Study on Volumetric, Compressibility and Viscometric Behavior of Cationic Surfactants (CTAB and DTAB) in Aqueous Glycyl Dipeptide: A Thermo-Acoustic Approach
Abstract
1. Introduction
2. Materials and Methods
2.1. Material
2.2. Methods
3. Result and Discussion
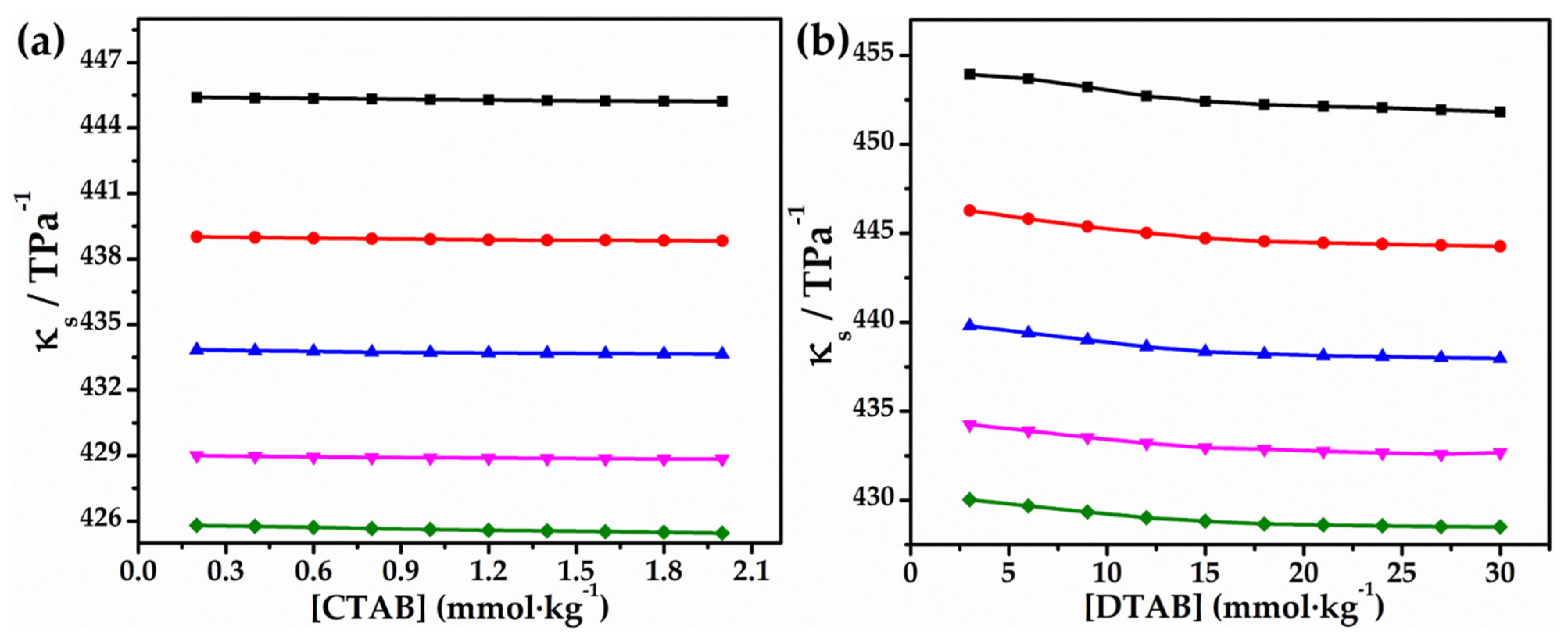
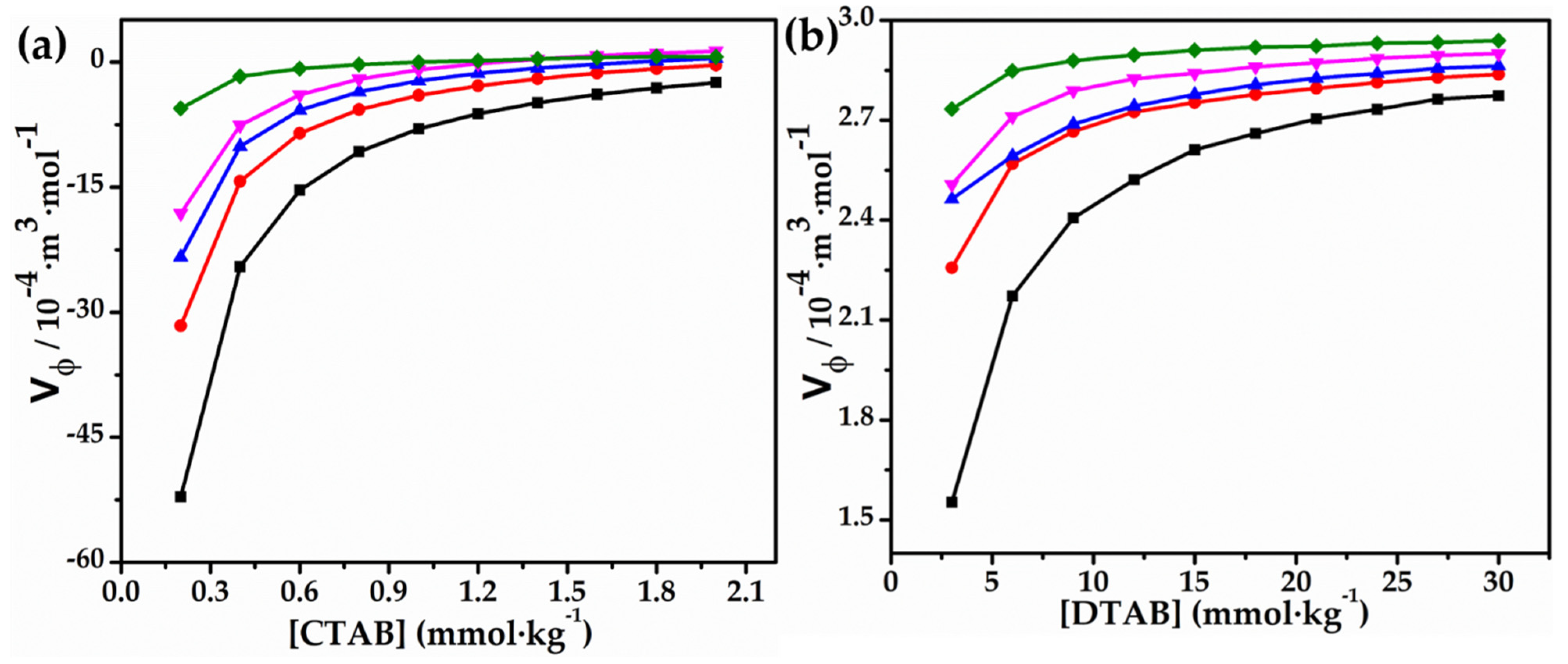
3.1. Volumetric and Compressibility Studies
| CTAB | DTAB | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| [CTAB] mmol∙kg−1 | 293.15 K | 298.15 K | 303.15 K | 308.15 K | 313.15 K | [DTAB] mmol∙kg−1 | 293.15 K | 298.15 K | 303.15 K | 308.15 K | 313.15 K |
| [Pure Water] | |||||||||||
| 0.2 | −477.80 | −197.71 | −617.39 | 265.41 | −343.65 | 3 | 2.80 | 2.81 | 2.84 | 2.89 | 2.90 |
| 0.4 | −61.39 | 76.34 | −135.79 | 298.29 | −74.52 | 6 | 2.84 | 2.86 | 2.88 | 2.91 | 2.93 |
| 0.6 | 74.06 | 159.31 | 19.70 | 310.93 | 65.97 | 9 | 2.86 | 2.87 | 2.89 | 2.92 | 2.93 |
| 0.8 | 138.03 | 204.56 | 99.97 | 319.79 | 134.94 | 12 | 2.87 | 2.88 | 2.90 | 2.92 | 2.94 |
| 1.0 | 178.41 | 231.71 | 150.14 | 327.12 | 176.32 | 15 | 2.88 | 2.88 | 2.90 | 2.92 | 2.94 |
| 1.2 | 208.68 | 252.33 | 185.28 | 331.17 | 206.45 | 18 | 2.88 | 2.89 | 2.91 | 2.92 | 2.95 |
| 1.4 | 229.58 | 267.05 | 206.77 | 335.50 | 225.79 | 21 | 2.89 | 2.89 | 2.91 | 2.93 | 2.95 |
| 1.6 | 244.63 | 276.21 | 223.52 | 338.12 | 242.21 | 24 | 2.89 | 2.90 | 2.91 | 2.93 | 2.95 |
| 1.8 | 254.11 | 285.57 | 237.10 | 337.91 | 254.41 | 27 | 2.89 | 2.90 | 2.92 | 2.93 | 2.95 |
| 2.0 | 262.19 | 292.55 | 245.95 | 337.74 | 264.16 | 30 | 2.89 | 2.90 | 2.92 | 2.94 | 2.95 |
| [Glycyl Dipeptide] = 0.001 mol∙kg−1 | |||||||||||
| 0.20 | −73.04 | −62.09 | −52.90 | −49.19 | −33.12 | 3 | 0.97 | 1.25 | 1.68 | 2.09 | 2.40 |
| 0.40 | −35.10 | −29.67 | −25.20 | −23.29 | −15.15 | 6 | 2.02 | 2.16 | 2.42 | 2.66 | 2.72 |
| 0.60 | −22.58 | −18.81 | −15.92 | −14.58 | −9.15 | 9 | 2.36 | 2.58 | 2.70 | 2.76 | 2.81 |
| 0.80 | −16.26 | −13.34 | −11.22 | −10.27 | −6.20 | 12 | 2.54 | 2.69 | 2.70 | 2.79 | 2.84 |
| 0.10 | −12.45 | −10.11 | −8.45 | −7.62 | −4.45 | 15 | 2.60 | 2.74 | 2.75 | 2.80 | 2.86 |
| 1.20 | −9.92 | −7.93 | −6.57 | −5.84 | −3.35 | 18 | 2.66 | 2.77 | 2.78 | 2.83 | 2.88 |
| 1.40 | −8.10 | −6.37 | −5.23 | −4.61 | −2.52 | 21 | 2.64 | 2.78 | 2.80 | 2.86 | 2.89 |
| 1.60 | −6.74 | −5.19 | −4.20 | −3.68 | −1.85 | 24 | 2.68 | 2.77 | 2.82 | 2.86 | 2.90 |
| 1.80 | −5.69 | −4.26 | −3.40 | −2.96 | −1.33 | 27 | 2.67 | 2.80 | 2.83 | 2.87 | 2.91 |
| 2.00 | −4.85 | −3.51 | −2.77 | −2.35 | −0.88 | 30 | 2.70 | 2.82 | 2.84 | 2.89 | 2.92 |
| [Glycyl Dipeptide] = 0.005 mol∙kg−1 | |||||||||||
| 0.20 | −67.33 | −42.27 | −32.28 | −21.86 | −11.96 | 3 | 1.28 | 1.49 | 1.95 | 2.40 | 2.56 |
| 0.40 | −32.12 | −19.96 | −14.79 | −9.55 | −4.40 | 6 | 2.16 | 2.27 | 2.51 | 2.72 | 2.82 |
| 0.60 | −20.39 | −12.64 | −8.96 | −5.36 | −1.91 | 9 | 2.45 | 2.52 | 2.63 | 2.77 | 2.87 |
| 0.80 | −14.53 | −8.87 | −6.06 | −3.28 | −0.61 | 12 | 2.54 | 2.62 | 2.70 | 2.81 | 2.88 |
| 0.10 | −11.02 | −6.63 | −4.33 | −2.02 | 0.18 | 15 | 2.59 | 2.66 | 2.74 | 2.84 | 2.89 |
| 1.20 | −8.67 | −5.11 | −3.19 | −1.15 | 0.75 | 18 | 2.63 | 2.70 | 2.77 | 2.85 | 2.90 |
| 1.40 | −7.00 | −3.95 | −2.37 | −0.52 | 1.13 | 21 | 2.66 | 2.74 | 2.80 | 2.87 | 2.91 |
