Enhanced Anti-Skin-Aging Activity of Yeast Extract-Treated Resveratrol Rice DJ526
Abstract
:1. Introduction
2. Results
2.1. Effect of Elicitors on Total Resveratrol Production and Antioxidant Activity of Elicitor-Treated DJ526 Seeds
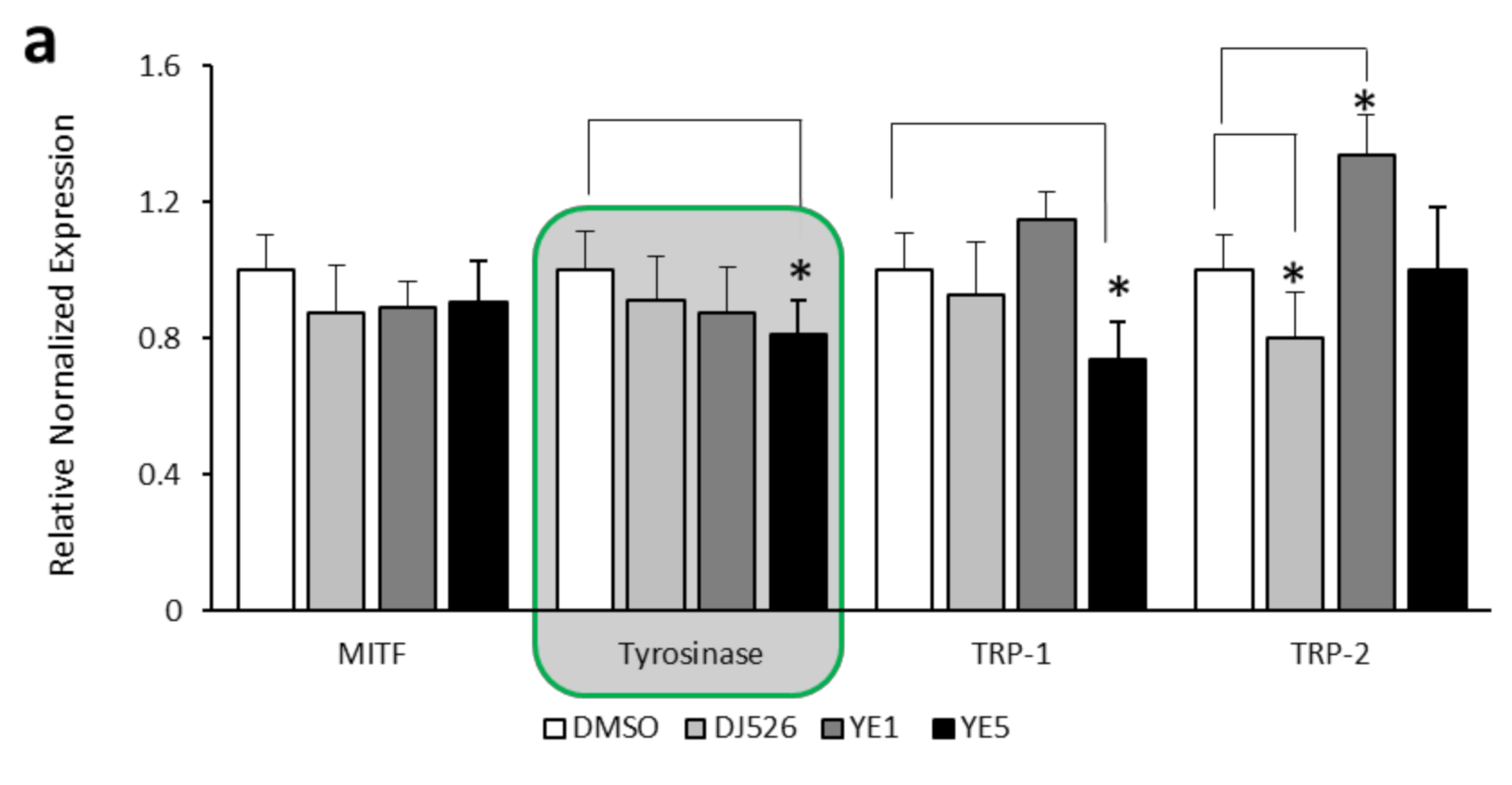
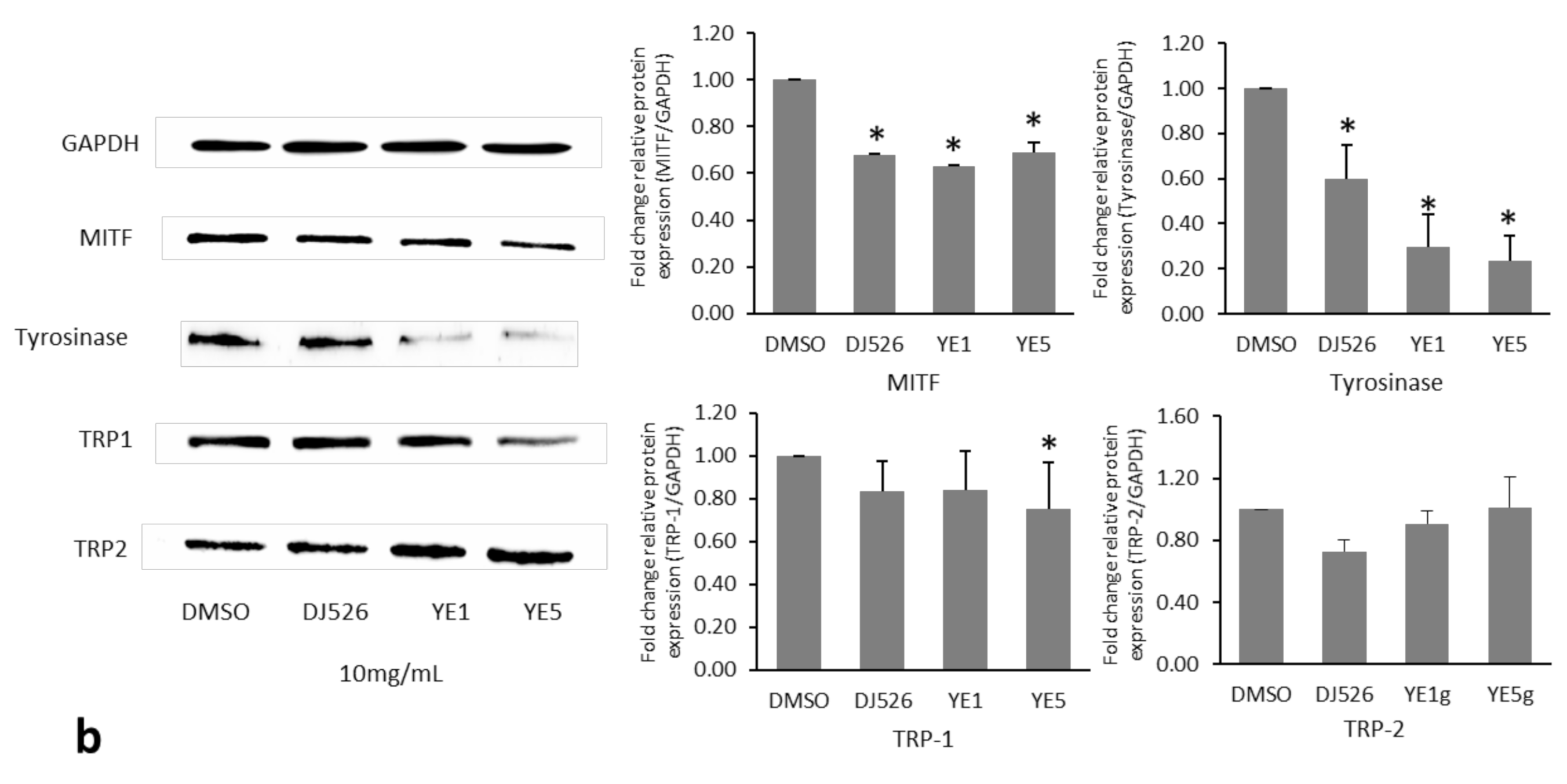
2.2. Effect of YE-Treated DJ526 Seeds on the Antimelanogenesis Activity of Malan-a Cells
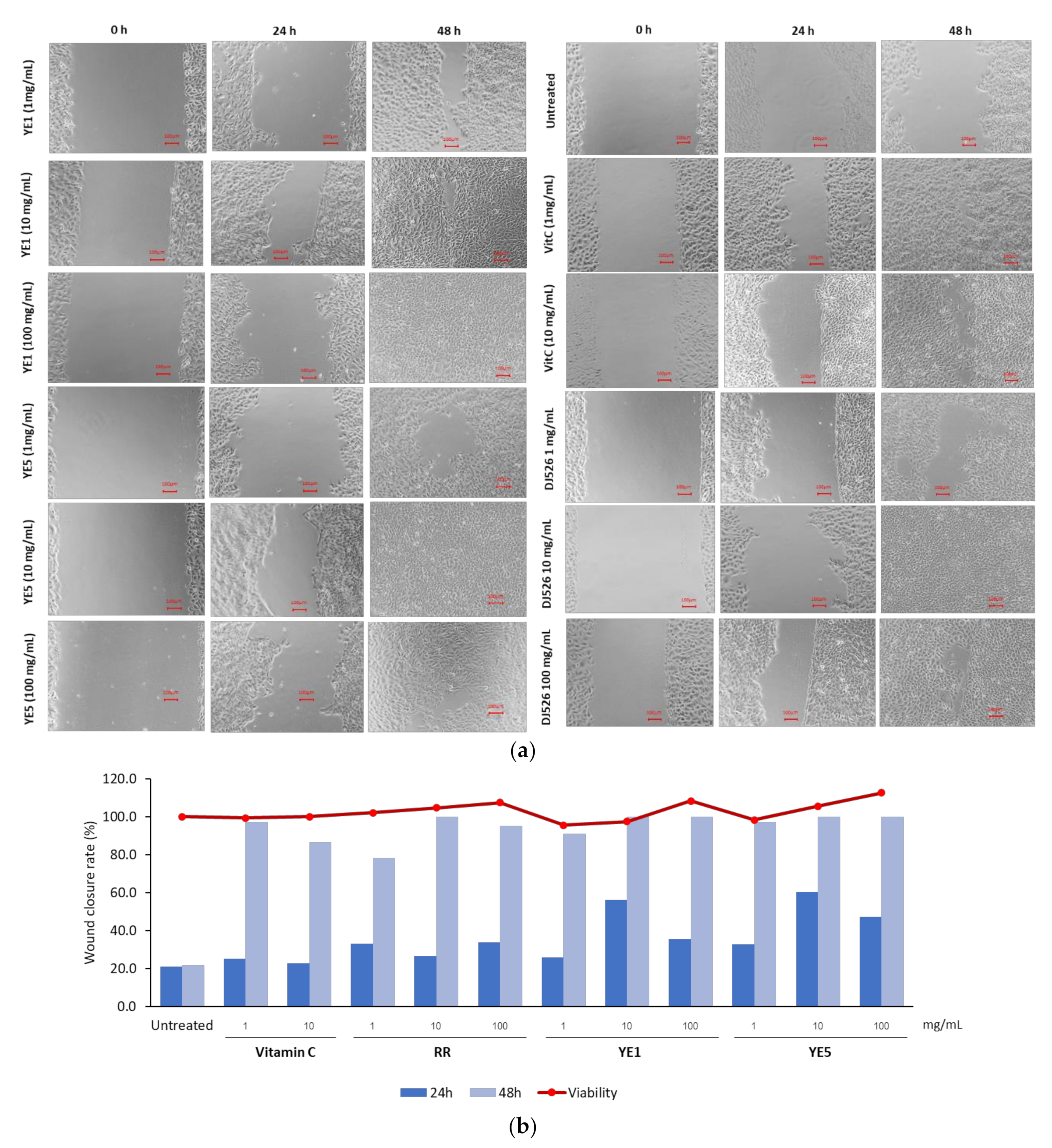
2.3. In Vitro Wound-Healing Effects of YE-Treated DJ526 Seed Extract on Human HaCaT Keratinocytes
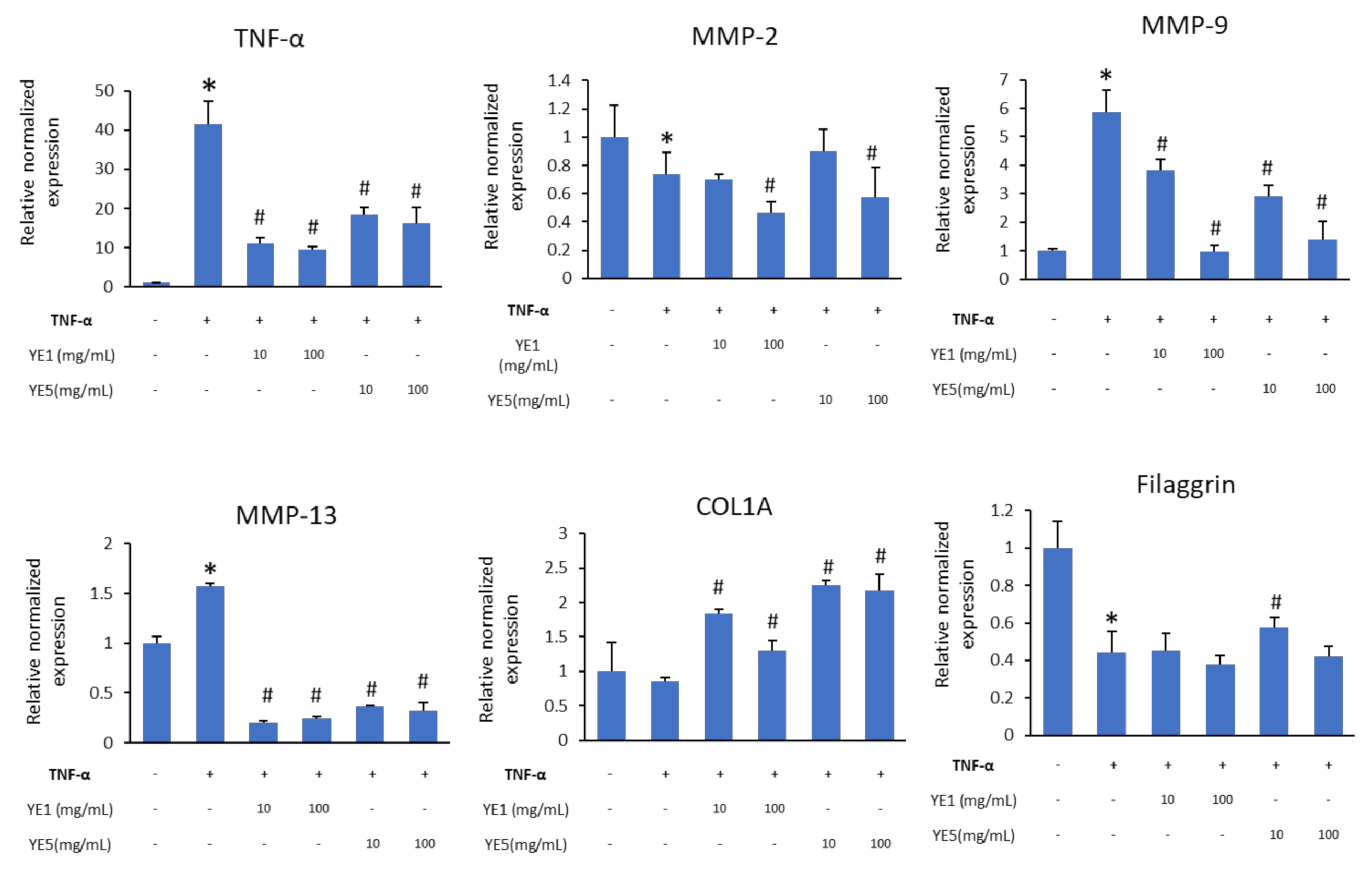
2.4. YE-Treated DJ526 Extracts Downregulate TNF-α in TNF-α-Induced HaCaT Cells
3. Discussion
4. Materials and Methods
4.1. Preparation of Elicitor-Treated DJ526 Seed Extract
4.2. Effect of Elicitation on Resveratrol and Piceid Content
4.3. Measurement of Antioxidant Activity of Elicitor-Treated DJ526 Seed Extract by 2,2′-Azino-bis (3-ethylbenzothiazoline-6-sulfonic acid) Diammonium Salt (ABTS) Scavenging Assay
4.4. In Vitro Cell Culture and Treatment
4.5. Cell Viability Assay
4.6. Measurement of Melanin Content and Cellular Tyrosinase Activity
4.7. Quantitative Polymerase Chain Reaction (qPCR) Analysis
4.8. Western Blot Analysis
4.9. Wound-Healing Scratch Assay
4.10. Statistic Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Tian, B.; Liu, J. Resveratrol: A review of plant sources, synthesis, stability, modification and food application. J. Sci. Food Agric. 2020, 100, 1392–1404. [Google Scholar] [CrossRef] [PubMed]
