable Zn batteries. Energy Storage Mater. 2020, 30, 104–112. [Google Scholar] [CrossRef]
- Wang, S.-B.; Ran, Q.; Yao, R.-Q.; Shi, H.; Wen, Z.; Zhao, M.; Lang, X.-Y.; Jiang, Q. Lamella-nanostructured eutectic zinc-aluminum alloys as reversible and dendrite-free anodes for aqueous rechargeable batteries. Nat. Commun. 2020, 11, 1634. [Google Scholar] [CrossRef]
- Zhang, C.; Holoubek, J.; Wu, X.; Daniyar, A.; Zhu, L.; Chen, C.; Leonard, D.P.; Rodriguez-Perez, I.A.; Jiang, J.-X.; Fang, C.; et al. A ZnCl2 water-in-salt electrolyte for a reversible Zn metal anode. Chem. Commun. 2018, 54, 14097–14099. [Google Scholar] [CrossRef]
- Ni, Q.; Jiang, H.; Sandstrom, S.; Bai, Y.; Ren, H.; Wu, X.; Guo, Q.; Yu, D.; Wu, C.; Ji, X. A Na3V2(PO4)2O1.6F1.4 cathode of Zn-ion battery enabled by a water-in-bisalt electrolyte. Adv. Funct. Mater. 2020, 30, 2003511. [Google Scholar] [CrossRef]
- Zhang, H.; Liu, X.; Qin, B.; Passerini, S. Electrochemical intercalation of anions in graphite for high-voltage aqueous zinc battery. J. Power Sources 2020, 449, 227594. [Google Scholar] [CrossRef]
- Song, X.; He, H.; Aboonasr Shiraz, M.H.; Zhu, H.; Khosrozadeh, A.; Liu, J. Enhanced reversibility and electrochemical window of Zn-ion batteries with an acetonitrile/water-in-salt electrolyte. Chem. Commun. 2021, 57, 1246–1249. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.; Borodin, O.; Gao, T.; Fan, X.; Sun, W.; Han, F.; Faraone, A.; Dura, J.A.; Xu, K.; Wang, C. Highly reversible zinc metal anode for aqueous batteries. Nat. Mater. 2018, 17, 543–549. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.-J.; Hao, J.; Luo, D.; Zhang, P.-F.; Zhang, B.; Davey, K.; Lin, Z.; Qiao, S.-Z. Dual-function electrolyte additive for highly reversible Zn anode. Adv. Energy Mater. 2021, 11, 2102010. [Google Scholar] [CrossRef]
- Wei, T.; Peng, Y.; Mo, L.e.; Chen, S.; Ghadari, R.; Li, Z.; Hu, L. Modulated bonding interaction in propanediol electrolytes toward stable aqueous zinc-ion batteries. Sci. China Mater. 2022, 65, 1156–1164. [Google Scholar] [CrossRef]
- Li, T.C.; Lim, Y.; Li, X.L.; Luo, S.; Lin, C.; Fang, D.; Xia, S.; Wang, Y.; Yang, H.Y. A universal additive strategy to reshape electrolyte solvation structure toward reversible Zn storage. Adv. Energy Mater. 2022, 12, 2103231. [Google Scholar] [CrossRef]
- Zhou, M.; Chen, H.; Chen, Z.; Hu, Z.; Wang, N.; Jin, Y.; Yu, X.; Meng, H. Nonionic surfactant coconut diethanol amide inhibits the growth of zinc dendrites for more stable zinc-ion batteries. ACS Appl. Mater. Interfaces 2022, 5, 7590–7599. [Google Scholar] [CrossRef]
- Cao, H.; Huang, X.; Liu, Y.; Hu, Q.; Zheng, Q.; Huo, Y.; Xie, F.; Zhao, J.; Lin, D. An efficient electrolyte additive of tetramethylammonium sulfate hydrate for Dendritic-Free zinc anode for aqueous Zinc-ion batteries. J. Colloid Interface Sci. 2022, 627, 367–374. [Google Scholar] [CrossRef]
- Bayaguud, A.; Luo, X.; Fu, Y.; Zhu, C. Cationic surfactant-type electrolyte additive enables three-dimensional dendrite-free zinc anode for stable zinc-ion batteries. ACS Energy Lett. 2020, 5, 3012–3020. [Google Scholar] [CrossRef]
- Xi, M.; Liu, Z.; Ding, J.; Cheng, W.; Jia, D.; Lin, H. Saccharin anion acts as a “traffic assistant” of Zn2+ to achieve a long-life and dendritic-free zinc plate anode. ACS Appl. Mater. Interfaces 2021, 13, 29631–29640. [Google Scholar] [CrossRef]