| 1.60 | −5.74 | −3.08 | −1.73 | −0.05 | 1.44 | 24 | 2.69 | 2.76 | 2.82 | 2.88 | 2.92 |
| 1.80 | −4.76 | −2.36 | −1.23 | 0.33 | 1.67 | 27 | 2.72 | 2.78 | 2.83 | 2.88 | 2.93 |
| 2.00 | −3.98 | −1.81 | −0.81 | 0.61 | 1.86 | 30 | 2.74 | 2.80 | 2.85 | 2.89 | 2.94 |
| [Glycyl Dipeptide] = 0.010 mol∙kg−1 | |||||||||||
| 0.20 | −52.15 | −31.60 | −23.37 | −18.12 | −5.54 | 3 | 1.55 | 2.26 | 2.46 | 2.51 | 2.73 |
| 0.40 | −24.55 | −14.30 | −10.11 | −7.58 | −1.69 | 6 | 2.17 | 2.57 | 2.59 | 2.71 | 2.85 |
| 0.60 | −15.35 | −8.52 | −5.74 | −3.88 | −0.75 | 9 | 2.41 | 2.67 | 2.69 | 2.79 | 2.88 |
| 0.80 | −10.74 | −5.66 | −3.55 | −2.02 | −0.28 | 12 | 2.52 | 2.72 | 2.74 | 2.82 | 2.90 |
| 0.10 | −7.99 | −3.96 | −2.23 | −0.92 | 0.00 | 15 | 2.61 | 2.75 | 2.78 | 2.84 | 2.91 |
| 1.20 | −6.17 | −2.83 | −1.32 | −0.17 | 0.19 | 18 | 2.66 | 2.78 | 2.81 | 2.86 | 2.92 |
| 1.40 | −4.87 | −1.97 | −0.71 | 0.35 | 0.40 | 21 | 2.70 | 2.80 | 2.83 | 2.87 | 2.92 |
| 1.60 | −3.88 | −1.30 | −0.23 | 0.78 | 0.55 | 24 | 2.73 | 2.81 | 2.84 | 2.89 | 2.93 |
| 1.80 | −3.10 | −0.78 | 0.14 | 1.08 | 0.67 | 27 | 2.76 | 2.83 | 2.86 | 2.89 | 2.93 |
| 2.00 | −2.46 | −0.34 | 0.47 | 1.32 | 0.67 | 30 | 2.77 | 2.84 | 2.86 | 2.90 | 2.94 |
| CTAB | DTAB | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| [CTAB] mmol∙kg−1 | 293.15 K | 298.15 K | 303.15 K | 308.15 K | 313.15 K | [DTAB] mmol∙kg−1 | 293.15 K | 298.15 K | 303.15 K | 308.15 K | 313.15 K |
| [Pure Water] | |||||||||||
| 0.20 | −1.53 | −1.36 | −1.21 | −1.05 | −0.68 | 3 | −0.09 | −0.06 | −0.02 | −0.01 | 0.00 |
| 0.40 | −0.90 | −0.79 | −0.65 | −0.56 | −0.44 | 6 | −0.07 | −0.04 | −0.01 | 0.00 | 0.01 |
| 0.60 | −0.72 | −0.66 | −0.46 | −0.40 | −0.32 | 9 | −0.06 | −0.03 | −0.01 | 0.00 | 0.02 |
| 0.80 | −0.59 | −0.54 | −0.38 | −0.31 | −0.26 | 12 | −0.05 | −0.02 | −0.01 | 0.00 | 0.02 |
| 1.00 | −0.55 | −0.46 | −0.30 | −0.25 | −0.22 | 15 | −0.04 | −0.02 | 0.00 | 0.01 | 0.02 |
| 1.20 | −0.47 | −0.39 | −0.24 | −0.20 | −0.17 | 18 | −0.02 | −0.01 | 0.02 | 0.02 | 0.03 |
| 1.40 | −0.42 | −0.35 | −0.21 | −0.17 | −0.14 | 21 | 0.01 | 0.01 | 0.03 | 0.04 | 0.05 |
| 1.60 | −0.38 | −0.31 | −0.18 | −0.14 | −0.12 | 24 | 0.01 | 0.02 | 0.04 | 0.05 | 0.06 |
| 1.80 | −0.34 | −0.27 | −0.15 | −0.12 | −0.10 | 27 | 0.02 | 0.03 | 0.05 | 0.06 | 0.07 |
| 2.00 | −0.32 | −0.25 | −0.14 | −0.11 | −0.08 | 30 | 0.03 | 0.04 | 0.06 | 0.07 | 0.07 |
| [Glycyl Dipeptide] = 0.001 mol∙kg−1 | |||||||||||
| 0.20 | −70.32 | −58.42 | −50.25 | −44.92 | −33.01 | 3 | −3.53 | −2.96 | −2.33 | −1.95 | −1.20 |
| 0.40 | −35.75 | −30.56 | −26.13 | −22.84 | −16.79 | 6 | −1.57 | −1.25 | −0.93 | −0.47 | −0.32 |
| 0.60 | −24.25 | −20.94 | −17.76 | −15.22 | −11.37 | 9 | −1.08 | −0.75 | −0.52 | −0.21 | −0.18 |
| 0.80 | −18.59 | −15.87 | −13.38 | −11.38 | −8.56 | 12 | −0.88 | −0.58 | −0.37 | −0.13 | −0.08 |
| 0.10 | −15.06 | −13.10 | −10.79 | −9.08 | −6.84 | 15 | −0.71 | −0.46 | −0.26 | −0.03 | 0.01 |
| 1.20 | −12.71 | −11.10 | −9.05 | −7.48 | −5.75 | 18 | −0.48 | −0.28 | −0.10 | 0.09 | 0.13 |
| 1.40 | −11.03 | −9.49 | −7.75 | −6.38 | −4.94 | 21 | −0.35 | −0.10 | 0.04 | 0.22 | 0.21 |
| 1.60 | −9.77 | −8.39 | −6.76 | −5.55 | −4.28 | 24 | −0.17 | 0.01 | 0.18 | 0.33 | 0.31 |