- Nawaz, W.; Zhou, Z.; Deng, S.; Ma, X.; Ma, X.; Li, C.; Shu, X. Therapeutic versatility of resveratrol derivatives. Nutrients 2017, 9, 1188. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Baek, S.-H.; Shin, W.C.; Ryu, H.-S.; Lee, D.-W.; Moon, E.; Seo, C.-S.; Hwang, E.; Lee, H.-S.; Ahn, M.-H.; Jeon, Y.; et al. Creation of resveratrol-enriched rice for the treatment of metabolic syndrome and related diseases. PLoS ONE 2013, 8, e57930. [Google Scholar] [CrossRef] [PubMed]
- Baek, S.-H.; Chung, H.-J.; Lee, H.-K.; D’Souza, R.; Jean, Y.-G.; Kim, H.-J.; Kweon, S.-J.; Hong, S.-T. Treatment of obesity with the resveratrol-enriched rice DJ526. Sci. Rep. 2014, 4, 3879. [Google Scholar] [CrossRef] [Green Version]
- Islam, M.S.; Jin, Y.Y.; Chung, H.-J.; Kim, H.-J.; Baek, S.-H.; Hong, S.-T. Effect of the Resveratrol Rice DJ526 on Longevity. Nutrients 2019, 11, 1804. [Google Scholar] [CrossRef] [Green Version]
- Lee, T.H.; Seo, J.O.; Do, M.H.; Ji, E.; Baek, S.H.; Kim, S.Y. Resveratrol-enriched rice down-regulates melanin synthesis in UVB-induced Guinea pigs epidermal skin tissue. Biomol. Ther. 2014, 22, 431–437. [Google Scholar] [CrossRef] [Green Version]
- Subedi, L.; Baek, S.H.; Kim, S.Y. Genetically engineered resveratrol-enriched rice inhibits neuroinflammation in Lipopolysaccharide-activated BV2 microglia via downregulating mitogen-activated protein kinase-nuclear factor kappa B signaling pathway. Oxid. Med. Cell Longev. 2018, 2018, 8092713. [Google Scholar] [CrossRef] [Green Version]
- Kantayos, V.; Kim, J.S.; Baek, S.H. Enhancing resveratrol bioproduction and anti-melanogenic activities through elicitation in DJ526 cell suspension. Plants 2021, 10, 1653. [Google Scholar] [CrossRef]
- Khan, M.; Park, S.-Y.; Kim, H.-J.; Lee, K.-J.; Kim, D.-H.; Baek, S.-H.; Hong, S.-T. The DJ526 seeds DJ526 callus significantly increase the lifespan of Drosophila (DJ526 seeds DJ526 callus for longevity). Nutrients 2019, 11, 983. [Google Scholar] [CrossRef] [Green Version]
