Fabrication and Characterization of Konjac Glucomannan/Oat β-Glucan Composite Hydrogel: Microstructure, Physicochemical Properties and Gelation Mechanism Studies
Abstract
1. Introduction
2. Results
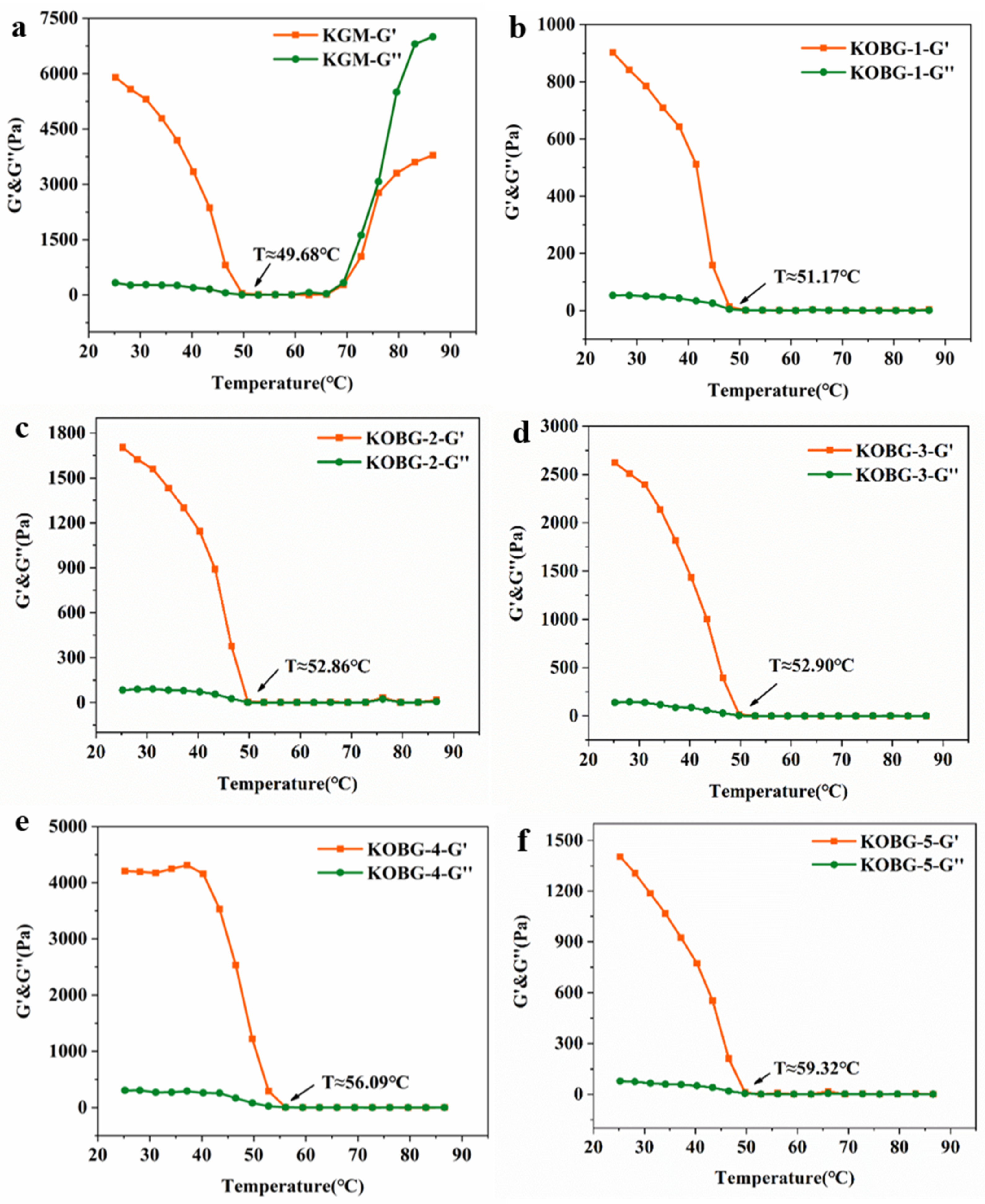
2.1. Gel Evolution Process
2.1.1. Temperature Sweep
2.1.2. Frequency Sweep
2.2. Gelling Force
2.2.1. Interaction Force Test
2.2.2. Fourier Transform Infrared Spectroscopy Analysis (FT-IR)
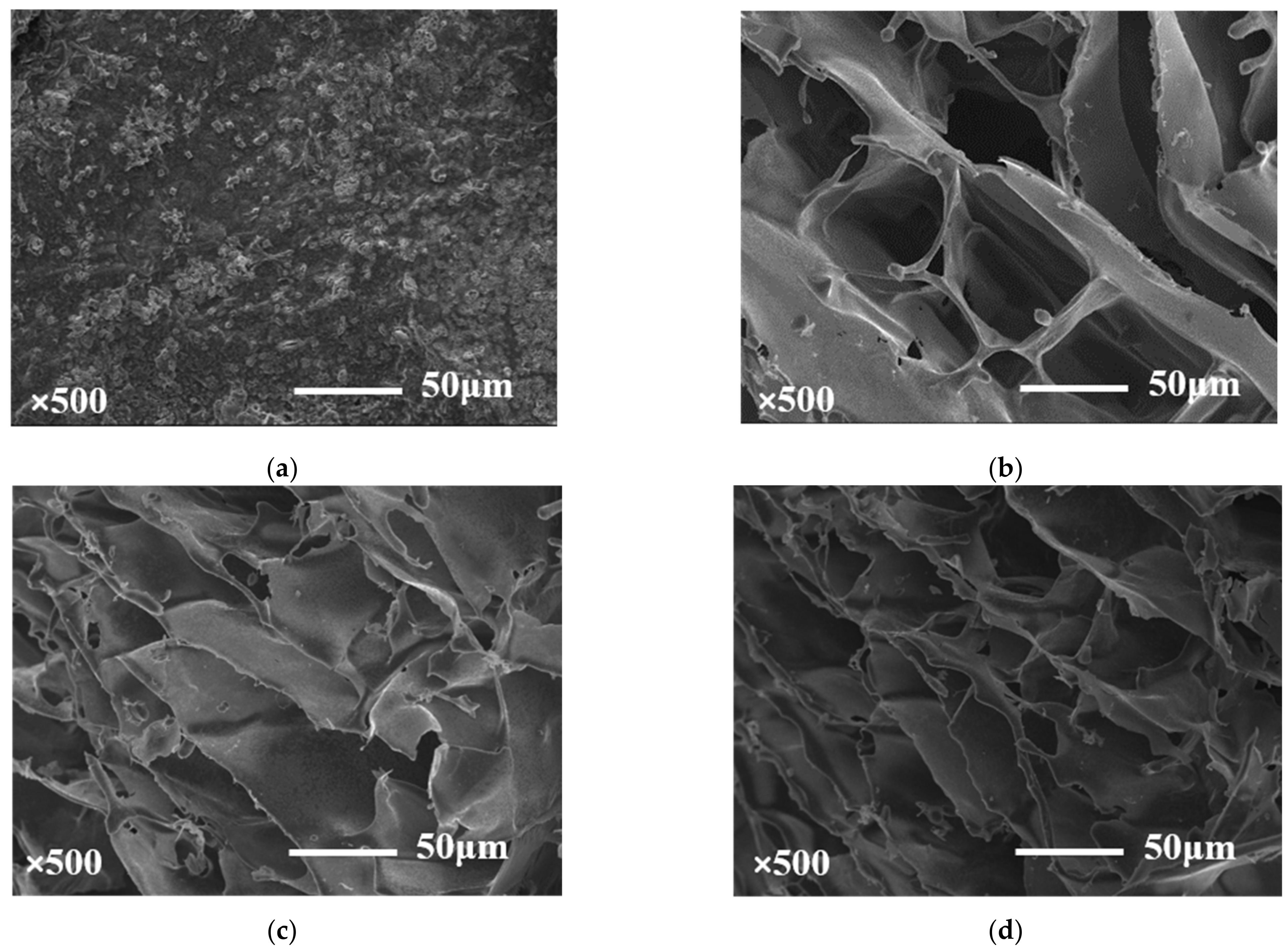
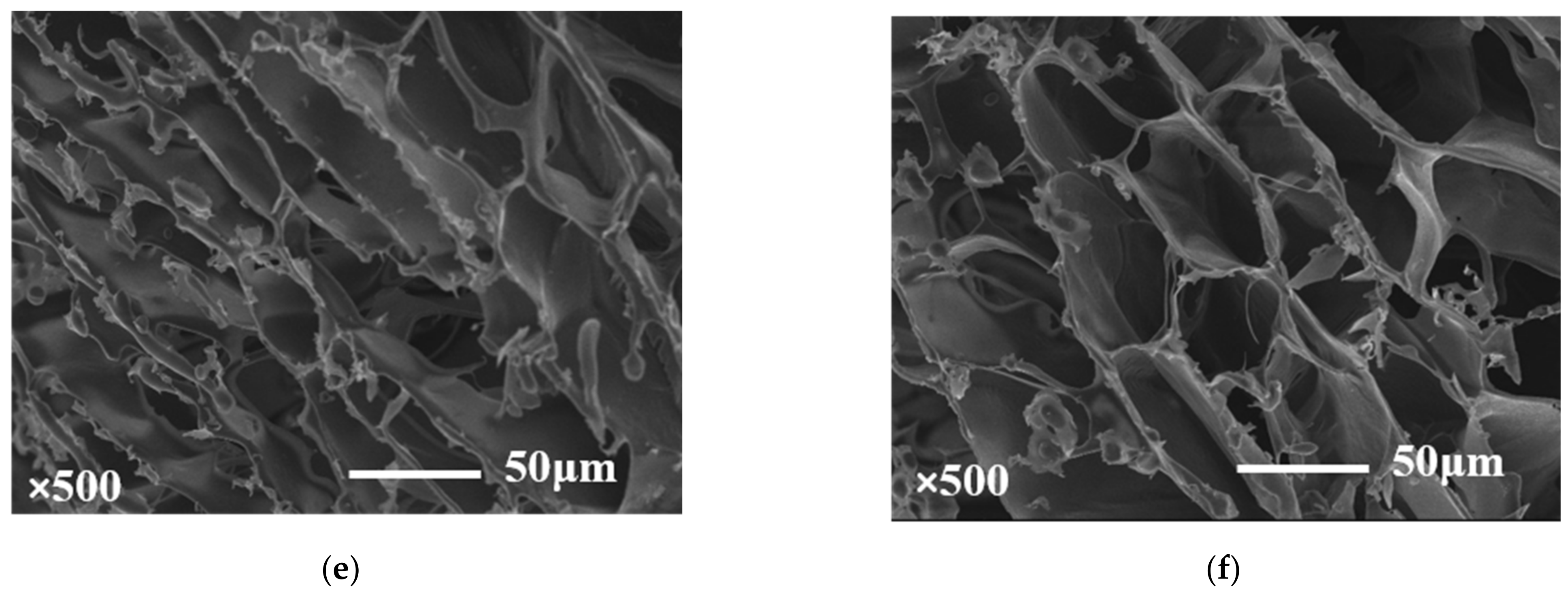
2.3. Scanning Electron Microscopy Observations (SEM)
2.4. Physicochemical Properties
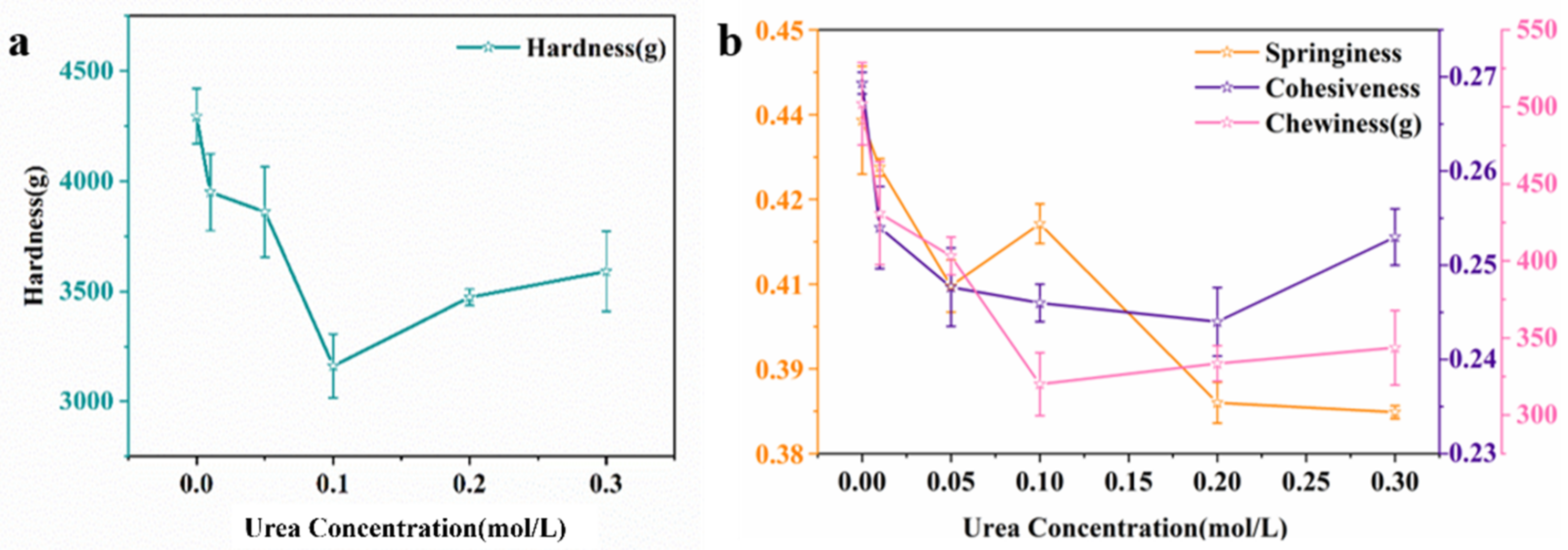
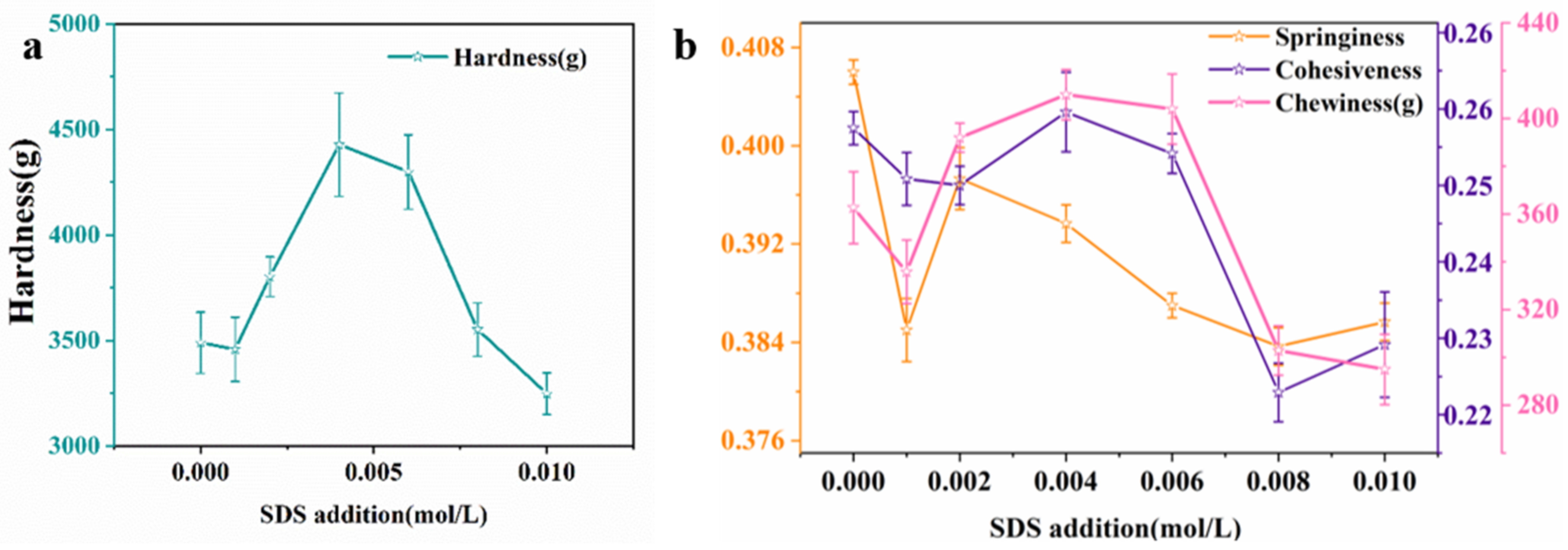
2.4.1. Textural Profile Analysis (TPA)
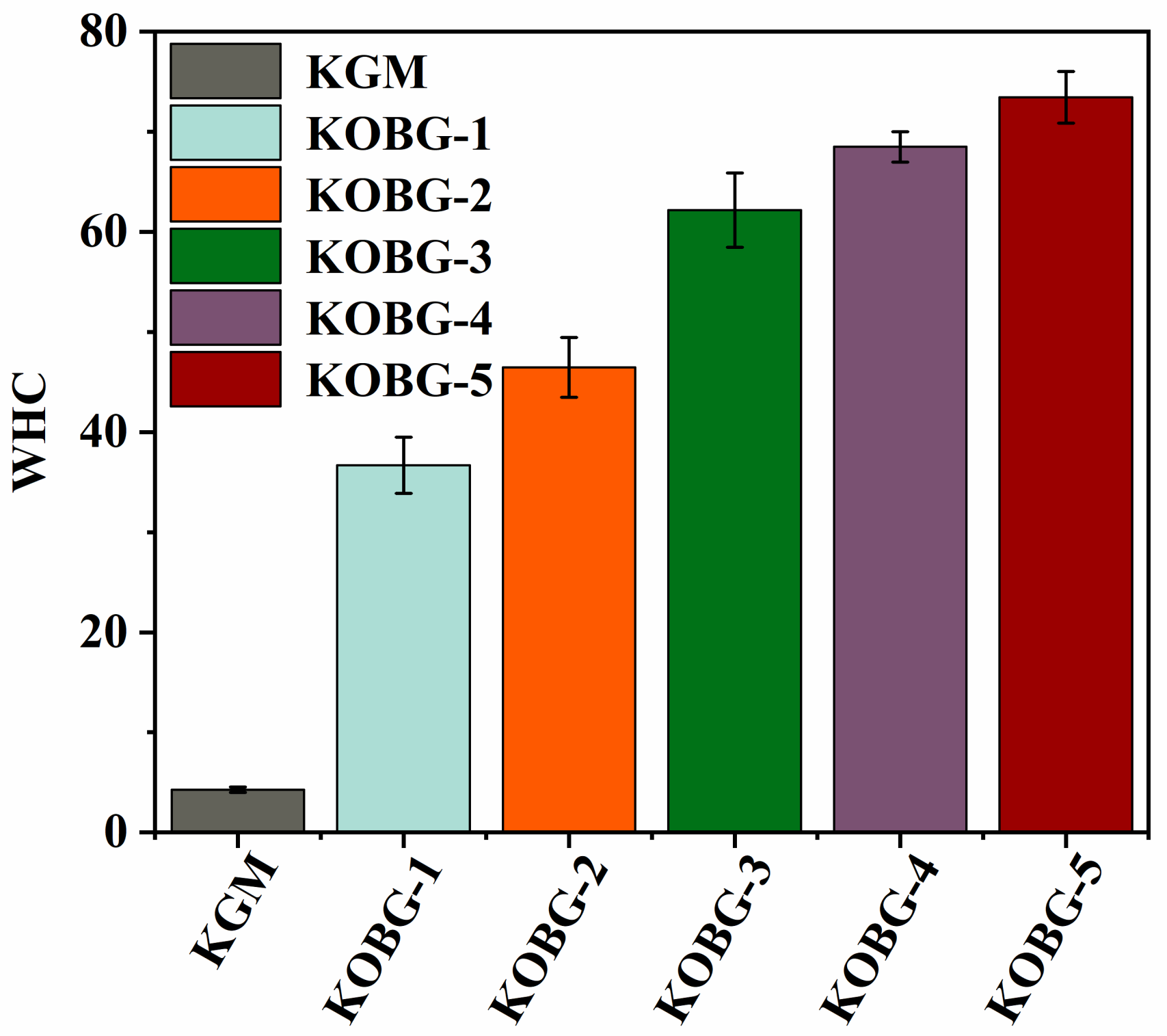
2.4.2. Water-Holding Capacity (WHC) of Composite Hydrogel
2.4.3. Thermogravimetric Analysis (TGA)
3. Materials and Methods
3.1. Materials
3.2. Fabrication of KGM/Oat β-Glucan Composite Hydrogel
3.3. Monitoring of Gel Evolution Process
3.3.1. Linear Viscoelastic Region Measurement (LVR)
3.3.2. Temperature Sweep
3.3.3. Frequency Sweep
3.4. Gelling Force
3.4.1. Interaction Force Test
3.4.2. Fourier Transform Infrared Spectroscopy Analysis (FT-IR)
3.5. Scanning Electron Microscopy Observations (SEM)
3.6. Physicochemical Properties
