1. Introduction
The prevalence of obesity continues to rise at an alarming rate worldwide, with nearly 40% of adults and almost one in five children and adolescents described as overweight or obese, according to the latest World Health Organization (WHO) Global Health observatory data collated in 2016 [
1]. Obesity increases the risk of a range of metabolic and cardiovascular diseases, some cancers, and severe illness and death from COVID-19 [
2]. Obesity affects nearly all age groups and constitutes a massive economic burden on healthcare systems. Dietary energy intake is a major contributor to and one of the modifiable risk factors for obesity. In most populations, fat intake accounts for 30% or more of the total energy intake, which exceeds the recommended intake of fats for the prevention of noncommunicable diseases [
3]. It is evident that energy intake and the risk of obesity increase as dietary fat content increases [
4,
5,
6].
The digestion and absorption of dietary fat, predominantly triacylglycerols (TAGs), consist of sequential physicochemical and enzymatic events through the gastrointestinal tract. TAGs are digested primarily by pancreatic lipase (triacylglycerol acyl hydrolase EC3.1.1.3) in the upper segment of the jejunum. The enzyme is secreted by the acinar glands of the pancreas into the pancreatic duct and then into the intestine in response to the ingestion of a fatty meal. At the oil–water interface, pancreatic lipase acts on the sn-1 and sn-3 positions of TAG molecules, resulting in the release of 2-monoacylglycerol and free fatty acids, which are taken up from the lumen into the polarized enterocytes that line the small intestine and used for the biosynthesis of neutral fat. Further hydrolysis of 2-monoacylglycerol by pancreatic lipase results in the formation of glycerol and free fatty acids [
7]. Pancreatic lipase is known as the single most important determinant of dietary fat absorption, as in its absence only 30% of ingested fats are absorbed [
8]. Hence, modulating human pancreatic lipase may represent an effective way to combat obesity.
Orlistat, a potent inhibitor of gastric and pancreatic lipases, is now the only medication approved for clinical use to aid weight loss, and the only anti-obesity medicine that does not act on the central nervous system or enter the bloodstream [
9]. However, orlistat has been associated with fat malabsorption [
10] and multiple gastrointestinal adverse effects, which compromise patients’ compliance, and drug interactions that affect its bioavailability and effectiveness [
11]. Moreover, since orlistat is indicated for patients with a body mass index (BMI) >30 or those with a BMI >27 and concomitant obesity-related risk factors or diseases [
10], it means that orlistat can hardly be used by healthy individuals on a regular basis for weight management purposes. Alternatively, bioactive compounds of natural origins that target pancreatic lipase have received substantial attention owing to their structural diversity, low toxicity, and wide range of sources.
During the process of fractioning sesame meal, a byproduct of sesame (Sesamum indicum L.) oil extraction, we fortuitously found that the ethanolic extract of sesame meal effectively inhibited the activity of porcine pancreatic lipase in in vitro assays. Therefore, we proceeded to identify the bioactive constituent(s) of sesame meal against pancreatic lipase, and to explore its/their effectiveness in reducing dietary fat absorption in vivo.
3. Discussion
Reducing dietary fat digestion and absorption by modulating the activity of pancreatic lipase has become a favorable strategy to tackle obesity, not only because pancreatic lipase is the single most important determinant of fat absorption, but targeting pancreatic lipase is also considered safe, as its inhibitors neither act on the central nervous system nor enter the bloodstream. At the present time, the only approved anti-obesity drug that acts by inhibiting pancreatic lipase is orlistat, which, however, is known to have multiple gastrointestinal side effects. As a safe alternative to orlistat, a number of natural product-derived compounds with varying degrees of inhibitory activity against pancreatic lipase have been identified. These compounds are derived from a wide range of plant and microbial extracts and belong to various chemical classes, including, but not limited to, alkaloids, carotenoids, polyphenols, glycosides, polysaccharides, terpenes, and saponins [
9,
12,
13]. In this regard, for instance, strictinin-rich white tea and green tea extracts were found to be effective in inhibiting pancreatic lipase in vitro (IC50 = 22 μg/mL and 35 μg/mL, respectively) [
14]; stephalagine (1,2-methylenedioxy-3-methoxyaporphine), an aporphine alkaloid isolated from
Annona crassiflora fruit peel, demonstrated high potency against pancreatic lipase, with an IC50 of 8.35 µg/mL (27.0 µM) [
15]; for the methanolic extracts of
Eucalyptus globulus and
Mentha viridis, the pancreatic lipase inhibitory activity (IC50 = 690 µg/mL and 1290 µg/mL, respectively) seemed to hinge on the contents of phenols and total flavonoids [
16]; the fractionation of
Murraya koenigii (L.) Spreng leaves led to the identification of four carbazole alkaloids that exhibited pancreatic lipase inhibitory activities, with IC50 values ranging from 17.9 µM to < 500 µM [
17].
