A Review on Gold Nanotriangles: Synthesis, Self-Assembly and Their Applications
Abstract
1. Introduction
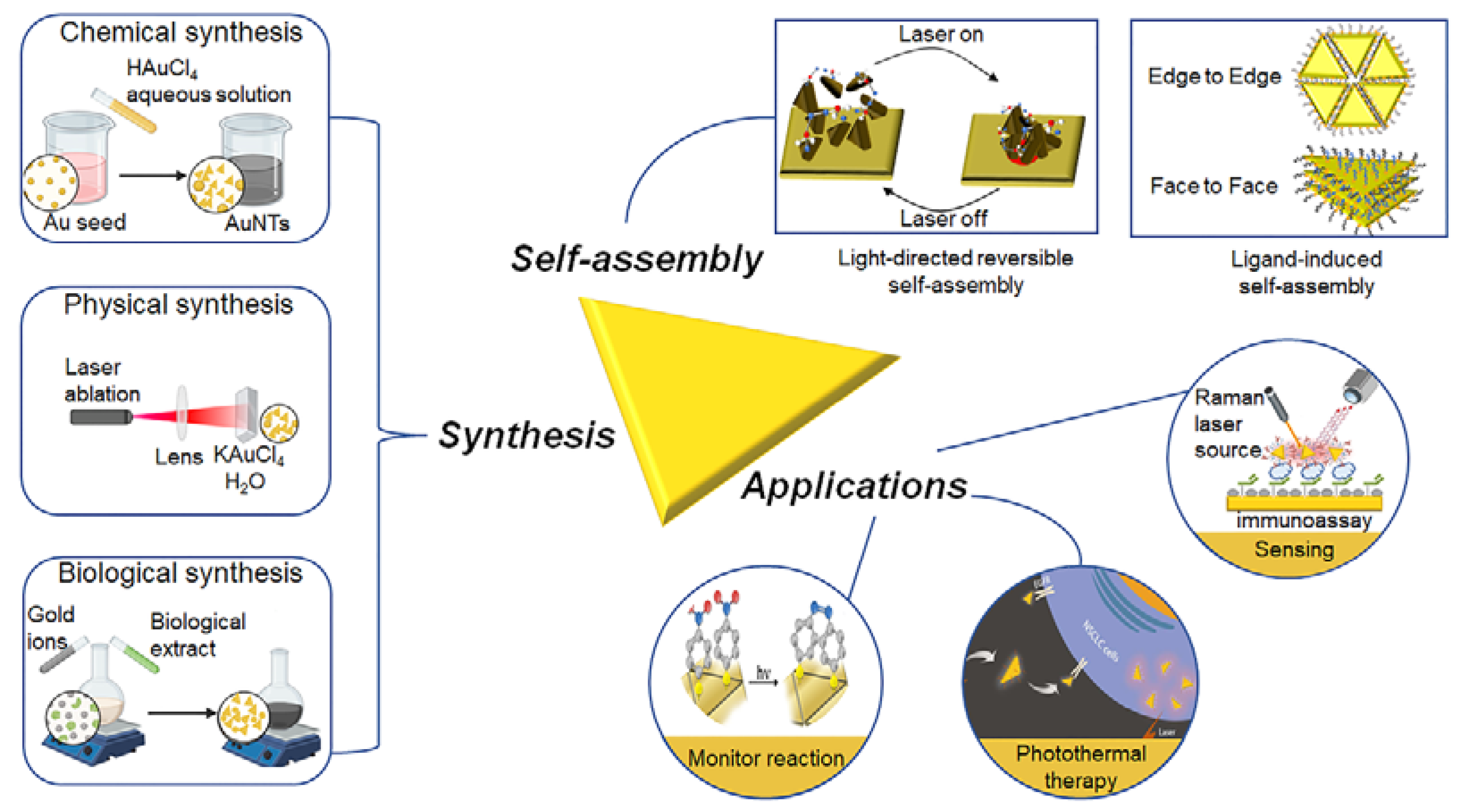
2. Synthesis of AuNTs
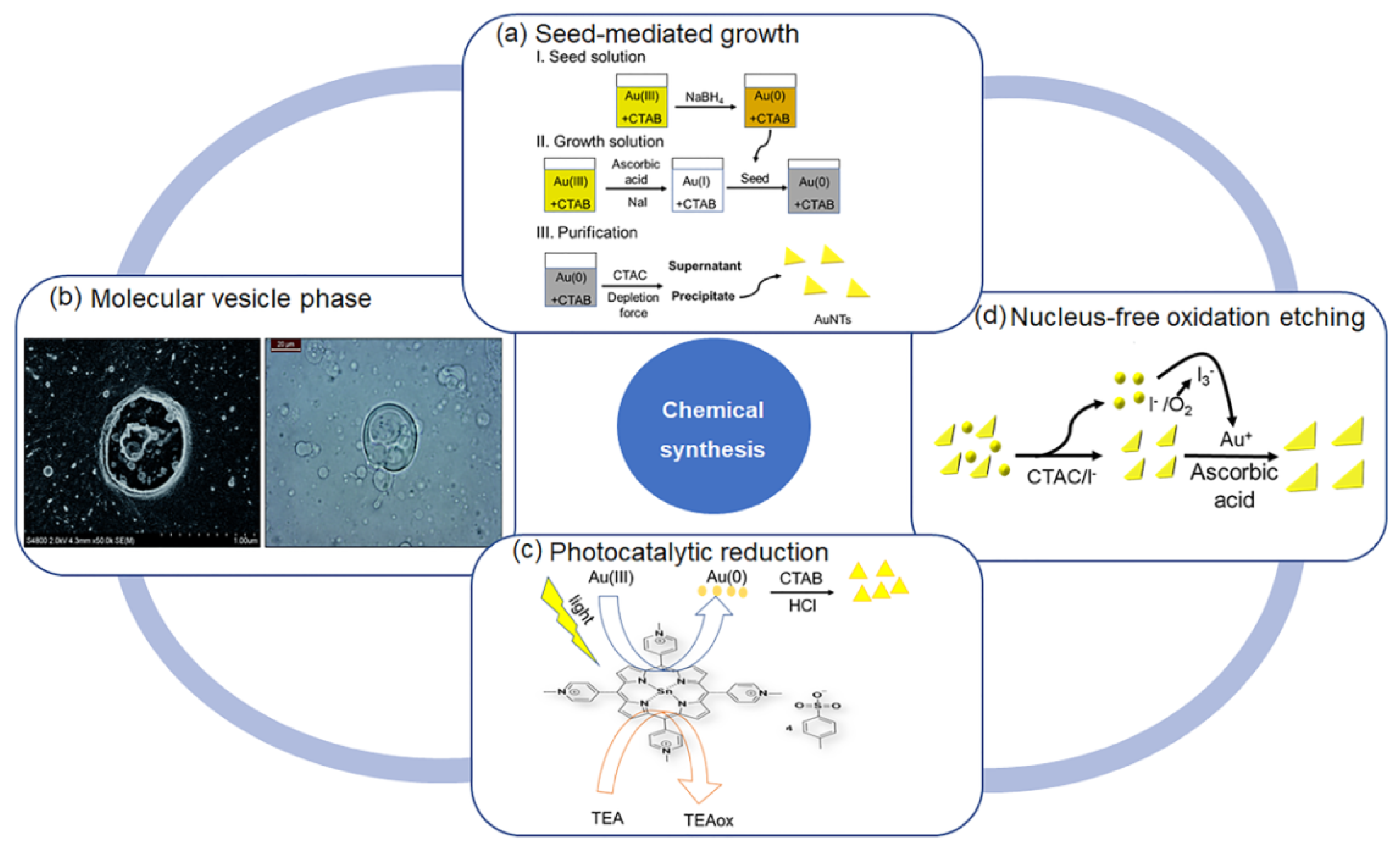
2.1. Chemical Synthesis
2.1.1. Seed-Mediated Growth Method
2.1.2. Molecular Vesicle Phase with Alternating Polyampholyte
2.1.3. Photocatalytic Reduction
2.1.4. Nucleus-Free Method
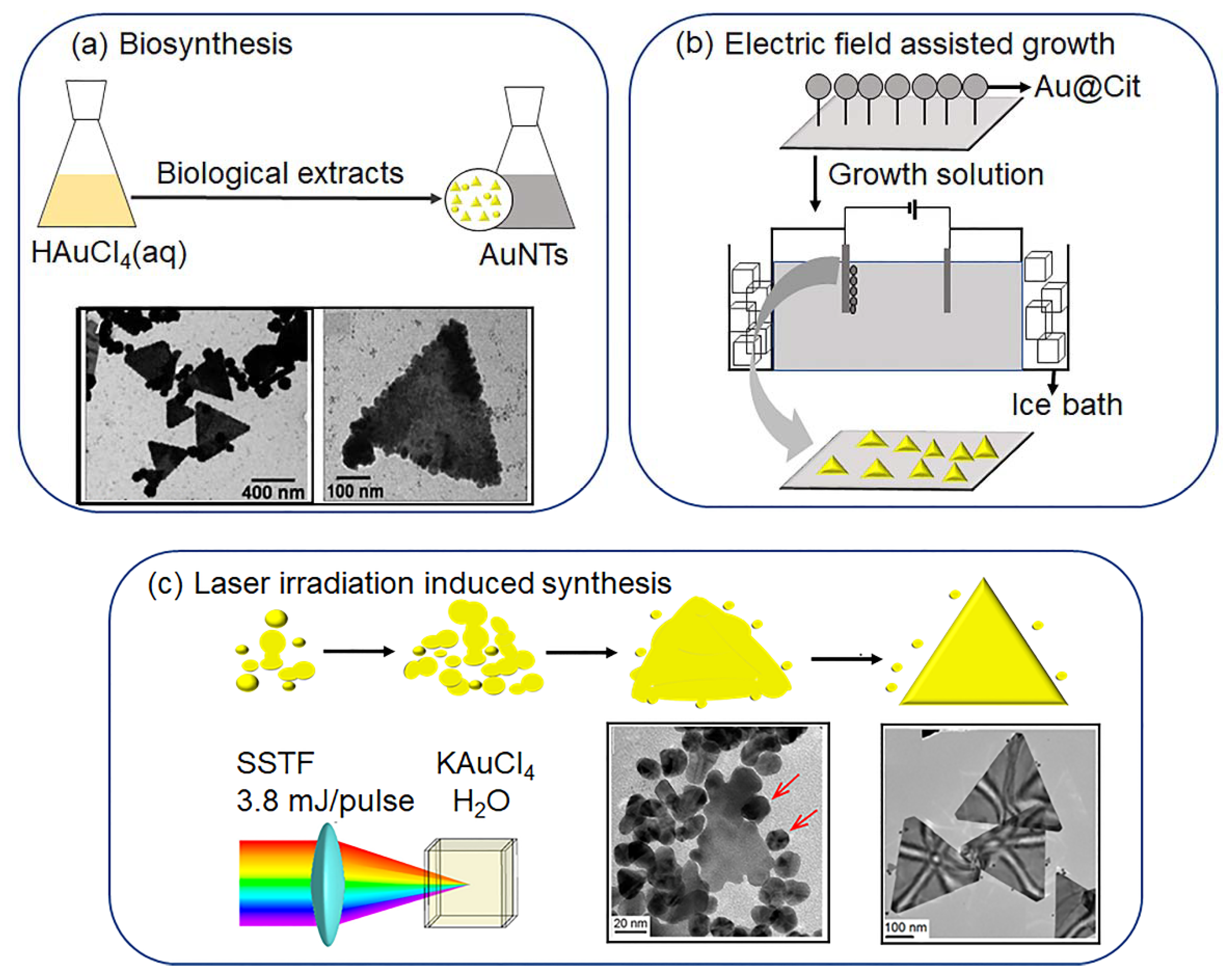
2.2. Biosynthesis
2.3. Physical-Stimulus-Induced Synthesis
2.3.1. Electric-Field-Assisted Growth
2.3.2. Laser Irradiation Induced AuNT Formation
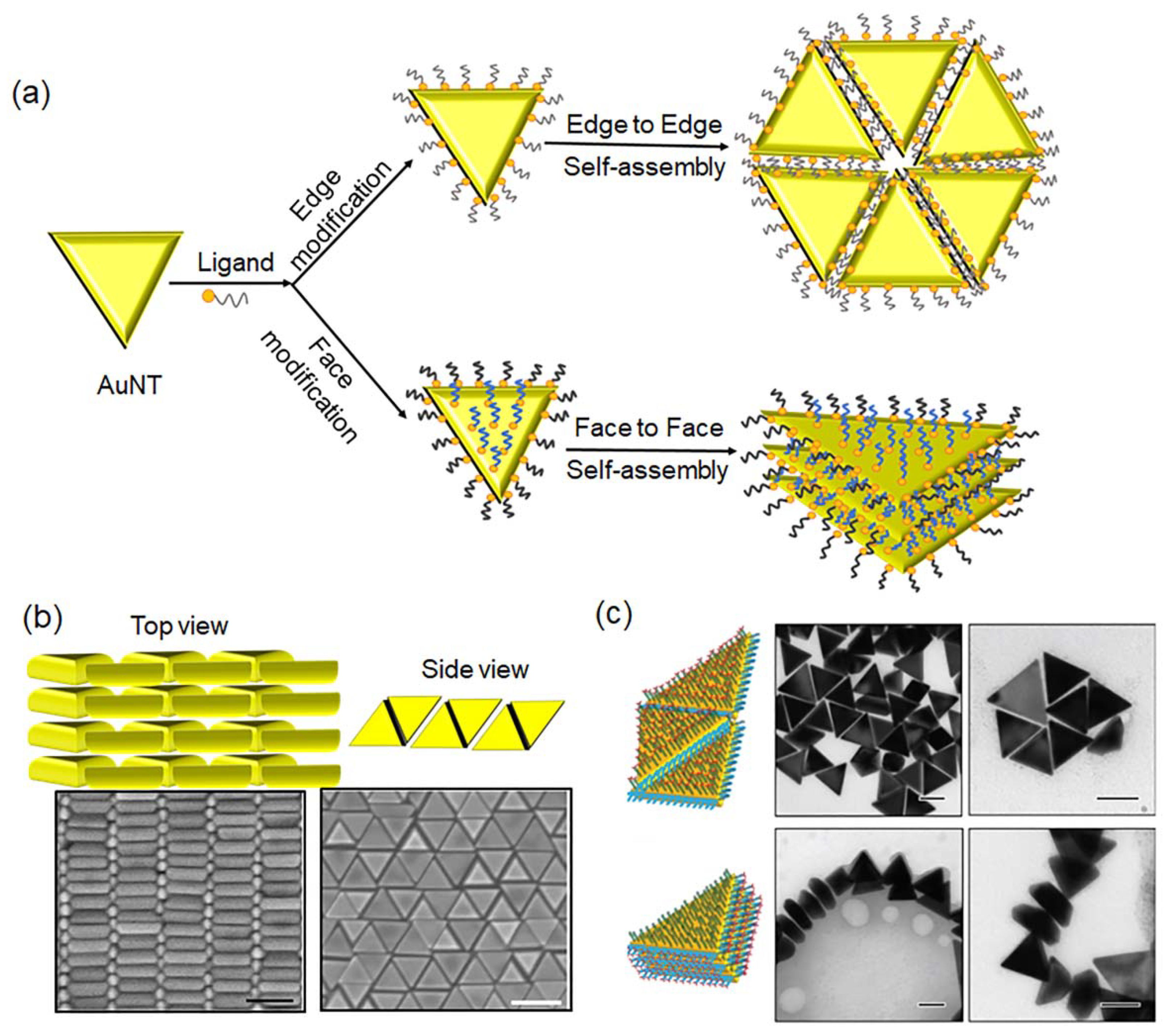
3. Self-Assembly of AuNTs
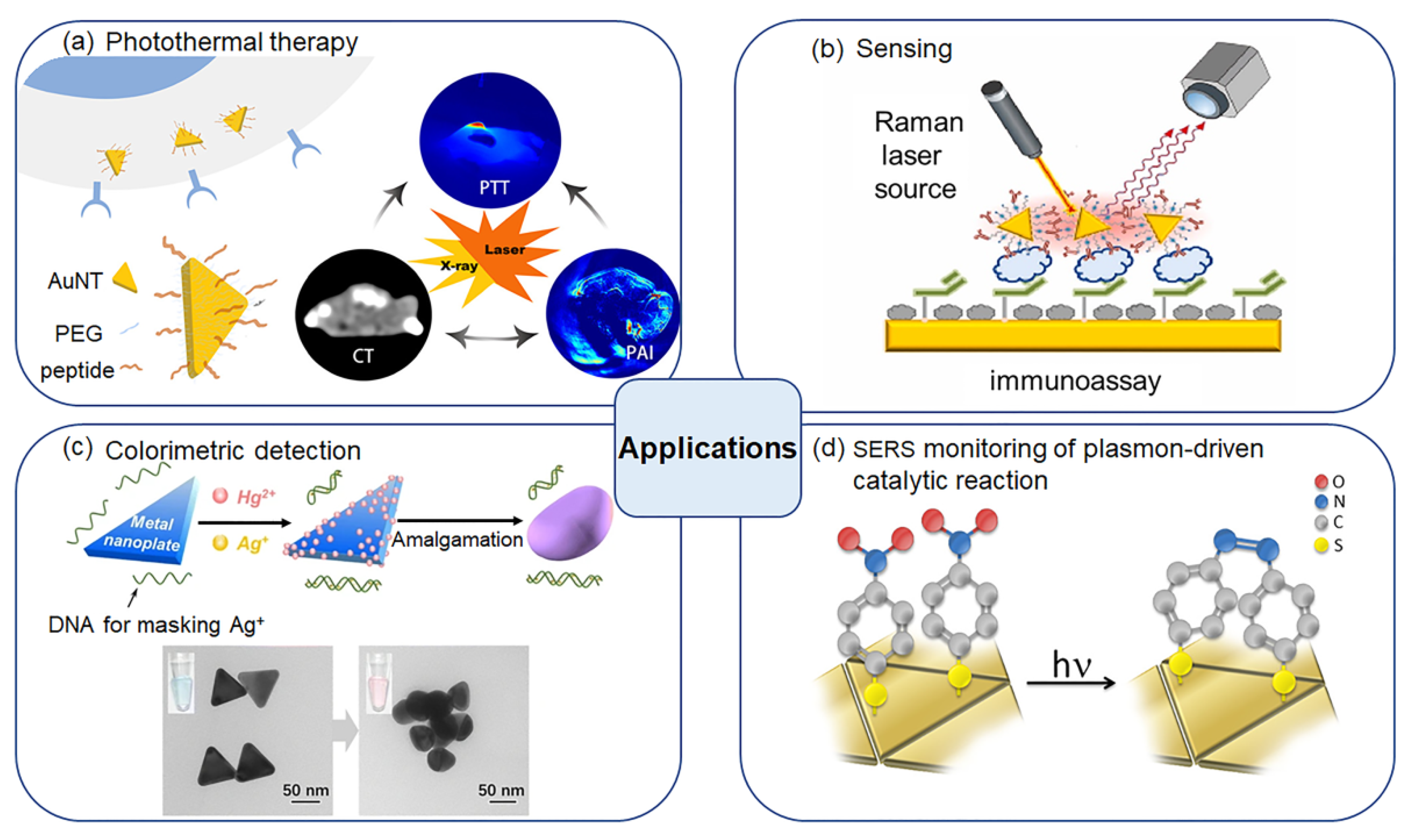
4. Applications
4.1. Biological Applications
4.2. Sensing
5. Summary and Outlook
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Fathima, R.; Mujeeb, A. Nonlinear Optical Investigations of Laser Generated Gold, Silver and Gold-Silver Alloy Nanoparticles and Optical Limiting Applications. J. Alloys Compd. 2021, 858, 157667. [Google Scholar] [CrossRef]
- Zhou, F.; Deng, Q.; Li, X.; Shao, L. Investigation of the Distinct Optical Property of Nanoporous Gold. Results Phys. 2019, 15, 102645. [Google Scholar] [CrossRef]
- Cheng, L.; Zhu, G.; Liu, G.; Zhu, L. FDTD Simulation of the Optical Properties for Gold Nanoparticles. Mater. Res. Express 2020, 7, 125009. [Google Scholar] [CrossRef]
- Cai, S.; Xiao, X.; Ye, X.; Li, W.; Zheng, C. Nonlinear Optical and Optical Limiting Properties of Ultra-Long Gold Nanowires. Mater. Lett. 2016, 166, 51–54. [Google Scholar] [CrossRef]
- Kato, R.; Uesugi, M.; Komatsu, Y.; Okamoto, F.; Tanaka, T.; Kitawaki, F.; Yano, T. Highly Stable Polymer Coating on Silver Nanoparticles for Efficient Plasmonic Enhancement of Fluorescence. ACS Omega 2022, 7, 4286–4292. [Google Scholar] [CrossRef]
- Fathima, R.; Mujeeb, A. Plasmon Enhanced Linear and Nonlinear Optical Properties of Natural Curcumin Dye with Silver Nanoparticles. Dye. Pigm. 2021, 189, 109256. [Google Scholar] [CrossRef]
- Jiang, Y.; Pillai, S.; Green, M. Realistic Silver Optical Constants for Plasmonics. Sci. Rep. 2016, 6, 30605. [Google Scholar] [CrossRef]
- Hamans, R.; Parente, M.; Garcia-Etxarri, A.; Baldi, A. Optical Properties of Colloidal Silver Nanowires. J. Phys. Chem. C 2022, 126, 8703–8709. [Google Scholar] [CrossRef]
- Bansal, A.; Sekhon, J.; Verma, S. Scattering Efficiency and LSPR Tunability of Bimetallic Ag, Au, and Cu Nanoparticles. Plasmonics 2013, 9, 143–150. [Google Scholar] [CrossRef]
- Li, R.; Zhao, Y.; Li, R.; Liu, H.; Ge, Y.; Xu, Z. Plasmonic Optical Trapping of Nanoparticles Using T-Shaped Copper Nanoantennas. Opt. Express 2021, 29, 9826–9835. [Google Scholar] [CrossRef]
- Han, X.; Rodriguez, R.; Haynes, C.; Ozaki, Y.; Zhao, B. Surface-Enhanced Raman Spectroscopy. Nat. Rev. Methods Prim. 2021, 1, 87. [Google Scholar] [CrossRef]
- Lin, L.; Wang, M.; Peng, X.; Lissek, E.N.; Mao, Z.; Scarabelli, L.; Adkins, E.; Coskun, S.; Unalan, H.E.; Korgel, B.A.; et al. Opto-Thermoelectric Nanotweezers. Nat. Photonics 2018, 12, 195–201. [Google Scholar] [CrossRef]
- Reguera, J.; Langer, J.; Aberasturi, D.J.; Liz-Marzán, L.M. Anisotropic Metal Nanoparticles for Surface Enhanced Raman Scattering. Chem. Soc. Rev. 2017, 46, 3866–3885. [Google Scholar] [CrossRef] [PubMed]