- Chung, H.-J.; Sharma, S.P.; Kim, H.-J.; Baek, S.-H.; Hong, S.-T. The resveratrol-enriched rice DJ526 boosts motor coordination and physical strength. Sci. Rep. 2016, 6, 23958. [Google Scholar] [CrossRef] [Green Version]
- Kantayos, V.; Kim, J.S.; Baek, S.H. Alteration of resveratrol-dependent glycosyltransferase activity by elicitation in DJ-526 rice. GM Crops Food 2020, 12, 242–250. [Google Scholar] [CrossRef] [PubMed]
- Moses, T.; Goossens, A. Plants for human health: Greening biotechnology and synthetic biology. J. Exp. Bot. 2017, 68, 4009–4011. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vuong, T.V.; Franco, C.; Zhang, W. Treatment strategies for high resveratrol induction in Vitis vinifera L. cell suspension culture. Biotechnol. Rep. 2014, 1–2, 15–21. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, M.H.; Kuo, C.H.; Hsieh, W.C.; Ku, K.L. Investigation of microbial elicitation of trans-resveratrol and trans-piceatannol in peanut callus led to the application of chitin as a potential elicitor. J. Agric. Food Chem. 2010, 8, 9537–9541. [Google Scholar] [CrossRef]
- Ribeiro, A.S.; Estanqueiro, M.; Oliveira, M.B.; Lobo, J.M.S. Main benefits and applicability of plant extracts in skin care products. Cosmetics 2015, 2, 48–65. [Google Scholar] [CrossRef] [Green Version]
- Nip, J.; Potterf, S.B.; Rocha, S.; Vora, S.; Bosko, C. The new face of pigmentation and aging. In Textbook of Aging Skin; Farage, M., Miller, K., Maibach, H., Eds.; Springer: Berlin/Heidelberg, Germany, 2017. [Google Scholar] [CrossRef]