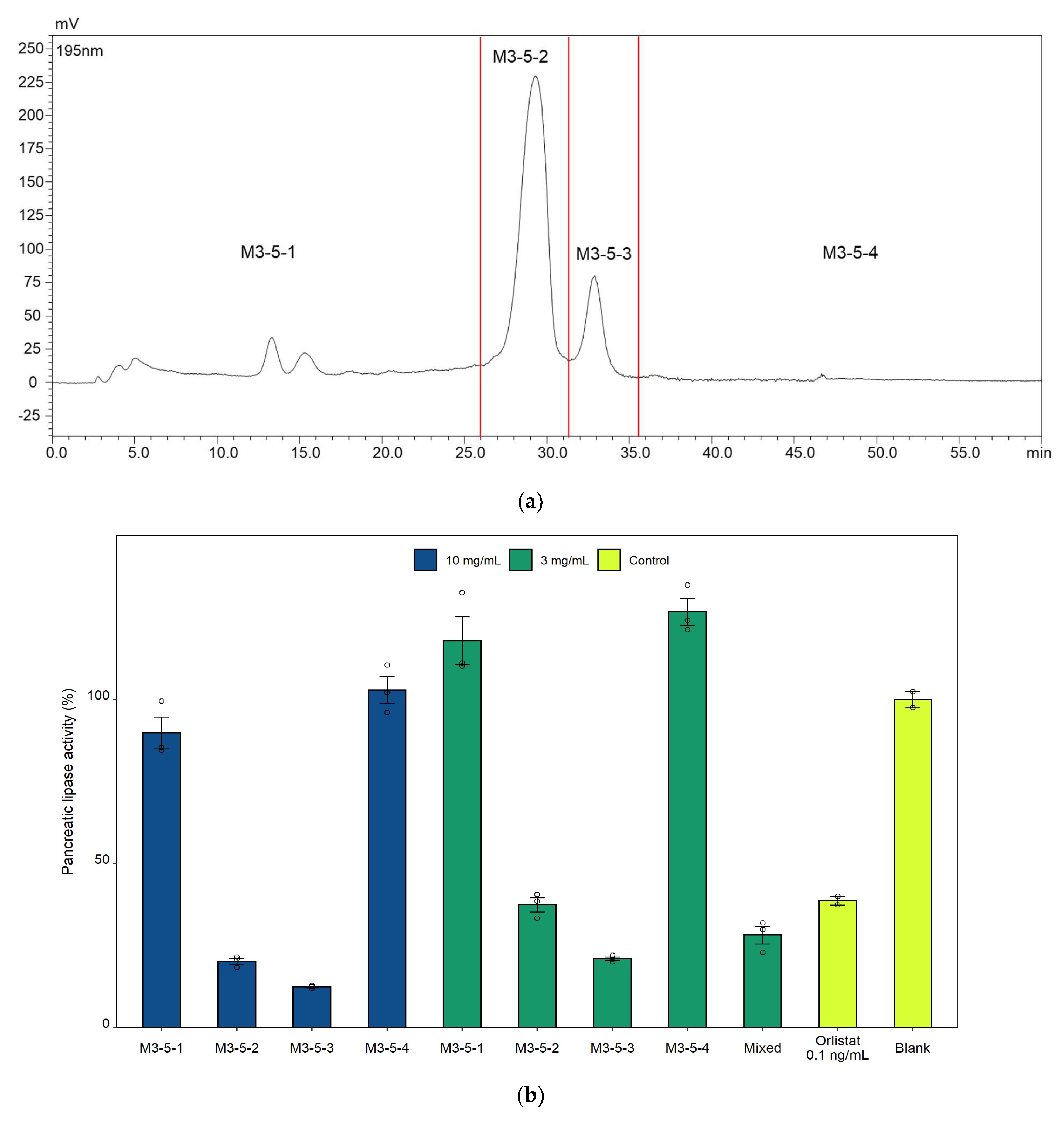
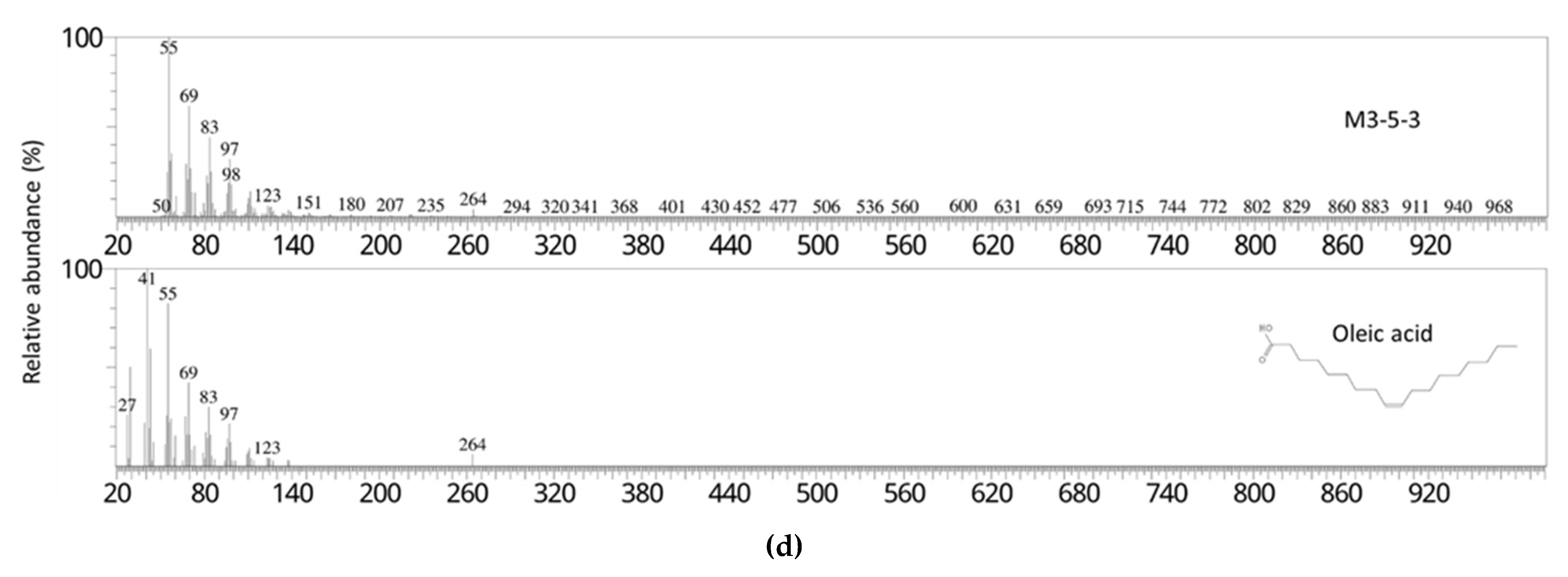
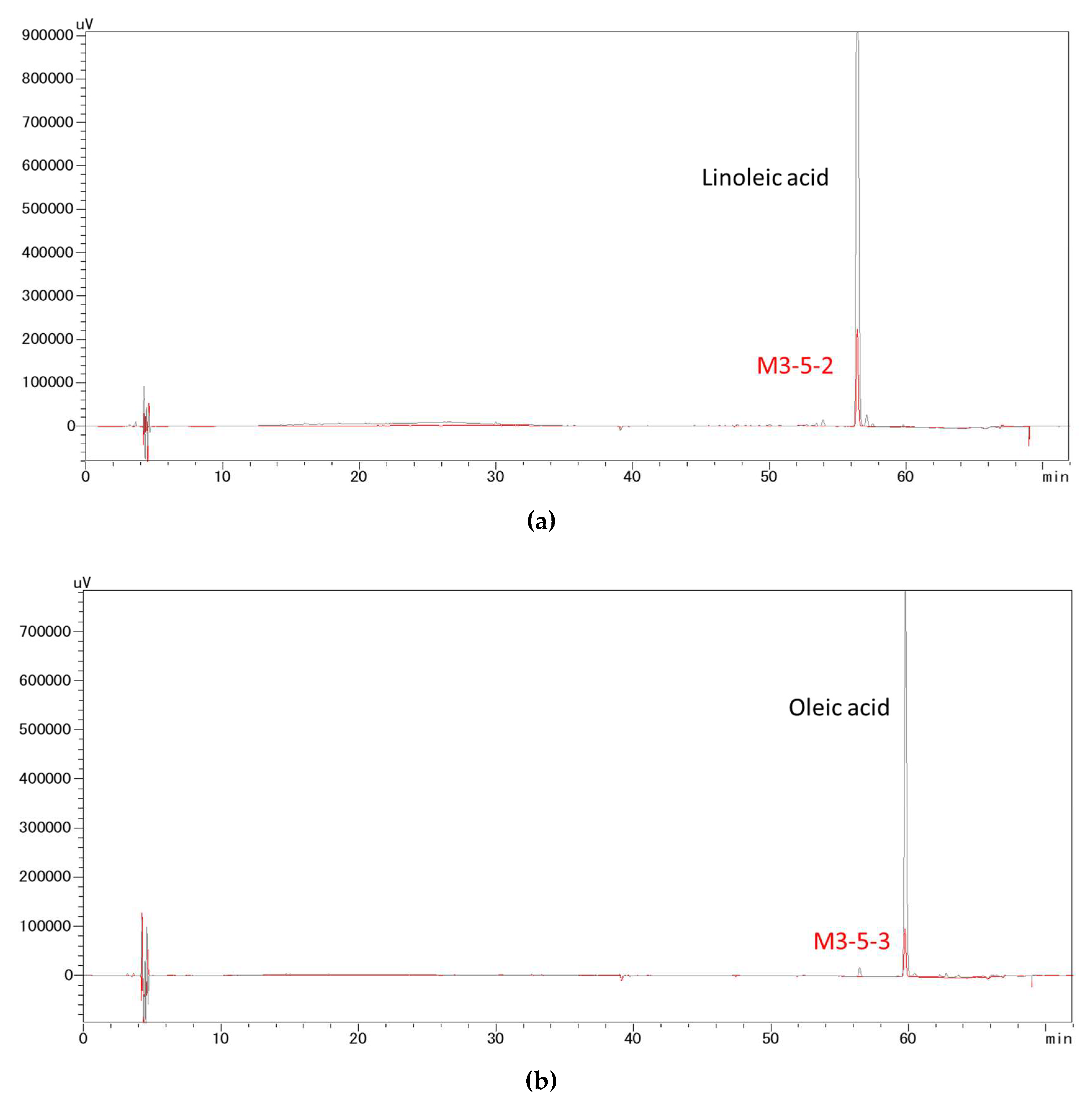
In this study, bioactivity-guided fractionation of the 40 °C 50% ethanol extract of sesame meal afforded two fractions that exerted the most potent in vitro inhibitory effects on pancreatic lipase. These fractions were suggested to comprise predominantly linoleic acid and oleic acid, respectively, by matching their fragmentation pattern and retention time with those of the pure analytical standards. As confirmed in the subsequent lipase inhibition assays, both linoleic acid and oleic acid demonstrated strong pancreatic lipase activities in vitro, which were comparable or superior to a number of pancreatic lipase inhibitors derived from natural sources, including the aforementioned ones. However, direct and rigorous comparison between these extracts or compounds is difficult due to the differences in the source of the enzyme and assay conditions. In addition to lipase inhibition assay, to further clarify the mechanism of action of free linoleic acid and oleic acid, molecular docking simulations and inhibition kinetic analysis will be needed in future studies.
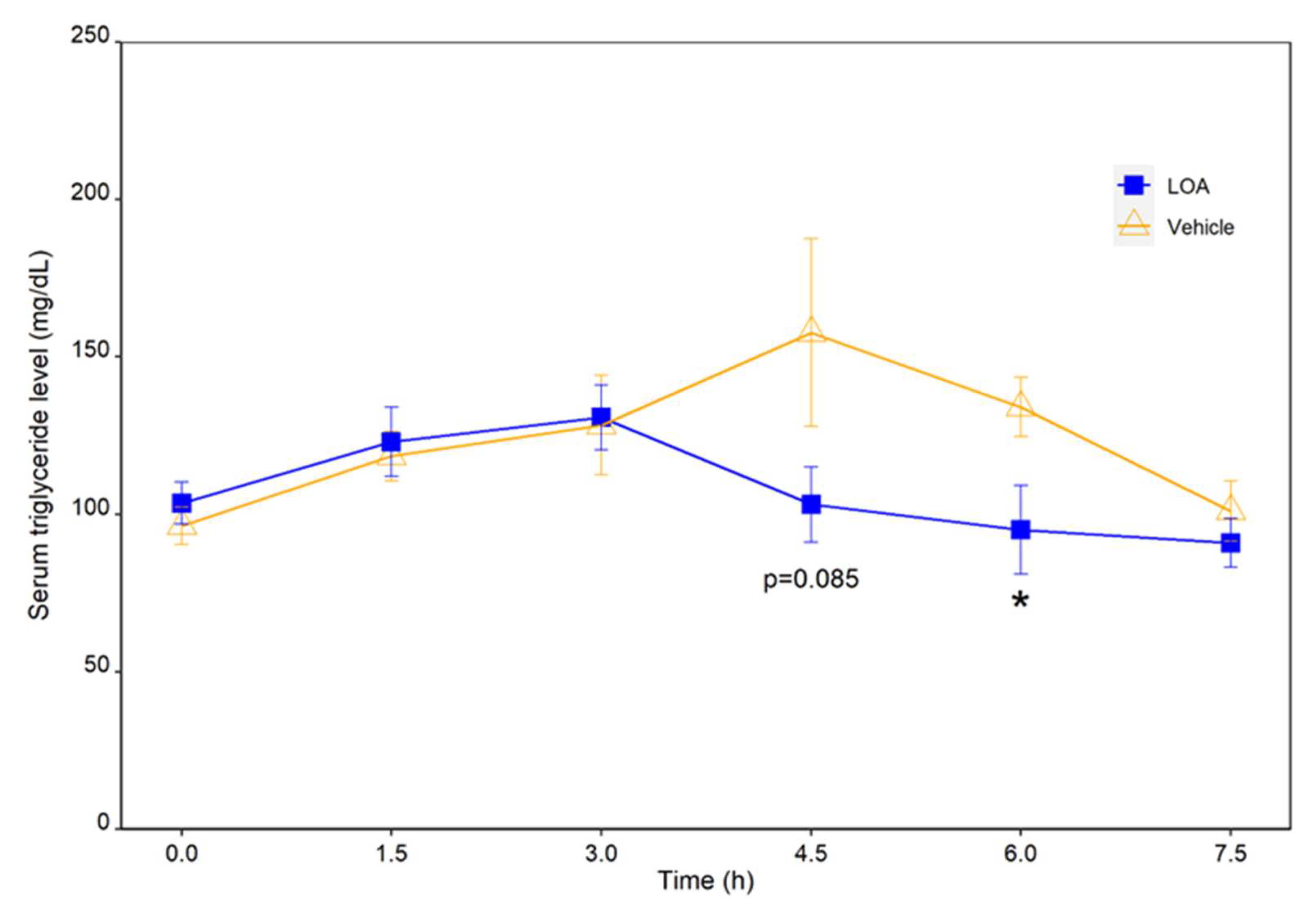