- Yang, P.; Zheng, J.; Xu, Y.; Zhang, Q.; Jiang, L. Colloidal Synthesis and Applications of Plasmonic Metal Nanoparticles. Adv. Mater. 2016, 28, 10508–10517. [Google Scholar] [CrossRef] [PubMed]
- Jauffred, L.; Samadi, A.; Klingberg, H.; Bendix, P.; Oddershede, L.B. Plasmonic Heating of Nanostructures. Chem. Rev. 2019, 119, 8087–8130. [Google Scholar] [CrossRef]
- Csáki, A.; Thiele, M.; Jatschka, J.; Dathe, A.; Zopf, D.; Stranik, O.; Fritzsche, W. Plasmonic Nanoparticle Synthesis and Bioconjugation for Bioanalytical Sensing. Eng. Life Sci. 2014, 15, 266–275. [Google Scholar] [CrossRef]
- Zhang, J.; Meng, L.; Zhao, D.; Fei, Z.; Lu, Q.; Dyson, P.J. Fabrication of Dendritic Gold Nanoparticles by Use of an Ionic Polymer Template. Langmuir 2008, 24, 2699–2704. [Google Scholar] [CrossRef]
- Xie, R.; Lu, S.; Deng, Y.; Mei, S.; Cao, X.; Zhou, L.; Lan, C.; Gu, H. Facile Synthesis of Sea-Urchin-Like Pt and Pt/Au Nanodendrites and Their Enhanced Electrocatalytic Properties. Inorg. Chem. 2019, 58, 5375–5379. [Google Scholar] [CrossRef]
- Feng, J.; Li, A.; Lei, Z.; Wang, A. Low-Potential Synthesis of “Clean” Au Nanodendrites and Their High Performance toward Ethanol Oxidation. ACS Appl. Mater. Interfaces 2012, 4, 2570–2576. [Google Scholar] [CrossRef]
- Li, M.; Feng, Q.; Zhou, Z.; Zhao, W.; Xu, J.; Chen, H. Plasmon-Enhanced Electrochemiluminescence for Nucleic Acid Detection Based on Gold Nanodendrites. Anal. Chem. 2017, 90, 1340–1347. [Google Scholar] [CrossRef]
- Kwon, K.; Lee, K.; Lee, Y.; Kim, M.; Heo, J.; Ahn, S.; Han, S. Controlled Synthesis of Icosahedral Gold Nanoparticles and Their Surface-Enhanced Raman Scattering Property. J. Phys. Chem. C 2006, 111, 1161–1165. [Google Scholar] [CrossRef]
- Xu, B.; Li, S.; Weng, J.; Wang, X.; Zhou, Z.; Yang, K.; Liu, M.; Chen, X.; Cui, Q.; Cao, M.; et al. Hydrothermal Syntheses of Gold Nanocrystals: From Icosahedral to Its Truncated Form. Adv. Funct. Mater. 2008, 18, 277–284. [Google Scholar] [CrossRef]
- Zhang, Q.; Xie, J.; Yang, J.; Lee, J.Y. Monodisperse Icosahedral Ag, Au, and Pd Nanoparticles: Size Control Strategy and Superlattice Formation. ACS Nano 2008, 3, 139–148. [Google Scholar] [CrossRef] [PubMed]
- Jiang, T.; Wang, X.; Tang, J.; Zhou, J.; Gua, C.; Tang, S. The Seeded-Synthesis of Core–Shell Au Dumbbells with Inbuilt Raman Molecules and Their SERS Performance. Anal. Methods 2017, 9, 4394–4399. [Google Scholar] [CrossRef]
- Hamon, C.; Henriksen-Lacey, M.; Porta, L.A.; Rosique, M.; Langer, J.; Scarabelli, L.; Montes, A.B.S.; González-Rubio, G.; Pancorbo, M.M.; Liz-Marzán, L.M.; et al. Tunable Nanoparticle and Cell Assembly Using Combined Self-Powered Microfluidics and Microcontact Printing. Adv. Funct. Mater. 2016, 26, 8053–8061. [Google Scholar] [CrossRef]
- Viarbitskaya, S.; Teulle, A.; Marty, R.; Sharma, J.; Girard, C.; Arbouet, A.; Dujardin, E. Tailoring and Imaging the Plasmonic Local Density of States in Crystalline Nanoprisms. Nat. Mater. 2013, 12, 426–432. [Google Scholar] [CrossRef]
- Li, N.; Zhao, P.; Astruc, D. Anisotropic Gold Nanoparticles: Synthesis, Properties, Applications, and Toxicity. Angew. Chem. Int. Ed. 2014, 53, 1756–1789. [Google Scholar] [CrossRef]
- Stangherlin, S.; Cathcart, N.; Sato, F.; Kitaev, V. Gold Nanoprisms: Synthetic Approaches for Mastering Plasmonic Properties and Implications for Biomedical Applications. ACS Appl. Nano Mater. 2020, 3, 8304–8318. [Google Scholar] [CrossRef]