- Alam, M.B.; Bajpai, V.K.; Lee, J.; Zhao, P.; Byeon, J.H.; Ra, J.S.; Majumder, R.; Lee, J.S.; Yoon, J.I.; Rather, I.A.; et al. Inhibition of melanogenesis by jineol from Scolopendra subspinipes mutilans via MAP-kinase mediated MITF downregulation and the proteasomal degradation of tyrosinase. Sci. Rep. 2017, 7, 45858. [Google Scholar] [CrossRef]
- Fu, C.; Chen, J.; Lu, J.; Yi, L.; Tong, X.; Kang, L.; Pei, S.; Ouyang, Y.; Jiang, L.; Ding, Y.; et al. Role of inflammation factors in melanogenesis (Review). Mol. Med. Rep. 2020, 21, 1421–1430. [Google Scholar] [CrossRef] [Green Version]
- Basholli-Salihu, M.; Schuster, R.; Mulla, D.; Praznik, W.; Viernstein, H.; Mueller, M. Bioconversion of piceid to resveratrol by selected probiotic cell extracts. Bioprocess. Biosyst. 2016, 39, 1879–1885. [Google Scholar] [CrossRef] [Green Version]
- Su, D.; Cheng, Y.; Liu, M.; Liu, D.; Cui, H.; Zhang, B.; Zhou, S.; Yang, T.; Mei, Q. Comparison of piceid and resveratrol in antioxidation and antiproliferation activities in vitro. PLoS ONE 2013, 8, e54505. [Google Scholar]
- Gambini, J.; Ingles, M.; Olaso, G.; Lopez-Grueso, R.; Bonet-Costa, V.; Gimeno-Mallench, L.; Mas-Bargues, C.; Abdelaziz, K.M.; Gomez-Cabrera, M.C.; Vina, J.; et al. Properties of resveratrol: In vitro and in vivo studies about metabolism, bioavailability, and biological effects in animal models and humans. Oxid. Med. Cell Longev. 2015, 2015, 837042. [Google Scholar] [CrossRef] [Green Version]
- Chen, F.; Qian, L.H.; Deng, B.; Liu, Z.M.; Zhao, Y.; Le, Y.Y. Resveratrol protects vascular endothelial cells from high glucose–induced apoptosis through inhibition of NADPH oxidase activation–driven oxidative stress. CNS Neuro. Sci. Ther. 2013, 19, 675–681. [Google Scholar] [CrossRef] [PubMed]