- Zhang, L.; Miao, L.; Xin, W.; Peng, H.; Yan, Z.; Zhu, Z. Engineering zincophilic sites on Zn surface via plant extract additives for dendrite-free Zn anode. Energy Storage Mater. 2022, 44, 408–415. [Google Scholar] [CrossRef]
- Zhao, F.; Jing, Z.; Guo, X.; Li, J.; Dong, H.; Tan, Y.; Liu, L.; Zhou, Y.; Owen, R.; Shearing, P.R.; et al. Trace amounts of fluorinated surfactant additives enable high performance zinc-ion batteries. Energy Storage Mater. 2022, 53, 638–645. [Google Scholar] [CrossRef]
- Wang, M.; Wu, X.; Yang, D.; Zhao, H.; He, L.; Su, J.; Zhang, X.; Yin, X.; Zhao, K.; Wang, Y.; et al. A colloidal aqueous electrolyte modulated by oleic acid for durable zinc metal anode. Chem. Eng.J. 2023, 451, 138589. [Google Scholar] [CrossRef]
- Krafft, M.P.; Riess, J.G. Selected physicochemical aspects of poly- and perfluoroalkylated substances relevant to performance, environment and sustainability: Part one. Chemosphere 2015, 129, 4–19. [Google Scholar] [CrossRef]
- Hao, J.; Long, J.; Li, B.; Li, X.; Zhang, S.; Yang, F.; Zeng, X.; Yang, Z.; Pang, W.K.; Guo, Z. Toward high-performance hybrid Zn-based batteries via deeply understanding their mechanism and using electrolyte additive. Adv. Funct. Mater. 2019, 29, 1903605. [Google Scholar] [CrossRef]
- Zhou, M.; Guo, S.; Li, J.; Luo, X.; Liu, Z.; Zhang, T.; Cao, X.; Long, M.; Lu, B.; Pan, A.; et al. Surface-preferred crystal plane for a stable and reversible zinc anode. Adv. Mater. 2021, 33, 2100187. [Google Scholar] [CrossRef]
- Martinez, L.; Andrade, R.; Birgin, E.G.; Martinez, J.M. Packmol: A package for building initial configurations for molecular dynamics simulations. J. Comput. Chem. 2009, 30, 2157–2164. [Google Scholar] [CrossRef]
- Jorgensen, W.L.; Chandrasekhar, J.; Madura, J.D.; Impey, R.W.; Klein, M.L. Comparison of simple potential functions for simulating liquid water. J. Chem. Phys. 1983, 79, 926–935. [Google Scholar] [CrossRef]
- Stote, R.H.; Karplus, M. Zinc binding in proteins and solution: A simple but accurate nonbonded representation. Proteins 1995, 23, 12–31. [Google Scholar] [CrossRef]
- Mamatkulov, S.; Schwierz, N. Force fields for monovalent and divalent metal cations in TIP3P water based on thermodynamic and kinetic properties. J. Chem. Phys. 2018, 148, 074504. [Google Scholar] [CrossRef]
- Williams, C.D.; Burton, N.A.; Travis, K.P.; Harding, J.H. The development of a classical force field to determine the selectivity of an aqueous Fe3+-EDA complex for TcO4- and SO42-. J. Chem. Theory Comput. 2014, 10, 3345–3353. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.U.; Jung, J.; Han, Y.K. Molecular dynamics study of the ionic conductivity of 1-n-butyl-3-methylimidazolium salts as ionic liquids. Chem. Phys. Lett. 2005, 406, 332–340. [Google Scholar] [CrossRef]
- Eastman, P.; Swails, J.; Chodera, J.D.; McGibbon, R.T.; Zhao, Y.; Beauchamp, K.A.; Wang, L.-P.; Simmonett, A.C.; Harrigan, M.P.; Stern, C.D.; et al. OpenMM 7: Rapid development of high performance algorithms for molecular dynamics. PLoS Comput. Biol. 2017, 13, e1005659. [Google Scholar] [CrossRef] [PubMed]
- Michaud-Agrawal, N.; Denning, E.J.; Woolf, T.B.; Beckstein, O. MDAnalysis: A toolkit for the analysis of molecular dynamics simulations. J. Comput. Chem. 2011, 32, 2319–2327. [Google Scholar] [CrossRef] [PubMed]