- Kelly, K.L.; Coronado, E.; Zhao, L.L.; Schatz, G.C. The Optical Properties of Metal Nanoparticles: The Influence of Size, Shape, and Dielectric Environment. J. Phys. Chem. B 2003, 107, 668–677. [Google Scholar] [CrossRef]
- Yaraki, M.T.; Tan, Y.N. Metal Nanoparticles-Enhanced Biosensors: Synthesis, Design and Applications in Fluorescence Enhancement and Surface-Enhanced Raman Scattering. Chem. Asian J. 2020, 15, 3180–3208. [Google Scholar] [CrossRef]
- Si, P.; Razmi, N.; Nur, O.; Solanki, S.; Pandey, C.; Gupta, R.K.; Malhotra, B.D.; Willander, M.; Zerda, A. Gold Nanomaterials for Optical Biosensing and Bioimaging. Nanoscale Adv. 2021, 3, 2679–2698. [Google Scholar] [CrossRef] [PubMed]
- Jiang, N.; Zhuo, X.; Wang, J. Active Plasmonics: Principles, Structures, and Applications. Chem. Rev. 2017, 118, 3054–3099. [Google Scholar] [CrossRef] [PubMed]
- Meng, L.; Zhang, J.; Li, H.; Zhao, W.; Zhao, T. Preparation and Progress in Application of Gold Nanorods. J. Nanomater. 2019, 2019, 4925702. [Google Scholar] [CrossRef]
- Sun, J.; Ren, Y.; Wei, K.; He, M.; Gao, B.; Qi, H. Photoacoustic Response Optimization of Gold Nanorods in the Near-Infrared Region. Results Phys. 2022, 34, 105209. [Google Scholar] [CrossRef]
- Haine, A.; Niidome, T. Gold Nanorods as Nanodevices for Bioimaging, Photothermal Therapeutics, and Drug Delivery. Chem. Pharm. Bull. 2017, 65, 625–628. [Google Scholar] [CrossRef]
- Meng, X.; Dyer, J.; Huo, Y.; Jiang, C. Greater SERS Activity of Ligand-Stabilized Gold Nanostars with Sharp Branches. Langmuir 2020, 36, 3558–3564. [Google Scholar] [CrossRef]
- Deveci, P. Synthesis, Optical Properties and Photherapy Applications of Gold Nanostars. J. Incl. Phenom. Macrocycl. Chem. 2020, 99, 23–31. [Google Scholar] [CrossRef]
- Luo, X.; Lai, C.; Lee, Y.; Kuo, C.; Chen, I. Investigating Metal-Enhanced Fluorescence Effect on Fluorescein by Gold Nanotriangles and Nanocubes Using Time-Resolved Fluorescence Spectroscopy. J. Chin. Chem. Soc. 2021, 69, 82–93. [Google Scholar] [CrossRef]
- Kuwahara, S.; Narita, Y.; Mizuno, L.; Kurotsu, H.; Yoshino, H.; Kuwahara, M. Localized Surface Plasmon Resonance-Induced Welding of Gold Nanotriangles and the Local Plasmonic Properties for Multicolor Sensing and Light-Harvesting Applications. ACS Appl. Nano Mater. 2020, 3, 5172–5177. [Google Scholar] [CrossRef]
- Losquin, A.; Zagonel, L.F.; Myroshnychenko, V.; Rodríguez-González, B.; Tencé, M.; Scarabelli, L.; Förstner, J.; Liz-Marzán, L.M.; García de Abajo, F.; Javier García de Abajo, F.; et al. Unveiling Nanometer Scale Extinction and Scattering Phenomena through Combined Electron Energy Loss Spectroscopy and Cathodoluminescence Measurements. Nano Lett. 2015, 15, 1229–1237. [Google Scholar] [CrossRef]
- Klekotko, M.; Olesiak-Banska, J.; Matczyszyn, K. Photothermal Stability of Biologically and Chemically Synthesized Gold Nanoprisms. J. Nanopart. Res. 2017, 19, 327. [Google Scholar] [CrossRef] [PubMed]
- Sun, Z.; Umar, A.; Zeng, J.; Luo, X.; Song, L.; Zhang, Z.; Chen, Z.; Li, J.; Su, F.; Huang, Y. Highly Pure Gold Nanotriangles with Almost 100% Yield for Surface-Enhanced Raman Scattering. ACS Appl. Nano Mater. 2022, 5, 1220–1231. [Google Scholar] [CrossRef]