- Baenas, N.; Garcia-Viguera, C.; Moreno, D.A. Elicitation: A tool for enriching the bioactive composition of foods. Molecules 2014, 19, 13541–13563. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kursvietiene, L.; Staneviciene, I.; Mongirdiene, A.; Bernatoniene, J. Multiplicity of effects and health benefits of resveratrol. Medicina 2016, 52, 148–155. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.; Ruan, Q.; Ye, Z.; Chu, Z.; Xi, M.; Li, M.; Hu, Q.; Guo, X.; Yao, P.; Xie, W. Resveratrol accelerates wound healing by attenuating oxidative stress-induced impairment of cell proliferation and migration. Burns 2021, 47, 133–139. [Google Scholar] [CrossRef] [PubMed]
- Silveira, D.; de Melo, A.F.; Magalhães, P.O.; Fonseca-Bazzo, Y.M. Tabernaemontana Species: Promising Sources of New Useful Drugs. In Studies in Natural Products Chemistry; Elsevier: Amsterdam, The Netherlands, 2017; Volume 54, pp. 227–289. [Google Scholar]
- Wang, W.; Wu, Z.; Wang, Y.; Wang, J.; Wang, Z. Evaluation of free radical scavenging and antioxidant capacity of resveratrol and polydatin. In Proceedings of the 2020 9th International Conference on Applied Science, Engineering and Technology (ICASET 2020), Qingdao, China, 25–26 May 2020. [Google Scholar]
- Li, Z.; Chen, X.; Liu, G.; Li, J.; Zhang, J.; Cao, Y.; Miao, J. Antioxidant activity and mechanism of resveratrol and polydatin isolated from mulberry (Morus alba L.). Molecules 2021, 26, 7574. [Google Scholar] [CrossRef] [PubMed]
- Akaberi, M.; Emami, S.A.; Vatani, M.; Tayarani-Najaran, Z. Evaluation of Antioxidant and Anti-Melanogenic Activity of Different Extracts of Aerial Parts of N. Sintenisii in Murine Melanoma B16F10 Cells. Iran. J. Pharm. Res. 2018, 17, 225–235. [Google Scholar] [PubMed]