3.6.1. Textural Profile Analysis (TPA)
3.6.2. Water-Holding Capacity (WHC)
3.6.3. Thermogravimetric Analysis (TGA)
3.7. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Roig-Sanchez, S.; Kam, D.; Malandain, N.; Sachyani-Keneth, E.; Shoseyov, O.; Magdassi, S.; Laromaine, A.; Roig, A. One-step double network hydrogels of photocurable monomers and bacterial cellulose fibers. Carbohydr. Polym. 2022, 294, 119778. [Google Scholar] [CrossRef]
- Ho, T.-C.; Chang, C.-C.; Chan, H.-P.; Chung, T.-W.; Shu, C.-W.; Chuang, K.-P.; Duh, T.-H.; Yang, M.-H.; Tyan, Y.-C. Hydrogels: Properties and Applications in Biomedicine. Molecules 2022, 27, 2902. [Google Scholar] [CrossRef]
- Kalia, S.; Roy Choudhury, A. Synthesis and rheological studies of a novel composite hydrogel of xanthan, gellan and pullulan. Int. J. Biol. Macromol. 2019, 137, 475–482. [Google Scholar] [CrossRef]
- Zhu, Q.; Han, K.; Wang, S.; Muhindo, E.M.; Wei, W.; Li, J.; Wu, T.; Fersht, V.; Zhang, M. Design and structural characterization of edible double network gels based on wheat bran arabinoxylan and pea protein isolate. Int. J. Biol. Macromol. 2022, 213, 824–833. [Google Scholar] [CrossRef] [PubMed]