- Soto-Cruz, J.; Conejo-Valverde, P.; Sáenz-Arce, G.; Dou, H.; Rojas-Carrillo, O. Biofabrication of Gold Nanotriangles Using Liposomes as a Dual Functional Reductant and Stabilizer. Langmuir 2021, 37, 3446–3455. [Google Scholar] [CrossRef]
- Liebig, F.; Sarhan, R.M.; Bargheer, M.; Schmitt, C.N.Z.; Poghosyan, A.H.; Shahinyan, A.A.; Koetz, J. Spiked Gold Nanotriangles: Formation, Characterization and Applications in Surface-Enhanced Raman Spectroscopy and Plasmon-Enhanced Catalysis. RSC Adv. 2020, 10, 8152–8160. [Google Scholar] [CrossRef] [PubMed]
- Yu, S.; Hachtel, J.A.; Chisholm, M.F.; Pantelides, S.T.; Laromaine, A.; Roig, A. Magnetic Gold Nanotriangles by Microwave-Assisted Polyol Synthesis. Nanoscale 2015, 7, 14039–14046. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Song, X.; Ji, F.; Luo, B.; Ice, N.F.; Liu, Q.; Zhang, Q.; Chen, Q. Polymorphic Assembly from Beveled Gold Triangular Nanoprisms. Nano Lett. 2017, 17, 3270–3275. [Google Scholar] [CrossRef] [PubMed]
- Szustakiewicz, P.; González-Rubio, G.; Scarabelli, L.; Lewandowski, W. Robust Synthesis of Gold Nanotriangles and Their Self-Assembly into Vertical Arrays. ChemistryOpen 2019, 8, 705–711. [Google Scholar] [CrossRef]
- Zhao, Y.; Liu, W.; Tian, Y.; Yang, Z.; Wang, X.; Zhang, Y.; Tang, Y.; Zhao, S.; Wang, C.; Liu, Y.; et al. Anti-EGFR Peptide-Conjugated Triangular Gold Nanoplates for Computed Tomography/Photoacoustic Imaging-Guided Photothermal Therapy of Non-Small Cell Lung Cancer. ACS Appl. Mater. Interfaces 2018, 10, 16992–17003. [Google Scholar] [CrossRef]
- Kim, W.; Bang, A.; Kim, S.; Lee, G.-J.; Kim, Y.-H.; Choi, S. Adiponectin-Targeted SERS Immunoassay Biosensing Platform for Early Detection of Gestational Diabetes Mellitus. Biosens. Bioelectron. 2022, 213, 114488. [Google Scholar] [CrossRef]
- Liebig, F.; Sarhan, R.M.; Sander, M.; Koopman, W.; Schuetz, R.; Bargheer, M.; Koetz, J. Deposition of Gold Nanotriangles in Large Scale Close-Packed Monolayers for X-ray-Based Temperature Calibration and SERS Monitoring of Plasmon-Driven Catalytic Reactions. ACS Appl. Mater. Interfaces 2017, 9, 20247–20253. [Google Scholar] [CrossRef]
- Kim, F.; Connor, S.; Song, H.; Kuykendall, T.; Yang, P. Platonic Gold Nanocrystals. Angew. Chem. Int. Ed. 2004, 43, 3673–3677. [Google Scholar] [CrossRef] [PubMed]
- Zhai, Y.; DuChene, J.S.; Wang, Y.-C.; Qiu, J.; Johnston-Peck, A.C.; You, B.; Guo, W.; DiCiaccio, B.; Qian, K.; Zhao, E.W.; et al. Polyvinylpyrrolidone-Induced Anisotropic Growth of Gold Nanoprisms in Plasmon-Driven Synthesis. Nat. Mater. 2016, 15, 889–895. [Google Scholar] [CrossRef] [PubMed]
- Millstone, J.E.; Park, S.; Shuford, K.L.; Qin, L.; Schatz, G.C.; Mirkin, C.A. Observation of a Quadrupole Plasmon Mode for a Colloidal Solution of Gold Nanoprisms. J. Am. Chem. Soc. 2005, 127, 5312–5313. [Google Scholar] [CrossRef]
- DuChene, J.S.; Niu, W.; Abendroth, J.M.; Sun, Q.; Zhao, W.; Huo, F.; Wei, W.D. Halide Anions as Shape-Directing Agents for Obtaining High-Quality Anisotropic Gold Nanostructures. Chem. Mater. 2012, 25, 1392–1399. [Google Scholar] [CrossRef]
- Ha, T.H.; Koo, H.-J.; Chung, B.H. Shape-Controlled Syntheses of Gold Nanoprisms and Nanorods Influenced by Specific Adsorption of Halide Ions. J. Phys. Chem. C 2006, 111, 1123–1130. [Google Scholar] [CrossRef]
- Sun, M.; Ran, G.; Fu, Q.; Xu, W. The Effect of Iodide on the Synthesis of Gold Nanoprisms. J. Exp. Nanosci. 2015, 10, 1309–1318. [Google Scholar] [CrossRef]