| 1.80 | −8.76 | −7.52 | −5.99 | −4.90 | −3.78 | 27 | −0.09 | 0.15 | 0.28 | 0.41 | 0.40 |
| 2.00 | −8.01 | −6.79 | −5.36 | −4.36 | −3.34 | 30 | 0.03 | 0.26 | 0.37 | 0.49 | 0.48 |
| [Glycyl Dipeptide] = 0.005 mol∙kg−1 | |||||||||||
| 0.20 | −93.33 | −69.34 | −35.68 | −21.53 | −17.12 | 3 | −3.23 | −2.77 | −1.97 | −1.68 | −1.31 |
| 0.40 | −47.59 | −35.03 | −18.61 | −11.22 | −9.11 | 6 | −1.43 | −1.19 | −0.71 | −0.45 | −0.18 |
| 0.60 | −31.96 | −23.89 | −13.01 | −7.80 | −6.19 | 9 | −0.99 | −0.82 | −0.52 | −0.33 | −0.05 |
| 0.80 | −24.44 | −18.29 | −10.37 | −6.04 | −4.83 | 12 | −0.84 | −0.65 | −0.34 | −0.22 | 0.06 |
| 0.10 | −20.05 | −14.77 | −8.40 | −5.08 | −3.88 | 15 | −0.66 | −0.48 | −0.22 | −0.13 | 0.16 |
| 1.20 | −16.88 | −12.42 | −7.18 | −4.37 | −3.32 | 18 | −0.45 | −0.28 | −0.07 | 0.03 | 0.25 |
| 1.40 | −14.78 | −10.70 | −6.32 | −3.77 | −2.93 | 21 | −0.26 | −0.09 | 0.06 | 0.19 | 0.36 |
| 1.60 | −13.13 | −9.34 | −5.68 | −3.36 | −2.58 | 24 | −0.10 | 0.04 | 0.18 | 0.31 | 0.46 |
| 1.80 | −12.02 | −8.31 | −5.15 | −3.03 | −2.33 | 27 | 0.01 | 0.17 | 0.28 | 0.40 | 0.54 |
| 2.00 | −10.29 | −7.46 | −4.65 | −2.70 | −2.20 | 30 | 0.11 | 0.26 | 0.37 | 0.47 | 0.60 |
| [Glycyl Dipeptide] = 0.010 mol∙kg−1 | |||||||||||
| 0.20 | −52.58 | −40.44 | −35.93 | −33.22 | −16.62 | 3 | −2.12 | −1.56 | −1.30 | −1.01 | −0.48 |
| 0.40 | −26.34 | −20.29 | −18.11 | −16.83 | −9.01 | 6 | −1.24 | −0.92 | −0.74 | −0.37 | −0.22 |
| 0.60 | −17.59 | −13.65 | −12.21 | −11.21 | −6.77 | 9 | −0.91 | −0.66 | −0.50 | −0.23 | −0.10 |
| 0.80 | −13.13 | −10.30 | −9.19 | −8.32 | −5.57 | 12 | −0.79 | −0.47 | −0.38 | −0.12 | −0.03 |
| 0.10 | −10.48 | −8.23 | −7.31 | −6.54 | −4.80 | 15 | −0.56 | −0.33 | −0.24 | −0.02 | 0.10 |
| 1.20 | −8.66 | −6.81 | −6.04 | −5.30 | −4.24 | 18 | −0.35 | −0.15 | −0.05 | 0.14 | 0.21 |
| 1.40 | −7.37 | −5.75 | −5.12 | −4.42 | −3.74 | 21 | −0.15 | 0.01 | 0.10 | 0.25 | 0.33 |
| 1.60 | −6.39 | −4.90 | −4.37 | −3.73 | −3.36 | 24 | 0.00 | 0.14 | 0.22 | 0.34 | 0.43 |
| 1.80 | −5.61 | −4.23 | −3.82 | −3.22 | −3.07 | 27 | 0.11 | 0.25 | 0.32 | 0.42 | 0.51 |
| 2.00 | −4.95 | −3.68 | −3.36 | −2.79 | −2.92 | 30 | 0.19 | 0.33 | 0.40 | 0.54 | 0.58 |
Effect of Glycyl Dipeptide
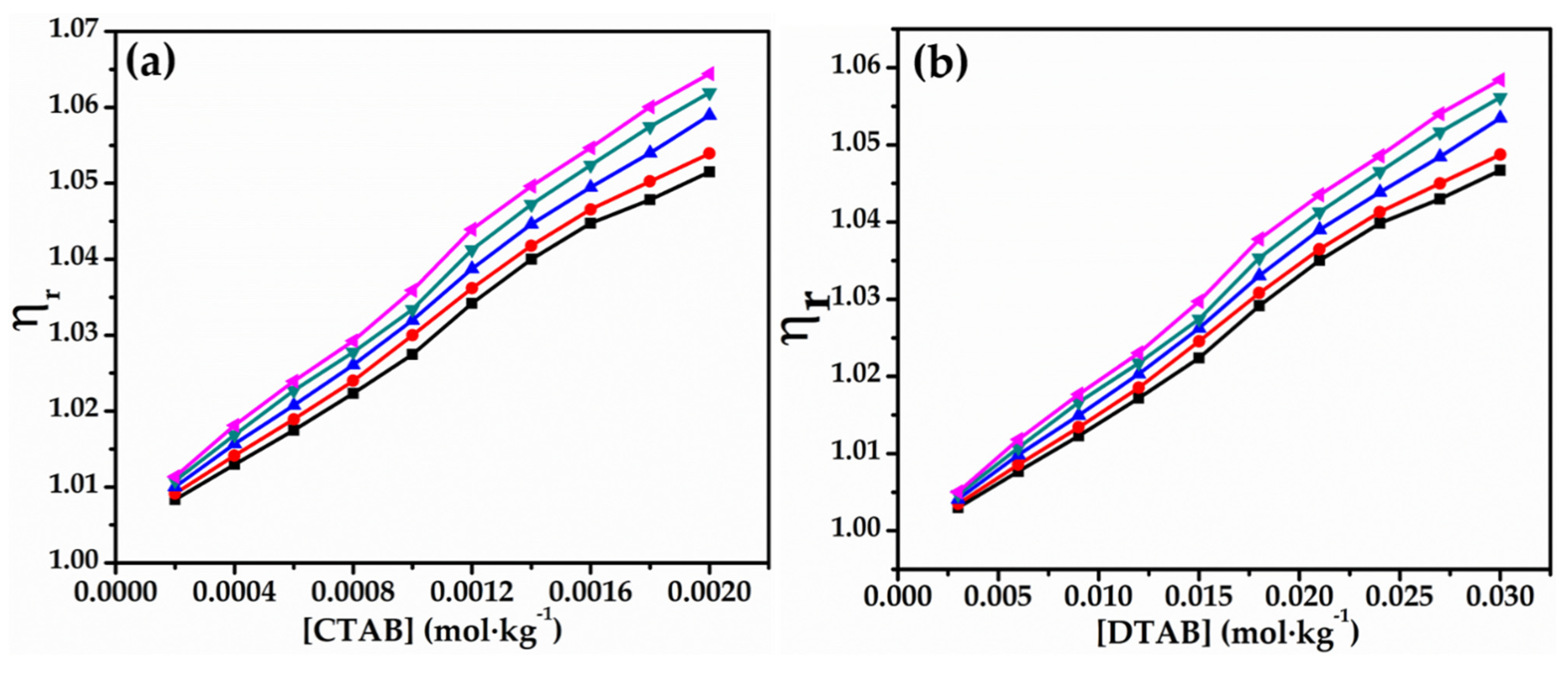
3.2. Viscometric Studies
4. Conclusions
- (i)
- The interactions between CTAB/DTAB and glycyl dipeptide have been found to be concentration dependent. The nature of interactions show remarkable changes in the micellar region of these surfactants, i.e., shifts from the hydrophilic to hydrophobic kind, and leads to the formation of complex aggregates of CTAB/DTAB and glycyl dipeptide.
- (ii)
- A substantial decrease has also been observed in the apparent molar volume and compressibility of the monomer with respect to its value in water. Both apparent molar volume and isentropic compressibility have been found to follow the same trend at higher surfactant concentrations for the surfactants, i.e., CTAB and DTAB.
- (iii)
- values and relative viscosity values also show concentration and temperature dependence; however, the results are more pronounced with CTAB than that of DTAB in aqueous solutions of glycyl dipeptide.