- Zhu, B.; Xin, C.; Li, J.; Li, B. Ultrasonic Degradation of Konjac Glucomannan and the Effect of Freezing Combined with Alkali Treatment on Their Rheological Profiles. Molecules 2019, 24, 1860. [Google Scholar] [CrossRef]
- Xu, Q.; Huang, W.; Jiang, L.; Lei, Z.; Li, X.; Deng, H. KGM and PMAA based pH-sensitive interpenetrating polymer network hydrogel for controlled drug release. Carbohydr. Polym. 2013, 97, 565–570. [Google Scholar] [CrossRef]
- Behera, S.S.; Ray, R.C. Konjac glucomannan, a promising polysaccharide of Amorphophallus konjac K. Koch in health care. Int. J. Biol. Macromol. 2016, 92, 942–956. [Google Scholar] [CrossRef]
- Zhou, Y.; Cao, H.; Hou, M.; Nirasawa, S.; Tatsumi, E.; Foster, T.J.; Cheng, Y. Effect of konjac glucomannan on physical and sensory properties of noodles made from low-protein wheat flour. Food Res. Int. 2013, 51, 879–885. [Google Scholar] [CrossRef]
- Li, Z.; Zhang, L.; Mao, C.; Song, Z.; Li, X.; Liu, C. Preparation and characterization of konjac glucomannan and gum arabic composite gel. Int. J. Biol. Macromol. 2021, 183, 2121–2130. [Google Scholar] [CrossRef]
- Liu, Z.; Ren, X.; Cheng, Y.; Zhao, G.; Zhou, Y. Gelation mechanism of alkali induced heat-set konjac glucomannan gel. Trends Food Sci. Technol. 2021, 116, 244–254. [Google Scholar] [CrossRef]