- Kumar, D.V.R.; Kulkarni, A.A.; Prasad, B.L.V. Synthesis of Triangular Gold Nanoplates: Role of Bromide Ion and Temperature. Colloid. Surfaces A 2013, 422, 181–190. [Google Scholar] [CrossRef]
- Scarabelli, L.; Coronado-Puchau, M.; Giner-Casares, J.J.; Langer, J.; Liz-Marzán, L.M. Monodisperse Gold Nanotriangles: Size Control, Large-Scale Self-Assembly, and Performance in Surface-Enhanced Raman Scattering. ACS Nano 2014, 8, 5833–5842. [Google Scholar] [CrossRef]
- Requejo, K.I.; Liopo, A.V.; Derry, P.J.; Zubarev, E.R. Improving the Shape Yield and Long-Term Stability of Gold Nanoprisms with Poly(Vinylpyrrolidone). Langmuir 2019, 35, 9777–9784. [Google Scholar] [CrossRef]
- Kuttner, C.; Mayer, M.; Dulle, M.; Moscoso, A.; López-Romero, J.M.; Förster, S.; Fery, A.; Pérez-Juste, J.; Contreras-Cáceres, R. Seeded Growth Synthesis of Gold Nanotriangles: Size Control, SAXS Analysis, and SERS Performance. ACS Appl. Mater. Interfaces 2018, 10, 11152–11163. [Google Scholar] [CrossRef]
- Liebig, F.; Sarhan, R.M.; Prietzel, C.; Reinecke, A.; Koetz, J. “Green” Gold Nanotriangles: Synthesis, Purification by Polyelectrolyte/Micelle Depletion Flocculation and Performance in Surface-Enhanced Raman Scattering. RSC Adv. 2016, 6, 33561–33568. [Google Scholar] [CrossRef]
- Schulze, N.; Koetz, J. Kinetically Controlled Growth of Gold Nanotriangles in a Vesicular Template Phase by Adding a Strongly Alternating Polyampholyte. J. Disper. Sci. Technol. 2016, 38, 1073–1078. [Google Scholar] [CrossRef][Green Version]
- Ramírez-Sánchez, K.; Alvarado-Hidalgo, F.; Zamora-Sequeira, R.; Sáenz-Arce, G.; Rojas-Carrillo, O.; Avedaño-Soto, E.; Ruepert, C.; Mena-Torres, F.; Starbird-Pérez, R. Biosensor Based on the Directly Enzyme Immobilization into a Gold Nanotriangles/Conductive Polymer Biocompatible Coat for Electrochemical Detection of Chlorpyrifos in Water. Med. Dev. Sens. 2019, 2, e10047. [Google Scholar] [CrossRef]
- Miranda, A.; Malheiro, E.; Skiba, E.; Quaresma, P.; Carvalho, P.A.; Eaton, P.; Castro, B.; Shelnutt, J.A.; Pereira, E. One-Pot Synthesis of Triangular Gold Nanoplates Allowing Broad and Fine Tuning of Edge Length. Nanoscale 2010, 2, 2209–2216. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Ji, F.; Xu, Y.; He, L.; Mi, Y.; Bao, F.; Sun, B.; Zhang, X.; Zhang, Q. High-Yield Seedless Synthesis of Triangular Gold Nanoplates through Oxidative Etching. Nano Lett. 2014, 14, 7201–7206. [Google Scholar] [CrossRef]
- Ramírez-Jiménez, R.; Artiga, Á.; Mitchell, S.G.; Martín-Rapún, R.; Fuente, J.M. Surfactant-Free Synthesis and Scalable Purification of Triangular Gold Nanoprisms with Low Non-Specific Cellular Uptake. Nanomaterials 2020, 10, 539. [Google Scholar] [CrossRef]
- Shankar, S.S.; Rai, A.; Ankamwar, B.; Singh, A.; Ahmad, A.; Sastry, M. Biological Synthesis of Triangular Gold Nanoprisms. Nat. Mater. 2004, 3, 482–488. [Google Scholar] [CrossRef]
- Shankar, S.S.; Rai, A.; Ahmad, A.; Sastry, M. Controlling the Optical Properties of Lemongrass Extract Synthesized Gold Nanotriangles and Potential Application in Infrared-Absorbing Optical Coatings. Chem. Mater. 2005, 17, 566–572. [Google Scholar] [CrossRef]
- Chandran, S.P.; Chaudhary, M.; Pasricha, R.; Ahmad, A.; Sastry, M. Synthesis of Gold Nanotriangles and Silver Nanoparticles Using Aloe Vera Plant Extract. Biotechnol. Progr. 2006, 22, 577–583. [Google Scholar] [CrossRef]