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Mazur, K.; Heisler, I.A.; Meech, S.R. Ultrafast Dynamics and Hydrogen-Bond Structure in Aqueous Solutions of Model Peptides. J. Phys. Chem. B 2010, 144, 10684–10691. [Google Scholar] [CrossRef] [PubMed]
- Akram, M.; Kumar, D.; Din, K. Catalytic effect of CTAB on the interaction of dipeptide glycyl-tyrosine (Gly-Tyr) with ninhydrin. J. Saudi. Chem. Soc. 2014, 18, 520–527. [Google Scholar] [CrossRef]
- Von Hippel, P.H.; Schleich, T. Structure and Stability of Biological Macromolecules; Marcel Dekker: New York, NY, USA, 1969; Volume 2. [Google Scholar]
- Yan, Z.; Li, Y.; Wang, X.; Dan, J.; Wang, J. Effect of glycyl dipeptides on the micellar behavior of gemini surfactant: A conductometric and fluorescence spectroscopic study. J. Mol. Liq. 2011, 161, 49–54. [Google Scholar] [CrossRef]
- Jencks, W.P. Catalysis in Chemistry and Enzymology; McGraw Hill: New York, NY, USA, 1969; p. 351. [Google Scholar]
- Yan, Z.; Zhang, Q.; Li, W.W.; Wang, J. Effect of Temperature on the Interactions of Glycyl Dipeptides with Sodium Dodecyl Sulfate in Aqueous Solution: A Volumetric, Conductometric, and Fluorescence Probe Study. J. Chem. Eng. Data 2010, 55, 3560–3566. [Google Scholar]
- Goddard, E.D.; Ananthapadmanabhan, K.P. Interactions of Surfactants with Polymers and Proteins; CRC Press: Boca Raton, FL, USA, 1993. [Google Scholar]
- Yan, Z.; Sun, X.; Li, W.W.; Li, Y.; Wang, J. Interactions of glutamine dipeptides with sodium dodecyl sulfate in aqueous solution measured by volume, conductivity, and fluorescence spectra. J. Chem. Thermodyn. 2011, 43, 1468–1474. [Google Scholar] [CrossRef]
- Iqbal, M.J.; Chaudhry, M.A. Thermodynamic study of three pharmacologically significant drugs: Density, viscosity and refractive index measurements at different temperatures. J. Chem. Thermodyn. 2009, 41, 221–226. [Google Scholar] [CrossRef]
- Yan, Z.; Wanga, X.; Xing, R.; Wang, J. Volumetric and conductometric studies on the interactions of dipeptides with sodium acetate and sodium butyrate in aqueous solutions at T = 298.15 K. J. Chem. Thermodyn. 2009, 41, 1343–1349. [Google Scholar] [CrossRef]
- Singh, S.K.; Kishore, N. Partial Molar Volumes of Amino Acids and Peptides in Aqueous Salt Solutions at 25 °C and a Correlation with Stability of Proteins in the Presence of Salts. J. Solut. Chem. 2003, 32, 117–135. [Google Scholar] [CrossRef]
- Wang, J.J.; Yan, Z.N.; Lu, J.S. Effect of sodium caproate on the volumetric and viscometric properties of glycine, DL-α-alanine, and DL-α-amino-n-butyric acid in aqueous solutions. J. Chem. Thermodyn. 2004, 36, 281–288. [Google Scholar] [CrossRef]
- Wang, J.J.; Yan, Z.N.; Zhuo, K.L.; Lu, J.S. Partial molar volumes of some α-amino acids in aqueous sodium acetate solutions at 308.15 K. Biophys. Chem. 1999, 80, 179–188. [Google Scholar] [CrossRef]
- Wang, J.J.; Yan, Z.N.; Zhuo, K.L.; Liu, D.Z. Standard Volumes of Transfer for Some α-Amino Acids from Water to Aqueous Sodium Acetate Solutions at 298.15 K. Z. Phys. Chem. 2000, 214, 333–345. [Google Scholar]
- Yan, Z.N.; Wang, X.G.; Zhao, Y.; Wang, J.J. Volumetric Properties of Glycyl Dipeptides in Aqueous Sodium Acetate Solutions at 298.15 K. Acta Chim. Sinica 2009, 67, 115–121. [Google Scholar]
- Yan, Z.N.; Zhao, Y.; Xing, R.H.; Wang, X.G.; Wang, J.J. Volumetric and Conductometric Behavior at T = 298.15 K of 2-[(2-Aminoacetyl)amino]acetic Acid, 2-[(2-Aminoacetyl)amino]-3-methylbutanoic Acid, and (2 S)-2-[(2-Aminoacetyl)amino]-4-methylpentanoic Acid with Sodium Hexanoate. J. Chem. Eng. Data 2010, 55, 759–764. [Google Scholar]
- Kumar, K.; Chauhan, S. Volumetric, compressibility and viscometric studies on sodium cholate/sodium deoxycholate–amino acid interactions in aqueous medium. Thermochim. Acta. 2015, 606, 12–24. [Google Scholar] [CrossRef]
- Yan, Z.N.; Li, W.W.; Zhang, Q.; Wang, X.G.; Wang, J.J. Effect of sodium caproate on the volumetric and conductometric properties of glycyl-l-glutamine and l-alanyl-l-glutamine in aqueous solution at 298.15K. Fluid Phase Equilib. 2011, 301, 156–162. [Google Scholar] [CrossRef]
- Singh, S.K.; Kundu, A.; Kishore, N. Interactions of some amino acids and glycine peptides with aqueous sodium dodecyl sulfate and cetyltrimethylammonium bromide at T=298.15 K: A volumetric approach. J. Chem. Thermodyn. 2004, 36, 7–16. [Google Scholar] [CrossRef]
- Han, G.; Wen, S.; Wang, H.; Feng, Q. Sulfidization regulation of cuprite by pre-oxidation using sodium hypochlorite as an oxidant. Int. J. Min. Sci. Technol. 2021, 1117, 31–1128. [Google Scholar] [CrossRef]
- Zhao, W.; Wang, M.; Yang, B.; Feng, Q.; Liu, D. Enhanced sulfidization flotation mechanism of smithsonite in the synergistic activation system of copper–ammonium species. Miner. Eng. 2022, 187, 107796. [Google Scholar] [CrossRef]
- Chauhan, S.; Chauhan, M.; Kaushal, D.; Syal, V.; Jyoti, J. Study of Micellar Behavior of SDS and CTAB in Aqueous Media Containing Furosemide—A Cardiovascular Drug. J. Solut. Chem. 2010, 39, 622–638. [Google Scholar]
- Chauhan, S.; Kumar, K. Partial Molar Volumes and Isentropic Compressibilities of Some Saccharides in Aqueous Solutions of Leucine at Different Temperatures. J. Chem. Eng. Data 2014, 59, 1375. [Google Scholar] [CrossRef]
- Singh, M. Survismeter for simultaneous viscosity and surface tension study for molecular interactions. J. Surf. Interface Anal. 2008, 40, 15–21. [Google Scholar] [CrossRef]