- Kim, D.H.; Shin, D.M.; Seo, H.G.; Han, S.G. Effects of konjac gel with vegetable powders as fat replacers in frankfurter-type sausage. Asian-Australas. J. Anim. Sci. 2019, 32, 1195–1204. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; Huang, X.; Wang, H.; Geng, F.; Nie, S. Effect of β-glucan on metabolic diseases: A review from the gut microbiota perspective. Curr. Opin. Food Sci. 2022, 47, 100907. [Google Scholar] [CrossRef]
- Mäkelä, N.; Rosa-Sibakov, N.; Wang, Y.-J.; Mattila, O.; Nordlund, E.; Sontag-Strohm, T. Role of β-glucan content, molecular weight and phytate in the bile acid binding of oat β-glucan. Food Chem. 2021, 358, 129917. [Google Scholar] [CrossRef]
- Shah, A.; Gani, A.; Masoodi, F.A.; Wani, S.M.; Ashwar, B.A. Structural, rheological and nutraceutical potential of β-glucan from barley and oat. Bioact. Carbohydr. Diet. Fibre 2017, 10, 10–16. [Google Scholar] [CrossRef]
- Lazaridou, A.; Vaikousi, H.; Biliaderis, C.G. Impact of mixed-linkage (1 → 3, 1 → 4) β-glucans on physical properties of acid-set skim milk gels. Int. Dairy J. 2008, 18, 312–322. [Google Scholar] [CrossRef]
- Yang, X.; Li, A.; Li, D.; Li, X.; Li, P.; Sun, L.; Guo, Y. Improved physical properties of konjac glucomannan gels by co-incubating composite konjac glucomannan/xanthan systems under alkaline conditions. Food Hydrocoll. 2020, 106, 105870. [Google Scholar] [CrossRef]