- Liebig, F.; Sarhan, R.M.; Schmitt, C.N.Z.; Thünemann, A.F.; Prietzel, C.; Bargheer, M.; Koetz, J. Gold Nanotriangles with Crumble Topping and Their Influence on Catalysis and Surface-Enhanced Raman Spectroscopy. ChemPlusChem 2020, 85, 519–526. [Google Scholar] [CrossRef]
- Soltani Nejad, M.; Khatami, M.; Shahidi Bonjar, G.H. Extracellular Synthesis Gold Nanotriangles Using Biomass of Streptomyces Microflavus. IET Nanobiotechnol. 2016, 10, 33–38. [Google Scholar] [CrossRef] [PubMed]
- Verma, V.C.; Singh, S.K.; Solanki, R.; Prakash, S. Biofabrication of Anisotropic Gold Nanotriangles Using Extract of Endophytic Aspergillus Clavatus as a Dual Functional Reductant and Stabilizer. Nanoscale Res. Lett. 2010, 6, 16. [Google Scholar] [CrossRef] [PubMed]
- Tangeysh, B.; Moore Tibbetts, K.; Odhner, J.H.; Wayland, B.B.; Levis, R.J. Triangular Gold Nanoplate Growth by Oriented Attachment of Au Seeds Generated by Strong Field Laser Reduction. Nano Lett. 2015, 15, 3377–3382. [Google Scholar] [CrossRef] [PubMed]
- Sajanlal, P.R.; Pradeep, T. Electric-Field-Assisted Growth of Highly Uniform and Oriented Gold Nanotriangles on Conducting Glass Substrates. Adv. Mater. 2008, 20, 980–983. [Google Scholar] [CrossRef]
- Tangeysh, B.; Tibbetts, K.M.; Odhner, J.H.; Wayland, B.B.; Levis, R.J. Gold Nanotriangle Formation through Strong-Field Laser Processing of Aqueous KAuCl4 and Postirradiation Reduction by Hydrogen Peroxide. Langmuir 2016, 33, 243–252. [Google Scholar] [CrossRef]
- Millstone, J.E.; Métraux, G.S.; Mirkin, C.A. Controlling the Edge Length of Gold Nanoprisms via a Seed-Mediated Approach. Adv. Funct. Mater. 2006, 16, 1209–1214. [Google Scholar] [CrossRef]
- Walker, D.A.; Browne, K.P.; Kowalczyk, B.; Grzybowski, B.A. Self-Assembly of Nanotriangle Superlattices Facilitated by Repulsive Electrostatic Interactions. Angew. Chem. 2010, 122, 6912–6915. [Google Scholar] [CrossRef]
- Wang, G.; Zhang, Y.; Liang, X.; Takarada, T.; Maeda, M. Regioselective DNA Modification and Directed Self-Assembly of Triangular Gold Nanoplates. Nanomaterials 2019, 9, 581. [Google Scholar] [CrossRef]
- Lee, Y.H.; Lee, C.K.; Tan, B.; Rui Tan, J.M.; Phang, I.Y.; Ling, X.Y. Using the Langmuir–Schaefer Technique to Fabricate Large-Area Dense SERS-Active Au Nanoprism Monolayer Films. Nanoscale 2013, 5, 6404–6412. [Google Scholar] [CrossRef]
- Lin, L.; Peng, X.; Wang, M.; Scarabelli, L.; Mao, Z.; Liz-Marzán, L.M.; Becker, M.F.; Zheng, Y. Light-Directed Reversible Assembly of Plasmonic Nanoparticles Using Plasmon-Enhanced Thermophoresis. ACS Nano 2016, 10, 9659–9668. [Google Scholar] [CrossRef]
- Otero, C.M.; Simal, G.B.; Scocozza, M.F.; Rubert, A.; Grillo, C.A.; Hannibal, L.; Lavorato, G.; Huergo, M.A.; Murgida, D.H.; Vericat, C. Optimized Biocompatible Gold Nanotriangles with NIR Absorption for Photothermal Applications. ACS Appl. Nano Mater. 2022, 5, 341–350. [Google Scholar] [CrossRef]
- Nambara, K.; Niikura, K.; Mitomo, H.; Ninomiya, T.; Takeuchi, C.; Wei, J.; Matsuo, Y.; Ijiro, K. Reverse Size Dependences of the Cellular Uptake of Triangular and Spherical Gold Nanoparticles. Langmuir 2016, 32, 12559–12567. [Google Scholar] [CrossRef] [PubMed]