We then proceeded to verify the combined effectiveness of linoleic acid and oleic acid in vivo on the digestion and absorption of dietary TAGs by monitoring the postprandial TAG responses in rats to an oral administration of these fatty acids along with an HFD. This experiment is based on the well-established notion that effective intestinal absorption of TAGs depends upon their hydrolysis catalyzed by pancreatic lipase. As a result, an oral administration of a mixture of linoleic acid and oleic acid at a dose of 0.46 mg/kg and 0.72 mg/kg body weight significantly suppressed the postprandial TAG increase, and led to a faster restoration of TAG to the preprandial level in HFD-challenged rats, as compared to the vehicle-treated group. These results suggested that the linoleic-oleic acid mixture used in the present study was effective in reducing the absorption of TAGs into circulation following an oral fat load. This coincided with the finding that these fatty acids were effective pancreatic lipase inhibitors in vitro, and thus it is reasonable to speculate that these fatty acids attenuate postprandial triglyceridemia by targeting pancreatic lipase, the single most important determinant of lipid absorption. Moreover, for the individual free fatty acids, as well as for the cocktail of these free fatty acids, the profile of safe and effective doses and the dose–response relationship remain to be established in future research.
Linoleic acid and oleic acid are the two most abundant dietary plant-derived fatty acids, and they collectively constitute over 80% of the total fatty acids of sesame seeds [
18]. Oleic acid is also the single most common monounsaturated fatty acid (MUFA) in daily nutrition, whereas linoleic acid is an essential n-6 polyunsaturated fatty acid (PUFA) widely found in plant oil. Recently, oleic acid has attracted much attention owing to its effectiveness in regulating food intake, body mass, and energy metabolism by modulating multiple cellular pathways, as reviewed by Tutunchi et al. [
19]. Linoleic acid has been reported to reduce blood cholesterol and to play a vital role in preventing cardiovascular diseases [
7]. Previously, fatty acids were suggested to be one of the bioactive components of the pancreatic lipase inhibitory
Arachis hypogaea nutshell extract [
20]. However, in this study, the authors did not specify which fatty acids were involved, in which form (esterified or free) these fatty acids existed and exhibited their inhibitory activity, or the individual effects of each fatty acid. Later, linoleic acid and oleic acid, along with other fatty acids and phytochemical compounds, were suggested to contribute to the pancreatic lipase inhibitory activity of
Elateriospermum tapos seed [
21]. Recently, linoleic acid (along with palmitic acid and linolenic acid) was identified in a free fatty acid-enriched dichloromethane extract of
Mimosa diplotricha bee pollen, which exhibited potent porcine pancreatic lipase inhibitory activity in vitro (IC50 of 52.6 µg/mL). However, in these studies, the inhibitory activities of individual fatty acids were not evaluated or verified, and the in vivo effectiveness of these fatty acids or the bioactive extracts in reducing fat digestion and absorption remained uninvestigated [
22]. In this study, we demonstrated for the first time that linoleic acid and oleic acid were not only potent inhibitors of pancreatic lipase in vitro, but also effective in reducing intestinal absorption of dietary TAGs in vivo, adding a new facet to the health benefits of these fatty acids. Moreover, it is worth noting that fatty acids are predominantly present in their esterified forms in dietary fats, whereas, as reported herein as well as in [
22], the fatty acids exerted their inhibitory effects on the digestion of fats in their non-esterified or free forms. In line with this, as intestinal digestion of dietary TAGs releases free fatty acids, it is possible that pancreatic lipase encounters increasing levels of inhibition by its products. Such product inhibition might affect the dynamics of pancreatic lipase-catalyzed fat digestion, which warrants further investigation.