- Gong, J.; Wang, L.; Wu, J.; Yuan, Y.; Mu, R.-J.; Du, Y.; Wu, C.; Pang, J. The rheological and physicochemical properties of a novel thermosensitive hydrogel based on konjac glucomannan/gum tragacanth. LWT 2019, 100, 271–277. [Google Scholar] [CrossRef]
- Yuan, C.; Zou, Y.; Cui, B.; Fang, Y.; Lu, L.; Xu, D. Influence of cyclodextrins on the gelation behavior of κ-carrageenan/konjac glucomannan composite gel. Food Hydrocoll. 2021, 120, 106927. [Google Scholar] [CrossRef]
- Zhou, P.; Liu, X.; Labuza, T.P. Moisture-Induced Aggregation of Whey Proteins in a Protein/Buffer Model System. J. Agric. Food Chem. 2008, 56, 2048–2054. [Google Scholar] [CrossRef]
- Yang, X.; Yuan, K.; Zhang, H.; Luo, S.; Wang, S.; Yang, X.; Guo, Y. Regulation on gel textures of Nicandra physalodes (Linn.) Gaertn. pectin by its synergistic interaction with sodium alginate. Food Hydrocoll. 2023, 134, 108047. [Google Scholar] [CrossRef]
- Xu, C.; Lv, J.; Lo, Y.M.; Cui, S.W.; Hu, X.; Fan, M. Effects of oat β-glucan on endurance exercise and its anti-fatigue properties in trained rats. Carbohydr. Polym. 2013, 92, 1159–1165. [Google Scholar] [CrossRef]
- Wang, J.; Liu, C.; Shuai, Y.; Cui, X.; Nie, L. Controlled release of anticancer drug using graphene oxide as a drug-binding effector in konjac glucomannan/sodium alginate hydrogels. Colloids Surf. B Biointerfaces 2014, 113, 223–229. [Google Scholar] [CrossRef]