- Kumar, K.; Patial, B.S.; Chauhan, S. Interactions of Saccharides in Aqueous Glycine and Leucine Solutions at Different Temperatures of (293.15 to 313.15) K: A Viscometric Stud. J. Chem. Eng. Data 2015, 60, 47–56. [Google Scholar] [CrossRef]
- Tanaka, M.; Kaneshinam, S.; Nishimoto, W.; Takabatake, H. The Viscosities of Aqueous Sodium Alkylsulfate and Alkyltrimethylammonium Bromide Solutions. Bull. Chem. Soc. Jpn. 1973, 46, 364–368. [Google Scholar] [CrossRef]
- Gill, D.S.; Chauhan, M.S.; Sekhri, M.B. Conductance and viscosity measurements of tetrabutylammonium tetraphenylboride in non-aqueous solvents at 25 °C. J. Chem. Soc. Faraday Trans. 1982, 78, 3461–3466. [Google Scholar] [CrossRef]
- Yan, Z.; Liu, R.; Wu, S.; Bai, X.; Wang, J. Effect of temperature on the interactions of glycyl dipeptides with sodium perfluorooctanoate in aqueous solution: Volumetric, conductometric, and spectroscopic study. J. Chem. Thermodyn. 2013, 57, 360–366. [Google Scholar] [CrossRef]
- Wang, J.X.; Zhu, Z.L.; Gao, Y.; Jiang, R.S.; Cao, P.X. Milling Performance of Fluorinated Surfactants-Based Coating Tools in Processing Particleboard. Sci. Adv. Mater. 2021, 13, 264–272. [Google Scholar] [CrossRef]
- Bhuiyan, M.M.H.; Hakin, A.W.; Liu, J.L. Densities, Specific Heat Capacities, Apparent and Partial Molar Volumes and Heat Capacities of Glycine in Aqueous Solutions of Formamide, Acetamide, and N,N-Dimethylacetamide at T=298.15 K and Ambient Pressure. J. Sol. Chem. 2010, 39, 877–896. [Google Scholar] [CrossRef]
- Zafarani-Moattar, M.T.; Sarmad, S. Apparent molar volumes, apparent isentropic compressibilities, and viscosity B-coefficients of 1-ethyl-3-methylimidazolium bromide in aqueous di-potassium hydrogen phosphate and potassium di-hydrogen phosphate solutions at T = (298.15, 303.15, 308.15, 313.15, and 318.15) K. J. Chem. Thermodyn. 2012, 54, 192–203. [Google Scholar]
- Chauhan, S.; Sharma, K.; Kumar, K.; Kumar, G. A Comparative Study of Micellization Behavior of an Ethoxylated Alkylphenol in Aqueous Solutions of Glycine and Leucine. J. Surfact. Deterg. 2014, 17, 161–168. [Google Scholar] [CrossRef]
- Gill, D.S.; Kaur, T.; Joshi, I.M.; Singh, J. Ultrasonic velocity, permittivity, density, viscosity and proton nuclear magnetic resonance measurements of binary mixtures of benzonitrile with organic solvents. J. Chem. Soc. Faraday Trans. 1993, 89, 1737–1740. [Google Scholar] [CrossRef]
- Chauhan, S.; Sharma, V.; Sharma, K. Maltodextrin–SDS interactions: Volumetric, viscometric and surface tension study. Fluid Phase Equilib. 2013, 354, 236–244. [Google Scholar] [CrossRef]
- Chauhan, S.; Chauhan, M.S.; Sharma, P.; Rana, D.S. Thermodynamics and micellization of cetyltrimethyl ammonium bromide in the presence of lysozyme. J. Mol. Liq. 2013, 187, 1–6. [Google Scholar] [CrossRef]
- Lee, Y.S.; Woo, K.W. Micellization of Aqueous Cationic Surfactant Solutions at the Micellar Structure Transition Concentration—Based upon the Concept of the Pseudophase Separation. Colloids Interface Sci. 1995, 169, 34–38. [Google Scholar] [CrossRef]
- Mehrian, T.; de Keizer, A.; Korteweg, A. Lyklema, Thermodynamics of adsorption of dodecylpyridinium chloride on Na-kaolinite. Colloids Surf. A Physicochem. Eng. 1993, 71, 255–267. [Google Scholar]
- Moren, A.K.; Khan, A. Surfactant Hydrophobic Effect on the Phase Behavior of Oppositely Charged Protein and Surfactant Mixtures: Lysozyme and Sodium Alkyl Sulfates. Langmuir 1998, 14, 6818–6826. [Google Scholar] [CrossRef]
- Rajagopal, K.; Gladson, S.E. Partial molar volume and partial molar compressibility of four homologous α-amino acids in aqueous sodium fluoride solutions at different temperatures. J. Chem. Thermodyn. 2011, 43, 852–867. [Google Scholar] [CrossRef]
- Singh, K.; Chauhan, S. Interactional Behavior of Sodium Cholate and Sodium Deoxycholate in the Presence of Ceftriaxone Sodium: Volumetric, Compressibility, Viscometric, and Proton Nuclear Magnetic Resonance Studies. J. Chem. Eng. Data 2020, 65, 4536–4546. [Google Scholar] [CrossRef]
- Cabani, S.; Conti, G.; Matteoli, E. Adiabatic and isothermal apparent molal compressibilities of organic compounds in water. I. Cyclic and open-chain secondary alcohols and ethers. J. Sol. Chem. 1979, 8, 11–23. [Google Scholar]
- Lindmann, B.H. Wennerstrom in Topics in Current Chemistry; Dewar, M.J.S., Hafner, K., Heilbronner, E., Ito, S., Lehn, J.M., Niedenzu, K., Rees, C.W., Schafer, K., Wittig, G., Eds.; Springer-Verlag: New York, NY, USA, 1980. [Google Scholar]
- Chauhan, S.; Jyoti, J.; Kumar, G. Non-ionic surfactant interactions in aqueous gelatin solution: A physico-chemical investigation. J. Mol. Liq. 2011, 159, 196–200. [Google Scholar] [CrossRef]
- Manna, K.; Chang, C.H.; Panda, A.K. Physicochemical studies on the catanionics of alkyltrimethylammonium bromides and bile salts in aqueous media. Colloids Surf. A Physicochem. Eng. 2012, 415, 10–21. [Google Scholar] [CrossRef]
- Iqbal, M.J.; Chaudhary, M.A. Volumetric and viscometric studies of antidepressant drugs in aqueous medium at different temperatures. J. Chem. Eng. Data 2009, 54, 2772–2776. [Google Scholar] [CrossRef]
- Kaur, K.; Kumar, H. Investigation on molecular interaction of amino acids in antibacterial drug ampicillin solutions with reference to volumetric and compressibility measurement. J. Mol. Liq. 2012, 177, 49–53. [Google Scholar]
- Naik, A.B. Densities, viscosities, speed of sound and some acoustical parameter studies of substituted pyrazoline compounds at different temperatures. Indian J. Pure Appl. Phys. 2015, 53, 27–34. [Google Scholar]