- Agarwala, R.; Singhb, V.; Jurneyb, P.; Shib, L.; Sreenivasanb, S.V.; Roy, K. Mammalian Cells Preferentially Internalize Hydrogel Nanodiscs over Nanorods and Use Shape-specific Uptake Mechanisms. Proc. Natl. Acad. Sci. USA 2013, 110, 17247–17252. [Google Scholar] [CrossRef] [PubMed]
- Jurney, P.; Agarwal, R.; Singh, V.; Choi, D.; Roy, K.; Sreenivasan, S.V.; Shi, L. Unique Size and Shape-Dependent Uptake Behaviors of Non-Spherical Nanoparticles by Endothelial Cells due to a Shearing Flow. J. Control. Release 2017, 245, 170–176. [Google Scholar] [CrossRef] [PubMed]
- Arnida; Janát-Amsbury, M.M.; Ray, A.; Peterson, C.M.; Ghandehari, H. Geometry and Surface Characteristics of Gold Nanoparticles Influence Their Biodistribution and Uptake by Macrophages. Eur. J. Pharm. Biopharm. 2011, 77, 417–423. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Yan, Y.; Müllner, M.; Ping, Y.; Cui, J.; Kempe, K.; Cortez-Jugo, C.; Caruso, F. Shape-Dependent Activation of Cytokine Secretion by Polymer Capsules in Human Monocyte-Derived Macrophages. Biomacromolecules 2016, 17, 1205–1212. [Google Scholar] [CrossRef] [PubMed]
- Dykman, L.A.; Khlebtsov, N.G. Uptake of Engineered Gold Nanoparticles into Mammalian Cells. Chem. Rev. 2013, 114, 1258–1288. [Google Scholar] [CrossRef]
- Nangia, S.; Sureshkumar, R. Effects of Nanoparticle Charge and Shape Anisotropy on Translocation through Cell Membranes. Langmuir 2012, 28, 17666–17671. [Google Scholar] [CrossRef]
- Xie, X.; Liao, J.; Shao, X.; Li, Q.; Lin, Y. The Effect of Shape on Cellular Uptake of Gold Nanoparticles in the Forms of Stars, Rods, and Triangles. Sci. Rep. 2017, 7, 3827. [Google Scholar] [CrossRef]
- Bhattarai, S.R.; Derry, P.J.; Aziz, K.; Singh, P.K.; Khoo, A.M.; Chadha, A.S.; Liopo, A.; Zubarev, E.R.; Krishnan, S. Gold Nanotriangles: Scale up and X-ray Radiosensitization Effects in Mice. Nanoscale 2017, 9, 5085–5093. [Google Scholar] [CrossRef]
- Jiang, Y.; Horimoto, N.N.; Imura, K.; Okamoto, H.; Matsui, K.; Shigemoto, R. Bioimaging with Two-Photon-Induced Luminescence from Triangular Nanoplates and Nanoparticle Aggregates of Gold. Adv. Mater. 2009, 21, 2309–2313. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhang, L.; Wang, L.; Wang, G.; Komiyama, M.; Liang, X. Colorimetric Determination of Mercury(II) Ion Based on DNA-Assisted Amalgamation: A Comparison Study on Gold, Silver and Ag@Au Nanoplates. Microchimica Acta 2019, 186, 713. [Google Scholar] [CrossRef] [PubMed]
- Yan, L.; Mu, J.; Ma, P.; Li, Q.; Yin, P.; Liu, X.; Cai, Y.; Yu, H.; Liu, J.; Wang, G.; et al. Gold Nanoplates with Superb Photothermal Efficiency and Peroxidase-like Activity for Rapid and Synergistic Antibacterial Therapy. Chem. Comm. 2021, 57, 1133–1136. [Google Scholar] [CrossRef] [PubMed]
- Dang, A.; Wang, Y.; Zhang, H.Q.; Panatdasirisuk, W.; Xia, Y.; Wang, Z.; Jariwala, D.; Li, T.; Yang, S. Electrospun Gold Nanoprism/Poly(vinyl alcohol) Nanofibers for Flexible and Free-Standing Surface-Enhanced Raman Scattering Substrates. ACS Appl. Nano Mater. 2022, 5, 6650–6658. [Google Scholar]
- Zhang, J.; Ma, X.; Wang, Z. Surface-Enhanced Raman Scattering-Fluorescence Dual-Mode Nanosensors for Quantitative Detection of Cytochrome c in Living Cells. Anal. Chem. 2019, 91, 6600–6607. [Google Scholar] [CrossRef]