- Su, Y.; Zhang, M.; Chang, C.; Li, J.; Sun, Y.; Cai, Y.; Xiong, W.; Gu, L.; Yang, Y. The effect of citric-acid treatment on the physicochemical and gel properties of konjac glucomannan from Amorphophallus bulbifer. Int. J. Biol. Macromol. 2022, 216, 95–104. [Google Scholar] [CrossRef]
- Zhang, T.; de Vries, R.; Xu, X.; Xue, Y.; Xue, C. Microstructural changes during alkali- and heat induced gelation of konjac glucomannan. Food Hydrocoll. 2021, 114, 106552. [Google Scholar] [CrossRef]
- Moschakis, T.; Lazaridou, A.; Biliaderis, C.G. Using particle tracking to probe the local dynamics of barley β-glucan solutions upon gelation. J. Colloid Interface Sci. 2012, 375, 50–59. [Google Scholar] [CrossRef]
- Jiang, S.; Shang, L.; Liang, H.; Li, B.; Li, J. Preparation of konjac glucomannan/xanthan gum/sodium alginate composite gel by freezing combining moisture regulation. Food Hydrocoll. 2022, 127, 107499. [Google Scholar] [CrossRef]
- Wang, L.-X.; Dao, L.-P.; Guo, Q.-Y.; Chen, T.-L.; Fu, L.-J.; Zhou, F.-C.; Yuan, Y. Investigation on the influence of AC electric filed and KCl on the structure and gel properties of Konjac glucomannan. Food Chem. 2022, 386, 132755. [Google Scholar] [CrossRef]