Through this study, we have widened the spectrum of pancreatic lipase inhibitors derived from natural sources to further include members of free mono- and poly- unsaturated fatty acids. In particular, we demonstrated that free linoleic acid and oleic acid were effective inhibitors of pancreatic lipase and modulators of fat digestion and absorption, and justified their potential use as regulators of energy intake in the management of overweight and obesity. Owing to their great abundance in food materials, not only do linoleic acid and oleic acid have minimal adverse effects in contrast to orlistat, but they are also readily incorporable into the diet and hence the effective dose is easily achievable. These advantages warrant further investigation on the effectiveness of free linoleic acid and oleic acid and the utilization of natural products rich in these fatty acids in reducing triglyceride absorption in rodent models and its translation to human research. Additionally, as free fatty acids have low solubility in water, nanotechnology formulations of these molecules may represent an effective strategy to facilitate their incorporation into aqueous matrices and to enhance their in vivo performance against the water-soluble pancreatic lipase.
4. Materials and Methods
4.1. Chemicals
All the chemicals used for this study were of analytical grade. Linoleic acid (18:2 cis-9,12), oleic acid (18:1 cis-9), anhydrous pyridine, ethanol, methanol, and formic acid were purchased from FUJIFILM Wako Pure Chemical Corporation (Kyoto, Japan). Acetonitrile was purchased from Nacalai Tesque (Kyoto, Japan).
4.2. Extraction and Fractionation of Sesame Meal
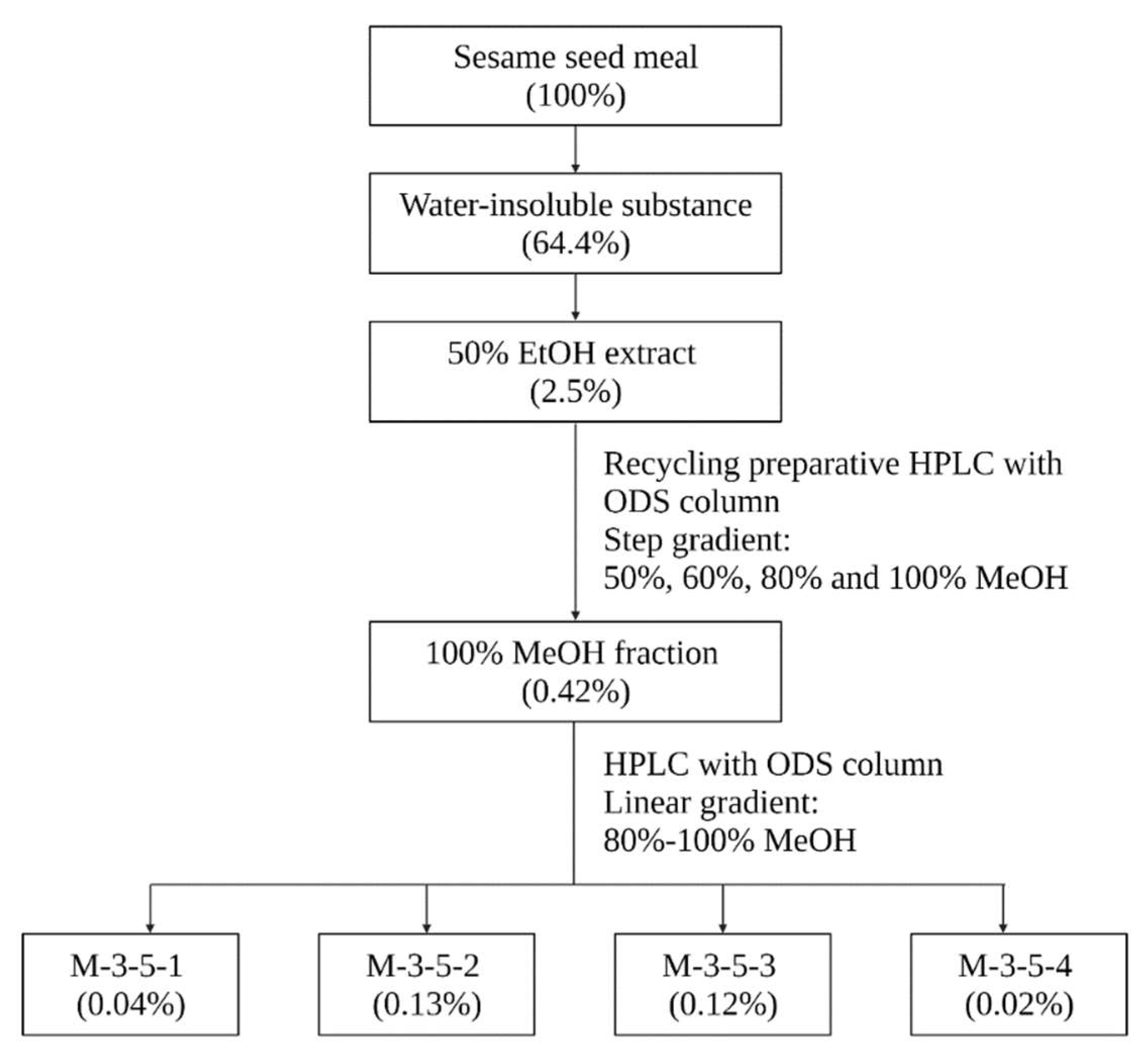