- Sinha, S.S.; Jones, S.; Demeritte, T.; Chavva, S.R.; Shi, Y.L.; Burrell, J.; Pramanik, A.; Ray, P.C. Multimodal Nonlinear Optical Imaging of Live Cells Using Plasmon-Coupled DNA-Mediated Gold Nanoprism Assembly. J. Phys. Chem. C 2016, 120, 4546–4555. [Google Scholar] [CrossRef]
- Xu, J.; Wang, J.; Kong, L.T.; Zheng, G.; Guo, Z.; Liu, J. SERS Detection of Explosive Agent by Macrocyclic Compound Functionalized Triangular Gold Nanoprisms. J. Raman Spectrosc. 2011, 42, 1728–1735. [Google Scholar] [CrossRef]
- Soares, L.; Csáki, A.; Jatschka, J.; Fritzsche, W.; Flores, O.; Franco, R.; Pereira, E. Localized Surface Plasmon Resonance (LSPR) Biosensing Using Gold Nanotriangles: Detection of DNA Hybridization Events at Room Temperature. Analyst 2014, 139, 4964–4973. [Google Scholar] [CrossRef]
- Wang, G.; Akiyama, Y.; Takarada, T.; Maeda, M. Rapid Non-Crosslinking Aggregation of DNA-Functionalized Gold Nanorods and Nanotriangles for Colorimetric Single-Nucleotide Discrimination. Chem. Eur. J. 2016, 22, 258–263. [Google Scholar] [CrossRef]
| Plasmonic Nanoparticles | Morphology | LSPR (nm) | Synthetic Methods |
|---|---|---|---|
| Cu spheres |  | 600~700 | Seed-mediated growth method; Photo-chemical synthesis; Biosynthesis |
| Ag spheres |  | 380~490 | |
| Au spheres |  | 510~570 | |
| Au stars |  | 520~630 | Seed-mediated growth method |
| Au cubes |  | 520~630 | Seed-mediated growth method |
| Au triangles |  | 600~1300 | Seed-mediated growth method; Biosynthesis; Physical stimulus induced synthesis |
| Au nanorods |  | 550~1100 | Template synthesis; Hydrothermal synthesis Seed-mediated growth method; |
| Au nanocages |  | 680~990 | Seed-mediated growth method; Template synthesis |
| Au dumbbells |  | 680~1090 | Seed-mediated growth method |
| Au nanodendrites |  | 550~600 | Electrochemical deposition |
| Au icosahedrons |  | 500~600 | Seed-mediated growth method; Hydrothermal synthesis |
| Synthetic Methods | Efficiency | Product Quality | Yield | Reproducibility | Scalability |
|---|---|---|---|---|---|
| Seed-mediated growth | High | Good | High | Relatively good | Wide |
| Molecular vesicle phase | Moderate | Good | Moderate | Good | Narrow |
| Photocatalysis | High | Good | High | Good | Narrow |
| Nucleus-free method | High | Moderate | High | Moderate | Narrow |
| Biosynthesis | Moderate | Moderate | Low | Low | Narrow |
| Physical synthesis | High | Good | Moderate | Good | Moderate |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yu, X.; Wang, Z.; Cui, H.; Wu, X.; Chai, W.; Wei, J.; Chen, Y.; Zhang, Z. A Review on Gold Nanotriangles: Synthesis, Self-Assembly and Their Applications. Molecules 2022, 27, 8766. https://doi.org/10.3390/molecules27248766
Yu X, Wang Z, Cui H, Wu X, Chai W, Wei J, Chen Y, Zhang Z. A Review on Gold Nanotriangles: Synthesis, Self-Assembly and Their Applications. Molecules. 2022; 27(24):8766. https://doi.org/10.3390/molecules27248766
Chicago/Turabian StyleYu, Xiaoxi, Zhengkang Wang, Handan Cui, Xiaofei Wu, Wenjing Chai, Jinjian Wei, Yuqin Chen, and Zhide Zhang. 2022. "A Review on Gold Nanotriangles: Synthesis, Self-Assembly and Their Applications" Molecules 27, no. 24: 8766. https://doi.org/10.3390/molecules27248766
APA StyleYu, X., Wang, Z., Cui, H., Wu, X., Chai, W., Wei, J., Chen, Y., & Zhang, Z. (2022). A Review on Gold Nanotriangles: Synthesis, Self-Assembly and Their Applications. Molecules, 27(24), 8766. https://doi.org/10.3390/molecules27248766