- Wu, H.; Wu, H.; Qing, Y.; Wu, C.; Pang, J. KGM/chitosan bio-nanocomposite films reinforced with ZNPs: Colloidal, physical, mechanical and structural attributes. Food Packag. Shelf Life 2022, 33, 100870. [Google Scholar] [CrossRef]
- Ju, Q.; Wu, C.; Yuan, Y.; Hu, Y.; Zhou, S.; Luan, G. Insights into the mechanism on Glucono-delta-lactone induced gelation of soybean protein at subunit level. Food Hydrocoll. 2022, 125, 107402. [Google Scholar] [CrossRef]
- Cortez-Trejo, M.C.; Loarca-Piña, G.; Figueroa-Cárdenas, J.D.; Manríquez, J.; Mendoza, S. Gel properties of acid-induced gels obtained at room temperature and based on common bean proteins and xanthan gum. Food Hydrocoll. 2022, 132, 107873. [Google Scholar] [CrossRef]



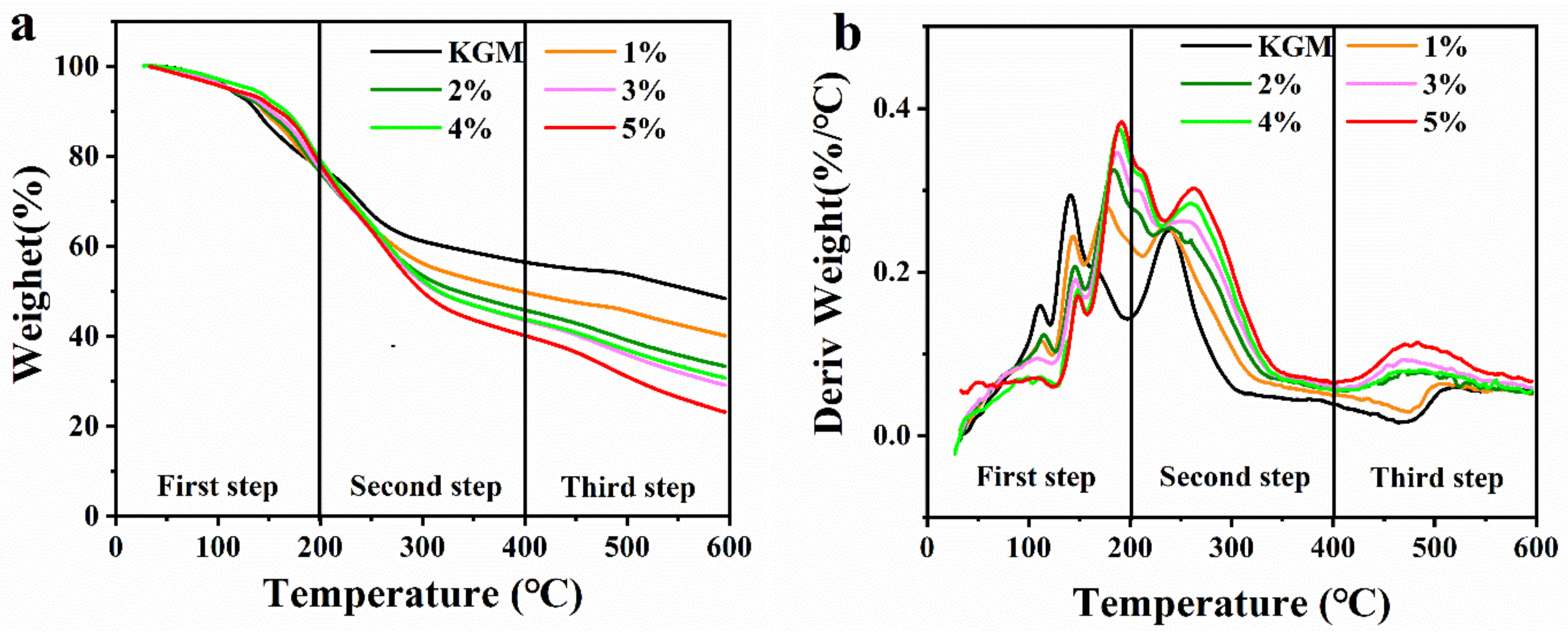
| Samples | First-Step Weight Loss (%) | Second-Step Weight Loss (%) | Third-Step Weight Loss (%) | Tmax (°C) |
|---|---|---|---|---|
| KGM gel | 22.51 | 20.94 | 8.09 | 238.75 |
| KOBG-1 | 22.51 | 27.74 | 9.55 | 239.13 |
| KOBG-2 | 22.51 | 31.50 | 12.39 | 240.37 |
| KOBG-3 | 22.51 | 33.76 | 12.80 | 244.73 |
| KOBG-4 | 22.51 | 33.78 | 14.35 | 259.49 |