- Abezgauz, L.; Kuperkar, K.; Hassan, P.A.; Ramon, O.; Bahadur, P.; Danino, D. Effect of Hofmeister anions on micellization and micellar growth of the surfactant cetylpyridinium chloride. J. Colloid Interface Sci. 2010, 342, 83–92. [Google Scholar] [CrossRef] [PubMed]
- Sharma, K.; Chauhan, S.; Priya, B. Surface, compressibility and viscometric measurements of binary system containing cationic surfactant with achiral amino acid at different temperatures. J. Mol. Liq. 2016, 222, 407–414. [Google Scholar] [CrossRef]
- George, J.; Nair, S.M.; Sreejith, L. Interactions of sodium dodecyl benzene sulfonate and sodium dodecyl sulfate with gelatin: A comparison. J. Surfact. Deterg. 2008, 11, 29–32. [Google Scholar] [CrossRef]
- Tamura, K.; Sonoda, T.; Murakami, S. Thermodynamic Properties of Aqueous Solution of 2-Isopropoxyethanol at 25 °C. J. Sol. Chem. 1999, 28, 777–789. [Google Scholar] [CrossRef]
- Ali, A.; Nain, A.K.; Kamil, M. Physico-chemical studies of non-aqueous binary liquid mixtures at various temperatures. Thermochim. Acta 1996, 274, 209–221. [Google Scholar] [CrossRef]
- Praharaj, M.K.; Mishra, S. Satapathy, Ultrasonic study of ternary liquid mixture containing substituted benzene. Arch. Phys. Res. 2012, 3, 192–200. [Google Scholar]
- Dhondge, S.S.; Zodape, S.P.; Parwate, D.V. Volumetric and viscometric studies of some drugs in aqueous solutions at different temperatures. J. Chem. 2012, 48, 207–212. [Google Scholar] [CrossRef]
- Sharma, P.; Chauhan, S.; Chauhan, M.S.; Syal, V.K. Ultrasonic velocity and viscosity studies of tramacip and parvodex in binary mixtures of alcohol+ water. Indian J. Pure Appl. Phys. 2013, 46, 839–843. [Google Scholar]
- Chauhan, S.; Kumar, K.; Patial, B.S. Study of acoustic parameters of proline in lecithin-ethanol mixture at varying temperature. Indian J. Pure Appl. Phys. 2013, 51, 531–541. [Google Scholar]
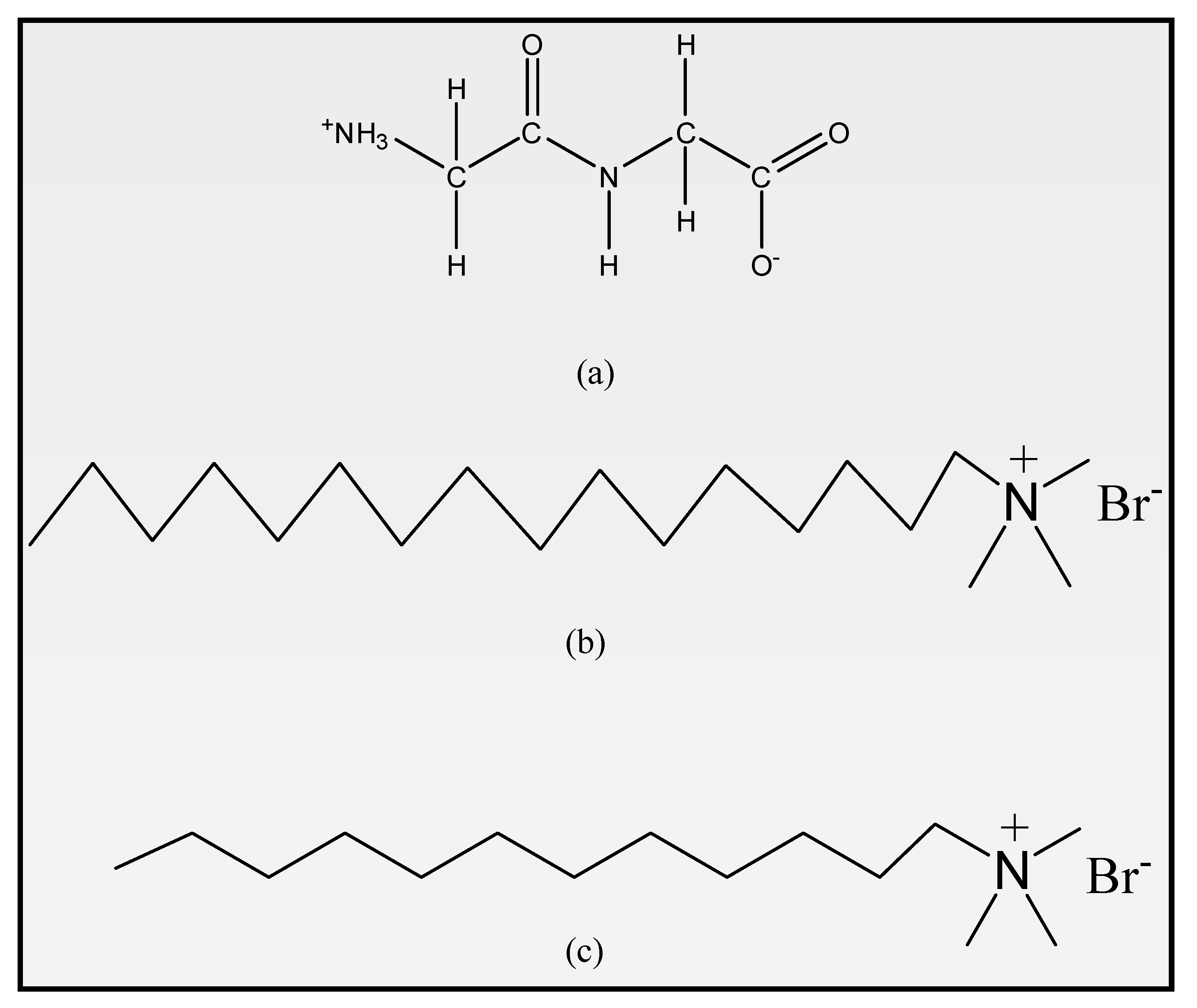

| Chemical Name | Source | Mol.Wt./kg∙mol−1 | Purification Method | Mass Fraction Purity a |
|---|---|---|---|---|
| Glycylglycine | Spectrochem Pvt. Ltd. (New Delhi, India) | 0.132 | None | 0.98 |
| Cetyltrimethylammonium Bromide | Himedia Pvt. Ltd. (Mumbai, India) | 0.364 | Recrystallization | 0.98 |
| Dodecyltrimethylammonium Bromide | Himedia Pvt. Ltd. (Mumbai, India) | 0.308 | Recrystallization | 0.98 |
| CTAB | DTAB | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| [CTAB] mmol∙kg−1 | 293.15 K | 298.15 K | 303.15 K | 308.15 K | 313.15 K | [DTAB] mmol∙kg−1 | 293.15 K | 298.15 K | 303.15 K | 308.15 K | 313.15 K |
| [Pure Water] | |||||||||||
| 0.2 | 455.79 | 447.77 | 440.89 | 435.50 | 431.13 | 3 | 455.27 | 447.34 | 440.64 | 435.17 | 430.87 |
| 0.4 | 455.71 | 447.70 | 440.84 | 435.46 | 431.07 | 6 | 454.72 | 446.91 | 440.25 | 434.79 | 430.55 |
| 0.6 | 455.61 | 447.59 | 440.80 | 435.41 | 431.03 | 9 | 454.23 | 446.48 | 439.87 | 434.44 | 430.25 |
| 0.8 | 455.54 | 447.52 | 440.74 | 435.37 | 430.98 | 12 | 453.76 | 446.07 | 439.45 | 434.10 | 429.94 |
| 1.0 | 455.42 | 447.46 | 440.72 | 435.34 | 430.94 | 15 | 453.33 | 445.65 | 439.20 | 433.78 | 429.65 |
| 1.2 | 455.37 | 447.42 | 440.69 | 435.32 | 430.92 | 18 | 453.30 | 445.43 | 439.10 | 433.71 | 429.57 |
| 1.4 | 455.32 | 447.38 | 440.67 | 435.30 | 430.90 | 21 | 453.28 | 445.38 | 439.08 | 433.71 | 429.58 |
| 1.6 | 455.27 | 447.34 | 440.64 | 435.28 | 430.88 | 24 | 453.05 | 445.33 | 439.06 | 433.70 | 429.59 |
| 1.8 | 455.23 | 447.32 | 440.62 | 435.25 | 430.86 | 27 | 452.94 | 445.27 | 439.04 | 433.70 | 429.60 |
| 2.0 | 455.17 | 447.27 | 440.59 | 435.23 | 430.84 | 30 | 452.87 | 445.23 | 439.02 | 433.72 | 429.62 |
| [Glycyl Dipeptide] = 0.001 mol∙kg−1 | |||||||||||
| 0.20 | 447.20 | 440.57 | 435.09 | 431.55 | 427.61 | 3 | 454.32 | 446.41 | 439.90 | 434.46 | 430.31 |
| 0.40 | 447.15 | 440.50 | 435.03 | 431.51 | 427.57 | 6 | 454.02 | 446.14 | 439.62 | 434.34 | 430.08 |
| 0.60 | 447.10 | 440.44 | 434.98 | 431.49 | 427.54 | 9 | 453.58 | 445.76 | 439.29 | 434.06 | 429.73 |
| 0.80 | 447.05 | 440.40 | 434.96 | 431.47 | 427.51 | 12 | 453.07 | 445.34 | 438.95 | 433.72 | 429.43 |
| 0.10 | 447.00 | 440.33 | 434.92 | 431.45 | 427.49 | 15 | 452.68 | 444.96 | 438.62 | 433.46 | 429.16 |
| 1.20 | 446.96 | 440.28 | 434.89 | 431.43 | 427.47 | 18 | 452.48 | 444.76 | 438.44 | 433.28 | 428.99 |
| 1.40 | 446.92 | 440.26 | 434.87 | 431.41 | 427.45 | 21 | 452.28 | 444.67 | 438.33 | 433.18 | 428.83 |
| 1.60 | 446.87 | 440.22 | 434.85 | 431.39 | 427.43 | 24 | 452.19 | 444.54 | 438.28 | 433.13 | 428.74 |
| 1.80 | 446.83 | 440.18 | 434.82 | 431.37 | 427.41 | 27 | 452.02 | 444.51 | 438.24 | 433.08 | 428.70 |
| 2.00 | 446.79 | 440.15 | 434.80 | 431.36 | 427.40 | 30 | 451.93 | 444.48 | 438.19 | 433.04 | 428.67 |
| [Glycyl Dipeptide] = 0.005 mol∙kg−1 | |||||||||||
| 0.20 | 446.65 | 440.08 | 434.30 | 430.37 | 427.04 | 3 | 454.14 | 446.27 | 439.83 | 434.32 | 430.08 |
| 0.40 | 446.59 | 440.05 | 434.25 | 430.33 | 427.00 | 6 | 453.84 | 445.98 | 439.59 | 434.16 | 429.75 |
| 0.60 | 446.55 | 440.00 | 434.19 | 430.28 | 426.96 | 9 | 453.39 | 445.55 | 439.17 | 433.77 | 429.43 |
| 0.80 | 446.48 | 439.95 | 434.12 | 430.24 | 426.92 | 12 | 452.89 | 445.13 | 438.85 | 433.42 | 429.17 |
| 0.10 | 446.41 | 439.91 | 434.09 | 430.19 | 426.89 | 15 | 452.54 | 444.81 | 438.55 | 433.12 | 428.97 |
| 1.20 | 446.36 | 439.88 | 434.04 | 430.15 | 426.85 | 18 | 452.34 | 444.64 | 438.36 | 432.98 | 428.79 |
| 1.40 | 446.29 | 439.84 | 434.00 | 430.12 | 426.81 | 21 | 452.22 | 444.56 | 438.22 | 432.94 | 428.72 |
| 1.60 | 446.23 | 439.82 | 433.95 | 430.08 | 426.78 | 24 | 452.12 | 444.47 | 438.15 | 432.89 | 428.68 |