- Ullah, S.; Park, C.; Ikram, M.; Kang, D.; Lee, S.; Yang, J.; Park, Y.; Yoon, S.; Chun, P.; Moon, H.R. Tyrosinase inhibition and anti-melanin generation effect of cinnamamide analogues. Bioorg. Chem. 2019, 87, 43–55. [Google Scholar] [CrossRef]
- Pignet, A.L.; Schellnegger, M.; Hecker, A.; Kohlhauser, M.; Kotzbeck, P.; Kamolz, L.P. Resveratrol-induced signal transduction in wound healing. Int. J. Mol. Sci. 2021, 22, 12614. [Google Scholar] [CrossRef]
- Kawano, Y.; Patrulea, V.; Sublet, E.; Borchard, G.; Iyoda, T.; Kageyama, R.; Morita, A.; Seino, S.; Yoshida, H.; Jordan, O.; et al. Wound healing promotion by hyaluronic acid: Effect of molecular weight on gene expression and in vivo wound closure. Phamarceuticals 2021, 14, 301. [Google Scholar] [CrossRef]
- Efron, P.A.; Moldawer, L.L. Cytokines and wound healing: The role of cytokine and anticytokine therapy in the repair response. J. Burn Care Rehabil. 2004, 25, 149–160. [Google Scholar] [CrossRef]
- Zhu, X.; Liu, Q.; Wang, M.; Liang, M.; Yang, X.; Xu, X.; Zou, H.; Qiu, J. Activation of Sirt1 by resveratrol inhibits TNF- α induced inflammation in fibroblasts. PLoS ONE 2011, 6, e27081. [Google Scholar] [CrossRef] [PubMed]
- Chen, G.; Yang, Z.; Wen, D.; Guo, J.; Xiong, Q.; Li, P.; Zhao, L.; Wang, J.; Wu, C.; Dong, L. Polydatin has anti-inflammatory and antioxidant effects in LPS-induced macrophages and improves DSS-induced mice colitis. Immune Inflamm. Dis. 2021, 9, 959–970. [Google Scholar] [CrossRef] [PubMed]
- Wikan, N.; Hankittichai, P.; Thaklaewphan, P.; Potikanond, S.; Nimlamool, W. Oxyresveratrol inhibits TNF-α-stimulated cell proliferation in human immortalized keratinocytes (HaCaT) by suppressing AKT activation. Pharmaceutics 2022, 14, 63. [Google Scholar] [CrossRef] [PubMed]