| KOBG-5 | 22.51 | 37.43 | 16.75 | 261.86 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Geng, X.; Zhao, N.; Song, X.; Wu, J.; Zhu, Q.; Wu, T.; Chen, H.; Zhang, M. Fabrication and Characterization of Konjac Glucomannan/Oat β-Glucan Composite Hydrogel: Microstructure, Physicochemical Properties and Gelation Mechanism Studies. Molecules 2022, 27, 8494. https://doi.org/10.3390/molecules27238494
Geng X, Zhao N, Song X, Wu J, Zhu Q, Wu T, Chen H, Zhang M. Fabrication and Characterization of Konjac Glucomannan/Oat β-Glucan Composite Hydrogel: Microstructure, Physicochemical Properties and Gelation Mechanism Studies. Molecules. 2022; 27(23):8494. https://doi.org/10.3390/molecules27238494
Chicago/Turabian StyleGeng, Xiaoyuan, Nuo Zhao, Xiwang Song, Jianfu Wu, Qiaomei Zhu, Tao Wu, Haixia Chen, and Min Zhang. 2022. "Fabrication and Characterization of Konjac Glucomannan/Oat β-Glucan Composite Hydrogel: Microstructure, Physicochemical Properties and Gelation Mechanism Studies" Molecules 27, no. 23: 8494. https://doi.org/10.3390/molecules27238494
APA StyleGeng, X., Zhao, N., Song, X., Wu, J., Zhu, Q., Wu, T., Chen, H., & Zhang, M. (2022). Fabrication and Characterization of Konjac Glucomannan/Oat β-Glucan Composite Hydrogel: Microstructure, Physicochemical Properties and Gelation Mechanism Studies. Molecules, 27(23), 8494. https://doi.org/10.3390/molecules27238494