The sesame meal used in this study was a byproduct of sesame oil pressing, and was provided by Mitsui Sugar Co., Ltd. The sesame meal was firstly extracted four times with 10 volumes (v/w) of distilled water by stirring at room temperature for 1 h. The resulting solid residues were collected and lyophilized. The lyophilized powder was then extracted twice with 10 volumes (v/w) of 50% ethanol at 40 °C for 3 h. The soluble part was recovered and lyophilized after the solvent was reduced using a rotary evaporator. The 50% ethanol extract was further fractionated by using recycling preparative high-performance liquid chromatography (HPLC) with an octadecyl silica (ODS) column. In this regard, the 50% ethanol extract was redissolved in 10 times (v/w) of 50% ethanol at room temperature, aliquoted into 1.5 mL tubes, and centrifuged at 10,000 rpm for 3 min. The supernatant was then clarified with 0.45 μm filter, and 3 mL of the filtrate was applied to the recycling preparative HPLC (LC908, Japan Analytical Industry). The chromatographic separation was performed on a Biotage® Sfär C18D Duo column (100 Å, 30 μm, 30 g, 45 mL). UV detection was carried out at 195 nm. The filtrate was recycled and eluted stepwise at 7.5 mL/min with 50% methanol (7.9 column volume (CV), 1CV = 45 mL), 60% methanol (5CV), 80% methanol (5CV), and 100% methanol (5CV). The 100% methanol fraction, which exerted the highest inhibitory activity against pancreatic lipase, as revealed by the in vitro lipase inhibition assay, was clarified and further separated with a Biotage® Sfär C18D Duo column (100 Å, 30 μm, 12 g) in a Shimadzu HPLC system with a linear methanol gradient from 80% to 100% over 30 min at a constant flow rate of 2.5 mL/min. UV detection was carried out at 195 nm. These fractions were then subject to the lipase inhibition assay.
4.3. Lipase Inhibition Assay
Pancreatic lipase solution was prepared by vigorously mixing 30 mg of lipase from porcine pancreas Type II (Sigma) with 30 mL 0.1 M citrate buffer (pH 6.0) in an ice bath for 1 h. The mixture was centrifuged at 4 °C, 10,000 rpm for 45 min, and the supernatant was recovered and diluted to 0.06 mg/mL as the working enzyme solution.