- Yao, R.; Qian, L.; Sui, Y.; Zhao, G.; Guo, R.; Hu, S.; Liu, P.; Zhu, H.; Wang, F.; Zhi, C.; et al. A versatile cation additive enabled highly reversible zinc metal anode. Adv. Mater. 2022, 12, 2102780. [Google Scholar] [CrossRef]
- Tian, H.; Yang, J.-L.; Deng, Y.; Tang, W.; Liu, R.; Xu, C.; Han, P.; Fan, H.J. Steel anti-corrosion strategy enables long-cycle Zn anode. Adv. Energy Mater. 2022, 13, 2202603. [Google Scholar] [CrossRef]
- Wang, H.; Ye, W.; Yin, B.; Wang, K.; Riaz, M.S.; Xie, B.-B.; Zhong, Y.; Hu, Y. Modulating cation migration and deposition with xylitol additive and oriented reconstruction of hydrogen bonds for stable zinc anodes. Angew. Chem. Int. Ed. 2023, 135, e202218872. [Google Scholar]
- Liu, Y.; Hu, J.; Lu, Q.; Hantusch, M.; Zhang, H.; Qu, Z.; Tang, H.; Dong, H.; Schmidt, O.G.; Holze, R.; et al. Highly enhanced reversibility of a Zn anode by in-situ texturing. Energy Storage Mater. 2022, 47, 98–104. [Google Scholar] [CrossRef]
- Chen, Z.; Chen, H.; Che, Y.; Cheng, L.; Zhang, H.; Chen, J.; Xie, F.; Wang, N.; Jin, Y.; Meng, H. Arginine cations inhibiting charge accumulation of dendrites and boosting Zn metal reversibility in aqueous rechargeable batteries. ACS Sustain. Chem. Eng. 2021, 9, 6855–6863. [Google Scholar] [CrossRef]
- Li, D.; Cao, L.; Deng, T.; Liu, S.; Wang, C. Design of a solid electrolyte interphase for aqueous Zn batteries. Angew. Chem. Int. Ed. 2021, 60, 13035–13041. [Google Scholar] [CrossRef]
- Zhang, W.; Dong, M.; Jiang, K.; Yang, D.; Tan, X.; Zhai, S.; Feng, R.; Chen, N.; King, G.; Zhang, H.; et al. Self-repairing interphase reconstructed in each cycle for highly reversible aqueous zinc batteries. Nat. Commun. 2022, 13, 5348. [Google Scholar] [CrossRef] [PubMed]
- Hao, J.; Yuan, L.; Zhu, Y.; Jaroniec, M.; Qiao, S.-Z. Triple-function electrolyte regulation toward advanced aqueous Zn-ion batteries. Adv. Mater. 2022, 34, 2206963. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.; Yan, W.; Xing, Y.; Li, L.; Liu, J.; Li, L.; Huang, P.; Lai, C.; Wang, C.; Chen, W.; et al. Tailoring the interfacial electric field using silicon nanoparticles for stable zinc-ion batteries. Adv. Funct. Mater. 2023, 23, 2213882. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Huang, J.; Fu, Z.; Sun, C.-F.; Deng, W. Surfactant Additives Containing Hydrophobic Fluorocarbon Chains and Hydrophilic Sulfonate Anion for Highly Reversible Zn Anode. Molecules 2023, 28, 4177. https://doi.org/10.3390/molecules28104177
Huang J, Fu Z, Sun C-F, Deng W. Surfactant Additives Containing Hydrophobic Fluorocarbon Chains and Hydrophilic Sulfonate Anion for Highly Reversible Zn Anode. Molecules. 2023; 28(10):4177. https://doi.org/10.3390/molecules28104177
Chicago/Turabian StyleHuang, Jinxian, Zhao Fu, Chuan-Fu Sun, and Wenzhuo Deng. 2023. "Surfactant Additives Containing Hydrophobic Fluorocarbon Chains and Hydrophilic Sulfonate Anion for Highly Reversible Zn Anode" Molecules 28, no. 10: 4177. https://doi.org/10.3390/molecules28104177
APA StyleHuang, J., Fu, Z., Sun, C.-F., & Deng, W. (2023). Surfactant Additives Containing Hydrophobic Fluorocarbon Chains and Hydrophilic Sulfonate Anion for Highly Reversible Zn Anode. Molecules, 28(10), 4177. https://doi.org/10.3390/molecules28104177