| 1.80 | 446.14 | 439.79 | 433.91 | 430.04 | 426.74 | 27 | 451.99 | 444.41 | 438.10 | 432.85 | 428.64 |
| 2.00 | 446.22 | 439.77 | 433.88 | 430.02 | 426.69 | 30 | 451.90 | 444.35 | 438.04 | 432.81 | 428.61 |
| [Glycyl Dipeptide] = 0.010 mol∙kg−1 | |||||||||||
| 0.20 | 445.41 | 439.02 | 433.83 | 429.00 | 425.81 | 3 | 453.93 | 446.27 | 439.80 | 434.25 | 430.04 |
| 0.40 | 445.38 | 438.99 | 433.80 | 428.96 | 425.76 | 6 | 453.69 | 445.80 | 439.39 | 433.90 | 429.67 |
| 0.60 | 445.35 | 438.95 | 433.77 | 428.94 | 425.70 | 9 | 453.23 | 445.38 | 439.01 | 433.53 | 429.34 |
| 0.80 | 445.33 | 438.92 | 433.74 | 428.91 | 425.66 | 12 | 452.71 | 445.03 | 438.62 | 433.21 | 429.01 |
| 0.10 | 445.30 | 438.90 | 433.72 | 428.89 | 425.61 | 15 | 452.42 | 444.72 | 438.34 | 432.95 | 428.82 |
| 1.20 | 445.28 | 438.88 | 433.70 | 428.88 | 425.57 | 18 | 452.24 | 444.55 | 438.21 | 432.86 | 428.66 |
| 1.40 | 445.27 | 438.86 | 433.68 | 428.87 | 425.55 | 21 | 452.14 | 444.46 | 438.13 | 432.75 | 428.60 |
| 1.60 | 445.25 | 438.86 | 433.67 | 428.86 | 425.52 | 24 | 452.07 | 444.39 | 438.07 | 432.66 | 428.57 |
| 1.80 | 445.23 | 438.85 | 433.65 | 428.85 | 425.49 | 27 | 451.94 | 444.33 | 438.01 | 432.58 | 428.52 |
| 2.00 | 445.22 | 438.84 | 433.64 | 428.84 | 425.45 | 30 | 451.83 | 444.26 | 437.96 | 432.67 | 428.51 |
| CTAB | DTAB | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| [CTAB] mmol∙kg−1 | 293.15 K | 298.15 K | 303.15 K | 308.15 K | 313.15 K | [DTAB] mmol∙kg−1 | 293.15 K | 298.15 K | 303.15 K | 308.15 K | 313.15 K |
| [Pure water] | |||||||||||
| 0.2 | 1.0042 | 1.0056 | 1.0066 | 1.0085 | 1.0096 | 3 | 1.002 | 1.004 | 1.005 | 1.006 | 1.007 |
| 0.4 | 1.0087 | 1.0103 | 1.0121 | 1.0147 | 1.0165 | 6 | 1.004 | 1.007 | 1.008 | 1.010 | 1.012 |
| 0.6 | 1.0136 | 1.0154 | 1.0171 | 1.0206 | 1.0226 | 9 | 1.006 | 1.010 | 1.012 | 1.014 | 1.016 |
| 0.8 | 1.0181 | 1.0201 | 1.0223 | 1.0260 | 1.0284 | 12 | 1.008 | 1.012 | 1.014 | 1.017 | 1.019 |
| 1.0 | 1.0228 | 1.0250 | 1.0274 | 1.0316 | 1.0342 | 15 | 1.010 | 1.014 | 1.016 | 1.020 | 1.022 |
| 1.2 | 1.0275 | 1.0299 | 1.0322 | 1.0369 | 1.0394 | 18 | 1.012 | 1.017 | 1.019 | 1.023 | 1.025 |
| 1.4 | 1.0320 | 1.0347 | 1.0371 | 1.0420 | 1.0446 | 21 | 1.015 | 1.020 | 1.022 | 1.026 | 1.028 |
| 1.6 | 1.0361 | 1.0390 | 1.0418 | 1.0468 | 1.0499 | 24 | 1.019 | 1.024 | 1.026 | 1.030 | 1.033 |
| 1.8 | 1.0409 | 1.0440 | 1.0463 | 1.0520 | 1.0549 | 27 | 1.021 | 1.026 | 1.028 | 1.032 | 1.035 |
| 2.0 | 1.0456 | 1.0489 | 1.0517 | 1.0564 | 1.0600 | 30 | 1.025 | 1.030 | 1.032 | 1.037 | 1.040 |
| [Glycyl Dipeptide] = 0.001 mol∙kg−1 | |||||||||||
| 0.20 | 1.014 | 1.014 | 1.006 | 1.010 | 1.012 | 3 | 0.864 | 0.867 | 0.882 | 0.896 | 0.858 |
| 0.40 | 1.028 | 1.026 | 1.020 | 1.026 | 1.027 | 6 | 0.869 | 0.873 | 0.887 | 0.899 | 0.861 |
| 0.60 | 1.039 | 1.037 | 1.038 | 1.042 | 1.045 | 9 | 0.875 | 0.898 | 0.891 | 0.905 | 0.865 |
| 0.80 | 1.050 | 1.053 | 1.053 | 1.056 | 1.061 | 12 | 0.881 | 0.884 | 0.896 | 0.910 | 0.868 |
| 0.10 | 1.064 | 1.069 | 1.068 | 1.075 | 1.080 | 15 | 0.886 | 0.890 | 0.899 | 0.914 | 0.874 |
| 1.20 | 1.075 | 1.077 | 1.086 | 1.090 | 1.098 | 18 | 0.890 | 0.896 | 0.902 | 0.919 | 0.878 |
| 1.40 | 1.084 | 1.088 | 1.103 | 1.105 | 1.114 | 21 | 0.897 | 0.902 | 0.908 | 0.920 | 0.883 |
| 1.60 | 1.093 | 1.101 | 1.119 | 1.121 | 1.128 | 24 | 0.901 | 0.908 | 0.910 | 0.924 | 0.884 |
| 1.80 | 1.101 | 1.117 | 1.133 | 1.138 | 1.143 | 27 | 0.907 | 0.912 | 0.914 | 0.925 | 0.889 |
| 2.00 | 1.113 | 1.134 | 1.148 | 1.158 | 1.164 | 30 | 0.911 | 0.918 | 0.918 | 0.928 | 0.891 |
| [Glycyl Dipeptide] = 0.005 mol∙kg−1 | |||||||||||
| 0.20 | 1.014 | 1.017 | 1.013 | 1.007 | 1.013 | 3 | 0.865 | 0.868 | 0.877 | 0.881 | 0.858 |
| 0.40 | 1.023 | 1.030 | 1.028 | 1.022 | 1.030 | 6 | 0.872 | 0.874 | 0.882 | 0.885 | 0.864 |
| 0.60 | 1.032 | 1.041 | 1.042 | 1.038 | 1.051 | 9 | 0.875 | 0.880 | 0.886 | 0.891 | 0.869 |
| 0.80 | 1.041 | 1.055 | 1.057 | 1.050 | 1.070 | 12 | 0.879 | 0.886 | 0.890 | 0.894 | 0.872 |
| 0.10 | 1.052 | 1.066 | 1.071 | 1.068 | 1.085 | 15 | 0.883 | 0.892 | 0.894 | 0.899 | 0.877 |
| 1.20 | 1.063 | 1.077 | 1.084 | 1.082 | 1.099 | 18 | 0.888 | 0.897 | 0.897 | 0.900 | 0.879 |
| 1.40 | 1.074 | 1.088 | 1.100 | 1.101 | 1.118 | 21 | 0.893 | 0.902 | 0.903 | 0.903 | 0.882 |
| 1.60 | 1.082 | 1.102 | 1.113 | 1.114 | 1.132 | 24 | 0.899 | 0.906 | 0.907 | 0.905 | 0.887 |
| 1.80 | 1.096 | 1.116 | 1.128 | 1.131 | 1.148 | 27 | 0.905 | 0.913 | 0.911 | 0.908 | 0.891 |
| 2.00 | 1.108 | 1.130 | 1.153 | 1.144 | 1.165 | 30 | 0.908 | 0.918 | 0.913 | 0.910 | 0.894 |
| [Glycyl Dipeptide] = 0.010 mol∙kg−1 | |||||||||||
| 0.20 | 1.011 | 1.010 | 1.013 | 1.013 | 1.007 | 3 | 0.868 | 0.879 | 0.889 | 0.865 | 0.853 |
| 0.40 | 1.021 | 1.023 | 1.034 | 1.025 | 1.021 | 6 | 0.879 | 0.887 | 0.895 | 0.872 | 0.860 |
| 0.60 | 1.035 | 1.032 | 1.050 | 1.041 | 1.035 | 9 | 0.888 | 0.893 | 0.901 | 0.877 | 0.865 |
| 0.80 | 1.050 | 1.046 | 1.067 | 1.053 | 1.052 | 12 | 0.895 | 0.899 | 0.906 | 0.883 | 0.870 |
| 0.10 | 1.058 | 1.059 | 1.091 | 1.069 | 1.066 | 15 | 0.901 | 0.903 | 0.912 | 0.887 | 0.875 |
| 1.20 | 1.069 | 1.072 | 1.109 | 1.084 | 1.073 | 18 | 0.910 | 0.909 | 0.917 | 0.894 | 0.880 |
| 1.40 | 1.081 | 1.086 | 1.124 | 1.096 | 1.086 | 21 | 0.913 | 0.915 | 0.922 | 0.897 | 0.884 |
| 1.60 | 1.093 | 1.099 | 1.141 | 1.109 | 1.100 | 24 | 0.916 | 0.918 | 0.927 | 0.902 | 0.889 |
| 1.80 | 1.104 | 1.110 | 1.159 | 1.121 | 1.114 | 27 | 0.919 | 0.921 | 0.933 | 0.907 | 0.892 |
| 2.00 | 1.114 | 1.119 | 1.175 | 1.133 | 1.128 | 30 | 0.924 | 0.924 | 0.938 | 0.909 | 0.898 |