- Ratz-Łyko, A.; Arct, J. Resveratrol as an active ingredient for cosmetic and dermatological applications: A review. J. Cosmet. Laser Ther. 2018, 21, 84–90. [Google Scholar] [CrossRef] [PubMed]
- Lin, M.H.; Hung, C.F.; Sung, H.C.; Yang, S.C.; Yu, H.P.; Fang, J.Y. The bioactivities of resveratrol and its naturally occurring derivatives on skin. J. Food Drug Anal. 2020, 29, 15–38. [Google Scholar] [CrossRef]
- Wen, S.; Zhang, J.; Yang, B.; Elias, P.M.; Man, M.Q. Role of resveratrol in regulating cutaneous functions. Evid. Based Complement. Alternat. Med. 2020, 2020, 2416837. [Google Scholar] [CrossRef] [Green Version]
- Yadang, S.A.F.; Sotoing, G.T.; Zouakeu, K.S.N.; Khan, M.A.; Agbor, G.A.; Ur-Rahman, N.; Bum, E.N. Quantification of bioactive compounds and evaluation of the antioxidant activity of Carissa edulis Valh (Apocynaceae) leaves. Sci. World J. 2019, 2019, 7549620. [Google Scholar]
- Fouche, M.; Willers, C.; Hamman, S.; Malherbe, C.; Steenekamp, J. Wound healing effects of Aloe muth-muth: In vitro investigations using immortailized human keratinocytes (HaCaT). Biology 2020, 9, 350. [Google Scholar] [CrossRef]


| Elicitor-Treated DJ526 Extracts | Concentration (mg/mL) | Antioxidant Activity (%) | Vitamin C Equivalents of Antioxidant Capacity (mg VEAC/g DW) | IC50 (mg/mL) |
|---|---|---|---|---|
| DJ526 | 1 | 3.9 ± 1.39 g | 1.04 ± 0.019 a | |
| 10 | 28.6 ± 0.11 f | 2.66 ± 0.007 c | 2.2 ± 0.015 | |
| 100 | 90.7 ± 0.92 a | 6.73 ± 0.061 i | ||
| YE1 | 1 | 6.3 ± 0.86 g | 1.20 ± 0.056 a,b | |
| 10 | 36.3 ± 0.76 d,e | 3.17 ± 0.05 d,e | 2.1 ± 0.006 | |