The activity of the extracts and fractions of sesame meal, linoleic acid, and oleic acid against pancreatic lipase was evaluated by using the Lipase Kit S (SB Bioscience, Osaka, Japan) per the manufacturer’s instructions. Specifically, the lyophilized extracts and fractions were dissolved in DMSO and then diluted 10 times with distilled water. 0.1 ng/mL of orlistat (prepared in 10% (v/v) DMSO) was used as a positive control. For blank measurements, 10% (v/v) DMSO was used. 100 μL of the sample, 237 μL of the color-developing agent (5,5′-dithiobis-(2-nitrobenzoic acid), DTNB), 9 μL of the prepared pancreatic lipase solution, and 4 μL of the esterase inhibitor (phenylmethylsulfonyl fluoride, PMSF) were mixed and incubated at 30 °C for 5 min, followed by the addition of 25 μL of substrate solution (2,3-dimercapto-1-propanol-tributyrate, BLAB) and incubation in darkness for 30 min. The reaction was quenched by adding 700 μL of stop solution, and the absorbance was measured at 412 nm. Background absorbance caused by the reaction components was determined by incubating 100 μL of the sample with 237 μL of the color-developing agent, 9 μL of the prepared lipase solution, and 4 μL of the esterase inhibitor in darkness for 35 min, followed by the addition of 700 μL of stop solution and 25 μL of substrate solution. The absorbance at 412 nm was then measured.
The percentage inhibition of pancreatic lipase activity by each sample was calculated using the following equations:
Pancreatic lipase activity (%) = (Sample OD412 − Background OD412) × 100/(Blank OD412 − Blank of background OD412);
Percentage inhibition of pancreatic lipase activity (%) = 100 − Pancreatic lipase activity (%).
4.4. Identification of the Bioactive Constituents against Pancreatic Lipase
The bioactive constituents of sesame meal against pancreatic lipase were identified by using gas chromatography-mass spectrometry (GC-MS) and HPLC, and by fragmentation pattern and retention time matching with the reference compounds.
For GC-MS analysis, 0.1 mg of the sample was dissolved in 20 μL of pyridine, and 1 μL was injected into a Shimadzu GCMS-QP2010 Plus system with a UA-5 (MS/HT) column (Ultra ALLOY) at an inlet split ratio of 1:50. Helium was used as the carrier gas, and the flow rate was 1.0 mL/min. The column temperature was programed to increase from 80 °C at 10 °C/min to 330 °C with a hold time of 5 min. A mass range of 50 m/z–1000 m/z was scanned.
HPLC analysis was performed on a Shimadzu HPLC system with a YMC-Pack ODS-A column (12 nm, 5 µm, 250 mm × 4.6 mm). A binary gradient elution was employed, i.e., 0.1% (v/v) formic acid aqueous solution and acetonitrile with 0.1% (v/v) formic acid, with the proportion of 0.1% formic acid increasing from 1% to 100% in 60 min at a flow rate of 0.8 mL/min and under 40 °C. UV detection was carried out at 195 nm.
4.5. Animal Experiments
All procedures involving experimental animals were performed in accordance with the “Basic guidelines for the conduct of animal experiments in implementing agencies under the jurisdiction of the ministry of health, labor and welfare”, and followed the protocols approved by the Committee on Animal Research and Ethics of Pharma Foods International Co., Ltd. (19KRD-008).
Six-week-old, male Wistar rats were purchased from Japan SLC, Inc. All rats were singly caged at 23 °C and 55% humidity with ad libitum access to water and a standard rodent diet (MF laboratory chow, Oriental Yeast Co., Tokyo, Japan) for the first 5 days following delivery to allow acclimation to the new environment. The rats were maintained on a 12:12-h light–dark cycle throughout the study. After acclimation, the rats were divided into two diet groups (7 rats per group; groups matched for body weight) and fasted overnight (16 h) prior to the HFD challenge.
The HFD used in the present study was a corn-oil-based lipid emulsion, which was prepared by ultrasonically emulsifying 30 mL of corn oil, 400 mg of cholic acid, 10 g of cholesterol oleate, and 30 mL of water.
Linoleic acid and oleic acid were dissolved in 400 μL of 20% (v/v) ethanol (vehicle), and administrated at a dose of 0.46 mg/kg and 0.72 mg/kg body weight, respectively. The LOA or the vehicle was administered to rats via gastrostomy tubes 15 min prior to the administration of the lipid emulsion (0 h). Blood samples were obtained from the tail vein at 0 h, 1.5 h, 3 h, 4.5 h, 6 h, and 7.5 h. Blood samples were centrifuged at 2500 rpm for 10 min, and the sera were stored at −20 °C until analysis. The quantification of TAGs in the serum was performed by using a LabAssayTM Triglyceride Kit (Wako) per the manufacturer’s instructions.
4.6. Statistical Analysis
Data are presented as mean ± standard error (S.E.), unless indicated otherwise. A two-tailed, Welch’s t-test was used to test for between-group differences. One-way ANOVA with Tukey’s HSD (honestly significant difference) tests were used for multiple comparisons. Differences at a probability level (p-value) of 0.05 were considered statistically significant.