| CTAB | DTAB | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| [CTAB] mmol∙kg−1 | 293.15 K | 298.15 K | 303.15 K | 308.15 K | 313.15 K | [DTAB] mmol∙kg−1 | 293.15 K | 298.15 K | 303.15 K | 308.15 K | 313.15 K |
| [Water] | |||||||||||
| 0.2 | 0.6110 | 0.5350 | 0.4720 | 0.4210 | 0.3550 | 3 | 0.609 | 0.533 | 0.471 | 0.420 | 0.354 |
| 0.4 | 0.6140 | 0.5370 | 0.4740 | 0.4240 | 0.3580 | 6 | 0.610 | 0.534 | 0.472 | 0.421 | 0.356 |
| 0.6 | 0.6170 | 0.5400 | 0.4770 | 0.4260 | 0.3600 | 9 | 0.610 | 0.535 | 0.473 | 0.422 | 0.357 |
| 0.8 | 0.6200 | 0.5420 | 0.4790 | 0.4290 | 0.3620 | 12 | 0.611 | 0.536 | 0.474 | 0.423 | 0.358 |
| 1.0 | 0.6220 | 0.5440 | 0.4810 | 0.4310 | 0.3640 | 15 | 0.612 | 0.537 | 0.475 | 0.424 | 0.359 |
| 1.2 | 0.6250 | 0.5470 | 0.4840 | 0.4330 | 0.3660 | 18 | 0.613 | 0.538 | 0.476 | 0.425 | 0.360 |
| 1.4 | 0.6280 | 0.5490 | 0.4860 | 0.4350 | 0.3680 | 21 | 0.614 | 0.539 | 0.477 | 0.427 | 0.361 |
| 1.6 | 0.6300 | 0.5520 | 0.4880 | 0.4370 | 0.3690 | 24 | 0.617 | 0.541 | 0.479 | 0.429 | 0.362 |
| 1.8 | 0.6330 | 0.5540 | 0.4900 | 0.4390 | 0.3710 | 27 | 0.618 | 0.542 | 0.480 | 0.430 | 0.363 |
| 2.0 | 0.6360 | 0.5570 | 0.4930 | 0.4410 | 0.3730 | 30 | 0.620 | 0.544 | 0.482 | 0.431 | 0.365 |
| [Glycyl Dipeptide] = 0.001 mol∙kg−1 | |||||||||||
| 0.20 | 0.850 | 0.747 | 0.656 | 0.619 | 0.526 | 3 | 0.715 | 0.649 | 0.623 | 0.605 | 0.555 |
| 0.40 | 0.861 | 0.755 | 0.664 | 0.629 | 0.534 | 6 | 0.719 | 0.653 | 0.626 | 0.606 | 0.557 |
| 0.60 | 0.870 | 0.763 | 0.676 | 0.639 | 0.543 | 9 | 0.724 | 0.671 | 0.628 | 0.610 | 0.558 |
| 0.80 | 0.880 | 0.775 | 0.686 | 0.647 | 0.551 | 12 | 0.728 | 0.660 | 0.632 | 0.613 | 0.560 |
| 0.10 | 0.891 | 0.786 | 0.696 | 0.659 | 0.562 | 15 | 0.731 | 0.663 | 0.633 | 0.615 | 0.563 |
| 1.20 | 0.900 | 0.792 | 0.707 | 0.668 | 0.571 | 18 | 0.734 | 0.668 | 0.635 | 0.618 | 0.566 |
| 1.40 | 0.908 | 0.800 | 0.718 | 0.677 | 0.579 | 21 | 0.740 | 0.672 | 0.639 | 0.619 | 0.569 |
| 1.60 | 0.915 | 0.810 | 0.729 | 0.687 | 0.586 | 24 | 0.743 | 0.676 | 0.641 | 0.621 | 0.570 |
| 1.80 | 0.922 | 0.822 | 0.738 | 0.697 | 0.594 | 27 | 0.747 | 0.679 | 0.643 | 0.622 | 0.572 |
| 2.00 | 0.932 | 0.834 | 0.747 | 0.709 | 0.605 | 30 | 0.750 | 0.684 | 0.646 | 0.624 | 0.574 |
| [Glycyl Dipeptide] = 0.005 mol∙kg−1 | |||||||||||
| 0.20 | 0.869 | 0.760 | 0.671 | 0.634 | 0.540 | 3 | 0.733 | 0.665 | 0.642 | 0.619 | 0.567 |
| 0.40 | 0.876 | 0.770 | 0.681 | 0.644 | 0.549 | 6 | 0.738 | 0.669 | 0.646 | 0.622 | 0.570 |
| 0.60 | 0.884 | 0.778 | 0.690 | 0.653 | 0.560 | 9 | 0.740 | 0.673 | 0.648 | 0.625 | 0.573 |
| 0.80 | 0.891 | 0.788 | 0.700 | 0.661 | 0.570 | 12 | 0.742 | 0.677 | 0.650 | 0.627 | 0.575 |
| 0.10 | 0.901 | 0.796 | 0.709 | 0.672 | 0.578 | 15 | 0.745 | 0.681 | 0.653 | 0.630 | 0.578 |
| 1.20 | 0.910 | 0.805 | 0.717 | 0.681 | 0.585 | 18 | 0.749 | 0.684 | 0.655 | 0.630 | 0.579 |
| 1.40 | 0.920 | 0.813 | 0.728 | 0.693 | 0.596 | 21 | 0.753 | 0.688 | 0.659 | 0.633 | 0.581 |
| 1.60 | 0.926 | 0.823 | 0.736 | 0.701 | 0.603 | 24 | 0.759 | 0.691 | 0.662 | 0.634 | 0.584 |
| 1.80 | 0.938 | 0.833 | 0.746 | 0.712 | 0.611 | 27 | 0.763 | 0.696 | 0.665 | 0.636 | 0.587 |
| 2.00 | 0.948 | 0.844 | 0.763 | 0.720 | 0.620 | 30 | 0.765 | 0.700 | 0.666 | 0.637 | 0.588 |
| [Glycyl Dipeptide] = 0.010 mol∙kg−1 | |||||||||||
| 0.20 | 0.875 | 0.771 | 0.684 | 0.647 | 0.556 | 3 | 0.751 | 0.693 | 0.673 | 0.625 | 0.576 |
| 0.40 | 0.884 | 0.781 | 0.698 | 0.655 | 0.563 | 6 | 0.760 | 0.699 | 0.677 | 0.630 | 0.580 |
| 0.60 | 0.895 | 0.788 | 0.709 | 0.665 | 0.571 | 9 | 0.767 | 0.703 | 0.681 | 0.633 | 0.583 |
| 0.80 | 0.908 | 0.798 | 0.721 | 0.673 | 0.580 | 12 | 0.772 | 0.707 | 0.685 | 0.637 | 0.586 |
| 0.10 | 0.916 | 0.808 | 0.736 | 0.682 | 0.588 | 15 | 0.777 | 0.709 | 0.688 | 0.640 | 0.589 |
| 1.20 | 0.925 | 0.818 | 0.748 | 0.692 | 0.591 | 18 | 0.785 | 0.714 | 0.692 | 0.644 | 0.593 |
| 1.40 | 0.935 | 0.828 | 0.758 | 0.700 | 0.599 | 21 | 0.787 | 0.719 | 0.696 | 0.646 | 0.595 |
| 1.60 | 0.946 | 0.839 | 0.770 | 0.708 | 0.606 | 24 | 0.789 | 0.721 | 0.700 | 0.650 | 0.598 |
| 1.80 | 0.956 | 0.847 | 0.782 | 0.716 | 0.614 | 27 | 0.792 | 0.723 | 0.704 | 0.653 | 0.600 |
| 2.00 | 0.964 | 0.854 | 0.793 | 0.724 | 0.622 | 30 | 0.796 | 0.726 | 0.708 | 0.655 | 0.604 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kumari, S.; Chauhan, S.; Singh, K.; Umar, A.; Fouad, H.; Akhtar, M.S. Study on Volumetric, Compressibility and Viscometric Behavior of Cationic Surfactants (CTAB and DTAB) in Aqueous Glycyl Dipeptide: A Thermo-Acoustic Approach. Molecules 2022, 27, 8767. https://doi.org/10.3390/molecules27248767
Kumari S, Chauhan S, Singh K, Umar A, Fouad H, Akhtar MS. Study on Volumetric, Compressibility and Viscometric Behavior of Cationic Surfactants (CTAB and DTAB) in Aqueous Glycyl Dipeptide: A Thermo-Acoustic Approach. Molecules. 2022; 27(24):8767. https://doi.org/10.3390/molecules27248767
Chicago/Turabian StyleKumari, Santosh, Suvarcha Chauhan, Kuldeep Singh, Ahmad Umar, Hassan Fouad, and Mohammad Shaheer Akhtar. 2022. "Study on Volumetric, Compressibility and Viscometric Behavior of Cationic Surfactants (CTAB and DTAB) in Aqueous Glycyl Dipeptide: A Thermo-Acoustic Approach" Molecules 27, no. 24: 8767. https://doi.org/10.3390/molecules27248767
APA StyleKumari, S., Chauhan, S., Singh, K., Umar, A., Fouad, H., & Akhtar, M. S. (2022). Study on Volumetric, Compressibility and Viscometric Behavior of Cationic Surfactants (CTAB and DTAB) in Aqueous Glycyl Dipeptide: A Thermo-Acoustic Approach. Molecules, 27(24), 8767. https://doi.org/10.3390/molecules27248767