| 100 | 93.2 ± 0.42 a | 6.90 ± 0.028 i | ||
| YE5 | 1 | 6.5 ± 0.57 g | 1.21 ± 0.037 a,b | |
| 10 | 94.1 ± 0.23 a | 6.95 ± 0.015 i | 1.7 ± 0.008 | |
| 100 | 94.3 ± 0.07 a | 6.97 ± 0.005 i | ||
| MJ 0.01 mM | 1 | 7.5 ± 0.13 g | 1.2 ± 0.009 a,b | |
| 10 | 34.4 ± 1.70 e,f | 3.04 ± 0.111 d | 3.2 ± 0.010 | |
| 100 | 42.6 ± 0.88 c,d | 3.58 ± 0.058 f | ||
| MJ 0.1 mM | 1 | 10.1 ± 0.38 g | 1.45 ± 0.025 b | |
| 10 | 51.6 ± 4.39 b | 4.17 ± 0.288 g | 2.5 ± 0.077 | |
| 100 | 56.5 ± 5.35 b | 4.49 ± 0.350 h | ||
| JA 0.01 mM | 1 | 7.4 ± 0.25 g | 1.27 ± 0.016 a,b | |
| 10 | 41.3 ± 7.92 c,d,e | 3.49 ± 0.519 ef | 3.2 ± 0.402 | |
| 100 | 41.7 ± 5.27 c,d,e | 3.52 ± 0.346 f | ||
| JA 0.1 mM | 1 | 7.6 ± 0.25 g | 1.29 ± 0.016 a,b | |
| 10 | 38.9 ± 4.73 b,c | 3.39 ± 0.237 e,f | 3.0 ± 0.299 | |
| 100 | 41.3 ± 1.21 c,d,e | 3.49 ± 0.079 e,f |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kantayos, V.; Kim, J.-S.; Baek, S.-H. Enhanced Anti-Skin-Aging Activity of Yeast Extract-Treated Resveratrol Rice DJ526. Molecules 2022, 27, 1951. https://doi.org/10.3390/molecules27061951
Kantayos V, Kim J-S, Baek S-H. Enhanced Anti-Skin-Aging Activity of Yeast Extract-Treated Resveratrol Rice DJ526. Molecules. 2022; 27(6):1951. https://doi.org/10.3390/molecules27061951
Chicago/Turabian StyleKantayos, Vipada, Jin-Suk Kim, and So-Hyeon Baek. 2022. "Enhanced Anti-Skin-Aging Activity of Yeast Extract-Treated Resveratrol Rice DJ526" Molecules 27, no. 6: 1951. https://doi.org/10.3390/molecules27061951
APA StyleKantayos, V., Kim, J.-S., & Baek, S.-H. (2022). Enhanced Anti-Skin-Aging Activity of Yeast Extract-Treated Resveratrol Rice DJ526. Molecules, 27(6), 1951. https://doi.org/10.3390/molecules27061951







